Abstract
Urachal adenocarcinoma represents the third most common histological type of non-urotelial bladder cancer. A very low incidence of this disease and the lack of prospective studies have led to a rich and heterogeneous treatment history. Currently, the standard of care for these patients is represented by partial cystectomy en bloc with resection of the urachal ligament and total omphalectomy. The aim of this article is to present our experience and results in the management of patients with urachal adenocarcinoma. Between 2005 and 2015, 16 patients have undergone surgical treatment for urachal adenocarcinoma in “Fundeni” Clinical Institute and Madrid University Hospital “Infanta Sofia.” Partial cystectomy was performed in 11 (68.76%) patients, while radical cystectomy en bloc with omphalectomy was performed in 5 (31.25%) patients, which were not amendable to a limited resection. The Sheldon classification was used, as it provides appropriate disease staging and is the most commonly utilized. Postoperative pathological results showed that 7 (43.75%) patients had localized tumors, and more than one-third (37.5%) of the patients had locally advanced Sheldon III disease, while 3 patients had distant metastasis at the time of surgery. Lymph node involvement was present in 3 patients (18.75%). Mean follow-up time was 2.5 years, ranging from 4 months to 7.6 years. Three patients (18.75%) were lost to follow-up, without any documented signs of local or systemic recurrence and were cancer free at the time of the last evaluation. In cases with lymph node involvement, local recurrence or distant metastasis, patients underwent cisplatin- or 5-fluorouracil-based salvage chemotherapy. Surgical treatment represents the gold standard, while adjuvant chemotherapy has a limited impact on overall survival. The utility of navel resection is questionable due to the rarity of direct invasion or local recurrence.
Keywords: Urachal adenocarcinoma, omphalectomy, partial cystectomy, radical cystectomy, bladder cancer
Introduction
Urachal adenocarcinoma, initially described by Hue and Jacquin in 1863, represents the third most common histological type of bladder tumors with non-urothelial origin. Data provided by the Surveillance, Epidemiology and End Results suggest that this histological variant accounts for about 10% of adenocarcinomas of the bladder,1 while the Netherlands Cancer Registry reports that 16.3% of bladder adenocarcinomas originate in the urachal ligament.2
The low incidence of this pathology and the lack of prospective studies have led to a rich and heterogeneous treatment history, which is still an intense focus of several ongoing studies. According to initial studies, the optimal surgical treatment is radical cystectomy and pelvic lymph node dissection (LND), similar to that performed in patients with muscle invasive bladder cancer. However, better understanding of local anatomy and the embryological origin of this particular type of tumor have allowed urologists to safely perform partial cystectomy while ensuring adequate control of the disease.3
Increased interest in bladder sparing techniques has been spurred by their ability to achieve adequate disease control as well as their avoidance of perioperative morbidity. Moreover, preserved bladder function, including both esthetic and functional outcomes, has helped establish partial cystectomy as the gold standard for treatment of urachal tumors. Currently, the standard of care for these patients is represented by en bloc partial cystectomy with resection of the urachal ligament and total omphalectomy. Omphalectomy was performed when the tumor had direct involvement of the urachal ligament or of the abdominal wall and in cases with a high index of suspicion for urachal adenocarcinoma.
The aim of this study was to present our experience and results in the management of patients with urachal adenocarcinoma.
Materials and methods
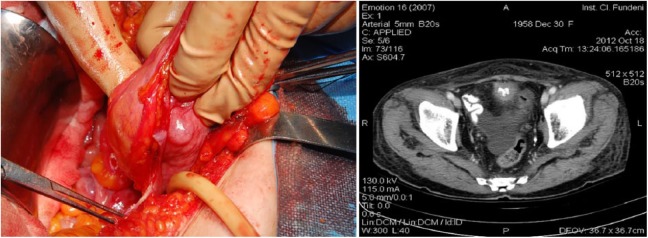
Data from patients who had undergone surgery for urachal adenocarcinoma at the “Fundeni” Clinical Institute, Bucharest, and Hospital “Infanta Sofia,” Madrid, between 2005 and 2015 were analyzed. The inclusion criteria were presence of a tumor located in the bladder dome on contrast-enhanced computed tomography (CT) or magnetic resonance imaging and the absence of cystic or glandular cystitis (Figure 1). We excluded patients with any concomitant neoplastic disease, a history of bladder cancer (urothelial and non-urothelial), or a history of colonic adenocarcinoma. A total of 16 patients who matched the inclusion and exclusion criteria were included in our study (Table 1).
Figure 1.
Intraoperative aspect and axial computed tomography scan of a Sheldon II urachal adenocarcinoma.
Table 1.
Preoperative data.
| Gender (M:F; ratio) | 9:4 | 2.25:1 |
| Age (median; min:max) | 54 | 51:74 |
| Symptoms (number; percentage) | ||
| Hypogastric pain | 3 | 18.75 |
| LUTS | 6 | 37.5 |
| Hematuria | 6 | 37.5 |
| Abdominal mass | 5 | 31.25 |
| Asymptomatic | 8 | 50 |
| Cystoscopy (number; percentage) | ||
| Anterior wall/dome | 5 | 31.25 |
| No anomalies | 10 | 62.50 |
| Not performed/not available | 1 | 6.25 |
| Imaging (number; percentage) | ||
| CT scan | 11 | 68.76 |
| MRI scan | 2 | 12.50 |
| Bladder ultrasound | 13 | 81.25 |
| IVP | 1 | 6.25 |
LUTS: lower urinary tract symptoms; CT: computed tomography; MRI: magnetic resonance imaging; IVP: intravenous pyelogram.
Partial cystectomy and pelvic LND extended to the aortic bifurcation was performed in 11 (68.76%) patients, while radical cystectomy with ileal conduit urinary diversion, and extended LND was performed in 5 (31.25%) patients not amendable to a limited resection. En bloc umbilical resection was performed in seven cases (43.75%; three partial cystectomy and four radical cystectomies). We did not record any macroscopic umbilical involvement, even in cases with large tumors. No major perioperative complications were recorded. Pelvic lymphoceles occurred in two patients and resolved completely after conservative treatment.
Follow-up periods ranged from 4 months to 7.6 years. Clinical and ultrasound evaluations were performed every 3 months for the first 2 years and contrast-enhanced CT scans every 6 months for the first 5 years and yearly thereafter. Nonsystematic cystoscopy was performed in patients with abnormal ultrasound or CT results.
Although several grading systems have been proposed for primary bladder adenocarcinoma, they have not been uniformly adopted. We opted for the Sheldon classification, as it provides appropriate disease staging and is the most commonly utilized.4
Results
Postoperative pathological results showed that 7 (43.75%) patients had localized tumors, 6 (37.5%) patients had locally advanced Sheldon III disease, and 3 patients had distant metastasis (2 in the liver, 1 in the lungs) at the time of surgery. Lymph node (LN) involvement was present in one patient with locally advanced tumor and in two with distant metastasis. None of the patients had positive margins. Supplementary perioperative and postoperative data can be found in Table 2.
Table 2.
Perioperative and postoperative data.
| Type of procedure (number; percentage) | ||
| Partial cystectomy | 11 | 68.76 |
| Total cystectomy | 5 | 31.25 |
| Omphalectomy | 7 | 43.75 |
| Tumor size, cm; (mean; IQR) | 4.1 (1.8–11.4) | |
| Sheldon tumor stage (number; percentage) | ||
| I (limited to urachal mucosa) | 5 | 31.25 |
| II (limited to the uracha) | 2 | 12.5 |
| III (locally advanced) | 6 | 37.5 |
| IIIA (bladder invasion) | 4 | 25 |
| IIIB (perivesical invasion) | 2 | 12.5 |
| IIIC (peritoneum involvement) | 0 | 0 |
| IIID (other) | 0 | 0 |
| IV (distant metastasis) | 3 | 18.75 |
| Lymph node involvement (number; percentage) | 3 | 18.75 |
| Histological type (number; percentage) | ||
| Pure adenocarcinoma | 12 | 75 |
| Adenocarcinoma and signet ring cells | 1 | 6.25 |
| Adenocarcinoma and mucinous cells | 2 | 12.5 |
| Mixed histology | 1 | 6.25 |
| Local recurrence (number; percentage) | ||
| Pelvic lymph nodes | 4 | 25 |
| Peritoneum | 2 | 12.5 |
| Umbilicus | 0 | 0 |
| Absent | 10 | 62.5 |
IQR: interquartile range.
The mean follow-up period was 2.5 years, ranging from 4 months to 7.6 years. A total of 3 patients (18.75%) were lost to follow-up, the first after 6 months, and the second and third after 3.5 years and 4 years, respectively. None had any documented signs of local or systemic recurrence and were cancer free at the time of the last evaluation.
Local recurrences were recorded in the pelvic LNs in 4 patients (25%) and the vesical peritoneum in 2 (12.5%). Mean time to local recurrence after surgery was 25 (6–36) months. Systemic progression was recorded in 2 patients (12.5%): one LN-positive patient developed bone metastasis at 14 months and one developed liver metastasis at 9 months. There were no cases of umbilical recurrence.
The median survival for Sheldon I patients was 45 months. The two Sheldon II patients had similar median survival to locally advanced cases at 30.6 months; however, both patients are currently receiving chemotherapy for local recurrence and one patient developed systemic progression at 6 months after surgery. Sheldon IIIA and IIIB patients had median survival rates of 30.5 and 33 months, respectively. Patients with LN involvement or metastasis had the lowest survival rates at 22.37 and 18.37 months, respectively.
Patients in Sheldon IV and those developing systemic progression had a poor prognosis with a 2-year cancer specific survival (CSS) of 0%. Sheldon I patients had favorable prognosis with a 100% CSS at 5 years, while Sheldon II and III patients had a poorer prognosis with a 2-year CSS of 50%.
The mean progression-free survival after salvage chemotherapy was 3.7 (1.5–5) years at the time of local recurrence detection.
Patients with LN involvement and those in Sheldon stages IIIB and IV received adjuvant chemotherapy based on 5-fluorouracil in association with doxorubicin, etoposide, cisplatin, and gemcitabine. In patients with either local recurrence or distant metastasis, cisplatin- or 5-fluorouracil-based salvage chemotherapy was administered (Table 3).
Table 3.
Chemotherapy data.
| Patient ID | Sheldon staging | LNI | Local recurrence | Systemic recurrence | Type of PCT | Chemotherapy regime | Response | Status at last follow-up |
|---|---|---|---|---|---|---|---|---|
| 4 | I | No | None | None | None | None | Stable | Lost to follow-up, disease free (6 months) |
| 5 | I | No | None | None | None | None | Stable | Lost to follow-up, disease free (49 months) |
| 6 | I | No | None | None | None | None | Stable | Alive, disease free (92 months) |
| 7 | I | No | None | None | None | None | Stable | Lost to follow-up, disease free (45 months) |
| 8 | I | No | None | None | None | None | Stable | Alive, disease free (9 months) |
| 9 | II | No | Pelvic lymph nodes | Lung | Salvage | Doxorubicin, cisplatin, and mitomycin C | Partial response (lung MTS at 9 months) | Deceased cancer related (19 months) |
| 10 | II | No | Pelvic lymph nodes | None | Salvage | Docetaxel and cisplatin | Partial response | Deceased cancer related (42 months) |
| 1 | IIIA | No | None | None | None | None | Stable | Alive, disease free (34 months) |
| 2 | IIIA | No | None | None | None | None | Stable | Alive, disease free (4 months) |
| 3 | IIIA | No | None | None | None | None | Stable | Alive, disease free (33 months) |
| 11 | IIIA | No | Pelvic lymph nodes | None | Adjuvant | 5-flourouracil, cisplatin, and gemcitabine | Partial response | Deceased cancer related (28 months) |
| 12 | IIIB | Yes | None | None | Adjuvant | 5-flourouracil, cisplatin, and gemcitabine | Partial response | Deceased cancer related (29 months) |
| 13 | IIIB | No | Vesical peritoneum | Bone | Salvage | Paclitaxel and cisplatin | Partial response (bone MTS at 14 months) | Deceased cancer related (37 months) |
| 14 | IVB (liver MTS + LNI) | Yes | None | None | Adjuvant | 5-fluorouracil, doxorubicin, and mitomycin C | Partial response | Deceased cancer related (22 months) |
| 15 | IVB (liver MTS + LNI) | Yes | Vesical peritoneum | None | Salvage | 5-fluorouracil, doxorubicin, and cisplatin | Partial response | Deceased cancer related (16 months) |
| Adjuvant | 5-fluorouracil, doxorubicin, and etoposide | Partial response | ||||||
| 16 | IVB (lung MTS) | No | Pelvic lymph nodes | None | Salvage | 5-fluorouracil, mitomycin c, and mitoxantrone | Partial response | Deceased cancer related (17 months) |
| Adjuvant | 5-fluorouracil, doxorubicin, and etoposide | Partial response |
LNI: lymph node metastasis; MTS: metastasis; PCT: pelvic computed tomography.
Patients with systemic disease had poor outcomes, with mean overall survival of 15.4 (12–17.2) months.
Discussion
The small number of reported cases has resulted in a lack of uniformity in the diagnostic criteria of urachal adenocarcinoma. Thus, the diagnosis is based on clinical and histological findings in most cases.
Three major staging systems have been proposed for urachal adenocarcinoma: Sheldon, Mayo, and Ontario. The TNM staging system does not apply because the tumor originates outside the bladder. The Sheldon grading system was the first to be introduced and is currently the most commonly utilized. Although this system has never been formally validated, the lack of randomized trials and the low incidence of this pathology have resulted in the other systems being seldom utilized.
Our results indicate that 37.5% of patients had hematuria at diagnosis, which correlates with previous evidence supporting that hematuria represents the most common symptom in urachal neoplasia.5
In most cases, the presumptive diagnosis of urachal tumor was established by exclusion, after cystoscopy and abdominal and pelvic CT scans. This diagnosis can be presumed in patients with normal findings on cystoscopy and the presence of a calcified mass in the bladder dome on the pelvic CT scan. However, in patients who present with bladder wall invasion (Sheldon >IIIA), the differential diagnosis is very difficult, and most patients are treated as if they had bladder adenocarcinoma with partial or total cystectomy, without omphalectomy. The definitive diagnosis is made at 1 month. Currently, there are no previous studies reporting navel recurrence in cases with urachal carcinoma, in which omphalectomy was not performed during partial/total cystectomy.
Positive surgical margins and tumor grade represent the most important prognostic factors impacting CSS and overall survival. High-grade tumors are most often associated with a poor prognosis; however, our study demonstrated good oncological results in patients with high-grade tumors limited to the urachal ligament or bladder.6 In this regard, all patients included in our cohort had high-grade disease, which may be considered a limitation of our study. The most common histological subtype in our study population was represented by pure adenocarcinoma, followed by mucinous and signet ring cell tumors. Patients with mucinous and signet cell tumors had a poorer prognosis due to their locally advanced or metastatic disease at diagnosis.
At the time of last follow-up (ranging between 4 and 92 months), 8 patients (50%) were disease free. This demonstrated that although patients with urachal adenocarcinoma frequently present with locally advanced tumors invading the perivesical and periurachal tissue, they may be candidates for curative resection if adequate partial cystectomy with en bloc resection of the urachal ligament and extended pelvic LND is performed. In contrast, tumors that extend to the peritoneal cavity or invade the abdominal organs are rarely amendable to radical treatment, even if aggressive surgery and adjuvant chemotherapy is performed.
Prognosis was modest in patients with locally advanced tumors, lymph node involvement (LNI), and metastasis.These patients had local/systemic progression irrespective of adjuvant/salvage therapy and all eventually succumbed to cancer-related death.
Chemotherapy is commonly recommended in high-risk patients and those with metastatic disease or positive LNs. Chemotherapy regimens are based on 5-fluorouracil, often in association with cisplatin, doxorubicin, etoposide, and mitomycin and have a limited impact on overall survival.7,8 There are two currently ongoing studies (one of which is based on 5-fluorouracil, leucovorin, gemcitabine, and cisplatin), which showed a partial response in most patients.9
Non-standardized 5-fluorouracil-based chemotherapy regimens were used in our study with modest results. The mean progression-free survival in patients who underwent adjuvant treatment was 9 (range: 4–14) months, and 4 months in patients receiving salvage chemotherapy for local/systemic progression. There were no cases of complete response after chemotherapy in our study population.
Henly et al.10 reported no significant difference between partial cystectomy and radical cystectomy in terms of oncological results in a study of 30 patients.
Ashley et al. evaluated 130 patients with urachal tumors. The authors reported macroscopic hematuria and age >55 years as significant negative prognostic factors. A total of 60 patients underwent surgical resection with no significant difference between partial and total cystectomy in terms of overall survival. An overall 5-year CSS of 49% was found in the surgical treatment cohort. The authors noted that urachal resection and omphalectomy were predictors of survival in a univariate analysis and subsequently recommended both procedures as standard of care. However, the study group included a large number of patients with advanced Sheldon stages (~85) with massive bladder involvement in 82% of patients.11
Umbilicus resection en bloc with the urachal ligament in the management of locally advanced urachal tumors was introduced by Sheldon et al.5 after finding navel invasion in 7% of autopsies performed on patients who died because of urachal tumors. However, the authors noted that omphalectomy may be omitted in patients with localized lesions.
One important aspect of our study was patient’s perception of their body image; while a postoperative scar may be acceptable in most cases, navel excision was considered unaesthetic by most of our patients. Several patients even referred to the postoperative results as a bodily deformity. Therefore, we aimed to preserve the umbilicus when it was technically feasible, as there are no studies reporting abdominal wall recurrence after partial cystectomy and omphalectomy. Although it has minor functional importance, the umbilicus is a major landmark in the aesthetical integrity of the anterior abdominal wall. The navel is the only physiological scar present on the human body, and its loss secondary to surgery may alter the patient’s self-image with severe psychological implications.12
Data in the literature reports a tendency for local recurrence in the pelvic area and progression to metastatic disease in the first 2 years.13 There were no umbilicus recurrences during the entire follow-up period. Local recurrence (pelvic LNs/peritoneum) occurred in five patients and required salvage chemotherapy, with poor outcomes. The utility of navel resection is questionable, with no therapeutic benefit, since there were no recorded cases of direct invasion or recurrence limited to the umbilicus, even in large tumors.
The main limitation of this study is the small number of included patients; consequently, we could not perform a multivariate analysis in order to evaluate additional risk factors. This drawback is present in most studies that focus on rare diseases. Larger and more robust studies are required; however, they may only be possible in a large multi-centric setting. A second limitation is the retrospective design of the study. A bias in the histological analysis must be taken into account, as the samples were evaluated by a single pathologist in each of the two centers.
Conclusion
Urachal adenocarcinoma is a rare condition with an often poor prognosis due to locally advanced or metastatic disease at the time of diagnosis, since most patients are asymptomatic. The most common symptoms are hematuria and the presence of a palpable hypogastric mass. Surgical treatment represents the gold standard, while adjuvant chemotherapy has a limited impact on overall survival. The utility of navel resection is questionable due to the rarity of direct invasion or local recurrence.
Footnotes
Authors’ contribution: C.P. wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Due to the retrospective nature of this study, ethical approval was not sought. This study was completed in accordance with the Helsinki Declaration as revised in 2013.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article, upon admission into each hospital.
ORCID iD: Cristian Pavelescu  https://orcid.org/0000-0002-2331-7604
https://orcid.org/0000-0002-2331-7604
References
- 1. Wright JL, Porter MP, Li CI, et al. Differences in survival among patients with urachal and nonurachal adenocarcinomas of the bladder. Cancer 2006; 107(4): 721–728. [DOI] [PubMed] [Google Scholar]
- 2. Ploeg M, Aben KK, Hulsbergen-van de Kaa CA, et al. Clinical epidemiology of nonurothelial bladder cancer: analysis of the Netherlands Cancer Registry. J Urol 2010; 183(3): 915–920. [DOI] [PubMed] [Google Scholar]
- 3. Soloway MS. Bladder cancer: lack of progress in bladder cancer—what are the obstacles? Nat Rev Urol 2013; 10(1): 5–6. [DOI] [PubMed] [Google Scholar]
- 4. Burnett AL, Epstein JI, Marshall FF. adenocarcinoma of urinary bladder: classification and management. Urology 1991; 37(4): 315–321. [DOI] [PubMed] [Google Scholar]
- 5. Sheldon CA, Clayman RV, Gonzalez R, et al. Malignant urachal lesions. J Urol 1984; 131(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 6. Herr HW, Bochner BH, Sharp D, et al. Urachal carcinoma: contemporary surgical outcomes. J Urol 2007; 178(1): 74–78; discussion 78. [DOI] [PubMed] [Google Scholar]
- 7. Wilson TG, Pritchett TR, Lieskovsky G, et al. Primary adenocarcinoma of bladder. Urology 1991; 38(3): 223–226. [DOI] [PubMed] [Google Scholar]
- 8. Siefker-Radtke AO, Gee J, Shen Y, et al. Multimodality management of urachal carcinoma: the M. D. Anderson Cancer Center experience. J Urol 2003; 169(4): 1295–1298. [DOI] [PubMed] [Google Scholar]
- 9. Siefker-Radtke A. Urachal carcinoma: surgical and chemotherapeutic options. Expert Rev Anticancer Ther 2006; 6(12): 1715–1721. [DOI] [PubMed] [Google Scholar]
- 10. Henly DR, Farrow GM, Zincke H. Urachal cancer: role of conservative surgery. Urology 1993; 42(6): 635–639. [DOI] [PubMed] [Google Scholar]
- 11. Ashley RA, Inman BA, Sebo TJ, et al. Urachal carcinoma: clinicopathologic features and long-term outcomes of an aggressive malignancy. Cancer 2006; 107(4): 712–720. [DOI] [PubMed] [Google Scholar]
- 12. Zietman A, Skinner E. Quality of life after radical treatment for invasive bladder cancer. Semin Radiat Oncol 2005; 15(1): 55–59. [DOI] [PubMed] [Google Scholar]
- 13. Grignon DJ, Ro JY, Ayala AG, et al. Primary adenocarcinoma of the urinary bladder: a clinicopathologic analysis of 72 cases. Cancer 1991; 67(8): 2165–2172. [DOI] [PubMed] [Google Scholar]