Abstract
Background
Despite policies for universal HIV testing and treatment (UTT) regardless of CD4 count, there are still 1.8 million new HIV infections and 1 million AIDS-related deaths annually. The UNAIDS 90-90-90 goals target suppression of HIV viral load in 73% of all HIV-infected people worldwide by 2030. However, achieving these targets may not lead to expected reductions in HIV incidence if the remaining 27% (persons with unsuppressed viral load) are the drivers of HIV transmission through high-risk behaviors. We aim to conduct a systematic review and meta-analysis to understand the demographics, mobility, geographic distribution, and risk profile of adults who are not virologically suppressed in sub-Saharan Africa in the era of UTT.
Methods
We will review the published and grey literature for study sources that contain data on demographic and behavioral strata of virologically suppressed and unsuppressed populations since 2014. We will search PubMed and Embase using four sets of search terms tailored to identify characteristics associated with virological suppression (or lack thereof) and each of the individual 90-90-90 goals. Record screening and data abstraction will be done independently and in duplicate. We will use random effects meta-regression analyses to estimate the distribution of demographic and risk features among groups not virologically suppressed and for each individual 90-90-90 goal.
Discussion
The results of our review will help elucidate factors associated with failure to achieve virological suppression in sub-Saharan Africa, as well as factors associated with failure to achieve each of the 90-90-90 goals. These data will help quantify the population-level effects of current HIV treatment interventions to improve strategies for maximizing virological suppression and ending the HIV epidemic.
Systematic review registration
PROSPERO CRD42018089505.
Electronic supplementary material
The online version of this article (10.1186/s13643-019-1024-6) contains supplementary material, which is available to authorized users.
Keywords: HIV, Care cascade, HIV diagnosis, Antiretroviral therapy, Viral suppression, Virological suppression, 90-90-90, Risk-factors, Systematic review, Meta-analysis, Africa
Background
In 2016, there were approximately 36.7 million people living with HIV (PLHIV), 1.8 million new HIV infections, and 1 million AIDS-related deaths [1]. In 2014, the Joint United Nations Programme on HIV/AIDS (UNAIDS) established the 90-90-90 goals for the HIV care cascade. The HIV care cascade and these goals aim for 90% of PLHIV to know their status, of whom 90% link to and initiate antiretroviral treatment (ART) and of whom 90% achieve virological suppression by 2020 [2]. UNAIDS modeling projections estimate that new HIV infections will be reduced by up to 90% if the global community achieves the ambitious UNAIDS 90-90-90 goals by 2030 [3].
A successful program for the 90-90-90 goals will achieve virological suppression in 73% (90% × 90% × 90%) of PLHIV, which will leave 27% of PLHIV—approximately 9.9 million people—without achieving virological suppression. Virological suppression occurs when an infected individual’s HIV RNA level drops below a particular threshold and results in improved health outcomes for the individual and reduced risk of onward transmission. If those who do not achieve HIV virological suppression are the drivers of HIV transmission by engaging in high-risk behaviors, then there is a strong risk that achieving the 90-90-90 targets will not lead to ending the HIV epidemic [4]. Therefore, incorporating data on the risk profile of the missing 27% who are not virologically suppressed may help to more accurately predict the impact of achieving the 90-90-90 targets [5]. This will also inform HIV programs to develop strategies, in addition to universal testing and treatment (UTT), that are most beneficial to ending the HIV epidemic [6].
Simulation studies, observational data, and randomized trials have had mixed results in estimating the population benefits of UTT. Mathematical modeling based on results of a cluster-randomized trial of UTT and circumcision in Zambia and South Africa estimated reductions of incidence between 25% and 62% over 10 years [6]. A large population-based cohort in South Africa found a considerable reduction in HIV incidence across communities with high levels of ART coverage [7]. However, completed randomized trials of UTT demonstrated limited population-level effectiveness, which supports the need for more research on the role that groups which are not virologically suppressed play in sustaining high levels of HIV incidence [8]. Empirical evidence is needed to determine the characteristics of people who are not reached through the 90-90-90 strategy, which may inform future strategies to curb onward HIV transmission.
We report herein our protocol for a systematic review and meta-analysis of the demographic, epidemiologic, sexual-risk behavior, and geographic heterogeneity across the HIV care cascade throughout sub-Saharan Africa.
Aims
The primary aim of this systematic review and meta-analysis is to characterize the HIV transmission potential (defined by demographic and other risk group characteristics) of populations not virologically suppressed in the era of UTT in sub-Saharan Africa. The second aim of this review is to quantify the demographic and risk group heterogeneity at each of the individual 90-90-90 goals—awareness of HIV infection, ART initiation, and HIV virological suppression. We will identify and use subpopulations and risk group strata that have been defined, find comparable strata by which to quantify heterogeneity across the 90-90-90 cascade, and calculate pooled point estimates of the proportion who do not achieve virological suppression within these strata.
Methods
This systematic review protocol follows the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines [9]. The PRISMA-P checklist can be reviewed as Additional file 1. This review protocol is registered on the International Prospective Register of Systematic Reviews database (PROSPERO CRD42018089505) [10].
Eligibility criteria
Study design and settings
We will include all observational cohort studies, case-control studies, cross-sectional studies, HIV surveillance reporting, and randomized control trials that report on one or more elements of the 90-90-90 HIV care cascade. We will include studies that report aggregate estimates or stratum-specific estimates. We will exclude studies that provide modeled estimates of testing, treatment, or virological suppression, systematic reviews and meta-analyses, and data collected exclusively prior to January 2014.
Population
We will include any study of adult populations, defined as PLHIV aged 15 years or older, located in sub-Saharan Africa. We will include strata defined in Table 1.
Table 1.
Demographic and risk group strata of interest
| Age | |
| Sex | |
| Subnational administrative unit | |
| Urban, peri-urban, or rural residence | |
| Occupation | |
| Active military | |
| Distance from facility | |
| Out of pocket payments | |
| Marital status | |
| Age at sexual debut | |
| CD4 count | |
| Timing of last HIV test | |
| Education attainment | |
| Income | |
| Migratory populations | |
| Recency of migration | |
| Mobile populations | |
| Depression status | |
| Anxiety status | |
| Opportunistic infections | |
| Number of concurrent sexual partners | |
| Number of previous sexual partners | |
| Age of sexual partners | |
| Condom use | |
| Pregnant women | |
| Discordant couples | |
| Injection drug users | |
| Transactional sex/commercial sex workers | |
| Gay, bisexual, and other men who have sex with men | |
| Transgender individuals |
Outcomes
The primary outcome is the prevalence of unsuppressed viral load by demographic and risk group, and risk factors associated with being unsuppressed. The labels and definitions of strata used in included studies will be recorded to document the diversity and similarity of strata categorization (e.g., mobile versus migratory populations). The secondary outcomes of interest are defined as the proportion, relative risk, or odds ratio of the following three groups: PLHIV who know their HIV status, PLHIV who know their status and are receiving ART, and PLHIV on ART who are virologically suppressed.
Information sources
Electronic databases
We will conduct four comprehensive literature searches, one for each of the 90-90-90 targets and a fourth search focusing on the entire HIV care cascade (Fig. 1). We will perform searches in PubMed and Embase and restrict the search to articles published in English between January 2014 and March 2018. We will limit our search to articles published after 2014 because the UNAIDS 90-90-90 targets were announced mid-2014 and the World Health Organization (WHO) universal test and treat guidelines were released in 2015 [11].
Fig. 1.
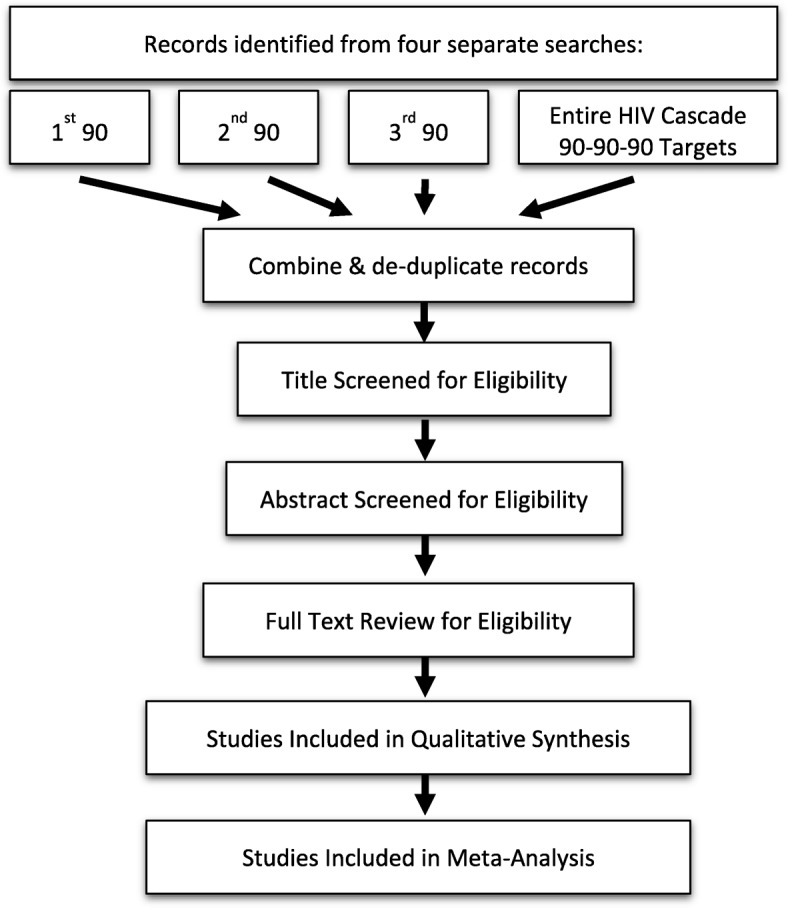
Search strategy
In addition to searching the published peer-review literature, we will conduct a comprehensive search of websites and databases for publicly available grey literature sources, such as WHO and UNAIDS reports, Ministries of Health websites and national surveillance reports, Médecins Sans Frontières (MSF) reports, the Integrated Bio-Behavioral Surveys (IBBS), U.S. President’s emergency plan for AIDS Relief (PEPFAR) country operational plans, the Analyzing Longitudinal Population-based HIV/AIDS data on Africa (ALPHA) Network, the National Technical Reports Library (NTRL), the Population-based HIV Impact Assessments (PHIA), AIDS Indicator Surveys (AIS), Demographic and Health Surveys (DHS), and other similar national HIV surveys (e.g., the South African National HIV Prevalence, Incidence and Behavior Survey) [12–22]. We will also search HIV conference abstracts including the International AIDS Society (IAS), the Conference on Retroviruses and Opportunistic Infections (CROI), and the International Conference on AIDS and STIs in Africa (ICASA) [23–25]. For eligible grey literature sources, we will review listed citations for additional data sources.
Search strategy
A detailed search strategy is presented in Additional file 2. In brief, MeSH and keywords will be included for HIV, such as “Human Immunodeficiency Virus” and “AIDS”, along with terms for each portion of the 90-90-90 cascade, and key terms for “Sub-Saharan Africa” and specific country names using Boolean “AND” and “OR” operators. For the first 90, we will include terms such as “testing”, “diagnosis”, and “serostatus”. The search for the second 90 will include terms such as “antiretroviral” and “treatment”, and the search for the third 90 will include terms such as “viral suppression” and “viral load”. Lastly, for our fourth search, we will include terms to capture the whole cascade, including “90-90-90”, “HIV care cascade”, and “universal test and treat”. Prior to publishing results, we will update our search to include any additional eligible papers that are published after March 2018.
Study records
Data management
Each of the separate four searches will be conducted and merged into a reference manager. We will record the number of duplicate records, which will then be removed prior to the selection process. We will use Microsoft Excel and Covidence, a systematic review management software, to document the outcome of the title screening, abstract screening, full text review, and data abstraction processes [26].
Selection process
We will first screen records for inclusion based on title only, duplicated independently by two reviewers. In cases of disagreement, the record in question proceeds to the abstract review stage. Abstract review will also include duplicate reviews. Full texts of the records will then be obtained and reviewed for inclusion and will be conducted independently and in duplicate. Discrepancies from the abstract and full text review will be resolved through consensus or in discussion with a third independent reviewer. Records will be excluded from consideration at title, abstract, and full text review stages if they satisfy any of the following exclusion criteria: study only among HIV-negative persons, study on persons outside of sub-Saharan Africa setting, study on children aged under 15, study of an inappropriate design, study data collected exclusively prior to January 2014, or study does not report on at least one of the 90-90-90 outcomes (Table 2).
Table 2.
Eligibility criteria
| Criteria | Variables |
|---|---|
| Inclusion criteria | Study includes HIV-positive persons |
| Study set in sub-Saharan Africa | |
| Study on adults aged 15 and older | |
| Study design is interventional, cohort, cross-sectional, case-control | |
| Study data collected at least partially from January 2014 onwards | |
| Study reports results on at least one 90-90-90 target | |
| Exclusion criteria | Study includes only HIV-negative persons |
| Study not set in sub-Saharan Africa | |
| Study includes children aged under 15 | |
| Study design is qualitative, mathematical model, systematic review, or editorial | |
| Study data collected exclusively prior to January 2014 | |
| Study does not report on any of the 90-90-90 targets |
Data collection process
We will develop a data extraction form and pilot test this form on ten randomly selected publications which have been selected for data abstraction. This form will guide the collection of strata-specific estimates of inclusion across the three 90-90-90 targets, as well as strata definitions. Data extraction will be done independently by two reviewers, with discrepancies resolved by consensus or in consultation with a third reviewer.
Data items
The data items for extraction are informed by the study aims. We will include the following information from all studies:
Study characteristics (authors, year of publication, study period, study design, geographic location, duration of follow-up, key findings)
Study setting, specifically differentiating between clinical, community, and population-based study populations
Definition and methods used to define the reported levels of the cascade, such as the threshold for virologic suppression
Definitions and inclusion criteria used for specific subgroups defined by age, sex, occupation, migratory/mobile status, and risk behavior as well as key population definitions used
For each population strata for which data are reported, the sample size, 95% confidence intervals, proportions, relative risk or odds ratio of PLHIV who know their HIV status (first 90), PLHIV who know their status and are on ART (second 90), and PLHIV on ART who are virologically suppressed (third 90)
Data synthesis
We will summarize information on virological suppression, and strata associated with being virologically suppressed. Strata reported, definitions used to define strata and levels of the HIV care cascade, and study characteristics including geography and study design will be summarized in the narrative. Study characteristics such as year and study period will be reported separately for each included study. Our qualitative assessment of the comparability of these definitions will inform our quantitative analysis.
We will summarize information on strata associated with knowledge of HIV serostatus, enrollment on ART, and virological suppression. This will include synthesizing odds ratios, relative risks, and proportions of persons virologically suppressed. We will hand calculate measures of association, odds ratio and risk ratio point estimates without confidence intervals, for studies which do not report or calculate measures of association but provide sufficient data for their calculation. Where appropriate and feasible, we will use random effects meta-regression analyses to estimate the distribution of demographic and risk features among groups of combinable strata who are not virologically suppressed. We hypothesize that national-level epidemic characteristics such as HIV prevalence and progress towards the 90-90-90 goals will be critical contextual features to consider in quantitative meta-analyses.
Missing or incomplete data
In the case of missing or incomplete data, we will contact corresponding authors to request relevant information or for additional clarification. If the corresponding authors fail to respond, then additional authors will be contacted. A description of the missing data for each included study will be provided, along with the possible implications of missing data. We will assess potential publication bias through the use of funnel plots and a subjective assessment of asymmetry [18].
Risk of bias in included studies
There will be three primary reviewers of included studies. At least two reviewers will assess the risk of selection bias, reporting bias, and attrition bias for each study included for data abstraction by using an adapted Cochrane Risk of Bias Tool including items for non-randomized studies [27]. Using this tool, reviewers will grade studies as having low, moderate, serious, or critical risk of bias. Conflicting ratings will be resolved through consultation of a third reviewer. We intend to assess the heterogeneity of study results by calculation of the I2 statistic [28]. Studies with critical risk of bias will not be considered for quantitative synthesis.
Discussion
The effectiveness of the UNAIDS 90-90-90 targets to curb the HIV epidemic by 2030 may depend on reaching individuals with the highest risk of onward HIV transmission. In addition, successful achievement of these targets will mean that 27% of PLHIV are not virologically suppressed. Little is known about the demographic profile of those accessing the HIV care cascade and perhaps more importantly those people who are falling off the HIV care cascade. This systematic review and meta-analysis will help identify key demographic, epidemiologic, and risk factor heterogeneity across the 90-90-90, with a specific focus on correlates of being among the 27% who are not virologically suppressed. This will include those who fail to test and get linked to care, in addition to those on ART who do not achieve suppression. Results from this review will be useful in more accurately predicting the impact of achieving the 90-90-90 targets for reducing global HIV incidence and guiding health intervention and prevention strategies to more effectively reach key populations.
Additional files
PRISMA-P 2015 checklist. (125 kb)
Search Terms. (19 kb)
Acknowledgements
None
Ethical approval and consent to participate
Not applicable
Funding
This work was funded by the Bill and Melinda Gates Foundation through an agreement with the University of Washington Strategic Analysis, Research and Training (START) Center. The funders had no role in the decision to publish or preparation of the manuscript.
Availability of data and materials
Not applicable
Abbreviations
- AIDS
Acquired immune deficiency syndrome
- AIS
AIDS Indicator Surveys
- ALPHA
Analyzing Longitudinal Population-based HIV/AIDS data on Africa
- ART
Antiretroviral treatment
- CD4
Cluster of differentiation 4
- CROI
The Conference on Retroviruses and Opportunistic Infections
- DHS
Demographic and Health Surveys
- HIV
Human immunodeficiency virus
- IAS
The International AIDS Society
- IBBS
Integrated Bio-behavioral Surveys
- ICASA
The International Conference on AIDS and STIs in Africa
- MSF
Médecins Sans Frontières
- NTRL
National Technical Reports Library
- PEPFAR
U.S. President’s Plan for AIDS Relief
- PHIA
Population-based HIV Impact Assessments
- PLHIV
People living with HIV
- PRISMA-P
Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols
- PROSPERO
International Prospective Register of Systematic Reviews database
- UNAIDS
Joint United Nations Programme on HIV/AIDS
- UTT
Universal test and treat
- WHO
World Health Organization
Authors’ contributions
AA and AB conceived the study. DG, BK, and DT prepared and registered the protocol and will conduct the literature search and data extraction under the supervision of PD, AD, AA, and AB. MM and GG provided content expertise. All authors contributed to the concept and design of the protocol. All authors provided critical revision of the protocol and read and approved the final manuscript.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dylan Green, Phone: +1-850-554-2394, Email: greend7@uw.edu.
Brenda Kharono, Email: bkharono@uw.edu.
Diana M. Tordoff, Email: dtordoff@uw.edu
Adam Akullian, Email: aakullian@idmod.org.
Anna Bershteyn, Email: abershteyn@idmod.org.
Michelle Morrison, Email: michelle.morrison@gatesfoundation.org.
Geoff Garnett, Email: Geoff.garnett@gatesfoundation.org.
Ann Duerr, Email: aduerr@fredhutch.org.
Paul Drain, Email: pkdrain@uw.edu.
References
- 1.UNAIDS . UNAIDS Data. 2017. [Google Scholar]
- 2.UNAIDS . 90-90-90 an ambitious treatment target to help end the AIDS epidemic. 2016. [Google Scholar]
- 3.UNAIDS . Fast-Track: ending the AIDS epidemic by 2030. 2014. [Google Scholar]
- 4.Huerga H, Venables E, Ben-Farhat J, van Cutsem G, Ellman T, Kenyon C. Higher risk sexual behaviour is associated with unawareness of HIV-positivity and lack of viral suppression – implications for treatment as prevention. Sci Rep. 2017;7(1):16117. doi: 10.1038/s41598-017-16382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akullian A, Bershteyn A, Jewell B, Camlin CS. The missing 27% AIDS. 2017;31(17):2427–2429. doi: 10.1097/QAD.0000000000001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cori Anne, Ayles Helen, Beyers Nulda, Schaap Ab, Floyd Sian, Sabapathy Kalpana, Eaton Jeffrey W., Hauck Katharina, Smith Peter, Griffith Sam, Moore Ayana, Donnell Deborah, Vermund Sten H., Fidler Sarah, Hayes Richard, Fraser Christophe. HPTN 071 (PopART): A Cluster-Randomized Trial of the Population Impact of an HIV Combination Prevention Intervention Including Universal Testing and Treatment: Mathematical Model. PLoS ONE. 2014;9(1):e84511. doi: 10.1371/journal.pone.0084511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell M-L. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science (80- ) 2013;339(6122):966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwuji Collins C, Orne-Gliemann Joanna, Larmarange Joseph, Balestre Eric, Thiebaut Rodolphe, Tanser Frank, Okesola Nonhlanhla, Makowa Thembisa, Dreyer Jaco, Herbst Kobus, McGrath Nuala, Bärnighausen Till, Boyer Sylvie, De Oliveira Tulio, Rekacewicz Claire, Bazin Brigitte, Newell Marie-Louise, Pillay Deenan, Dabis François, Bärnighausen Till, Herbst Kobus, Iwuji Collins, Makowa Thembisa, Naidu Kevi, Newell Marie-Louise, Okesola Nonhlanhla, de Oliveira Tulio, Pillay Deenan, Rochat Tamsen, Tanser Frank, Viljoen Johannes, Zuma Thembelihle, McGrath Nuala, Balestre Eric, Dabis François, Karcher Sophie, Orne-Gliemann Joanna, Plazy Melanie, Prague Mélanie, Thiébaut Rodolphe, Tiendrebeogo Thierry, Boyer Sylvie, Donfouet Hermann, Gosset Andrea, March Laura, Protopopescu Camelia, Spire Bruno, Calmy Alexandra, Larmarange Joseph, Inghels Maxime, Diallo Hassimiou, Calvez Vincent, Derache Anne, Marcelin Anne-Geneviève, Dray-Spira Rosemary, Lert France, El Farouki Kamal, Lessells Richard, Freedberg Kenneth, Imrie John, Chaix Marie-Laure, Newell Colin, Hontelez Jan, Bazin Brigitte, Rekacewicz Claire. Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial. The Lancet HIV. 2018;5(3):e116–e125. doi: 10.1016/S2352-3018(17)30205-9. [DOI] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/BMJ.B2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth A, Clarke M, Ghersi D, Moher D, Petticrew M, Stewart L. An international registry of systematic-review protocols. Lancet (London, England) 2011;377(9760):108–109. doi: 10.1016/S0140-6736(10)60903-8. [DOI] [PubMed] [Google Scholar]
- 11.WHO . Guideline on when to start antiretroviral therapy and pre-exposure prophylaxis for HIV. 2015. [PubMed] [Google Scholar]
- 12.PEPFAR. Country Operational Plans - Fiscal Years 2007-2017. https://www.pepfar.gov/countries/cop/index.htm. Accessed 15 Feb 2018.
- 13.WHO. WHO IRIS: Home. http://apps.who.int/iris/. Accessed 15 Feb 2018.
- 14.UNAIDS. Publications | UNAIDS. http://www.unaids.org/en/resources/publications/all/. Accessed 15 Feb 2018.
- 15.MSF. Publications and Reports | Médecins Sans Frontières (MSF) International. http://www.msf.org/en/aggregator-group-resources/publications. Accessed 15 Feb 2018.
- 16.ICAP. Resources – PHIA. http://phia.icap.columbia.edu/resources/. Accessed 15 Feb 2018.
- 17.LSHTM. ALPHA Network Data. http://alpha.lshtm.ac.uk/data/. Accessed 15 Feb 2018.
- 18.NTRL. National Technical Reports Library. https://ntrl.ntis.gov/NTRL/. Accessed 15 Feb 2018.
- 19.DHS. STATcompiler. https://www.statcompiler.com/en/. Accessed 16 Feb 2018.
- 20.DHS. The DHS Program - AIDS Indicators Survey (AIS). https://dhsprogram.com/What-We-Do/Survey-Types/AIS.cfm. Accessed 16 Feb 2018.
- 21.HSRC . HSRC - South African national HIV prevalence, incidence and behavior survey. 2012. [Google Scholar]
- 22.UCSF. Integrated HIV Bio-behavioral Surveillance Toolbox. http://globalhealthsciences.ucsf.edu/resources/integrated-hiv-bio-behavioral-surveillance-toolbox. Accessed 16 Feb 2018.
- 23.International AIDS Soceity . Conference Programme IAS. 2017. [Google Scholar]
- 24.ICASA. ICASA 2017 Côte d’Ivoire. http://icasa2017cotedivoire.org/. Accessed 16 Feb 2018.
- 25.CROI. CROI Conference. http://www.croiconference.org/. Accessed 16 Feb 2018.
- 26.Covidence - Accelerate your systematic review. https://www.covidence.org/. Accessed 4 Mar 2018.
- 27.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/BMJ.I4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA-P 2015 checklist. (125 kb)
Search Terms. (19 kb)
Data Availability Statement
Not applicable


