Abstract
Endometrial polyps are overgrowths of endometrial glands that typically protrude into the uterine cavity. Endometrial polyps are benign in nature and affect both reproductive age and postmenopausal women. Although endometrial polyps are relatively common and may be accompanied by abnormally heavy bleeding at menstruation. In asymptomatic women, endometrial polyps may regress spontaneously, in symptomatic women endometrial polyps can be treated safely and efficiently with hysteroscopic excision.
Keywords: Polyps, endometrial, uterine
Introduction
An endometrial polyp or uterine polyp is an abnormal growth containing glands, stroma and blood vessels projecting from the lining of the uterus (endometrium) that occupies spaces small or large enough to fill the uterine cavity. They are found during both reproductive and postmenopausal phases of life.1 The majority of polyps are located in the fundus, often in the corneal area, and in this area there are obvious technical difficulties for removal by curettage.2 They range in size from about 5 mm to as large as filling the whole uterine cavity3 can be found in all age groups, however, most common between age 40 and 49.4
If an endometrial polyp is attached to the uterine surface by a narrow elongated pedicle, then it is known as pedunculated, however, if they have a large flat base, absence of a stalk, they are known as sessile5 (Figure 1). Gross morphological appearance is smooth, spherical or cylindrical in structure and is tan to yellow in colour. The endometrium varies from normal cycling endometrium to simple or complex hyperplasia in the presence of endometrial polyps, and rarely endometrial cancer can be found.
Figure. 1.
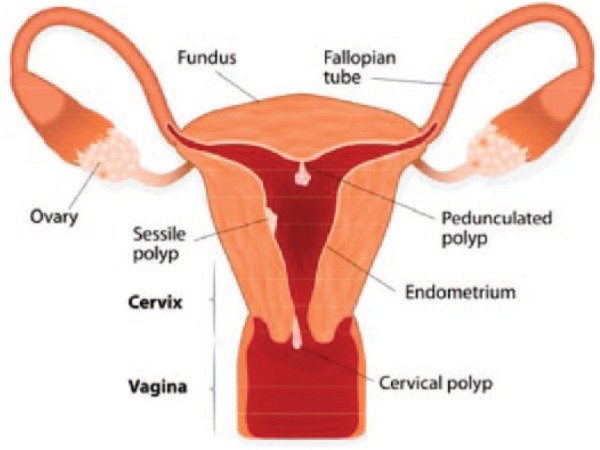
Illustration showing positions of sessile and pedunculated endometrial (uterine) polyps.
Used with permission Artist: Designua. http://www.Shutterstock.com.
Endometrial polyps are the most frequently observed pathological finding in the uterus and are usually benign lesions.6 The exact prevalence of endometrial polyps is not known, however, Dreisler et al.7 reported 82% of the women who had histopathology verified polyps were asymptomatic. Nevertheless, endometrial polyps have been implicated in about 50% of cases of abnormal uterine bleeding8 and 35% of infertility.9
Aetiology and pathogenesis
The pathogenesis and natural history of endometrial polyps are not very clear,10 exact cause of endometrial polyps is unknown, however, there are several theories proposed relating to the aetiology and pathogenesis of these lesions. They are believed to be related to oestrogen stimulation, this may be as a result of an increased concentration of oestrogen receptors (ERs), predominantly ER-alpha in polyp glandular cells compared with normal endometrium, and a decreased expression of progesterone receptors (PRs) A and B in polyps compared with normal endometrium.11,12 Endometrial polyps contain both ERs and PRs, and the concentration of these receptors have been found to be much higher in the glandular epithelium in endometrial polyps in comparison with the normal epithelium.13,14 The concentration of ERs and PRs has been observed to have decreased in the stromal cells of endometrial polyps,15 which may prevent the stroma of the polyp from undergoing decidual changes and menstrual shedding which is seen in the rest of the endometrium.
The balance between mitotic activity and apoptosis appears to play a role in regulating the development of normal endometrium during the menstrual cycle. The role of B-cell lymphoma-2 (Bcl-2) marker, which is an inhibitor of apoptosis, and Ki67 protein, which is a cellular marker for proliferation and cell mitotic activity, has been reported. 16 Taylor et al.,16 observed a marked increase in the expression of Bcl-2 in the proliferative phase polyps in both the glandular epithelium and stroma compared with the proliferative endometrium, however, this increase was not observed in any of the polyps in the secretory phase. A localised elevation of Bcl-2 expression in endometrial polyps may explain the failure of polyps to undergo normal cyclical apoptosis, and therefore not be shed during the menstrual cycle.17 Other studies have similarly observed that there is decreased apoptosis in endometrial polyp tissue.18–20 Glandular, menopause-independent AB DNA fragmentation factor 40, 45 (DFF40), (DFF45) and Bcl-2 overexpression may play an important role in the pathogenesis of endometrial polyps.21
Ki67 expression was observed to occur predominately in the proliferative phase, with glandular epithelium exhibiting the strongest expression. Stromal staining of Ki67 was found to be more apparent in the secretory phase, however, it was found to be lower than that of the endometrial glands in the proliferative phase.16 Miranda et al.22 reported that the expression of Ki-67 were significantly higher in the polyp samples from tamoxifen-treated women compared with those samples from women using no hormone.
Cytogenetic studies have suggested that chromosomal abnormalities may have a role in the development of endometrial polyps.23 Endometrial polyps arise as a result of chromosomal rearrangements (translocation) in the stromal cells. Dal Cin et al.24 distinguished three major cytogenetically abnormal subgroups involving 6p21-22, 12q13-15 or 7q22 regions, a normal karyotype was found in a fourth subgroup.
The expression of p63, aromatase P450 (P450 arom) and steroidogenic factor-1 (SF-1) may play a role in the formation of endometrial polyps Su and Sui.25 Stewart et al.26 concluded that stromal p16 immunoreactivity is characteristic of endometrial polyps which may be reflective of the pathogenesis of polyp formation.
The risk factors for endometrial polyp formation include increased endogenous oestrogen and exogenous oestrogen administration. Tamoxifen (a uterine oestrogen agonist used to treat breast cancer in pre- and postmenopausal women) have an increased likelihood of developing endometrial polyp.27
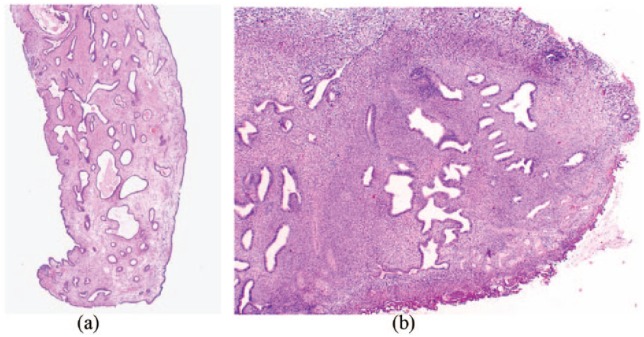
Tamoxifen has oestrogenic effects on the uterus, and the incidence of endometrial polyps, hyperplasia and endometrial cancer in women taking Tamoxifen is higher than non-users.28,29 Tamoxifen-related polyps (Figure 2(a)) differ histologically from polyps in non-tamoxifen users (Figure 2(b)). McGurgan et al.27 observed that the use of Tamoxifen decreases the levels of ER and increases the levels of PR in these polyps, and decreases the level of apoptotic cells. These results were able to support their hypothesis that Tamoxifen promoted polyp growth by inhibiting apoptosis. Gokmen Karasu et al.30 reported the possibility that there is a direct effect of Tamoxifen on apoptosis or an indirect effect through a progesterone-related mechanism. Gokmen Karasu et al.30 also observed that a low expression of the anti-apoptotic marker survives in tamoxifen-exposed polyps; however, it was found to be illustrative that diverse mechanisms are responsible in the pathogenesis of endometrial polyps, if there is a high expression of the anti-apoptotic marker in other polypoid endometrium.
Figure 2.
(a) Tamoxifen-associated polyp with fibrotic stroma stained with hematoxylin and eosin (H&E), magnification (100×). (b) Benign endometrial polyp with hematoxylin and eosin (H&E), magnification (2×).
Similarly, postmenopausal women on hormone replacement therapy (HRT) have been found to have a higher incidence of endometrial polyps.31 This may be due to the continuous stimulation of the endometrium by oestrogen. Obesity is associated with increased endogenous oestrogen production1 via increased levels of aromatase (oestrogen converter enzyme) which converts androgens in fat to oestrogen. A systematic semi-quantitative review has reported causative, non-causative, protective and unclear links regarding the factors involved in the pathogenesis of endometrial polyps.10
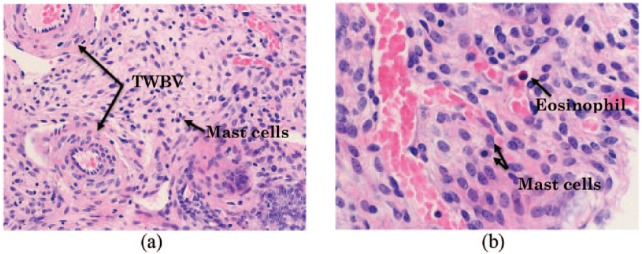
Endometrial polyp formation may be the result of localised chronic inflammation in the endometrium. Mast cells (Figure 3(a) and (b)) are known to initiate and control inflammation through their secretion of cytokines and growth factors.32 Cyclooxygenase-2 (COX-2), a key enzyme involved in the production of prostaglandin in mast cells, was also found to be significantly higher in polyps compared with normal endometrium.33 Inflammation results in the formation of new blood vessels and growth of tissue.
Figure 3.
(a) Mixed inflamed polyp with mast cells and thick walled blood vessels (TWBV) stained with hematoxylin and eosin (H&E), magnification (200×), (b) Mast cells and eosinophil under higher magnification stained with hematoxylin and eosin (H&E), magnification (400×).
The quantity of mast cells were found to be seven-fold higher in endometrial polyps compared with normal endometrium,34 and the majority of these mast cells were found to be activated.35 Secreting mast cells are capable of inducing or enhancing angiogenesis36 and as a result, an increase in blood vessel density would be expected. It is generally considered that polyp biology is similar throughout the human body regardless of the specific type (e.g. endometrial, nasal and colorectal).34 An increased expression of vascular endothelial growth factor (VEGF) occurs in nasal polyps,37 which is likely to result in increased blood vessel density. Angiogenic factors such as VEGF and basic fibroblast growth factor (bFGF) in turn stimulate mast cell migration.38
VEGF and transforming growth factor beta-1 (TGF beta-1) were found to be significantly higher in endometrial polyps compared with normal endometrium.39 VEGF is angiogenic, while TGF beta-1 is associated with fibrotic tissue formation, both of which are characteristic of endometrial polyps. This is supported by a higher concentration of Ki67 (tissue proliferative factor) in endometrial polyps compared with normal endometrium.40
Inflammation may result in an overreaction, or an attack on the host resulting in tissue damage. It has been speculated that this may be via proliferation of fibrin and blood vessels during the inflammatory repair process resulting in metaplasia and potentially, in tumour growth.41,42 The inflammatory process could result in a shift in homeostatic set points leading to development of disease. The difference in the expressions of growth factors may have an implication on the existence of two different kinds of endometrial polyps, one being hormonal dependent and the other of an inflammatory nature. This nature of variation may cause different symptoms, rise of a relapse, effects on reproduction and oncology.43
Clinical characteristics
Polyp lesions are usually benign; however, a small minority may have atypical or malignant features. For the basic classification system, polyps are categorised as being either present or absent, as defined by one or a combination of ultrasound and hysteroscopic imaging with or without histopathology.44,45
Bleeding
Endometrial polyps are mostly asymptomatic lesions, although they can present with abnormal uterine bleeding.46 Abnormal uterine bleeding is the most common symptom of endometrial polyps, occurring in approximately 68% of both pre- and postmenopausal women with the condition.47 The bleeding may be due to stromal congestion within the polyp leading to venous stasis and apical necrosis.48 The abnormal uterine bleeding appears to increase with age: bleeding in premenopausal women is observed 6% less than in postmenopausal counterparts.7 This finding could also be as a result of selection bias as postmenopausal women are more likely to be investigated when presented with vaginal bleeding.7 Driesler et al.7 further noted that, contrary to what one might expect, the size of the polyp, number of polyps and anatomical location of the polyp(s) did not appear to correlate with bleeding symptoms.
In women complaining of abnormal uterine bleeding, polyps account for 13%–50% in premenopausal women49 and 30% in postmenopausal women.50 Postmenopausal uterine bleeding and menstrual disorders were significant clinical symptoms in 44% post- and in 82% of premenopausal women. The other 56% post- and 18% premenopausal women were asymptomatic. Multiple endometrial polyps were present in 26% of postmenopausal and in 15% premenopausal women.1
Infertility
Endometrial polyps have been associated with infertility; the incidence of this disease in primary infertility is 3.8%–38.5%, and 1.8%–17% in secondary infertility.51 It has a combined infertility incidence of 1.9%–24%.52 Endometrial polyps can also result in infertility, due to recurrent implantation failure.53 The actual causal relationship remains uncertain,51 although some hypotheses have been proposed. The first way that endometrial polyps can cause infertility is by mechanical obstruction. This may result in a number of consequences such as hindering sperm transport47 by blocking the cervical canal or entrance into the fallopian tube. The physical surface area of the polyp could also prevent implantation of the embryo into the endometrium acting as a space occupying lesion. Furthermore, polyps may create an inflammatory endometrial response similar to an intrauterine device disturbing implantation of the embryo. In addition, hysteroscopic polypectomy appears to improve pregnancy rate in formerly infertile patients irrespective of their size or number of polyps,54 restoration of reproductive ability is not dependent on the size of polyp excised.55 Another study involving in vitro fertilization (IVF) patients found that hysteroscopic polypectomy prior to IVF resulted to a better chance of becoming pregnant and that pregnancy after polypectomy was frequently obtained spontaneously while waiting for treatment. Irregular vaginal bleeding may also cause infertility by reducing mating frequency.56
A second way that polyps may cause infertility is through biochemical effects.57 Endometrial polyps have increased levels of matrix metalloproteinases (MMPs) and cytokines such as interferon gamma, and glycodelin compared with normal endometrium, while placenta protein 14 is lower in polyps compared with normal endometrium.57 An increased level of MMP in endometrial polyps causes an imbalance in the endometrium of these women inhibiting implantation of the embryo.58 Interferon gamma has toxic effects on sperm and inhibitory consequences on embryonic development. Glycodelin is known to obstruct sperm-oocyte interaction.59 Placenta protein 14, an immune suppressor favouring acceptance of the allogenic embryo, has been found to be lower in endometrial polyp endometrium compared with normal endometrium.60 Infertile patients with endometriosis were reported to have a higher prevalence of endometrial polyps, and these polyps were often combined with simple hyperplasia.61
Pregnancy
Post polypectomy, pregnancy rates improved two-fold in intrauterine inseminated patients.56 Stamatellos et al.54 conducted a retrospective study and reported that hysteroscopic polypectomies appeared to increase pregnancy rates and ultimately improved fertility in women who were previously infertile with no known cause. The hysteroscopic removal of polyps prior to intrauterine insemination (IUI) can increase the chance of a clinical pregnancy compared with simple diagnostic hysteroscopy and polyp biopsy.62,63 The removal of endometrial polyps in subfertile women is commonly being performed in many countries with an aim to improve the reproductive outcome. Jayaprakasan et al.64 could not identify any analysable randomised trials which would allow the reaching of any sound scientific conclusions on the efficacy of endometrial polypectomy in subfertile women.
Hysterosalpingography has been described as still being the main method to commence with when studying the causes of female impossibility to conceive.65 Covali65 further reported as conducting largest study involving Hysterosalpingography and the most successful pregnancy rate outcomes in Romania.
Malignancy
A very small fraction of polyps, about 1.0%, may become hyperplastic or show malignant transformation.66 The most common cancer subtypes are endometrioid adenocarcinoma and serous adenocarcinoma. The prognosis is variable and for endometrioid adenocarcinoma, associated with pre-existing hyperplasia, is often predicted by stage. In contrast, serous adenocarcinomas typically arising in an inactive endometrium in postmenopausal patients may behave in a highly aggressive fashion despite low stage in the uterus. The risk of developing malignancy appears to be associated with the following: symptoms, age, obesity, hypertension, the size of the polyp, use of Tamoxifen and HRT. Both symptomatic vaginal bleeding and postmenopausal status in women with endometrial polyps are associated with an increased risk of malignancy.67 The incidence of malignant transformation in endometrial polyp increases with age.68 The lower incidence in younger women may be due to spontaneous regression mechanism that is characteristic of the cycling endometrium in women of reproductive age.69
Karakaya et al.70 reported a prevalence of endometrial cancer among 9% of geriatric women with endometrial polyps. Consequently, it is important to conduct a pathological evaluation of endometrial polyps in such patients in that age group.
Obesity is another risk factor for malignancy and as alluded to previously in the aetiology of polyps, may be as a result of the excess oestrogenic effect, due to aromatization of androgens in fat into oestrogen, on the uterus. Hypertension is a risk factor, which may be a correlation rather than causative, because most high blood pressure patients have increased body mass index (BMI).69
The size of polyps may be relevant, and those above 15 mm are thought more like to lead to malignant transformation.71 However, this is controversial and others Gregoriou et al.69 have found no link between the size of polyps, hypertension, abnormal uterine bleeding and malignant transformation.
Tamoxifen has also been implicated in the development of malignancy in endometrial polyps.72 Hachisuga et al.73 reported malignant transformations in endometrial polyps of women on Tamoxifen were found to be due to mutations of the codon 12 of K-RAS (Kirsten Rat Sarcoma viral oncogene homolog). HRT is also associated with the occurrence of malignancy in endometrial polyps.74 This may be due to the oestrogenic effect on the uterus.
The mechanism of malignant transformation in endometrial polyps is generally thought to be due to COX-2, an enzyme responsible for prostaglandin synthesis. The quantity of COX-2 was significantly elevated in the cytoplasm of tumour cells in all cases, independent of grade, stage of development, histological subtype and aggressiveness.66 Giordano et al.66 also found that Phosphoprotein p53 (P53) and Ki67, both of which are associated with abnormal cell proliferation, were significantly higher in tumour cells, and associated with more advanced stage, grading, subtype and deep invasion of the myometrium, but there was, however, no correlation with COX-2 immuno-reactivity.
Diagnosis
Macroscopic diagnosis
There are several options available for the macroscopic diagnosis of endometrial polyps.
Transvaginal ultrasonography
The primary tool for initial diagnosis of endometrial polyps is transvaginal ultrasonography (TVUS) (Figure 4). This is achieved by inserting an ultrasound probe through the vagina in order to visualise the uterine cavity. Endometrial polyps appear as a hyperechogenic lesion with regular contours.75 Cystic glands may be visible within the polyp.
Figure 4.
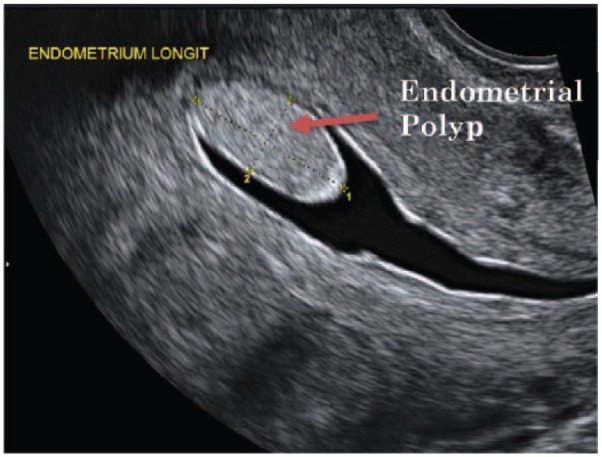
Transvaginal ultrasonography (TVUS) image showing an endometrial polyp. Used with permission from Associate Professor Kirsten Black, Discipline of Obstetrics, Gynaecology and Neonatology, The University of Sydney via Dr Philippa Ramsay, Ultrasound Care, Sydney, NSW, Australia.
Endometrial polyps are seen as a focal mass or nonspecific thickening. These findings, however, are not specific to polyps as leiomyomas (fibroids) particularly submucosal forms may have the same features.76 Imaging is best on the 10th day of the menstrual cycle, when the endometrium is thinnest, to minimise false positive and false negative results. TVUS has a reported sensitivity of 19%–96%, specificity of 53%–100%, positive predicative value (PPV) of 75%–100% and negative predictive value (NPV) of 87%–97% to diagnose endometrial polyps.
TVUS was found to be representative of a practical approach for the initial evaluation of uterine pathologies, however, hysteroscopy appeared to offer a better diagnostic value for uterine pathologies in general, and for uterine polyps in particular.77
Colour-flow or power Doppler
The addition of ‘Colour-Flow or Power Doppler’ (Figure 5) may improve the diagnostic capability of TVUS. Colour-flow Doppler may demonstrate the single feeding vessel typical of endometrial polyps. Power Doppler has been reported to increase sensitivity to around 97%, while specificity and NPV may be increased to 95% and 94%, respectively. The addition of intrauterine contrast by Sonohysterography (SHG) may outline small endometrial polyps missed on greyscale TVUS and is likely to improve diagnostic accuracy.79
Figure 5.
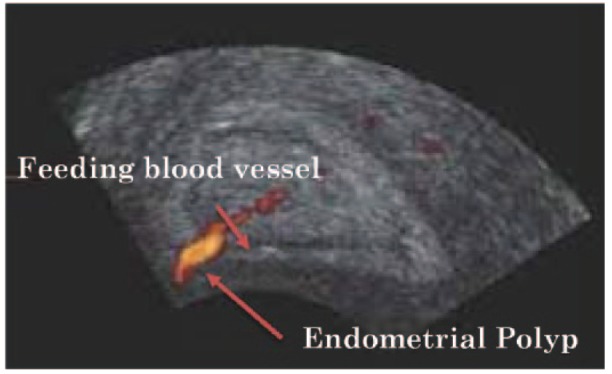
Power Doppler or colour-flow ultrasound image showing the feeding blood vessel characteristic of an endometrial polyp. Adapted from Lieng et al.78 with permission from Professor Marit Lieng, Department of Gynaecology, RESearch Centre for Obstetrics and Gynaecology (RESCOG), Oslo University Hospital, Norway.
Doppler sonography can aid in the identification of the characteristic single vessel pattern of endometrial polyps as opposed to the multiple vessel pattern seen in hyperplasia and malignant lesions with a sensitivity of 89% and specificity of 87%.80 Although TVUS is not specific for diagnosing endometrial polyps, it is the method of choice for the screening of endometrial diseases in women with abnormal uterine bleeding.76 Nogueira et al.76 added, however, that endometrial thickness of greater than 5 cm in postmenopausal women or hyperechogenic focal image in symptomatic women of reproductive age should raise the possibility of endometrial polyp or malignancy and deserve further investigation. The following year, Lieng et al.79 reported that examination via transvaginal colour Doppler enhanced by intravenous contrast may aid in the discrimination between benign endometrial polyps and cancer.
Transvaginal colour Doppler examination enhanced by intravenous contrast may aid in the discrimination between benign endometrial polyps and cancer, however, larger studies are required to confirm these findings.81
Saline infusion sonography or Sonohysterography
Saline infusion sonography (SIS) or SHG (Figure 6) is the gold standard for diagnosing endometrial polyps82 increases contrast of the endometrial cavity enabling the viewing size, location and other features of endometrial polyps. Endometrial polyps appear as echogenic smooth masses.83 Schwarzler et al.83 reported that SIS or SHG method improved diagnosing accuracy, picking up small polyps missed on TVUS. Differentiating endometrial polyps from submucosal fibroid is also difficult using SIS.84 SIS, however, has the advantage of assessing both the uterine cavity and other pelvic structures visualising potential myometrial and adnexal abnormalities. The main disadvantage is its ability to determine final endometrial disease as it does not allow for a histological diagnosis.85 Exalto et al.85 observed patient discomfort due to fluid leakage or pain with the use of a balloon catheter. Furthermore, saline solution infusion may enhance sonographic details.86
Figure 6.
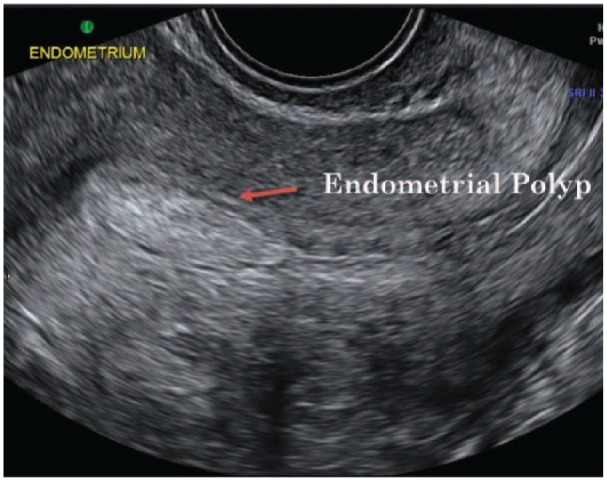
Saline infusion sonography (SIS) or Sonohysterography (SHG) ultrasound image showing an endometrial polyp. Used with permission from Associate Professor Kirsten Black, Discipline of Obstetrics, Gynaecology and Neonatology, The University of Sydney via Dr Philippa Ramsay, Ultrasound Care, Sydney, NSW, Australia.
Saline infusion SHG has been reported Fadl et al.87 to provide a better diagnostic accuracy for the detection and exclusion of endometrial polyps than TVUS, even in cases where the diagnostic confidence for the presence of polyps is high. Saline infusion SHG may still be required for the confirmation of a TVUS diagnosis for polyps to provide a limit on the number of negative hysteroscopies.
Histological diagnosis
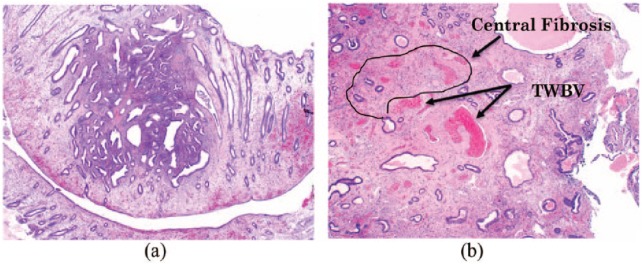
Endometrial polyps can be diagsuspected hysteroscopically by the treating clinician, however, must be confirmed microscopically by the pathologist. The initial clue that a polyp is present, under microscopic examination utilising low power objective, is that there is often a cocktail of fragments that are morphologically different from normal cyclical endometrium. The stroma is dense fibrous tissue compared with the surrounding endometrium, parallel arrangement of endometrial gland long axis to the surface epithelium which is characteristic for the disease, glandular structural anomalies and glands are often dilated, spaced closely together and are unusual in shape, extracellular connective tissue and other features include thick-walled stromal blood vessels76,88 (Figure 7(a) and (b)). Proliferative activity is common in endometrial polyps, even when activity is arrested in the surrounding endometrium.89
Figure 7.
(a) Microscopic appearance of hematoxylin and eosin (H&E) stained endometrial polyp, magnification (100×). (b) Hematoxylin and eosin (H&E) image of endometrial polyp illustrating central fibrosis and thick walled blood vessels (TWBV), magnification (100×).
The majority of endometrial polyps are composed of immature endometrium, which does not respond to hormonal stimuli. These endometrial polyps have the appearance of cystic hyperplasia during all stages of the menstrual cycle90 and are not shed at the time of menstruation.91 Less commonly endometrial polyps consist of functional endometrium which undergoes cyclic histological changes.
Treatment
Management of endometrial polyps depends on symptoms, risk of malignancy and fertility issues. It can be grouped under conservative surgical, radical surgery and conservative non-surgical. Small asymptomatic polyps may resolve spontaneously, in these cases watchful waiting can be the treatment of choice.92 However, in women suffering from infertility, the majority of EPs do not appear to regress spontaneously and surgical intervention is usually required.
Post hysteroscopic progesterone hormone therapy was reported to have encouraging clinical effects in the treatment of endometrial polyps as it is shown to have effectively prevented the recurrence of endometrial polyps, and both a reduction of haemoglobin levels and endometrial thickness.93 Progesterone appeared to be a valid therapeutic alternative for the management of endometrial polyps.94
Conservative surgery
Hysteroscopy
Hysteroscopic polypectomy has been recommended to be the optimal treatment for the removal of endometrial polyps.95 Hysteroscopy polypectomy still remains the gold standard for surgical treatment. Evidence regarding the cost and efficacy of different methods for hysteroscopic resection of endometrial polyps in the office and outpatient surgical settings has begun to emerge.96 It is usually the preferred therapy, and removal of the endometrial basalis at the endometrial polyp origin appears to prevent recurrence of further endometrial polyps.1 The resection of polyps by surgical treatments has resulted in being highly satisfactory with a reduction in patients’ bleeding symptoms. Daniele et al.97 reported it to be a good tool to predict malignancy of the epithelial layer of endometrial polyps.
Hysteroscopy has a higher accuracy than other imaging and also allows for directed sampling and removal of endometrial polyps.98 Satisfactory outcomes of hysteroscopic polypectomy treatments remain the same regardless of menopausal status, size and number of polyps.49 Hysteroscopic resection of endometrial polyps results to the definitive treatment of the disease.95 Whether or not to use the resectoscope or the bipolar electric probe is dependent upon the size and site of the polyp.99 However, there is no recurrence of endometrial polyps when the loop resectoscope is used. Large (>20 mm) diameter and those located at the fundus are better removed by the resectoscope. Sessile polyps are best removed with an electrosurgical loop, while, small (<20 mm) diameter and pedunculated polyps (non-fundal) may be excised using an operating hysteroscope under direct vision with a pair of scissors.100
Hysteroscopic polypectomy has a low complication rate and recurrence rate and technically feasible for practicing gynaecologists which do not need much training and is also cost-effective.101 Although a low complication rate, there are still some possible complications of a hysteroscopic polypectomy that need be taken into consideration; these may include the following: infection, bleeding, pelvic inflammatory disease, tearing of the uterus (rare) or damage to the cervix and complications from fluid or gas used to expand the uterus. There also may be slight vaginal bleeding and cramps for a day or two after the procedure. There may also be other risks based on the patient’s condition, these include the following: pelvic inflammatory disease, vaginal discharge, inflamed cervix and bloated bladder.
Hysteroscopic surgery is still indicated for the treatment of serval intrauterine disorders.102 Giacobbe et al.’s102 experiential observational recommendations for medical specialists were the reduction of operating times; monitoring of fluid balances; monitoring electrolyte levels and kinetic heart rates; and the monitoring of symptoms that include otorrhagia and nosebleeds, for the identification and the possible prevention of Intravascular Absorption Syndrome (IAS) due to an overload of low-viscosity fluids. Vitale et al.103 reported that hysteroscopic tissue removal systems (HTRS) appeared to be a feasible surgical option in terms of operative times and complications.
Dilation and curettage
Dilation and curettage (D&C) combined with the use of polypectomy forceps used to be the standard method for investigating abnormalities. However, there is a potential for polyps to be missed. This procedure is known as a ‘blind procedure’ as its limitations are well known, and polyps have the potential to be missed in excess of 50%–85% of cases due to their mobility.104 In order to reduce the risk of missing a polyp, the uterus should be investigated prior to the procedure using grasping forceps. However, there are also possible risks and complications of a D&C procedure: the risks associated with anaesthesia such as an adverse reaction to medication and breathing problems, haemorrhage or heavy bleeding, infection in the uterine or other pelvic organs, perforation or puncture to the uterus, laceration or weakening of the cervix, scarring of the uterus or cervix, which may require further treatment, incomplete procedure that requires another procedure to be performed.
Conservative non-surgical treatment
Symptom free women with small polyps (<10 mm) have a high regression rate over a 1-year period, and a low chance of malignancy.105 These women could be managed conservatively by observation alone. In postmenopausal women, hormone combined treatment may reduce the development of endometrial polyps. Reasonable effects have been observed with Tibolone, a synthetic steroid with estrogenic progestogenic and weak androgenic action that has the highest anti-oestrogenic activity.106 Zhang et al.107 study was indicative that ovarian hyperthecosis with its resultant risk factor of hyperestrinism may be contributive to the pathogenesis of endometrial polyps in postmenopausal women.
Levonorgestrel intrauterine system
The levonorgestrel intrauterine system (LNG-IUS) has been used to prevent the development of endometrial polyps and hyperplasia (Figure 8) in women on oestrogen replacement therapy and Tamoxifen.28 Fraser108 suggested that the use of LNG-IUS was useful to prevent the development of EPs.109 Wada-Hiraike et al. introduced the oral contraceptive (OC) for the treatment of endometrial polyps. The rate of regression was higher in the group of sessile polyps compared with those of pedunculated polyps. The observed regression could be due to a number of factors (1) endometrial apoptosis and decrease in cell proliferation in epithelial and stromal cells were noticed to occur after exposure to OC;110 (2) anti-inflammatory effects of progesterone, given that mast cell associated inflammation could be involved in endometrial polyp growth;34 and (3) endometrial quiescence, established by steady levels of oestrogen and progesterone.
Figure 8.
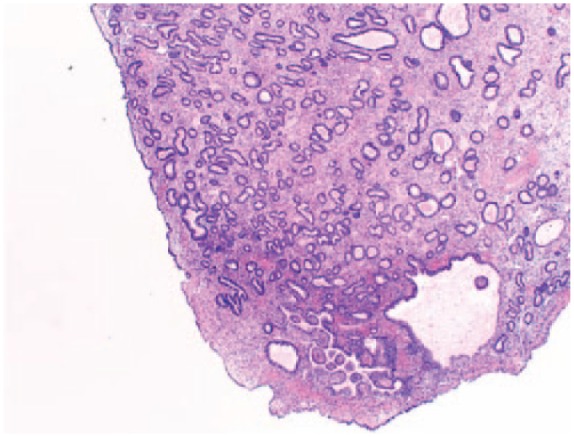
Hematoxylin and eosin (H&E) image of endometrial polyp with complex hyperplasia, magnification (20×).
Van Dijk et al.111 concluded that there was no evidence available on LNG-IUS as a treatment for heavy menstrual bleeding (HMB) in women with an endometrial polyp. They were able to hypothesise that levonorgestrel intrauterine device (LNG-IUD) could be a beneficial alternative to transcervical polyp resection (TCRP) for the treatment of HMB in premenopausal women with a polyp; however, further evidence would be required.
HRT
The effect of HRT may be due to reduced endometrial thickness through oestrogen suppression and thus reduction of polyp development. Mittal et al.12 demonstrated that there was no effect of HRT in reducing polyp size probably because of lack of PRs in endometrial polyps.
Dienogest and danazol
Lagana et al.112 reported on a comparative study of desogestrel and danazol as a preoperative endometrial preparation for hysteroscopic surgery. Their observations were that desogestrel caused less side effects and presented obvious outcomes in inducing endometrial atrophy, this allowed for a better intraoperative management and was shown to cause less side effects during treatment. Dienogest, respective of danazol has been reported to be more effective for the preparation of the endometrium in patients whom have to undergo hysteroscopic surgery for submucous myomas and has been found to cause less side effects.113 The usage of dienogest has been further reported to be effective in reducing the thickness of the endometrium, and the severity of bleeding and also of operative time, with a reduced number of side effects in comparison with other pharmacological preparations or no treatment.114
Conclusion
A common, thorough understanding of the aetiology and development of endometrial polyps is still in its infancy. The literature surrounding the topic of polyps is complex and at times contradictory. Although endometrial polyps are often overlooked, they can offer valuable insights into biological processes at play in the uterus. And, as a cause of uterine bleeding, as a significant subset of all causes of female infertility and as a possible precursor to malignancy, they remain a frequent reason for presentation to health care professionals.
Acknowledgments
We wish to thank Associate Professor Kirsten Black, Discipline of Obstetrics, Gynaecology and Neonatology, The University of Sydney for providing us with Figures 4 and 6 via Dr Philippa Ramsay, Ultrasound Care, Sydney, NSW, Australia. Figure 5 was adapted with permission from Professor Marit Lieng, Department of Gynaecology, RESearch Centre for Obstetrics and Gynaecology (RESCOG), Oslo University Hospital, Norway.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Frank Manconi  https://orcid.org/0000-0002-4980-1656
https://orcid.org/0000-0002-4980-1656
References
- 1. Reslová T, Tosner J, Resl M, et al. Endometrial polyps. A clinical study of 245 cases. Arch Gynecol Obstet 1999; 262(3–4): 133–139. [DOI] [PubMed] [Google Scholar]
- 2. Peterson WP, Novak ER. Endometrial polyps. Obstet Gynecol 1956; 8(1): 40–49. [PubMed] [Google Scholar]
- 3. Moon SH, Lee SE, Jung IK, et al. A giant endometrial polyp with tamoxifen therapy in postmenopausal woman. Korean J Obstet Gynecol 2011; 54(12): 836–840. [Google Scholar]
- 4. Hamani Y, Eldar I, Sela HY, et al. The clinical significance of small endometrial polyps. Eur J Obstet Gynecol Reprod Biol 2013; 170(2): 497–500. [DOI] [PubMed] [Google Scholar]
- 5. Weschler T. Taking charge of your fertility. Revised ed. New York: Harper Collins, 2002. [Google Scholar]
- 6. Chaudhry S, Reinhold C, Guermazi A, et al. Benign and malignant diseases of the endometrium. Top Magn Reson Imaging 2003; 14(4): 339–357. [DOI] [PubMed] [Google Scholar]
- 7. Dreisler E, Stampe Sorensen S, Ibsen PH, et al. Prevalence of endometrial polyps and abnormal uterine bleeding in a Danish population aged 20–74 years. Ultrasound Obstet Gynecol 2009; 33(1): 102–108. [DOI] [PubMed] [Google Scholar]
- 8. Tjarks M, Van Voorhis BJ. Treatment of endometrial polyps. Obstet Gynecol 2000; 96(6): 886–889. [DOI] [PubMed] [Google Scholar]
- 9. Check JH, Bostick-Smith CA, Choe JK, et al. Matched controlled study to evaluate the effect of endometrial polyps on pregnancy and implantation rates following in vitro fertilization-embryo transfer (IVF-ET). Clin Exp Obstet Gynecol 2011; 38(3): 206–208. [PubMed] [Google Scholar]
- 10. Indraccolo U, DiIorio R, Matteo M, et al. The pathogenesis of endometrial polyps: a systematic semi-quantitative review. Eur J Gynaecol Oncol 2013; 34(1): 5–22. [PubMed] [Google Scholar]
- 11. Peng X, Li T, Xia E, et al. A comparison of oestrogen receptor and progesterone receptor expression in endometrial polyps and endometrium of premenopausal women. J Obstet Gynaecol 2009; 29(4): 340–346. [DOI] [PubMed] [Google Scholar]
- 12. Mittal K, Schwartz L, Goswami S, et al. Estrogen and progesterone receptor expression in endometrial polyps. Int J Gynecol Pathol 1996; 15(4): 345–348. [DOI] [PubMed] [Google Scholar]
- 13. Inceboz US, Nese N, Uyar Y, et al. Hormone receptor expressions and proliferation markers in postmenopausal endometrial polyps. Gynecol Obstet Invest 2006; 61(1): 24–28. [DOI] [PubMed] [Google Scholar]
- 14. Lopes RG, Baracat EC, de Albuquerque Neto LC, et al. Analysis of estrogen- and progesterone-receptor expression in endometrial polyps. J Minim Invasive Gynecol 2007; 14(3): 300–303. [DOI] [PubMed] [Google Scholar]
- 15. Mittal K, Schwartz L, Goswami S, et al. Estrogen and progesterone receptor expression in endometrial polyps. Int J Gynecol Pathol 1996; 15(4): 345–348. [DOI] [PubMed] [Google Scholar]
- 16. Taylor LJ, Jackson TL, Reid JG, et al. The differential expression of oestrogen receptors, progesterone receptors, Bcl-2 and Ki67 in endometrial polyps. BJOG 2003; 110(9): 794–798. [PubMed] [Google Scholar]
- 17. McLennan CE, Rydell AH. Extent of endometrial shedding during normal menstruation. Obstet Gynecol 1965; 26(5): 605–621. [PubMed] [Google Scholar]
- 18. Altaner S, Gucer F, Tokatli F, et al. Expression of Bcl-2 and Ki-67 in tamoxifen-associated endometrial polyps: comparison with postmenopausal polyps. Onkologie 2006; 29(8–9): 376–380. [DOI] [PubMed] [Google Scholar]
- 19. Antunes A, Jr, Andrade LA, Pinto GA, et al. Is the immunohistochemical expression of proliferation (Ki-67) and apoptosis (Bcl-2) markers and cyclooxigenase-2 (COX-2) related to carcinogenesis in postmenopausal endometrial polyps? Anal Quant Cytopathol Histpathol 2012; 34(5): 264–272. [PubMed] [Google Scholar]
- 20. Mourits MJE, Hollema H, De Vries EG, et al. Apoptosis and apoptosis-associated parameters in relation to tamoxifen exposure in postmenopausal endometrium. Hum Pathol 2002; 33(3): 341–346. [DOI] [PubMed] [Google Scholar]
- 21. Banas T, Pitynski K, Mikos M, et al. Endometrial polyps and benign endometrial hyperplasia have increased prevalence of DNA fragmentation factors 40 and 45 (DFF40 and DFF45) together with the antiapoptotic B-cell lymphoma (Bcl-2) protein compared with normal human endometria. Int J Gynecol Pathol 2018; 37(5): 431–440. [DOI] [PubMed] [Google Scholar]
- 22. Miranda SP, Traiman P, Candido EB, et al. Expression of p53, Ki-67, and CD31 proteins in endometrial polyps of postmenopausal women treated with tamoxifen. Int J Gynecol Cancer 2010; 20(9): 1525–1530. [DOI] [PubMed] [Google Scholar]
- 23. Dal Cin P, DeWolf F, Klerckx P, et al. The 6p21 chromosome region is nonrandomly involved in endometrial polyps. Gynecol Oncol 1992; 46(3): 393–396. [DOI] [PubMed] [Google Scholar]
- 24. Dal Cin P, Vanni R, Marras S, et al. Four cytogenetic subgroups can be identified in endometrial polyps. Cancer Res 1995; 55(7): 1565–1568. [PubMed] [Google Scholar]
- 25. Su T, Sui L. Expression and significance of p63, aromatase P450 and steroidogenic factor-1 in endometrial polyp. Zhonghua Fu Chan Ke Za Zhi 2014; 49(8): 604–608. [PubMed] [Google Scholar]
- 26. Stewart CJ, Bharat C, Crook M. p16 immunoreactivity in endometrial stromal cells: stromal p16 expression characterises but is not specific for endometrial polyps. Pathology 2015; 47(2): 112–117. [DOI] [PubMed] [Google Scholar]
- 27. McGurgan P, Taylor LJ, Duffy SR, et al. Does tamoxifen therapy affect the hormone receptor expression and cell proliferation indices of endometrial polyps? An immunohistochemical comparison of endometrial polyps from postmenopausal women exposed and not exposed to tamoxifen. Maturitas 2006; 54(3): 252–259. [DOI] [PubMed] [Google Scholar]
- 28. Chan SS, Tam WH, Yeo W, et al. A randomised controlled trial of prophylactic levonorgestrel intrauterine system in tamoxifen-treated women. BJOG 2007; 114(12): 1510–1515. [DOI] [PubMed] [Google Scholar]
- 29. Kraft JK, Hughes T. Polypoid endometriosis and other benign gynaecological complications associated with Tamoxifen therapy: a case to illustrate features on magnetic resonance imaging. Clin Radiol 2006; 61(2): 198–201. [DOI] [PubMed] [Google Scholar]
- 30. Gokmen Karasu AF, Sonmez FC, Aydin S, et al. Survivin expression in simple endometrial polyps and tamoxifen-associated endometrial polyps. Int J Gynecol Pathol 2018; 37(1): 27–31. [DOI] [PubMed] [Google Scholar]
- 31. Inceboz US, Nese N, Uyar Y, et al. Hormone receptor expressions and proliferation markers in postmenopausal endometrial polyps. Gynecol Obstet Invest 2006; 61(1): 24–28. [DOI] [PubMed] [Google Scholar]
- 32. Metz M, Grimbaldeston MA, Nakae S, et al. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev 2007; 217: 304–328. [DOI] [PubMed] [Google Scholar]
- 33. Erdemoglu E, Guney M, Karahan N, et al. Expression of cyclooxygenase-2, matrix metalloproteinase-2 and matrix metalloproteinase-9 in premenopausal and postmenopausal endometrial polyps. Maturitas 2008; 59(3): 268–274. [DOI] [PubMed] [Google Scholar]
- 34. Al-Jefout M, Black K, Schulke L, et al. Novel finding of high density of activated mast cells in endometrial polyps. Fertil Steril 2009; 92(3): 1104–1106. [DOI] [PubMed] [Google Scholar]
- 35. El-Hamarneh T, Hey-Cunningham AJ, Berbic M, et al. Cellular immune environment in endometrial polyps. Fertil Steril 2013; 100(5): 1364–1372. [DOI] [PubMed] [Google Scholar]
- 36. Norrby K. Mast cells and angiogenesis. APMIS 2002; 110(5): 355–371. [DOI] [PubMed] [Google Scholar]
- 37. Coste A, Brugel L, Maitre B, et al. Inflammatory cells as well as epithelial cells in nasal polyps express vascular endothelial growth factor. Eur Respir J 2000; 15(2): 367–372. [DOI] [PubMed] [Google Scholar]
- 38. Gruber BL, Marchese MJ, Kew R. Angiogenic factors stimulate mast-cell migration. Blood 1995; 86(7): 2488–2493. [PubMed] [Google Scholar]
- 39. Xuebing P, TinChiu L, Enlan X, et al. Is endometrial polyp formation associated with increased expression of vascular endothelial growth factor and transforming growth factor-beta1. Eur J Obstet Gynecol Reprod Biol 2011; 159(1): 198–203. [DOI] [PubMed] [Google Scholar]
- 40. Miranda SP, Traiman P, Candido EB, et al. Expression of p53, Ki-67, and CD31 proteins in endometrial polyps of postmenopausal women treated with tamoxifen. Int J Gynecol Cancer 2010; 20(9): 1525–1530. [DOI] [PubMed] [Google Scholar]
- 41. Ribatti D, Crivellato E. Chapter 4: the controversial role of mast cells in tumor growth. In: Kwang WJ. (ed.) International review of cell and molecular biology, vol. 275 New York: Academic Press, 2009, pp. 89–131. [DOI] [PubMed] [Google Scholar]
- 42. Kuwano T, Nakao S, Yamamoto H, et al. Cyclooxygenase 2 is a key enzyme for inflammatory cytokine-induced angiogenesis. FASEB J 2004; 18(2): 300–310. [DOI] [PubMed] [Google Scholar]
- 43. Resta L, Cicinelli E, Lettini T, et al. Possible inflammatory origin of endometrial polyps. Arch Reprod Med Sex Health 2018; 1(2): 8–16. [Google Scholar]
- 44. Munro MG, Critchley HO, Broder MS, et al. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet 2011; 113(1): 3–13. [DOI] [PubMed] [Google Scholar]
- 45. Munro MG. Practical aspects of the two FIGO systems for management of abnormal uterine bleeding in the reproductive years. Best Pract Res Clin Obstet Gynaecol 2017; 40: 3–22. [DOI] [PubMed] [Google Scholar]
- 46. Brenner PF. Differential diagnosis of abnormal uterine bleeding. Am J Obstet Gynecol 1996; 175(3 Part 2): 766–769. [DOI] [PubMed] [Google Scholar]
- 47. Salim S, Won H, Nesbitt-Hawes E, et al. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol 2011; 18(5): 569–581. [DOI] [PubMed] [Google Scholar]
- 48. Jakab A, Ovari L, Juhasz B, et al. Detection of feeding artery improves the ultrasound diagnosis of endometrial polyps in asymptomatic patients. Eur J Obstet Gynecol Reprod Biol 2005; 119(1): 103–107. [DOI] [PubMed] [Google Scholar]
- 49. Tjarks M, Van Voorhis BJ. Treatment of endometrial polyps. Obstet Gynecol 2000; 96(6): 886–889. [DOI] [PubMed] [Google Scholar]
- 50. Cohen MA, Sauer MV, Keltz M, et al. Utilizing routine sonohysterography to detect intrauterine pathology before initiating hormone replacement therapy. Menopause 1999; 6(1): 68–70. [PubMed] [Google Scholar]
- 51. Shokeir TA, Shalan HM, El-Shafei MM. Significance of endometrial polyps detected hysteroscopically in eumenorrheic infertile women. J Obstet Gynaecol Res 2004; 30(2): 84–89. [DOI] [PubMed] [Google Scholar]
- 52. Valle RF. Hysteroscopy for gynecologic diagnosis. Clin Obstet Gynecol 1983; 26(2): 253–276. [DOI] [PubMed] [Google Scholar]
- 53. Alansari LM, Wardle P. Endometrial polyps and subfertility. Hum Fertil 2012; 15(3): 129–133. [DOI] [PubMed] [Google Scholar]
- 54. Stamatellos I, Apostolides A, Stamatopoulos P, et al. Pregnancy rates after hysteroscopic polypectomy depending on the size or number of the polyps. Arch Gynecol Obstet 2008; 277(5): 395–399. [DOI] [PubMed] [Google Scholar]
- 55. Rackow BW, Jorgensen E, Taylor HS. Endometrial polyps affect uterine receptivity. Fertil Steril 2011; 95(8): 2690–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Perez-Medina T, Bajo-Arenas J, Salazar F, et al. Endometrial polyps and their implication in the pregnancy rates of patients undergoing intrauterine insemination: a prospective, randomized study. Hum Reprod 2005; 20(6): 1632–1635. [DOI] [PubMed] [Google Scholar]
- 57. Richlin SS, Ramachandran S, Shanti A, et al. Glycodelin levels in uterine flushings and in plasma of patients with leiomyomas and polyps: implications for implantation. Hum Reprod 2002; 17(10): 2742–2747. [DOI] [PubMed] [Google Scholar]
- 58. Jokimaa V, Oksjoki S, Kujari H, et al. Altered expression of genes involved in the production and degradation of endometrial extracellular matrix in patients with unexplained infertility and recurrent miscarriages. Mol Hum Reprod 2002; 8(12): 1111–1116. [DOI] [PubMed] [Google Scholar]
- 59. Seppala M, Koistinen H, Koistinen R, et al. Glycosylation related actions of glycodelin: gamete, cumulus cell, immune cell and clinical associations. Hum Reprod Update 2007; 13(3): 275–287. [DOI] [PubMed] [Google Scholar]
- 60. Afifi K, Anand S, Nallapeta S, et al. Management of endometrial polyps in subfertile women: a systematic review. Eur J Obstet Gynecol Reprod Biol 2010; 151(2): 117–121. [DOI] [PubMed] [Google Scholar]
- 61. Zhang YN, Zhang YS, Yu Q, et al. Higher prevalence of endometrial polyps in infertile patients with endometriosis. Gynecol Obstet Invest 2018; 83(6): 558–563. [DOI] [PubMed] [Google Scholar]
- 62. Bosteels J, Kasius J, Weyers S, et al. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database Syst Rev 2013; 1: CD009461. [DOI] [PubMed] [Google Scholar]
- 63. Bosteels J, Kasius J, Weyers S, et al. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. The Cochrane Database of Systematic Review 2015; 1: CD009461. [DOI] [PubMed] [Google Scholar]
- 64. Jayaprakasan K, Polanski L, Sahu B, et al. Surgical intervention versus expectant management for endometrial polyps in subfertile women. Cochrane Database Syst Rev 2014; 8: CD009592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Covali R. Correlation between the wide range of tubal pathology discovered by routine hysterosalpingography in a university hospital in Romania and the successful pregnancy rate. J Med Life 2017; 10(4): 232–236. [PMC free article] [PubMed] [Google Scholar]
- 66. Giordano G, Gnetti L, Merisio C, et al. Postmenopausal status, hypertension and obesity as risk factors for malignant transformation in endometrial polyps. Maturitas 2007; 56(2): 190–197. [DOI] [PubMed] [Google Scholar]
- 67. Lee SC, Kaunitz AM, Sanchez-Ramos L, et al. The oncogenic potential of endometrial polyps: a systematic review and meta-analysis. Obstetrics & Gynecology 2010; 116(5): 1197–1205. [DOI] [PubMed] [Google Scholar]
- 68. Hileeto D, Fadare O, Martel M, et al. Age dependent association of endometrial polyps with increased risk of cancer involvement. World J Surg Oncol 2005; 3(1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gregoriou O, Konidaris S, Vrachnis N, et al. Clinical parameters linked with malignancy in endometrial polyps. Climacteric 2009; 12(5): 454–458. [DOI] [PubMed] [Google Scholar]
- 70. Karakaya BK, Ozkan NT, Kansu-Celik H, et al. Malignancy risk of endometrial polyps among geriatric women. Int J Gerontol 2018; 12(3): 215–217. [Google Scholar]
- 71. Ben-Arie A, Goldchmit C, Laviv Y, et al. The malignant potential of endometrial polyps. Eur J Obstet Gynecol Reprod Biol 2004; 115(2): 206–210. [DOI] [PubMed] [Google Scholar]
- 72. Yamazawa K, Matsui H, Ishikura H, et al. Clear cell adenocarcinoma within a tamoxifen-related polyp. Acta Obstet Gynecol Scand 2002; 81(10): 985–987. [DOI] [PubMed] [Google Scholar]
- 73. Hachisuga T, Miyakawa T, Tsujioka H, et al. K-ras mutation in tamoxifen-related endometrial polyps. Cancer 2003; 98(9): 1890–1897. [DOI] [PubMed] [Google Scholar]
- 74. Van den Bosch T, Van Schoubroeck D, Ameye L, et al. Ultrasound assessment of endometrial thickness and endometrial polyps in women on hormonal replacement therapy. Am J Obstet Gynecol 2003; 188(5): 1249–1253. [DOI] [PubMed] [Google Scholar]
- 75. LaTorre R, De Felice C, De Angelis C, et al. Transvaginal sonographic evaluation of endometrial polyps: a comparison with two dimensional and three dimensional contrast sonography. Clin Exp Obstet Gynecol 1999; 26(3–4): 171–173. [PubMed] [Google Scholar]
- 76. Nogueira AA, Dos Reis FJC, Silva JCRE, et al. Endometrial polyps: a review. J Gynecol Surg 2007; 23(3): 111–116. [Google Scholar]
- 77. Babacan A, Gun I, Kizilaslan C, et al. Comparison of transvaginal ultrasonography and hysterosocpy in the diagnosis of uterine pathologies. Int J Clin Exp Med 2014; 7(3): 764–769. [PMC free article] [PubMed] [Google Scholar]
- 78. Lieng M, Qvigstad E, Dahl GF, et al. Flow differences between endometrial polyps and cancer: a prospective study using intravenous contrast-enhanced transvaginal color flow Doppler and three-dimensional power Doppler ultrasound. Ultrasound Obstet Gynecol 2008; 32(7): 935–940. [DOI] [PubMed] [Google Scholar]
- 79. Metello J, Jimenez J. Hysteroscopy and infertility. In: Shawki O, Deshmukh S, Pacheco LA. (eds) Mastering the techniques in hysteroscopy. New Delhi, India: Jaypee Brothers, 2017, p. 454. [Google Scholar]
- 80. Goldstein SR, Monteagudo A, Popiolek D, et al. Evaluation of endometrial polyps. Am J Obstet Gynecol 2002; 186(4): 669–674. [DOI] [PubMed] [Google Scholar]
- 81. Lieng M, Qvigstad E.G.F. D, Lstre O Flow differences between endometrial polyps and cancer: a prospective study using intravenous contrast-enhanced transvaginal color flow Doppler and three-dimensional Doppler ultrasound. Ultrasound Obstet Gynecol 2008; 32(7): 935–940. [DOI] [PubMed] [Google Scholar]
- 82. Valle RF. Hysteroscopy for gynecologic diagnosis. Clin Obstet Gynecol 1983; 26(2): 253–276. [DOI] [PubMed] [Google Scholar]
- 83. Schwarzler P, Concin H, Bosch H, et al. An evaluation of sonohysterography and diagnostic hysteroscopy for the assessment of intrauterine pathology. Ultrasound Obstet Gynecol 1998; 11(5): 337–342. [DOI] [PubMed] [Google Scholar]
- 84. Shipp TD. Does ultrasound have a role in the evaluation of postmenopausal bleeding and among postmenopausal women with endometrial cancer. Menopause 2005; 12(1): 8–11. [DOI] [PubMed] [Google Scholar]
- 85. Exalto N, Stappers C, van Raamsdonk LA, et al. Gel instillation sonohysterography: first experience with a new technique. Fertil Steril 2007; 87(1): 152–155. [DOI] [PubMed] [Google Scholar]
- 86. Goldstein SR, Zeltser I, Horan CK, et al. Ultrasonography-based triage for perimenopausal patients with abnormal uterine bleeding. Am J Obstet Gynecol 1997; 177(1): 102–108. [DOI] [PubMed] [Google Scholar]
- 87. Fadl SA, Sabry AS, Hippe DS, et al. Diagnosing polyps on transvaginal sonography: is sonohysterography always necessary. Ultrasound Q 2018; 34(4): 272–277. [DOI] [PubMed] [Google Scholar]
- 88. Kim KR, Peng R, Ro JY, et al. A diagnostically useful histopathologic feature of endometrial polyp: the long axis of endometrial glands arranged parallel to surface epithelium. Am J Surg Pathol 2004; 28(8): 1057–1062. [DOI] [PubMed] [Google Scholar]
- 89. McCluggage WG. My approach to the interpretation of endometrial biopsies and curettings. J Clin Pathol 2006; 59(8): 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dolan MS, Hill C, Valea FA. Benign gynecologic lessons: vulva, vagina, cervix, uterus, oviduct, ovary, ultrasound imaging of pelvic structures. In: Gershenson DM, Lentz GM, Fidel VA. (eds) Comprehensive gynecology. Amsterdam: Elsevier, 2017, pp. 1–936. [Google Scholar]
- 91. Khaund A, Lumsden AM. Benign disease of the uterus. In: Edmonds K. (ed.) Dewhurst’s textbook of obstetrics and gynaecology. 8th ed. Hoboken, NJ: Wiley-Blackwell, 2012, pp. 715–726. [Google Scholar]
- 92. DeWaay DJ, Syrop CH, Nygaard IE, et al. Natural history of uterine polyps and leiomyomata. Obstet Gynecol 2002; 100(1): 3–7. [DOI] [PubMed] [Google Scholar]
- 93. Li F, Wei S, Yang S, et al. Post hysteroscopic progesterone hormone therapy in the treatment of endometrial polyps. Pak J Med Sci 2018; 34(5): 1267–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Venturella R, Miele G, Cefali K, et al. Subcutaneous progesterone for endometrial polyps in premenopausal women: a preliminary retrospective analysis. J Minim Invasive Gynecol 2019; 26(1): 143–147. [DOI] [PubMed] [Google Scholar]
- 95. Abdollahi Fard S, Mostafa Gharabaghi P, et al. Hysteroscopy as a minimally invasive surgery, a good substitute for invasive gynecological procedures. Iran J Reprod Med 2012; 10(4): 377–382. [PMC free article] [PubMed] [Google Scholar]
- 96. Pereira N, Petrini AC, Lekovich JP, et al. Surgical management of endometrial polyps in infertile women: a comprehensive review. Surg Res Pract 2015; 2015: 914390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Daniele A, Ferrero A, Maggiorotto F, et al. Suspecting malignancy in endometrial polyps: value of hysteroscopy. Tumori 2013; 99(2): 204–209. [DOI] [PubMed] [Google Scholar]
- 98. Lieng M, Qvigstad E, Sandvik L, et al. Hysteroscopic resection of symptomatic and asymptomatic endometrial polyps. J Minim Invasive Gynecol 2007; 14(2): 189–194. [DOI] [PubMed] [Google Scholar]
- 99. Muzii L, Bellati F, Pernice M, et al. Resectoscopic versus bipolar electrode excision of endometrial polyps: a randomized study. Fertil Steril 2007; 87(4): 909–917. [DOI] [PubMed] [Google Scholar]
- 100. Lieng M, Istre O, Qvigstad E. Treatment of endometrial polyps: a systematic review. Acta Obstet Gynecol Scand 2010; 89(8): 992–1002. [DOI] [PubMed] [Google Scholar]
- 101. Kanthi J, Remadevi C, Sumathy S, et al. Clinical study of endometrial polyp and role of diagnostic hysteroscopy and blind avulsion of polyp. J Clin Diagn Res 2016; 10(6): QC01-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Giacobbe V, Rossetti D, Vitale SG, et al. Otorrhagia and nosebleed as first signs of intravascular absorption syndrome during hysteroscopy: from bench to bedside. KUMJ 2016; 14(53): 87–89. [PubMed] [Google Scholar]
- 103. Vitale SG, Sapia F, Rapisarda AMC, et al. Hysteroscopic morcellation of submucous myomas: a systematic review. Biomed Res Int 2017; 2017: 6848250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Timmermans A, Gerritse MB, Opmeer BC, et al. Diagnostic accuracy of endometrial thickness to exclude polyps in women with postmenopausal bleeding. J Clin Ultrasound 2008; 36(5): 286–290. [DOI] [PubMed] [Google Scholar]
- 105. Lieng M, Istre O, Sandvik L, et al. Prevalence, 1-year regression rate, and clinical significance of asymptomatic endometrial polyps: cross-sectional study. J Minim Invasive Gynecol 2009; 16(4): 465–471. [DOI] [PubMed] [Google Scholar]
- 106. Oguz S, Sargin A, Kelekci S, et al. The role of hormone replacement therapy in endometrial polyp formation. Maturitas 2005; 50(3): 231–236. [DOI] [PubMed] [Google Scholar]
- 107. Zhang C, Sung CJ, Quddus MR, et al. Association of ovarian hyperthecosis with endometrial polyp, endometrial hyperplasia, and endometrioid adenocarcinoma in postmenopausal women: a clinicopathological study of 238 cases. Hum Pathol 2017; 59: 120–124. [DOI] [PubMed] [Google Scholar]
- 108. Fraser IS. The promise and reality of the intrauterine route for hormone delivery for prevention and therapy of gynecological disease. Contraception 2007; 75(6 Suppl): S112–S117. [DOI] [PubMed] [Google Scholar]
- 109. Wada-Hiraike O, Osuga Y, Hiroi H, et al. Sessile polyps and pedunculated polyps respond differently to oral contraceptives. Gynecol Endocrinol 2011; 27(5): 351–355. [DOI] [PubMed] [Google Scholar]
- 110. Meresman GF, Auge L, Baranao RI, et al. Oral contraceptives suppress cell proliferation and enhance apoptosis of eutopic endometrial tissue from patients with endometriosis. Fertil Steril 2002; 77(6): 1141–1147. [DOI] [PubMed] [Google Scholar]
- 111. Van Dijk MM, van Hanegen N, de Lange ME, et al. Treatment of women with an endometrial polyp and heavy menstrual bleeding: a levonorgestrel-releasing intrauterine device or hysteroscopic polypectomy? J Minimally Invasive Gynecol 2015; 22(7): 1153–1162. [DOI] [PubMed] [Google Scholar]
- 112. Lagana AS, Palmara V, Granese R, et al. Desogestrel versus danazol as preoperative treatment for hysteroscopic surgery: a prospective, randomized evaluation. Gynecol Endocrinol 2014; 30(11): 794–797. [DOI] [PubMed] [Google Scholar]
- 113. Lagana AS, Giacobbe V, Triolo O, et al. Dienogest as preoperative treatment of submucous myomas for hysteroscopic surgery: a prospective, randomized study. Gynecol Endocrinol 2016; 32(5): 408–411. [DOI] [PubMed] [Google Scholar]
- 114. Lagana AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet 2017; 295(3): 661–667. [DOI] [PubMed] [Google Scholar]