Abstract
The case of a male patient is reported, who presented with renal carcinoma and tumor thrombus in the inferior vena cava (IVC) extending from the right atrium (RA) to the bifurcation of IVC, common and external right iliac vein thrombosis, common and deep right femoral vein thrombosis, right popliteal vein thrombosis, with pulmonary and hepatic metastasis, treated with sorafenib. Renal cell carcinoma (RCC), the most common form of kidney cancer, occurs in 90% of cases and is nearly twice as common in men as in women. The diagnosis of RCC is accompanied by intravascular tumor thrombus in 10% of cases, and further extension of the tumor reaching RA is detected in approximately 1% of all patients. Therapy for advanced renal cell cancer has evolved considerably in the past decade, with new agents greeted like “buried treasure.” Before 2005, the widely used systemic agents were cytokine interferon alfa and interleukin-2, which yielded modest efficacy and substantial toxicity. Tyrosine kinase inhibitors (TKIs) increase progression-free survival and/or overall survival as both first-line and second-line treatments for metastatic RCC. Sorafenib is an oral multikinase inhibitor with activity against Raf-1 serine/threonine kinase, B-Raf, vascular endothelial growth factor receptor-2 (VEGFR-2), platelet-derived growth factor receptor (PDGFR), FMS-like tyrosine kinase 3 (FLT-3), and c-KIT.
Keywords: renal cell carcinoma, inferior vena cava, right atrium, thrombus
Renal cell carcinoma (RCC) represents the most common form of kidney cancer, with a peak incidence in the sixth and seventh decade of life and a 1.5:1 male predominance. Risk factors include smoking, obesity, and hypertension, as well as acquired cystic kidney disease associated with end-stage renal disease (Chow, Gridley, Fraumeni, & Jarvholm, 2000).
The classic presentation triad of flank pain, hematuria, and palpable abdominal mass is rare and correlates with aggressive histology and advanced disease. Widespread use of sophisticated imaging modalities has resulted in an increase in the incidental detection of kidney tumors and now more than 70% of RCCs are detected incidentally by noninvasive imaging used to investigate various nonspecific symptoms (Chen & Uzzo, 2011).
Venous migration and tumor thrombus formation are unique aspects of RCC and along with the presence of metastases, they are a significant adverse prognostic factor (Reese, Whitson, & Meng, 2013). Intravascular tumor growth along the renal vein into the inferior vena cava (IVC) occurs in up to 10% of all patients with RCC (Quencer, Friedman, Sheth, & Oklu, 2017), and further extension of the tumor reaching the right atrium (RA) is detected in approximately 1% of all patients (Schimmer, Hillig, Riedmiller, & Elert, 2004).
Current guidelines (the European Association of Urology [EAU] Guidelines on Renal Cell Cancer, 2016) recommend an aggressive surgical approach with excision of the kidney tumor and caval thrombus in patients with nonmetastatic disease, irrespective of the extent of tumor thrombus at presentation, since a higher level of thrombus was not found to be correlated with increased tumor dissemination to lymph nodes, perinephric fat, or distant metastasis. For most patients with metastatic disease, the proposed treatment consists of a palliative cytoreductive nephrectomy, along with systemic treatments (Ljungberg et al., 2016).
Case Report
A 70-year-old male, long-time smoker with no prior medical history, presented to the Department of Internal Medicine with progressive pain and tumefaction of the right leg over the past 2 months. The patient also reported a considerable unintentional weight loss of 15 kg (33 pounds) in 3 months, anorexia, and macroscopic hematuria.
Clinical examination revealed bilateral but asymmetric lower limb edema, with cyanosis and a positive Homan’s sign in the right leg, hepatomegaly, and extensive bilateral venous collaterals of the abdominal wall (Figure 1).
Figure 1.
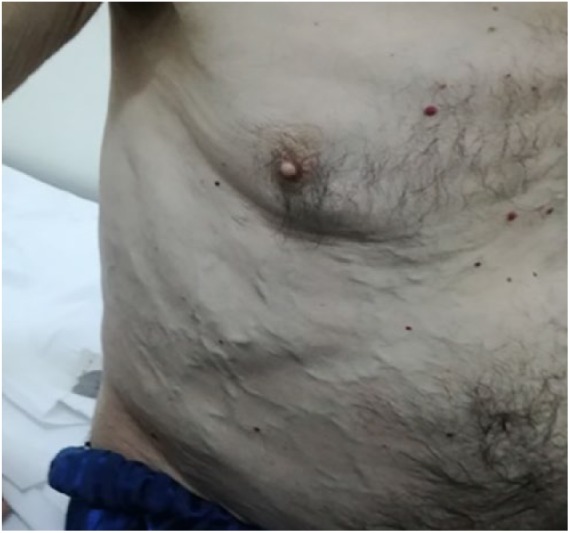
Collateral veins on abdomen, determined by obstruction of the inferior vena cava.
ECG upon admission was in sinus rhythm, with neither conduction disturbances nor signs of ischemia.
Laboratory tests revealed normocytic anemia, thrombocytosis, elevated D-dimer levels, inflammatory syndrome, high alkaline phosphatase, elevated gamma-glutamyl transferase (GGT) and lactate dehydrogenase (LDH), and glomerular filtration rate (GFR) 60 ml/min/1.73 m2.
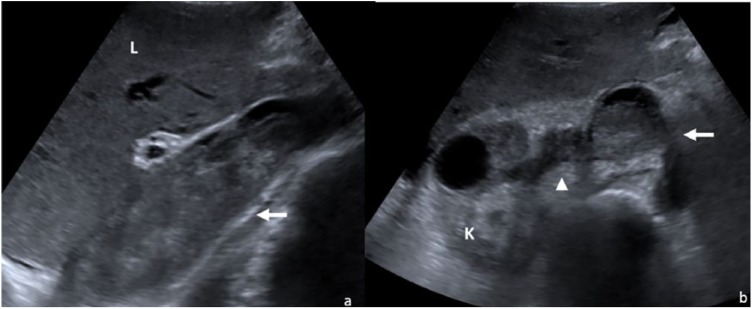
Duplex ultrasonography revealed massive venous thrombosis extending from the right popliteal fossa (to the common and deep femoral veins, common and external right iliac veins) to the IVC, inducing a complete caval obstruction and expansion of the IVC. Abdominal ultrasonography (Figure 2) identified a complicated right renal cyst, hepatomegaly with microgranular structure and irregular surface, perihepatic and perisplenic ascites, splenomegaly, biliary calculus, and prostatic hypertrophy.
Figure 2.
Abdominal ultrasonography—inferior vena cava thrombosis. (a) Longitudinal section: thrombus in the inferior vena cava (arrow); (b) axial section: thrombus in the inferior vena cava (arrow); thrombus in the renal vein (arrow head).
Transthoracic echocardiography showed concentric left ventricular hypertrophy with preserved ejection fraction and extension of the IVC thrombus to the RA (Figure 3).
Figure 3.
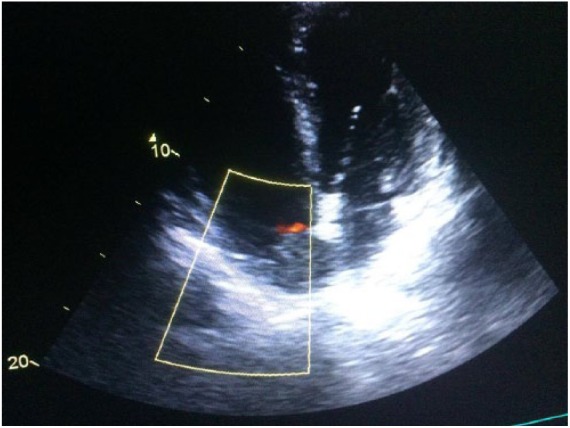
Apical four-chamber view by transthoracic echocardiography showing the large mobile mass (white arrow) into right atrium—the thrombus in the right atrium.
The suspicion of a renal carcinoma with extensive thrombosis was raised.
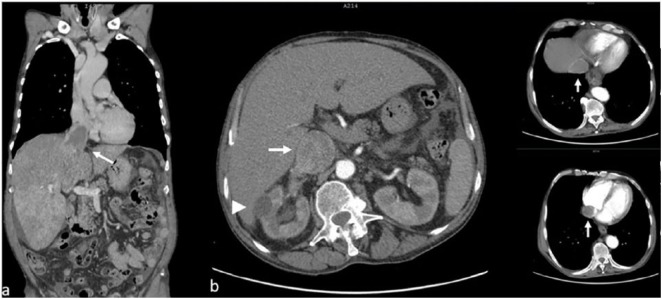
The thoraco-abdominopelvic computed tomography (CT) scan revealed a right kidney mass measuring 30/22 mm with significant uptake of contrast suggestive of RCC, with tumor thrombus extending into the right renal vein, the infrarenal IVC, the common, internal, and external iliac veins bilaterally, as well as cephalic extension of the thrombosis to the suprarenal IVC and RA (Figure 4). Multiple pulmonary and liver metastases were identified; there was minimal pericardial and right pleural collection, as well as ascites.
Figure 4.
Thoraco-abdominal CT. (a) Frontal section: thrombus in the inferior vena cava extending to the right atrium (arrow), multiple pulmonary and liver metastases, ascites. (b) Axial section: small cystic renal tumor (arrow head), thrombus in the inferior vena cava and right atrium (arrows).
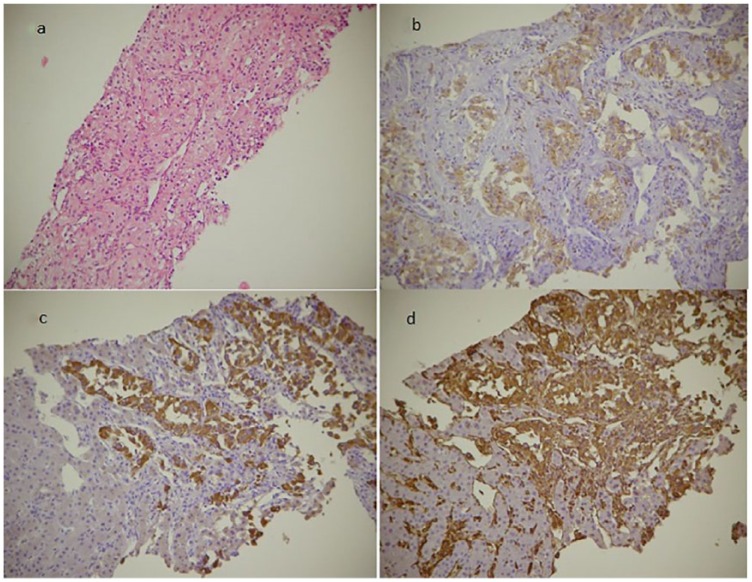
Contrast-enhanced ultrasound of the focal liver lesions and percutaneous hepatic biopsy were performed. Histopathologic examination of the lesions confirmed the diagnosis of clear cell RCC (Figure 5).
Figure 5.
Histological images from liver biopsy. (a) Tumor proliferation formed by nests and trabeculae of cubic cells with abundant pale eosinophilic or foamy cytoplasm with clear vacuoles and small nuclei with minimal atypia, arranged in a fine connective vascular stroma (Hematoxylin and eosin 10×). Tumor cells in immunohistochemical staining are positive for CD10 (b), CKAE1/AE3 (c), and vimentin (d; 20×).
The patient was hospitalized and anticoagulation with unfractionated heparin/low-molecular-weight heparin was started.
The patient was further referred to the oncologist. Radical nephrectomy with IVC thrombectomy was proposed, but the patient refused the surgical intervention. Systemic therapy was thus employed. After allocating the patient to the Memorial Sloan Kettering Cancer Center (MSKCC) intermediate prognostic group, treatment with sunitinib was intended. However, after a thorough risk/benefit assessment, sorafenib was preferred, mainly due to its lower risk for thrombotic events and better toxicity profile related to patient’s comorbidities. Long-term anticoagulation was pursued with dalteparin.
The 8-month evaluation of the patient showed stationary disease in terms of clinical and imaging findings.
Discussion
The classic diagnosis of RCC is established based on the classic triad: hematuria, flank pain, and a palpable mass in the flank only in 10% of cases (Doshi, Saab, & Singh, 2007) This patient did not present with the classic triad but with the clinical picture of deep venous thrombosis in the right lower limb, complicated by IVC syndrome. Imaging detected extension of thrombosis from the IVC to the RA. This was reported in only 1% of RCC cases (Schimmer et al., 2004). The differential diagnosis of a mass in the RA should include, in the first place, the primary or secondary tumor, thrombus, and tricuspid valve vegetation. An important characteristic of RCC is the tendency to renal vein invasion, with extension to IVC and sometimes to RA, like in the case of this patient (Kumar, Abbas, & Aster, 2015).
This is why in the presence of a thrombus in RA, the diagnosis of RCC should always be considered. A similar presentation of RCC with intracardiac thrombosis was described in a series of case reports (Schimmer et al., 2004; Yoon, Jeon, & Yang, 2013; Posacioglu et al., 2008). At the time of diagnosis, almost 25% of patients have distant metastases, the common sites of metastases being the lungs, adrenal glands, intestine, brain, and the majority of intra-abdominal organs (Sountoulides, Metaxa, & Cindolo, 2011). This patient was diagnosed in an advanced stage with liver and lung metastases, IVC thrombosis extending to RA, and right ileo-femoro-popliteal thrombosis. The most frequent histological form of RCC is clear cell carcinoma (70%–80%), followed by the papillary type (10%; Sountoulides et al., 2011). This patient underwent biopsy from a liver metastasis, and diagnosis was confirmed by immunohistochemistry: clear cell RCC, the form of RCC known to have the most severe prognosis.
Surgical treatment of RCC with thrombosis extending to RA is a challenge and there still is controversy regarding the safety of this strategy. Radical surgery of RCC would include extracorporeal circulation with cardiopulmonary bypass (CPB) and deep hypothermic circulatory arrest (DHCA), with removal of the tumor and intracardiac thrombosis (Schimmer et al., 2004). This patient refused surgery, which would have been risky given the presence of liver and lung metastases.
The prognosis of patients with RCC in the metastatic stage, like this patient, is unfavorable, and survival is limited to months even with the availability of immunotherapy (Sountoulides et al., 2011).
In patients with metastatic disease at presentation, cytoreductive nephrectomy and tumor thrombectomy may be safely performed with simultaneous metastasectomy if possible. However, in patients with significant risk factors for postoperative complications and mortality, and especially in those with metastatic disease, consultation with medical oncology and frontline targeted therapy may be considered (Psutka & Leibovich, 2015). Two randomized trials, one in the United States and one in Europe, have reported that nephrectomy followed by systemic therapy is associated with a superior overall survival rate, as compared with systemic therapy alone. Important considerations include the patient’s overall health and functional status, the extent of local disease, and the extent of metastatic disease (Psutka & Leibovich, 2015). Patients with vena cava involvement require surgery of much greater magnitude, often resulting in a prolonged postoperative stay in the intensive care unit. If these patients have coexisting metastatic disease, like this patient, long-term survival is often poor and does not justify the extreme nature of the surgery (Michaelson et al., 2008).
If left untreated, RCC with venous tumor thrombus (VTT) is associated with a median survival of 5 months, with increased risk of cancer-specific mortality in the setting of pT3b and pT3c disease, as well as the presence of concurrent metastases (Psutka & Leibovich, 2015). Most data on overall treatment outcomes are from studies involving patients with clear cell RCC, which makes up approximately 70% of RCC (Choueiri & Motzer, 2017). Clear cell carcinomas are associated with a worse prognosis than the other histological subtypes of renal carcinomas are (Michaelson et al., 2008).
Therapeutic agents that target tumor angiogenesis also affect the endothelium of non-tumor vessels and induce venous and arterial thromboembolic events. Preventing thrombosis in patients who receive these agents is complicated because the drugs also may cause bleeding; therefore, antiplatelet and anticoagulant therapy could be harmful. Until more is known about the effects of vascular endothelial growth factor (VEGF) receptor inhibitors on the vessel walls, patients’ risk factors for thrombosis should be carefully assessed and perhaps the doses of these agents should be adjusted (Choueiri, Schutz, Je, Rosenberg, & Bellmunt, 2010). Data showing increased risk of thromboembolic events associated with the use of tyrosine kinase inhibitors (TKIs) in mRCC mostly consist of retrospective and nonsignificant results; however, as there is a clear rationale for this, a more comprehensive risk/benefit assessment should be done.
It appears that patients aged over 65 years benefit as much from targeted therapies as younger patients and do not experience more frequent or severe toxicity. In the field of oncology as a whole, elderly patients are at risk of receiving suboptimal therapy (Bellmunt, Négrier, Escudier, Awada, & Aapro, 2009). In the Treatments Against RA and Effect on FDG PET-CT (TARGET) trial of sorafenib versus placebo, the effect of sorafenib treatment on health-related quality of life, symptomatology, and time to deterioration of health status was similar in the over-65-year-old and under-65-year-old groups (Bellmunt et al., 2009). The safety and efficacy of sorafenib was evaluated in a trial as part of a North American expanded access program. Response rates seen in this elderly group were said to be comparable with those seen in younger patients. Two toxicities showed greater frequency with age, rash/desquamation and fatigue (Bellmunt et al., 2009).
Clinical trials of sunitinib, sorafenib, and temsirolimus in patients with advanced RCC show how promising treatments can emerge from an understanding of the molecular genetics and biology of tumors. It will be important to evaluate how these drugs work through the analysis of both responsive and resistant tumors. Elucidating how these drugs inhibit tumor growth is paramount for the development of the next generation of drugs and for their rational combination (Brugarolas, 2007).
The immune responsiveness of RCC provides an opportunity for the development and optimization of vaccines and other immune therapies. Preservation of as much renal function as possible and reduced rates of complications are two goals of new minimally invasive approaches to RCC; other goals are to identify early markers of disease, prognosis, and responsiveness to therapy (Ljungberg et al., 2016).
Particularities of the Case
Given the particularities of the case, several aspects can be considered. The diagnosis of renal carcinoma in the presence of one of its complications, deep venous thrombosis in the lower limb, is one. Another element is the discordance between the reduced size of the renal tumor and tumor extension to the venous system, as well as distant metastases. A third aspect is the particularity of thrombosis extension both in ascending direction up to the RA and in descending direction up to the femoral vein.
Footnotes
Ethical Statements: Informed consent was obtained from the patient included in this case report.
Authors’ Contributions: All the authors contributed to the conception and design of the article. All of the authors approved the final version submitted for publication.
The article has not been submitted/published elsewhere in the same form, in English or in any other language.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Angela Cozma  https://orcid.org/0000-0002-3989-2291
https://orcid.org/0000-0002-3989-2291
References
- Bellmunt J., Négrier S., Escudier B., Awada A., Aapro M. (2009). The medical treatment of metastatic renal cell cancer in the elderly: Position paper of a SIOG taskforce. Critical Reviews in Oncology/Hematology, 69(1), 64–72. doi: 10.1016/j.critrevonc.2008.08.002 [DOI] [PubMed] [Google Scholar]
- Brugarolas J. (2007). Renal-cell carcinoma—Molecular pathways and therapies. New England Journal of Medicine, 356(2), 185–187. doi: 10.1056/nejme068263 [DOI] [PubMed] [Google Scholar]
- Chen D. Y. T., Uzzo R. G. (2011). Evaluation and management of the renal mass. Medical Clinics of North America, 95(1), 179–189. doi: 10.1016/j.mcna.2010.08.021 [DOI] [PubMed] [Google Scholar]
- Choueiri T. K., Motzer R. J. (2017). Systemic therapy for metastatic renal-cell carcinoma. New England Journal of Medicine, 376(4), 354–366. doi: 10.1056/nejmra1601333 [DOI] [PubMed] [Google Scholar]
- Choueiri T. K., Schutz F. A. B., Je Y., Rosenberg J. E., Bellmunt J. (2010). Risk of arterial thromboembolic events with Sunitinib and Sorafenib: A systematic review and meta-analysis of clinical trials. Journal of Clinical Oncology, 28(13), 2280–2285. doi: 10.1200/jco.2009.27.2757 [DOI] [PubMed] [Google Scholar]
- Chow W.-H., Gridley G., Fraumeni J. F., Järvholm B. (2000). Obesity, hypertension, and the risk of kidney cancer in men. New England Journal of Medicine, 343(18), 1305–1311. doi: 10.1056/nejm200011023431804 [DOI] [PubMed] [Google Scholar]
- Doshi D., Saab M., Singh N. (2007). Atypical presentation of renal cell carcinoma: A case report. Journal of Medical Case Reports, 1, 26. doi: 10.1186/1752-1947-1-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Abbas A. K., Aster J. C. (2015). Robbins & Cotran pathologic basis of disease (9th ed., p. 955). Amsterdam: Elsevier. [Google Scholar]
- Ljungberg B., Bensalah K., Bex A., Canfield S., Debestani S., Giles R., … Volpe A. (2016). Guidelines on renal cell carcinoma. Retrieved from https://uroweb.org/wp-content/uploads/EAU-Guidelines-Renal-Cell-Carcinoma-2016.pdf
- Michaelson M. D., Iliopoulos O., McDermott D. F., McGovern F. J., Harisinghani M. G., Oliva E. (2008). Case 17-2008. New England Journal of Medicine, 358(22), 2389–2396. doi: 10.1056/nejmcpc0802449 [DOI] [PubMed] [Google Scholar]
- Posacioglu H., Ayik M. F., Zeytunlu M., Amanvermez D., Engin C., Apaydin A. Z. (2008). Management of renal cell carcinoma with intracardiac extension. Journal of Cardiac Surgery, 23(6), 754–758. doi: 10.1111/j.1540-8191.2008.00664.x [DOI] [PubMed] [Google Scholar]
- Psutka S. P., Leibovich B. C. (2015). Management of inferior vena cava tumor thrombus in locally advanced renal cell carcinoma. Therapeutic Advances in Urology, 7(4), 216–229. doi: 10.1177/1756287215576443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quencer K. B., Friedman T., Sheth R., Oklu R. (2017). Tumor thrombus: Incidence, imaging, prognosis and treatment. Cardiovascular Diagnosis and Therapy, 7(S3), S165–S177. doi: 10.21037/cdt.2017.09.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese A. C., Whitson J. M., Meng M. V. (2013). Natural history of untreated renal cell carcinoma with venous tumor thrombus. Urologic Oncology: Seminars and Original Investigations, 31(7), 1305–1309. doi: 10.1016/j.urolonc.2011.12.006 [DOI] [PubMed] [Google Scholar]
- Schimmer C., Hillig F., Riedmiller H., Elert O. (2004). Surgical treatment of renal cell carcinoma with intravascular extension. Interactive Cardiovascular and Thoracic Surgery, 3(2), 395–397. doi: 10.1016/j.icvts.2004.02.014 [DOI] [PubMed] [Google Scholar]
- Sountoulides P., Metaxa L., Cindolo L. (2011). Atypical presentations and rare metastatic sites of renal cell carcinoma: A review of case reports. Journal of Medical Case Reports, 5(1), 429. doi: 10.1186/1752-1947-5-429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S.-J., Jeon D. W., Yang J. Y. (2013). A huge thumb in the heart. Heart Asia, 5(1), 228. doi: 10.1136/heartasia-2013-010443 [DOI] [PMC free article] [PubMed] [Google Scholar]