Abstract
Type 1 diabetes (T1D) is caused by autoimmune destruction of insulin-producing β cells located in the endocrine pancreas in areas known as islets of Langerhans. The current standard-of-care for T1D is exogenous insulin replacement therapy. Recent developments in this field include the hybrid closed-loop system for regulated insulin delivery and long-acting insulins. Clinical studies on prediction and prevention of diabetes-associated complications have demonstrated the importance of early treatment and glucose control for reducing the risk of developing diabetic complications. Transplantation of primary islets offers an effective approach for treating patients with T1D. However, this strategy is hampered by challenges such as the limited availability of islets, extensive death of islet cells, and poor vascular engraftment of islets post-transplantation. Accordingly, there are considerable efforts currently underway for enhancing islet transplantation efficiency by harnessing the beneficial actions of stem cells. This review will provide an overview of currently available therapeutic options for T1D, and discuss the growing evidence that supports the use of stem cell approaches to enhance therapeutic outcomes.
Keywords: Type 1 diabetes, insulin therapy, islet transplant, immune therapies, mesenchymal stem cells, induced pluripotent stem cells, endothelial colony forming cells
Introduction
Type 1 (T1D) and type 2 (T2D) diabetes are the 2 major forms of diabetes, although a recent study proposed that there are actually 5 types of diabetes.1 While T2D accounts for >85% of global cases, there has been a steady increase (3%-5% annually) in cases of T1D (IDF Diabetes Atlas 8th Edition). Tight glucose control during early stages can decrease the susceptibility for progressing towards multisystem complications of microvascular and macrovascular endpoints.2 The pathogenesis of T1D is driven by destruction of insulin releasing pancreatic beta cells, accompanied by cellular invasion by both CD4+ and CD8+ T cells, which results in reduction of beta cell mass. Although genetic predisposition is believed to play a role, T1D is mainly a polygenic disease where genetic factors are controversial.3 As such, the rise in global incidences cannot be completely attributed to genetic susceptibility, and exposure to environmental factors also has an important role in progression from islet autoimmunity to clinical T1D. Nevertheless, there are currently more than 50 genetic loci that have been identified using genome-wide association studies and meta-analysis.4 Out of many genetic variabilities, polymorphisms within the HLA (human leucocyte antigen) located on chromosome 6 have been implicated in nearly 40% to 50% of cases of T1D.5 Other widely reported genetic variations are polymorphisms within the insulin gene (Ins-VNTR, IDDM2) located at chromosome 2,6 CTLA-4 gene (cytotoxic T lymphocyte antigen-4) located on chromosome 2,7 and (Arg620Trp) PTPN22 (protein tyrosine phosphatase non-receptor type 22) located on chromosome 1.8 There is a small percentage of T1D patients (<10%) who display no evidence of autoimmune response and as such are categorised as type 1B diabetic or idiopathic diabetic population.9
According to IDF Diabetes Atlas (8th Edition), the number of young people (<20 years) living with T1D worldwide is more than 1 million, and if current trends continue, the number of cases is expected to increase by more than 100 000 every year. In the United Kingdom, there are currently 400 000 cases of patients diagnosed with T1D, out of which 29 000 are children (IDF Diabetes Atlas 8th Edition). In 1986, a model was proposed by Eisenbarth10 to understand pathophysiology of T1D. This model described clinical T1D development as a multi-step pathological process. This work also highlighted opportunities for therapeutic interventions at various stages of disease progression, including primary prevention prior to autoimmune activation, secondary treatment after the immune destruction of pancreatic islets, and tertiary therapy after the clinical onset of T1D.10 As a result, there have been many advancements made in the past decade for the treatment of T1D. This article will focus on recent progress made for the development of novel therapies for T1D.
Insulin Replacement Therapy
Type 1 diabetes is a chronic disease that eventually leads to complete loss of insulin due to destruction of β cells. Also, the lack of appropriate islet cell repair mechanisms which ultimately affects glycaemic control. As a result, insulin replacement therapy is currently the first-line therapeutic option for treating T1D. The use of exogenous insulin as a therapy for T1D was first described by Banting and Best in 1921, who used crude extracts of animal pancreas to achieve glucose-lowering actions.11 Soon after in 1922, crude insulin preparations of animal origins were commercialised for clinical use. However, there were problems associated with pharmacokinetics of insulin, prominently due to insulin absorption. In addition, inefficient action of administered insulin was also reported, which led to either inconsistent glucose-lowering effects or prolonged periods of hypoglycaemia. These remained hindrances for achieving long-term glycaemic control and for prevention of diabetic complications.12 Since then, many humanised insulin analogues have emerged (Table 1), which not only mimic the biologic actions of endogenous insulin but also have enhanced the pharmacokinetic profile.13 Nevertheless, clinical insights in the past decades have highlighted limitations of insulin replacement therapy, especially the failure of insulin preparations to fully replicate biological actions of endogenous insulin.14 Together with increasing number of T1D cases, there is a need to identify novel therapeutic approaches to restore normoglycaemia.
Table 1.
List of different categories of insulin available in the National Health Service formulary.
| Type | Brand names | Time action profile | Dose |
|---|---|---|---|
| Rapid acting | Insulin aspart (Novorapid, Fiasp), insulin lispro (Humalog), insulin glulisine (Apidra) | Usually 4-20 min after s.c. injection with peak at 20-30 min | Three times a day up to 15 min before food intake |
| Short acting | Actrapid (Novo Nordisk), Humulin S (Lilly), Insuman Rapid (Aventis) | Begins from 30 min after s.c. injection with peak action reaching 2-4 h | Three times a day, 30 min before food intake |
| Long acting | Levemir (Novo Nordisk), ABASAGLAR (Lilly), Lantus (Aventis), Toujeo (Aventis), Tresiba (Novo Nordisk) | Beyond 24 h and up to 36 h | Once daily s.c., usually at the same time everyday with minimum 8 h interval between consecutive doses |
| Intermediate acting | Insulatard (Novo Nordisk), Insuman Basal (Aventis) | Peak onset from 4-6 h, with duration of action until 14-16 h | Once or twice daily s.c. |
Abbreviation: s.c., subcutaneous.
The current treatment regimen for T1D focusses on combining intensive diet treatments coupled with lifelong exogenous insulin administration, either using multiple daily injections or by insulin pumps.15 In addition, there have been advances in the development of genetically modified insulin analogues (aspart, lispro, and Delgludec insulins), which are fast acting and long acting. These provide a more physiological glycometabolic control compared with traditional insulins.16 The current insulin centric therapeutic approach renders a T1D patient susceptible to severe episodes of hypoglycaemia, lifelong dependency on exogenous insulin, insulin resistance, mild obesity, and psychiatric conditions.15,17,18 Such observations highlight the importance of developing alternative strategies to restore glycaemic control and complete insulin independence.
Future perspectives on insulin replacement therapy
The widespread use of self-monitoring devices for measuring blood glucose and non-enzymatically glycosylated haemoglobin A1C (HbA1c) has enhanced the therapeutic applicability of commercial insulin preparations.19 This has resulted in the generation of a range of insulin analogues (Table 1). These modified insulins are rapid acting (biological activity begins from 4 min and lasts for 30 min), short acting (regular insulin – biological activity beginning from 30 min and lasting for 4 h), intermediate acting (Insulatard, Insuman – peak onset from 4 to 6 h), long acting (Glargine and Detemir – biological activity from 24 to 36 h), and ultra-long acting (Degludec – onset from 30 to 90 min and lasts until 42 h). However, even such preparations are dependent on delivery systems, including syringes, glucose sensor-augmented insulin infusion pumps, supersonic injectors, and pens.20 The use of these traditional delivery systems involves an invasive procedure and treatment fails to provide long-term insulin independence.14 Consequently, research is being carried out to identify alternative means of insulin replacement therapy. A novel approach for oral insulin delivery utilises an ingestible self-orienting millimetre-scale applicator (SOMA).21 The device autonomously positions itself to engage with gastro-intestinal tissue and deploys milliposts directly through the gastric mucosa while avoiding perforation.21 The results obtained from diabetic rodent studies demonstrate stable plasma insulin levels comparable with those achieved with subcutaneous millipost administration.21 Such approach has potential to enhance the clinical outcomes of exogenous insulin replacement therapies.
Artificial pancreas
Despite successful implementation of multiple insulin delivery devices, maintaining normoglycaemia without frequent episodes of hypoglycaemia remains a considerable challenge for health care providers. As a result, the clinical practice in recent years has gradually moved towards using continuous insulin infusion systems for insulin delivery. Indeed, the National Institute of Health and Care Excellence (NICE) recommends the use of continuous insulin infusion over bolus insulin injection to enable greater control over HbA1c and lower incidences of hypoglycaemia. In addition, a meta-analysis carried out with 19 clinical trials demonstrated superior glycaemic control with continuous insulin infusion pumps as compared with multiple insulin injections (Table 2).15,22
Table 2.
List of prominent clinical trials utilising different interventions.
| Intervention | Trial | Prominent findings/ongoing trial |
|---|---|---|
| Insulin | Open-label trial comparing insulin glargine plus insulin glulisine with biphasic insulin aspart (LanScape) (NCT00965549) | Patients, who received a combination of once daily fast-acting and basal insulin, demonstrated a similar HbA1c level and significantly better treatment satisfaction as compared with basal insulin alone |
| Insulin pump | Randomised controlled trial to determine the REPOSE in adult patients with T1DM (ISRCTN61215213) | Long-lasting reduction in HbA1c and improved psychosocial responses observed in patients using insulin pump |
| Artificial pancreas | Randomised trial of a dual-hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor-augmented pump therapy in T1DM patients (NCT02241889) | Adjusting insulin and glucagon delivery using dual-hormone artificial pancreas at exercise onset significantly reduced hypoglycaemia |
| Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes (NCT02189694) | Delivering insulin and glucagon using dual-hormone artificial pancreas demonstrated better nocturnal glycaemic control | |
| Immune modulation/incretins | Clinical proof-of-concept trial to evaluate therapeutic applicability of IL-21 antibody – NNC01144-0006 (NCT02443155) and liraglutide on β cell function in recently diagnosed T1DM patients | Ongoing |
| Immune modulation | Trial to determine the role of B-lymphocyte depletion using rituximab in T1DM patients | Four-dose course of rituximab partially preserved beta cell function over a period of 1 year |
| Randomised controlled CD3-antibody trial in recent-onset T1DM patients (NCT00627146) | Treatment with ChAglyCD3 for 6 days suppressed the rise in insulin requirements over 48 months. | |
| Trial of regulatory T cells in renal transplantation for immunosuppression minimisation (The ONE study UK Treg Trial–NCT02129881) | Ongoing | |
| Safety and tolerability trial to evaluate umbilical cord derived Treg for T1DM (NCT02932826) | Ongoing | |
| A study to determine safety and tolerability of immune targeting using a combination of polyclonal Tregs and IL-2 antibody (NCT02772679) | Ongoing | |
| Randomised trial to evaluate the therapeutic applicability of Antithymocyte globulin in T1DM patients (NCT00515099) | Administration of antithymocyte globulin for 8 weeks significantly depleted the number of regulator T cells and preserved C-peptide secretion. However, no islet preservation was observed after 24 months follow-up | |
| SGLT2 inhibition | Efficacy and safety study of DEPICT-1 (NCT02268214) | Reduction in HbA1c levels (0.4%-0.5%) and daily insulin requirements coupled with weight loss was observed |
| Tandem3 trial to evaluate therapeutic applicability of Sotagliflozin in combination with insulin (NCT02531035) | Significant reduction in HbA1c levels with no severe hypoglycaemia or diabetic ketoacidosis was observed in T1DM patients | |
| Stem cell mobilisation | A randomised open-labelled trial to evaluate Plerixafor for treating T1DM (NCT03182426) | Ongoing |
| β cell encapsulation | Safety, tolerability, and efficacy trial of VC-01 in T1D patients | Ongoing |
| Open label trial to assess the safety and efficacy of transplanted macroencapsulated human islets within bio-artificial βAir device in T1DM patients (NCT02064309) | Insignificant increase in C-peptide levels, no impact on glycaemic control, and glucose stimulated insulin secretory response | |
| Microencapsulation | Open label investigation of safety and effectiveness of DIABECELL in T1D patients | Marginal reduction in HbA1c and less frequent hypoglycaemia |
| Stem cells | Safety, tolerability, and efficacy study of VC-01 combination product in T1DM patients (NCT02239354) | The PEC-Encap product candidate was safe and tolerable. Also, when delivered at a subtherapeutic dose, the device also protected the implanted cells from alloimmune and autoimmune rejection and the patient from sensitisation |
| Incretins | Randomised, double-blind, placebo-controlled trial to evaluate the efficacy of liraglutide as an add-on therapy to insulin for overweight T1DM patients | Liraglutide treatment was associated with reductions in hypoglycaemic events, bolus and total insulin dose, body weight, and increased heart rate. |
Abbreviations: DEPICT-1, Dapagliflozin Evaluation in Patients with Inadequately Controlled Type 1 Diabetes; HbA1c, haemoglobin A1C; IL, interleukin; REPOSE, Relative Effectiveness of Pumps Over MDI and Structured Education; SGLT2, sodium–glucose co-transporter 2; T1DM, type 1 diabetes mellitus; Tregs, regulatory T cells.
The widespread clinical use of continuous glucose sensors and insulin pumps has allowed a steady progress for the development of artificial pancreas.23 Also referred to as closed-loop system in the literature, this is an emerging treatment option based on using an algorithm that takes into account the continuous glucose monitoring to determine the most appropriate insulin infusion rate needed.24 The system continuously monitors blood glucose and only sufficient amount of insulin is injected. This provides a better glycaemic control, precise insulin dosing, reduced insulin spikes, and less episodes of hypoglycaemia.25 However, even with computer-assisted insulin delivery, achievement of desired glycaemic control targets remains challenging. The most prominent reason is the variable pharmacokinetics and absorption rates of traditional insulin analogues. Currently available insulin analogues have relatively restricted onset and prolonged duration of action, with onset time of 10 to 15 min, with peak glucose excursion of 40 to 60 min and an overall duration of action of 4 to 6 h (Table 1). As such, the inherent pharmacokinetic profile of insulin remains a hindrance for preventing episodes of hypoglycaemia. This has led to the development of dual-hormone artificial pancreas system, where glucagon can also be administered simultaneously, thereby providing tight glucose regulation as well as mimicking the physiological action of the endocrine pancreas.26
Therefore, when compared with traditional and sensor-augmented insulin pumps, a closed-loop system alleviates much of the patient’s discomfort by adjusting the amount of insulin entering the circulation. Accordingly, there have been many artificial pancreas systems developed which have undergone safety and efficacy tests in a number of clinical studies and have shown beneficial effects.27 Recently, a meta-analysis of randomised controlled trials was carried out which compared artificial pancreas systems (insulin or insulin plus glucagon) with traditional insulin pumps in adults and children T1D (Table 2).28 The findings demonstrated significantly improved glucose control in patients using the artificial pancreas systems in an outpatient setting.28 Furthermore, patients treated with the dual-hormone artificial pancreas systems demonstrated a greater improvement in time and target range compared with single-hormone systems.28 Considering the growing evidence for metabolic benefits, the Food and Drug Administration (FDA) recently approved the first artificial pancreas system for use by patients with T1D.29 However, some concerns have been raised in a recent systematic review and meta-analysis pointing out inconsistencies in relation to inaccurate outcome reporting, relatively small sample size, and short follow-up duration of individual trials in patients using closed-loop systems.30 In addition, there are a number of other concerns such as high costs associated with equipment procurement and high sensor replacement costs, buildup of scar tissue due to repeated micro-needle insertion, and premature sensor failure.30
Immune Therapies
The rise in the number of cases with T1D cannot be explained solely by genetic predisposition. It is accepted that an interplay between genetic susceptibility and environment influences is responsible for activating self-reactive immune cells. Accordingly, the pathogenesis of T1D involves a complex interaction between the β cells and the components of both innate and adaptive immune systems. The activated immune system then destroys the β cells through many cell types and multiple pathways. Therefore, there are many immunomodulatory strategies proposed for the treatment of T1D. In the late 1980s, a large clinical trial was carried out investigating the therapeutic utility of cyclosporin A (Table 2).31 Although cyclosporin A treatment increased T1D remission, this was only for short duration and studies reported progressive increase in daily insulin requirement.32 Similarly, there have been many clinical interventional studies carried out using anti-CD3 (Table 2)33 and anti-CD2034 monoclonal antibodies; however, only transient preservation in c-peptide levels was observed.35 Furthermore, a study investigating safety and efficacy of antithymocyte globulin (ATG) failed to preserve β cell function after 2 years.36 A randomised controlled trial was also performed to evaluate the clinical utility of known inhibitors of interleukin-1 (IL-1), namely, Canakinumab (human monoclonal antibody for IL-1) and Anakinra (human IL-1 receptor antagonist).37 Results reported that both Canakinumab and Anakinra were safe but not effective as single immunomodulatory drugs for recent-onset T1D.37 The repeated failures observed in clinical trials underscore a knowledge gap for effectively translating immunotherapies for the benefit of patients with T1D (Table 2).
Studies are currently underway to develop the next generation of immunotherapies. A phase II clinical trial is being currently conducted by Novo Nordisk using anti-IL-21 (NCT02443155). An alternative strategy to modulate the activity of regulatory T cells (Tregs) is also being worked upon (Table 2).38 Regulatory T cells are classically known to negatively modulate the function of other immune cells, including dendritic and cytotoxic T cells. The immunomodulatory activity of Tregs has also been harnessed to enhance islet graft survival. Preclinical studies have demonstrated prolonged islet survival and function in mice co-transplanted with islets and Tregs without immune suppression.39 Studies carried out in humans represent Tregs as a biological alternative for chemical immunosuppression (Table 2) and as a novel approach for modulating immune responses in T1D patients undergoing islet transplant.40 Indeed, a small clinical trial carried out in 12 newly diagnosed T1D children demonstrated safety and tolerability of autologous expanded Tregs. In addition, patients treated with Tregs showed partially controlled inflammation and preservation of insulin dosage for the duration of 2 years.41 The currently ongoing open-label, controlled, and dose finding pilot clinical study will shed further light on potential direct effects of ex-vivo expanded autologous Tregs on β cell function and survival (NCT03444064).
Recent research has demonstrated a robust selection technique based on expression of CD4, CD25, and CD127 to isolate and expand Tregs from T1D patients.42 The cells were grown in vitro and Tregs demonstrated enhanced functional activity.42 The ex vivo expanded Tregs were then administered back into T1D patients in a phase I trial (Table 2).42 The result demonstrated survival of injected Tregs for up to 1 year in a quarter of patients and preserved C-peptide responses in all cohorts for up to 1 year and in 2 cohorts after 2 years.42 Furthermore, the same research group is also currently pursuing a phase I clinical trial of Tregs therapy in combination with commercially available form of IL-2 called aldesleukin (NCT02772679; Table 2). Another novel therapeutic strategy is to expose the immune system to nanoparticles coated with pancreatic peptides, which are bound with major histocompatibility complex class–II (MHC-II) protein.43 The strategy involves systemic delivery of nanoparticles coated with autoimmune-disease-relevant pancreatic peptides bound to MHC-II molecules. Administration of coated nanoparticles triggers generation and expansion of antigen-specific regulatory CD4(+) T cell type 1 (TR1)-like cells in rodents.44 These coated nanoparticles were demonstrated to enhance cellular differentiation of peptide primed autoreactive T cells into type 1 regulatory (TR1) cells. Once activated, TR1 cells suppress autoantigen-presenting cells and drive the differentiation of cognate B cells into disease-suppressing regulatory B cells.44 The approach appears particularly attractive as it aims to selectively target pancreas-specific immune response while keeping the remaining immune system intact.
Sodium–glucose co-transporter 2 Inhibitors
The use of sodium–glucose co-transporter-2 (SGLT2) inhibition for the treatment of T2D was evaluated in experimental models45 and in patients.46 Regulation of glycaemic control with selective inhibition of SGLT2 was also reported in a streptozotocin-induced T1D model.47 Interestingly, research demonstrated direct beneficial actions of SGLT2 inhibitors by protecting and preserving β cell regenerative capacity.48 In addition, SGLT2 inhibitors are also reported to lower body weight, which in a combination therapy with insulin might prevent mild obesity associated with long-term insulin mono therapy. The recent phase II (Dapagliflozin Evaluation in Patients with Inadequately Controlled Type 1 Diabetes [DEPICT-1]) and a 24-week phase III (Tandem3) trial provided clinical evidence of additional reduction of HbA1c levels (0.4%-0.5%) accompanied by weight loss and reduction of daily insulin doses with SGLT2 inhibitors (dapagliflozin and sotagliflozin) in combination with insulin (Table 2).49 These findings support the inclusion of SGLT2 inhibitors in the therapeutic regimen for T1D in combination with insulin replacement therapy. However, long-term clinical trials and observational studies are needed to study potential adverse effects and to further understand additional benefits of this combination approach.
Peptide Hormone–Based Therapies
Gut-derived peptide hormone mimetics, especially glucagon-like peptide 1 (GLP-1), are being widely used for the treatment of T2D and obesity. The clinical acceptance has been a result of intensive preclinical assessment carried out in the past 2 decades. The preclinical data generated strongly suggest beneficial actions of many peptide hormones, which also hold potential for the treatment of T1D. This includes potent regulation of glucose excursions, insulin secretion, preservation of β cell mass, inhibition of β cell apoptosis, and increased insulin sensitivity.50
There have been many preliminary clinical studies exploring the therapeutic utility of GLP-1 receptor agonists as adjunct to insulin therapy in T1D patients (Table 2). Findings demonstrate significant reduction of postprandial glucose excursions, decreased glucagon production, and delayed gastric emptying in patients taking insulin in combination with GLP-1R agonists exenatide and liraglutide as compared with patients on insulin monotherapy.51,52 In addition, a study demonstrated tight glycaemic control in diabetic mice after combination therapy with anti-IL-21 antibody and liraglutide.53 Indeed, a clinical proof-of-principle trial (Table 2) in newly diagnosed T1D to investigate the effect of anti-IL-21 in combination with liraglutide is currently underway (NCT02443155). Therefore, the use of GLP-1-based agonists appears beneficial as an add-on therapy in patients with uncontrolled HbA1c and mild obesity. However, the impact of GLP-1-based drugs on long-term glycaemic control and on secondary complications remains to be explored.
Oxyntomodulin (Oxm) is another gut-derived hormone, which acts through simultaneous activation of GLP-1 and glucagon receptors.54 Evidence has also suggested a direct role of Oxm in regulating β cell function.55 As a result of concomitant glucagon receptor activation, the use of Oxm as an adjunct therapy with insulin seems counterintuitive for treating T1D. However, a rodent study demonstrated a significant lowering of circulating blood glucose and increasing in number of smaller sized islets after Oxm monotherapy.56 Similarly, glucagon-like peptide 2 (GLP-2) is peptide hormone released from intestinal endocrine L cells in response to food intake. Studies have suggested a protective role of GLP-2 on endocrine pancreas, especially in response to islet stress.57 Furthermore, GLP-2 expression was also observed in cultured rodent and human beta cells, rodent alpha cells, and isolated mouse islets.57 This represents an interesting finding that requires further investigations into the role of GLP-2 in maintaining islet cell health, especially as ablation of GLP-2 is known to diminish stress-induced adaptive response of islets.57
Peptide YY (PYY), which is released by intestinal L cells, is known to be co-localised with pro-glucagon-derived peptides GLP-1 and GLP-2. Interestingly, PYY expression has been observed in islet α, pancreatic polypeptide, and δ cells.58 It is therefore suggested that PYY may play a role in direct regulation of islet cell function, including control of insulin secretion and preservation of β cell mass.58 Taken together, these studies with peptide hormones demonstrate their essential role in regulating islet cell function and islet stress responses. These observations highlight the therapeutic potential of various approaches based on peptide hormones for T1D.
Xenotransplantation of Islets
Transplantation of whole pancreas and islets from humans offers an alternative for providing lifelong insulin independence; however, there are practical challenges pertaining to donor shortages. To alleviate the scarcity of donors, islet xenotransplantation offers exciting prospects. The first reported attempt to transplant porcine islets into human patients of T1D was carried out in 1994.59 Results demonstrated detectable C-peptide levels in patient urine for up to 300 days after transplant. However, these were without significant effects on blood glucose levels.59 Since then, there have been small clinical studies, which reported benefits of using pig-derived islets for the treatment of T1D.60,61 These studies provided strong clinical benefits for treating T1D. However, a number of challenges limit the clinical implementation of this approach, including a reliable source of pig islets, developing strategy for immune isolation of xeno-islets, and identifying suitable sites for transplantation.
Preclinical studies have reported additional benefits of using neonatal porcine islet-like cell pancreatic cell clusters (NPCCs) as opposed to mature islets. This includes robust survival during pre-transplant islet isolation procedures, especially in the ischaemic ex vivo environment.62 In addition, after transplant, the encapsulated NPCCs are reported to proliferate and differentiate into mature β cells, providing robust glucose control.62 Unlike mature islet cells, NPCCs are also resistant to cytotoxic effects of the host pro-inflammatory cytokines, including tumour necrosis factor–alpha (TNF-α), interleukin-1β (IL-1β), and interferon-gamma (IFN-γ).63 Regarding potential zoonosis from porcine graft, there are variety of designated pathogen-free pig breeds available, including the Chicago Medical School miniature pigs, New Zealand Auckland Island pigs, and transgenic pigs targeting porcine endogenous retrovirus (PERV).62
There are also studies, which have demonstrated stable glycaemic control using transplantation of genetically modified xeno-islet grafts. For instance, islets genetically altered to overexpress anti-apoptotic gene Bcl-2 have been transplanted and demonstrate stable functional profile with minimal islet loss post-transplant.64 Furthermore, pigs expressing the human antithrombotic or anticoagulant gene, such as thrombomodulin, tissue factor pathway inhibitor, or CD39, are available to minimise instant blood-mediated inflammatory reaction (IBMIR) and provide better transplantation outcomes.65 In the future, there is potential for using genetically modified pigs with engineered expression profiles for genes responsible for immune modulation, survival, and function to optimise transplantation outcomes. However, there are many concerns such as potential genetic stability in transgenic pigs and ethical justification, which require attention before widespread implementation of xenotransplantation of islets.
Islet Transplantation
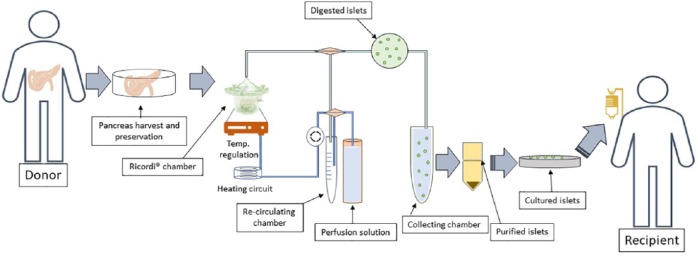
Islet transplantation provides an alternative to exogenous insulin treatment. The first attempt at xenotransplantation predates the discovery of insulin and was carried out in 1893. The concept was revisited in 1972, when Ballinger and Lacy66 successfully restored glycaemic control by infusing isolated islets through the intraportal vein in streptozotocin-induced diabetic rats. This was followed by successful intraportal transplant in patients with own islets in 1980.67 The researchers demonstrated that 3 patients achieved complete insulin independence for 1, 9, and 38 months, respectively.67 In addition, the development of a semi-automated method of islet isolation using the Ricordi chamber significantly optimised islet isolation protocol (Figure 1), allowing for greater efficiency in islet transplantation with minimal islet loss.68 The use of Ricordi chamber has since become a gold standard method for isolating islets from human pancreas (Figure 1).
Figure 1.
Procedure for human pancreatic islet isolation from a donor and transplantation into the recipient: Donor pancreas are harvested and preserved in a temperature regulated preservation chamber prior to collagenase digestion in a Ricordi chamber. The chamber consists of silicon beads that are constantly agitated and perfused with the perfusion solution via a peristaltic pump. The digested islets are collected and purified using density gradient centrifugation. Prior to transplantation into the recipient, the islets are cultured in in vitro to assess viability and insulin secretion.
The efficiency and consistency in islet isolation procedure and care post-transplantation were further optimised by Edmonton protocol. The protocol was published in 2000 and demonstrated effective insulin-free glycaemic regulation after islet transplantation in all seven T1D patients treated.69 These results have led to worldwide clinical implementation of Edmonton protocol as a standard and establishment of 34 centres for Collaborative Islet Transplant Registry (CITR) as of 2018. As a result, islet transplantation has considerably improved in the past 2 decades, with many refinements carried out to optimise pre-transplant and post-transplant procedures.70,71 This can also be partially attributed to the use of safer and effective anti-inflammatory interventions. However, there are still limitations, including the occurrence of IBMIR immediately after transplant, loss of islet numbers and islet mass due to ischaemia, apoptosis of islet cells, and detrimental side effects of immunosuppressive agents.72,73 Therefore, efforts are currently underway to enhance islet transplant outcomes by optimising islet isolation method48 and pre-transplant islet care.48,49 In addition, novel strategies are also being tested to enhance islet graft survival.50 Such novel approaches are discussed in detail elsewhere.74,75 Nevertheless, the increasing number of newly diagnosed T1D cases and the scarcity of pancreatic donors highlights the need for alternative approaches such as cellular replacement therapies.
Encapsulation strategies
This is an emerging technique where islets for transplant are physically protected from patient’s immune response, thereby not only sustaining islet survival without the need for chronic immune suppression but also avoiding stem cell dissemination.76 Currently, there are many encapsulation approaches that are being tested to allow both islet survival and efficient islet function.77,78 A phase I/II clinical trial being conducted by Viacyte, Inc, is currently underway to demonstrate safety and tolerability of their PEC-Encap combination product (Table 2). The product comprises stem cell–derived pancreatic progenitor cells (PEC-01) encapsulated in a delivery device called the Encaptra cell delivery system (STEP ONE clinical trial – NCT02239354). The results indicate safety and tolerability of PEC-Encap product candidate when delivered at a subtherapeutic dose. Such constructs allow for maturation of cells post-transplant and enable embryonic stem cell-derived β cells to efficiently vascularise. However, because β cells are protected by a physical barrier, there are risks for β cell death due to hypoxia, especially during early stages post-transplantation. In addition, the PEC-Encap cells are expected to vascularise externally with the recipient vascular network, which would allow for consistent engraftment and glucose sensing but will still require sustained immunosuppression for long-term survivability and function of encapsulated β cells.79
Recently, an open-labelled phase I clinical trial was conducted by using encapsulation device named βAir (Table 2).80 The device alleviates the problem of insufficient oxygen supply to the encapsulated β cells by incorporating a refillable oxygen tank in the design.80 Four patients were transplanted with 1-2 βAir devices each comprising nearly 155 000 to 180 000 islet equivalents (ie, 1800-4600 islet equivalents per kg body weight). The results revealed minimal increase in C-peptide levels, no impact on glycaemic control, and glucose stimulated insulin secretory response.80 However, the assessments from recovered grafts demonstrated complete survival of β cells for up to 6 months post-transplant.80 Although the trial did not demonstrate therapeutic efficacy of βAir device, it adds to the growing evidence for developing novel strategies of islet/β cell encapsulation for preventing immune rejection of the transplanted islets.
Retrievable device such as βAir offers novel approaches to enhance islet transplantation outcomes. However, translation of such approaches into the clinic requires scaling down of device size. In Evron et al’s study,81 this was achieved by stable maintenance of high islet surface density through modulation of partial pressure of oxygen (pO2) of the gas chamber. When diabetic rats were implanted with this device, normoglycaemia and glucose tolerance were achieved up until 7 months post implant.81 This finding demonstrated preclinical feasibility of developing retrievable macroencapsulated devices small enough for clinical use. The study by Farina et al82 further demonstrated the therapeutic utility of encapsulation system for subcutaneous engraftment of islets by using 3D printed and functionalised encapsulated islets. In keeping with this, researchers at Cornell University recently developed implantable, removable device: an ionised calcium-releasing, nano-porous polymer ‘thread’ dubbed TRAFFIC (Thread-Reinforced Alginate Fiber for Islets enCapsulation).83 The development involved encapsulation of rat or human islets in an alginate hydrogel which forms a cylindrical device. The researchers demonstrate significant reduction in blood glucose for up to 4 weeks in diabetic mice and, importantly, did not induce tissue damage and cellular overgrowth, fibrosis, and inflammation.83 In collaboration with Novo Nordisk, the device TRAFFIC has now received patent protection.
Furthermore, the concept of microencapsulation is also beginning to receive attention in recent years. A candidate product called DIABECELL consists of neonatal pig islets encapsulated in alginate microcapsules (NCT01739829). According to the manufacturers, Living Cell Technologies and Diatranz Otsuka Ltd, the device has been successfully tested in healthy and diabetic mice, rats, rabbits, dogs, and non-human primates. The results in humans indicated marginal reduction in HbA1c and less frequent hypoglycaemia (Table 2). Another approach utilises microencapsulation of insulin-producing Melligen cell line in cellulose-based microcapsule. In a small clinical trial, the device was demonstrated to be safe and tolerable (PharmaCyte Biotech, California, USA). These advances offer multiple approaches for efficient clinical transplant outcomes.
Stem Cell–Based Therapies
Stem cells have gained attention due to their potential for providing a limitless source of glucose responsive insulin-producing β cells as well as their ability to enhance the survival and function of transplanted islets. This holds the potential to solve the problem of limited availability of suitable donor islets, and can also enhance therapeutic outcome of islet transplantation in T1D patients.
Human embryonic stem cells
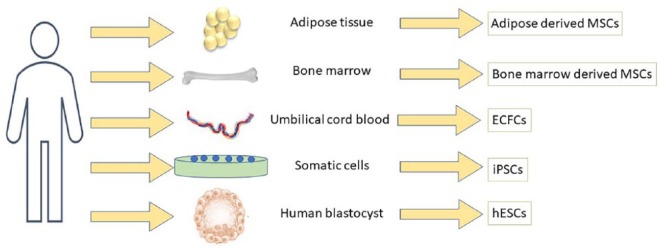
Human embryonic stem cells (hESCs) are pluripotent cells that give rise to all somatic cells in a developing embryo (Figure 2). Therefore, hESCs can potentially be used to generate new β cells for transplantation into T1D patients. Research has identified molecular cues that mimic stages of β cell development.84 Subsequently, researchers are now able to differentiate hESCs into pancreatic progenitor, endocrine progenitor, and insulin-producing β cell by forced expression of pancreatic transcription factors (TFs) such as Pdx,85 Mafa, Neurod1, Neurog3,86 and Pax4.87 This approach has been utilised in many studies88,89 to generate functional cells that are comparable with mature human insulin-producing β cells.90 These studies also provide proof-of-concept that hESCs can be specifically engineered to generate functional glucose responsive insulin-producing beta-like cells for transplantation in T1D patients. Indeed, a phase 1/2 clinical trial for T1D patients has already been started in the United States to evaluate the use of hESC-derived pancreatic progenitors (NCT02239354; Table 2). Nevertheless, there are still critical obstacles associated with the clinical implementation of this approach, including maintenance of homogeneous culture conditions to generate a genetically stable population of cells, variability in cell survival rate, and functional glucose responsive potential of differentiated cells.91 In addition, there are also ethical considerations of using embryo-derived stem cells, which also need to be considered.
Figure 2.
Sources of progenitor/stem cells: Mesenchymal stem cells also known as adult stem cells are highly multipotent and are known to differentiate into several specialised cells, including the vascular pericytes. Studies have suggested adipocytes and the bone marrow as an efficient source of MSCs, and remain attractive option for allogenic MSC co-transplantation with islets. Endothelial colony forming cells are vascular stem cells isolated from mononuclear fraction of umbilical cord blood. While somatic cells can be programmed to generate iPSCs, human blastocyst is the source of hESCs. ECFCs indicate endothelial colony forming cells; hESCs, human embryonic stem cells; iPSCs, induced pluripotent stem cells; MSCs, mesenchymal stem cells.
Induced pluripotent stem cells
Induced pluripotent stem cells (iPSCs) are specialised pluripotent stem cells generated from somatic cells (Figure 2). The emergence of autologous iPSC technology has demonstrated the potential to differentiate large numbers of patient-specific iPSCs from adult somatic cells into functional β cells. Indeed, by using the Yamanaka factors Oct3/4, Sox2, c-myc, and Klf4, T1D-specific iPSCs have been generated from patients with T1D.92,93 In addition, there has been substantial progress made towards elucidating important signalling pathways and regulators controlling cell fate.94 Such efforts have led to generation of glucose responsive and insulin-producing pancreatic progenitor cells which have been transplanted in mice.95 Furthermore, iPSC-derived cells express mature β cell-like markers, including PDX1, NKX6.1, MAFA, PCSK1, and PCSK2, and provide protection from T1D progression in diabetic animals.92
These iPSCs, with their unlimited replicative capability and potential to differentiate into functional β-like cells, bring exciting prospects for generating glucose responsive allogenic β cells for transplantation in T1D patients. The use of iPSCs for T1D therapy has additional benefits by serving as a source of autologous transforming growth factor beta 1 (TGFβ-1) and IL-10 producing Tregs.96 Therefore, the intense ongoing research will establish the clinical utility of generating patient’s own β cells using the iPSC method (Table 2). Furthermore, there are other benefits of using iPSC approach over hESCs such as fewer ethical considerations. In addition, at this stage, the iPSC protocols have progressed to a point where patient-specific iPSCs can function as an important source of autologous cells for cell therapy, without immune rejection. This, together with precise genome editing for gene correction, holds promising potential for T1D treatment. However, generation of β cells from iPSC is a complex procedure involving forced expression of transcription factors to mimic the normal developmental stages of pancreas.97 Also, gene expression analysis has revealed that iPSC-derived β cells do not accurately represent features of mature adult β-cells and more closely resemble embryonic β cells.98 There is also widely reported vulnerability of iPSC-derived transplanted graft to teratoma formation in vivo. Furthermore, the associated costs for good manufacturing practices for stem cell generation per patient and reprogramming could limit its general feasibility. These all remain prominent obstacles in the clinical translation of iPSC-derived β cell therapy.
Stem Cell Strategies to Enhance Islets Survival
The overall rate of β cell loss soon after intraportal transplantation ranges from 5% to 47%.99 This occurs as a result of stresses encountered during islet isolation and transplantation, including the IBMIR, impaired vascularisation, hypoxia, and nutrient deprivation.100 Furthermore, there are additional reasons such as alloimmune rejection,101 toxicity induced by immunosuppressive drugs,102 and glucolipotoxicity-induced β cell death.103 To increase the rates of islet cell survival and their function, many clinical trials (Table 2) are currently underway which propose the use of stem cells to overcome loss of transplanted islet cell mass.
Co-transplantation with mesenchymal stem cells
Co-culture of isolated islets and their co-transplantation with mesenchymal stem cells (MSCs) have been explored due to the anti-apoptotic and pro-angiogenic effects of MSCs.104 These cells are multipotent stromal cells capable of differentiating into a variety of cell types and can be isolated from various tissues (Figure 2), and also have been reported to differentiate into pericytes, which have the potential to further stabilise the vascular network around the islet graft.105 Importantly, MSCs have potent immunomodulatory properties and can modulate the activity of immune cells, including dendritic cells, NK cells, cytotoxic T cells, and B cells,106 thereby reducing the presence of pro-inflammatory cytokines in the vicinity of the graft area. In addition, MSCs are also shown to release many paracrine factors, which promote the growth and function of neighbouring cells. Taken together, these beneficial attributes have been shown to improve islet graft outcome in mice.107
Co-transplantation of islets with bone marrow–derived MSCs (BM-MSCs) has been well examined and demonstrated to reduce islet apoptosis, increased rates of revascularisation, and better islet function in streptozotocin-induced diabetic mice.108 In addition, mitigation of hypoxia-induced damage to the islets was reported after their exposure to MSC pre-conditioned media, demonstrating that paracrine factors are responsible for improved islet functioning by MSCs.109 As such, paracrine factors, including the vascular endothelial growth factor (VEGF), insulin-like growth factor 1, transforming growth factor beta 1, hepatocyte growth factor, heme oxygenase 1, IL-6, tissue inhibitor of metalloproteinase–1 (TIMP-1), and indoleamine 2,3-dioxygenase, are all known to be released by MSCs after co-transplantation with islets.110 In contrast, some studies have suggested that a direct physical contact between islets and MSCs is critical to improve islet survival, structural integrity, and insulin function.111,112 A recent meta-analysis suggests that viability of islets is higher in islets co-cultured with MSCs than islets cultured alone.113
Transplantation of adipose tissue–derived MSCs (Ad-MSCs; Figure 2) with islets has also been recently reported;114 this represents an attractive source of MSCs for autologous transplantation in patients with T1D.114 Islets that were co-cultured with Ad-MSCs demonstrated decreased cell death, superior viability, better membrane integrity, improved glucose stimulated insulin secretion, and reduced apoptosis compared with control islets.115 Importantly, the researchers showed superior glucose control in mice transplanted with co-cultured islets and Ad-MSCs as compared with both islets cultured/transplanted alone and islets co-transplanted with Ad-MSCs without prior co-culturing.115 Furthermore, a recent study evaluated islet survival and function after co-transplantation of Ad-MSCs from chronic pancreatitis patients.116 The findings reported enhanced islet function, reduced macrophage infiltration, inhibition of β-cell apoptosis, suppressed expression of TNF-α, and upregulated expressions of insulin like growth factor 1 (IGF-1) in islet grafts when they were co-transplanted with Ad-MSCs.116
Another advancement in this field has been the emergence of CRISPR-Cas9 gene editing technology. Through the use of CRISPR-Cas9 technique, researchers have been able to target insulin gene promoter and induce its activation in HEK293T, Hela, and human fibroblasts.117 The use of specific guide RNAs in this approach can be further utilised for sustained activation of insulin gene for prolonged periods in islets prior to their transplant into the recipient. This could potentially offer additional benefits by stably maintaining sufficient amounts of releasable insulin pool in transplanted islets, thereby allowing better post transplant islet function. On the other hand, CRISPR-Cas9 technique can also be utilised to target the transcription of endogenous genes involved in pancreatic development such as Pdx-1, Neurod1, and MafA in hESCs and iPSCs. This together with selective activation of MSC genes involved in immunomodulation can further develop successful MSCs–islet-based therapies.
An important facet of in vivo studies with MSCs in rodents is the use of renal subcapsular graft site as it facilitates anatomical co-localisation of islet graft and that islet–MSC grafts can easily be retrieved for studies through nephrectomy. On the contrary, clinical islet transplants are almost exclusively carried out via delivery into hepatic portal vein. It is therefore suggested that the much smaller MSCs of size, range from 15 to 30 µm in diameter, can almost freely pass through hepatic portal vein and has indeed been demonstrated to end up in lung capillaries.118 This represents a barrier for successful clinical implementation of experimental knowledge with islets and MSC co-transplantation.
Co-transplantation with endothelial colony forming cells
The pancreas is a highly vascularised organ. The vast network of blood vessels is critical for detecting subtle changes in circulating blood glucose and corresponding release of insulin from β cells. Islet isolation procedure severs the connection between the islet vasculature and systemic vessels. In whole organ pancreatic transplantation, the obstacle is circumvented by quickly re-connecting pancreatic vessels with arterial and venous vessels of the recipient. On the contrary, reinstating blood flow around and within the transplanted islet requires selective angiogenesis and vasculogenesis. In this regard, endothelial colony forming cells (ECFCs; Figure 2) exhibit ideal phenotypical characteristics which may be suitable for efficient re-establishment of vascular network around and within the graft. Also known as late endothelial progenitor cells (late-EPCs) or blood outgrowth endothelial cells (BOECs), ECFCs can be isolated from mononuclear fraction and cultured in established endothelial cell culture medium (Figure 2). Endothelial colony forming cells are positive for endothelial markers CD31, CD146, VEGFR2,119 and CD201.120 It has been demonstrated that ECFCs represent a vascular stem/progenitor cell with significant proliferative and vasoreparative potential.121 These cells have demonstrated efficacy in several in vivo preclinical models such as the ischaemic heart, retina, brain, limb, lung, and kidney.122
Based on their unique abilities to promote vascular repair, ECFCs have also been investigated to promote islet graft survival and islet function. Transplantation of porcine islets coated with ECFCs under the kidney capsule provided better protection against xenogenic IBMIR as compared with mature ECs.123 Furthermore, intraportal transplantation of ECFC-coated porcine islets demonstrated better graft survival and glycaemic control in nude mice.124 Cell tracing analysis has revealed that composite ECFC–islet grafts remain in the liver sections and were associated with higher insulin-positive immunostaining.124 These studies support the use of allogeneic ECFCs in translational studies that aim to improve current islet transplantation protocols for the treatment of T1D.
Stem Cell Mobilisation Strategies
Chronic hyperglycaemia is detrimental to many organs, and studies have revealed that prolonged diabetes also damages the bone microenvironment.125 As a result, mobilisation of haematopoietic stem cells (HSCs) is drastically affected in response to stimuli such as ischaemia.126 This is suggested to severely affect the clinical outcomes in diabetic patients undergoing islet or stem cell transplant.127 Furthermore, bone marrow is a rich source for circulating vascular progenitor cells, whose mobilisation is also known to be negatively modulated in diabetes.128 Considering that the overall HSC numbers in circulation under diabetes are reduced, strategies to mobilise HSCs are being researched as potential therapies for diabetes-related complications.129
The primary factor for recruitment and mobilisation of HSCs from the bone marrow is granulocyte-colony stimulating factor (G-CSF).130 The mobilisation involves reduction in intramedullary concentrations of CXCL12, which is a ligand for CXCR4 and is a known chemoattractant of leucocytes. Plerixafor is a CXCR4 antagonist, which is used as a therapy for mobilisation of HSCs in cancer patients.130 Furthermore, there is an ongoing clinical trial to evaluate the potential of Plerixafor in combination with long-acting GLP-1 receptor agonist for mobilising CD34+ HSCs for the treatment of T1D (NCT03182426). If successful, this study will pave the way for novel treatment strategies for treating T1D in the future. In addition, a clinical study has demonstrated an increase in numbers of circulating EPCs (cEPCs) and pro-angiogenic cells after treatment with metformin.131 Interestingly, the observed increase in cell populations was independent of reduction in HbA1c.131 Nevertheless, the study demonstrates beneficial actions on stem cell mobilisation of known pharmacotherapy beyond traditional glycaemic regulatory effects.
Future Perspectives
Type 1 diabetes remains one of the major causes of blindness, kidney failure, and stroke. Over the past 4 decades, the number of cases has steadily increased. Although the recent emergence of fast-acting and long-acting insulin analogues has improved the quality of life in T1D patients, many challenges still remain. Transplantation of primary islets offers exciting prospects for treating patients with T1D. However, limited availability of islets remains one of the most prominent obstacle for widespread use of islets transplantation. To overcome the shortage of donors, xenotransplantation of islets has been explored, but there remains ethical considerations and concerns about genetic stability of transgenic islets. Therefore, in the current state, insulin replacement therapy and transplantation of islets from humans remain a practical and financially feasible option to treat T1D.
Development of effective human islet transplantation strategies is also hampered by many barriers, such as the extensive death of islet cells during the immediate period post-transplantation. This inevitably does increase the requirement for number of islets needed to achieve glycaemic control and insulin independence. In addition, disruption of normal islet architecture and morphology, as well as poor vascular engraftment during the post-transplantation period, also significantly contribute to deterioration of islet graft function. During the immediate period at both pre-transplant and post-transplant stages, isolated islets had to undergo considerable shear stresses, which leads to loss in beta cell number and function, thereby also contributing to graft failure.
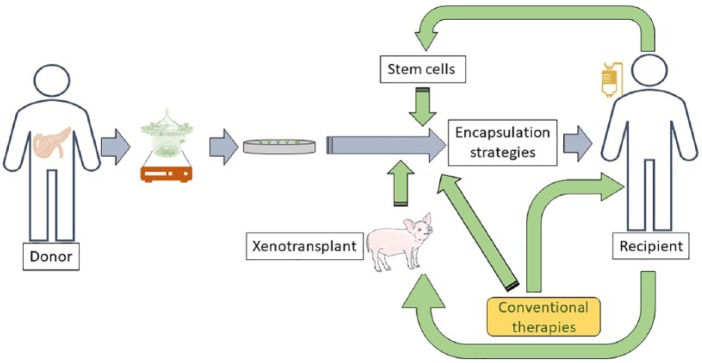
Clearly, new approaches are needed for successful therapeutic outcomes and complete insulin independence (Figure 3). Emergence of β cell encapsulation strategies and stem cell approaches such as mobilisation strategies and implantation of islets in combination with ECFCs and/or MSCs could improve graft survival, not only by aiding revascularisation but also by providing both pre-transplanted and post-transplanted islets with paracrine growth factors needed for proliferation and function (Figure 3). Furthermore, conventional T1D therapeutic approaches, including insulin replacement, SGLT2 inhibitors, immune therapies, and peptide agonists, also need to be considered either alone or in combination with emerging approaches for optimum clinical therapeutic outcomes (Figure 3).
Figure 3.
Future strategies for successful outcome of human islet transplantation: While the pre-transplant islet isolation stages have been carefully optimised, there is more than 1 strategy to enhance the efficiency, viability, and biological function of the transplanted islets. Once purified islets are cultured in vitro, they can be co-transplanted with recipient’s own stem cells to enhance vascularisation, overcoming islet graft loss, and dysfunction post-transplantation. Another strategy is to use transgenic neonatal pigs as a source of islets. The overall survival during the pre-transplant period and post-transplant function of purified islets can be further enhanced using encapsulation strategies either alone or in combination with conventional therapies.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: R.J.M. is funded by grants from the Juvenile Diabetes Research Foundation (JDRF), the Leverhulme Trust, and the National Eye Research Centre.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: VP, NMP, CO, JG-F, and RM: manuscript writing and final approval of manuscript.
References
- 1. Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361–369. [DOI] [PubMed] [Google Scholar]
- 2. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–188. [DOI] [PubMed] [Google Scholar]
- 3. Egro FM. Why is type 1 diabetes increasing? J Mol Endocrinol. 2013;51:R1–R13. [DOI] [PubMed] [Google Scholar]
- 4. Størling J, Pociot F. Type 1 diabetes candidate genes linked to pancreatic islet cell inflammation and beta-cell apoptosis. Genes (Basel). 2017;8:E72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nguyen C, Varney MD, Harrison LC, Morahan G. Definition of high-risk type 1 diabetes HLA-DR and HLA-DQ types using only three single nucleotide polymorphisms. Diabetes. 2013;62:2135–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jerram ST, Leslie RD. The genetic architecture of type 1 diabetes. Genes (Basel). 2017;8:E209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang J, Liu L, Ma J, Sun F, Zhao Z, Gu M. Common variants on cytotoxic T lymphocyte antigen-4 polymorphisms contributes to type 1 diabetes susceptibility: evidence based on 58 studies. PLoS ONE. 2014;9:e85982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prezioso G, Comegna L, Di Giulio C, Franchini S, Chiarelli F, Blasetti A. C1858T polymorphism of protein tyrosine phosphatase non-receptor type 22 (PTPN22): an eligible target for prevention of type 1 diabetes? Expert Rev Clin Immunol. 2017;13:189–196. [DOI] [PubMed] [Google Scholar]
- 9. Guarnotta V, Vigneri E, Pillitteri G, Ciresi A, Pizzolanti G, Giordano C. Higher cardiometabolic risk in idiopathic versus autoimmune type 1 diabetes: a retrospective analysis. Diabetol Metab Syndr. 2018;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–1368. [DOI] [PubMed] [Google Scholar]
- 11. Banting FG, Best CH. Pancreatic extracts. 1922. J Lab Clin Med. 1990;115:254–272. [PubMed] [Google Scholar]
- 12. Richter B, Neises G. ‘Human’ insulin versus animal insulin in people with diabetes mellitus. Cochrane Database Syst Rev. 2002;3:CD003816. [DOI] [PubMed] [Google Scholar]
- 13. Evans M, Schumm-Draeger PM, Vora J, King AB. A review of modern insulin analogue pharmacokinetic and pharmacodynamic profiles in type 2 diabetes: improvements and limitations. Diabetes Obes Metab. 2011;13:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen O, Filetti S, Castañeda J, Maranghi M, Glandt M. When intensive insulin therapy (MDI) fails in patients with type 2 diabetes: switching to GLP-1 receptor agonist versus insulin pump. Diabetes Care. 2016;39:S180–S186. [DOI] [PubMed] [Google Scholar]
- 15. Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157:336–347. [DOI] [PubMed] [Google Scholar]
- 16. Franek E, Haluzík M, Canecki Varžić S, et al. Twice-daily insulin degludec/insulin aspart provides superior fasting plasma glucose control and a reduced rate of hypoglycaemia compared with biphasic insulin aspart 30 in insulin-naïve adults with type 2 diabetes. Diabet Med. 2016;33:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacobson AM, Braffett BH, Cleary PA, Gubitosi-Klug RA, Larkin ME; DCCT/EDIC Research Group. The long-term effects of type 1 diabetes treatment and complications on health-related quality of life: a 23-year follow-up of the diabetes control and complications/epidemiology of diabetes interventions and complications cohort. Diabetes Care. 2013;36:3131–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Priya G, Kalra S. A review of insulin resistance in type 1 diabetes: is there a place for adjunctive metformin? Diabetes Ther. 2018;9:349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vora J, Cohen N, Evans M, Hockey A, Speight J, Whately-Smith C. Intensifying insulin regimen after basal insulin optimization in adults with type 2 diabetes: a 24-week, randomized, open-label trial comparing insulin glargine plus insulin glulisine with biphasic insulin aspart (LanScape). Diabetes Obes Metab. 2015;17:1133–1141. [DOI] [PubMed] [Google Scholar]
- 20. Shah RB, Patel M, Maahs DM, Shah VN. Insulin delivery methods: past, present and future. Int J Pharm Investig. 2016;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abramson A, Caffarel-Salvador E, Khang M, et al. An ingestible self-orienting system for oral delivery of macromolecules. Science. 2019;363:611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. REPOSE Study Group. Relative effectiveness of insulin pump treatment over multiple daily injections and structured education during flexible intensive insulin treatment for type 1 diabetes: cluster randomised trial (REPOSE). BMJ. 2017;356:j1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Slover RH, Tryggestad JB, DiMeglio LA, et al. Accuracy of a fourth-generation continuous glucose monitoring system in children and adolescents with type 1 diabetes. Diabetes Technol Ther. 2018;20:576–584. [DOI] [PubMed] [Google Scholar]
- 24. Kambe N, Kawahito S, Mita N, et al. Impact of newly developed, next-generation artificial endocrine pancreas. J Med Invest. 2015;62:41–44. [DOI] [PubMed] [Google Scholar]
- 25. Boughton CK, Hovorka R. Is an artificial pancreas (closed-loop system) for Type 1 diabetes effective? Diabet Med. 2019;36:279–286. [DOI] [PubMed] [Google Scholar]
- 26. Jacobs PG, El Youssef J, Reddy R, et al. Randomized trial of a dual-hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor-augmented pump therapy. Diabetes Obes Metab. 2016;18:1110–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haidar A, Legault L, Matteau-Pelletier L, et al. Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3:595–604. [DOI] [PubMed] [Google Scholar]
- 28. Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:501–512. [DOI] [PubMed] [Google Scholar]
- 29. Bergenstal RM, Garg S, Weinzimer SA, et al. Notably, the US Food and Drug Administration has recently approved the first artificial pancreas system for use by people with type 1 diabetes over 14 years of age, based on a safety outpatient study. JAMA. 2018;316:1407–1408. [Google Scholar]
- 30. Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361:k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. The Canadian-European Randomized Control Trial Group. Cyclosporin-induced remission of IDDM after early intervention. Association of 1 yr of cyclosporin treatment with enhanced insulin secretion. Diabetes. 1988;37:1574–1582. [PubMed] [Google Scholar]
- 32. Martin S, Pawlowski B, Greulich B, Ziegler AG, Mandrup-Poulsen T, Mahon J. Natural course of remission in IDDM during 1st yr after diagnosis. Diabetes Care. 1992;15:66–74. [DOI] [PubMed] [Google Scholar]
- 33. Keymeulen B, Walter M, Mathieu C, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53:614–623. [DOI] [PubMed] [Google Scholar]
- 34. Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Type 1 Diabetes TrialNet Anti-CD20 Study Group. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ludvigsson J. Therapies to preserve β-cell function in type 1 diabetes. Drugs. 2016;76:169–185. [DOI] [PubMed] [Google Scholar]
- 36. Gitelman SE, Gottlieb PA, Felner EI, et al. ; ITN START Study Team. Antithymocyte globulin therapy for patients with recent-onset type 1 diabetes: 2 year results of a randomised trial. Diabetologia. 2016;59:1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moran A, Bundy B, Becker DJ, et al. ; Type 1 Diabetes TrialNet Canakinumab Study Group and Pickersgill L, de Koning E, Ziegler AG, et al. ; AIDA Study Group. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381:1905–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Visperas A, Vignali DA. Are regulatory T cells defective in type 1 diabetes and can we fix them? J Immunol. 2016;197:3762–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takemoto N, Konagaya S, Kuwabara R, Iwata H. Coaggregates of regulatory T cells and islet cells allow long-term graft survival in liver without immunosuppression. Transplantation. 2015;99:942–947. [DOI] [PubMed] [Google Scholar]
- 40. Duggleby R, Danby RD, Madrigal JA, Saudemont A. Clinical grade regulatory CD4+ T cells (Tregs): moving toward cellular-based immunomodulatory therapies. Front Immunol. 2018;9:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marek-Trzonkowska N, Myśliwiec M, Iwaszkiewicz-Grześ D, et al. Factors affecting long-term efficacy of T regulatory cell-based therapy in type 1 diabetes. J Transl Med. 2016;14:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bluestone JA, Buckner JH, Fitch M, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singha S, Shao K, Yang Y, et al. Peptide-MHC-based nanomedicines for autoimmunity function as T-cell receptor microclustering devices. Nat Nanotechnol. 2017;12:701–710. [DOI] [PubMed] [Google Scholar]
- 44. Clemente-Casares X, Blanco J, Ambalavanan P, et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530:434–440. [DOI] [PubMed] [Google Scholar]
- 45. Millar PJ, Pathak V, Moffett RC, et al. Beneficial metabolic actions of a stable GIP agonist following pre-treatment with a SGLT2 inhibitor in high fat fed diabetic mice. Mol Cell Endocrinol. 2016;420:37–45. [DOI] [PubMed] [Google Scholar]
- 46. Van Baar MJB, van Ruiten CC, Muskiet MHA, van Bloemendaal L, IJzerman RG, van Raalte DH. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care. 2018;41:1543–1556. [DOI] [PubMed] [Google Scholar]
- 47. Tahara A, Kurosaki E, Yokono M, et al. Effects of sodium-glucose cotransporter 2 selective inhibitor ipragliflozin on hyperglycaemia, oxidative stress, inflammation and liver injury in streptozotocin-induced type 1 diabetic rats. J Pharm Pharmacol. 2014;66:975–987. [DOI] [PubMed] [Google Scholar]
- 48. Cheng STW, Chen L, Li SYT, Mayoux E, Leung PS. The effects of empagliflozin, an SGLT2 inhibitor, on pancreatic β-cell mass and glucose homeostasis in type 1 diabetes. PLoS ONE. 2016;11:e0147391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McCrimmon RJ, Henry RR. SGLT inhibitor adjunct therapy in type 1 diabetes. Diabetologia. 2018;61:2126–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. The enteroinsular axis – contribution to obesity-diabetes and its treatments. In: Opara EC, Dagogo-Jack S, eds Nutrition and Diabetes: Pathophysiology and Management. 2nd ed. Boca Raton, FL: CRC Press; 2019:41–56. [Google Scholar]
- 51. Traina AN, Lull ME, Hui AC, Zahorian TM, Lyons-Patterson J. Once-weekly exenatide as adjunct treatment of type 1 diabetes mellitus in patients receiving continuous subcutaneous insulin infusion therapy. Can J Diabetes. 2014;38:269–272. [DOI] [PubMed] [Google Scholar]
- 52. Frandsen CS, Dejgaard TF, Holst JJ, Andersen HU, Thorsteinsson B, Madsbad S. Twelve-week treatment with liraglutide as add-on to insulin in normal-weight patients with poorly controlled type 1 diabetes: a randomized, placebo-controlled, double-blind parallel study. Diabetes Care. 2015;38:2250–2257. [DOI] [PubMed] [Google Scholar]
- 53. Rydén AK, Perdue NR, Pagni PP, et al. Anti-IL-21 monoclonal antibody combined with liraglutide effectively reverses established hyperglycemia in mouse models of type 1 diabetes. J Autoimmun. 2017;84:65–74. [DOI] [PubMed] [Google Scholar]
- 54. Pocai A. Action and therapeutic potential of oxyntomodulin. Mol Metab. 2014;3:241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pathak NM, Pathak V, Lynch AM, Irwin N, Gault VA, Flatt PR. Stable oxyntomodulin analogues exert positive effects on hippocampal neurogenesis and gene expression as well as improving glucose homeostasis in high fat fed mice. Mol Cell Endocrinol. 2015;412:95–103. [DOI] [PubMed] [Google Scholar]
- 56. Irwin N, Pathak V, Pathak NM, Gault VA, Flatt PR. Sustained treatment with a stable long-acting oxyntomodulin analogue improves metabolic control and islet morphology in an experimental model of type 1 diabetes. Diabetes Obes Metab. 2015;17:887–895. [DOI] [PubMed] [Google Scholar]
- 57. Khan D, Vasu S, Moffett RC, Irwin N, Flatt PR. Differential expression of glucagon-like peptide-2 (GLP-2) is involved in pancreatic islet cell adaptations to stress and beta-cell survival. Peptides. 2017;95:68–75. [DOI] [PubMed] [Google Scholar]
- 58. Khan D, Vasu S, Moffett RC, Irwin N, Flatt PR. Islet distribution of peptide YY and its regulatory role in primary mouse islets and immortalised rodent and human beta-cell function and survival. Mol Cell Endocrinol. 2016;436:102–113. [DOI] [PubMed] [Google Scholar]
- 59. Groth CG, Korsgren O, Tibell A. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344:1402–1404. [DOI] [PubMed] [Google Scholar]
- 60. Sykes M, Cozzi E, D’Apice AJ, et al. Response to Valdes-Gonzalez ‘Clinical trial of islet xenotransplantation in Mexico’. Xenotransplantation. 2007;14:90–91. [DOI] [PubMed] [Google Scholar]
- 61. Wynyard S, Nathu D, Garkavenko O, Denner J, Elliott R. Microbiological safety of the first clinical pig islet xenotransplantation trial in New Zealand. Xenotransplantation. 2014;21:309–323. [DOI] [PubMed] [Google Scholar]
- 62. Foster JL, Williams G, Williams LJ, Tuch BE. Differentiation of transplanted microencapsulated fetal pancreatic cells. Transplantation. 2007;83:1440–1448. [DOI] [PubMed] [Google Scholar]
- 63. Bai L, Tuch BE, Hering B, et al. Fetal pig β cells are resistant to the toxic effects of human cytokines. Transplantation. 2002;73:714–722. [DOI] [PubMed] [Google Scholar]
- 64. Contreras JL, Xie D, Mays J, et al. A novel approach to xenotransplantation combining surface engineering and genetic modification of isolated adult porcine islets. Surgery. 2004;136:537–547. [DOI] [PubMed] [Google Scholar]
- 65. Ekser B, Cooper DKC. Overcoming the barriers to xenotransplantation: prospects for the future. Expert Rev Clin Immunol. 2010;6:219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ballinger WF, Lacy PE. Transplantation of intact pancreatic islets in rats. Surgery. 1972;72:175–186. [PubMed] [Google Scholar]
- 67. Sutherland DE, Matas AJ, Goetz FC, Najarian JS. Transplantation of dispersed pancreatic islet tissue in humans: autografts and allografts. Diabetes. 1980;29:31–44. [DOI] [PubMed] [Google Scholar]
- 68. Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–420. [DOI] [PubMed] [Google Scholar]
- 69. Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. [DOI] [PubMed] [Google Scholar]
- 70. Koh A, Senior P, Salam A, et al. Insulin-heparin infusions peritransplant substantially improve single-donor clinical islet transplant success. Transplantation. 2010;9:465–471. [DOI] [PubMed] [Google Scholar]
- 71. Johansson H, Lukinius A, Moberg L, et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54:1755–1762. [DOI] [PubMed] [Google Scholar]
- 72. Bottino R, Knoll MF, Knoll CA, Bertera S, Trucco MM. The future of islet transplantation is now. Front Med (Lausanne). 2018;5:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McCall M, Shapiro AMJ. Update on islet transplantation. Cold Spring Harb Perspect Med. 2012;2:a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gamble A, Pepper AR, Bruni A, Shapiro AMJ. The journey of islet cell transplantation and future development. Islets. 2018;10:80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pellegrini S, Cantarelli E, Sordi V, Nano R, Piemonti L. The state of the art of islet transplantation and cell therapy in type 1 diabetes. Acta Diabetol. 2016;53:683–691. [DOI] [PubMed] [Google Scholar]
- 76. Korsgren O. Islet encapsulation: physiological possibilities and limitations. Diabetes. 2017;66:1748–1754. [DOI] [PubMed] [Google Scholar]
- 77. O’Sullivan ES, Vegas A, Anderson DG, Weir GC. Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocr Rev. 2011;32:827–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rattananinsruang P, Dechsukhum C, Leeanansaksiri W. Establishment of insulin-producing cells from human embryonic stem cells underhypoxic condition for cell based therapy. Front Cell Dev Biol. 2018;6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kopan C, Tucker T, Alexander M, Mohammadi MR, Pone EJ, Lakey JRT. Approaches in immunotherapy, regenerative medicine, and bioengineering for type 1 diabetes. Front Immunol. 2018;9:1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Carlsson PO, Espes D, Sedigh A, et al. Transplantation of macroencapsulated human islets within the bioartificial pancreas βAir to patients with type 1 diabetes mellitus. Am J Transplant. 2018;18:1735–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Evron Y, Colton CK, Ludwig B, et al. Long-term viability and function of transplanted islets macroencapsulated at high density are achieved by enhanced oxygen supply. Sci Rep. 2018;8:6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Farina M, Ballerini A, Fraga DW, et al. 3D printed vascularized device for subcutaneous transplantation of human islets. Biotechnol J. 2017;12:1700169. [DOI] [PubMed] [Google Scholar]
- 83. An D, Chiu A, Flanders JA, et al. Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes. Proc Natl Acad Sci U S A. 2018;115:E263–E272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pagliuca FW, Melton DA. How to make a functional β-cell. Development. 2013;140:2472–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Miyazaki S, Yamato E, Miyazaki J. Regulated expression of pdx-1 promotes in vitro differentiation of insulin-producing cells from embryonic stem cells. Diabetes. 2004;53:1030–1037. [DOI] [PubMed] [Google Scholar]
- 86. Xu H, Tsang KS, Chan JC, et al. The combined expression of Pdx1 and MafA with either Ngn3 or NeuroD improve the differentiation efficiency of mouse embryonic stem cells into insulin-producing cells. Cell Transplant. 2013;22:147–158. [DOI] [PubMed] [Google Scholar]
- 87. Blyszczuk P, Czyz J, Kania G, et al. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci U S A. 2003;100:998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kelly OG, Chan MY, Martinson LA, et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29:750–756. [DOI] [PubMed] [Google Scholar]
- 89. Schulz TC, Young HY, Agulnick AD, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS ONE. 2012;7:e37004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Robert T, De Mesmaeker I, Stangé GM, et al. Functional beta cell mass from device-encapsulated hESC-derived pancreatic endoderm achieving metabolic control. Stem Cell Reports. 2018;10:739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vazin T, Freed WJ. Human embryonic stem cells: derivation, culture, and differentiation: a review. Restor Neurol Neurosci. 2010;28:589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rezania A, Bruin JE, Riedel MJ, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61:2016–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kunisada Y, Tsubooka-Yamazoe N, Shoji M, Hosoya M. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. 2012;8:274–284. [DOI] [PubMed] [Google Scholar]
- 94. Millman JR, Xie C, Van Dervort A, Gürtler M, Pagliuca FW, Melton DA. Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nat Commun. 2016;7:11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. [DOI] [PubMed] [Google Scholar]
- 96. Song J. Development of auto antigen-specific regulatory T cells for diabetes immunotherapy. Immune Netw. 2016;16:281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Toyoda T, Mae S, Tanaka H, et al. Cell aggregation optimizes the differentiation of human ESCs and iPSCs into pancreatic bud-like progenitor cells. Stem Cell Res. 2015;14:185–197. [DOI] [PubMed] [Google Scholar]
- 98. Hrvatin S, O’Donnell CW, Deng F, et al. Differentiated human stem cells resemble fetal, not adult, β cells. Proc Natl Acad Sci U S A. 2014;111:3038–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Potter KJ, Westwell-Roper CY, Klimek-Abercrombie AM, Warnock GL, Verchere CB. Death and dysfunction of transplanted β-cells: lessons learned from type 2 diabetes? Diabetes. 2014;63:12–19. [DOI] [PubMed] [Google Scholar]
- 100. Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant. 2014;14:428–437. [DOI] [PubMed] [Google Scholar]
- 101. Delaune V, Toso C, Benhamou PY, et al. Alloimmune monitoring after islet transplantation: a prospective multicenter assessment of 25 recipients. Cell Transplant. 2016;25:2259–2268. [DOI] [PubMed] [Google Scholar]
- 102. Vallabhajosyula P, Hirakata A, Shimizu A, et al. Assessing the effect of immunosuppression on engraftment of pancreatic islets. Transplantation. 2013;96:372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sharma RB, Alonso LC. Lipotoxicity in the pancreatic beta cell: not just survival and function, but proliferation as well? Curr Diab Rep. 2014;14:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Shafiee A, Patel J, Lee JS, Hutmacher DW, Fisk NM, Khosrotehrani K. Mesenchymal stem/stromal cells enhance engraftment, vasculogenic and pro-angiogenic activities of endothelial colony forming cells in immunocompetent hosts. Sci Rep. 2017;7:13558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Deschepper M, Oudina K, David B, et al. Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J Cell Mol Med. 2011;15:1505–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kerby A, Jones ES, Jones PM, King AJ. Co-transplantation of islets with mesenchymal stem cells in microcapsules demonstrates graft outcome can be improved in an isolated-graft model of islet transplantation in mice. Cytotherapy. 2013;15:192–200. [DOI] [PubMed] [Google Scholar]
- 108. Borg DJ, Weigelt M, Wilhelm C, et al. Mesenchymal stromal cells improve transplanted islet survival and islet function in a syngeneic mouse model. Diabetologia. 2014;57:522–531. [DOI] [PubMed] [Google Scholar]
- 109. Brandhorst D, Brandhorst H, Acreman S, Schive SW, Bjornson Scholz H, Johnson PRV. Hypoxia-induced damage in human islets is reduced with the use of mesenchymal stem cell-preconditioned medium. Transplant Proc. 2017;49:2330–2332. [DOI] [PubMed] [Google Scholar]
- 110. Bell GI, Meschino MT, Hughes-Large JM, Broughton HC, Xenocostas A, Hess DA. Combinatorial human progenitor cell transplantation optimizes islet regeneration through secretion of paracrine factors. Stem Cells Dev. 2012;21:1863–1876. [DOI] [PubMed] [Google Scholar]
- 111. Rackham CL, Dhadda PK, Chagastelles PC, et al. Pre-culturing islets with mesenchymal stromal cells using a direct contact configuration is beneficial for transplantation outcome in diabetic mice. Cytotherapy. 2013;15:449–459. [DOI] [PubMed] [Google Scholar]
- 112. Jun Y, Kang AR, Lee JS, et al. Microchip-based engineering of super-pancreatic islets supported by adipose-derived stem cells. Biomaterials. 2014;35:4815–4826. [DOI] [PubMed] [Google Scholar]
- 113. De Souza BM, Bouças AP, Oliveira FD, et al. Effect of co-culture of mesenchymal stem/stromal cells with pancreatic islets on viability and function outcomes: a systematic review and meta-analysis. Islets. 2017;9:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gamble A, Pawlick R, Pepper AR, et al. Improved islet recovery and efficacy through co-culture and co-transplantation of islets with human adipose-derived mesenchymal stem cells. PLoS ONE. 2018;13:e0206449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Schive SW, Mirlashari MR, Hasvold G, et al. Human adipose-derived mesenchymal stem cells respond to short-term hypoxia by secreting factors beneficial for human islets in vitro and potentiate antidiabetic effect in vivo. Cell Med. 2017;9:103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Song L, Sun Z, Kim DS, et al. Adipose stem cells from chronic pancreatitis patients improve mouse and human islet survival and function. Stem Cell Res Ther. 2017;8:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Giménez CA, Ielpi M, Mutto A, Grosembacher L, Argibay P, Pereyra-Bonnet F. CRISPR-on system for the activation of the endogenous human INS gene. Gene Ther. 2016;23:543–547. [DOI] [PubMed] [Google Scholar]
- 118. Eggenhofer E, Benseler V, Kroemer A, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Medina RJ, O’Neill CL, Sweeney M, et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics. 2010;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yu QC, Song W, Wang D, Zeng YA. Identification of blood vascular endothelial stem cells by the expression of protein C receptor. Cell Res. 2016;26:1079–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Medina RJ, Barber CL, Sabatier F, et al. Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med. 2017;6:1316–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. O’Neill CL, McLoughlin KJ, Chambers SEJ, Guduric-Fuchs J, Stitt AW, Medina RJ. The vasoreparative potential of endothelial colony forming cells: a journey through pre-clinical studies. Front Med (Lausanne). 2018;5:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kim JH, Oh BJ, Lee HN, Park HS, Park SG, Park KS. Endothelial colony-forming cell coating of pig islets prevents xenogeneic instant blood-mediated inflammatory reaction. Cell Transplant. 2011;20:1805–1815. [DOI] [PubMed] [Google Scholar]
- 124. Jung HS, Kim MJ, Hong SH, et al. The potential of endothelial colony-forming cells to improve early graft loss after intraportal islet transplantation. Cell Transplant. 2014;23:273–283. [DOI] [PubMed] [Google Scholar]
- 125. Spinetti G, Cordella D, Fortunato O, et al. Global remodeling of the vascular stem cell niche in bone marrow of diabetic patients: implication of the microRNA-155/FOXO3a signaling pathway. Circ Res. 2013;112:510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Fadini GP, Avogaro A. Diabetes impairs mobilization of stem cells for the treatment of cardiovascular disease: a meta-regression analysis. Int J Cardiol. 2013;168:892–897. [DOI] [PubMed] [Google Scholar]
- 127. Stocks BT, Thomas AB, Elizer SK, et al. Hematopoietic stem cell mobilization is necessary but not sufficient for tolerance in islet transplantation. Diabetes. 2017;66:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Westerweel PE, Teraa M, Rafii S, et al. Impaired endothelial progenitor cell mobilization and dysfunctional bone marrow stroma in diabetes mellitus. PLoS ONE. 2013;8:e60357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Albiero M, Avogaro A, Fadini GP. Restoring stem cell mobilization to promote vascular repair in diabetes. Vascul Pharmacol. 2013;58:253–258. [DOI] [PubMed] [Google Scholar]
- 130. Winkler IG, Pettit AR, Raggatt LJ, et al. Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia. 2012;26:1594–1601. [DOI] [PubMed] [Google Scholar]
- 131. Ahmed FW, Rider R, Glanville M, Narayanan K, Razvi S, Weaver JU. Metformin improves circulating endothelial cells and endothelial progenitor cells in type 1 diabetes: MERIT study. Cardiovasc Diabetol. 2016;15:116. [DOI] [PMC free article] [PubMed] [Google Scholar]