Abstract
Glioblastoma (GBM), the most common and aggressive brain tumor in adults, shows resistance to treatment, particularly radiotherapy. One method for effective treatment is using a group of radiosensitizers that make tumor cells responsive to radiotherapy. A class of molecules whose expression is affected by radiotherapy is the microRNAs (miRNAs) that present promising regulators of the radioresponse. Eighteen miRNAs (miR-26a, -124, -128, -135b, -145, -153, -181a/b, -203, -21, -210, -212, -221/222, -223, -224, -320, and -590), involved in the pathogenesis of GBM and its radioresponsive state, were reviewed to identify their role in GBM and their potential as radiosensitizing agents. MicroRNAs-26a, -124, -128, -145, -153, -181a/b, -203, -221/222, -223, -224, -320, and -590 promoted GBM radiosensitivity, while microRNAs-135b, -21, -210, and -212 encouraged radioresistance. Ectopic overexpression of the radiosensitivity promoting miRNAs and knockdown of the radioresistant miRNAs represent a prospective radiotherapy enhancement opportunity. This offers a glimmer of hope for a group of the most unfortunate patients known to medicine.
Keywords: Glioblastoma, microRNAs, radioresistance, radiosensitivity, review
Introduction
Glioblastoma (GBM) is the most common and lethal brain tumor in adults.1,2 It is a grade IV tumor characterized by a heterogeneous population of cells that are genetically unstable, highly infiltrative, angiogenic, and resistant to chemotherapy and radiotherapy.3 Exploring the mechanisms underlying tumor resistance and recurrence is warranted to design future molecularly targeted therapies.2
The current first-line therapy is surgical resection, followed by a combination of the chemotherapeutic agent temozolomide (TMZ) and regional fractionated ionizing radiation (IR).4 In addition, personalized therapeutic modalities against molecular deregulated targets that drive tumor growth have been tried in several clinical trials. However, almost all patients with GBM undergo inevitable tumor recurrence.1,3 This could be attributed to the incomplete resection of the infiltrative tumor tissues, as well as the extensive hypoxic nature of GBM tumors that limits the efficiency of chemotherapy and radiotherapy.5,6
Multiple factors can affect resistance to radiotherapy, for example, tumor location, size, microenvironment, and most importantly, genetic influences.7 Some of the major genetic elements regulating tumorogenesis as well as the radioresistance are microRNAs (miRNAs). In the following sections, we will address the role of miRNAs in GBM and the differential miRNA genomic landscape of pre- and posttreated GBM cells for a better understanding of the miRNA-related tumor regression and resistance.
MicroRNAs: Key Players in Glioblastoma
MicroRNAs are small noncoding RNA molecules that regulate gene expression at a posttranscriptional level by either cleaving or repressing the translation of messenger RNA targets via binding to complementary sequence.8 They control various cellular processes including apoptosis, proliferation, cell cycle, invasion, and angiogenesis.9 Several miRNAs have been recently reported to be involved in modulation of GBM development and progression.10 The miRNAs can function as oncogenes or tumor suppressors according to the genes or pathways that they target.11 Emerging evidence demonstrated the potential role of miRNAs in the response of chemotherapy and radiotherapy, a finding that opens new avenues for identifying potentially more effective therapeutic targets in an attempt of improving patient survival.12
Role of MicroRNAs in Modulation of Radiosensitivity in Glioblastoma
Radiotherapy is widely used in cancer treatment and biological studies where the IR damages cancer cells through producing free radicals and intermediate ions that cause single- or double-stranded breaks in the DNA. This usually triggers activation of the DNA damage response, which is one of the main reasons of radioresistance.13
DNA, however, is not the only component affected by radiation, where growing evidence suggests that radiation can disturb the expression of miRNAs. Since miRNAs regulate DNA repair,14 cellular homeostasis,15 and response to stress,16 they can modulate the radiosensitivity of GBM tumor cells. In that context, miRNAs could have the potential to be either radiosensitizers or radioprotectors.17
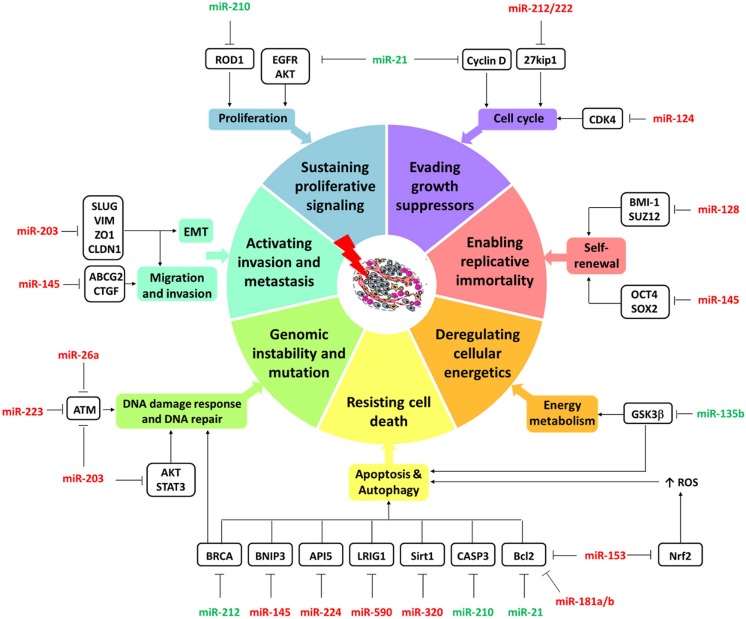
Multiple miRNAs deregulated in GBM were found to affect the tumor response to radiotherapy. The mechanisms of those miRNAs in GBM are displayed in Figure 1 and reviewed below together with their association with IR.
Figure 1.
Mechanisms of radioresistance in glioblastoma (GBM). The figure shows deregulated microRNAs following irradiation of GBM. They are involved in hallmarks of cancer (colored donut). Micro-RNAs-26a, -124, -128, -145, -153, -181a/b, -203, -221/222, -223, -224, -320, and -590 (red) promoted GBM radiosensitivity, while microRNAs-135b, -21, -210 and -212 (green) encouraged radioresistance via targeting various cancer-related genes (black box).
MicroRNAs Involved in Radiosensitivity Enhancement in GBM
MicroRNA-26a
In GBM, miRNA-26a (miR-26a) was frequently amplified at the DNA level in human glioma.18 It was found to promote low expression of the tumor suppressors phosphatase and tensin homolog (PTEN) and retinoblastoma 1.19 Furthermore, Guo et al showed that overexpression of miR-26a can enhance radiosensitivity and reduce the DNA repair ability of cells by targeting ataxia–telangiectasia mutated (ATM) gene with subsequent inhibition of the homologous recombination repair pathway. In contrast, miR-26a knockdown in U87 GBM radiosensitive cells reverses this phenotype.20
MicroRNA-124
MicroRNA-124 (miR-124), a brain-specific miRNA, was identified to be markedly downregulated in human brain glioma. Aberrant expression of miR-124 resulted in cyclin-dependent kinase 4 (CDK4) upregulation, which in turn caused radioresistance and disease relapse. Ectopic expression of miR-124 and knockdown of CDK4 could confer radiosensitivity in glioma cell lines and animal models.7
MicroRNA-128
MicroRNA-128 (miR-128), which is also enriched in brain cells, has previously been observed to be underexpressed in GBM.21,22 It could function as a tumor suppressor in glioma stem cells (GSCs) by negatively regulating tumor cell proliferation and invasion.23 Downregulation of miR-128, frequently encountered in GBM cells, could mediate tumorigenesis, promote cancer stem cell self-renewal, and enhance radiation resistance through targeting the oncogenes Bmi-1 and SUZ12, 2 members of the polycomb repressor complex (PRC).24,25 Ye et al25 reported that low expression of miR-128 in U87 GBM cells following high doses of irradiation enhanced the escape of cells from radiation-induced senescence resulting in radioresistance. However, miR-128 upregulation decreased the expression of PRC genes and rendered GSC more sensitive to radiation.26
MicroRNA-145
Another tumor suppressor, microRNA-145 (miR-145), has been reported to be downregulated in GBM. The recovery of its expression level can induce apoptosis via targeting Bcl2/adenovirus E1b 19-kDa interacting protein 327 and inhibit migration and invasion of GSC via interaction with ATP-binding cassette subfamily G member 228 and connective tissue growth factor.29 In GBM-CD133 (+) cells, delivery of miR-145 using a therapeutic vehicle can inhibit the malignant phenotype and cancer stem cell–like abilities by targeting octamer-binding transcription factor 4 (Oct4) and SRY-box 2 (Sox2). This was found to effectively suppress the expression of drug resistance and antiapoptotic genes and synergistically increase the sensitivity of the cells to radiation both in vivo and in vitro.30
MicroRNA-153
MicroRNA-153 (miR-153), a brain-enriched miRNA, is abnormally downregulated in GBM. This miRNA has the ability to reverse stem cell properties and induce apoptosis via targeting B-cell lymphoma/leukemia-2 (Bcl-2) and myeloid cell leukemia sequence 1 proteins.31 Upregulation of miR-153 suppressed the oxidative stress transcription factor nuclear factor erythroid 2-related factor 2 and increased reactive oxygen species level, with subsequent enhancement of apoptosis, differentiation, and radiosensitivity in GSCs in vitro and increased survival in mice bearing human GSCs.26,32
MicroRNA-181a/b
MicroRNA-181a/b (miR-181a/b), members of the miR-181 family, was one of the downregulated miRNAs in U87GBM cells.33 In response to radiation treatment, the radiation-responsive miR-181a was significantly overexpressed transiently, leading to malignant glioma (MG) cell sensitization to radiotherapy via targeting the apoptotic regulator Bcl-2 protein.33
MicroRNA-203
MicroRNA-203 (miR-203), another known cancer-associated miRNA, was downregulated in glioma and correlated with prognosis.34 Overexpression of miR-203 increased the radiation sensitivity in U251, U373, and T98G human MG cell lines, prolonged radiation-induced γ-H2AX (H2A Histone Family Member X) foci formation, which is an indicator of double-strand DNA damage, and inhibited DNA damage repair by downregulating ATM and modulating AKT and STAT3 signaling pathways.35 Moreover, upregulated miR-203 suppressed invasion, epithelium–mesenchyme transition, and migration potentials via inhibiting prosurvival signaling, neural crest transcription factor SLUG (a member of the Snail family of zinc finger transcriptional repressors), has been implicated in the acquisition of invasive behavior during tumor progression, and the intermediate filament protein (Vimentin) and increasing the expression of the senescence-associated epithelial membrane protein 1 (Claudin-1) and the tight junction protein (Zona Occludens 1).26,35,36 Therefore, miR-203 could potentially contribute to the modulation of radiation sensitivity in MG cell lines.26
MicroRNAs-221/222
MicroRNAs-221/222 (miR-221/222) play key roles in modulating DNA damage response.26 In GBM tissues and cells, miR-221/222 were found to be upregulated and correlated with the stage of disease.37 They regulate glioma tumorigenesis and invasiveness through the control of protein phosphate PTPμ.37 In contrast, transfection with miR-221/222 antisense oligonucleotides halted GBM proliferation in the U251 human glioblastoma cell line and U251 glioma subcutaneous mice.38 Knocked down cells exhibited cell cycle arrest via increasing the cell cycle inhibitor p27Kip1 which in turn suppressed G1/S shift in the cell cycle in vitro and in vivo38 and reduced tumor volume in a GBM xenograft mouse model.26 Combining anti-miR-221/222 with tumor irradiation synergistically enhanced mitotic cell death and decreased S-phase fraction.38
MicroRNA-223
MicroRNA-223 (miR-223) was identified as a key regulator in cancer cell differentiation, proliferation, adhesion, and motility via targeting several proteins as forkhead box O 1 transcription factor and insulin-like-growth factor 1 receptor, expression of erythrocyte membrane protein band 4.1.-like 3, and F-box/WD repeat-containing protein 7. In U87 MG cells, miR-223 overexpression downregulates the serine/threonine kinase ATM expression and sensitizes U87 cells to radiation in vitro and in vivo, thus highlighting its putative utility as a cancer-targeting therapy.39
MicroRNA-224
The influence of microRNA-224 (miR-224) on cell growth has been well characterized in GBM cell lines and primary GBM tumor tissues.40 Low expression level in patients with GBM was associated with poor prognosis. Exogenous introduction of miR-224 reduced clonogenic potential of U87GBM cells by 30% to 55% with a more synergistic effect reaching 85% to 90% upon combination with irradiation with a dose of 6G that when applied solely produced a 50% reduction.40 Therefore, miR-224 increased radiation sensitivity in GBM tumor partially by targeting apoptosis inhibitor 5 gene.
MicroRNA-320
MicroRNA-320 (miR-320) has been demonstrated to be closely correlated with the development of glioma. Downregulated miR-320 along with upregulated forkhead box protein M1 was encountered in radioresistant glioma tissues and cells. However, miR-320 overexpression dramatically enhanced radiosensitivity, promoted apoptosis, and improved γ H2AX expression and caspase 3 activity in glioma cells through downregulation of sirtuin 1.41
MicroRNA-590-3p
MicroRNA-590-3p (miR-590-3p) was reported as a mediator for glioma initiation and development. Its upregulation was observed in high-grade glioma tissues and radioresistant human GBM cells (U251 R) through targeting leucine-rich repeats and immunoglobulin-like domains protein 1 (LRIG1). Inhibiting miR-590-3p promoted radiosensitivity of U251 R cells by enhancing apoptosis and suppressing cell viability.42
MicroRNAs Involved in Radioresistance in GBM
MicroRNA-21
MicroRNA-21 (miR-21) profile was observed to be significantly increased in MG cell lines and tissues, promoting cell survival, tumor growth, and chemo- and radioresistance.43 It is one of the major players in glioma radioresistance through the regulation of autophagy.44 It was upregulated 1.4.9-fold in radioresistant cell line SHG-44 (R) relative to the SHG-44 cells.45 Application of anti-miR-21 resulted in radiosensitization of U373 and U87 cells, whereas overexpression of miR-21 led to a decrease in radiosensitivity of LN18 and LN428 cells.44 Moreover, knockdown of miR-21 combined with tumor irradiation synergistically enhanced mitotic death and apoptosis in glioma cell lines and xenograft tumor models.44 Blocking miR-21 decreased the expression of the epidermal growth factor receptor,46 phospho-AKT,44 caspase-3,45 cyclin D, and Bcl-2, as well as induced cell cycle arrest and autophagy.26
MicroRNA-210
High expression of microRNA-210 (miR-210) was found in some types of cancer, especially in GBM.46 It is involved in cell survival, stemness maintenance, and hypoxia adaptation,47-49 and its expression is modulated by hypoxia inducible factor and nuclear factor κB.50 It regulates cell proliferation and apoptosis via targeting regulator of differentiation 1 in GBM cells.51 Knockdown of miR-210 increased the apoptotic rate, reduced the antioxidant capacity, and sensitized hypoxic GSCs following irradiation,52 thus suggesting the putative beneficial role of combining miR-210 inhibition and radiotherapy to halt GBM cells.26
MicroRNA-212
MicroRNA-212 (miR-212) revealed inconsistent altered expression patterns in various types of tumors. However, in GBM, it functioned as a tumor suppressor whose expression was significantly downregulated.53 Overexpression of miR-212 decreased viability of GBM cells in vitro and suppressed tumor growth in vivo by directly targeting serum and glucocorticoid-inducible kinase 3 (SGK3).53 In human U251 and SHG-44 GBM cells, miR-212 was identified as negatively associated radiation-induced miRNA that was downregulated following γ-ray exposure.17 Transfection of miR-212 mimic in irradiated U251 and SHG-44GBM cell lines has been shown to attenuate radiation-induced apoptosis, alter the expression of apoptosis-related proteins (downregulation of Bcl-2 and upregulation of cleaved-caspase-3), and increase colony formation ability in response to radiation, hence suggesting its contribution in radioresistance via targeting breast cancer susceptibility gene 1.17
MicroRNA-135b
The tumor suppressor microRNA-135b (miR-135b) was recognized to play a critical role in GBM development. It was the most downregulated miRNA in patient-derived GBM stem-like cells, and its restoration decreased tumorigenic potentiality and reduced brain infiltration in GBM animal models.54 However, miR-135b expression was found to be upregulated in the U87R radio-GBM cell line compared to parent U87 cells. Its knockdown increased radiosensitivity by direct regulation of glycogen synthase kinase-3b (GSK3β), a negative regulator of cell growth.54 Similarly, in patients with GBM, miR-135b overexpression and GSK3β downregulation were encountered in recurrent tumors compared to primary ones after treatment with IR, highlighting the correlation of miR-135b/ GSK3β axis with radiotherapy.26,55
Functional Enrichment Analysis
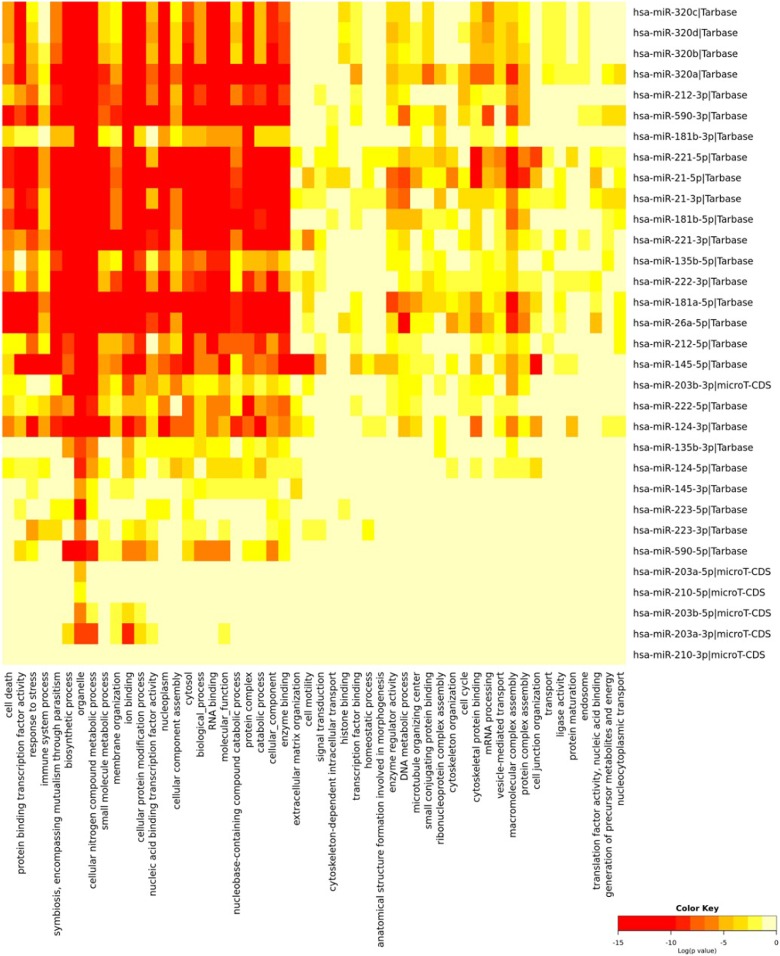
Taking into consideration the “off-target phenomenon” associated with miRNA therapy, we used DIANA tools56 to investigate the miRNA molecular target pathways and gene ontology and define how each miRNA can influence the downstream signaling pathway in each target. Radiotherapy-related miRNAs were analyzed, and ontology terms were filtered according to the significance of the interaction (P < .01) and intersected as depicted in Figure 2. Among the 18 selected miRNAs, miR-320 and miR-210, modulators for radiosensitivity and radioresistance, respectively, were involved in few biological processes, which could indicate minimal off-target effects upon using these miRNAs in therapeutic purpose.
Figure 2.
Functional enrichment analysis of glioblastoma-related microRNAs (miRNAs). Gene ontology analysis of 18 miRNAs (miR-26a, -124, -128, -135b, -145, -153, -181a/b, -203, -21, -210, -212, -221/222, -223, -224, -320 and -590) involved in the pathogenesis of glioblastoma (GBM), and its radioresponsive state was carried out by DIANA-miRPath v3.0 tool using experimentally validated gene targets stored in TarBase v7.0 database.56
MicroRNA Delivering System in Glioblastoma
Micro-RNA-based therapies (miRNA mimics or antagonists) could be delivered locally or systemically in the form of naked or modified nucleic acids, conjugated with lipids and other molecules or carried in various forms of nanoparticles and vectors.57 However, such therapies present several challenges including inadequate penetration to tumor cells, miRNA degradation and reduced half-life, undesired effects on other genes, and systemic toxicities.57 Although the blood–brain barrier, which limits passage of miRNAs to the brain tissue, represents another major hurdle in GBM, some studies have described effective miRNA therapy techniques.58 For instance, Shatsberg et al used miR-34a nanogels in mice with human GBM cell line U-87 MG and succeeded in suppressing tumor progression.59 Malhotra et al adopted cyclic arginine-glycine-aspartic-targeted poly(lactic-co-glycolic acid) nanoparticles in GBM mouse models and reported an enhancement in therapeutic response to TMZ.60 Also noted was the in vitro and in vivo inhibition of GBM growth after using ribonucleoprotein containing anti-miR-21.61 Delivery through mesenchymal stem cells has also proved worthwhile in GBM.62,63 Further clinical trials are required to test the side effects of such therapies.
Conclusions and Prospectives
Taken together, miR-26a, miR-124, miR-128, miR-145, miR-153, miR-181a/b, miR-203, miR-221/222, miR223, miR-224, miR-320, and miR-590-3p increase the radiosensitivity of GBM cells, while miR-21, miR-210, miR-212, and miR-135b decrease it. Therefore, overexpressing the former group or knocking down the latter group could help increase the response of GBM cancer cells to radiotherapy, ultimately contributing to cancer control and improving patient survival.
Acknowledgments
The authors thank the science and technology development fund (STDF) for providing the facilities for ongoing work.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Manal S. Fawzy  https://orcid.org/0000-0003-1252-8403
https://orcid.org/0000-0003-1252-8403
References
- 1. Osuka S, Van Meir E. Overcoming therapeutic resistance in glioblastoma: the way forward. J Clin Invest. 2017;127(2):415–426. doi:10.1172/JCI89587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis D.N, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. doi:10.1007/s00401-016-1545 -1. [DOI] [PubMed] [Google Scholar]
- 3. Wen P, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi:10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 4. Ramirez Y, Weatherbee J, Wheelhouse R, Ross A. Glioblastoma multiforme therapy and mechanisms of resistance. Pharmaceuticals [Basel]. 2013;6(12):1475–1506. doi:10.3390/ph6121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hall E, Giaccia A. Radiobiology for the Radiologist. 7th ed New York, NY; Philadelphia, PA: Lippincott; Williams & Wilkins; 2012. https://trove.nla.gov.au/version/51355121 [Google Scholar]
- 6. Kesari S. Understanding glioblastoma tumor biology: the potential to improve current diagnosis and treatments. Semin Oncol. 2011;38(suppl 4):2–10. doi:10.1053/j.seminoncol.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 7. Deng X, Ma L, Wu M, et al. miR-124 radiosensitizes human glioma cells by targeting CDK4. J Neurooncol. 2013;114(3):263–274. doi:10.1007/s11060-013-1179-2. [DOI] [PubMed] [Google Scholar]
- 8. Toraih EA, Ibrahiem AT, Fawzy MS, Hussein MH, Al-Qahtani SAM, Shaalan AAM. MicroRNA-34a: a key regulator in the hallmarks of renal cell carcinoma. Oxid Med Cell Longev. 2017;2017:3269379 doi:10.1155/2017/3269379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fawzy MS, Toraih EA, Ibrahiem A, Abdeldayem H, Mohamed AO, Abdel-Daim MM. Evaluation of miRNA-196a2 and apoptosis-related target genes: ANXA1, DFFA and PDCD4 expression in gastrointestinal cancer patients: a pilot study. PLoS One. 2017;12(11):e0187310 doi:10.1371/journal.pone.0187310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toraih EA, Aly NM, Abdallah HY, et al. MicroRNA-targets cross-talks: key players in glioblastoma multiforme. Tumour Biol. 2017;39(11):1–22. doi:10.1177/1010428317726842. [DOI] [PubMed] [Google Scholar]
- 11. Toraih EA, Mohammed EA, Farrag S, Ramsis N, Hosny S. Pilot study of serum microRNA-21 as a diagnostic and prognostic biomarker in Egyptian breast cancer patients. Mol Diagn Ther. 2015;19(3):179–190. doi:10.1007/s40291-015-0143-6. [DOI] [PubMed] [Google Scholar]
- 12. Zhao L, Bode A, Cao Y, Dong Z. Regulatory mechanisms and clinical perspectives of miRNA in tumor radiosensitivity. Carcinogenesis. 2012;33(11):2220–2227. doi:10.1093/carcin/bgs235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ni J, Bucci J, Chang L, Malouf D, Graham P, Li Y. Targeting microRNAs in prostate cancer radiotherapy. Theranostics. 2017;7(13):3243–3259. doi:10.7150/thno.19934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Natarajan V. Regulation of DNA repair by non-coding miRNAs. Noncoding RNA Res. 2016;1(1):64–68. doi:10.1016/j.ncrna.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galatenko V, Galatenko A, Samatov T, et al. Comprehensive network of miRNA-induced intergenic interactions and a biological role of its core in cancer. Scientific Reports. 2018;8:2418 doi:10.1038/s41598-018-20215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leung A, Sharp P. MicroRNA functions in stress responses. Mol Cell. 2010;40(2):205–215. doi:10.1016/j.molcel.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He X, Fan S. Hsa-miR-212 modulates the radiosensitivity of glioma cells by targeting BRCA1. Oncol Rep. 2018;39(3):977–984. doi:10.3892/or.2017.6156. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Huse J, Brennan C, Hambardzumyan D, Wee B. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009;23(11):1327–1337. doi:10.1101/gad.1777409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim H, Huang W, Jiang X, Pennicooke B, Park P, Johnson M. Integrative genome analysis reveals an oncomir/onco gene cluster regulating glioblastoma survivorship. Proc Nat Acad Sci USA. 2010;107(5):2183–2188. doi:10.1073/pnas.0909896107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo P, Lan J, Ge J, et al. MiR-26a enhances the radiosensitivity of glioblastoma multiforme cells through targeting of ataxia-telangiectasia mutated. Exp Cell Res. 2014;320(2):200–208. doi:10.1016/j.yexcr.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 21. Ciafrè S, Galardi S, Mangiola A, et al. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun. 2005;334(4):1351–1358. doi:10.1016/j.bbrc.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 22. Silber J, Lim D, Petritsch C, et al. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;24:14 doi:10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen PH, Cheng CH, Shih CM, et al. The inhibition of microRNA-128 on IGF-1-activating mTOR signaling involves in temozolomide-induced glioma cell apoptotic death. PLoS One. 2016;11(11):e0167096 doi:10.1371/journal.pone.0167096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peruzzi P, Bronisz A, Nowicki M, et al. MicroRNA-128 coordinately targets Polycomb repressor complexes in glioma stem cells. Neuro oncol. 2013;15(9):1212–1224. doi:10.1093/neuonc/not055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ye L, Yu G, Wang C, et al. MicroRNA-128a, BMI1 polycomb ring finger oncogene, and reactive oxygen species inhibit the growth of U-87 MG glioblastoma cells following exposure to X-ray radiation. Mol Med Rep. 2015;12(4):6247–6254. doi:10.3892/mmr.2015.4175. [DOI] [PubMed] [Google Scholar]
- 26. Han X, Xue X, Zhou H, Zhang G. A molecular view of the radioresistance of gliomas. Oncotarget. 2017;8(59):100931–100941. doi:10.18632/oncotarget.21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Du Y, Li J, Xu T, Zhou DD, Zhang L, Wang X. MicroRNA-145 induces apoptosis of glioma cells by targeting BNIP3 and Notch signaling. Oncotarget 2017;8(37):61510–61527. doi:10.18632/oncotarget.18604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shi L, Wang Z, Sun G, Wan Y, Guo J, Fu X. miR-145 inhibits migration and invasion of glioma stem cells by targeting ABCG2. Neuromolecular Med. 2014;16(2):517–528. doi:10.1007/s12017-014-8305-y. [DOI] [PubMed] [Google Scholar]
- 29. Lee H, Bier A, Cazacu S, et al. MicroRNA-145 is downregulated in glial tumors and regulates glioma cell migration by targeting connective tissue growth factor. PLoS One 2013;8(2):e54652 doi:10.1371/journal.pone.0054652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang Y, Chien Y, Chiou G, et al. Inhibition of cancer stem cell-like properties and reduced chemoradioresistance of glioblastoma using microRNA145 with cationic polyurethane-short branch PEI. Biomaterials. 2012;33(5):1462–1476. doi:10.1016/j.biomaterials.2011.10.071. [DOI] [PubMed] [Google Scholar]
- 31. Agrawal R, Pandey P, Jha P, Dwivedi V, Sarkar C, Kulshreshtha R. Hypoxic signature of microRNAs in glioblastoma: insights from small RNA deep sequencing. BMC Genomics. 2014;15:686 doi:10.1186/1471-2164-15-686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang W, Shen Y, Wei J, Liu F. MicroRNA-153/Nrf-2/GPx1 pathway regulates radiosensitivity and stemness of glioma stem cells via reactive oxygen species. Oncotarget. 2015;6(26):22006–22027. doi:10.18632/oncotarget.4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen G, Zhu W, Shi D, et al. MicroRNA-181a sensitizes human malignant glioma U87MG cells to radiation by targeting Bcl-2. Oncol Rep. 2010;23(4):997–1003. doi:10.3892/or_00000725 [DOI] [PubMed] [Google Scholar]
- 34. He J, Li Y, Xie X, Wang L, Chun S, Cheng W. hsa-miR-203 enhances the sensitivity of leukemia cells to arsenic trioxide. Exp Ther Med. 2013;5(5):1315–1321. doi:10.3892/etm.2013.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang J, Hwang Y, Lee D, et al. MicroRNA-203 Modulates the radiation sensitivity of human malignant glioma cells. Int J Radiat Oncol Biol Phys. 2016;94(2):412–420. doi:10.1016/j.ijrobp.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 36. Zhou Y, Wan G, Spizzo R, et al. miR-203 induces oxaliplatin resistance in colorectal cancer cells by negatively regulating ATM kinase. Mol Oncol. 2014;8(1):83–92. doi:10.1016/j.molonc.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Quintavalle C, Garofalo M, Zanca C, et al. miR-221/222 overexpression in human glioblastoma increases invasiveness by targeting the protein phosphate PTPμ. Oncogene 2012;31(7):858 doi:10.1038/onc.2011.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang C, Kang C, You Y, et al. Co-suppression of miR-221/222 cluster suppresses human glioma cell growth by targeting p27kip1 in vitro and in vivo. Int J Oncol. 2009;34(6):1653–1660. doi:10.3892/ijo_00000296. [DOI] [PubMed] [Google Scholar]
- 39. Liang L, Zhu J, Zaorsky N, et al. MicroRNA-223 enhances radiation sensitivity of U87MG cells in vitro and in vivo by targeting ataxia telangiectasia mutated. Int J Radiat Oncol Biol Phys. 2014;88:955–960. doi:10.1016/j.ijrobp.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 40. Upraity S, Kazi S, Padul V, Shirsat N. MiR-224 expression increases radiation sensitivity of glioblastoma cells. Biochem Biophys Res Commun. 2014;448:225–230. doi:10.1016/j.bbrc.2014.04.095. [DOI] [PubMed] [Google Scholar]
- 41. Li T, Ma J, Han X, et al. MicroRNA-320 enhances radiosensitivity of glioma through down-regulation of sirtuin type 1 by directly targeting forkhead box protein M1. Transl Oncol. 2018;11:205–212. doi:10.1016/j.tranon.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen L, Wang W, Zhu S, et al. MicroRNA-590-3p enhances the radioresistance in glioblastoma cells by targeting LRIG1. Exp Ther Med. 2017;14:1818–1824. doi:10.3892/etm.2017.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chao T, Xiong H, Liu W, Chen Y, Zhang J. MiR-21 mediates the radiation resistance of glioblastoma cells by regulating PDCD4 and hMSH2. J Huazhong Univ Sci Technolog Med Sci. 2013;33:525–529. doi:10.1007/s11596-013-1153-4. [DOI] [PubMed] [Google Scholar]
- 44. Gwak H, Kim T, Jo G. Silencing of microRNA-21 confers radio-sensitivity through inhibition of the PI3K/AKT pathway and enhancing autophagy in malignant glioma cell lines. PLoS One. 2012;7:e47449 doi:10.1371/journal.pone.0047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou J, Zhou C, Wang L, Xu X, Qin S. Influence of knock-down of miR-21 expression on the radiosensitivity of glioma SHG-44 cells. Zhonghua Zhong Liu Za Zhi. 2011;33:747-751. doi:10.3760/cma.j.issn.0253-3766.2011.10.006. [PubMed] [Google Scholar]
- 46. Zhou X, Ren Y, Moore L, et al. Downregulation of miR-21 inhibits EGFR pathway and suppresses the growth of human glioblastoma cells independent of PTEN status. Lab Invest. 2010;90:144–155. doi:10.1038/labinvest.2009.126. [DOI] [PubMed] [Google Scholar]
- 47. Shang C, Hong Y, Guo Y, Liu Y, Xue Y. MiR-210 up-regulation inhibits proliferation and induces apoptosis in glioma cells by targeting SIN3A. Med Sci Monit. 2014;20:2571–2577. doi:10.12659/MSM.892994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu Y, Han Y, Zhang H, et al. Synthetic miRNA-mowers targeting miR-183–96–182 cluster or miR-210 inhibit growth and migration and induce apoptosis in bladder cancer cells. PLoS One. 2012;7:e52280 doi:10.1371/journal.pone.0052280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim J, Park S, Song S, Kim J, Sung J. Reactive oxygen species-responsive miR-210 regulates proliferation and migration of adipose-derived stem cells via PTPN2. Cell Death Dis. 2013;4:e588 doi:10.1038/cddis.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang X, Le Q, Giaccia A. MiR-210-micromanager of the hypoxia pathway. Trends Mol Med. 2010;16:230–237. doi:10.1016/j.molmed.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang S, Lai N, Liao K, Sun J, Lin Y. MicroRNA-210 regulates cell proliferation and apoptosis by targeting regulator of differentiation 1 in glioblastoma cells. Folia Neuropathol. 2015;53:236–244. doi:10.5114/fn.2015.54424. [DOI] [PubMed] [Google Scholar]
- 52. Yang W, Wei J, Guo T, Shen Y, Liu F. Knockdown of miR-210 decreases hypoxic glioma stem cells stemness and radioresistance. Exp Cell Res. 2014;326:22–35. doi:10.1016/j.yexcr.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 53. Liu H, Li C, Shen C, et al. MiR-212-3p inhibits glioblastoma cell proliferation by targeting SGK3. J Neurooncol. 2015;122:431–439. doi:10.1007/s11060-015-1736-y. [DOI] [PubMed] [Google Scholar]
- 54. Lulli V, Buccarelli M, Martini M, et al. miR-135b suppresses tumorigenesis in glioblastoma stem-like cells impairing proliferation, migration and self-renewal. Oncotarget. 2015;6:37241–37256. doi:10.18632/oncotarget.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xiao S, Yang Z, Lv R, et al. miR-135b contributes to the radioresistance by targeting GSK3β in human glioblastoma multiforme cells. PLoS One. 2014;9:e108810 doi:10.1371/journal.pone.0108810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vlachos IS, Zagganas K, Paraskevopoulou MD, et al. DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res. 2015;43:W460–W466. doi:10.1093/nar/gkv403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen Y, Gao DY, Huang L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev. 2014;81:128–141. doi:10.1016/j.addr.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Purow B. The elephant in the room: do microRNA-based therapies have a realistic chance of succeeding for brain tumors such as glioblastoma?. J Neurooncol. 2010;103:429–436. doi:10.1007/s11060-010-0449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shatsberg Z, Zhang X, Ofek P, et al. Functionalized nanogels carrying an anticancer microRNA for glioblastoma therapy. J Control Release. 2016;239:159–168. doi:10.1016/j.jconrel.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 60. Malhotra M, Sekar TV, Ananta JS, et al. Targeted nanoparticle delivery of therapeutic antisense microRNAs presensitizes glioblastoma cells to lower effective doses of temozolomide in vitro and in a mouse model. Oncotarget. 2018;9:21478–21494. doi:10.18632/oncotarget.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee TJ, Yoo JY, Shu D, et al. RNA Nanoparticle-Based Targeted Therapy for Glioblastoma through Inhibition of Oncogenic miR-21. Mol Ther. 2017;25:1544–1555. doi:10.1016/j.ymthe.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee HK, Finniss S, Cazacu S, Xiang C, Brodie C. Mesenchymal stem cells deliver exogenous miRNAs to neural cells and induce their differentiation and glutamate transporter expression. Stem Cells Dev. 2014;23:851–861. doi:10.1089/scd.2014.0146. [DOI] [PubMed] [Google Scholar]
- 63. Sharif S, Ghahremani MH, Soleimani M. Delivery of exogenous miR-124 to glioblastoma multiform cells by Wharton’s jelly mesenchymal stem cells decreases cell proliferation and migration, and confers chemosensitivity. Stem Cell Rev. 2018;14:236–246. doi:10.1007/s12015-017-9788-3. [DOI] [PubMed] [Google Scholar]