Abstract
Background
Oral and anal sexual behaviours are increasingly reported among adolescents and adults reporting heterosexual sex in peer-reviewed journals in high income countries, but less is known about these behaviours in low and middle-income countries, especially in sub-Saharan Africa. The aim of this systematic review is to describe the prevalence of, and motivations for, oral and anal sex among adolescents and adults reporting heterosexual sex in sub-Saharan Africa.
Methods
A systematic review of published articles that reported oral and or anal sex in sub-Saharan Africa was conducted from seven databases up to and including 30th August 2018.
Results
Of 13,592 articles, 103 met the inclusion criteria. The prevalence of reporting ever practising oral sex among adolescents, university students and a combined population of adolescents/adults ranged from 1.7–26.6%, 5.0–46.4% and 3.0–47.2% respectively. Similarly, prevalences of reported ever practising anal sex ranged from 6.4–12.4% among adolescents, 0.3–46.5% among university students and 4.3–37.8% amongst combined population of adolescents and adults. Higher prevalences of oral and anal sex were reported among populations at high-risk for sexually transmitted infections and HIV and university students and, in most studies, both behaviours were more commonly reported by males than females. Heterosexual oral and anal sexual acts were associated with some high-risk behaviours such as inconsistent condom use and multiple sexual partners.
Conclusion
Reported oral and anal sex between men and women are prevalent behaviours in sub-Saharan Africa. Health professionals and policy makers should be aware of these behaviours and their potential associated health risks.
Electronic supplementary material
The online version of this article (10.1186/s12978-019-0722-9) contains supplementary material, which is available to authorized users.
Keywords: Oral/anal sex, Sexual behaviour, Heterosexual, Adolescent, Adult, Sub-Saharan Africa
Plain English summary
Oral and anal sexual acts are increasingly reported in peer reviewed journals, especially among adolescents and young adults in high income countries. These behaviours are associated with negative health outcomes such as sexually transmitted infections (STIs). Oral and anal sex may be important unrecognised modes of transmission for STIs, contributing to onward transmission. In addition, STIs in the oropharynx and anus may result in poor health outcomes such as oral and anal cancers; however, oral and anal sex are not always regarded as ‘hetero-normative sexual intercourse’, and are often disregarded by researchers, programmers and policy makers. Importantly, both sexual acts are sometimes perceived by some people to be safer than vaginal sex against pregnancy and STIs, and are associated with lower reported use of condoms to prevent HIV and STIs.
We conducted a systematic review of published scientific papers reporting these behaviours in sub-Saharan Africa between 1946 and 30th August 2018. We investigated the prevalences of oral and anal sex, and factors that influenced these behaviours.
Our findings showed that oral and anal sex were commonly reported among adolescents and adults as well as female sex workers. We found that more boys/men reported oral and anal sex than girls/women in most of the studies, and that a substantial number of those engaging in oral and anal sex did not use barrier methods during those sexual acts.
In summary, oral and anal sexual behaviours are commonly reported in sub-Saharan Africa among people reporting heterosexual sex. While testing for oropharyngeal and anal infections may not be feasible in resource-constrained settings, these data are important for researchers, programmers and policy makers to raise awareness of these potential modes of STI transmission. Information about the risk of STI transmission for oral and anal sex should be included in information, education and counseling programmes for both general and key populations at risk for STIs.
Background
Condomless heterosexual oral and anal intercourse have been associated with extragenital sexually transmitted infections (STI) such as Chlamydia trachomatis, Neisseria gonorrhoea, syphilis, hepatitis, herpes simplex virus (HSV) and human papillomavirus (HPV) infections in the anal and oropharyngeal niches [1–6]. Although oral and anal STIs are frequently asymptomatic, they remain an important source of onward transmission [1–3, 5]. Clinical sequelae of oral and anal STIs include pain, anal discharge, ulcerative proctitis, HPV-associated premalignant lesions and cancers [2, 3, 6, 7]. The comparative risk of HIV infection transmission between condomless anal sex and vaginal sex is higher than oral sex, and also, the risk is higher among those engaging in receptive anal sex than insertive anal sex when other HIV prevention methods such as anti-retroviral treatment or preexposure prophylaxis are not used [8, 9].
Several studies have reported a higher prevalence of oral and penile-anal sex among key affected populations’ such as female sex workers (FSWs) [10, 11], men who have sex with men (MSM) [12], entertainment outlet workers, long distance drivers and people who inject drugs, compared to general populations [13]. Most studies report low or inconsistent condom use during oral and anal sex. For example, in Lima, Peru (2010), 98.4% of FSWs aged 18–26 years had performed oral sex in their lifetime and only 20.0% reported condom use during the sexual act [11]. Another study in Peru (2013) showed that 21.2% of FSWs performed oral sex with clients in the previous month, and only 37.6% used condoms consistently while performing oral sex [14]. A study conducted in India (2009–2010) reported that 12.3% of 18–60 year old FSWs engaged in receptive penile-anal intercourse in the past 6 months, and only 48.4% used condoms consistently [15]. In the Netherlands (2016), the prevalence of anal sex in the past 6 months among FSWs aged 18 years and above was 20.0%, and only 31.0% of these FSWs always used condoms with clients [16]. In the USA, a systematic review of anal sex that included published articles between 1987 and 2013 reported that the prevalence of anal sex among FSWs ranged between 0 and 18.0% and that 14.0–82.0% of these FSWs always used condoms during anal sex [17].
Oral and anal sexual behaviours are increasingly reported among general population adolescents and adults reporting heterosexual sex in both developed and developing countries [18–21]. For example, a recent systematic review among young people aged less than 25 years worldwide showed that the average life time prevalence of reported heterosexual anal sex was 22.0%, and this behaviour accounted for 3.0–24.0% of all reported sexual acts [22]. Three sexual behaviour surveys conducted between 1990 and 2012 among men and women aged 16–44 years in the United Kingdom showed that the prevalence of reported heterosexual penetrative anal sex by men in the past year increased from 7.0% in the 1990–1991 survey to 12.2% in the 1999–2001 survey to 17.0% in 2010–2012 [21]. Similarly, the proportion of women that reported any receptive anal sex in the past year also increased from 6.5 to 11.3% to 15.1% over the same periods [21]. Between the first and last surveys, the proportion of those giving or receiving oral sex in the preceding year increased from 65.6 to 75.0% among women and from 69.7 to 77.1% among men [21]. National surveys in Australia in 2001–2002 (aged 16–59 years) and 2012–2013 (aged 16–69 years) also showed a moderate increase in prevalence of reported oral and penile-anal sex over time in both genders [18, 23].
In sub-Saharan Africa (SSA), many studies reporting sexual behaviours in heterosexual relationships have focused on penile-vaginal sex and associated negative health outcomes [24–27]. This has influenced sexual health policies and programmes in many countries within the region [28, 29]. The role of heterosexual oral and anal sexual acts within the spectrum of sexual behaviours needs to be documented within the region, in order to appreciate their potential impact on STI transmission and other associated morbidities such as oral and anal cancers. We conducted a systematic review of the prevalence of, and motivations for, practising heterosexual oral and penile-anal sex, and the cultural interpretations of these behaviours in SSA.
Methods
This review was conducted in accordance with Preferred Items for Reporting of Systematic Reviews and Meta-analyses (PRISMA) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [30, 31]. The protocol was registered in PROSPERO database with registration number CRD42015025311 [32].
Search strategy
The search was conducted in English using seven databases: Medline; Embase; African-Wide Information; Cinahl; Global Health; Scopus; and Popline databases. We used medical subject headings (MeSH) and text words related to oral and anal sex for the search. The terms used for oral sex were oral (sex OR sexual behaviour OR sexual practices), cunnilingus, oral vaginal contact, fellatio, oral penile contact, anilingus, and oral anal contact. The search terms for anal sex included anal (anus OR anal cavity) sex OR anal (sexual behaviour OR sexual practice) or ano-genital (sex OR intercourse). The search was restricted to SSA by using “AND” before adding different search terms for sub-regions (West Africa OR East Africa OR Central Africa OR Southern Africa), and by specific country names. Multi-continent studies that had separate data from any country in SSA were also considered. The search included published articles from 1946 up to and including the final search of 30th August 2018. We also conducted manual searches of bibiliography of relevant publications on the subject. All titles retrieved from the search were compiled and reviewed with Endnote X 8.0 (Thompson Reuters) by one author (IMB); duplications were removed using the Endnote automated system and through a manual check.
Eligibility criteria
The eligibility criteria were determined apriori in the registered protocol [32], and only published original research articles that reported on oral or anal sex with a partner of the opposite sex in adolescents and adults in SSA were considered. The review excluded articles that focused exclusively on non-consensual heterosexual intercourse and MSM, even if men reported sex with both men and women. Commentaries or review articles, letter to editors, editorials, case series and case reports were also excluded. Oral sex was defined as oral contact with the vulva and or vagina (cunnilingus) or penile shaft (fellatio) or anus (analingus). Anal sex was defined as penetration (insertion) of a man’s penis into the woman’s anus or, for women, reception of the penile shaft into the the woman’s anus.
Two authors (IMB, SK) independently screened the titles and abstracts using the eligibility criteria. Thereafter, full-text of selected articles were independently reviewed again by IMB and SK. Discrepancies at each stage of review were resolved through discussion and consensus. DWJ and SCF served as arbitrators for cases that could not be resolved by discussion.
Data extraction
Data were extracted by IMB into pre-specified data extraction form prepared in Microsoft Excel 2010, and verified by SK. The data extracted included author and journals’ name, sampling methods, study location, definition of oral and anal sex, prevalence/proportion of those that reported oral and anal sex, including reasons/motivations and risk factors associated with these behaviours. Prevalence was defined as the proportion of those that reported oral/anal sex by the total number of individuals in the study population.
For reporting periods, studies that used “ever had” or “ever experienced” or “life-time experience” for oral and or anal sex were classified as “ever practiced”. Other specific look back reporting periods recorded were “past 12 months”, “past 3 months”, “past 1 month”, “last sexual act” and the “first sexual act”. We classified studies that used any form of random sampling as “probability sampling” while others that used non-probability techniques were categorised as “convenience”or “snowball” or “venue-based” or “volunteer” sampling. Studies that had participants with increased risk of STI were categorised as key affected populations (e.g. FSWs, HIV positive men and women, recreational facility workers such as bar and guesthouse workers, long distance truck drivers and participants described as “high-risk” in the methods sections of eligible publications).
Assessment of quality of eligible studies
Separate risk of bias tools were used to assess the quality of papers reporting quantitative and qualitative data. For papers reporting quantitative data, a validated tool for observational studies was modified (Additional file 1: Figure S3) [33] by developing a list of methodological features of the eligible studies that could bias the prevalence and risk factor estimates for oral and anal sex. For each study, we assessed documentation of the following items to classify the study as being either at lower or higher risk of bias: description of study population (Yes/No); type of sampling techniques (probability sampling [Yes] or non-probability sampling/not reported [No]); response rate (Yes/No); eligibility criteria (Yes/No); definition of oral sex (Yes/No); definition of anal sex (Yes/No); sexual behaviour roles reported as giving/insertive or receptive/received (Yes/No); risk factor estimates controlled for potential confounders (Yes/No); and inclusion of a statement on the ethical approval (Yes/No). For papers reporting qualitative data, a critical appraisal skill programme tool was used for qualitative studies (Additional file 1: Figure S4) [34]. Each tool has ten fields for assessment; studies with five or more “Yes” fields were considered to be of lower risk of bias.
Data synthesis
Due to the heterogeneity in study populations, study design, sampling strategy and definitions of exposure of interest in the eligible studies, we performed a descriptive analysis of both quantitative and qualitative studies without providing a pooled estimates by meta-analysis. In the descriptive analysis, data were disaggregated by exposure of interest (oral sex, anal sex or both), gender, population category (key affected or general population), country locations and regions. We presented prevalences of outcomes (oral and anal sex) and risk factors for oral and anal sex in quantitative studies. We also used Minitab 18.0 statistical software (Minitab, Inc.) to graphically present individual value plot of prevalences of oral and anal sex by sub-region, study population, population category and risk of bias to visualise the range in prevalences compared by population type, geographical area and risk of bias. Key findings from qualitative studies were summarised and categorised into the following themes: cultural meaning; interpretations; and reported personal experiences.
Results
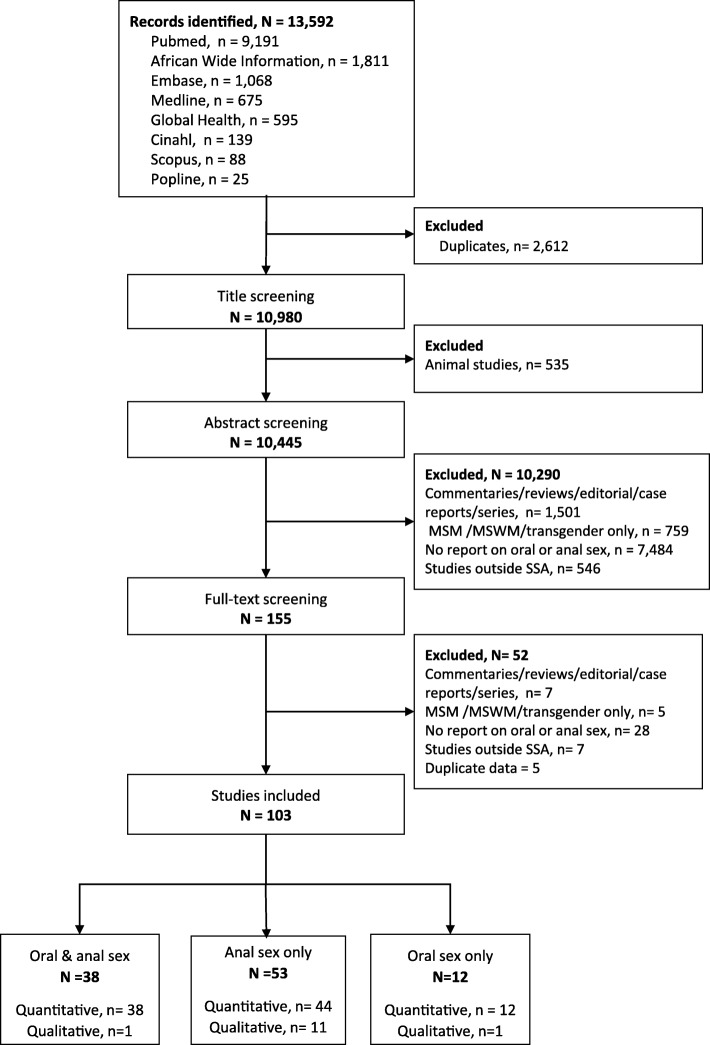
Out of the 13,592 articles retrieved, 155 full texts were reviewed and 103 were included in the descriptive analysis of heterosexual oral and or anal sexual behaviours (Fig. 1). Among the 103 articles reviewed, 38 reported on both oral and anal sex [35–73], 53 reported on anal sex only [74–125], and 12 reported on oral sex only [126–137]. One Nigerian publication out of the 38 articles reported the prevalence of oral and anal sex as a combined outcome [43]. Six articles were mixed method studies (a study each from Ethiopia, Kenya, Nigeria, Tanzania, Zambia and another was conducted in Kenya and Rwanda) [39, 66, 70, 90, 120, 133]. Fifty-nine studies were from Southern Africa, 38 were from East Africa, 20 were from West Africa and four were from Central Africa (Additional file 2: Table S1).
Fig. 1.
PRISMA Flow for the systematic review
Only nine quantitative studies presented the operational definitions of reported oral sex [37, 38, 52, 54, 66, 71–73, 137] and six studies presented operational definitions of reported anal sex [38, 52, 54, 66, 73, 90] in the methods section of the papers. For oral sex, definitions included oral stimulation of either the external female genitalia (i.e. clitoris, vulva and vagina) or the penis [37]; ejaculation during oral stimulation of the male genitalia [38]; contact between the mouth and penis or vagina or anus [54]; and putting one’s mouth on their partner’s penis or vagina or letting their partner put his or her mouth on one’s penis or vagina [52]. For anal sex, definitions included were ejaculation during anal sex [38]; introduction of the penis into the anus or back passage of the partner [54, 66, 73, 90]; and putting the penis into the partner’s anus or letting the partner insert his penis to her anus [52].
Twelve out of 13 articles presented qualitative data investigating motivations, cultural interpretations and personal experiences of anal sex [66, 90, 100–104, 120, 122–125] (Table 1). Of these, seven studies (two from South Africa [100, 101] and three from Tanzania [103, 104, 124], and two multi-country studies conducted in Kenya, Tanzania and Uganda [102] and Uganda, South Africa and Zimbabwe [123]) used qualitative methods only. Six qualitative studies each on anal sex were conducted among key affected populations [90, 102–104, 124, 133] and general populations [66, 100, 101, 120, 122, 123]. No studies included adolescents. One article reported on perception of FSWs on the use of condom for oral sex [133].
Table 1.
Selected data from qualitative studies reporting on heterosexual oral and anal sex in sub-Saharan Africa by year of publication
| Author; Year | Country | Study design | Sampling; Data collection methods | Study population | Gender (M/F); Age (yrs) | Summary of Key findings on oral sex | Assessment of risk of bias |
| Gathece et al. 2000 [133] | Kenya | Mixed methods | Purposive sampling; NS Focus group discussions and In-depth interviews | FSW (KAP) | F: 30.5 | Participants said that there was no need to use condom during oral sex because they felt that oral sex is “safer” than vaginal sex. | high-risk of bias |
| Author; Year | Country | Study design | Sampling; Data collection methods | Study population | Gender (M/F); Age (yrs) | Summary of Key findings on Anal sex | Assessment of risk of bias |
| Stadler et al. 2007 [100] | South Africa | Qualitative | Purposive sampling; Focus group discussion | Adult women in community (GP) | F; NS | Some of the local terminologies of anal sex that were mentioned by participants included: “pata pata”, “matanyula” and “dog style”. Participants had open discussions on anal sex. They mentioned pornographic films and television as sources of information of anal sex. The reported reasons for engaging in anal sex that were discussed included: a form of partner punishment and coercion, sexual experimentation and partner desire or pleasure. Some of the misconceptions that emerged during discussion were: anal sex is safer than penile-vaginal sex, anal sex was regarded as a form of contraception and it could prevent STIs/HIVs. | low risk of bias |
| Mavhu et al. 2008 [122] | Zimbabwe | Qualitative | Purposive sampling; 65 In-depth interviews | Adolescent and Adult in the community (GP) | M and F:18–40 | Participants were uncomfortable with “anal sex” as a question. They preferred anal sex to be referred to as “sex at a place where wastes (faeces) comes out” or “sex at backside”. Some referred to anal sex as “doggy style”. They described anal sex as “homosexual do”. | low risk of bias |
| Ndinda et al. 2008 [101] | South Africa | Qualitative | Purposive sampling; 11 Focus group discussions | Adult men and women in community (GP) | MandF; NS | Participants were reluctant to talk about anal sex. They largely used proxy names in Zulu to describe anal sex. There was poor understanding about the meaning of anal sex. They expressed shock and disbelief when the facilitator told them the meaning of anal sex as “having sex in the faecal passage”. Some participants felt that anal sex is practiced only by MSM. They expressed shock and disbelief when the facilitator told them the meaning of anal sex as “having sex in the feacal passage”. Some participant felt that anal sex is practiced only by MSM. | low risk of bias |
| Ambaw et al. 2010 [66] | Ethiopia | Mixed methods | Purposive sampling; 6 Focus group discussions | University students (GP) | M and F: 17–45 | Participants interpreted sexual intercourse to mean heterosexual and vaginal sex. They believed only “whites” (ferenges) accept anal sex as a form of sexual intercourse | low risk of bias |
| Veldhuijzen et al. 2011 [90] | Kenya and Rwanda | Mixed methods | Purposive sampling; 7 Focus group discussions and 4 In-depth interviews | FSW in Kigali only (KAP) | F; 22–30 | Some FSWs had strong negative perceptions about clients requesting anal sex. They believed that anal sex was mostly requested by non-Rwandans. The most common reported reason for engaging in anal sex was a financial incentive. Some participants said that anal sex practices are associated with alcohol use. Some FSWs reported that their clients do not use condoms for anal sex. | low risk of bias |
| Duby et al. 2014 [102] | Kenya, Tanzania and Uganda | Qualitative | Purposive sampling; 40 Focus group discussions and 54 In-depth interviews | Adult men and women in community/clinics (KAP) | M and F; NS | Participants demonstrated a good knowledge of anal sex, but they were reluctant to discuss their personal experiences. They also regarded anal sex as a taboo. Reported reasons for engaging in anal sex that were discussed included: preserving virginity, contraception, financial incentives, as an alternative when vaginal sex was not feasible (e.g. pregnancy and menstruation), to satisfy their male partner’s pleasure, adventure and novelty. Participants had the misconception that anal sex is safer than vaginal sex, is associated with lesser risk of HIV/STIs and hence there is no reason to use condoms. | low risk of bias |
| Beckham et al. 2015 [103] | Tanzania | Qualitative | Purposive sampling; 3 Focus group discussions and 30 In-depth interviews | FSW (KAP) | F; 20–40 | Participants had open discussion about anal sex including their personal experiences with clients. The main reported reason for engaging anal sex was receiving higher financial incentives than for penile-vaginal sex. Clients paid more for requesting unprotected anal sex. | low risk of bias |
| Mtenga et al. 2015 [124] | Tanzania | Qualitative | Purposive sampling; 24 Focus group discussions and 81 In-depth interviews | Adult men and women in community (KAP) | M and F; NS | Truck drivers and their female partners said heterosexual anal sex is increasingly being practiced in Tanzania. Reasons for engaging in anal sex by men included: seeking better sexual pleasure than vaginal sex, to avoid pregnancy, to prove to their female partners that they are sexually strong and for sexual adventure. Women mentioned receiving greater financial gains with anal sex compared to vaginal sex, that anal sex helped to retain their sexual partners, that it could prevent pregnancy, was an alternative during menstruation and fattening their buttocks to improve their beauty to suitors as reasons for practicing anal sex. Both men and women said a barrier method is not needed during anal sex because the sexual act is safer than vaginal sex, sexual pleasure will be reduced and there is a fear of losing the condom inside the anus. | low risk of bias |
| Wamoyi et al. 2015 [104] | Tanzania | Qualitative | Purposive sampling; 4 Focus group discussions and 16 In-depth interviews | Adult men and women in community/clinics (KAP) | MandF; 20–30 | There was culture of silence about anal sex and participants used proxy names for the practice. Participants believed that anal sex was mostly practiced by foreigners, young people, FSWs and Arabs. Reported reasons for engaging in anal sex that were described by participants included: adventure, ‘trending’, financial incentives and male partner desire. Women felt that anal sex was associated with delivery complications, and anal incontinence. Male participants reported that engaging in anal sex could cause blockage of the anus and urinary system, and cancer. Both male and female participants believed that anal sex is risky and there was a need to use condom during anal sexual act. | low risk of bias |
| Duby et al. 2016 [123, 159] | South Africa; Uganda; Zimbabwe | Qualitative | Purposive sampling; 88 In-depth interviews | Adult women in community (GP) | F: NS | Some participants expressed “shock”, “disbelief”, “disgust”, “embarrassment” and “denial” about anal sex. Some believed that anal sex should not be openly discussed. There were misconceptions that the back side vaginal sex and anal sex were the same sexual act by the participants until it was explained by the researcher with annotated diagram. A number of participants preferred to use alternative name for anal sex e.g. “hidden part”, “down there” and “where faeces comes out”. Women reported engaging in anal sex in order to satisfy their partners’ sexual pleasure/ They considered anal sex to be safer than vaginal sex and as an alternative sexual act when vaginal sex was not feasible. Some engaged in anal sex for better financial gains. | low risk of bias |
| Mazeingia et al. 2017 [125] | Ethiopia | Qualitative | Snow ball Sampling; 18 In-depth interviews | FSW (KAP) | F:18–39 | Participants engaged in anal sex with their clients for financial gains, for personal sexual pleasure, due to coercion by clients and to satisfy their lovers (husband or boyfriends). | low risk of bias |
| Shayo et al. 2017 [120] | Tanzania | Mixed methods | Purposive sampling; 20 Focus group discussions | Adolescent and Adult in the community (GP) | F:> = 15 | Heterosexual anal sex was perceived to be more culturally acceptable especially among the younger population and it is regarded as part of love making. Some women said men force them to practice anal sex against their wish while other women engage in anal sex to protect their sexual relationships. Some women suffered discrimination among their peers for refusing anal sex. A number of participants believed that anal sex is safe without using any barrier methods. Lubricants such as “jelly” were believed to enhance anal sex pleasure and to minimise risk of injury. | low risk of bias |
GP General population, KAP Key affected population, M Male, F Female, FSW Female sex worker, NS Not stated, Yrs Years
The majority of the studies (90 out of 103) focused on participants aged 10 to 49 years (Additional file 2: Table S1). Eleven out of 91 studies included only adolescents (aged ≤19 years) [40, 46–48, 54–56, 127]. Seven studies did not indicate the study population age [35, 81, 100–102]. Overall, 46 studies included both male and females [35, 37–48, 51–56, 59, 64–66, 68, 70, 73, 78, 80, 84, 87, 92, 94, 101, 102, 111, 114, 120, 126–129, 134, 136], 51 studies included women/girls only [36, 49, 50, 57, 58, 60–63, 67, 69, 71, 72, 74, 76, 77, 79, 81–83, 85–91, 96–99, 105, 106, 109, 110, 112, 113, 115–119, 121, 132, 133, 135], and eight studies included men/boys only [75, 93, 95, 107, 108, 130, 131, 137].
Reported condom use during heterosexual oral and anal sex
Condom use was reported in 19 studies during heterosexual anal sex [38, 39, 52, 53, 56, 59, 61, 62, 73, 75, 77, 79, 84, 87, 90, 106, 112, 113, 115, 120], five studies during oral sex [38, 52, 53, 56, 61], and 29 studies for combined vaginal, oral and anal sexual experience [40, 47, 54, 57, 58, 60, 62, 63, 65, 67–69, 76, 80, 82, 85, 88, 92, 97, 98, 105, 107, 111, 116–119, 135]. (Additional file 2: Table S2).
Four (three from South Africa and one from East Africa) out of six studies that reported condomless anal sex were among key affected populations [53, 77, 87, 90], and one study was a general population study in South Africa [77]. Condomless oral sex was also reported in a study among HIV positive men and women in South Africa [53]. Reported condom use tended to be higher during heterosexual anal sex than during oral sex, and was more frequently reported among key affected populations than general populations during heterosexual anal sex. The range of any condom use during oral sex was 1.7–16.5% among three general population studies [38, 52, 56]. Of these, consistent condom use during oral sex was reported by 13.2% of Nigerian and 16.5% of Zimbabwean students [38], and by 12.2% of high school students in Ethiopia [52]. A study in South Africa showed that 54.8% of FSWs reported consistent condom use during oral sex [61].
The range of any condom use during heterosexual anal sex was 6.7–73.1% among eight general population studies [38, 39, 52, 56, 59, 73, 77, 120]. Consistent condom use during anal sex was reported by 22.5 and 27.5% of Nigerian and Zimbabwean students respectively [38], 26.1% of Ethiopian high school students [52] and 36.4% of adolescents and young adults in Tanzania [120]. Among five key affected population studies, the range of condom use during heterosexual anal sex was 13.2.0–67.0% [61, 75, 79, 84, 112]. Two of these studies reported consistent condom use of 45.0% among Kenyan FSWs with their clients [79] and 50% among HIV positive women in a South African city [61]. The range of condomless heterosexual anal sex among four key affected population studies was 7.0–54.3% [53, 79, 87, 90].
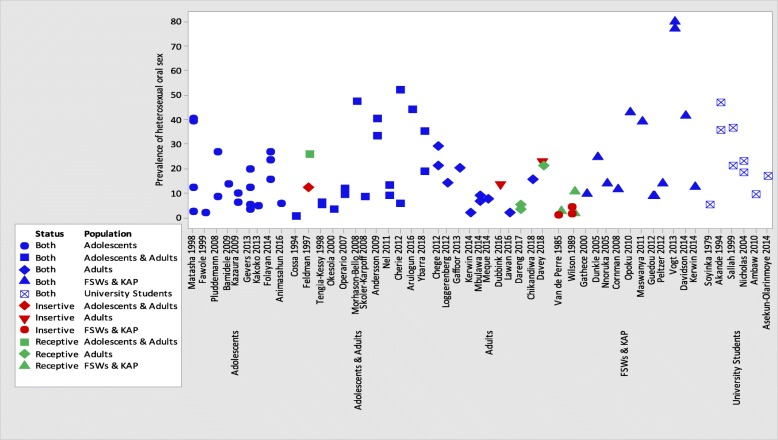
Prevalence of reported oral sex
Only six (two from South Africa and one each from Nigeria, Rwanda, Zambia and Zimbabwe) out of the 51 oral sex studies described the prevalence of reported oral sex as either giving or receiving oral sex [36, 37, 39, 69, 71, 72]. Most studies that reported on prevalence of oral sex were from South African region, among adolescents and young adults, and key affected populations (Fig. 2, Additional file 2: Table S1 and Additional file 1: Figures S1 and S2).
Fig. 2.
Prevalence of oral sex by study population
Reported prevalence of ever practiced oral sex
Twenty-eight general population studies reported on the prevalence of ever practising oral sex (Table 1), of which only two studies (Nigeria and Zambia) described the prevalence of giving and receiving oral sex separately [39, 71]. However, the Zambian study combined these data by gender [39]. Of the remaining 26 general population studies reporting oral sex prevalence, 14 described this by gender: five among adolescents and adults [50, 63, 65, 73, 126]; four among adolescents [46, 48, 54, 135]; two studies among adult women [60, 132]; two studies among university students [41, 66]; and one study among an adult men only [130]. Men/boys tended to report a higher prevalence of ever practising oral sex compared to women/girls across these populations. For example, 26.2% of boys reported ever practising oral sex compared to 8.1% of girls in a cross-sectional study in South Africa [46], and 22.8% of males reported ever practising oral sex compared to 18.2% of female South African university students [41]. The range of reported oral sex prevalence in the remaining studies was 5.0–46.4% among university students [35, 38, 64, 68]; 1.7–26.6% among adolescents [47, 55, 56, 127]; 3.0–47.2% among a combined population of adolescents and adults [45, 52, 128, 136], and 1.7–40.8% in three studies among adult populations [130–132]. In all but adult populations, higher prevalences of ever practicing oral sex were recorded after 2000 compared to before 2000. Studies conducted among university students reported a relatively higher prevalence of oral sex compared with other groups within the general population.
Ten studies amongst key affected populations described prevalence of ever practising oral sex [36, 42, 49, 57, 61, 70, 129, 131, 133, 137]. Three studies were among FSWs, of which only a Rwandan study described prevalence of giving and receiving oral sex separately [36, 57, 133]. The Rwandan study showed that 1.8 and 0.5% of FSWs reported that they ever received or gave oral sex respectively [36]. Prevalence of ever practiced oral sex in the other two studies among FSWs were 9.0% in Kenya [133] and 24.1% in South Africa [57]. The prevalences of ever practiced oral sex in two Nigerian studies were reported to be 1.5% among adult men and women in the general community [70] and 13.3% among HIV positive men and women [42]. In Ghana, 42.3% of ‘women considered to be at risk of STIs’ (working in food and recreational facilities) ever practiced oral sex [49]. Three studies from South Africa reported prevalences of ever practising oral sex among key affected population [61, 129, 137]. A study showed that higher proportion of HIV positive men (79.4%) in the community/clinic reported ever practising oral sex than HIV positive women (76.5%) [129] while the two other studies reported prevalences of ever practiced oral sex of 13.9% [57] among HIV positive women [61] and 15.0% among HIV positive adult men [137] in the community.
Prevalence of oral sex by other reporting periods
Data on the prevalence of oral sex using other reporting periods came from 11 general population studies [40, 51, 52, 54, 56, 58, 59, 62, 69, 72, 134]. Three of these studies found that men/boys generally reported a higher prevalence of oral sex than in women/girls. For example, any oral sex in the past 3 months was reported by 29.0 and 21.0% of 18–34 year old Kenyan men and women respectively [51], and by 4.8 and 2.8% of 12–15 year old South African boys and girls respectively [54]. In contrast, a Tanzanian study showed that having had oral sex during their first sexual experience was reported by 39.0 and 40.0% of primary school boys and girls, respectively [40]. Among a combined population of adolescents and adults in Addis Ababa, the prevalence of reported oral sex in the past 12 months among the 5.4% of the study population that had ever practiced oral sex was 51.6% [52]. Two South African studies reported the prevalence of oral sex in the past 3 months as 8.0% among girls/women above 16 years [58] and 19.9% among women aged 18 years and above [62]. Reported prevalence of oral sex in the past 6 months ranged between 32.9 and 40.0% among adult men and women with their casual and steady partners, respectively in Soweto [59], 13.4% among women in rural Mopani District [69], and 6.2% of men and 8.7% of women in Cape Town [134]. Finally, 22.8 and 21.0% of pregnant and postpartum women in Cape Town described either giving or receiving oral sex in the past 12 months respectively respectively [72].
Among four key affected population studies, two studies among HIV positive men and women described the prevalence of reported oral sex in the past 3 months to be 11.0% in KwaZulu-Natal and 13.4% in Mpumalanga in South Africa [44, 53]. A third study among FSWs and their clients in Harare, Zimbabwe, described higher reporting by men of receiving oral sex from FSWs during their last sexual act than by FSWs giving oral sex during their last sexual encounters (10.0% vs 1.0%) [37]. In the same study, 4.0 and 1.0% of men and FSWs respectively reported giving/receiving oral sex during their last sexual act [37]. Another study among FSWs from South Africa, Uganda and Benin reported that the prevalence of oral sex in the past month with clients was 8.3% [67].
Factors associated with engaging in oral sex
Eight studies investigated factors associated with reported oral sex (Table 2). Five of these studies reported unadjusted estimates [48, 51, 54, 56, 133]. In 2000, a study in Kenya found that older FSWs were less likely to have ever engaged in oral sex than younger FSW [133]. In 2012, another study in Kenya, showed that men tended to report oral sex in the past 3 months more than women (29.0% vs 21.0%) [51]. Similarly, adolescent boys in Tanzania were more likely to report ever practicing oral sex compared with adolescent girls (9.4% vs 5.8%) [48]. In Nigeria, girls were more likely to report oral sex as their last sexual act compared with boys (23.5% vs 15.1%) [56]. Girls in South Africa aged 12–15 years who were “currently dating” compared to girls that were not currently dating were more likely to have reported ever having oral sex with their partners (8.1% versus 0.6%) and also having oral sex in the last 3 months (6.5% versus 0%,) [54]. Similar results on dating status were also reported among boys (20.2% versus 8.1%) [54].
Table 2.
Factors reported to be associated with engaging in heterosexual oral and anal sex among adolescents and adults in sub-Saharan Africa
| Author; Year; Country | Study population (no of individuals) | Test of association | Reported associated risk factor for Oral sex | Summary of results |
| Gathece et al. 2000; Kenya [133] | FSW (322) | Unadjusted, Chi Square | Age | Older respondents were more likely to engage in oral sex (X2 = 18.847, p = 0.002) |
| Kazaura et al. 2009; Tanzania [48] | Adolescents in schools/community (885) | Unadjusted, Chi Square | gender | Male vs female (9.4% vs 5.8% p = 0.07) |
| Chege et al. 2012; Kenya [51] | Adult men and women in community (846) | Unadjusted, Chi Square | gender | Male vs female (29% vs 21%, p = 0.03) |
| Gevers et al. 2013; South Africa [54] | Adolescents in schools/community (474) | Unadjusted, Chi Square and Fisher’s Exact Test | type of sexual relationship by gender |
For Girls
In the past 3 months: currently dating vs not currently dating (6.5% vs 0%; p < 0.01) Ever practiced: currently dating vs not currently dating (8.1% vs 0.6%; p < 0.01) For Boys In the past 3 months: currently dating vs not currently dating (7.8% vs 2.0%; p < 0.06 Ever practiced: currently dating vs not currently dating (20.2% vs 8.1%; p = 0.01) |
| Folayan et al. 2014; Nigeria [56] | Adolescents in schools/community (357) | Unadjusted, Chi Square | gender | female vs male (23.5% vs 15.1%, p = 0.01) |
| Ambaw et al. 2010; Ethiopia [66] | University students (1945) | Adjusted, Logistic regression | Gender; level of education; faculty; place of residence; marital status | Male (AOR = 1.6, 95%CI1.02–2.57); protestant (AOR = 0.59, 95%CI 0.39–0.9); year one student (AOR = 2.14, 95%CI1.23–3.66); business and economics faculty (AOR = 5.47, 95%CI3.09–9.67), technology faculty (AOR = 6.23, 95%CI3.32–11.67), humanities faculty (AOR = 3.15, 95%CI1.92–5.18), social sciences faculty (AOR = 2.96, 95%CI1.61–5.42) and education faculty (AOR = 3.91, 95%CI2.01–7.61); out of campus (AOR = 1.85, 95%CI1.09–3.14); have boy/girlfriend (AOR = 1.81, 95%CI1.17–2.8) |
| Cherie et al. 2012; Ethiopia [52] | Adolescents in schools/community (3543) | Adjusted, Logistic regression | age; gender; attitude to oral sex; aspiration for college education; self-esteem; maternal education; partner education; perception of peer oral sexual activity and living arrangement | Younger age (AOR = 3.2, 95%CI 1.9–5.3); female (AOR = 1.3, 95%CI 1.1–2.2); positive attitude to oral sex (AOR = 2.3, 95%CI 1.7–4.5); low aspiration to attend college education (AOR = 3.1, 95%CI = 2.8–5.9); low self-esteem (AOR = 2.1, 95%CI 1.7–3.9); illiterate mother (AOR = 11.5, 95%CI 6.4–18.5); illiterate father (AOR = 1.4, 95%CI 0.9–3.2); friends that engage in oral sex (AOR = 5.7, 95%CI 3.6–11.2); living with both parents (AOR = 0.4, 95%CI 0.2–0.9) |
| Kerwin et al. 2014; Malawi [131] | Adult men in community (2753) | Adjusted, Logistic regression | no of lifetime sex partners; ever used condom for oral sex; history of spending in the past 3 months | higher total lifetime sex partner (AOR = 1.04, 95%CI 1.02–1.06); ever used condom (AOR = 3.16, 95%CI 1.47–6.8); history of spending in the last 3 months (AOR = 1.94, 95%CI 1.55–2.42) |
| Author; Year; Country | Study population (no of individuals) | Test of association | Reported associated risk factor for Anal sex | Summary of results |
| Chege et al. 2012; Kenya [51] | Adult men and women in community (846) | Unadjusted, Chi Square | Gender | female vs male (25% vs 16%, p = 0.03) |
| Gevers et al. 2013; South Africa [54] | Adolescents in schools/community (474) | Unadjusted, Chi Square and Fisher’s Exact Test | type of sexual relationship by gender |
For Girls
In the past 3 months: currently dating vs not currently dating (2.4% vs 0%, p = 0.09) Ever practiced: currently dating vs not currently dating (3.3% vs 0%, p = 0.04) For Boys In the past 3 months: currently dating vs not currently dating (13.3% vs 2.0%, p < 0.01) Ever practiced: currently dating vs not currently dating (15.6% vs 6.0%, p = 0.01) |
| Priddy 2011; Kenya [89] | FSW (200) | Unadjusted, Chi Square | type of sexual relationship | regular vs casual vs primary (35% vs 29% vs 9%, p < 0.01) |
| Kalichman et al. 2009; South Africa [84] | Adult men and women in community /clinic (M = 2593; F = 1818) |
Adjusted, Logistic regression | age; sexual relationship; history of condom use; history of STI; transactional sex; alcohol and cannabis abuse use in past 3 months; HIV test and status; number of sexual partners | Older age (AOR = 0.97, 95%CI 0.96–0.98); married/living with partner (AOR = 0.62, 0.5–0.77); never used condom (AOR = 1.79, 1.49–2.17); history of STI (AOR = 1.64, 1.46–1.85); received gift for sex (AOR = 1.77, 1.57–1.99); given gift for sex (AOR = 1.7, 1.51–1.9); alcohol use in past 3 months (AOR = 2.16, 1.77–2.63); cannabis use in past 3 months (AOR = 1.97, 1.62–2.4); had HIV test (AOR = 1.44, 1.19–1.73); test HIV positive (AOR = 2.62, 1.89–3.63); increased number of sexual partners (AOR = 1.26, 1.2–1.32); unprotected vaginal intercourse (AOR = 0.97, 0.94–0.98); previous vaginal intercourse with condom (AOR = 1.04, 1.03–1.05); increasing percentage condom use during vaginal intercourse (AOR = 5.61, 4.27–7.37) |
| Ambaw et al. 2010; Ethiopia [66] | University students (1921) | Adjusted, Logistic regression | Faculty; marital status | Business and economics faculty (AOR = 6.3, 95%CI2.64–15.05), technology faculty (AOR = 7.5, 95%CI2.96–18.99), humanities faculty (AOR = 4.59, 95%CI2.19–10.05), social sciences law faculty (AOR = 3.02, 95%CI1.15–7.94) and education faculty (AOR = 5.85, 95%CI2.26–15.1); ever married (AOR = 4.06, 95%CI1.53–10.79) |
| Veldhuijzen et al. 2011; Rwanda and Kenya [90] | FSW Kigal = 800; Momb = 820 |
Adjusted, Logistic regression | inconsistent condom use; number of sexual partner; alcohol use before sex; year of sex work and history of genital symptoms |
For the Kigali cohorts - inconsistent condom use with casual partner (AOR = 5.9, 95%CI 1.4–24.7); had more than 5 sexual partners in last week (AOR = 4.34, 95%CI 1.52–12.36); regular use of alcohol before sex (AOR = 2.83, 95%CI 1.37–5.84). For Mombasa cohorts - more than 5 years of sex work (AOR = 2.44, 95%CI 1.22–4.89); inconsistent condom use with casual partner or client (AOR = 2.1, 95%CI 1.10–4.20); condom not used in the last sex (AOR = 3.40, 95%CI 1.70–6.80); had more than 5 sexual partners in last week (AOR = 2.20, 95%CI 1.10–4.30); had genital symptoms (AOR = 3.60, 95%CI 1.70–7.90). |
| Kalichman et al. 2011; South Africa [87] | Men and women attending bar/night clubs (4965) | Adjusted, Logistic regression | age; education; type of sexual relationship; meeting sexual partner in Shebeen in past months | Age (AOR = 0.97, 95%CI 0.96–0.98); primary sexual partner (AOR = 1.56, 1.19–2.05); casual sexual partner (AOR = 2.33, 1.92–2.83); meeting sexual partner in Shebeen (drinking spot) in the past month (AOR = 1.81, 95%CI 1.47–2.22) |
| Cherie et al. 2012; Ethiopia [52] | Adolescents in schools/community (3543) |
Adjusted, Logistic regression | age; gender; attitude to oral sex; aspiration for college education; self-esteem; maternal education; partner education; perception of peer oral/anal sexual activity and living arrangement | Younger age (AOR = 1.7, 95%CI 1.3–3.1), female (AOR = 2.9, 1.6–4.7), having positive attitude towards anal sex (AOR = 6.2, 95%CI 3.8–12.4), having low aspiration for college education (AOR = 4.2, 95%CI 2.8–8.1), having low self-esteem (AOR = 1.6, 95%CI 1.2–3.1); illiterate mother (AOR = 11.6, 95%CI 7.8–19.6); illiterate father (AOR = 7.8, 95%CI 5.3–14.9); friends that engage in oral/anal sex (AOR = 9.7, 95%CI 5.4–17.7); living with both parents (AOR = 0.4, 95%CI 0.2–0.9) |
AOR Adjusted odds ratio, CI Confidence interval, M Male, F Female
Two studies from Ethiopia and a study from Malawi reported adjusted estimates for the association between potential risk factors and reported oral sex [52, 66, 131]. A study among 3543 adolescents in high schools in Ethiopia showed that reporting oral sex was associated with having an illiterate mother, being younger (15–16 years compared to 17 years and older), being female, having a perception of oral sexual activity in peers, having a positive attitude to oral sex and low self-esteem [52]. Of the 5.4% who reported ever having had oral sex, 13.5% had initiated oral sex before the age of 10 years [52]. Another Ethiopian study found that ever practising oral sex among university students was associated with male gender; being a first year undergraduate; being a student in faculties of business and economics, technology, humanities social sciences and education; living off campus; being Protestant Christian denomination; and having boy/girl friends [66]. A study among Malawian men that reported ever practicing oral sex had three times odds of ever using condoms, two times odds of spending money in the last 3 months and having higher number of lifetime sexual partners than men with no history of oral sex [131].
Motivations for engaging in oral sex
Only one study reported on motivations for engaging in oral sex. This study was conducted among Ethiopian school boys and girls aged 15–24 years in Addis Ababa [52]. The main motivations reported by participants were preventing pregnancy (95.9%), minimizing risk of HIV acquisition (86.5%), preserving virginity (85.8%) and reducing the risk of STIs (80.4%). Of those having oral sex within the past 12 months, 48.0% had received a gift in exchange for practising oral sex.
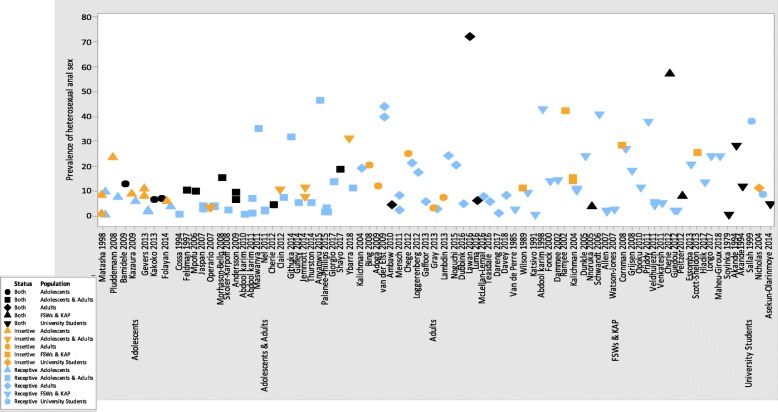
Prevalence of reported heterosexual anal sex
Sixty-five out of 82 studies distinguished the prevalence of reported anal sex into ‘insertive’ by male participants or ‘receptive’ by female participants [36, 37, 40, 41, 44, 46, 48–51, 54, 56–58, 60–67, 69, 71–77, 79–99, 105–113, 115–119, 121]. Most studies that described anal sex were from Southern and East African countries, among adolescents and young adults, and key affected populations (Fig. 3, Additional file 2: Table S1 and Additional file 1: Figures S4 and S5). We used the same reporting periods as oral sex in presenting prevalences of heterosexual anal sex in different populations.
Fig. 3.
Prevalence of heterosexual anal sex by study population
Reported prevalence of ever practiced heterosexual anal sex
Fifty-one out of 82 studies reported on the prevalence of ever practising heterosexual anal sex. Twenty-three out of 51 studies were conducted among key affected populations [36, 42, 49, 57, 61, 70, 74–76, 79, 82, 89, 93, 105–108, 110, 112, 114, 117, 118]. Seventeen of the 28 general population studies described reported prevalence of receptive or insertive heterosexual anal sex separately [41, 46, 48, 50, 54, 60, 63–66, 71, 73, 95–97, 109, 113]. Of these, seven studies were among adolescents and adults [50, 60, 65, 73, 95, 96, 113], five studies were conducted among adolescents only in schools/communities (South Africa [46, 54, 97], Tanzania [48] and Mozambique [63]), three studies among university students in South Africa [41, 64, 66] and two studies among adult women [71, 109]. In seven studies, the proportion of boys that reported ever practising heterosexual insertive anal sex was higher than proportion of girls that reported ever engaging in receptive anal sex [41, 46, 48, 54, 65, 66, 73]. For example, in Tanzania, 8.5 and 5.4% of boys and girls reported ever having insertive or receptive anal sex, respectively [48]. In South Africa, 10.8 and 8.4% of male and female university students reported ever engaging in insertive or receptive anal sex, respectively [41]. In the three studies that were conducted among girls/women only, the reported prevalence of receptive anal sex among adolescents and adults was 34.8% in Tanzania [50] and 7.7–11.3% in South Africa [95], and in a study from South Africa a prevalence of 1.5–1.9% was reported among adult women [60].
In the remaining 11 general population studies that reported prevalence of ever practiced anal sex among boys/men and girls/women: three were among university students [35, 38, 68] and four studies each were among adolescents and adults in schools/community [39, 45, 52, 78] or adolescents in schools [47, 55, 56, 80]. The reported prevalence of ever practising anal sex among university students in Nigeria ranged between 0.3–4.4% [35, 68]. In another similar study, 35.2% among university students in Zimbabwe, and 46.4% among university students in Nigeria reported ever practising in anal sex [38]. Studies that combined adolescent and adult populations reported prevalences between 4.3–34.8% from Zambia, South Africa, Nigeria and Ethiopia [39, 45, 52, 78]. Studies amongst adolescents in schools/communities showed that the prevalence of ever practicing anal sex was 6.7–12.4% in Nigeria [47, 56], 2.4–3.8% in South Africa [80] and 6.4% in Tanzania [55].
In key affected populations, the reported prevalence of ever practising receptive heterosexual anal sex in nine different studies among FSWs was 2.3–42.8% in Rwanda [36], Cameroun [110], Uganda [118], Kenya [74, 79, 89] and South Africa [57, 76, 106]; 42.0% among male truck drivers in South Africa [75]; 25.4% among men attending bars/night clubs in South Africa [93]; 11.5% [49], 2.4% [82] and 0.4% [105] among women working in food and recreational facilities in Ghana, Tanzania and Kenya respectively. Other reported prevalences of anal sex were 3.3–17.1% among HIV positive men and women in Nigeria [42] and South Africa [61], 5.7–72.0% among adult men and women in community/clinics in Nigeria [70] and Cameroun [114], 11.7–20.0% among adult men only in Nigeria [108] and Angola [107], and 13.4% among adolescents and adults women in South Africa [117].
Prevalence of heterosexual anal sex by other reporting periods
Six key affected population studies reported specifically on insertive or receptive anal sex within the past 3 months [44, 53, 83, 84, 90, 91]. Higher prevalences of insertive anal sex in the past 3 months were reported by men compared to receptive anal sex reported by women among key affected populations [44, 84]. Similarly, two key affected population studies in South Africa (one study each among HIV positive men and women [44] and adult men and women [84]) showed higher prevalences among men that reported practising insertive anal than women that engaged in receptive anal sex. Three other studies among HIV positive men and women in Kenya, South Africa and Zimbabwe described the prevalence of heterosexual anal sex in the past 3 months to be 18.0% [83], 7.7% [53] and 4.8% [91] respectively.
Four studies among key affected populations presented prevalences of anal sex in the past month [67, 81, 87, 92]. One study compared prevalences of anal sex in the past month by method of data collection and found that women in food and recreational centres in Tanzania reported a higher prevalence of anal sex using daily diaries (2.1%) to record their sexual behaviours compared to face-to-face interviews (1.4%) [81]. The other two studies from South Africa reported the prevalence of insertive anal sex in the past month among men attending bars/night clubs and men patronising alcohol drinking points to be 15.0% [87] and 10.6% respectively [92]. In the same studies, 11.0% of women in bars/night clubs [87] and 6.9% women at alcohol drinking points reported receiving anal sex in the past month [92]. Another study reported that 2.2% of FSWs in South Africa, Uganda and republic of Benin received anal sex in the past month [67]. Other reporting periods used to describe prevalence for anal sex among key affected populations were past 12 months in 3 studies (two from Tanzania and Cote-d’ivoire) [111, 120, 121], and during the most recent sexual acts in 2 studies from Zimbabwe [37] and the Central African Republic [119].
Seven general population studies, including six from South Africa, reported on insertive or receptive anal sex in the past 3 months [51, 54, 58, 62, 77, 98, 99]. The range of reported prevalence of receptive anal sex in three studies among South African women was 5.6–20.3% [62, 77, 98]. Two studies among adolescents and adults reported a prevalence of 2–3.0% [58, 99]. In the same country, a study showed that 1.1% of girls reported receptive anal sex while 7.4% of boys reported insertive anal sex during the same period [54]. A Kenyan study showed that 25.0% men reported insertive anal sex while 21.0% women reported receptive anal sex in the preceding 3 months [51].
Twelve studies used different reporting periods (past 12 months [52, 72, 115], past 6 months [59, 69, 94], past month [85, 86, 88], during first sexual act [40] and last sexual act [56, 116]). Within these reporting periods, the highest reported prevalence of anal sex was 57.1% among anal sex experienced Ethiopian high school students in the past 12 months [52], 9.2% among South African young men and women in the past 6 months [59], 7.8% of South African adult women in the past month [88] and 5.6% of Nigerian boys during their last sexual act [56].
Factors associated with engaging in anal sex
Eight studies explored factors associated with practising heterosexual anal sex (Table 2). Two of the three studies that presented unadjusted estimates showed that the reported prevalence of heterosexual anal sex was associated with type of sexual relationship [54, 89]. A study amongst South African adolescents found that the prevalence of having ever practised heterosexual anal sex was higher among ‘currently dating’ girls (3.3% vs 0%) and boys (15.6% vs 6.0%) than those with ‘no dating partners’ [54]. A similar result was also described in the same study among boys that reported prevalence of anal sex act in the past 3 months [54]. In Kenya the frequency of anal sex in past month among FSWs was higher among those with regular and casual partners than those with primary partner only [89]. In the same study, the frequency of condom use among FSWs was lower during anal sex than during vaginal sex (data not shown). Unlike the general gender pattern observed in other studies, women in Kenya reported a higher prevalence of heterosexual anal sex than men (25.0% vs 16.0%) [51].
Five studies reported adjusted estimates on factors associated with reported anal sex [52, 66, 84, 87, 90]: A Kenyan study among FSWs showed that the odds of reporting heterosexual anal sex was about four times higher among those with current genital symptoms versus no genital symptoms, three times with inconsistent condom use during last sex compared to those reporting consistent condom use, two times with at least 5 years of sex compared to those with more than 5 years, six times with inconsistence condom use with casual partners than those that used condom, and higher number of sexual partners than those with lower number [90]. A study among FSWs in Rwanda found that inconsistent condom use with casual sex partners (adjusted odds ratio (AOR) = 5.9) and a higher number of sexual partners (AOR = 4.3) were also identified as risk factors for reporting heterosexual anal sex [90]. In addition, the odds ratio for those with regular use of alcohol before sex was about three times associated with reporting anal sex than FSWs that did not regularly use alcohol before sex. A study among men and women attending bars/night clubs in South Africa found the following factors to be associated with reported anal sex in the past month: younger age, having casual sexual partners compared to regular partner, having sex with only one sexual partner compared to having multiple recent sexual partners, and meeting their sexual partners in shebeens (alcohol drinking venues) in the past month [87].
Other risk factors associated with engaging heterosexual anal sex that were reported among men and women in the community and special treatment clinics in South Africa included the following [84]: never using condoms; previous transactional sex; cannabis use in the past 3 months; previously tested for HIV and being HIV positive. Being older, married or living with a partner and previous condomless vaginal intercourse reduced the risk of reporting anal sex. Factors associated with ever practising heterosexual anal sex among Ethiopian school boys and girls included younger age, being a boy, having a positive attitude towards anal sex, having low aspirations for college education, having low self-esteem, having a perception of peer engagement in anal sex and having an illiterate mother or father [52]. However, adolescents living with both parents were less likely to engage in anal sex [52]. In another Ethiopian study, reporting ever having had anal sex experience by university students was associated with enrolment in non-medical university faculties compared with students enrolled in a medical faculty [66]. In the same study, university students that had ever married were more likely to report previous anal sex experience than single students [66].
Motivations for engaging in anal sex
A study in Ethiopia described the motivations for engaging in anal sex among school-attending boys and girls aged 15–24 years [52]. The motivations included minimizing the risk of pregnancy (92.1%), preserving virginity (85.5%), minimising the risk of STIs (82.9%), and minimising risk of acquiring HIV (77.6%). Other motivations reported were desire by the sexual partner, increasing sexual pleasure, and self-preference for anal sex. Amongst the 57.0% who had reported in anal sex in the previous 12 months, 52.3% had received money or gifts for engaging in anal sex.
Four studies (two from Tanzania [103, 104], one from South Africa [100] and one multi-site study from Kenya, Tanzania and Uganda [102]) explored motivations for engaging in anal sex using qualitative research. Their findings showed that motivations differed between men and women (Table 1). Reasons mentioned by women were preserving their virginity, to promote a sexual relationship or to avoid a domestic quarrel, to prevent pregnancy, as an alternative during menstruation or during pregnancy or when there is evidence of a sexually transmitted infection, and in exchange for money [101, 102]. Motivations reported by men included adventure, influence from their peers, to avoid unwanted pregnancy, to enjoy enhanced sexual pleasure and to show sexual supremacy over women [104].
Cultural meaning, interpretations and personal experiences of anal sex
Twelve qualitative studies explored interpretations of anal sex (Table 1). Five qualitative studies reported on the culture of silence and reluctance to openly discuss heterosexual anal sex [90, 100, 103, 122, 123]. For example, a study in rural South Africa among men and women found that some participants considered heterosexual anal sex to be too sensitive for discussion and some even expressed shock, disappointment and threatened to abandon a focus group discussion when it was brought up for discussion by the facilitator [101]. In the same study, men were reported to be more willing to discuss anal sex than women. In another study in Kenya, men in two counties reported that they were reluctant to discuss anal sex among themselves as it was regarded as a cultural taboo to claim knowledge of, or practice, anal sex in their community [102].
Two studies (Rwanda and South Africa) reported that women, including FSWs, perceived anal sex as punishment, and they only engaged in it to avoid quarrel from their partners/clients and for financial benefits [90, 100]. Other reasons mentioned by participants for engaging in anal sex included adventure, and coercion [100, 120, 124, 125]. There was some evidence of misunderstanding the definition of anal sex; for example, one South African study reported that participants in rural part of Soweto believed anal sex to mean “penile-vaginal penetration from behind” [100]. In several other studies, interviewees believed that anal sex is “foreign” to the African culture [66, 90, 101] or that it is exclusively practiced by men who have sex with men [101]. Some believed anal sex is safer than penile-vaginal sex [120, 124].
Seven qualitative studies presented personal experiences of men and women about heterosexual anal sex [90, 100, 101, 103, 104, 120, 124]. Findings from three studies showed that men expressed more desire for anal sex than women as they often regarded the act as a sign of manhood, and women were reported not to be receptive to openly discussing or demanding anal sex [100, 103, 124]. Tanzanian and South African studies reported that both men and women sometimes used proxy names such as slang or colloquial terms to describe anal sex [101, 104]. A study among FSWs in Kigali reported extreme resentment towards clients who asked for anal sex as they regarded the practice to be uncomfortable, emotionally painful and associated with STIs and faecal and urinary incontinence [90]. However, some young women in Tanzania said that anal sex was more acceptable and enjoyable when performed with “jelly” lubricants [120].
Assessment of quality of studies
The detailed assessment of risk of bias are presented in Additional file 2: Table S3 and Additional file 1: Figures S3 and S6. Overall, 53 out of 94 quantitative studies assessed had low risk of bias in their methods: 51 of these described heterosexual anal sex [40, 52, 54–57, 59, 60, 62, 64–67, 69–76, 79, 80, 82–84, 86–88, 90–93, 95, 96, 98, 99, 107–112, 115–121] while 24 studies described oral sex [40, 52, 54–60, 62, 64–67, 69–73, 131, 132, 135–137]. The majority of articles assessed to be low risk were from Southern and Eastern Africa. Most studies with a high risk of bias used convenience sampling techniques to recruit study participants, had unclear eligibility criteria, did not include operational definition of outcome measure in the methods, control for potential confounders and present prevalence of oral or anal sex by the reported sexual behaviour role, and gave no indication as to whether ethical approvals were obtained (data not shown – Additional file 2: Table S3). All the nine qualitative studies assessed had low risk of bias (Additional file 2: Table S4).
Discussion
This is the first systematic review of reported prevalence of oral and anal sex among adolescents and adults reporting heterosexual sex in SSA. The review showed a large range of prevalences for both behaviours. Generally, the range of prevalences were similar among key affected and general populations. However, reported prevalences of oral and anal sex among FSWs and university students tended to be higher than other population groups of adolescents and adults. In addition, reported prevalences of both oral and anal sex tended to be higher among males than females. Few studies reported the use of condoms or other barrier methods with oral and anal sex with a number of them reporting low or inconsistent condom use during oral and anal sex. Factors associated with these behaviours showed that those who engaged in oral and anal sex often also engaged in other high-risk activities such as having frequent and multiple sexual partners, illegal substance or alcohol use, and inconsistent condom use. Oral and anal sex are important modes of transmission for STIs, and these data are vital for understanding sexual behaviours in populations at high risk for STIs including HIV, oral and anal cancers.
Findings from three qualitative studies provided possible explanations for engaging in unprotected anal sex [100, 102, 103] including that it was regarded as less risky than vaginal sex, and could prevent STIs including HIV. Anal sex was also associated with exchange of money or gifts among FSWs. Studies from USA also showed that the belief that oral sex is without health risk, and not regarding oral and anal sex as “having sex” might have accounted for low condom use [138, 139]. Qualitative research also illustrated a culture of silence in discussing anal sex, and many of those that discussed it expressed shock and disbelief that such sexual act existed within heterosexual relationships. This, combined with the belief by some boys/men that performing heterosexual anal sex demonstrates a supremacy over girls/women, may further create a culture of secrecy, shame, and transgression. Indeed, women reported coercion for anal sex and a perception that men engaged in it to punish them. In such an environment, discussion about safety and condom use may be undermined, and strategies for STI control may be challenging. More research is needed to further understand motivations for and meaning of engaging in oral and anal sex in SSA, as well as opportunities and challenges for addressing violence, safety and STI/HIV risk.
The comparatively higher prevalences of oral and anal sex among some key affected populations compared to general populations in this review has also been widely reported in high income countries. Some studies among key affected populations suggested that these behaviours were associated with increased use of alcohol and substance abuse during sexual activity, increased frequency of sexual acts and multiple sexual partners [140–144]. Men who engaged in anal sex with FSWs were more likely to have consumed alcohol and be a frequent customer [140]. Apart from these reasons, it has also been reported that FSWs engaged more in oral and anal sex to satisfy their clients’ requests and for financial gain [140, 145]. This was corroborated by two qualitative studies in this review where FSWs reported economic gain as the main reason for engaging in anal sex [90, 103]. Adolescents in Ethiopia also reported receiving gifts or money for practicing oral and anal sex [52].
We observed high prevalences of oral and anal sex among university students, and this is similar to finding from studies in high-income countries. In the UK NATSAL survey, young people were more likely to report oral and anal sex than older adults, irrespective of gender [21]. The increased reporting of oral and anal sex among young people has been associated with changing perception of sexual activity among younger generation and the influence of social media including pornography amongst others [146–149]. An Australian study that was conducted among young people found that previous anal sex experience was associated with frequent use of pornography [149]. It will be important that adolescent health providers are aware that these behaviours are now being practiced by young people across the continent and education programmes will need to be tailored to addressing the risk associated with these behaviours and how these can be prevented.
Although routine testing for oropharyngeal and anal infections is recommended in high-income countries for sexually active MSM [150, 151], some argue that exclusion of sexually active women with history of receptive oral and anal sex from routine testing will lead to missed opportunities for early detection of STI and the prevention of onward transmission [150, 151]. The cost-effectiveness of routine testing for asymptomatic pharyngeal and anal infection is unclear [151]. Routine testing strategy is not yet a feasible option for people reporting heterosexual oral and anal sex in SSA. However, raising awareness on the risk of STIs during unprotected oral and anal sex through information, education and counselling programmes could be a practicable strategy in the region. It is also imperative that policy makers in the region expand the concept of hetero-normative sexual act to include oral and anal sex.
The higher prevalence of reported oral and anal sex by males in this review should be interpreted within a context of reporting bias. Studies in SSA and other regions have shown that during self-reported sexual behaviour interviews, males tend to report a higher number of sexual partners, non-marital partners and concurrent relationships than females, and females may under-report numbers of sexual partnerships [152–154]. Reporting differences by gender may also vary according to the specific sexual behaviour. Available evidence from population studies in UK and USA showed that more men or boys reported receiving oral sex than women or girls in heterosexual relationship [21, 155]. In the same report, more girls were reported to give oral sex than boys. Some researchers argued that gender differences in the reporting of sexual activity might be influenced by the perception of sexual pleasure, health risks and beliefs [156, 157]. A qualitative study further showed that men preferred to receive oral sex from their partners than to give their partners because they perceived receiving oral sex to be less risky and giving oral sex to be a dirty and dangerous practice [158].
The strengths of this review are the range of prevalences across geographic sub-regions, populations and ages within SSA. However there are a number of limitations. There were very few population-based estimates of the oral and anal sex prevalence such as prevalences reported in the population-based studies in the UK and Australia [18, 21]. Instead, there was a wide range of prevalences of oral and anal sex observed in various population sub-groups which are unlikely to be generalizable. In addition, there were several methodological weaknesses in the studies reviewed including consistent operational definitions of oral and anal sex. Furthermore, studies also used different reporting periods making it challenging to pool and compare results across population sub-groups and settings in SSA.
In addition to reporting gender reporting bias discussed above, there is likely to be under-reporting of these sensitive behaviours due to social desirability bias, especially for heterosexual anal sex, since this practice is not well accepted in some communities [101, 102, 159]. Many studies were assessed as having a high-risk of bias and did not provide information on how their sample sizes were determined. Lastly, restricting our inclusion criteria to only published peer reviewed articles, limiting our search to seven databases and exclusion of MSM that also engaged in heterosexual oral and anal sex could have missed other studies that reported on oral and anal sex in the region.
Conclusion
In summary, oral and anal sex are commonly practiced among adolescents and adults reporting heterosexual sex in SSA, often without condom use. Future sexual reproductive health research investigating risks for STIs should incorporate questions on oral and anal sex using clear definitions of these behaviours. Well powered and rigorous population based study designs similar to studies in United Kingdom and Australia [18, 21, 160] are needed to understand population estimates of these behaviours, their associated morbidities, and changes in sexual behaviour trends over time. Researchers should also consider using qualitative research methods, complimentary tools such as pictures/drawings and other visual aids to elicit more accurate responses from participants [159]. Accurate data are needed to inform reproductive and sexual health policies, and information on oral and anal sex and their health risks should be included in information, education and counselling messages for both the key affected and general populations.
Additional files
Figure S1. Prevalence of oral sex by sub-region. Figure S2. Prevalence of oral sex by population category. Figure S3. Prevalence of oral sex by risk of bias. Figure S4. Prevalence of anal sex by sub-region. Figure S5 Prevalence of anal sex by population category. Figure S6. Prevalence of anal sex by risk of bias. (DOCX 122 kb)
Table S1. Selected data from quantitative studies reporting on heterosexual oral and anal sex in sub-Saharan Africa by year of publication. Table S2. Reported condom use during penetrative heterosexual sex (oral, anal and vaginal). Table S3. Risk of bias assessment for quantitative studies. Table S4. Risk of bias assessment of qualitative studies. (XLSX 41 kb)
Acknowledgements
IMB received grant support from the University of Ibadan, Ibadan, Nigeria for his PhD programme at the London School of Hygiene and Tropical Medicine, London. SCF (MR/N023692/1 and MR/R010161/1) and KB (MR/R010161/1) were funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement and is also part of the EDCTP2 programme supported by the European Union.
Funding
Not applicable.
Availability of data and materials
Interested parties can obtain all available data by contacting the corresponding author.
Authors’ contributions
IMB participated in the design, conducted literature search, undertook screening of literature, extracted data and wrote the first draft. SK participated in screening of literature, verified data extraction and revised the manuscript. DWJ, SCF and KB participated in the design, supervised the literature search, data extraction and synthesis of results, including revision of the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Imran O. Morhason-Bello, Email: imranmorhasonbello@gmail.com
Severin Kabakama, Email: skabaka@yahoo.com.
Kathy Baisley, Email: Kathy.baisley@lshtm.ac.uk.
Suzanna C. Francis, Email: Suzanna.francis@lshtm.ac.uk
Deborah Watson-Jones, Email: Deborah.watson-jones@lshtm.ac.uk.
References
- 1.van Liere G, Dukers-Muijrers N, Levels L, Hoebe C. High proportion of anorectal chlamydia trachomatis and Neisseria gonorrhoeae after routine universal urogenital and anorectal screening in women visiting the sexually transmitted infection clinic. Clin Infect Dis. 2017;64(12):1705–1710. doi: 10.1093/cid/cix243. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Lopez C, Morales-Angulo C. Otorhinolaryngology manifestations secondary to oral sex. Acta Otorrinolaringol Esp. 2017;68(3):169–180. doi: 10.1016/j.otorri.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Taylor S, Bunge E, Bakker M, Castellsague X. The incidence, clearance and persistence of non-cervical human papillomavirus infections: a systematic review of the literature. BMC Infect Dis. 2016;16:293. doi: 10.1186/s12879-016-1633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velicko I, Ploner A, Sparen P, Marions L, Herrmann B, Kuhlmann-Berenzon S. Sexual and testing behaviour associated with chlamydia trachomatis infection: a cohort study in an STI clinic in Sweden. BMJ Open. 2016;6(8):e011312. doi: 10.1136/bmjopen-2016-011312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan PA, Robinette A, Montgomery M, Almonte A, Cu-Uvin S, Lonks JR, Chapin KC, Kojic EM, Hardy EJ. Extragenital infections caused by chlamydia trachomatis and Neisseria gonorrhoeae: a review of the literature. Infect Dis Obstet Gynecol. 2016;2016:5758387. doi: 10.1155/2016/5758387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chancellor James A., Ioannides Sally J., Elwood James M. Oral and oropharyngeal cancer and the role of sexual behaviour: a systematic review. Community Dentistry and Oral Epidemiology. 2016;45(1):20–34. doi: 10.1111/cdoe.12255. [DOI] [PubMed] [Google Scholar]
- 7.Assi R, Hashim PW, Reddy VB, Einarsdottir H, Longo WE. Sexually transmitted infections of the anus and rectum. World J Gastroenterol. 2014;20(41):15262–15268. doi: 10.3748/wjg.v20.i41.15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Leary Ann, DiNenno Elizabeth, Honeycutt Amanda, Allaire Benjamin, Neuwahl Simon, Hicks Katherine, Sansom Stephanie. Contribution of Anal Sex to HIV Prevalence Among Heterosexuals: A Modeling Analysis. AIDS and Behavior. 2017;21(10):2895–2903. doi: 10.1007/s10461-016-1635-z. [DOI] [PubMed] [Google Scholar]
- 9.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. Aids. 2014;28(10):1509–1519. doi: 10.1097/QAD.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owen BN, Baggaley RF, Elmes J, Harvey A, Shubber Z, Butler AR, Silhol R, Anton P, Shacklett B, van der Straten A, Boily MC. What Proportion of Female Sex Workers Practise anal Intercourse and How Frequently? A Systematic Review and Meta-analysis. AIDS Behav. 2019. 10.1007/s10461-019-02477-w. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 11.Brown B, Blas MM, Cabral A, Carcamo C, Gravitt PE, Halsey N. Oral sex practices, oral human papillomavirus and correlations between oral and cervical human papillomavirus prevalence among female sex workers in Lima, Peru. Int J STD AIDS. 2011;22(11):655–658. doi: 10.1258/ijsa.2011.010541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng W, Tang W, Zhong F, Babu GR, Han Z, Qin F, Gao K, Mai H, Zhao Y, Liang C, et al. Consistently high unprotected anal intercourse (UAI) and factors correlated with UAI among men who have sex with men: implication of a serial cross-sectional study in Guangzhou, China. BMC Infect Dis. 2014;14:696. doi: 10.1186/s12879-014-0696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halperin DT. Heterosexual anal intercourse: prevalence, cultural factors, and HIV infection and other health risks, part I. AIDS Patient Care STDs. 1999;13(12):717–730. doi: 10.1089/apc.1999.13.717. [DOI] [PubMed] [Google Scholar]
- 14.Ma Q, Jiang J, Pan X, Cai G, Wang H, Zhou X, Jiang T, Chen L. Consistent condom use and its correlates among female sex workers at hair salons: a cross-sectional study in Zhejiang province, China. BMC Public Health. 2017;17(1):910. doi: 10.1186/s12889-12017-14891-12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramanathan Shreena, Nagarajan Karikalan, Ramakrishnan Lakshmi, Mainkar Mandar K, Goswami Prabuddhagopal, Yadav Diwakar, Sen Shrabanti, George Bitra, Rachakulla Harikumar, Subramanian Thilakavathi, Paranjape Ramesh S. Inconsistent condom use by male clients during anal intercourse with occasional and regular female sex workers (FSWs): survey findings from southern states of India. BMJ Open. 2014;4(11):e005166. doi: 10.1136/bmjopen-2014-005166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marra E, Kroone N, Freriks E, van Dam CL, Alberts CJ, Hogewoning AA, Bruisten S, van Dijk A, Kroone MM, Waterboer T, et al. Vaginal and anal human papillomavirus infection and seropositivity among female sex workers in Amsterdam, the Netherlands: Prevalence, concordance and risk factors. J Infect. 2018;76(4):393–405. doi: 10.1016/j.jinf.2017.1012.1011. [DOI] [PubMed] [Google Scholar]
- 17.Paz-Bailey G, Noble M, Salo K, Tregear SJ. Prevalence of HIV among U.S. female sex workers: systematic review and meta-analysis. AIDS Behav. 2016;20(10):2318–2331. doi: 10.1007/s10461-016-1332-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rissel C, Badcock PB, Smith AM, Richters J, de Visser RO, Grulich AE, Simpson JM. Heterosexual experience and recent heterosexual encounters among Australian adults: the second Australian study of health and relationships. Sex Health. 2014;11(5):416–426. doi: 10.1071/SH14105. [DOI] [PubMed] [Google Scholar]
- 19.Gindi RM, Ghanem KG, Erbelding EJ. Increases in oral and anal sexual exposure among youth attending sexually transmitted diseases clinics in Baltimore, Maryland. J Adolesc Health. 2008;42(3):307–308. doi: 10.1016/j.jadohealth.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Visser RO, Smith AM, Rissel CE, Richters J, Grulich AE. Sex in Australia: heterosexual experience and recent heterosexual encounters among a representative sample of adults. Aust N Z J Public Health. 2003;27(2):146–154. doi: 10.1111/j.1467-842x.2003.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 21.Mercer CH, Tanton C, Prah P, Erens B, Sonnenberg P, Clifton S, Macdowall W, Lewis R, Field N, Datta J, et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of sexual attitudes and lifestyles (Natsal) Lancet. 2013;382(9907):1781–1794. doi: 10.1016/S0140-6736(13)62035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owen Branwen N., Brock Patrick M., Butler Ailsa R., Pickles Michael, Brisson Marc, Baggaley Rebecca F., Boily Marie-Claude. Prevalence and Frequency of Heterosexual Anal Intercourse Among Young People: A Systematic Review and Meta-analysis. AIDS and Behavior. 2015;19(7):1338–1360. doi: 10.1007/s10461-015-0997-y. [DOI] [PubMed] [Google Scholar]
- 23.Rissel C, Badcock PB, Smith AM, Richters J, de Visser RO, Grulich AE, Simpson JM. Corrigendum to: heterosexual experience and recent heterosexual encounters among Australian adults: the second Australian study of health and relationships. Sex Health. 2015;12(6):568. doi: 10.1071/SH14105. [DOI] [PubMed] [Google Scholar]
- 24.Tarkang EE. Sexual risk behaviours of high school female learners in Mbonge subdivision of rural Cameroon. Pan Afr Med J. 2015;20:49. doi: 10.11604/pamj.2015.20.49.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salih NA, Metaferia H, Reda AA, Biadgilign S. Premarital sexual activity among unmarried adolescents in northern Ethiopia: a cross-sectional study. Sex Reprod Healthc. 2015;6(1):9–13. doi: 10.1016/j.srhc.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Naidoo S, Taylor M. HIV health literacy, sexual behaviour and self-reports of having tested for HIV among students. Afr J AIDS Res. 2015;14(2):107–115. doi: 10.2989/16085906.2015.1040808. [DOI] [PubMed] [Google Scholar]
- 27.Akinsoji AA, Olufunmilola AA, Idowu AA, Pius AO. Sexual and contraceptive practices among female undergraduates in a Nigerian tertiary institution. Ethiop J Health Sci. 2015;25(3):209–216. doi: 10.4314/ejhs.v25i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Geugten J, van Meijel B, den Uyl MH, de Vries NK. Evaluation of a sexual and reproductive health education programme: students’ knowledge, attitude and behaviour in Bolgatanga municipality, northern Ghana. Afr J Reprod Health. 2015;19(3):126–136. [PubMed] [Google Scholar]
- 29.Muntean N, Kereta W, Mitchell KR. Addressing the sexual and reproductive health needs of young people in Ethiopia: an analysis of the current situation. Afr J Reprod Health. 2015;19(3):87–99. [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009. 10.1016/jjclinepi200906005. [PMC free article] [PubMed]
- 31.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 32.Morhason-Bello I, Francis S, Kabakama S, Watson-Jones D. A systematic review on oral and anal sexual behaviour among heterosexually active adolescents and adults in sub-Saharan Africa. In: PROSPERO:CRD42015025311; 2015. Available from http://www.crd.york.ac.uk/PROSPERO/DisplayPDF.php?ID=CRD42015025311. Accessed 3 May 2019.
- 33.Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS) BMJ Open. 2016;6:e011458. doi: 10.1136/bmjopen-2016-011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Critical appraisal skills programme (CASP) qualitative research checklist. 2017. Available at: https://casp-uk.net/wp-content/uploads/2018/01/CASP-Qualitative-Checklist-2018.pdf. Accessed 3 May 2019.
- 35.Soyinka F. Sexual behavior among university students in Nigera. Arch Sex Behav. 1979;8(1):15–26. doi: 10.1007/BF01541209. [DOI] [PubMed] [Google Scholar]
- 36.Van de Perre P, Clumeck N, Carael M, Nzabihimana E, Robert-Guroff M, De Mol P, Freyens P, Butzler JP, Gallo RC, Kanyamupira JB. Female prostitutes: a risk group for infection with human T-cell lymphotropic virus type III. Lancet. 1985;2(8454):524–527. doi: 10.1016/s0140-6736(85)90462-3. [DOI] [PubMed] [Google Scholar]
- 37.Wilson D, Chiroro P, Lavelle S, Mutero C. Sex worker, client sex behaviour and condom use in Harare, Zimbabwe. AIDS Care. 1989;1(3):269–280. doi: 10.1080/09540128908253032. [DOI] [PubMed] [Google Scholar]
- 38.Akande A. AIDS-related beliefs and behaviours of students: evidence from two countries (Zimbabwe and Nigeria) Int J Adolesc Youth. 1994;4(34):285–303. doi: 10.1080/02673843.1994.9747742. [DOI] [PubMed] [Google Scholar]
- 39.Feldman DA, O’Hara P, Baboo KS, Chitalu NW, Lu Y. HIV prevention among Zambian adolescents: developing a value utilization/norm change model. Soc Sci Med. 1997;44(4):455–468. doi: 10.1016/s0277-9536(96)00164-5. [DOI] [PubMed] [Google Scholar]
- 40.Matasha E, Ntembelea T, Mayaud P, et al. Sexual and reproductive health among primary and secondary school pupils in Mwanza, Tanzania: need for intervention. AIDS Care. 1998;10(5):571–582. doi: 10.1080/09540129848433. [DOI] [PubMed] [Google Scholar]
- 41.Nicholas LJ. The association between religiosity, sexual fantasy, participation in sexual acts, sexual enjoyment, exposure, and reaction to sexual materials among black south Africans. J Sex Marital Ther. 2004;30(1):37–42. doi: 10.1080/00926230490247264. [DOI] [PubMed] [Google Scholar]
- 42.Nnoruka EN, Ezeoke AC. Evaluation of syphilis in patients with HIV infection in Nigeria. Trop Med Int Health. 2005;10(1):58–64. doi: 10.1111/j.1365-3156.2004.01344.x. [DOI] [PubMed] [Google Scholar]
- 43.Okafor II, Obi SN. Sexual risk behaviour among undergraduate students in Enugu, Nigeria. J Obstet Gynaecol. 2005;25(6):592–595. doi: 10.1080/01443610500239511. [DOI] [PubMed] [Google Scholar]
- 44.Cornman DH, Kiene SM, Christie S, Fisher WA, Shuper PA, Pillay S, Friedland GH, Thomas CM, Lodge L, Fisher JD. Clinic-based intervention reduces unprotected sexual behavior among HIV-infected patients in KwaZulu-Natal, South Africa: results of a pilot study. J Acquir Immune Defic Syndr. 2008;48(5):553–556. doi: 10.1097/QAI.0b013e31817bebd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morhason-Bello IO, Oladokun A, Enakpene CA, Fabamwo AO, Obisesan KA, Ojengbede OA. Sexual behaviour of in-school adolescents in Ibadan, south-West Nigeria. Afr J Reprod Health. 2008;12(2):89–97. [PubMed] [Google Scholar]
- 46.Plüddemann A, Flisher AJ, Mathews C, Carney T, Lombard C. Adolescent methamphetamine use and sexual risk behaviour in secondary school students in Cape Town, South Africa. Drug Alcohol Rev. 2008;27(6):687–692. doi: 10.1080/09595230802245253. [DOI] [PubMed] [Google Scholar]
- 47.Bamidele JO, Abodunrin OL, Adebimpe WO. Sexual behavior and risk of HIV/AIDS among adolescents in public secondary schools in Osogbo, Osun state, Nigeria. Int J Adolesc Med Health. 2009;2009(21):387–394. doi: 10.1515/ijamh.2009.21.3.387. [DOI] [PubMed] [Google Scholar]
- 48.Kazaura MR, Masatu MC. Sexual practices among unmarried adolescents in Tanzania. BMC Public Health. 2009;9:373. doi: 10.1186/1471-2458-9-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Opoku B. Contraceptive use among ‘at-risk’ women in a metropolitan area in Ghana. Acta Obstet Gynecol Scand. 2010;89(8):1105–1107. doi: 10.3109/00016341003801672. [DOI] [PubMed] [Google Scholar]
- 50.Maswanya ES, Moji K, Aoyagi K, Takemoto T. Sexual behavior and condom use in female students in Dar-es-Salaam, Tanzania: differences by steady and casual partners. East Afr J Public Health. 2011;8(2):69–76. [PubMed] [Google Scholar]
- 51.Chege W, Pals SL, McLellan-Lemal E, Shinde S, Nyambura M, Otieno FO, Gust DA, Chen RT, Thomas T. Baseline findings of an HIV incidence cohort study to prepare for future HIV prevention clinical trials in Kisumu, Kenya. J Infect Dev Ctries. 2012;15(6):870–880. doi: 10.3855/jidc.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cherie A, Berhane Y. Oral and anal sex practices among high school youth in Addis Ababa, Ethiopia. BMC Public Health. 2012;12:5. doi: 10.1186/1471-2458-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peltzer K. Correlates of HIV infection among people visiting public HIV counseling and testing clinics in Mpumalanga, South Africa. Afr Health Sci. 2012;12(1):8–16. [PMC free article] [PubMed] [Google Scholar]
- 54.Gevers A, Mathews C, Cupp P, Russell M, Jewkes R. Illegal yet developmentally normative: a descriptive analysis of young, urban adolescents’ dating and sexual behaviour in Cape Town, South Africa. BMC Int Health Hum Rights. 2013;13:31. doi: 10.1186/1472-698X-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kakoko DC. Reported heterosexual intercourse and related behaviours among primary school pupils in Kinondoni district, Dar es Salaam, Tanzania. Cult Health Sex. 2013;15(2):235–245. doi: 10.1080/13691058.2012.738829. [DOI] [PubMed] [Google Scholar]
- 56.Folayan MO, Odetoyinbo M, Brown B, Harrison A. Differences in sexual behaviour and sexual practices of adolescents in Nigeria based on sex and self-reported HIV status. Reprod Health. 2014;11:83. doi: 10.1186/1742-4755-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dunkle KL, Beksinska ME, Rees VH, Ballard RC, Htun Y, Wilson ML. Risk factors for HIV infection among sex workers in Johannesburg, South Africa. Int J STD AIDS. 2005;16(3):256–261. doi: 10.1258/0956462053420220. [DOI] [PubMed] [Google Scholar]
- 58.Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, Friedland B, Govender S, De Kock A, Cassim N, Palanee T, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9654):1977–1987. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 59.Andersson KM, Van Niekerk RM, Niccolai LM, Mlungwana ON, Holdsworth I, Bogoshi M, McIntyre JA, Gray GE, Vardas E. Sexual risk behaviour of the first cohort undergoing screening for enrollment into phase I/II HIV vaccine trials in South Africa. Int J STD AIDS. 2009;20(2):95–101. doi: 10.1258/ijsa.2008.008207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nel A, Louw C, Hellstrom E, Braunstein SL, Treadwell I, Marais M, de Villiers M, Hugo J, Paschke I, Andersen C, et al. HIV prevalence and incidence among sexually active females in two districts of South Africa to determine microbicide trial feasibility. PloS One. 2011;6(8):e21528. doi: 10.1371/journal.pone.0021528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Loggerenberg F, Dieter AA, Sobieszczyk ME, Werner L, Grobler A, Mlisana K, Team CAIS HIV prevention in high-risk women in South Africa: condom use and the need for change. PLoS One. 2012;7(2):e30669. doi: 10.1371/journal.pone.0030669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaffoor Z, Wand H, Daniels B, Ramjee G. High risk sexual behaviors are associated with sexual violence among a cohort of women in Durban, South Africa. BMC Res Notes. 2013;6:532. doi: 10.1186/1756-0500-6-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cossa HA, Gloyd S, Vaz RG, Folgosa E, Simbine E, Diniz M, Kreiss JK. Syphilis and HIV infection among displaced pregnant women in rural Mozambique. Int J STD AIDS. 1994;5(2):117–123. doi: 10.1177/095646249400500208. [DOI] [PubMed] [Google Scholar]
- 64.Sallah ED, Grunitzky-Bekele M, Bassabi K, Dodzro K, Sadzo A, Balogou AK, Grunitzky EK, Gaudreau L. The sexual behavior, knowledge and attitudes towards aids and sexually transmitted diseases of students at the University of Benin (Togo) Cahiers Sante. 1999;9(2):101–109. [PubMed] [Google Scholar]
- 65.Operario D, Pettifor A, Cluver L, MacPhail C, Rees H. Prevalence of parental death among young people in South Africa and risk for HIV infection. J Acquir Immune Defic Syndr. 2007;44(1):93–98. doi: 10.1097/01.qai.0000243126.75153.3c. [DOI] [PubMed] [Google Scholar]
- 66.Ambaw F, Mossie A, Gobena T. Sexual practices and their development pattern among Jimma university students. Ethiopian J Health Sci. 2010;20(3):159–167. doi: 10.4314/ejhs.v20i3.69445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guedou FA, Damme L, Mirembe F, Solomon S, Becker M, Deese J, Crucitti T, Alary M. Intermediate vaginal flora is associated with HIV prevalence as strongly as bacterial vaginosis in a cross-sectional study of participants screened for a randomised controlled trial. Sex Transm Infect. 2012;88(7):545–551. doi: 10.1136/sextrans-2011-050319. [DOI] [PubMed] [Google Scholar]
- 68.Asekun-Olarinmoye OS, Asekun-Olarinmoye EO, Adebimpe WO, Omisore AG. Effect of mass media and internet on sexual behavior of undergraduates in Osogbo metropolis, Southwestern Nigeria. Adolesc Health Med Ther. 2014;5:15–23. doi: 10.2147/AHMT.S54339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dubbink JH, van der Eem L, McIntyre JA, Mbambazela N, Jobson GA, Ouburg S, Morre SA, Struthers HE, Peters RP. Sexual behaviour of women in rural South Africa: a descriptive study. BMC Public Health. 2016;16:557. doi: 10.1186/s12889-016-3207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lawan UM, Amole GT, Shuaib MJ. Sexual health of prison inmates: a case study of Kano central prison, north western Nigeria. Afr J Reprod Health. 2016;20(1):98–103. doi: 10.29063/ajrh2016/v20i1.10. [DOI] [PubMed] [Google Scholar]
- 71.Dareng EO, Adebamowo SN, Eseyin OR, Odutola MK, Pharoah PP, Adebamowo CA. Test–retest reliability of self-reported sexual behavior history in urbanized Nigerian women. Front Public Health. 2017;5:172. doi: 10.3389/fpubh.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davey DJ, Farley E, Gomba Y, Coates T, Myer L. Sexual risk during pregnancy and postpartum periods among HIV-infected and -uninfected South African women: Implications for primary and secondary HIV prevention interventions. PLoS One. 2018;13(3):e0192982. doi: 10.1371/journal.pone.0192982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ybarra M, Price-Feeney M, Mwaba K. Prevalence and correlates of anal sex among secondary school students in Cape Town, South Africa. AIDS Care. 2018;30(7):821–829. doi: 10.1080/09540121.2018.1426824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fonck K, Kaul R, Kimani J, et al. A randomized, placebo-controlled trial of monthly azithromycin prophylaxis to prevent sexually transmitted infections and HIV-1 in Kenyan sex workers: study design and baseline findings. Int J STD AIDS. 2000;11(12):804–811. doi: 10.1258/0956462001915327. [DOI] [PubMed] [Google Scholar]
- 75.Ramjee G, Gouws E. Prevalence of HIV among truck drivers visiting sex workers in KwaZulu-Natal, South Africa. Sex Transm Dis. 2002;29(1):44–49. doi: 10.1097/00007435-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 76.Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, Sirivongrangson P, Mukenge-Tshibaka L, Ettiègne-Traoré V, Uaheowitchai C, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360(9338):971–977. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 77.Kalichman SC, Simbayi LC. Sexual assault history and risks for sexually transmitted infections among women in an African township in Cape Town, South Africa. AIDS Care. 2004;16(6):681–689. doi: 10.1080/09540120410331269530. [DOI] [PubMed] [Google Scholar]
- 78.Mpofu E, Flisher AJ, Bility K, Onya H, Lombard C. Sexual partners in a rural south African setting. AIDS Behav. 2006;10(4):399–404. doi: 10.1007/s10461-005-9037-7. [DOI] [PubMed] [Google Scholar]
- 79.Schwandt M, Morris C, Ferguson A, Ngugi E, Moses S. Anal and dry sex in commercial sex work, and relation to risk for sexually transmitted infections and HIV in Meru, Kenya. Sex Transm Infect. 2006;82(5):392–396. doi: 10.1136/sti.2006.019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jaspan HB, Flisher AJ, Myer L, Mathews C, Seebregts C, Berwick JR, Wood R, Bekker LG. Brief report: methods for collecting sexual behaviour information from south African adolescents--a comparison of paper versus personal digital assistant questionnaires. J Adolesc. 2007;30(2):353–359. doi: 10.1016/j.adolescence.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 81.Allen CF, Lees SS, Desmond NA, et al. Validity of coital diaries in a feasibility study for the Microbicides Development Programme trial among women at high risk of HIV/AIDS in Mwanza, Tanzania. Sex Transm Infect. 2007;83(6):490–496. doi: 10.1136/sti.2007.024810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Watson-Jones D, Weiss H, Rusizoka M, Baisley K, Mugeye K, Changalucha J, Everett D, Balira R, Knight L, Ross D, Hayes RJ. Risk factors for herpes simplex virus type 2 and HIV among women at high risk in northwestern Tanzania: preparing for an HSV-2 intervention trial. J Acquir Immune Defic Syndr. 2007;46(5):631–642. doi: 10.1097/QAI.0b013e31815b2d9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grijsen ML, Graham SM, Mwangome M, et al. Screening for genital and anorectal sexually transmitted infections in HIV prevention trials in Africa. Sex Transm Infect. 2008;84(5):364–370. doi: 10.1136/sti.2007.028852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kalichman SC, Simbayi LC, Cain D, Jooste S. Heterosexual anal intercourse among community and clinical settings in Cape Town, South Africa. Sex Transm Infect. 2009;85(6):411–415. doi: 10.1136/sti.2008.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karim QA, Kharsany AB, Frohlich JA, Werner L, Mashego M, Mlotshwa M, Madlala BT, Ntombela F, Abdool Karim SS. Stabilizing HIV prevalence masks high HIV incidence rates amongst rural and urban women in KwaZulu-Natal, South Africa. Int J Epidemiol. 2011;40(4):922–930. doi: 10.1093/ije/dyq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalichman SC, Pinkerton SD, Carey MP, et al. Heterosexual anal intercourse and HIV infection risks in the context of alcohol serving venues, Cape Town, South Africa. BMC Public Health. 2011;11:807. doi: 10.1186/1471-2458-11-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mensch BSHP, Abbott S, Rankin J, Littlefield S, Ahmed K, Cassim N, Patel SRG, Palanee T, Mierzwa S, Skoler-Karpoff S. Assessing the reporting of adherence and sexual activity in a simulated microbicide trial in South Africa: an interview mode experiment using a placebo gel. AIDS Behav. 2011;15(2):407–421. doi: 10.1007/s10461-010-9791-z. [DOI] [PubMed] [Google Scholar]
- 89.Priddy FH, Wakasiaka S, Hoang TD, et al. Anal sex, vaginal practices, and HIV incidence in female sex workers in urban Kenya: implications for the development of intravaginal HIV prevention methods. AIDS Res Hum Retrovir. 2011;27(10):1067–1072. doi: 10.1089/aid.2010.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Veldhuijzen NJ, Ingabire C, Luchters S, Bosire W, Braunstein S, Chersich M, van de Wijgert J. Anal intercourse among female sex workers in East Africa is associated with other high-risk behaviours for HIV. Sex Health. 2011;8(2):251–254. doi: 10.1071/SH10047. [DOI] [PubMed] [Google Scholar]
- 91.Venkatesh KK, de Bruyn G, Mayer KH, et al. Changes in sexual risk behavior before and after HIV seroconversion in southern African women enrolled in a HIV prevention trial. J Acquir Immune Defic Syndr. 2011;57(5):435–441. doi: 10.1097/QAI.0b013e318220379b. [DOI] [PubMed] [Google Scholar]
- 92.Cain D, Pare V, Kalichman SC, Harel O, Mthembu J, Carey MP, Carey KB, Mehlomakulu V, Simbayi LC, Mwaba K. HIV risks associated with patronizing alcohol serving establishments in South African townships, Cape Town. Prev Sci. 2012;13(2):627–634. doi: 10.1007/s11121-012-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scott-Sheldon LA, Carey MP, Carey KB, et al. HIV testing is associated with increased knowledge and reductions in sexual risk behaviours among men in Cape Town, South Africa. Afr J AIDS Res. 2013;12(4):195–201. doi: 10.2989/16085906.2013.863219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gray GE, Metch B, Churchyard G, Mlisana K, Nchabeleng M, Allen M, Moodie Z, Kublin J, Bekker LG, HVTN 503 team Does participation in an HIV vaccine efficacy trial affect risk behaviour in South Africa? Vaccine. 2013;31(16):2089–2096. doi: 10.1016/j.vaccine.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jemmott JB, 3rd, Jemmott LS, O’Leary A, Ngwane Z, Icard LD, Heeren GA, Mtose X, Carty C. Cluster-randomized controlled trial of an HIV/sexually transmitted infection risk-reduction intervention for south African men. Am J Public Health. 2014;104(3):467–473. doi: 10.2105/AJPH.2013.301578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guffey MB, Richardson B, Husnik M, Makanani B, Chilongozi D, Yu E, Ramjee G, Mgodi N, Gomez K, Hillier SL, et al. HPTN 035 phase II/IIb randomised safety and effectiveness study of the vaginal microbicides BufferGel and 0.5% PRO 2000 for the prevention of sexually transmitted infections in women. Sex Transm Infect. 2014;90(5):363–369. doi: 10.1136/sextrans-2014-051537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thurston IB, Dietrich J, Bogart LM, Otwombe KN, Sikkema KJ, Nkala B, Gray GE. Correlates of sexual risk among sexual minority and heterosexual south African youths. Am J Public Health. 2014;104(7):1265–1269. doi: 10.2105/AJPH.2013.301865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Noguchi LM, Richardson BA, Baeten JM, Hillier SL, Balkus JE, Chirenje ZM, Bunge K, Ramjee G, Nair G, Palanee-Phillips T, et al. Risk of HIV-1 acquisition among women who use diff erent types of injectable progestin contraception in South Africa: a prospective cohort study. Lancet HIV. 2015;2(7):e279–e287. doi: 10.1016/S2352-3018(15)00058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Palanee-Phillips T, Schwartz K, Brown ER, Govender V, Mgodi N, Kiweewa FM, Nair G, Mhlanga F, Siva S, Bekker LG, et al. Characteristics of women enrolled into a randomized clinical trial of Dapivirine vaginal ring for HIV-1 prevention. PLoS One. 2015;10(6):e0128857. doi: 10.1371/journal.pone.0128857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stadler JJ, Delany S, Mntambo M. Sexual coercion and sexual desire: ambivalent meanings of heterosexual anal sex in Soweto, South Africa. AIDS Care. 2007;19(10):1189–1193. doi: 10.1080/09540120701408134. [DOI] [PubMed] [Google Scholar]
- 101.Ndinda C, Chimbwete C, McGrath N, Pool R. Perceptions of anal sex in rural South Africa. Cult Health Sex. 2008;10(2):205–212. doi: 10.1080/13691050600988416. [DOI] [PubMed] [Google Scholar]
- 102.Duby Z, Colvin C. Conceptualizations of heterosexual anal sex and HIV risk in five east African communities. J Sex Res. 2014;51(8):863–873. doi: 10.1080/00224499.2013.871624. [DOI] [PubMed] [Google Scholar]
- 103.Beckham SW, Shembilu CR, Winch PJ, Beyrer C, Kerrigan DL. ‘If you have children, you have responsibilities’: motherhood, sex work and HIV in southern Tanzania. Cult Health Sex. 2015;17(2):165–179. doi: 10.1080/13691058.2014.961034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wamoyi J, Mongi A, Sally M, Kakoko D, Shamba D, Geubbels E, Kapiga S. A qualitative study of discourses on heterosexual anal sexual practice among key, and general populations in Tanzania: implications for HIV prevention. BMC Public Health. 2015;15:417. doi: 10.1186/s12889-015-1768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Katsivo MN, Muthami LN. Social characteristics and sexual behaviour of women at high risk of HIV infection in a town in Central Province of Kenya. Arch AIDS Res. 1991;5(1–2):25–27. [PubMed] [Google Scholar]
- 106.Karim SSA, Ramjee G. Anal sex and HIV transmission in women. Am J Public Health. 1998;88(8):1265–1266. doi: 10.2105/ajph.88.8.1265-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bing EG, Cheng KG, Ortiz DJ, Ovalle-Bahamon RE, Ernesto F, Weiss RE, Boyer CB. Evaluation of a prevention intervention to reduce HIV risk among Angolan soldiers. AIDS Behav. 2008;12(3):384–395. doi: 10.1007/s10461-008-9368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Adoga MP, Banwat EB, Forbi JC, Nimzing L, Pam CR, Gyar SD, Agabi YA, Agwale SM. Human immunodeficiency virus, hepatitis B virus and hepatitis C virus: sero-prevalence, co-infection and risk factors among prison inmates in Nasarawa state, Nigeria. J Infect Dev Ctries. 2009;3(7):539–547. doi: 10.3855/jidc.472. [DOI] [PubMed] [Google Scholar]
- 109.van der Elst EM, Okuku HS, Nakamya P, Muhaari A, Davies A, McClelland RS, Price MA, Smith AD, Graham SM, Sanders EJ. Is audio computer-assisted self-interview (ACASI) useful in risk behaviour assessment of female and male sex workers, Mombasa, Kenya? PloS One. 2009;4(5):e5340. doi: 10.1371/journal.pone.0005340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Essomba EN, Kollo B, Kouoh Ngambi M, Owona Manga LJ, Mbunya S, Bita Fouda A, Dissongo JI, Mikendeffo D, Lehman L. Sex risk behaviour and prevalence of HIV of sex workers in Douala at 2011. Mali Med. 2013;2:24–28. [PubMed] [Google Scholar]
- 111.Lambdin BH, Bruce RD, Chang O, Nyandindi C, Sabuni N, Zamudio-Haas S, McCurdy S, Masao F, Ivo Y, Msami A, et al. Identifying programmatic gaps: inequities in harm reduction service utilization among male and female drug users in Dar es Salaam, Tanzania. PLoS One. 2013;8(6):e67062. doi: 10.1371/journal.pone.0067062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Githuka G, Hladik W, Mwalili S, Cherutich P, Muthui M, Gitonga J, Maina WK, Kim AA. Populations at increased risk for HIV infection in Kenya: results from a national population-based household survey, 2012. (special issue: Kenya AIDS Indicator survey 2012.) J Acquir Immune Defic Syndr. 2014;66(Suppl. 1):S46–S56. doi: 10.1097/QAI.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Anyanwu PE, Fulton J. Knowledge and perception of young adults in Nigeria on effectiveness of condom use in prevention of sexually transmitted infections. Int J Adolesc Med Health. 2015. 10.1515/ijamh-2015-0050. [DOI] [PubMed]
- 114.Luma HN, Eloumou SAFB, Atemlefeh FE, Malongue A, Temfack E, Lekpa FK, Donfack-Sontsa O, Ndip L, Ditah IC. Anorectal pathology amongst HIV infected patients attending the Douala General Hospital: a cross-sectional study. Int J STD AIDS. 2016;0:1–8. doi: 10.1177/0956462416650817. [DOI] [PubMed] [Google Scholar]
- 115.McLellan-Lemal E, Gust DA, Gvetadze R, Furtado M, Otieno FO, Desai M, Zeh C, Samandari T, Nyagol B, Makanga EM. Characteristics of women screened for a contraceptive intravaginal ring study in Kisumu, Kenya, 2014. Res J Womens Health. 2016;3:1–23. doi: 10.7243/2054-9865-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Teasdale Chloe A., Abrams Elaine J., Chiasson Mary Ann, Justman Jessica, Blanchard Kelly, Jones Heidi E. Sexual Risk and Intravaginal Practice Behavior Changes During Pregnancy. Archives of Sexual Behavior. 2016;46(2):539–548. doi: 10.1007/s10508-016-0818-z. [DOI] [PubMed] [Google Scholar]
- 117.Giorgio M, Townsend L, Zembe Y, Cheyip M, Guttmacher S, Kapadia F, Mathews C. The relationship between social support, HIV Serostatus, and perceived likelihood of being HIV positive among self-settled female, foreign migrants in Cape Town, South Africa. J Immigr Minor Health. 2017;19(4):883–890. doi: 10.1007/s10903-016-0514-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hladik W, Baughman AL, Serwadda D, Tappero JW, Kwezi R, Nakato ND, Barker J. Burden and characteristics of HIV infection among female sex workers in Kampala, Uganda - a respondent-driven sampling survey. BMC Public Health. 2017;17(1):565. doi: 10.1186/s12889-017-4428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Longo JD, Simaleko MM, Diemer HS, Gresenguet G, Brucker G, Belec L. Risk factors for HIV infection among female sex workers in Bangui, Central African Republic. PLoS One. 2017;12(11):e0187654. doi: 10.1371/journal.pone.0187654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shayo EH, Kalinga AA, Senkoro KP, Msovela J, Mgina EJ, Shija AE, Materu G, Kilima SP, Mboera LEG, Massaga JJ. Prevalence and risk factors associated with female anal sex in the context of HIV/AIDS in the selected districts of Tanzania. BMC Res Notes. 2017;10:140. doi: 10.1186/s13104-017-2452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maheu-Giroux M, Baral S, Vesga JF, Diouf D, Diabate S, Alary M, Abo K, Boily MC. Anal intercourse among female sex Workers in Cote d'Ivoire: prevalence, determinants, and model-based estimates of the population-level impact on HIV transmission. Am J Epidemiol. 2018;187(2):287–297. doi: 10.1093/aje/kwx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mavhu W, Langhaug L, Manyonga B, Power R, Cowan F. What is ‘sex’ exactly? Using cognitive interviewing to improve the validity of sexual behaviour reporting among young people in rural Zimbabwe. Cult Health Sex. 2008;10(6):573–585. doi: 10.1080/13691050801999071. [DOI] [PubMed] [Google Scholar]
- 123.Duby Z, Hartmann M, Montgomery ET, Colvin CJ, Mensch B, Straten A. Sexual scripting of heterosexual penile-anal intercourse amongst participants in an HIV prevention trial in South Africa, Uganda and Zimbabwe. Cult Health Sex. 2016;18(1):30–44. doi: 10.1080/13691058.2015.1064165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mtenga S, Shamba D, Wamoyi J, Kakoko D, Haafkens J, Mongi A, Kapiga S, Geubbels E. How long-distance truck drivers and villagers in rural southeastern Tanzania think about heterosexual anal sex: a qualitative study. Sex Transm Infect. 2015;91(8):576–580. doi: 10.1136/sextrans-2015-052055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mazeingia YT, Olijjira L, Dessie Y. Anal sexual experience and HIV risk awareness among female sex workers in Dire Dawa, eastern Ethiopia. Glob Health Res Policy. 2017;2:27. doi: 10.1186/s41256-41017-40047-41256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tengia-Kessy A, Msamanga GI, Moshiro CS. Assessment of behavioural risk factors associated with HIV infection among youth in Moshi rural district, Tanzania. East Afr Med J. 1998;75(9):528–532. [PubMed] [Google Scholar]
- 127.Fawole OI, Ajayi IO, Babalola TD, Oni AA, Asuzu MC. Socio-demographic characteristics and sexual behaviour of adolescents attending the STC, UCH, Ibadan: a 5 year review. West Afr J Med. 1999;18(3):165–169. [PubMed] [Google Scholar]
- 128.Okesola AO, Fawole OI. Prevalence of human papilloma virus genital infections in sexually transmitted diseases clinic attendees in Ibadan. West Afr J Med. 2000;19(3):195–199. [PubMed] [Google Scholar]
- 129.Vogt SL, Gravitt PE, Martinson NA, Hoffmann J, D’Souza G. Concordant Oral-genital HPV infection in South Africa couples: evidence for transmission. Front Oncol. 2013;3:303. doi: 10.3389/fonc.2013.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Davidson CL, Richter KL, Van der Linde M, Coetsee J, Boy SC. Prevalence of oral and oropharyngeal human papillomavirus in a sample of south African men: a pilot study. S Afr Med J. 2014;104(5):358–361. doi: 10.7196/samj.7542. [DOI] [PubMed] [Google Scholar]
- 131.Kerwin JT, Thornton RL, Foley SL. Prevalence of and factors associated with oral sex among rural and urban Malawian men. Int J Sexual Health. 2014;26(1):66–77. doi: 10.1080/19317611.2013.830671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Meque I, Dube K, Feldblum PJ, Clements AC, Zango A, Cumbe F, Chen PL, Ferro JJ, van de Wijgert JH. Prevalence, incidence and determinants of herpes simplex virus type 2 infection among HIV-seronegative women at high-risk of HIV infection: a prospective study in Beira, Mozambique. PloS One. 2014;9(2):e89705. doi: 10.1371/journal.pone.0089705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gathece LW. Prevalence of oral sex and wet kissing among female sex workers in two areas of Nairobi, Kenya. Afr J Oral Health Sci. 2000;1(1):17–18. [Google Scholar]
- 134.Mbulawa ZZA, Johnson LF, Marais DJ, Coetzee D, Williamson AL. Risk factors for oral human papillomavirus in heterosexual couples in an African setting. J Infect. 2014;68:185–189. doi: 10.1016/j.jinf.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 135.Animasahun VJ, Sholeye OO, Oduwole AD. Promoting the sexual and reproductive health of adolescent females in Ijebu-Ode, southwest, Nigeria: a study of sexual risk-taking. Int J Adolesc Med Health. 2016;29(6):09. doi: 10.1515/ijamh-2016-0021. [DOI] [PubMed] [Google Scholar]
- 136.Arulogun OS, Ogbu IA, Dipeolu IO. Influence of internet exposure on sexual behaviour of young persons in an urban district of Southwest Nigeria. Pan Afr Med J. 2016;25:261. doi: 10.11604/pamj.2016.25.261.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chikandiwa A, Pisa PT, Chersich MF, Muller EE, Mayaud P, Delany-Moretlwe S. Oropharyngeal HPV infection: prevalence and sampling methods among HIV-infected men in South Africa. Int J STD AIDS. 2018;29(8):776–780. doi: 10.1177/0956462418755882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sanders SA, Reinisch JM. Would you say you “had sex” if...? JAMA. 1999;281(3):275–277. doi: 10.1001/jama.281.3.275. [DOI] [PubMed] [Google Scholar]
- 139.Sanders SA, Hill BJ, Yarber WL, Graham CA, Crosby RA, Milhausen RR. Misclassification bias: diversity in conceptualisations about having ‘had sex’. Sex Health. 2010;7(1):31–34. doi: 10.1071/SH09068. [DOI] [PubMed] [Google Scholar]
- 140.Wong William C.W., Yim Yuet Lin, Lynn Henry. Sexually Transmitted Infections Among Female Sex Workers in Hong Kong: The Role of Migration Status. Journal of Travel Medicine. 2011;18(1):1–7. doi: 10.1111/j.1708-8305.2010.00453.x. [DOI] [PubMed] [Google Scholar]
- 141.Lim RB, Wong ML, Tan PH, Govender M. Heterosexual men who patronise entertainment establishments versus brothels in an Asian urban setting – which group practises riskier sexual behaviours? BMC Public Health. 2015;15:777. doi: 10.1186/s12889-015-2132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.World Health Organisation. Alcohol use and sexual risk behaviour: a cross-cultural study in eight countries. 2005. Available from: https://www.who.int/substance_abuse/publications/alcohol_sexual_risk_crosscultural.pdf. Accessed 3 May 2019.
- 143.Mao YX, Xiao CC, Wang T, Li SY, Yan H. One-night-stand behavior and associated factors among young men who have sex with men in Wuhan, China. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38(6):746–749. doi: 10.3760/cma.j.issn.0254-6450.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 144.Calsyn DA, Hatch-Maillette MA, Meade CS, Tross S, Campbell AN, Beadnell B. Gender differences in heterosexual anal sex practices among women and men in substance abuse treatment. AIDS Behav. 2013;17(7):2450–2458. doi: 10.1007/s10461-012-0387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Beattie TS, Bradley JE, Vanta UD, Lowndes CM, Alary M. Vulnerability re-assessed: the changing face of sex work in Guntur district, Andhra Pradesh. AIDS Care. 2013;25(3):378–384. doi: 10.1080/09540121.2012.701726. [DOI] [PubMed] [Google Scholar]
- 146.Lewis R, Tanton C, Mercer CH, Mitchell KR, Palmer M, Macdowall W, Wellings K. Heterosexual practices among young people in Britain: evidence from three national surveys of sexual attitudes and lifestyles. J Adolesc Health. 2017;61(6):694–702. doi: 10.1016/j.jadohealth.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pizzol D, Bertoldo A, Foresta C. Adolescents and web porn: a new era of sexuality. Int J Adolesc Med Health. 2016;28(2):169–173. doi: 10.1515/ijamh-2015-0003. [DOI] [PubMed] [Google Scholar]
- 148.Smith Lucy Watchirs, Liu Bette, Degenhardt Louisa, Richters Juliet, Patton George, Wand Handan, Cross Donna, Hocking Jane S., Skinner S. Rachel, Cooper Spring, Lumby Catharine, Kaldor John M., Guy Rebecca. Is sexual content in new media linked to sexual risk behaviour in young people? A systematic review and meta-analysis. Sexual Health. 2016;13(6):501. doi: 10.1071/SH16037. [DOI] [PubMed] [Google Scholar]
- 149.Lim MSC, Agius PA, Carrotte ER, Vella AM, Hellard ME. Young Australians’ use of pornography and associations with sexual risk behaviours. Aust NZ J Public Health. 2017;41:438–443. doi: 10.1111/1753-6405.12678. [DOI] [PubMed] [Google Scholar]
- 150.Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. 2015. Available from: https://www.cdc.gov/std/tg2015/default.htm. Accessed 20 Nov 2018. [DOI] [PubMed]
- 151.Dukers-Muijrers NH, Schachter J, van Liere GA, Wolffs PF, Hoebe CJ. What is needed to guide testing for anorectal and pharyngeal Chlamydia trachomatis and Neisseria gonorrhoeae in women and men? Evidence and opinion. BMC Infect Dis. 2015;15:533. doi: 10.1186/s12879-015-1280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Curtis SL, Sutherland EG. Measuring sexual behaviour in the era of HIV/AIDS: the experience of demographic and health surveys and similar enquiries. Sex Transm Infect. 2004;80(2):22–27. doi: 10.1136/sti.2004.011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Plummer M, Ross D, Wight D, Changalucha J, Mshana G, Wamoyi J, Todd J, Anemona A, Mosha F, Obasi A, et al. “A bit more truthful”: the validity of adolescent sexual behaviour data collected in rural northern Tanzania using five methods. Sex Transm Infect. 2004;80(2):45–56. doi: 10.1136/sti.2004.011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Doyle AM, Plummer ML, Weiss HA, Changalucha J, Watson-Jones D, Hayes RJ, Ross DA. Concurrency and other sexual partnership patterns reported in a survey of young people in rural northern Tanzania. PLoS One. 2017;12(8):e0182567. doi: 10.1371/journal.pone.0182567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Child Trends Databank. Oral sex behaviors among teens. 2015. Available from: https://www.childtrends.org/wp-content/uploads/2015/11/indicator_1446940280.638.pdf. Accessed 3 May 2019.
- 156.Lefkowitz ES, Vasilenko SA, Leavitt CE. Oral vs. vaginal sex experiences and consequences among first-year college students. Arch Sex Behav. 2016;45(2):329–337. doi: 10.1007/s10508-015-0654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brady SS, Halpern-Felsher BL. Adolescents’ reported consequences of having oral sex versus vaginal sex. Pediatrics. 2007;119(2):229–236. doi: 10.1542/peds.2006-1727. [DOI] [PubMed] [Google Scholar]
- 158.Lewis Ruth, Marston Cicely. Oral Sex, Young People, and Gendered Narratives of Reciprocity. The Journal of Sex Research. 2016;53(7):776–787. doi: 10.1080/00224499.2015.1117564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Duby Z, Hartmann M, Mahaka I, Munaiwa O, Nabukeera J, Vilakazi N, Mthembu F, Colvin CJ, Mensch B, van der Straten A. Lost in translation: language, terminology, and understanding of penile-anal intercourse in an HIV prevention trial in South Africa, Uganda, and Zimbabwe. J Sex Res. 2016;53(9):1096–1106. doi: 10.1080/00224499.2015.1069784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mercer CH, Wellings K, Johnson AM. What’s new about Natsal-3? Sex Transm Infect. 2014;90(2):80–81. doi: 10.1136/sextrans-2013-051292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Prevalence of oral sex by sub-region. Figure S2. Prevalence of oral sex by population category. Figure S3. Prevalence of oral sex by risk of bias. Figure S4. Prevalence of anal sex by sub-region. Figure S5 Prevalence of anal sex by population category. Figure S6. Prevalence of anal sex by risk of bias. (DOCX 122 kb)
Table S1. Selected data from quantitative studies reporting on heterosexual oral and anal sex in sub-Saharan Africa by year of publication. Table S2. Reported condom use during penetrative heterosexual sex (oral, anal and vaginal). Table S3. Risk of bias assessment for quantitative studies. Table S4. Risk of bias assessment of qualitative studies. (XLSX 41 kb)
Data Availability Statement
Interested parties can obtain all available data by contacting the corresponding author.