Abstract
Background
In women with evidence of ischemia and no obstructive coronary artery disease the underlying mechanism is most often attributed to coronary microvascular dysfunction. Higher rates of adverse cardiovascular events, specifically heart failure with preserved ejection fraction, are present in women with coronary microvascular dysfunction, leading to the hypothesis that coronary microvascular dysfunction may contribute to the progression of heart failure with preserved ejection fraction.
Case summary
A 55-year-old, Caucasian woman with a past medical history of chest pain and shortness of breath was referred to our tertiary care center and diagnosed as having coronary microvascular dysfunction by invasive coronary reactivity testing. After 10 years of follow-up care for coronary microvascular dysfunction, she presented to an emergency room in acute heart failure and was diagnosed as having heart failure with preserved ejection fraction.
Discussion
The current case report provides a specific example in support of existing studies that demonstrate that coronary microvascular dysfunction may be a precursor of heart failure with preserved ejection fraction. Further research is needed to establish causality and management.
Trial registration
Clinical Trial Registration: ClinicalTrials.gov Identifier: NCT02582021.
Keywords: Coronary microvascular dysfunction, Heart failure with preserved ejection fraction, Non-obstructive coronary artery disease, Cardiac magnetic resonance imaging
Introduction
Cardiovascular disease remains the number one cause of death in the USA [1]. Coronary microvascular dysfunction (CMD) is common in women with evidence of ischemia and no obstructive coronary artery disease (INOCA) [2]. Previous studies have shown that these women have a higher risk of fatal and non-fatal cardiovascular events in comparison to asymptomatic healthy women and are more likely to be readmitted for angina or for acute coronary syndrome within 180 days of having normal coronary angiography [3]. These women also experience unexpectedly higher rates of heart failure hospitalizations [3]. Remarkably, a previous report from the Women’s Ischemia Syndrome Evaluation (WISE) study revealed that in women with signs and symptoms of INOCA hospitalized for heart failure, 90% had preserved left ventricular ejection fraction (LVEF). Although the prevalence of CMD in heart failure with preserved ejection fraction (HFpEF) is currently unknown, exercise studies have indicated that vascular stiffness, impaired exercise vasodilation, and impaired diastolic reserve may be related to endothelial and microvascular dysfunction [4]. This has led to the hypothesis that CMD may contribute to the development of HFpEF [5]. The following case highlights some of the first empirical evidence supporting this hypothesis.
This case demonstrates that progression to HFpEF may occur parallel with common pathophysiological mechanisms or risk factors of CMD. To date, there has been indirect and limited clinical evidence of CMD as a novel mechanism underlying the pathogenesis of HFpEF. This case underscores the importance of investigating the link between these two conditions. Understanding the correlates associating CMD in HFpEF could provide additional insight into the mechanisms of HFpEF, improve health outcomes for patients with CMD, and help in the design and development of future preventative pharmacotherapies.
Case presentation
Initial presentation
A 55-year-old, Caucasian woman was referred to our tertiary women’s heart center for persistent chest pain, palpitations, and dyspnea. Her medical history included hypertension, dyslipidemia, chronic anxiety, and bilateral non-obstructive carotid atherosclerosis. She had no prior history of diabetes mellitus, tobacco smoking, alcohol or substance abuse, or adverse pregnancy outcomes. Her family history was significant for premature coronary artery disease. Her father had a history of hypertension and had a myocardial infarction (MI) and coronary artery bypass grafting at the age of 39. Her brother had a history of coronary artery disease and also had a MI at the age of 40. Her occupational history indicated that she had been working in the field of psychology and was still an employee in the same job at the time of the hospital visit and follow-up care.
Table 1 summarizes the general symptoms and characteristic signs of our patient for the onset of CMD and her progression to HFpEF. She had undergone an exercise treadmill test which revealed ischemic ECG changes and dyspnea. Her initial echocardiogram demonstrated a LVEF of 67%, mild diastolic dysfunction, mild left ventricular (LV) hypertrophy, no significant valvular heart disease, and no pulmonary hypertension. Subsequent invasive left heart catheterization was performed and it showed normal epicardial coronary arteries without angiographic evidence of atherosclerotic plaque. She continued to have exertional symptoms and angina-like chest pain and was subsequently referred to our center for further evaluation of suspected INOCA. During her evaluation and treatment she continued to experience stable angina and exertional dyspnea despite initial management with atorvastatin 20 mg daily, lisinopril 20 mg daily, aspirin 81 mg daily, and sublingual nitroglycerin as needed. She had a poor clinical response to sublingual nitroglycerin. Due to her persistent symptoms and abnormal stress testing, she was referred for coronary reactivity testing (CRT) to establish the diagnosis of CMD.
Table 1.
Timeline of coronary microvascular disease onset, progression to heart failure with preserved ejection fraction, and therapy
| Time | Visit type | Symptoms | Medications | Vital signs | Laboratory assessments | Diagnostic testing |
|---|---|---|---|---|---|---|
| December 2006 (baseline) | Initial evaluation for symptoms of ischemic heart disease | 5-month history of dyspnea both at rest and at exertion, squeezing chest pain on a daily basis, intermittent palpitations | Lipitor (atorvastatin; 20 mg, daily), lisinopril (20 mg, daily), aspirin (81 mg, daily) and sublingual nitroglycerin (0.4 mg, as needed) | Blood pressure, 120/75 mmHg; pulse, 60 bpm |
Sodium, 143 (reference range, 135–145 mmol/L); potassium, 3.8 (reference range, 3.5–5.0 mmol/L), creatinine, 0.5 (0.6–1.1 mg/dL) |
Diagnostic coronary reactivity testing demonstrating coronary endothelial dysfunction. Previous right left heart catheterization showed normal coronary arteries and was negative for any shunt. CMRI showed normal left ventricular structure and function. MPRI borderline normal 1.8 |
| November 2016 (10-year follow-up) | Emergency Department visit for heart failure and initial diagnosis for heart failure with preserved ejection fraction | Increased orthopnea, dyspnea, mild diffuse headache, lower extremity edema, and elevated blood pressure | Eplerenone (25 mg, daily), lisinopril (40 mg, daily), aspirin (81 mg, daily), pravastatin (40 mg, daily), spironolactone (100 mg, daily), nitroglycerin (0.4 mg, as needed) | Blood pressure, 152/77 mmHg; pulse, 70 bpm; respiratory rate, 18; temperature, 36.9 °C (98.4 °F) |
BNP, 406 (reference range, < 100 pg/mL); troponin, < 0.01 (reference range, < 0.04 ng/mL); hemoglobin, 9.9 (reference range, 10.6–13.5 g/dL); sodium, 139 (reference range, 135–145 mmol/L); potassium, 4.3 (reference range, 3.5–5.0 mmol/L); creatinine, 0.5 (0.6–1.1 mg/dL) |
Follow-up CMRI revealed worsening ischemia with MPRI 1.1 and increased wall thickness, with evidence of myocardial steatosis |
bpm beats per minute, BNP brain natriuretic peptide, CMRI cardiac magnetic resonance imaging, MPRI myocardial perfusion reserve index
Diagnosis of CMD
Our patient underwent invasive CRT, as previously published [6]. Testing demonstrated normal coronary flow reserve (CFR) in response to intra-coronary adenosine (CFR 3.1; normal ≥ 2.5), abnormal macrovascular endothelial function to intra-coronary acetylcholine (− 6% change in coronary diameter, constriction; normal, dilation), abnormal microvascular endothelial function (coronary blood flow change 48%; normal ≥ 50%), and abnormal non-endothelial function to intra-coronary nitroglycerin (coronary diameter change + 0%; normal dilation) (Table 2). She also underwent cardiac magnetic resonance imaging (CMRI) with perfusion imaging at rest and with adenosine stress (140 μg/kg per minute) which showed circumferential subendocardial perfusion defect at stress, normal LV end-diastolic volume indexed to body surface area (EDVi) of 56.4 mL/m2, LV mass index 42.3 grams/m2, and no LV hypertrophy (septum 7.2 mm and lateral wall 6.0 mm). The myocardial perfusion reserve index (MPRI) was 1.8 which was considered borderline abnormal [7] (Table 3). There was no evidence of myocardial scar.
Table 2.
Results of coronary reactivity testing
| Coronary microvascular dysfunction pathways | Microvascular dysfunction | Macrovascular dysfunction |
|---|---|---|
| Non-endothelial dependent |
aCoronary flow reserve in response to adenosine 2.8 (normal ≥ 2.5) |
bChange in coronary diameter in response to nitroglycerin 0% (normal ≥ 20%) |
| Endothelial dependent |
cChange in coronary blood flow in response to acetylcholine 48% (normal ≥ 50%) |
dChange in coronary diameter in response to acetylcholine − 6% (normal ≥ 5%) |
a Non-endothelial-dependent microvascular dysfunction with coronary flow reserve 2.8 (adenosine). b Non-endothelial-dependent macrovascular dysfunction with 0% (nitroglycerin) coronary diameter change. c Endothelial-dependent microvascular dysfunction with coronary blood flow change 48%. d Endothelial-dependent macrovascular dysfunction with coronary diameter change − 6% in response to acetylcholine
Table 3.
Changes in left ventricular morphology
| Cardiac magnetic resonance imaging parameters | Baseline | 10 years later |
|---|---|---|
| LV EDVi (mL/m2) | 56.4 | 69 |
| LV ESVi (mL/m2) | 20.8 | 23.6 |
| LVMi (g/m2) | 42.3 | 48.5 |
| LV mass-to-volume ratio (g/mL) | 0.75 | 0.70 |
| Wall thickness | ||
| Septum (cm) | 7.15 | 9.34 |
| Lateral wall (cm) | 6.06 | 7.06 |
| Scar (g) | 0 | 0 |
| LVEF (%) | 67 | 64 |
| LGE | Yes | Yes |
| MRPI | 1.8 | 1.1 |
EDVi end-diastolic volume indexed to body surface area, ESVi end-systolic volume indexed to body surface area, LGE late gadolinium enhancement, LV left ventricular, LVEF left ventricular ejection fraction, LVMi left ventricular mass indexed to body surface area, MPRI myocardial perfusion reserve index
The diagnosis of CMD was established by the coronary endothelial dysfunction observed with invasive CRT, and carvedilol and eplerenone 25 mg daily were added to her regimen. She was followed regularly in clinic with good control of her blood pressure and serum lipid levels. She reported improvement of her angina and dyspnea along with reduction in the duration and frequency of these episodes.
Diagnosis of heart failure
Ten years after her initial diagnosis of CMD, our patient was hospitalized due to symptoms of dyspnea. She was found to have elevated brain natriuretic peptide (BNP) levels of 406 pg/mL and normal LVEF. She had a computed tomography (CT) angiogram of her chest to evaluate for pulmonary embolism, which was negative but revealed bilateral pulmonary edema. She was treated with intravenously administered furosemide for pulmonary edema and diagnosed as having HFpEF. Subsequently, she was discharged with instructions to increase her eplerenone.
She continued to experience worsening dyspnea on exertion, orthopnea, and paroxysmal nocturnal dyspnea. A repeat echocardiogram demonstrated normal LV systolic function with an LVEF of 64%, and diastolic dysfunction as evidenced by decreased lateral E′ velocity (4.2 cm/s, indicating impaired myocardial relaxation) and elevated E/E′ ratio 12.9 (suggestive of increased LV filling pressure). She underwent coronary CT angiography which showed absence of coronary atherosclerotic plaque and a coronary calcium score of 0. She was diagnosed as having HFpEF based on clinical symptoms, preserved ejection fraction of 64%, elevated BNP, and evidence of diastolic dysfunction.
As part of the WISE – Coronary Vascular Dysfunction (WISE-CVD) Continuation Study (NCT00832702), she underwent a repeat rest-stress CMRI to assess myocardial structure, function, perfusion, and scar, and 13C magnetic resonance (CMR) spectroscopy. Compared to her prior CMRI 7 years ago, she had an increase in LV wall thickness in both the septum and lateral wall (Table 3). On CMR spectroscopy, the myocardial triglyceride content was elevated (0.83%) compared to normal control women (mean 0.43%), suggesting myocardial steatosis which is consistent with an ischemia-induced metabolic shift and HFpEF phenotype [8]. Adenosine stress first pass-perfusion CMRI again showed circumferential subendocardial hypoperfusion (Fig. 1) and her MPRI worsened from 1.8 to 1.1, consistent with severe CMD [7]. There was no evidence of scar on late gadolinium enhancement imaging.
Fig. 1.
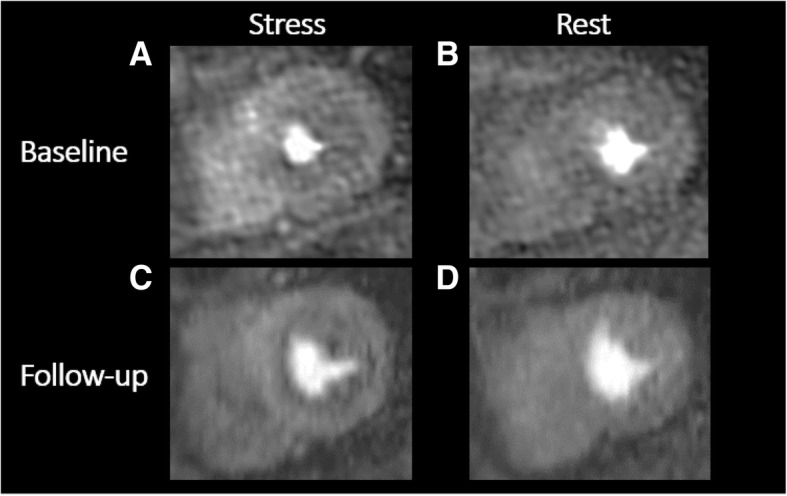
Baseline (a, b) and 10-year follow-up (c, d) adenosine stress first-pass perfusion cardiac magnetic resonance imaging showing evidence of circumferential subendocardial hypoperfusion at stress, consistent with coronary microvascular dysfunction-related ischemia. Myocardial perfusion reserve index decreased from 1.8 to 1.1 over 10-year period, indicating worsened ischemia
Discussion
This case documents, in a 55-year-old woman, the progression of CMD diagnosed with invasive CRT to HFpEF over a span of 10 years despite well-controlled hypertension and dyslipidemia. Although we and others have previously hypothesized that CMD may contribute to progression to HFpEF [9, 10], we are not aware of case examples of CMD progressing to HFpEF reported in the literature. While existing cross-sectional registry studies cannot determine whether primary CMD leads to ventricular remodeling, diastolic dysfunction, and HFpEF or whether these findings observed in HFpEF lead to secondary CMD [11], the current case example provides evidence that CMD may contribute to the development of HFpEF.
HFpEF has often been referred to as “diastolic heart failure” and is characterized by impaired LV relaxation and elevated LV filling pressures [12]. The diagnosis of HFpEF is challenging and currently accounts for approximately half of all cases of heart failure [12], with morbidity and mortality rates similar to those with heart failure with reduced ejection fraction [13, 14]. Previous research has observed an increased incidence of CMD as evidenced by abnormal CFR and index of microvascular resistance (IMR) after adenosine administration in patients with HFpEF compared with normal reference control individuals [15, 16].
At present, there are insufficient prospective studies to confirm that CMD contributes to progression to HFpEF, nor putative mechanistic pathways. Previous studies have identified common inflammatory markers as contributing to the progression to heart failure [17]. Systemic inflammation may lead to reduced nitric oxide bioavailability, expression of growth factor-β, activation of cardiac fibroblasts, and an increase in collagen type 1 formation [18]. In addition, this process may lead to interstitial fibrosis contributing to high diastolic LV stiffness and eventual progression to HFpEF [18]. There are currently no evidence-based treatments for CMD or HFpEF. Understanding the links and disease pathophysiology between CMD and HFpEF may lead to the development of preventive and treatment strategies [19].
Conclusions
This case report of a patient with HFpEF and antecedent CMD is in line with our hypothesis that CMD may contribute to the development of HFpEF [5, 8]. In an individual patient, whether these are causally related, whether they are simply related to similar underlying risk factors, or whether they represent unrelated presences of two common disorders is the subject of current research. Further evaluation of the association between CMD and HFpEF will be necessary to prove our hypothesis.
Acknowledgments
Funding
Research reported in this publication was supported by the National Heart, Lung and Blood Institute (NHLBI) under grant numbers N01HV68161, N01HV68162, N01HV68163, N01HV68164, U01HL64829, U01HL64914, U01HL64924, K23HL105787, T32HL69751, R01HL090957, R01HL33610, R01HL56921, and UM1HL087366; the National Institute on Aging (NIA) under grant number R03AG032631; the National Center for Research Resources (NCRR) under grant number M01RR000425; the National Center for Advancing Translational Sciences (NCATS) under grant numbers UL1TR000124, UL1TR000064, and UL1TR001427. This work was also supported by grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ; The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA; The Society for Women’s Health Research (SWHR), Washington, D.C.; QMED, Inc., Laurence Harbor, NJ; The Women’s Guild of Cedars-Sinai, the Edythe L. Broad, the Constance Austin Women’s Heart Research Fellowships, the Barbra Streisand Women’s Cardiovascular Research and Education Program, the Linda Joy Pollin Women’s Heart Health Program, the Erika J. Glazer Women’s Heart Research Initiative, and The Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, CA; the Gatorade Trust and the PCORnet-One Florida Clinical Research Consortium CDRN-1501-26692, University of Florida, Gainesville, FL.
Availability of data and materials
The datasets used during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BNP
Brain natriuretic peptide
- CFR
Coronary flow reserve
- CMD
Coronary microvascular dysfunction
- CMR
13C magnetic resonance
- CMRI
Cardiac magnetic resonance imaging
- CRT
Coronary reactivity testing
- CT
Computed tomography
- HFpEF
Heart failure with preserved ejection fraction
- IMR
Index of microvascular resistance
- INOCA
Ischemia and no obstructive coronary artery disease
- LV
Left ventricular
- LVEF
Left ventricular ejection fraction
- MI
Myocardial infarction
- MPRI
Myocardial perfusion reserve index
- WISE
Women’s Ischemia Syndrome Evaluation
- WISE-CVD
Women’s Ischemia Syndrome Evaluation – Coronary Vascular Dysfunction
Authors’ contributions
CNBM made substantial contributions to the conception or design of the work. MDN, BT, CS, LT, and DB participated in the acquisition, analysis, or interpretation of data. SJ, JW, and HA are responsible for drafting the work and all authors are responsible for revising critically for important intellectual content. All authors have reviewed the manuscript and have given final approval of the version submitted. All authors agree to be responsible for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
All studies have been approved by Cedars-Sinai Medical Center’s Institutional Review Board (IRB).
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sandy Joung, Email: Sandy.Joung@cshs.org.
Janet Wei, Email: Janet.Wei@cshs.org.
Michael D. Nelson, Email: michael.nelson3@uta.edu
Haider Aldiwani, Email: Haider.Aldiwani@cshs.org.
Chrisandra Shufelt, Email: Chrisandra.Shufelt@cshs.org.
Balaji Tamarappoo, Email: Balaji.Tamarappoo@cshs.org.
Daniel Berman, Email: Daniel.Berman@cshs.org.
Louise E. J. Thomson, Email: Louise.Thomson@cshs.org
C. Noel Bairey Merz, Phone: 310-423-9680, Email: Noel.BaireyMerz@cshs.org, Email: noel.baireymerz@cshs.org.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JHY, Alger HM, Wong SS, Muntner P. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation. 2017;135:1075–1092. doi: 10.1161/circulationaha.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ, Merz CN. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843–850. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakir M, Nelson MD, Jones E, Li Q, Wei J, Sharif B, Minissian M, Shufelt C, Sopko G, Pepine CJ, Merz CN. Heart failure hospitalization in women with signs and symptoms of ischemia: A report from the women's ischemia syndrome evaluation study. Int J Cardiol. 2016;223:936–939. doi: 10.1016/j.ijcard.2016.07.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, Azarbal B, Petersen J, Sharaf B, Handberg E, Shufelt C, Kothawade K, Sopko G, Lerman A, Shaw L, Kelsey SF, Pepine CJ, Merz CN. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women's Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv. 2012;5:646–653. doi: 10.1016/j.jcin.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson LE, Wei J, Agarwal M, Haft-Baradaran A, Shufelt C, Mehta PK, Gill EB, Johnson BD, Kenkre T, Handberg EM, Li D, Sharif B, Berman DS, Petersen JW, Pepine CJ, Bairey Merz CN. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc imaging. 2015;8 10.1161/circimaging.114.002481. [DOI] [PMC free article] [PubMed]
- 8.Wei J, Nelson MD, Szczepaniak EW, Smith L, Mehta PK, Thomson LE, Berman DS, Li D, Bairey Merz CN, Szczepaniak LS. Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. Am J Physiol Heart Circ Physiol. 2016;310:H14–H19. doi: 10.1152/ajpheart.00612.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pepine CJ, Petersen JW, Bairey Merz CN. A Microvascular-Myocardial Diastolic Dysfunctional State and Risk for Mental Stress Ischemia: A Revised Concept of Ischemia During Daily Life∗. JACC Cardiovasc Imaging. 2014;7:362–365. doi: 10.1016/j.jcmg.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Crea F, Bairey Merz CN, Beltrame JF, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Camici PG. The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J. 2017;38:473–477. doi: 10.1093/eurheartj/ehw461. [DOI] [PubMed] [Google Scholar]
- 11.Nelson MD, Wei J, Bairey Merz CN. Coronary microvascular dysfunction and heart failure with preserved ejection fraction as female-pattern cardiovascular disease: the chicken or the egg? Eur Heart J. 2018;39:850–852. doi: 10.1093/eurheartj/ehx818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 13.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 14.Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of Diastolic Stress Testing in the Evaluation for Heart Failure With Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation. 2017;135:825–838. doi: 10.1161/circulationaha.116.024822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giamouzis G, Schelbert EB, Butler J. Growing Evidence Linking Microvascular Dysfunction With Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc. 2016;5 10.1161/jaha.116.003259. [DOI] [PMC free article] [PubMed]
- 16.Wei J, Nelson MD, Sharif B, Shufelt C, Bairey Merz CN. Why do we care about coronary microvascular dysfunction and heart failure with preserved ejection fraction: addressing knowledge gaps for evidence-based guidelines. Eur Heart J. 2018;39:3451–3453. doi: 10.1093/eurheartj/ehy558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AlBadri A, Lai K, Wei J, Landes S, Mehta PK, Li Q, Johnson D, Reis SE, Kelsey SF, Bittner V, Sopko G, Shaw LJ, Pepine CJ, Bairey Merz CN. Inflammatory biomarkers as predictors of heart failure in women without obstructive coronary artery disease: A report from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) PLoS One. 2017;12:e0177684. doi: 10.1371/journal.pone.0177684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohanyan V, Sisakian H, Peketi P, Parikh A, Chilian W. A chicken and egg conundrum: coronary microvascular dysfunction and heart failure with preserved ejection fraction. Am J Phys Heart Circ Phys. 2018;314:H1262–H1263. doi: 10.1152/ajpheart.00154.2018. [DOI] [PubMed] [Google Scholar]
- 19.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during and/or analyzed during the current study are available from the corresponding author on reasonable request.


