Abstract
Parasites and parasitic lifestyles have evolved from free-living organisms multiple times. How such a key evolutionary transition occurred remains puzzling. Facultative parasites represent potential transitional states between free-living and fully parasitic lifestyles because they can be either free-living or parasitic depending on environmental conditions. We suggest that facultative parasites with phenotypically plastic life-history strategies may serve as evolutionary stepping-stones towards obligate parasitism. Pre-adaptations provide a starting point for the transition towards opportunistic or facultative parasitism, but what evolutionary mechanism underlies the transition from facultative to obligate parasitism? In this Opinion Piece, we outline how facultative parasites could evolve towards obligate parasites via genetic assimilation, either alone or in combination with the Baldwin effect. We further describe the key predictions stemming from each of these evolutionary pathways. The importance of genetic assimilation in evolution has been hotly debated. Studies on facultative parasites may not only provide key insights regarding the evolution of parasitism, but also provide ideal systems in which to test evolutionary theory on genetic accommodation.
Keywords: evolution of parasitism, genetic assimilation, genetic accommodation, Baldwin effect, opportunistic pathogens, phenotypic plasticity
1. Introduction
Parasitism has evolved independently over 200 times [1], yet the processes leading to its evolution remain elusive [2]. In this Opinion Piece, we point out that current hypotheses for the evolution of obligate parasitism are incomplete. Pre-adaptations create opportunities for facultative parasitism, but do not account for the transition from facultative to obligate parasitism. Here, we develop hypotheses for the evolution of obligate parasites from facultative parasites based on genetic assimilation. We propose that facultative parasites provide excellent model systems to study the evolution of parasitism, and, more generally, to test evolutionary theory of genetic assimilation.
2. Current hypotheses for the evolution of parasitism
Several hypotheses have been proposed for the evolutionary transition to a parasitic lifestyle, including those arising from fungal associations, predator–prey interactions, close co-habitation and ‘pre-adaptation’ [3]. Although these hypotheses are not mutually exclusive, ‘pre-adaptation’ is the most commonly cited pathway for the evolution of parasitism among multicellular parasites, the primary focus of this article. The ‘pre-adaptation’ hypothesis posits that adaptations for free-living lifestyles can facilitate the evolution of parasitism because these traits may also permit new functions in a novel environment. Incipient parasites that possess the pre-adaptations needed to opportunistically locate, obtain nutrients from, survive and reproduce on a host would be favoured by natural selection, assuming that the fitness gains are greater for the exploiter than for strictly free-living conspecifics.
While numerous examples fall within the ‘pre-adaptation’ framework, we focus here on two of the most commonly studied pre-adaptations for parasitism: phoresy and the formation of dauer larvae [4,5]. Phoresy is a symbiotic association between two organisms in which one (the phoront) is transported by another for the purpose of dispersal. Initially, a phoretic organism uses the carrier for dispersal only and does not feed on it. Necromeny, a type of phoresy, occurs when the phoront waits for the carrier to die from other causes before feeding on the bacteria and other microbes that grow on the decaying carcass [4]. Such intermediate associations with other organisms favour suites of adaptations that could eventually be co-opted for life as a parasite (i.e. pre-adaptations). For example, sensory systems initially used to locate hosts for dispersal may be pre-adapted to locate hosts for parasitic interactions [6], and tolerance for high toxicity or low oxygen environments that allow phoretic animals to feed on dead and decaying matter could be pre-adaptations to combat the immune systems and digestive enzymes of hosts, as well as the anaerobic conditions within host intestines [5].
Another hypothesized pathway to parasitism based on pre-adaptation is the ‘dauer hypothesis’ [4]. Dauers are the arrested third-stage larvae of nematodes that form in response to environmental stress. The formation of dauer larvae may serve as a pre-adaptation to parasitism; mechanisms involved in dauer development are later co-opted by incipient parasites to form infective juveniles. Among extant free-living and facultatively parasitic species, the dauer larvae are conditionally expressed, and the formation of the dauer stage involves similar physiological changes that respond to a similar set of chemical cues [7].
Our thesis assumes that the ancestral phenotype is free-living, an assumption that is well supported by phylogenetic analyses [3]. Under certain ecological conditions, a previously free-living organism exhibits a novel phenotype and opportunistically exploits a host. Pre-adaptations may make such novel inductions more likely, but the initial production of the parasitic phenotype requires particular environmental conditions. Indeed, this is the case for facultative parasites. Facultative parasites can complete their life cycle without a host, but may exploit hosts (i.e. become parasitic) under certain conditions. In facultatively parasitic nematodes, infective juveniles (analogous to dauer larvae) are formed under stressful conditions (e.g. high nematode density, low food availability and heat stress), but these nematodes can also complete their life cycle without a host under favourable conditions [8,9]. In other types of facultative parasites (e.g. mites), environmental conditions dictating whether organisms will behave parasitically include host condition (e.g. reproductive status and injury), host availability and parasite condition (e.g. level of starvation) [10]. The conditions favouring the evolution of facultative parasitism appear to be met frequently, as facultative parasitism has been reported in a range of taxonomic groups [9–13].
Explanations based on pre-adaptations address the processes underlying the evolutionary changes that move a lineage from free-living towards facultative (or conditional) parasitism. But what drives the evolutionary transition from facultative to obligate parasite? Below, we outline how a specific type of genetic accommodation, called genetic assimilation, may underlie this evolutionary change.
3. From facultative to obligate parasitism via genetic accommodation
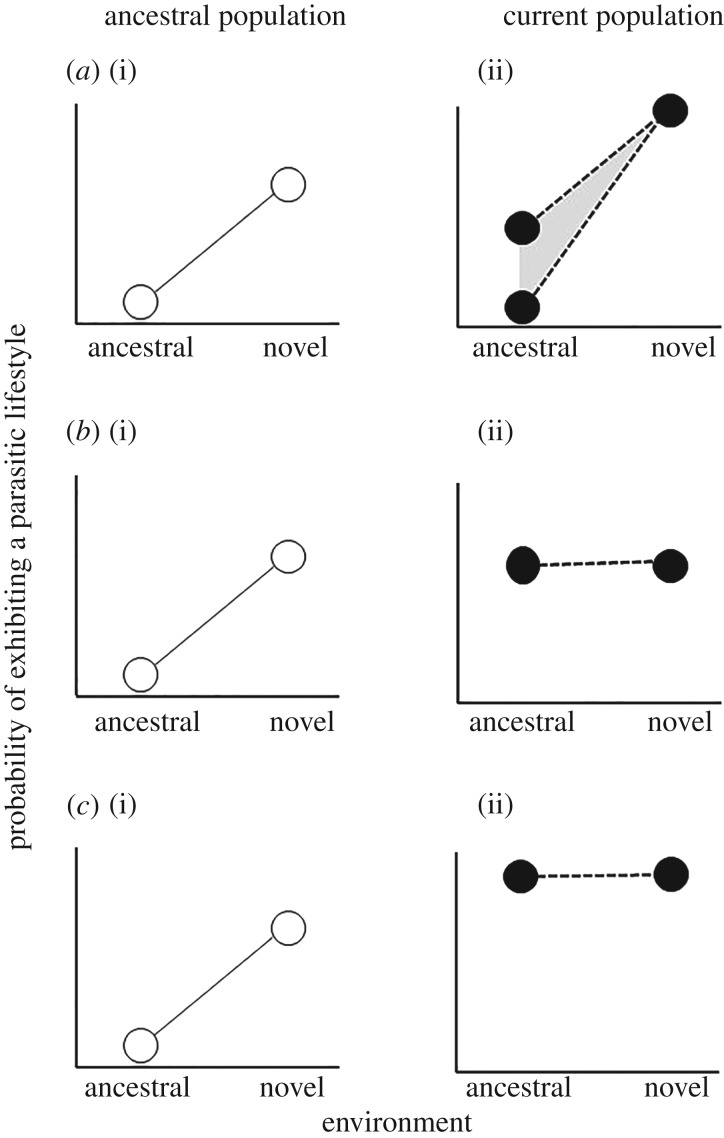
Genetic accommodation is a process in which novel phenotypic induction results in heritable changes [14]. Two related, but distinct, evolutionary theories have been proposed that invoke genetic accommodation: the Baldwin effect and genetic assimilation. The Baldwin effect posits that if phenotypic plasticity increases survival under novel environmental conditions, it will provide the opportunity for natural selection to act on heritable variations that arise later on (figure 1a) [15].
Figure 1.
Illustration of three scenarios of genetic accommodation in facultative parasites. (a(i), b(i) and c(i)) Ancestral, free-living populations that exhibit an increased probability of exhibiting a parasitic lifestyle when exposed to novel environmental conditions. (a(ii), b(ii) and c(ii)) Current populations following genetic accommodation. (a) The Baldwin effect. Expected phenotypes for the population if phenotypic plasticity exhibited in the ancestral population was adaptive, but was less than the optimal level of parasitism. Plasticity provided the opportunity for natural selection to act on heritable variants such that the mean phenotypic value further increased in the novel environment. Whether selection for higher trait expression in the novel environment is associated with concurrent changes in the mean trait expression in the ancestral environment will depend on the extent of genetic correlation between intercept and slope (illustrated by the grey region). Note that the Baldwin effect alone (shown in c(ii)) would not lead to complete loss of the free-living lifestyle. (b) Genetic assimilation. Plasticity exhibited by the ancestral population produced the optimal phenotype in the novel environment, in this case, 100% chance of adopting a parasitic lifestyle. Selection then acted to reduce phenotypic plasticity, such that the novel trait (parasitism) no longer requires the novel environmental cue to induce its expression. The current population is exclusively parasitic across all environments (i.e. obligate parasites). (c) Genetic assimilation and the Baldwin effect. The evolution of obligate parasitism occurred via selection on mean propensity to behave parasitically in the novel environment, coincident with selection for reduced plasticity.
By contrast, genetic assimilation is a process through which environmentally induced phenotypic variation ceases to require the environmental signal for its expression [15,16]. Novel environmental conditions induce novel phenotypes via previously unexpressed phenotypic plasticity. The capacity for phenotypic plasticity was preexistent, but unexpressed, because the environmental conditions had not previously been encountered. However, this pre-existing capacity for phenotypic plasticity allows organisms to persist in the novel environmental conditions. Subsequent selection in the novel environment leads to the novel phenotype becoming genetically fixed (i.e. assimilated) through a loss in phenotypic plasticity (figure 1b).
We propose that genetic assimilation may provide an evolutionary mechanism underlying the transition from facultative to obligate parasitism. Once facultative parasitism arises, the evolutionary transition to obligate parasite may occur via genetic assimilation (figure 1b), or a combination of the Baldwin effect and genetic assimilation (figure 1c). Genetic assimilation requires that phenotypic plasticity has the capacity to evolve. That is, a genotype by the environment (G × E) interaction must be present for the propensity for facultative parasites to adopt a parasitic lifestyle [17,18]. Several experimental studies have demonstrated heritable variation in reaction norms for the propensity to follow an infective developmental path (i.e. parasitic lifestyle), including studies documenting responses to selection [8,19,20]. Second, given heritable variation in phenotypic plasticity, genetic assimilation of parasitic behaviour requires that conditions normally required to induce parasitic phenotypes persist over multiple generations. Although this would not be the case for all known triggers of condition-dependent expression of parasitic behaviour (e.g. host condition or condition of the facultative parasite), temporal auto-correlation may be expected for others, including conspecific density, food availability and host availability.
Finally, for genetic assimilation to underlie the transition from facultative to obligate parasite, plasticity must be either costly to maintain or maladaptive [15]. Empirical demonstrations of the cost of plasticity are rare, and the generality of such costs have been heavily debated [21]. Thus, the loss of plasticity may be more likely to arise via mutation accumulation in parts of the genome required for the expression of a free-living lifestyle rather than selection for reduced plasticity per se [21]. Indeed, the evolutionary transition from free-living to parasitic is typically associated with the loss of key functions required for a free-living state [22,23].
Alternatively, if the Baldwin effect occurs coincident with sustained selection (i.e. constant exposure to the novel environment across generations) for expression of the parasitic form, changing trait means would produce phenotypes that are better adapted for a parasitic lifestyle. This would increase the propensity to express the parasitic phenotype. Furthermore, if the adaptations for parasitism resulted in the parasitic phenotype achieving higher fitness than the free-living phenotype under a wider array of conditions, this could make plasticity maladaptive and result in selection for reduced plasticity (i.e. genetic assimilation).
4. Case studies and future directions
Comparative studies can be used to test our hypothesis that facultative parasites are an evolutionary stepping-stone towards obligate parasitism. Specifically, we predict that in clades that contain free-living, facultative and obligate parasites, facultative parasites will be ancestral to obligate parasites. Mites and nematodes fulfil this criterion, as parasitic lifestyles have evolved independently in both lineages multiple times [24,25]. Free-living predatory mites are thought to be the ancestral form, giving rise to obligate parasitism via some transitional state. Phoresy has been proposed as a possible mechanism for the evolution of parasitism in mites [6]. For example, the mite Hemisarcoptes cooremani has free-living stages that prey on scale insects (Coccoidea). Juvenile stages called deutonymphs feed and increase in size while attached to coccinelid beetles, and are unable to develop or survive in the absence of a host [11]. Interestingly, deutonymphs represent only 6% of the population, suggesting that the production of this stage is facultative. This mite appears to be in the process of transitioning from a free-living lifestyle towards a fully parasitic life. Hence, H. cooremani could serve as a case study for investigating the hypothesis that facultative parasites constituted stepping-stones in the evolutionary transition to parasitism.
Nematodes have also evolved parasitic lifestyles across numerous independent lineages [24]. Some members of the family Strongyloididae are facultative parasites of mammals [7,8]. These nematodes present challenges for genetic and evolutionary studies because they have relatively long generation times and require mammalian hosts to be maintained in the laboratory. By contrast, facultative parasites of invertebrate hosts typically have shorter life cycles and are relatively easy to maintain in vivo under laboratory conditions. One candidate is a facultative parasite of slugs and terrestrial snails, Phasmarhabditis hermaphrodita. This nematode has a circumglobal distribution and is currently being marketed in the UK and Europe as a biological control agent against pestiferous slugs in gardens and various agricultural crops [26]. The nematode is easy to isolate from the wild, easy to grow in the laboratory and is gastropod-specific (i.e. does not infect earthworms or other soil invertebrates) [27]. More importantly, P. hermaphrodita resides in a clade with other species that exhibit a range of host-exploitation strategies, including three obligate slug parasites (Agfa flexilis, Angiostoma limacis and Acacia dentifera) and the free-living nematode Caenorhabditis spp. [28].
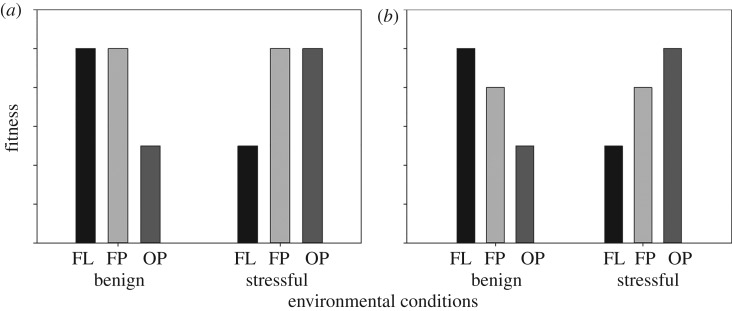
There are several open questions related to our hypothesis that genetic assimilation may underlie the evolutionary transition from facultative to obligate parasite. For example, how common is heritable variation in reaction norms for the propensity to follow an infective lifestyle? Are there costs for maintaining the capacity for plasticity in facultative parasites, or can loss of plasticity only occur when there is coincident selection on trait means and/or via accumulation of mutations? If facultative parasites are evolutionary stepping-stones towards obligate parasitism, it should be possible to evolve populations towards obligate parasitism if populations are experimentally exposed to conditions favouring the expression of parasitic behaviour over multiple generations. Experimental evolution studies (i.e. studying the response of populations to experimentally imposed selection) would allow for quantification of the costs of plasticity (figure 2), and whether facultative parasitic strategies evolve towards obligate parasitism via selection against plasticity, selection on trait means, mutation accumulation or any combination of these (figure 1).
Figure 2.
Illustration of how facultative parasites could be used to address questions related to the costs of plasticity by studying clades that contain free-living species (FL), facultative parasites (FP) and obligate parasites (OP). FL exhibit the free-living phenotype and OP exhibit the parasitic phenotype regardless of environmental conditions. However, FP can be induced to exhibit either a free-living phenotype (typically under benign environmental conditions) or a parasitic phenotype (typically under stressful conditions). (a) No costs to plasticity: the FP exhibiting a free-living phenotype achieve equal fitness to FL under benign environmental conditions, and FP exhibiting the parasitic phenotype achieve the same fitness as OP under stressful environmental conditions. (b) Costs to plasticity: FP always achieve lower fitness than the non-plastic species exhibiting the same phenotype.
5. Concluding remarks
Pre-adaptations allow for the expression of condition-dependent plasticity (i.e. facultative parasitism). However, we point out that pre-adaptations alone do not explain the loss of capacity to express parasitism in a plastic manner. Genetic assimilation offers a means by which obligate parasitism can evolve from facultative parasitism. Although theoretically plausible, this hypothesis requires empirical scrutiny. We suggest that the future directions laid out in this article provide a useful starting point for researchers interested in the evolution of parasitism, as well as those interested more generally in the role of genetic assimilation in evolutionary processes.
Acknowledgements
We thank R. Palmer and C. Lagrue for their thoughtful and constructive feedback on this manuscript. Thank you to the anonymous referees for providing useful comments and suggestions.
Data accessibility
This article has no additional data.
Authors' contributions
L.T.L. conceived of the study, and L.T.L. and K.J.M. co-wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
L.T.L. received funding from an NSERC Discovery Grant. K.J.M. received funding from an NSERC Discovery Grant and the Canada Research Chair program.
References
- 1.Blaxter M, Koutsovoulos G. 2015. The evolution of parasitism in Nematoda. Parasitology 142, S26–S39. ( 10.1017/s0031182014000791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viney M. 2017. How can we understand the genomic basis of nematode parasitism? Trends Parasitol. 33, 444–452. ( 10.1016/j.pt.2017.01.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poulin R. 2007. Evolutionary ecology of parasites. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Crook M. 2014. The Dauer hypothesis and the evolution of parasitism: 20 years on and still going strong. Int. J. Parasitol. 44, 1–8. ( 10.1016/j.ijpara.2013.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dieterich C, Sommer RJ. 2009. How to become a parasite—lessons from the genomes of nematodes. Trends Genet. 25, 203–209. ( 10.1016/j.tig.2009.03.006) [DOI] [PubMed] [Google Scholar]
- 6.Athias-Binche F, Morand S. 1993. From phoresy to parasitism: the example of mites and nematodes. Res. Rev. Parasitol. 53, 73–79. [Google Scholar]
- 7.Harvey SC, Gemmill AW, Read AF, Viney ME. 2000. The control of morph development in the parasitic nematode Strongyloides ratti. Proc. R. Soc. Lond. B 267, 2057–2063. ( 10.1098/rspb.2000.1249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stasiuk SJ, Scott MJ, Grant WN. 2012. Developmental plasticity and the evolution of parasitism in an unusual nematode, Parastrongyloides trichosuri. Evodevo 3, 14 ( 10.1186/2041-9139-3-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viney ME. 1996. Developmental switching in the parasitic nematode Strongyloides ratti. Proc. R. Soc. Lond. B 263, 201–208. ( 10.1098/rspb.1996.0032) [DOI] [PubMed] [Google Scholar]
- 10.Luong LT, Brophy T, Stolz E, Chan SJ. 2017. State-dependent parasitism by a facultative parasite of fruit flies. Parasitology 144, 1468–1475. ( 10.1017/s0031182017000890) [DOI] [PubMed] [Google Scholar]
- 11.Houck MA, Cohen AC. 1995. The potential role of phoresy in the evolution of parasitism: radiolabelling (tritium) evidence from an astigmatid mite. Exp. Appl. Acarol. 19, 677–694. ( 10.1007/bf00052079) [DOI] [Google Scholar]
- 12.Washburn JO, Gross ME, Mercer DR, Anderson JR. 1988. Predator-induced trophic shift of a free-living ciliate—parasitism of mosquito larvae by their prey. Science 240, 1193–1195. ( 10.1126/science.3131877) [DOI] [PubMed] [Google Scholar]
- 13.Morin L, Auld BA, Brown JF. 1993. Interaction between Puccinia xanthii and facultative parasitic fungi on Xanthium occidentale. Biol. Control 3, 288–295. ( 10.1006/bcon.1993.1038) [DOI] [Google Scholar]
- 14.Schlichting CD, Wund MA. 2014. Phenotypic plasticity and epigeneic marking: an assessment of evidence for genetic accomodation. Evolution 68, 656–672. ( 10.1111/evo.12348) [DOI] [PubMed] [Google Scholar]
- 15.Crispo E. 2007. The Baldwin effect and genetic assimilation: revisiting two mechanisms of evolutionary change mediated by phenotypic plasticity. Evolution 61, 2469–2479. ( 10.1111/j.1558-5646.2007.00203.x) [DOI] [PubMed] [Google Scholar]
- 16.Waddington CH. 1953. The ‘Baldwin effect,’ ‘genetic assimilation’ and ‘homeostasis’. Evolution 7, 386–387. ( 10.1111/j.1558-5646.1953.tb00099.x) [DOI] [Google Scholar]
- 17.Via S, Lande R. 1985. Genotype–environment interaction and the evolution of phenotypic plasticity. Evolution 39, 505–522. ( 10.2307/2408649) [DOI] [PubMed] [Google Scholar]
- 18.Scheiner SM, Lyman RF. 1989. The genetics of phenotypic plasticity I. Heritability. J. Evol. Biol. 2, 95–107. ( 10.1046/j.1420-9101.1989.2020095.x) [DOI] [Google Scholar]
- 19.Durkin ES, Luong LT. 2018. Experimental evolution of infectious behaviour in a facultative ectoparasite. Evolution 31, 362–370. ( 10.5061/dryad.9p0nm) [DOI] [PubMed] [Google Scholar]
- 20.Harvey SC, Shorto A, Viney ME. 2008. Quantitative genetic analysis of life-history traits of Caenorhabditis elegans in stressful environments. BMC Evol. Biol. 8, 15 ( 10.1186/1471-2148-8-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murren CJ, et al. 2015. Constraints on the evolution of phenotypic plasticity: limits and costs of phenotype and plasticity. Heredity 115, 293 ( 10.1038/hdy.2015.8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poulin R, Randhawa HS. 2013. Evolution of parasitism along convergent lines: from ecology to genomics. Parasitology 142, S6–S15. ( 10.1017/S0031182013001674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moran NA, Wernegreen JJ. 2000. Lifestyle evolution in symbiotic bacteria: insights from genomics. Trends Ecol. Evol. 15, 321–326. ( 10.1016/s0169-5347(00)01902-9) [DOI] [PubMed] [Google Scholar]
- 24.Blaxter ML, et al. 1998. A molecular evolutionary framework for the phylum Nematoda. Nature 392, 71–75. ( 10.1038/32160) [DOI] [PubMed] [Google Scholar]
- 25.Dowling A. 2015. The evolution of parasitism and host associations in mites. In Parasite diversity and diversification: evolutionary ecology meets phylogenetics (eds Morand S, Krasnov B, Littlewood D), pp. 265–288. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 26.Rae R. 2017. Phasmarhabditis hermaphrodita—a new model to study the genetic evolution of parasitism. Nematology 19, 375–387. ( 10.1163/15685411-00003053) [DOI] [Google Scholar]
- 27.Wilson MJ, Glen DM, Hamacher GM, Smith JU. 2004. A model to optimise biological control of slugs using nematode parasites. Appl. Soil Ecol. 26, 179–191. ( 10.1016/j.apsoil.2004.01.005) [DOI] [Google Scholar]
- 28.Ross JL, Ivanova ES, Spiridonov SE, Waeyenberge L, Moens M, Nicol GW, Wilson MJ. 2010. Molecular phylogeny of slug-parasitic nematodes inferred from 18S rRNA gene sequences. Mol. Phylogenet. Evol. 55, 738–743. ( 10.1016/j.ympev.2010.01.026) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.