Abstract
Decreased cerebral blood flow (CBF) after cardiac arrest (CA) contributes to secondary ischemic injury in infants and children. We previously reported cortical hypoperfusion with tissue hypoxia early in a pediatric rat model of asphyxial CA. In order to identify specific alterations as potential therapeutic targets to improve cortical hypoperfusion post-CA, we characterize the CBF alterations at the cortical microvascular level in vivo using multiphoton microscopy. We hypothesize that microvascular constriction and disturbances of capillary red blood cell (RBC) flow contribute to cortical hypoperfusion post-CA. After resuscitation from 9 min asphyxial CA, transient dilation of capillaries and venules at 5 min was followed by pial arteriolar constriction at 30 and 60 min (19.6 ± 1.3, 19.3 ± 1.2 µm at 30, 60 min vs. 22.0 ± 1.2 µm at baseline, p < 0.05). At the capillary level, microcirculatory disturbances were highly heterogeneous, with RBC stasis observed in 25.4% of capillaries at 30 min post-CA. Overall, the capillary plasma mean transit time was increased post-CA by 139.7 ± 51.5%, p < 0.05. In conclusion, pial arteriolar constriction, the no-reflow phenomenon and increased plasma transit time were observed post-CA. Our results detail the microvascular disturbances in a pediatric asphyxial CA model and provide a powerful platform for assessing specific vascular-targeted therapies.
Keywords: Cardiac arrest, microcirculation, mean transit time, no-reflow phenomenon, pediatric, cerebral blood flow
Introduction
Cardiac arrest (CA) is a significant cause of mortality in the pediatric population. Approximately 16,000 pediatric patients suffer from CA every year in the United States, accounting for up to one-quarter of all pediatric mortalities.1 Thirty-five percent of the surviving patients at discharge have unfavorable neurological outcome.2 To date, no pharmacological therapy has demonstrated neuroprotective benefit in the treatment of post-CA brain injury.
Cerebral hypoperfusion in the initial minutes to hours post-CA contributes to the overall ischemic injury, as described in animal models.3,4 Previously, we established a clinically relevant pediatric asphyxial CA model in rats.5 We noted marked regional and temporal variation in cerebral blood flow (CBF) characterized using arterial spin label magnetic resonance imaging (ASL-MRI).4 Cortical hypoperfusion was observed 30–120 min after resuscitation and prolonged cortical hypoperfusion was associated with poor long-term behavioral outcome. Subsequently, we reported cortical tissue hypoxia, using brain tissue oxygen (PbO2) monitoring, which regionally and temporally mimicked the CBF decrement.6 While the presence of post-CA hypoperfusion is well established, the pathophysiological underpinnings of the cortical hypoperfusion post-CA have not been completely elucidated or detailed at the microcirculation level. A thorough understanding of the status of the cerebral microcirculation early after CA is essential for the development of CBF targeted therapies. Two major mechanisms have been identified to date that contribute to decreased CBF post-CA: intravascular stasis, also known as the no-reflow phenomenon, and vasoconstriction. These pathophysiological processes have been largely characterized ex vivo using histological methods or have been ascertained from experiments using pharmacological manipulations.
The no-reflow phenomenon was first described histologically in a global ischemia model in 1968.7 Rabbits subjected to ischemia had incomplete capillary filling upon reperfusion and developed occlusive lesions at the particle size of red blood cells (RBCs) in the cerebrum. Subsequent studies questioned the existence of the no-reflow phenomenon—suggesting that it may have resulted from an artifact of inadequate perfusion pressure in the experimental system used.8,9 However, others suggested that it can develop, and may result from astrocyte swelling, platelet and leukocyte adherence, increased blood viscosity, and/or post-insult hypotension, among other mechanisms.10,11 The no-reflow phenomenon has yet to be characterized in vivo after CA.
Cerebral vasoconstriction represents another microvascular disturbance postulated to cause post-ischemic hypoperfusion, as cerebral microvessels play a key role in regulating CBF and contribute significantly to cerebral vascular resistance.12,13 Various mediators of vasoconstriction such as thromboxane, calcium, endothelin (ET), or reactive oxygen species have been targeted experimentally and improved post-resuscitation CBF.14,15 In our model, inhibition of 20-Hydroxyeicosatetraenoic acid (20-HETE) formation, a vasoconstrictor produced in the cerebral microcirculation improved neurological outcome.16 Ristagno et al.17 suggested that microcirculatory disturbances post resuscitation after experimental CA in adult pigs contribute to cortical hypoxia, and suggested a possible contribution of epinephrine. However, there is limited understanding of cerebral microcirculation and RBC flow post-CA, especially at the level of pial vessels, penetrating vessels, and capillaries.
We hypothesize that microvessel constriction and disturbances of capillary RBC flow lead to cortical hypoperfusion post-CA. In this study, we modified our pediatric asphyxia CA model in rats to characterize the cortical microvasculature using in vivo multiphoton microscopy. We observed and quantified pial arteriolar constriction, along with RBC stasis and increased transit time of plasma in cortical capillaries post-CA. Our findings suggest that the microcirculation is a key target for optimizing current and future therapies directed at mitigating cortical hypoperfusion and tissue hypoxia, and improving long-term outcome after CA in infants and children.
Material and methods
Animals
This study was performed in accordance with Guide for the Care and Use of Laboratory Animals (8th Ed), published by National Research Council and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. All experiments were reported according to the Animal Research: Reporting of in Vivo Experiments (ARRIVE) guidelines. Male Sprague-Dawley, post-natal day (PND), 16—18 rats (30–45 g) were used in this study. Four cohorts of rats were used for the measurement of microvascular diameter (n = 8), assessment of capillary RBC flow (n = 12), RBC velocity and density (n = 8), and cortical plasma mean transit time (MTT) (Sham and CA group, n = 5/group).
Surgical preparation and asphyxial CA
We utilized an established pediatric asphyxial CA rat model5 with slight modifications for CBF assessment.4 Rats were initially anesthetized via inhalation of 3% isoflurane in N2O and oxygen (50:50), administered via a nasal cone. The trachea was then intubated, mechanical ventilation was initiated, and isoflurane was reduced to 2.5%. After the placement of femoral arterial and venous catheters, to reduce the effect of isoflurane on CBF, we discontinued the isoflurane and administered fentanyl intravenously at a constant rate of 50 µg/kg/h for the entire duration of the experiment. Neuromuscular blockade using vecuronium was administered intravenously at a rate of 5 mg/kg/h. Body temperature was maintained at 37℃ with a heating pad.
To prepare for in vivo microscopy, the rat was placed prone on the surgical table and the head was stabilized in a stereotactic frame. The skull was then exposed through a midline scalp incision. A circular craniotomy of 3–4 mm was performed over the sensory-motor cortex. After removing the bone, an optical chamber was created. A solution of 1% agarose in normal saline was applied to the intact dura. A coverslip of 5 mm diameter was applied over the agarose and was secured with tissue glue and dental cement.
Rats were subjected to 9 min asphyxial CA, which produces cortical hypoperfusion as assessed by ASL-MRI.4 The FiO2 was reduced to 0.21 (room air) for 1 min before asphyxia to prevent hyperoxygenation. CA was induced by disconnecting the tracheal tube from the ventilator for 9 min after neuromuscular blockade. Resuscitation was started by reconnecting the ventilator at a FiO2 of 1.0 and was followed by the administration of epinephrine (0.005 mg/kg, iv), sodium bicarbonate (1 mEq/kg, iv), and manual chest compressions until return of spontaneous circulation (ROSC). A FiO2 of 1.0 was maintained until the end of the experiment.
Cerebrovascular imaging using multi-photon microscopy
To assess the microcirculation, we used in vivo multiphoton microscopy. A Nikon A1R MP microscope was configured with the Nikon Ni-E (Nikon Instruments, Tokyo, Japan) upright motorized system. The microscope was equipped with a Chameleon Laser Vision (Coherent, Inc., CA, USA), an APO LWD 25 × water immersion objective with 1.1 NA, a high-speed (30 frames/s) resonant scanning mode and a galvano-mode scanning suitable for linescanning acquisitions. The Nano-Drive system was used to acquire fine high speed control of z-plane selection. The fluorescence detection unit of the microscope consisted of four detectors (photo multiplying tubes). We used detector 2 for FITC 525/50 nm (green channel). The microscope components including the laser, stage, resonant scanning head, detectors and acquisition were controlled using NIS Elements software (Nikon).
To delineate the vasculature, we administered Fluorescein isothiocyanate–dextran (FITC, Sigma, wt 2,000,000) 3% (w/v) 0.1 mL at baseline. Additional doses of FITC were given as needed to maintain adequate delineation of the microvasculature. The specific sequence of FITC administration for the MTT experiment is detailed below. Post-acquisition image processing was achieved using NIS Elements and MATLAB (R2015a, The MathWorks, Inc., Natick, MA, USA). The microscope stage was enclosed in a temperature controlled black plexiglass chamber, which prevented interference by the ambient light. We used an excitation wavelength of 850 nm and a laser power of 1–2%.
Assessment of cerebral microvessel diameter
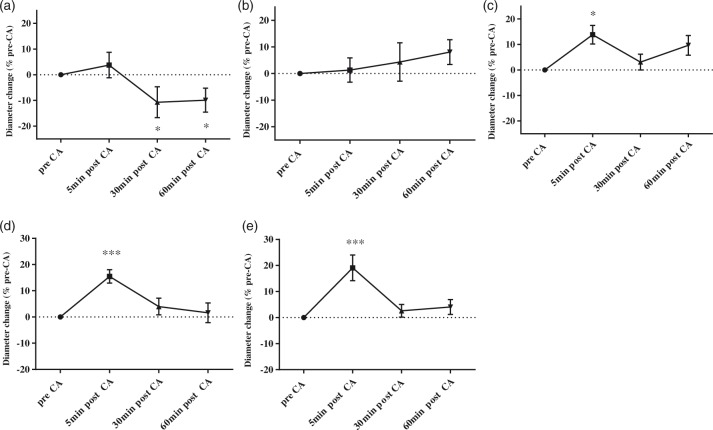
Image stacks were acquired at baseline, and at 5, 30 and 60 min post-CA in eight rats. The following parameters were used: field view of 512 × 512 pixels at a resolution of 1 µm/ pixel using resonant mode, 16 times average. Cortical microvessels were categorized by size and morphology into five types. Data were collected from 8 rats and 39 pial arterioles, 134 pial venules, 16 penetrating arterioles, 42 penetrating venules, and 75 capillaries. Pial arterioles were identified by their location on the pial layer, and morphologically by their relatively straight appearance and fewer branches. Pial venules were identified as vessels also located on the pia surface but with tortuous contours and more branches than the arterioles. Penetrating arterioles and penetrating venules were identified as vessels branching from the parent pial arterioles and draining into pial venules, respectively, and perpendicular to the pia. Capillaries were identified as microvessels located in the parenchyma and with diameter of less than 10 µm at baseline (Figure 1(a)). For each animal, three to five locations with adequate contrast resolution were identified within the imaging window at baseline. The image stacks reached to a depth ranging from 250 to 400 µm from the pia. All the locations were saved in the software such that the system was able to automatically obtain imaging stacks at the same location post-CA. At each location, the baseline imaging stacks were set as reference stacks to which post-CA stacks (5, 30 and 60 min post-CA) were overlaid in the x-y-z dimensions (Figure 1(b)). The vessel diameters at each time point were then quantified from a single location per segment from the maximum projection of the aligned image stacks using the Annotation and Measurement function in NIS Element. The accuracy of the measured shape is subject to the point-spread function of the system which is roughly 0.29 µm in-plane. Simulations at this resolution show that under noiseless conditions, vessel diameter can be estimated with sub-pixel precision with error <10% for vessels >3 μm, error <7% for vessels >4 μm, and error <4% for vessels >5 μm. Only the vessels with acquired data at all four time points were included.
Figure 1.
(a) A representative 3D image stack of the cortical microvasculature. The z depth is about 250 µm. (b) Representative images of a pial arteriole, pial venule, penetrating arteriole, penetrating venule, and cortical capillary at baseline and post-CA coded with pseudo colors. We observed pial arteriolar constriction at 60 min post-CA compared with baseline, indicated by white arrows. The pial and penetrating venule and capillary dilations are visualized at 5 min post-CA as indicated by white triangles.
Assessment of capillary RBC flow (no-reflow phenomenon)
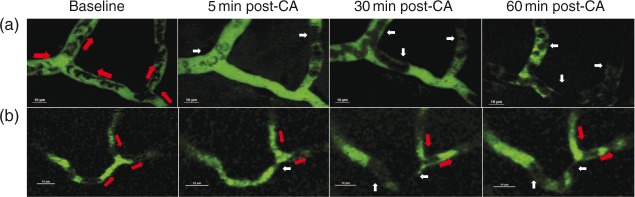
The live time-series at baseline and post-CA were acquired to assess the presence of RBC flow vs. stasis in the capillaries, defined as microvessels in the parenchyma with diameter of less than 10 µm. With FITC delineating the plasma, RBCs appear as oval negative signals and a size of 3–6 µm. The time-series were acquired at the speed of 30 frames/s with resonant mode. To capture the movement of RBCs, two to three time-series were identified per animal at baseline and one to three capillaries with adequate contrast were included in each time-series. The locations for each time-series were recorded so that the same capillaries could be repeatedly assessed post-CA. All capillaries captured at baseline had continuous RBC flow. Absent RBC flow was defined as no RBC flow during the time series (15–30 s). Some capillaries were observed to have stagnant RBCs that appeared fixed in place in the vessel, while others did not have any RBCs or RBC flow visualized, such that only plasma-filled vessels were seen. A total of 59 capillaries from 12 rats were evaluated.
Assessment of capillary RBC velocity and density
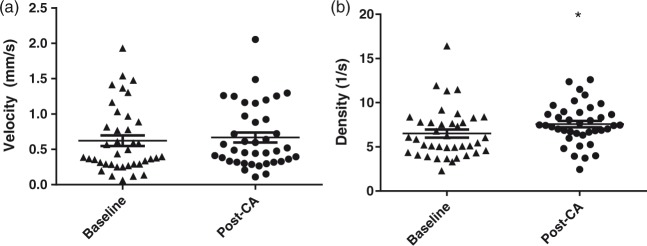
To quantify the capillary RBC velocity and density, the linescan method was applied, as previously described.18 Briefly, a straight section of a capillary with adequate contrast was identified at baseline. The scan path was then placed along the capillary lumen. In linescan mode, the movement of RBCs is detected as an angled streak. For a known scan speed, the velocity of the RBC movement is proportional to the slope of the streak. Linescans were collected at a speed of 1000 lines/s for 10–15 s on capillaries at baseline and post-CA. To quantify and compare the effect of CA on RBC velocity, only capillaries with both baseline and post-CA reliable measurements were included. Capillaries with stagnant RBCs flow were not included due to unreliable linescan reading. The RBC velocity was quantified by analyzing the angle of RBC streaks in contiguous 150–250 lines using MATLAB. In total, we measured the RBC velocity and density in 39 capillaries from eight rats at baseline and post-CA. To assess the effect of anesthesia over time in our preparation on RBC velocity, we also collected measurements from 33 capillaries of 4 sham-operated rats and found no change in either RBC velocity or density during the 60-min measurements (data not shown).
Assessment of mean transit time of plasma through the cortical microcirculation
The MTT was assessed to describe the time required for plasma to traverse the cortical microcirculation. A pial artery and adjacent pial vein supply regional cortical blood flow, therefore the plasma transit time from the pial artery to the pial vein indicates the plasma flow that traverses the cortical capillary bed. A longer MTT indicates slower capillary plasma flow which suggests capillary flow disturbances. MTT was assessed at baseline and at 30 and 60 min after CA or sham surgery (n = 5/group) using a previously described method.19,20 Briefly, a pial artery and an adjacent pial vein were identified in the imaging field (512 × 512 µm) at baseline. Time-series were recorded at the speed of 30 frames/s with resonant mode during a bolus of FITC dextran (3% w/v, 0.03 mL) iv injection at baseline, 30 and 60 min post-CA. The time-series were fitted with a median filter and smooth function to reduce the motion artifact. Two regions of interests (ROIs), one on a pial arteriole and the other on a pial venule were identified (Figure 5(b)). The intensity of the fluorescent tracer in the selected ROIs was then plotted over time using NIS Element software. The standard γ function was fit to the intensity-time curves in MATLAB (Figure 5(c)). Time-to-peak (TTP) was defined as the time required for intensity change from baseline to the peak in the fit curves for the artery and vein. MTT was defined as the difference of TTP between the pial artery and the pial venule ROIs (Figure 5(c)) at each time point. In addition, to evaluate the MTT for the entire pial vessel network within the imaging field, the TTP for each pixel in the time-series was calculated and normalized to the TTP of the arterial ROI. Image processing was implemented in MATLAB. All the post-measurements were normalized to the corresponding baseline values.
Figure 5.
Mean transit time of plasma through cortical microcirculation. (a) MTT increased post-CA at 30 and 60 min vs. sham (*p < 0.05). Each line represents an individual rat, n = 5/group. (b) The cortical pial vasculature of a representative rat: the yellow square represents a region of interest (ROI) on a pial vein, while the white square represents a ROI on a pial artery. The intensity of the fluorescent tracer was repeatedly measured in the same ROIs at baseline, and at 30 and 60 min post-CA. (c) Intensity-time curves and γ fit curves at baseline, 30, 60 min post-CA (left to right). MTT increased at 30 and 60 min post-CA, indicated by the black arrows. (d) The time-to-peak (TPP) for each pixel on the pia layer was calculated and normalized to mean arterial TPP. Increased MTT was observed at 30, 60 min post-CA in most of the pial veins, indicated by brighter color.
Statistical analysis
Descriptive statistics were tabulated to summarize the measurements. Means and standard error of the means (SEM) were presented for the continuous variables. Data were analyzed by using SPSS (IBM Corp. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.) and figures were generated using Graphpad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Physiological parameters were tested for time effect within each experiment using repeated measurement ANOVA followed by Dunnett’ post hoc comparison. For the MTT experiments, physiological data were analyzed using repeated measure ANOVA. Microvessel diameters were log transformed to normalize the distribution. In each type of vessel, we analyzed the diameter measurements in a mixed linear model. In this statistical model, repeated measurements for each vessel were nested within vessel number, and vessel numbers were nested in each animal. Similarly, RBC velocity and density were also log transformed and analyzed in a mixed linear model. MTT measurements were normalized to the baseline value for each rat and analyzed using two-way ANOVA. For all tests, p-value < 0.05 was considered statistically significant.
Results
Physiological parameters, including mean arterial pressure (MAP), end-tidal (Et) CO2 and temperature, were recorded throughout the experiment. All physiologic parameters were within normal limits at baseline. All animals included in the study were successfully resuscitated from CA and had return of systemic circulation for at least 60 min post-CA. The physiologic parameters for each experimental group are summarized in Table 1. As expected, rats subjected to CA had a modest temperature reduction at 5 min post-CA compared to baseline as normothermia was not actively maintained during the no-flow period—to mimic clinical CA. Also as anticipated, given the resuscitation, for the group in which capillary RBC flow was assessed, EtCO2 was lower at 5 and 30 min post-CA, and MAP higher at 5 min post-CA compared with baseline. Similarly, for the group in which MTT was assessed, the CA group had lower EtCO2 compared with shams. No other significant differences were observed in the physiological data.
Table 1.
Physiology parameters for each group of rats.
| Temperature(℃) |
EtCO2 (mmHg) |
MAP (mmHg) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experiment groups | N | Baseline | 5 min | 30 min | 60 min | Baseline | 5 min | 30 min | 60 min | Baseline | 5 min | 30 min | 60 min |
| Diameter | 8 | 37.3 ± 0.3 | 36.2 ± 0.2# | 37.3 ± 0.4 | 36.8 ± 0.2 | 35.7 ± 1.9 | 31.5 ± 1.1 | 29.7 ± 3.1 | 27.0 ± 2.4 | 56.4 ± 3.2 | 66.1 ± 6.4 | 52.57 ± 3.5 | 55.0 ± 3.8 |
| RBC flow | 12 | 37.1 ± 0.3 | 35.6 ± 0.2# | 36.9 ± 0.3 | 37.1 ± 0.2 | 36.3 ± 1.1 | 30.9 ± 1.2# | 28.3 ± 2.0# | 29.5 ± 2.1 | 54.5 ± 3.0 | 68.5 ± 4.4# | 50.7 ± 3.7 | 51.5 ± 3.7 |
| RBC velocity | 8 | 37.2 ± 0.4 | 35.7 ± 0.2# | 36.4 ± 0.3 | 37.2 ± 0.3 | 36.1 ± 1.0 | 36.3 ± 2.9 | 30.1 ± 2.0 | 32.6 ± 1.6 | 56.4 ± 4.0 | 59.0 ± 4.5 | 55.0 ± 3.2 | 50.0 ± 4.1 |
| MTT (CA) | 5 | 37.9 ± 0.4 | 35.6 ± 0.3*# | 36.7 ± 0.4 | 37.2 ± 0.5 | 33.6 ± 2.6* | 32.0 ± 2.1* | 32.0 ± 3.4* | 30.3 ± 2.3* | 65.0 ± 2.2 | 61.0 ± 2.4 | 59.6 ± 4.8 | 57.0 ± 9.4 |
| MTT (Sham) | 5 | 37.6 ± 0.5 | 37.2 ± 0.4 | 37.6 ± 0.2 | 37.4 ± 0.1 | 36.1 ± 1.6 | 38.7 ± 2.1 | 36.4 ± 1.7 | 37.0 ± 3.7 | 59.2 ± 1.9 | 56.3 ± 3.8 | 56.3 ± 2.4 | 55.0 ± 4.6 |
p < 0.05 vs. sham, #p < 0.05 vs. baseline.
RBC: red blood cell; MTT: mean transit time.
Assessment of microvessel diameter
Figure 1(a) illustrates images of microvessels from one representative rat at baseline. The diameter change post-CA for representative microvessels is illustrated in Figure 1(b). Vascular dynamics post-CA was different in different types of vessels. The diameter of pial arterioles was similar to baseline at 5 min post-CA and decreased at 30 and 60 min post-CA (19.6 ± 1.3, 19.3 ± 1.2 vs. 22.0 ± 1.2 µm, 30, 60 min post-CA vs. baseline, respectively, p < 0.05) (Figure 2(a)). No difference was found in the diameter of penetrating arterioles after CA vs. baseline (Figure 2(b)). Capillaries had increased diameter early post-CA at 5 min and returned to baseline value at 30, 60 min post-CA (5.5 ± 0.2 vs. 5.0 ± 0.2 µm, 5 min post-CA vs. baseline, p < 0.05) (Figure 2(c)). Penetrating venules had increased diameter at 5 min post-CA and returned to baseline value at 30 and 60 min post-CA (20.6 ± 1.2 vs. 18.0 ± 1.1 µm, 5 min post-CA vs. baseline, respectively, p < 0.001) (Figure 2(d)). Similarly, the diameter of pial venules increased at 5 min post-CA and returned to baseline at 30 and 60 min post-CA (20.0 ± 1.0 vs. 17.2 ± 0.8 µm, 5 min post-CA vs. baseline, p < 0.001) (Figure 2(e)).
Figure 2.
Mircovascular diameter changes post-CA. Diameter changes for pial arterioles (a), penetrating arterioles (b), capillaries (c), penetrating venules (d) and pial venules (e). Post-CA values are normalized to pre-CA values for each vessel. Data were collected from eight post-natal day 16–18 rats at baseline and post-CA. The diameters of pial arterioles decreased at 30 and 60 min post CA vs. baseline (*p < 0.05). The diameters of capillaries increased at 5 min post-CA compared with baseline (*p < 0.05). The diameters of the pial and penetrating venules increased at 5 minutes post-CA (***p < 0.001).
RBC flow (no reflow phenomenon)
We assessed the continuity of RBC flow in the cortical capillaries at baseline and post-CA. At baseline, RBC flow was continuous in all capillary branches (Supplementary video V1). In capillaries, stagnant RBCs were seen early as 5 min post-CA (Figure 3(a) and supplementary video V2). Stagnant RBCs were observed in several bifurcated branches of a capillary loop (Figure 3(a)), or more often stagnant flow was observed in only one of the branches, while in the other branches, RBC flow remained continuous (Figure 3(b)). Stagnant RBCs appeared to be attached to the capillary wall (Supplementary video V2 and V3).
Figure 3.
Capillary RBC flow after CA. Upper panel: Illustration of RBC stasis (no-reflow phenomenon) in representative capillaries in vivo. Panels A and B represent two individual capillaries with stagnant RBCs post-CA from two rats. Both capillaries were continuously perfused with red blood cells (RBCs) prior to CA. Red arrows indicate the flow direction in every capillary branch. White arrows indicate stagnant RBCs. In panel A, RBC flow was stagnant in all branches at 5, 30 and 60 min post-CA. In panel B, RBC flow in the left branch was stagnant at 5, 30 and 60 min post-CA, while the RBC flow in the other two branches was continuous.
Among 59 capillary branches from 12 rats with continuous RBC flow at baseline, 25.4% of capillaries (15/59) were observed to have no RBC flow at 30 min post CA. At 60 min post-CA, 13.5% capillaries (8/59) were found with no RBC flow. Out of the eight capillaries with no RBC flow at 60 min post-CA, seven capillaries had no RBC flow at 30 min post-CA.
Overall, no reflow was captured in 7 out of 12 rats at 30 or 60 min after CA. In four rats, the no reflow was observed at 30 min and was not observed at 60 min in the capillaries studies. In two rats, no reflow was observed at both time points, and in one rat, it was observed only at 60 min post-CA.
Assessment of capillary RBC velocity and density
To quantify the movement of RBCs in the capillaries with continuous RBC flow post-CA, we examined the RBC velocity and density using linescan. Capillaries with stagnant RBCs were not included in this evaluation due to the unreliable linescan quantification. Capillary RBC flow was highly heterogeneous at both baseline and post-CA (Figure 4(a) and (b)). No change was seen in the capillary RBC velocity at baseline and post-CA (0.62 ± 0.08 and 0.67 ± 0.07 mm/s, p = 0.195). RBC density increased post-CA in the perfused capillaries (7.57 ± 0.37 vs. 6.50 ± 0.46 RBC/s, post-CA vs. baseline, p < 0.05).
Figure 4.
RBC velocity and density in patent capillaries. RBCs velocity (a) and density (b) were calculated from capillaries with continuous flow at baseline and post-CA (39 capillaries from 8 CA rats). RBC density was increased post-CA compared with baseline. Each symbol represents a single vessel. *p < 0.05.
Assessment of capillary mean transit time of plasma through the cortical microcirculation
Overall MTT increased post-CA compared with sham (200.6 ± 29.2 vs. 90.0 ± 9.1%, post-CA vs. sham, p < 0.05). At 30 min post-CA, MTT increased compared to baseline (p < 0.001), while at 60 min post-CA, MTT showed a non-significant trend towards increase compared to baseline (p = 0.08) (Figure 5(a)). No change in MTT for sham rats was observed at any time points.
Figure 5(b) shows the pial vascular network in a representative rat with pial artery and pial vein ROIs (white and yellow squares, respectively). Figure 5(c) presents intensity-time curves at baseline, 30, and 60 min post-CA.
Prolonged MTT was also observed when the entire pial network was evaluated (Figure 5(d)). In the representative example illustrated, brighter color indicates relatively longer latency compared with arterioles, and the brightness appeared in almost all the branches of pial venules. The most prolonged MTT appeared at 30 min post-CA, and partially recovered at 60 min post-CA.
Discussion
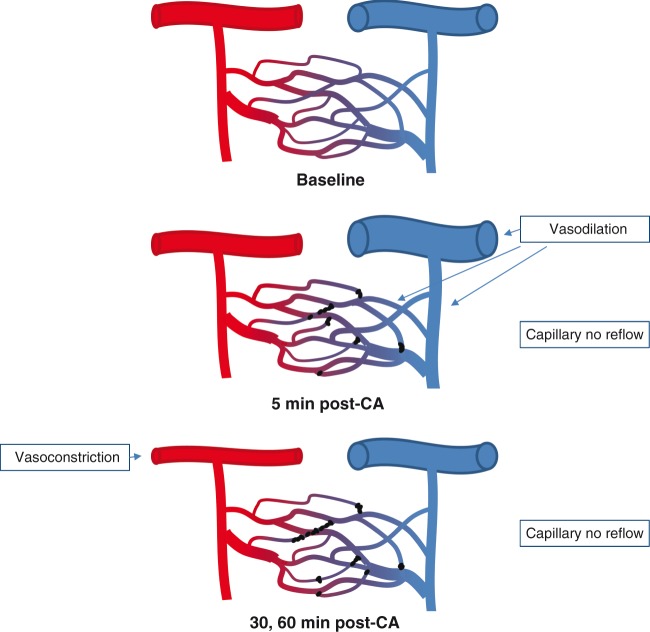
Using in vivo microscopy, we characterized cortical microcirculatory disturbances during the first 60 min after CA in a pediatric rat model of CA and resuscitation. Immediately after resuscitation, brief transient dilation of capillaries and venules is followed by prolonged pial arteriolar constriction. At the capillary level, the microcirculatory disturbances are heterogeneous; a no-reflow phenomenon is present in a quarter of capillary branches post-CA, while the RBC velocity is maintained in the patent capillary branches, where a higher density of RBCs is observed. Overall, the time required for plasma to travel from the pial arterioles through capillaries and into the pial venules is increased after CA (Figure 6). Our results detail the cortical microvascular alterations post-CA in the developing rat brain, and should serve as a platform to assess both the impact of standard of care interventions and novel vascular-targeted therapies on perfusion after CA.
Figure 6.
A schematic summary of our finding. The capillaries are perfused with RBC flow at baseline. At 5 min post-CA, capillary no reflow is present in some capillaries, and pial venules and penetrating venules are dilated. At 30, 60 min post-CA, pial arterioles are constricted while the diameter of venules returned to baseline level. No reflow is present in a fourth of capillaries at 30 min post-CA.
We observed constriction at the level of the pial arterioles and not penetrating arterioles at 30 and 60 min after resuscitation, suggesting that pial arterioles are responsible in part for the decreased cortical CBF after CA. Arteriolar smooth muscle plays an important role in regulating local CBF and maintaining blood pressure autoregulation.12,21 Under physiological conditions, pial arterioles on the surface of the brain are regarded as the major site of flow control, especially during functional hyperemia when upstream pial arteriolar dilation was observed away from the site of brain activity.22 According to the Poiseuille’s equation, arterial constriction of 10% is expected to produce a CBF reduction of ∼36%. The arteriolar constriction observed thus correlates with the 30–50% decrease in cortical CBF assessed using ASL-MRI in our model in a prior report.4 Our results are also consistent with previous reports of arteriolar constriction in gerbils after bilateral carotid artery occlusion and reperfusion.19 The pathophysiological mechanisms governing post-ischemic arteriolar vasoconstriction are not fully elucidated. It has been suggested that disturbances in the balance of vasoconstrictors and vasodilators are responsible for arteriolar vasoconstriction post-CA. Vasoconstrictive mediators such as ET, reactive oxygen species, and some products of arachidonic acid metabolism are increased after global or focal ischemia.14,16 Vasodilator mediators such as nitric oxide or selected metabolites of arachidonic acid are decreased after ischemia and might facilitate vasoconstriction.16,23 Our observations further support the potential benefit of therapies promoting vasodilatation to optimize CBF after CA.24 Indeed, several vasodilator strategies such as use of the calcium channel blockers,25 or thromboxane inhibitors26 proved beneficial in improving CBF and in some instances outcome after CA in preclinical models. In our model, inhibiting the vasoconstrictive eicosanoid 20-HETE improved CBF early after CA, and decreased neuronal degeneration and neurological deficits.16 Similarly, in other models, the ET(A) receptor antagonist BQ123 improved functional outcome and CO2 reactivity after CA14 and administration of the vasodilator nitrite improved neurological outcome and was accompanied with reduced reactive oxygen species production.23 A more complete characterization of the effects of therapies targeting arteriolar vasodilation in cortex is warranted and supported by our findings. The impact of these interventions on the observed derangements in the microcirculation could provide additional insight to conventional CBF assessments.
Capillary and venular dilation was observed at 5 min after resuscitation. At this early time, some of the capillaries had evidence of RBC stasis, despite a lack of pial and penetrating arteriolar constriction. The role of capillaries in regulating CBF is controversial. The dilatation of capillaries could represent either a passive compensatory mechanism to accommodate blood flow in patent capillaries, or an active process in response to tissue acidosis and/or hypoxia, and merits further study. Hall, et al.13 suggest that 84% of CBF regulation occurs at the capillary level, and the capillaries responded to whisker-pad stimulation earlier than the arterioles. Conversely, other studies suggest that arterioles rather than capillaries are responsible for CBF regulation.12 Similarly, we found that the capillary diameter increased only transiently at 5 min post-CA and returned to baseline afterwards. At 5 min post-CA, the increased MAP in the absence of arteriolar constriction may have produced increased hemodynamic pressure in the capillaries and venules, leading to capillary dilation if autoregulation in impaired. The increased MAP could be secondary to a transient increase in cardiac output and the hypertensive effect of epinephrine post-resuscitation,27 or may be due to a transient increase of blood volume secondary to the osmolar effect of bicarbonate administrated at resuscitation. At 30 and 60 min post-CA, we observed constriction of the pial arterioles with concomitant return of MAP to baseline, which could explain the return of the diameter of capillaries and venules to baseline levels.
We observed RBC stasis in a fourth of the capillary branches. This observation, referred to as the no-reflow phenomenon, was previously described ex-vivo and was suggested as a mechanism underlying cerebral hypoperfusion after global ischemia.28 In a model of CA, the no-reflow phenomenon was observed post-CA in 30% of brain regions.29 Our in-vivo evaluation provides specific morphological data regarding the no reflow phenomenon, supporting its existence and further defining its appearance at the microcirculatory level. Our data suggest that individual capillary branches are affected, and flow is diverged from the affected branches towards the surrounding capillaries. Some branches with no RBC flow regain perfusion by 60 min after CA. Thrombocyte and leukocyte aggregation, as well as decreased RBC deformability post-CA may play a role in the no-reflow phenomenon.30 Thrombolytic therapy using plasminogen activator and heparin reduced the no-reflow post-CA31 and blood flow promotion therapy with hypertension and hemodilution instituted upon resuscitation from CA normalized post-resuscitation CBF and improved neurological outcome in dogs.3 Pentoxifylline, a hemorrheologic agent which can reduce RBC deformability, has been shown to ameliorate the hypoperfusion after CA.32 Similarly, we qualitatively observed that RBCs appeared to be less deformable after CA. Given that decreased RBC deformability has been associated with acidosis,33 the change in RBC membrane elasticity in the acidotic milieu post-CA might contribute to the RBC stasis. Additionally, arteriolar constriction may exacerbate the no-reflow phenomenon, as arteriolar constriction in a model of focal ischemia was thought to contribute to hypoperfusion, thrombosis and distal microvascular occlusions.12
We observed increased RBC density in the patent capillaries after CA. The increased density of RBC could represent their passive redistribution diverted from the blocked capillaries towards patent capillaries, or active recruitment of oxygen carrying RBCs to areas affected by the no-reflow via an alternative capillary route. In patent capillaries, RBC velocity was similar to baseline. Obstruction of ∼25% of capillaries without an increase in RBC velocity in the capillaries that remain patent contributes to the increased plasma MTT.
The transit time of plasma in the cortex provides an overall assessment of microvascular dynamics after CA and integrates the microcirculatory components assessed, including vascular diameter, capillary patency, and velocity. Similar to our data, Crumrine and LaManna34 calculated MTT based on measurements of CBF and cerebral blood volume (CBV) and reported a four-fold increased transit time at 30 and 60 min after CA. The peak increase in MTT in our study was observed at 30 min post-CA, coinciding with the peak of the capillary obstruction, suggesting that the longer duration required for plasma to traverse the capillary bed is secondary to the capillary obstructions. Pial arterial vasoconstriction, along with stasis at the level of capillaries likely contribute to increased MTT and together represent the underlying vascular mechanism for cortical hypoperfusion observed in our model.
We assessed alterations in cerebral microcirculation in a pediatric model of CA in immature rats. In pediatric CA, asphyxia is the predominant mechanism, in contrast to adult CA where most cases result from ventricular fibrillation (VF).1 In comparison with VF CA, the unique pathophysiology of asphyxial CA includes hypoxia, bradycardia, hypovolemia, and acidosis leading to different CBF disturbances post-CA,35 and most likely, distinct alterations in cerebral microcirculation. Indeed, the CBF alterations observed in our model using ASL-MRI differ from the adult model of CA, especially at early time points after resuscitation, where cortical hyperemia is observed after adult CA.35 The alterations observed in our study might be specific to the pediatric brain, especially in light of recent data that unveiled that the vascular response to neuronal stimulation was different in neonatal vs. adult rats, specifically functional hyperemia was uncoupled from neuronal activity in developing rats from 7 to 13 days postnatally.36 This difference in the vascular response in pediatric vs. adult brain may be explained by myriad processes occurring at the neurovascular unit in the developing brain, such as angiogenesis, vascular pruning, and gradual maturation of astrocytes until PND 21.37 The vast majority of studies of microcirculation in global cerebral ischemia or CA used adult models,17 and thus the current characterization of microcirculatory changes in a pediatric model is important for the understanding of pediatric specific post-CA pathophysiology and ultimately for the development of pediatric specific therapies.
We observed a modest decrease of temperature at 5 min post-CA compared to baseline due to spontaneous cooling during CA. Clinically, the same extent of cooling may not develop during CA in humans, due to significantly different body surface area and metabolic rates. This is a limitation of the rodent model that may affect vascular changes at early time points (5 min). Sodium bicarbonate was given in this experiment to correct the acidosis post-CA. This could potentially limit the translational significance of the current study. Post-CA hypocarbia may also contribute to the reduced CBF post-CA. We used male rats in our study, and although PND 16-18 is a pre-pubertal age, innate sex differences have been reported cytotoxicity and programmed cell death.38,39 Further experiments using female rats are warranted.
In conclusion, we characterized in vivo the dynamic changes of the cortical microcirculation in an established pediatric CA model that produces cortical hypoperfusion and tissue hypoxia in rats. Our study identifies several vascular-specific targets in the microcirculation to mitigate these derangements. Characterization of the effects of both standard post-resuscitation therapies along with novel approaches on the microcirculation after pediatric CA could optimize post-resuscitation perfusion and ultimately improve neurological outcome.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grants R01 HD075760, R01NS084604, 1S10RR028478-01, R01NS094404, and Alzheimer's Association grant 305086.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Lingjue Li designed and participated in data collection, analysis, and manuscript writing. Samuel Poloyac participated in experiment design and manuscript writing. Simon Watkins and Claudette St Croix helped design the experiment and participated in writing the manuscript. Henry Alexander prepared and conducted animal surgery and participated in experimental design. Gregory Gibson and Patricia Loughran assisted with data collection and manuscript preparation. Robert Clark and Patrick Kochanek assisted with the experiment design, data analysis, and manuscript writing. Levent Kirisci assisted with statistical analysis and manuscript preparation. Alberto Vazquez participated in data analysis, study design, and manuscript preparation. Mioara Manole participated in experiment design, data analysis, and manuscript writing.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Rossano JW, Naim MY, Nadkarni VM, et al. Epidemiology of pediatric cardiac arrest. In: Cruz EM, Ivy D, Jaggers J (eds). Pediatric and congenital cardiology, cardiac surgery and intensive care, London: Springer, 2014, pp. 1275–1287. [Google Scholar]
- 2.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA 2006; 295: 50–57. [DOI] [PubMed] [Google Scholar]
- 3.Leonov Y, Sterz F, Safar P, et al. Hypertension with hemodilution prevents multifocal cerebral hypoperfusion after cardiac arrest in dogs. Stroke 1992; 23: 45–53. [DOI] [PubMed] [Google Scholar]
- 4.Manole MD, Foley LM, Hitchens TK, et al. Magnetic resonance imaging assessment of regional cerebral blood flow after asphyxial cardiac arrest in immature rats. J Cereb Blood Flow Metab 2009; 29: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fink EL, Alexander H, Marco CD, et al. An experimental model of pediatric asphyxial cardiopulmonary arrest in rats. Pediatr Crit Care Med 2004; 5: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manole MD, Kochanek PM, Bayır H, et al. Brain tissue oxygen monitoring identifies cortical hypoxia and thalamic hyperoxia after experimental pediatric cardiac arrest. Pediatr Res 2014; 75: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ames A, Wright RL, Kowada M, et al. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol 1968; 52: 437. [PMC free article] [PubMed] [Google Scholar]
- 8.Levy DE, Brierley JB, Plum FR. Ischaemic brain damage in the gerbil in the absence of 9no-reflow9. J Neurol Neurosurg Psychiatry 1975; 38: 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer EG, Ames AI, Hedley-Whyte ET, et al. Reassessment of cerebral capillary changes in acute global ischemia and their relationship to the” no-reflow phenomenon”. Stroke 1977; 8: 36–39. [DOI] [PubMed] [Google Scholar]
- 10.Bai J, Lyden PD. Revisiting cerebral postischemic reperfusion injury: new insights in understanding reperfusion failure, hemorrhage, and edema. Int J Stroke 2015; 10: 143–152. [DOI] [PubMed] [Google Scholar]
- 11.del Zoppo GJ. Virchow’s triad: the vascular basis of cerebral injury. Rev Neurol Dis 2008; 5(Suppl 1): S12. [PMC free article] [PubMed] [Google Scholar]
- 12.Hill RA, Tong L, Yuan P, et al. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 2015; 87: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krep H, Brinker G, Schwindt W, et al. Endothelin type A-antagonist improves long-term neurological recovery after cardiac arrest in rats. Crit Care Med 2000; 28: 2873–2880. [DOI] [PubMed] [Google Scholar]
- 15.Itoh Y, Suzuki N. Control of brain capillary blood flow. J Cereb Blood Flow Metab 2012; 32: 1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaik JS, Poloyac SM, Kochanek PM, et al. 20-Hydroxyeicosatetraenoic acid inhibition by HET0016 offers neuroprotection, decreases edema, and increases cortical cerebral blood flow in a pediatric asphyxial cardiac arrest model in rats. J Cereb Blood Flow Metab 2015; 35: 1757–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ristagno G, Tang W, Huang L, et al. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit Care Med 2009; 37: 1408–1415. [DOI] [PubMed] [Google Scholar]
- 18.Kamoun WS, Chae SS, Lacorre DA, et al. Simultaneous measurement of RBC velocity, flux, hematocrit and shear rate in vascular networks. Nat Meth 2010; 7: 655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauck EF, Apostel S, Hoffmann JF, et al. Capillary flow and diameter changes during reperfusion after global cerebral ischemia studied by intravital video microscopy. J Cereb Blood Flow Metab 2004; 24: 383–391. [DOI] [PubMed] [Google Scholar]
- 20.Stefanovic B, Hutchinson E, Yakovleva V, et al. Functional reactivity of cerebral capillaries. J Cereb Blood Flow Metab 2008; 28: 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams DL, Piserchia V, Economides JR, et al. Vascular supply of the cerebral cortex is specialized for cell layers but not columns. Cereb Cortex 2014; 25: 3673–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nature Rev Neurosci 2004; 5: 347. [DOI] [PubMed] [Google Scholar]
- 23.Dezfulian C, Kenny E, Lamade A, et al. Mechanistic characterization of nitrite‐mediated neuroprotection after experimental cardiac arrest. J Neurochem 2016; 139: 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan RW, Kilbaugh TJ, Shoap W, et al. A hemodynamic-directed approach to pediatric cardiopulmonary resuscitation (HD-CPR) improves survival. Resuscitation 2017; 111: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forsman M, Aarseth HP, Nordby HK, et al. Effects of nimodipine on cerebral blood flow and cerebrospinal fluid pressure after cardiac arrest: correlation with neurologic outcome. Anesth Analg 1989; 68: 436–443. [PubMed] [Google Scholar]
- 26.Itoh Y. Blockade of thromboxane A2 receptor ameliorates delayed postischemic hypoperfusion of the brain in cats. Keio J Med 1994; 43: 88–93. [DOI] [PubMed] [Google Scholar]
- 27.Lee JK, Brady KM, Mytar JO, et al. Cerebral blood flow and cerebrovascular autoregulation in a swine model of pediatric cardiac arrest and hypothermia. Crit Care Med 2011; 39: 2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smrcka M, Maca K, Juran V, et al. “No-reflow” phenomenon as a cause of hypoperfusion after severe head injury? Bratisl Lek Listy 2003; 104: 236–238. [PubMed] [Google Scholar]
- 29.Fischer M, Hossmann KA. No-reflow after cardiac arrest. Intens Care Med 1995; 21: 132–141. [DOI] [PubMed] [Google Scholar]
- 30.Pluta R, Lossinsky AS, Walski M, et al. Platelet occlusion phenomenon after short-and long-term survival following complete cerebral ischemia in rats produced by cardiac arrest. J Hirnforsch 1993; 35: 463–471. [PubMed] [Google Scholar]
- 31.Fischer M, Böttiger BW, Hossmann KA, et al. Thrombolysis using plasminogen activator and heparin reduces cerebral no-reflow after resuscitation from cardiac arrest: an experimental study in the cat. Intens Care Med 1996; 22: 1214–1223. [DOI] [PubMed] [Google Scholar]
- 32.Tanahashi N, Fukuuchi Y, Tomita M, et al. Pentoxifylline ameliorates postischemic delayed hypoperfusion of the cerebral cortex following cardiac arrest in cats. J Neurol Sci 1995; 132: 105–109. [DOI] [PubMed] [Google Scholar]
- 33.Kuzman D, Žnidarčič T, Gros M, et al. Effect of pH on red blood cell deformability. Pflügers Archiv 2000; 440: r193–r194. [DOI] [PubMed] [Google Scholar]
- 34.Crumrine RC, LaManna JC. Regional cerebral metabolites, blood flow, plasma volume, and mean transit time in total cerebral ischemia in the rat. J Cereb Blood Flow Metab 1991; 11: 272–282. [DOI] [PubMed] [Google Scholar]
- 35.Drabek T, Foley LM, Janata A, et al. Global and regional differences in cerebral blood flow after asphyxial versus ventricular fibrillation cardiac arrest in rats using ASL-MRI. Resuscitation 2014; 85: 964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozberg MG, Chen BR, DeLeo SE, et al. Resolving the transition from negative to positive blood oxygen level-dependent responses in the developing brain. Proc Natl Acad Sci 2013; 110: 4380–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seregi A, Keller M, Hertting G. Are cerebral prostanoids of astroglial origin? Studies on the prostanoid forming system in developing rat brain and primary cultures of rat astrocytes. Brain Res 1987; 404: 113–120. [DOI] [PubMed] [Google Scholar]
- 38.Du L, Bayir H, Lai Y, et al. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem 2004; 279: 38563–38570. [DOI] [PubMed] [Google Scholar]
- 39.Armstead WM, Riley J, Vavilala MS. K channel impairment determines sex and age differences in epinephrine‐mediated outcomes after brain injury. J Neurosci Res 2017; 95: 1917–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.