Abstract
Individuals often associate socially with those who behave the same way. This principle, homophily, could structure populations into distinct social groups. We tested this hypothesis in a bottlenose dolphin population that appeared to be clustered around a specialized foraging tactic involving cooperation with net-casting fishermen, but in which other potential drivers of such social structure have never been assessed. We measured and controlled for the contribution of sex, age, genetic relatedness, home range and foraging tactics on social associations to test for homophily effects. Dolphins tended to group with others having similar home ranges and frequency of using the specialized foraging tactic, but not other traits. Such social preferences were particularly clear when dolphins were not foraging, showing that homophily extends beyond simply participating in a specific tactic. Combined, these findings highlight the need to account for multiple drivers of group formation across behavioural contexts to determine true social affiliations. We suggest that homophily around behavioural specialization can be a major driver of social patterns, with implications for other social processes. If homophily based on specialized tactics underlies animal social structures more widely, then it may be important in modulating opportunities for social learning, and therefore influence patterns of cultural transmission.
Keywords: fission–fusion, behaviour specialization, network, social structure, Tursiops truncatus
1. Introduction
Social animals seeking to maximize foraging benefits face crucial decisions—whom to group with and for how long? Members of foraging groups pay the cost of intragroup competition [1]. One possible solution is to specialize in foraging tactics that reduce resource-use overlap [2,3]. Simultaneously, there are benefits of being social—information sharing, predation avoidance, to name but two. To maintain group cohesion and facilitate group decisions it may be benefitial to behave as the other group members do [4]. Individuals, therefore, may be prone to group with those that behave in the same way, as posited by the homophily principle [5]. When social interactions occur at higher rates among similar than dissimilar individuals, homophily arranges social ties into cohesive, distinct social groups [6], as recently illustrated by modular animal social networks constructed around repertoires of foraging specializations (e.g. [7,8]).
However, multiple processes modulate the opportunities for animals to interact socially, which complicates determining whether individuals are indeed inclined to group with those who behave similarly. While extrinsic factors can aggregate or disperse individuals in time and space—e.g. habitat complexity [9], predation risk [10], demographic changes [11]—individuals can be passively or actively assorted by biological traits, such as age, sex and kin [12,13]. Disentangling the effects of these drivers from the contribution of homophily in clustering individuals remains challenging, but important because by controlling for such effects preferred associations can be discriminated from passive assortment or chance [14]. Here, we evaluated the multiple drivers of the associations of Lahilles' bottlenose dolphins (Tursiops truncatus gephyreus) that specialize in foraging with artisanal fishermen [15] to evaluate the extent to which homophily around behavioural specializations can shape social structures over and above other factors that influence grouping formation.
In southern Brazil, bottlenose dolphins herd fish schools towards a line of net-casting fishermen ashore, who cast their nets in response to the dolphins’ stereotyped foraging cues [16]. Not all dolphins of this small population use this tactic with the same frequency [17], and their social organization reflects such variation: dolphins that frequently forage with fishermen are more often seen together than with dolphins that forage independently [15]. However, it remains unclear whether this clustering depicts a spatial assortment in different foraging areas or assortment around the above-mentioned biological traits. If homophily underlies this social structure, then active choice of companions (i.e. social affiliations [14]) should occur among dolphins that consistently use the same foraging tactic.
We tested the hypothesis that homophily around a specialized behaviour—here dolphins foraging with the assistance of artisanal fishermen—underlies social preferences and can structure animal populations. First, we quantified the influence of multiple structural factors on dyadic associations, namely the frequency of use of the specialized foraging tactic, home range overlap, and assortment by genetic relatedness, sex and age classes. Next, we removed the effects of the confounding variables from the associations to test for active social preferences that could create modules in the dolphin social network. By accounting for different behavioural contexts that include or not the specialized foraging, we found that social patterns at the population-level result from active individual choices rather than from passive assortment.
2. Material and methods
Our study area comprised the 200 km2 lagoon system in Laguna (28°20′ S, 48°50′ W), southern Brazil (electronic supplementary material, figure S1), where approximately 60 bottlenose dolphins reside [18]. We carried out boat surveys during 2007–2009 following a pre-defined route covering the area evenly [19]. Upon encountering a group of dolphins—i.e. all individuals in close proximity to each other (within approx. 50 m radius) engaged in the same behavioural state [20]—we attempted to photograph all individuals and recorded the time, location, group size and behavioural state. Following photo-identification protocols [21], we then identified individuals based on natural long-lasting marks on the dorsal fin, excluding unmarked individuals such as calves [15,20]. We collected skin samples (n = 13 using a remote biopsy system; n = 3 from stranded photo-identified individuals) to determine sex and pairwise relatedness through molecular analyses and microsatellite genotyping (electronic supplementary material, S1).
To account for behaviour-specific associations (e.g. [22]), we assigned dolphin groups to the following behavioural contexts. The ‘cooperative foraging’ context defined the specialized foraging tactic whereby dolphins herd fish schools towards a line of artisanal fishermen ashore who cast nets in response to the dolphin stereotyped behavioural cues [16]. The ‘non-cooperative foraging’ context represented typical dolphin foraging characterized by frequent, asynchronous dives in various directions [23], away from fishermen. The ‘non-foraging’ context included three other distinctive behavioural states: travelling, socializing and resting [24], away from fishermen. Finally, ‘all behaviour’ combined all these contexts.
We considered group members to be associated [25], and calculated the simple-ratio index (SRI) to quantify the proportion of time each pair of individuals spent associated [25] in each behavioural context. To avoid spurious associations with juveniles and eventual transient individuals, we restricted our dataset (41 individuals and 503 groups) to adults resighted more than 25 times (34 individuals and 497 groups).
We quantified five structural factors that could affect social patterns: the proportion of use of ‘cooperative’ and ‘non-cooperative’ foraging tactics (fp), home range overlap (HRO), genetic relatedness, and sex and age classes (full methods in electronic supplementary material, S2). To quantify the contribution of such factors in driving associations, we used the multiple regression quadratic assignment procedure (MRQAP) and double-semi-partialling methods [26]. The dependent variables were each of the four context-dependent SRI matrices, and the independent variables were matrices representing pairwise similarity of each of the five structural factors. We performed three independent MRQAPs, dropping non-significant structural factors that constrained sample size. The first MRQAP included 12 individuals for which all factors were known; the second included 30 individuals for which all but the genetic relatedness factor were known; the third included all 34 individuals (electronic supplementary material, S3, tables S1–S3). We evaluated the empirical coefficients using a randomized distribution of regression coefficients (20 000 iterations), and the influence of structural variables on associations using the adjusted R2.
We used generalized affiliation indices (GAI) to remove the effect of the significant structural factors from the associations, and so represent active association preferences among individuals (i.e. social affiliations [14]). To develop a GAI for each behavioural context, we fitted a binomial generalized linear model with the corresponding SRI matrix as the dependent variable, and the significant structural factors identified by the MRQAP as independent variables [14]: SRI(all behaviour) ∼ FP + HRO; SRI(cooperative foraging) ∼ HRO; SRI(non-cooperative foraging) ∼ FP + HRO; SRI(non-foraging) ∼ FP + HRO, where SRIx = context-dependent SRI matrix, FP = Euclidean distance of fp (electronic supplementary material, table S3). GAI are the deviance residuals of such models, which represent the assortment of individuals not explained by the significant factors in each context [14].
To test for social preferences, social division and assortativity around these structural factors, we used null models that permuted individuals among groups (constraining the empirical group sizes, number of groups and individuals, and frequency of observation) to create an ensemble of 20 000 randomized SRI matrices, and 20 000 randomized GAI as done for the empirical data. We tested for social preferences between dyads by comparing the standard deviation (s.d.) of the observed context-dependent SRI and GAI with randomized s.d. distributions, considering preferences when the observed s.d. was higher than the null expectancy [25]. We tested for population-level social division (i.e. strongly connected modules of individuals) by plotting the context-dependent SRI and the non-negative GAI matrices as networks and comparing their empirical modularity (Q) [25] with that of the randomized matrices. Finally, we evaluated whether the specialized foraging tactic (i.e. high fp, low home range size, hr [17]) was coupled to social preferences and social division by testing the network assortativity [27] by fp () and by hr (), and evaluating the within-module fp and hr values. We considered empirical s.d., Q, , values statistically significant when they fell outside the 95% confidence interval of their corresponding benchmark distributions (full methods in the electronic supplementary material, S4–S7).
3. Results
We analysed social associations, foraging and ranging behaviours of the 34 well-sampled adult dolphins (mean resightings = 34.60 ± 5.97 s.d., range = 26–48), corresponding to approximately 56% of the population [18,28]. We observed 497 groups (mean size = 2.37 ± 0.86 s.d.; 74–83% individuals identified [19]): 120 in the cooperative foraging, 219 in the non-cooperative foraging, and 158 in the non-foraging contexts (electronic supplementary material, S8). The most significant predictors of dolphin associations in all behavioural contexts were the foraging tactic with fishermen and home range overlap, explaining 46% of the total variance in SRI (35% in non-cooperative and 31% in non-foraging contexts). In the cooperative context, however, only home range overlap influenced (18%) the associations (full MRQAP results in electronic supplementary material, tables S1–S3).
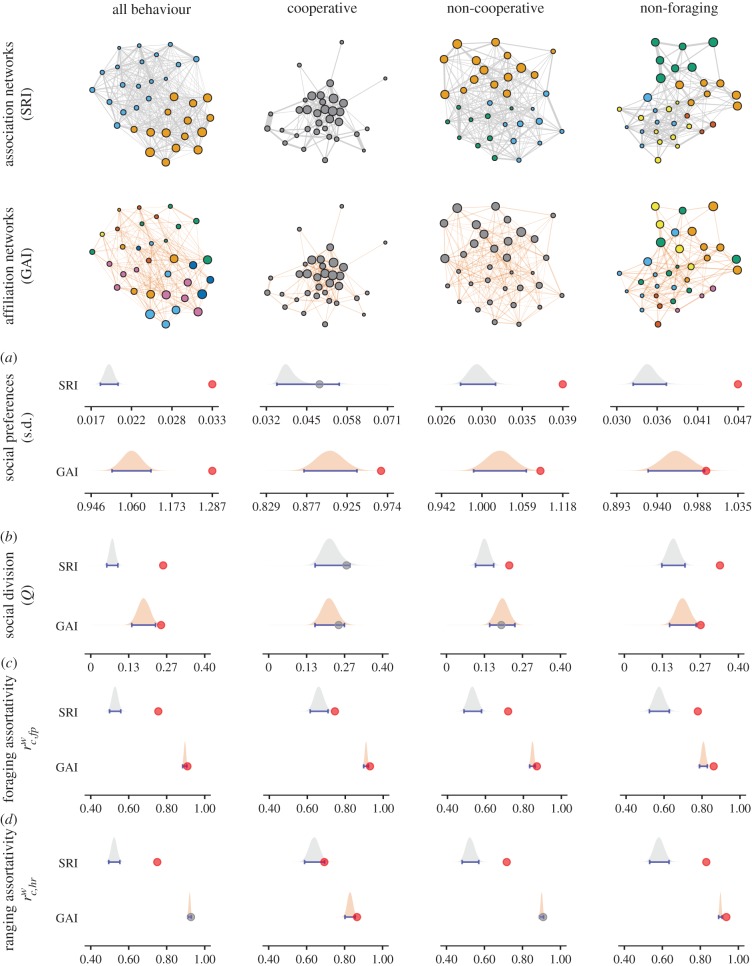
We rejected the null hypothesis that dolphins associate randomly in all but the cooperative foraging context, as the observed s.d. of SRI was higher than the random s.d. However, when GAI removed the influence of frequency of foraging and home range overlap from SRI, we rejected the null hypothesis of random affiliations in all behavioural contexts (figure 1a). Therefore, preferred social affiliations were always detected, even when dolphins were foraging with fishermen.
Figure 1.
Dolphin social preferences at the individual and population level across behavioural contexts. Nodes representing photo-identified individuals are proportional to the frequency of use of the specialized foraging tactic and colour-coded by social modules; individuals are connected by links whose thicknesses are proportional to SRI in the association networks and to GAI removing confounding factors (electronic supplementary material, tables S1–S3) in the affiliation networks (for better visualization, only positive GAIs were plotted). In the density plots, red dots denote statistically significant observed values; grey dots indicate non-significant values; shaded distributions indicate null expectancy; and blue whiskers indicate 95% confidence intervals. The specialized foraging tactic underlies social patterns as shown by (a) significant standard deviations (s.d.) of SRI and GAI indicating social preferences; (b) significant modularity (Q) indicating social division; and significant assortativity by both (c) frequency of foraging with fishermen and (d) home range size. Values in the scales are rounded. (Online version in colour.)
At the population level, the modularity (Q) of the association (SRI) networks was higher than expected in all but the cooperative context. However, the modularity of the affiliation networks (GAI) revealed that social modules are not detectable in foraging contexts (figure 1b). Social modules were typically distinguished between those composed of dolphins that often interact with fishermen and have small home ranges, and those of dolphins that rarely interact and forage over large areas (figure 1; electronic supplementary material, table S4). All association and affiliation networks were to some degree assorted by the similarity in frequency of foraging with fishermen (figure 1c). The affiliation networks suggested that individuals can also be spatially assorted during the cooperative and non-foraging contexts (figure 1d).
4. Discussion
We found that homophily around specialized behaviour in wild dolphins underlies their active dyadic social preferences and leads to social differentiation at the population level. By parsing out many of the potential drivers of social associations, we show that the combined influence of small home range and high frequency of foraging with artisanal fishermen is superior to the influence of genetic relatedness, sex and age on social structure; by separating these social patterns into behavioural contexts, we further show that the effect of this specialized foraging tactic is not simply a passive or spatial assortment of dolphins performing the same tactic. These findings support that behavioural specializations can define social affiliations and thus shape animal social structures.
Do dolphins associate because they forage with fishermen or do they forage with fishermen because they associate? More than reinforcing this foraging specialization as a driver of the population-level social structure [15], our findings reveal that dolphins prefer to affiliate with others that forage with fishermen at similar frequencies, but not in order to perform such a foraging tactic. This is strikingly clear when dolphins are engaged in activities other than foraging—when travelling, socializing or resting, the dolphin social preferences are evident and structure their social networks into distinctive social modules. The social division based on different foraging tactics indicates that active choice of companions extends beyond the context of their foraging interactions with fishermen. Although dolphins still show dyadic social preferences when foraging, they spatially aggregate at specific fishing spots when interacting with fishermen [17], which temporarily dismantles the modular social structure.
The key finding is that dolphins actively seek to be with similar others outside of the specialized foraging context. Such social affiliations among dolphins that forage in the same way may be reinforced by the differences in vocal repertoires of their social modules [29], which could function in mediating the recognition of preferred affiliates. The homophily around the specialized foraging with net-casting fishermen in Laguna closely resembles the Shark Bay population where the dolphins' associations are structured by homophily around the tactic of using marine sponges as foraging tools [7]. Both studies combined strengthen the evidence for socially learned behavioural specializations as important drivers of non-human societies [6,7].
Homophily is widely observed in nature and can influence a range of social processes, from social clustering to the evolution of cooperation [30]. The socially distinctive modules that can result from homophily narrow the pathways for the transmission of genes, diseases, parasites, as well as for the transmission of socially learned information related to behaviour specializations [7]. Given that dolphins often copy and emulate their related and unrelated peers [8], social learning is likely to be involved in the spread and maintenance of the specialized foraging tactic among dolphins in Laguna [31]. When dolphins actively associate with few similar others, learning is facilitated within social modules rather than between modules [32]. Thus, by modulating opportunities for social learning, homophily can reinforce social differentiation and broaden behavioural divergence [6], thereby influencing the evolution of specializations and their transmission patterns. Revealing the causes of social differentiation among animals is crucial for understanding the ecological drivers of behavioural specializations and the extent to which intraspecific competition, individual learning, cultural transmission and genetics are involved [7,33].
Supplementary Material
Acknowledgements
We thank all fieldwork volunteers, P. Rosel for assistance with molecular analyses, L. Rendell, M. Silk and two anonymous referees for insightful comments.
Ethics
Research approved by the Brazilian Ministry of Environment (permit SISBio 47876-1).
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.20vd145 [27] and R codes at https://bitbucket.org/alexandremarcelsm/botonet/src/master/.
Authors' contributions
F.G.D.-J. and M.C. conceived and coordinated the research. F.G.D.-J., C.B., A.M.S.M. and B.P.H.R. collected data. A.M.S.M., M.C., J.V.S.V.-P. and F.G.D.-J. performed statistical analyses. A.P.B.C. and B.P.H.R. performed molecular analyses. P.C.S.-L. and P.V.C. provided field infrastructure and secondary data. A.M.S.M., M.C. and F.G.D.-J. wrote the manuscript with contributions from the co-authors. All authors critically revised the manuscript, approved the final version of the manuscript and agreed to be held accountable for its content.
Competing interests
We have no competing interests.
Funding
This study was partially financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brasil) (A.M.S.M. and C.B.). A.M.S.M. received Animal Behaviour Society and Society for Marine Mammalogy grants. M.C. was supported by CNPq-Brasil (153797/2016-9) and PMP/BS (46/2016); P.V.C. by FAPESC-PAP/UDESC (2017TR744); and F.G.D.-J. by CNPq (407190/2012-0) and FAPESC (TR2012000295).
References
- 1.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML. 2003. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28. ( 10.1086/343878) [DOI] [PubMed] [Google Scholar]
- 3.Sheppard CE, Inger R, McDonald RA, Barker S, Jackson AL, Thompson FJ, Vitikainen EIK, Cant MA, Marshall HH. 2018. Intragroup competition predicts individual foraging specialisation in a group-living mammal. Ecol. Lett. 21, 665–673. ( 10.1111/ele.12933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conradt L, Roper TJ. 2003. Group decision-making in animals. Nature 421, 155–158. ( 10.1038/nature01294) [DOI] [PubMed] [Google Scholar]
- 5.McPherson M, Smith-Lovin L, Cook JM. 2001. Birds of a feather: homophily in social networks. Annu. Rev. Sociol. 27, 415–444. ( 10.1146/annurev.soc.27.1.415) [DOI] [Google Scholar]
- 6.Cantor M, Whitehead H. 2013. The interplay between social networks and culture: theoretically and among whales and dolphins. Phil. Trans. R. Soc. B 368, 20120340 ( 10.1098/rstb.2012.0340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mann J, Stanton MA, Patterson EM, Bienenstock EJ, Singh LO. 2012. Social networks reveal cultural behaviour in tool-using dolphins. Nat. Commun. 3, 980 ( 10.1038/ncomms1983) [DOI] [PubMed] [Google Scholar]
- 8.Whitehead H, Rendell L. 2014. The cultural lives of whales and dolphins. Chicago, IL: University of Chicago Press. [Google Scholar]
- 9.Leu ST, Farine DR, Wey TW, Sih A, Bull CM. 2016. Environment modulates population social structure: experimental evidence from replicated social networks of wild lizards. Anim. Behav. 111, 23–31. ( 10.1016/j.anbehav.2015.10.001) [DOI] [Google Scholar]
- 10.Kelley JL, Morrell LJ, Inskip C, Krause J, Croft DP. 2011. Predation risk shapes social networks in fission-fusion populations. PLoS ONE 6, e24280 ( 10.1371/journal.pone.0024280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantor M, Wedekin LL, Guimarães PR, Daura-Jorge FG, Rossi-Santos MR, Simões-Lopes PC. 2012. Disentangling social networks from spatiotemporal dynamics: the temporal structure of a dolphin society. Anim. Behav. 84, 641–651. ( 10.1016/j.anbehav.2012.06.019) [DOI] [Google Scholar]
- 12.Carter KD, Brand R, Carter JK, Shorrocks B, Goldizen AW. 2013. Social networks, long-term associations and age-related sociability of wild giraffes. Anim. Behav. 86, 901–910. ( 10.1016/j.anbehav.2013.08.002) [DOI] [Google Scholar]
- 13.Frère CH, Krützen M, Mann J, Watson-capps JJ, Tsai YJ, Patterson EM, Connor RC, Bejder L, Sherwin WB. 2010. Home range overlap, matrilineal and biparental kinship drive female associations in bottlenose dolphins. Anim. Behav. 80, 481–486. ( 10.1016/j.anbehav.2010.06.007) [DOI] [Google Scholar]
- 14.Whitehead H, James R. 2015. Generalized affiliation indices extract affiliations from social network data. Methods Ecol. Evol. 6, 836–844. ( 10.1111/2041-210X.12383) [DOI] [Google Scholar]
- 15.Daura-Jorge FG, Cantor M, Ingram SN, Lusseau D, Simões-Lopes PC. 2012. The structure of a bottlenose dolphin society is coupled to a unique foraging cooperation with artisanal fishermen. Biol. Lett. 8, 702–705. ( 10.1098/rsbl.2012.0174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simões-Lopes PC, Fabián ME, Menegheti JO. 1998. Dolphin interactions with the mullet artisanal fishing on southern Brazil: a qualitative and quantitative approach. Rev. Bras. Zool. 15, 709–726. ( 10.1590/S0101-81751998000300016) [DOI] [Google Scholar]
- 17.Cantor M, Simões-Lopes PC, Daura-Jorge FG. 2018. Spatial consequences for dolphins specialised in foraging with fishermen. Anim. Behav. 139, 19–27. ( 10.1016/j.anbehav.2018.03.002) [DOI] [Google Scholar]
- 18.Bezamat C, Simões-Lopes PC, Castilho PV, Daura-Jorge FG. In press. The influence of cooperative foraging with fishermen on the dynamics of a bottlenose dolphin population. Mar. Mammal Sci. ( 10.1111/mms.12565) [DOI] [Google Scholar]
- 19.Daura-Jorge FG, Ingram SN, Simões-Lopes PC. 2013. Seasonal abundance and adult survival of bottlenose dolphins (Tursiops truncatus) in a community that cooperatively forages with fishermen in southern Brazil. Mar. Mammal Sci. 29, 293–311. ( 10.1111/j.1748-7692.2012.00571.x) [DOI] [Google Scholar]
- 20.Irvine AB, Scott MD, Wells RS, Kaufmann JH. 1981. Movements and activities of the Atlantic bottlenose dolphin, Tursiops truncatus, near Sarasota, Florida. Fish. Bull. 79, 671–688. [Google Scholar]
- 21.Urian K, et al. 2015. Recommendations for photo-identification methods used in capture-recapture models with cetaceans. Mar. Mammal Sci. 31, 298–321. ( 10.1111/mms.12141) [DOI] [Google Scholar]
- 22.Gero S, Bejder L, Whitehead H, Mann J, Connor RC. 2005. Behaviourally specific preferred associations in bottlenose dolphins, Tursiops spp. Can. J. Zool. 83, 1566–1573. ( 10.1139/z05-155) [DOI] [Google Scholar]
- 23.Shane SH, Wells RS, Würsig B. 1986. Ecology, behavior and social organization of the bottlenose dolphin: a review. Mar. Mammal Sci. 2, 34–63. ( 10.1111/j.1748-7692.1986.tb00026.x) [DOI] [Google Scholar]
- 24.Smolker RA, Richards AF, Connor RC, Pepper JW. 1992. Sex differences in patterns of association among Indian Ocean bottlenose dolphins. Behaviour 123, 38–69. ( 10.1163/156853992X00101) [DOI] [Google Scholar]
- 25.Whitehead H. 2008. Analyzing animal societies: quantitative methods for vertebrate social analysis. Chicago, IL: University of Chicago Press. [Google Scholar]
- 26.Dekker D, Krackhardt D, Snijders TAB. 2007. Sensitivity of MRQAP tests to collinearity and autocorrelation conditions. Psychometrika 72, 563–581. ( 10.1007/s11336-007-9016-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farine DR. 2014. Measuring phenotypic assortment in animal social networks: weighted associations are more robust than binary edges Anim. Behav. 89, 141–153. ( 10.1016/j.anbehav.2014.01.001) [DOI] [Google Scholar]
- 28.Machado AMS, Cantor M, Costa APB, Righetti BPH, Bezamat C, Valle-Pereira JVS, Simões-Lopes PC, Castilho PV, Daura-Jorge FG. 2019. Data from: Homophily around specialized foraging underlies dolphin social preferences. Dryad Digital Repository. ( 10.5061/dryad.20vd145) [DOI] [PMC free article] [PubMed]
- 29.Romeu B, Cantor M, Bezamat C, Simões-Lopes PC, Daura-Jorge FG. 2017. Bottlenose dolphins that forage with artisanal fishermen whistle differently. Ethology 123, 906–915. ( 10.1111/eth.12665) [DOI] [Google Scholar]
- 30.Fu F, Nowak MA, Christakis NA, Fowler JH. 2012. The evolution of homophily. Sci. Rep. 2, 845 ( 10.1038/srep00845) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simões-Lopes PC, Daura-Jorge FG, Cantor M. 2016. Clues of cultural transmission in cooperative foraging between artisanal fishermen and bottlenose dolphins, Tursiops truncatus (Cetacea: Delphinidae). Zoologia 33, e20160107 ( 10.1590/s1984-4689zool-20160107) [DOI] [Google Scholar]
- 32.Cantor M, Shoemaker LG, Cabral RB, Flores CO, Varga M, Whitehead H. 2015. Multilevel animal societies can emerge from cultural transmission. Nat. Commun. 6, 8091 ( 10.1038/ncomms9091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitehead H. 2009. How might we study culture? A perspective from the ocean. In The question of animal culture, pp. 125–151. Cambridge, MA: Harvard University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.20vd145 [27] and R codes at https://bitbucket.org/alexandremarcelsm/botonet/src/master/.