Abstract
The ischemic penumbra is both a concept in understanding the evolution of cerebral tissue injury outcome of focal ischemia and a potential therapeutic target for ischemic stroke. In this review, we examine the evidence that angiogenesis can contribute to beneficial outcomes following focal ischemia in model systems. Several studies have shown that, following cerebral ischemia, endothelial proliferation and subsequent angiogenesis can be detected beginning four days after cerebral ischemia in the border of the ischemic core, or in the ischemic periphery, in rodent and non-human primate models, although initial signals appear within hours of ischemia onset. Components of the neurovascular unit, its participation in new vessel formation, and the nature of the core and penumbra responses to experimental focal cerebral ischemia, are considered here. The potential co-localization of vascular remodeling and axonal outgrowth following focal cerebral ischemia based on the definition of tissue remodeling and the processes that follow ischemic stroke are also considered. The region of angiogenesis in the ischemic core and its surrounding tissue (ischemic periphery) may be a novel target for treatment. We summarize issues that are relevant to model studies of focal cerebral ischemia looking ahead to potential treatments.
Keywords: Ischemic penumbra, ischemic core, remodeling, angiogenesis, axonal outgrowth
Introduction
The vascular events occurring within the ischemic penumbra following focal cerebral ischemia (ischemic stroke) have not been well worked out, yet their participation holds promise for new treatment approaches that could preserve cerebral tissue function. Among these events are potential contribution(s) of changes in the microvasculature, including focal “no-reflow,” new vessel formation, and vascular remodeling to tissue recovery. Here we address the development of new vessels in the setting of focal ischemia. Angiogenesis after ischemia could be either a pathological process contributing to the post-ischemic injury to the neuropil, or an attempt to limit injury, or both, or perhaps to recover tissue function. It may also have little benefit.
In this review, we will examine the recent evidence that angiogenesis can contribute to beneficial outcomes following focal ischemia in model systems. We also describe the co-localization of vascular remodeling and axonal outgrowth following focal cerebral ischemia based on the definition of tissue remodeling and the processes that follow ischemic stroke (the “maturation phenomenon,” reviewed by Ito et al.).1,2 We also discuss evidence supporting the notion that post-ischemic angiogenesis might contribute to cerebral tissue recovery.
Hypotheses
It has been proposed, based on observations in brain development,3,4 that angiogenesis is necessary for functional recovery after cerebral ischemia. However, the purpose and outcomes of angiogenesis may be different in the ischemic core and the penumbra. Hypotheses central to this notion that could be tested are that (1) the contributions of angiogenesis to tissue and functional preservation might differ with the region and degree of ischemic injury, (2) while apparently coincident, angiogenesis and axonal recovery may indeed be discoordinated, (3) angiogenesis may appear to occur in the ischemic core, but in fact is associated with mini-penumbras within these core regions, and/or (4) axonal recovery may occur independent of angiogenesis along established microvessel supply routes.
Ischemic core and penumbra
The ischemic penumbra was first defined by Astrup et al.5 as a zone of metabolically compromised tissue around the more densely affected ischemic center or core, with limited neuronal damage if the regional cerebral blood flow (rCBF) was restored by rapid therapeutic intervention (in that case, restitution of blood volume). This concept originated from electrophysiological studies in non-human primates with induced graded reduction in rCBF by partial exsanguination.6 That maneuver revealed CBF thresholds distinguishing: (i) a lower threshold, due to ion-pump failure, that was associated with tissue infarction, and (ii) an upper threshold, denoted by electrical failure, that was associated with preserved tissue structures.6 The zones of complete electrical failure and K+ release with functional inactivation, but not yet cell death, define the ischemic penumbra in the neocortex. Clinically, the diffusion/perfusion mismatch using magnetic resonance (MR) imaging or functional impairment, biochemical disturbances, tissue damage, and the duration of critical perfusion by positron emission tomography (PET) provide evidence of the putative ischemic penumbra.7–9
More recently, both experimental and clinical settings of focal cerebral ischemia have demonstrated that early after onset, the ischemic core and penumbra appear heterogeneous, each containing islands of “mini-cores” and “mini-penumbras.”10 Although not fully characterized, it is a fair assessment that these may represent varying spatiotemporal susceptibilities to injury and cell death.10
Definitions of the ischemic penumbra
Attempting to identify the “penumbra” in different model systems has led to the invention of several non-exclusive definitions of the ischemic penumbra that represent possible secondary features of the developing injury that may be reversible. The most general practical use of the term “ischemic penumbra” is that of a peri-infarct region salvaged by any treatment (Table 1).5 Hossmann11 described alterations in protein synthesis in the cerebrum following focal cerebral ischemia. Within the ischemic core, protein synthesis decreased early and was associated with ATP loss, whereas in the ischemic penumbra, ATP levels remained normal. Protein synthesis was initially depressed, yet recovered over time after reperfusion.
Table 1.
Definitions of ischemic penumbra in use.
| Year | Authors | The definition |
|---|---|---|
| 1981 | Astrup et al.5 | Zones of non-functioning, but viable, tissue that may recover electrophysiological function |
| 1993 | Hossmann11 | Regions where protein synthesis is depressed and ATP levels remain normal |
| 2000 | Heiss et al.7 | Regions within the thresholds for maintenance of function |
| 2006/2018 | Albers et al.8,9 | Regions of diffusion/perfusion mismatch as interpreted from functional images |
| 2000 | Sharp et al.12 | Regions consisting of selected molecule susceptibilities |
| 2011 | del Zoppo et al.10 | Ischemic cores and penumbral regions that heterogeneously develop (are not homogeneous), e.g. “mini-penumbras” |
The term “ischemic penumbra” has also been used for a region consisting of stratified layers or zones, some of which exhibit high levels of specific molecules of interest that might contribute to injury. These include the zone of selective cell death with alterations in heat shock protein (HSP)-70 expression or hypoxia inducible factor (HIF) related to the zone of spreading depression.12 In the HIF zone, for instance, HIF stimulates vascular endothelial growth factor (VEGF), inducible nitric oxide synthase (iNOS), and erythropoietin (EPO). VEGF and EPO have been associated with vascular remodeling in vitro and in vivo.13,14 In model studies in rodents, the “ischemic penumbra” has been taken by some investigators as regions peripheral to the core, whether in the cortex or in subcortical tissues (e.g. the basal ganglia), without a clear reference to recovery. However, care must be taken here as (i) the structures do not receive a comparable cerebral vascular supply to that of humans or non-human primates and (ii) often no proof is given that the peripheral regions have metabolic features of the “penumbra” as defined by Astrup et al.
Definitions of the ischemic core
Although the ischemic core is defined as a region or regions below perfusion threshold that correlate with characteristic electrophysiological changes, for practical reasons, in animal models, the core has also generally been defined as regions lacking microtubule-associated protein (MAP)-2 (MAP-2-negative region),15,16 regions displaying deoxyuridine triphosphate (dUTP)-positive cells or lesions,17 or terminal deoxyribonucleotidyl transferase-mediated dUTP-digoxigenin nick end labeling (TUNEL)-positive lesions18 depending upon the setting and model system used. Furthermore, several groups have demonstrated that the MAP-2-negative core or dUTP/TUNEL-positive ischemic core is not a homogeneous entity.19,20 Evidence of new vessel formation (called “angiogenesis” by some) has been observed in MAP-2-negative or dUTP+ regions.18,20–24
Abumiya et al.19 were among the first to demonstrate that markers associated with angiogenesis can appear within hours of the onset of focal ischemia in the ischemic core and periphery, in the awake non-human primate, and are highly significantly associated with activated microvessels, independent of time, after focal ischemia.
Periphery versus core
Experimental data from modeling studies, as well as patient imaging work, indicate that the core and penumbra are dynamic.10 The definitions of the ischemic core and penumbra, in particular of the ischemic penumbra, may be different for each study. The border of the ischemic core may involve the core itself, the penumbra, or both. The definition of the “border” of the ischemic core can overlap with the ischemic penumbra depending upon the experimental setting. Without metabolic features of the “penumbra,” the “ischemic periphery” is assumed to be a suitable expression that refers to tissue surrounding the ischemic core. However, importantly, with this understanding, the notion of “mini-cores” and “mini-penumbras” may be lost.
The neurovascular unit
The “neurovascular unit” is a conceptual anatomic framework that connects microvessels (endothelium, the basal lamina matrix, astrocyte end-feet, and pericytes) via intervening astrocytes to neurons and their axons, as supported by other cells (e.g. microglia, oligodendroglia).25–35 These are arrayed in networks. Efferent aspects of this arrangement are encompassed by “neurovascular coupling,” while the afferent activities have so far best been displayed by the consequences of restricted nutrient delivery, increased microvessel permeability, matrix protease expression, adhesion receptor change, and the consequences of vascular injury.30,36 Regional differences in the arrangements of neurovascular units in the CNS are likely, that could reflect endothelial cell function, matrix composition, and neuron vulnerability. Although, there is still little normative information about this in three-dimensions among various species.
It has been hypothesized that acute or chronic disruption of microvessel flow and structure can have irreversible effects on astrocyte and neuron function and survival.37 In the core, for instance, neurovascular units can be irreversibly damaged due to flow cessation and its consequences, while in the penumbra, a proportion of neurovascular units seem viable.10
Heterogeneity of the microvascular responses
The responses of the microvasculature in the territory-at-risk (e.g. in the striatum) to focal ischemia are very rapid, nearly as rapid as those of the neurons they supply. Within the core regions, in the first minutes after the onset of ischemia, changes in endothelial cell receptor presentation, matrix degradation, and detachment of astrocyte end-feet occur heterogeneously in pockets (“mini-cores”).10,38 Somewhat later, within 4 h after ischemia onset and large artery recanalization, microvessel obstruction can occur.39 The initial microvessel responses are neither simultaneous nor homogeneous. This suggests that in the early moments following arterial occlusion, the entire “core” is studded with pockets of “penumbra,” and that these “mini-cores” and their “mini-penumbras” evolve dynamically and heterogeneously depending upon local differences of microvessel perfusion.10 This implies that if not arrested, these mini-cores can grow into their respective mini-penumbras and consolidate to encompass a larger region of injury. In this evolution, signals are generated early that are consistent with new microvessel formation.19
Vascular remodeling
New blood vessels form by three related mechanisms: (i) angiogenesis, which refers to a combination of the sprouting of new vessels from pre-existing capillaries during a reactive phase, and longitudinal extension of pre-existing vessels in a process termed intussusception,40–42 (ii) vasculogenesis, which refers to initial events of vascular growth in which endothelial cell precursors (angioblasts) differentiate and assemble into primitive vessels during embryogenesis,43 or (iii) arteriogenesis, which refers to the formation of mature arteries from pre-existent interconnecting arterioles after an arterial occlusion.44 These forms of new vessel formation have been reviewed elsewhere.45,46 During embryology, vasculogenesis is essential for organ development. Leptomeningeal arteriogenesis may be inducible after cerebral ischemia,47 although anatomical features of the baseline collateral circulation are a potentially important consideration in the response to focal ischemia.48,49 Angiogenesis and arteriogenesis have been observed after ischemic stroke. Therefore, these two processes can be considered reactive to the signal ischemic event.
Angiogenesis and its quantitation
Studies conducted with animal models, as well as human patients, have been undertaken with the notion that angiogenesis following focal cerebral ischemia could facilitate functional recovery.
To evaluate angiogenesis, some investigators have measured the density of microvessels (microvessel number/unit area) in territories of interest. Microvessel density increases by 14 days after cerebral ischemia in the border of the ischemic core and has been reported to be associated with increased numbers of macrophages.50,51 The presence of a higher density of new vessels in the ischemic periphery and its persistence in the ischemic brain from 4 to 28 days following arterial occlusion in rat and mouse models have been observed with functional recovery following ischemic stroke upon administration of VEGF.52,53 Although the definition of the peri-infarct region is not clear in many studies, increased microvessel density in the peri-infarct region has been reported and has been attributed to or correlated with longer survival times in ischemic stroke patients.54–56
Another approach to the quantitation of new vessel formation following focal ischemia has employed the demonstration of immunoreactive collagen IV, as a measure of microvessel formation and/or presence, and the association with cell-specific BrdU incorporation.57
Others have evaluated endothelial activation using cell proliferation markers, and subsequent endothelial and microvessel surface marker immunoreactivities. Abumiya et al.19 demonstrated the highly coordinated appearance of VEGF and of integrin αv subunits in cerebral microvessels with endothelial cells expressing proliferating cell nuclear antigen (PCNA) beginning within 1–2 h following focal ischemia in the non-human primate within both the ischemic core and penumbra. Kanazawa et al.20 demonstrated that endothelial cells expressing the proliferation marker (Ki67) and subsequent angiogenesis involving CD31+ endothelial cells within the border in the ischemic core, but not in the MAP-2-positive ischemic periphery from seven days after cerebral ischemia in a rat model of focal cerebral ischemia using both the suture and thromboembolic techniques.20 Angiogenesis has been observed primarily in the border of the developing infarction, for instance as defined by MAP-2-negative regions or dUTP-positive regions (see Table 2).21–24 In addition, another marker of angiogenesis, endocan,58 was up-regulated in the MAP-2-negative ischemic core and not in the periphery (Supplementary Figure 1). Chen et al.57 also reported that new vessel formation, defined as collagen IV structures with BrdU+ endothelial cells, appeared in peri-infarct regions 14 days after ischemic stroke. Angiogenesis was also defined as increases in BrdU+CD31+ vessels59 or BrdU+vWF+ vessels following ischemic stroke.13,14 In contrast, astrocyte proliferation and gliosis occur in the ischemic periphery, but not in the ischemic core.18,20,22,24,60–62
Table 2.
Cell responses to cerebral ischemia within the neurovascular unit.
| Cell | Ischemic core | Ischemic periphery | References | |
|---|---|---|---|---|
| With treatment | Neuron | Loss | Axonal outgrowth | Li et al.60 Ishizaka et al.23 Kanazawa et al.20 |
| Endothelial cell | Angiogenesis18–24,52,99 (border area) | Angiogenesis23,57,146,147 | Abumiya et al.19 Zhang et al.52 Marti et al.21 Hill et al.22 Taguchi et al.147 Li et al.18,99 Wang et al.146 Ishizaka et al.201323 Chen et al.57 Jiang et al.24 Kanazawa et al.20 | |
| Pericyte | Proliferation and coverage of capillaries very increased | Proliferation and coverage of capillaries increased | Fernández-Klett et al.62 Hall et al.105 Kanazawa et al.20 | |
| Without treatment | Microglia | Very increased density | Increased density | Mabuchi et al.131 Li et al.18 Kanazawa et al.132 Jiang et al.24 Kanazawa et al.20 Zarruk et al.133 |
| Astrocyte | No change | Proliferation/gliosis | Hill et al.22 Li et al.60 Kanazawa et al.61 Li et al.18 Fernández-Klett et al.62 Jiang et al.24 Kanazawa et al.20 |
The locations of cellular responses within the neurovascular unit appear to be different for each cell type (references in Table 2). In addition, the responses of integrins to hypoxia versus ischemia also can be different with each stimulation (Table 3).
Table 3.
Responses of microvessel integrin expression to ischemia or hypoxia.
| Integrin | Ischemia | Hypoxia |
|---|---|---|
| α1β1 | Decreased (Tagaya et al.95) | ? |
| α3β1 | Decreased (Tagaya et al.95) | ? |
| αVβ3 | Increased (Abumiya et al.19 Huang et al.98) | ? |
| α5β1 | Increased (Li et al.18) | Increased (Milner et al.97) Increased (Li et al.99) Increased (Huang et al.98) |
| α5 | Increased (Milner et al.97) | Increased (Li et al.164) |
| α6β1 | Decreased (Li et al.18) | |
| α6β4 | Decreased (Tagaya et al.95) | Increased (Li et al.164) |
Angiogenesis: Receptors and stimulators
Angiogenesis involves the coordinated remodeling of the basal lamina matrix with extension of endothelial cells behind tip cells,63 in conjunction with the investiture by pericytes, to generate new blood vessels.35 A coordinated set of stimuli and receptors are involved.
VEGF has been implicated in endothelial cell proliferation, permeability, and angiogenesis (reviewed by Adams and Alitalo).64–66 In mammals, the VEGF family comprises five members: VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placenta growth factor. These members have different functions. While VEGF-A (VEGF) participates in endothelial cell proliferation, permeability, and angiogenesis,66 VEGF-B mediates embryonic angiogenesis in myocardial tissue, and VEGF-C predominantly mediates lymphangiogenesis.66 VEGF plays roles in angiogenesis in cerebral ischemia.43,67 In vitro studies have demonstrated that after oxygen–glucose deprivation, VEGF derives from endothelial cells,68 neurons,69 astrocytes,20,70 and microglia.20 Thus, after cerebral ischemia, both VEGF and the VEGF receptor (VEGFR) are found on endothelial cells,20,21,61,71 neurons,20,61,69,71 astrocytes,20,61 and microglia.20,61,72 The role of pericytes in this respect is fully unknown. Binding of VEGF to the receptor tyrosine kinase VEGFR-2 (also known as KDR or Flk1) promotes endothelial proliferation and angiogenesis.73,74 This function is counteracted by the receptor VEGFR-1 (also known as Flt1), which has higher affinity for VEGF, but weak tyrosine-kinase activity.73–75 VEGFR-1 also exists in a secreted, catalytically inactive and, therefore, inhibitory isoform.74,75 The interactions of VEGF with VEGFR-2 induce angiogenic sprouting by guiding filopodial extension from endothelial cells at the tips of the vascular sprouts in vitro.76 Integrin αvβ3 binds directly to VEGFR-2 for receptor activation and downstream signaling of angiogenesis in the presence of VEGF.77 VEGF also mediates increased vascular permeability and blood–brain barrier disruption via increased intracellular calcium,78 src activation,79 and unknown mechanisms.52,80 Incidentally, the participation of matrix metalloproteinase-9 up-regulation has been suggested61; however, the exact involvement is still unclear. Hence, VEGF may be a double-edged sword after cerebral ischemia.
During development, VEGFR expression precedes the appearance of angiopoietin receptors on nascent blood vessels.81 Here the tyrosine kinase receptors Tie-1 and Tie-2 are expressed in relation to the ligands angiopoietin-1 (Ang-1) and Ang-2.45,82 Tie-1 and Tie-2 are expressed on endothelial cells. In the CNS, capillary integrity is maintained by the constitutive interaction of Ang-1 from astrocytes with the Tie-2 receptor on endothelial cells.83 Null preparations for Tie-2 and Ang-1 fail to develop perivascular cells, although vasculogenesis occurs during development.84 Tie-1 is required for vascular network formation. Murine Tie-2−/− constructs fail to establish the integrity of the developing endothelium resulting in edema and hemorrhage,85 consistent with the premise that Ang-1 and Tie-2 regulate capillary tubule formation during vasculogenesis.86
Reports have described that by two days after cerebral ischemia, both VEGFRs are induced in microvessels in the peri-infarct regions with strong expression at three days post-ischemia.21 By seven days after cerebral ischemia, expression levels of VEGFR-1 and -2 return to normal. A separate group also demonstrated that both VEGFR-1 and -2 are induced in endothelial cells at the border of the ischemic core and peripheral regions at one and three days post-ischemia.87 Moreover, increasing levels of Ang-1 mRNA were detected at 24 h of ischemia and persisted for 28 days. Ang-2 mRNA was not detected until 24 h after ischemia onset, and the increased Ang-2 mRNA persisted for 28 days after ischemia.88 Ang-2 has been shown to increase blood–brain barrier compromise, vascular permeability, and injury volume.89 Tie-1 and Tie-2 were expressed in the ischemic cortex, with Tie-2 appearing in the outer cortical layers and Tie-1 detected in layers II-IV on capillary-like structures.90 As with brain development, angiogenesis is a response to cerebral hypoxia and contributes to the pathology of injury development.
Notable also are the signaling responses of microvessel pericytes to ischemia and innate inflammation.35,91 Pluripotent cells embedded in the basal lamina matrix of cerebral capillaries, pre-capillary arterioles and post-capillary venules contribute to the integrity of the blood–brain barrier; these cells undergo transformation and migrate in response to injury.92–94 Pericyte migration and proliferation are required for the branching and generation of new microvessels during angiogenesis.63 This is also stimulated during hypoxia.
Integrin receptors and changes in the setting of new vessel formation
It is noteworthy that in the awake non-human primate model, by 2 h after middle cerebral artery (MCA) occlusion in the basal ganglia, where microvessel-associated β1-integrin antigen decreased rapidly in the core and significantly, β1-integrin transcripts and αv integrin subunits were up-regulated within the boundaries between individual ischemic minicores.19,95 Loss of VLA-1, -3, and -6 occurred acutely relative to the loss of matrix protein immunoreactivity from the microvessel basal lamina within hours after ischemia onset.95 In contrast, the fibronectin receptor integrin αvβ3 expression in cerebral microvessels that was minimal under normoxia, increased significantly in the early hours following MCA occclusion.19,96
α5β1-integrin expression under conditions of ischemia18 and under hypoxia97–99 has been studied. The α5-integrin subunit, which is paired with the β1-integrin subunit in vascular remodeling, is expressed by capillary endothelial cells in dUTP+-positive tissue following cerebral ischemia in the mouse seven days after ischemia onset.18 Increased α5-integrin subunit expression after cerebral ischemia was closely correlated with the number of Ki67-positive endothelial cells, in the border of the ischemic core.18 Integrin α5β1 participates in angiogenesis in the border of the ischemic core following the onset of focal cerebral ischemia,18 while integrin-α5β1 is required for angiogenesis during embryogenesis.97,100 The integrin α5β1 (i) appears transiently during embryogenesis of the brain vasculature, and (ii) underlies the vasculogenesis associated with cerebral tissues exposed to chronic hypoxia.97 Angiogenesis induced by tumor necrosis factor-α (TNF-α) and VEGF could occur via up-regulation of α5-integrin and binding between integrin α5β1 and Ang-2 in endothelial cells.98,101 Finally, endothelial cell/microvessel proliferation and subsequent angiogenesis are detected in the border of the MAP-2-negative ischemic core, but not in the ischemic periphery, following cerebral ischemia alone (i.e. without therapeutic intervention) (Figure 1(a)).18–21
Figure 1.
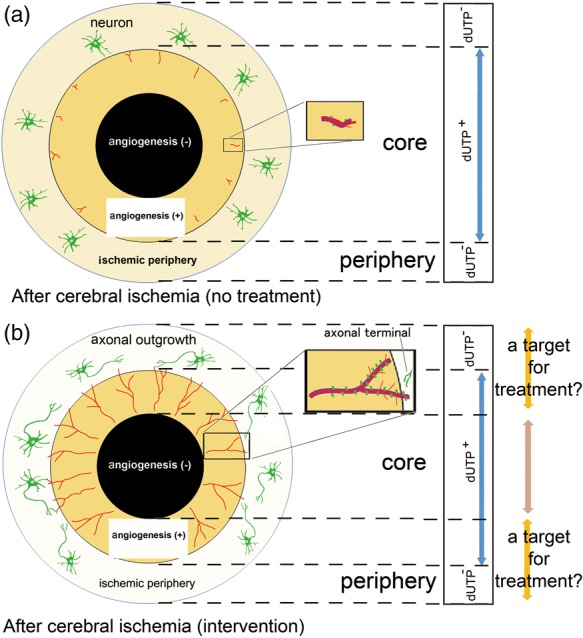
A scheme for angiogenic responses following focal cerebral ischemia (modified from reference Kanazawa et al.20). (a) A proposed scheme depicting the location of angiogenesis after cerebral ischemia. The ischemic core can be defined as the deoxyuridine triphosphate (dUTP)-positive or microtubule-associated protein 2 (MAP-2)-negative ischemic tissue. Angiogenesis (red lines) occurs at the border of the ischemic core (orange) and the peripheral regions. Endothelial sprouting occurs prior to pericyte coverage of capillaries (purple lines). Angiogenic endothelial cells following cerebral ischemia express integrin α5β1 (green columns) in capillaries. Thus, the dUTP-positive or MAP-2-negative ischemic core consists of the angiogenesis-positive ischemic core and angiogenesis-negative ischemic core (black) which can proceed to irreversible change. (b) A proposed depiction of angiogenesis and axonal outgrowth (green cells: neurons and axons) in the setting of treatment interventions after cerebral ischemia. Interventions have been proposed to promote angiogenesis (red lines), with integrin α5β1 expression (green columns) and pericyte coverage of capillaries (purple lines) in angiogenesis vessels in the border of ischemic core. Axonal outgrowth by neuronal cells (green) in association with interventions could be found in the ischemic periphery.
In addition, up-regulation of integrin α5β1 has been associated with the up-regulation of the tight junction proteins claudin-5 and ZO-1, and is associated with Ang-1 in vascular remodeling.102 Fibronectin, α5β1-integrin, and αvβ3-integrin contribute to endothelial sprouting that is distinct from VEGF-mediated angiogenesis, yet important for the growth of tumors in the cancer setting.103
Angiogenesis in response to focal cerebral ischemia
New blood vessels that form after focal ischemia appear by days 4 to 7 in peri-infarct regions.102 Tsutsumi et al.40,41 have demonstrated capillary bud formation by seven days in the cerebral ischemic region in a canine model. Endothelial sprouting and increased capillary density 10 days after cerebral ischemia have been detected prior to the coverage of endothelial cells by pericytes.104 At the same time, there is a highly significantly coordinated increased expression of VEGF and αv-integrin subunits in the same microvessels that also display proliferative signals, as indicated by the presence of PCNA.19 The significance of these interactions is emphasized by the fact that they are independent of time from MCA occlusion, occurring from 2 h through seven days post-ischemia. These events occur in 5 to 20-µm diameter microvessels, primarily in the ischemic core and much less in the peripheral regions. Based on the diameter, it seems that the site of angiogenesis is most consistently located in the capillaries.105 Hence, elements known to be associated with angiogenesis/new vessel formation, including VEGF and integrin subunit αv, appear early following occlusion of the MCA in the awake non-human primate.19
Interactions with oligodendrocytes
As the concept of neurovascular unit suggests, oligodendrocytes do not stand alone. Rather they actively interact with neighboring cells to allow the integrated control of white-matter function as demonstrated in subcortical ischemia106 and traumatic brain injury.107 In addition, oligodendrocyte precursor cell (OPC) migration appears to occur along vascular tracks, and does not require the participation of pericytes during vasculogenesis in the developing CNS.108 Crosstalk among axons, microvessels, and oligodendrocytes may be important for remodeling and plasticity in post-ischemic injury zones. Proof of cross-cellular communication requires the further teasing apart of complex inter-relationships. This may be accomplished with high-quality ex vivo tissue slice and in vivo preparations.
Return of axons
Axonal regeneration stands in contrast to “axonal sprouting,” which is axonal outgrowth from uninjured neurons in response to injury.109,110 Regeneration of axons following injury in the CNS is limited.110–113 Methodologically, distinguishing between regenerating and sprouting axons is important, because while regeneration failure is the norm in the adult mammalian CNS, axonal sprouting might explain the partial spontaneous recovery of function that is observed following an ischemic event.114,115
Pioneering work by David and Aguayo and Benfey and Aguayo et al.116,117 demonstrated that adult mammalian CNS neurons, which normally do not regenerate, are able to grow for long distances into the permissive environment of a peripheral nerve graft. More recently, Izumi et al.118 demonstrated that, in vitro, dopaminergic neurites can extend along striatal neurons in the paired-cultures of mesencephalic cells with striatal cells, and that these extensions appear to depend on integrin α5β1.
Therefore, the term “axonal outgrowth” may be suitable for recovery in ischemic settings. Axonal outgrowth in the absence of intervention does not appear until 14 to 28 days after cerebral ischemia even in the ischemic periphery (Table 2).20,119 Most studies have evaluated axonal outgrowth by limited immunohistological means and not functional responses. As the ischemic core is a MAP-2-negative region in experimental settings, axonal outgrowth within the core would not be expected to be observed. However, when angiogenic vessels are in the core, it would be proposed that these could occur in mini-penumbras. This possibility has not yet been tested. Studies generally have not simultaneously evaluated microvessel responses, and the relationship between angiogenesis and axonal outgrowth.
Angiogenesis and axonal outgrowth/neurogenesis
It might be expected that angiogenesis would be essential for ischemic brain repair as this event allows increased blood flow and metabolic nutrients to the stricken regions. Angiogenesis has been purported to be associated with neurogenesis,59,120,121 and the assertion that neurogenesis is dependent on new vessel formation for long-term survival.122 However, it is speculative whether angiogenesis and axonal outgrowth/neurogenesis could occur together and be coordinated in space and time in vivo.
Experimental systems
Using ex vivo co-culture systems, possible interactions between angiogenesis and neurogenesis have been examined.123,124 For instance, recently, some characteristics of a three-dimensional culture model, including neurons, endothelial cells, and extracellular matrix, have been described.124 In three dimensions in vivo, features of angiogenesis and neurogenesis appear to be coupled.59 Furthermore, recently it has been reported that oligodendrocyte precursor cells (OPCs) require vasculature for their extensive migration through the brain and spinal cord during development in whole tissue preparations.108 After ischemia, VEGF from vessel components might prompt axonal outgrowth.68,125,126 Recently, Lei et al.127 and Tan et al.128 reported that axons bound to laminin expressed a β1-integrin, and that laminin/β1-integrin signaling might contribute to axon development and promote axonal outgrowth in vitro. However, it is not known whether β1-integrins behave in this way in brain tissue. It is known for endothelial cells that integrin expression is matrix-dependent in vivo and in vitro.129,130 Angiogenesis in the ischemic core may be associated with axonal outgrowth via laminin/β1-integrin from the periphery. It raises the possibility that angiogenesis and neurogenesis are coupled by VEGF and laminin/β1-integrin signaling, although proof that this is relevant in vivo has not appeared. In typical immunohistochemical preparations, neurons do not display β1-integrins.
Microglia, axons, and angiogenesis
Microglial proliferation and/or monocyte infiltration have been observed mainly in and around the ischemic core, in the same regions in which angiogenesis has been seen.18,20,131,132 One hypothesis suggests that newborn vessels in conjunction with activated microglia and/or macrophages contribute to the clearing up of cellular debris.50,51 While this hypothesis is still unproven, animals in which macrophages were not seen in ischemic brain regions also did not display increased microvessel density compared to naïve animals.50 Microglial and monocyte/macrophage phenotypes have been defined as either classic (pro-inflammatory; M1-like) or alternative (anti-inflammatory or protective; M2-like) under pathophysiological conditions. For instance, increased numbers of microglia and monocyte/macrophages have been detected in the borders between the ischemic core and peripheral regions, especially on the side of the ischemic core, several days after focal ischemia (Table 2).20,131–133 Although several studies have demonstrated that pro-inflammatory M1-like microglia might expand cerebral infarction volume in the early (i.e. several days) phase,131,134 anti-inflammatory M2-like microglia might facilitate angiogenesis and axonal outgrowth, due to the secretion of remodeling factors, such as VEGF and TGF-β in the subacute and chronic phases.20,134–136 Jin et al.137 demonstrated that metformin treatment induced M2-like microglial polarization, which was associated with increasing numbers of microvessels following stroke (Table 4). Those observations are also consistent with the notion that angiogenesis that follows cerebral ischemia might be localized in the border between ischemic core and the periphery, perhaps in conjunction with microglia and/or macrophages.20 However, causal relationships have not yet been reported.
Table 4.
The effects of discrete interventions on neuronal/axonal and microvessel components of the neurovascular unit after focal cerebral ischemia.
| Citation | Ischemic duration | The timing for intervention after ischemia | Intervention | Outcome measures | After intervention | Results | What happened to neurons/ axons | What happened to microvessels (assay for measurement) |
|---|---|---|---|---|---|---|---|---|
| Moriyama et al.148 | Microsphere-embolized | 7 days | Injection ofneural stem cells | Not examined | Up to 28 days | No data | No data | Angiogenesis(proliferation, surface marker, density) |
| Taguchi et al.147 | Permanently ligated | 48 h | Injection of CD34 + bone marrow cells | Locomotion test | Up to 90 days | 160% of functional improvement | Increasing number of neuronsReduced infarct volume | Angiogenesis(proliferation, surface marker, density) |
| Ishizaka et al.23 | 75 min | 1 or 4 days | Injection of mesenchymal stem cells | Cylinder test | Up to 21 days | 125% of functional improvement | Reduced infarct volume | Angiogenesis (density) |
| Kanazawa et al.20 | 90 min | 7 days | Injection of M2-like microglia | Corner test | Up to 28 days | 400% of functional improvement | Axonal outgrowth | Angiogenesis(proliferation, surface marker, density) |
| Zhang et al.52 | Permanently occluded by a single clot | 48 h | Injection of VEGF | Rotarod test | Up to 28 days | 130% of functional improvement | No data | Angiogenesis (density) |
| Wang et al.13 | 30 min | 15 min | Injection of VEGF | Not examined | Up to 56 days | No data | Reduced infarct volumeAxonal outgrowth | Angiogenesis(proliferation, surface marker, density) |
| Wang et al.146 | 60 min | For up to 14 days | Injection of valproate | Rotarod test | Up to 14 days | 160% of functional improvementInduction of VEGF | Reduced infarct volume | Angiogenesis(surface marker, density) |
| Jin et al.137 | 60 min | 24 h | Injection of metformin | NSS Corner test Rotarod | Up to 30 days | 130% of functional improvement M2 polarization | Increased numbers of neurons | Angiogenesis (proliferation, surface marker, density) |
| Chen et al.57 | Permanently ligated | 30 min | Intranasal delivery of apelin-13 | Walking test Hanging test | Up to 21 days | 200% of functional improvement Induction of VEGF | Reduced infarct volume | Angiogenesis (proliferation, surface marker, density) |
| Jin et al.149 | 35 min | 3 to 8 days | Oral administration of Sonic Hedgehog agonist | Locomotion test Barnes maze test | Up to 30 days | 250% of functional improvement | Neuronal proliferation Reduced infarct volume | Angiogenesis (surface marker, density) |
Ischemic core versus periphery in vitro and in vivo
If one defines the ischemic core as a region or regions below adequate tissue perfusion threshold and the ischemic periphery as surrounding the ischemic core tissue, it is technically difficult to distinguish between ischemic core and periphery in in vitro models. However, the levels of O2 deprivation and/or nutrient supply can have differential effects on cell survival in vitro that could mimic aspects of the core and penumbra. To date, focal cerebral ischemic models in vivo would be the appropriate setting for this evaluation. However, the study of molecular and microcirculatory communication between the ischemic core and periphery is very relevant and may be facilitated by in vitro modeling.
Angiogenesis and recovery in the subacute to chronic phase of cerebral ischemia
It has been demonstrated that angiogenesis is a necessary early process for recovery after cardiac ischemia138 or peripheral artery disease.139 This raises the possibility that enhancement of angiogenesis could be a potential strategy to facilitate functional recovery after ischemic stroke.120,121
Theoretically, the vasculature adjacent to the injury zones may play roles in new vessel formation. The brain employs a low pressure/high flow vascular system in which the established Circle of Willis and pial collateral vasculature provide protection from significant focal ischemic injury.140,141 The volume of ischemic injury is in part determined by collateral protection from arterial segments and venous outflow separate from the arterial supply to the territory-at-risk.142 While the pial anastomoses that provide potential collateral protection of the cortex are under genetic control,49 the vascular network for the protection of the striatum in humans and higher mammals is currently unknown. Furthermore, in small animal species, it has been shown that anesthesia can increase the cerebral blood volume (CBV) by 10 to 36% from latent channels.143,144
Important to the processes of recovery and limitation of injury is capillary density. In a rat model of focal cerebral ischemia, arteriolar collateral growth and new capillaries restored perfusion in the borders of the ischemic regions 30 days after ligation of the MCA branches.145 Additionally, in a model of focal cerebral ischemia in the male Sprague-Dawley rat, increased capillary density in the cortical peri-infarct region was associated with partial functional recovery by 7 and 14 days post-MCA occlusion.146
Interventions
A number of reports using rodent models of focal ischemia have described the effects of pharmacological and cell-based treatments on the ischemic brain tissue of animals that appeared to increase angiogenesis in the border of the ischemic core. VEGF and/or cell-based interventions appeared to induce angiogenesis at the border of the ischemic core, and axonal outgrowth in the ischemic periphery (Figure 2(b)).20,126 Several groups have demonstrated that in ischemic brain tissue of animals receiving cell-based or pharmacological treatments, mediators might be associated with a 130–400% improvement in functional outcome compared to controls (Table 4).13,20,23,52,57,137,146–149 However, the direct causal relationships between angiogenesis and functional recovery have not yet been proven.
Figure 2.
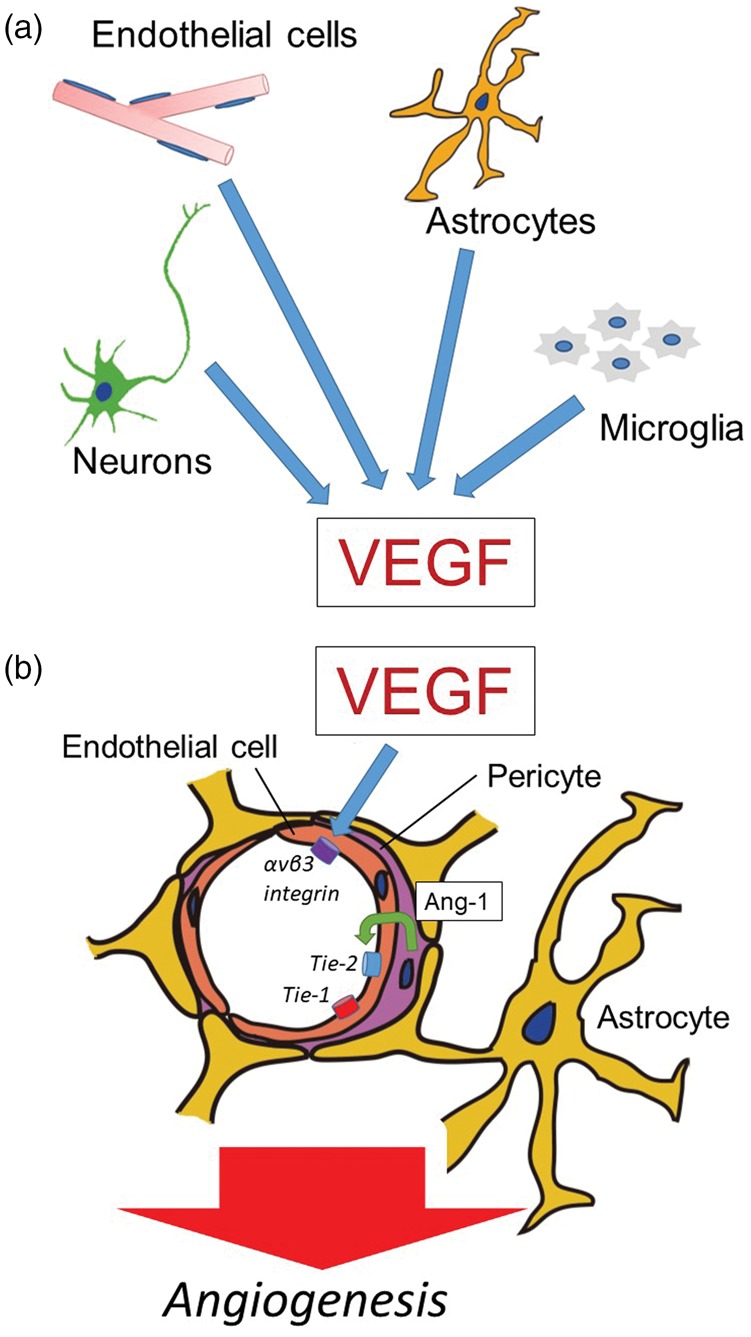
Roles of VEGF in angiogenesis of cerebral microvessels. (a) After focal ischemia, vascular endothelial growth factor (VEGF) is secreted from endothelial cells, astrocytes, neurons, and/or microglia. However, there is no direct evidence whether VEGF from neurons enhances angiogenesis because axonal outgrowth and angiogenesis can differ in the location after cerebral ischemia in vivo. (b) VEGF participates in angiogenesis in the brain following focal ischemia. The integrin αvβ3 is expressed in coordination with VEGF. Capillary integrity is maintained by the constitutive interaction of angiopoietin-1 (Ang-1) from pericytes with the Tie-1 and Tie-2 receptor on endothelial cells.
Intra-arterial injection of cultured mesenchymal stem cells appeared to enhance angiogenesis and associated axonal outgrowth in the peri-infarct regions during the chronic post-ischemic period following MCA occlusion in rats (Table 4).23 In a separate study, systemic administration of human cord blood-derived CD34+ cells or neural progenitor cells following ischemic stroke also enhanced angiogenesis in the ischemic border and suggested a favorable environment for axonal outgrowth.147,148 Axonal outgrowth was, in turn, observed to have effects around angiogenesis, although a causal relationship was not identified.
Systemic administration of valproate (VPA), a histone deacetylase (HDAC) inhibitor commonly used to treat seizures and bipolar disorder, was observed to further increase capillary density at 14 days post-MCA occlusion and to improve functional recovery in a rat model.146 No other times were examined. In contrast, 2-methoxyestradiol (2ME2), a natural metabolite of estradiol, was shown to inhibit hypoxia-dependent HIF-1 stabilization and appeared to inhibit the effects of VPA on capillary density and on rotarod performance.146 Separately, it has also been reported that new vessel formation, defined as collagen IV structures with BrdU+ endothelial cells, was significantly increased in peri-infarct regions by apelin-13, and was associated with the appearance of VEGF 14 days after ischemic stroke.57 A Sonic Hedgehog agonist delivered post-stroke enhanced angiogenesis in the border of the ischemic core and also increased functional recovery in a mouse focal cerebral ischemia model.149 The observation that increased vascular density can improve functional recovery following several unrelated interventions after cerebral ischemia have not yet proven causality.
Direct interactions between angiogenesis in the ischemic core and axonal outgrowth in the ischemic periphery have not been studied. It is unknown whether angiogenesis in the ischemic periphery could aid in shrinking the ischemic core. However, angiogenesis might provide a suitable environment that triggers axonal outgrowth, although this is not conclusively known. In separate studies, axonal outgrowth with functional recovery was observed after spinal cord injury by delivered chondroitinase ABC111 and grafted Schwann cells112 or human neural stem cells.113 Those observations raise the possibility that agents and/or cell-therapies that prompt angiogenesis at the border of the ischemic core, and axonal outgrowth in the ischemic periphery, may offer opportunities for treatment although the precise mechanisms have yet to be clearly identified (Figure 1(b)).
Quality of the evidence so far
A significant limitation of these studies so far is the lack of clear evidence for a direct link mechanistically among angiogenesis, axonal outgrowth, and functional recovery. To date, the majority of reports that have examined the interaction of an intervention with the development of additional angiogenesis in association with cortical focal ischemic lesions, have been correlational or associational, and have not shown causality with regard to outcome improvement.
After focal cerebral ischemia, specific interventions including the administration of cell-based treatments or VEGF appear to (i) induce axonal outgrowth following angiogenesis, or (ii) have been accompanied by proliferation of pericytes that cover the endothelial cells on the border of the ischemic core, for instance (Figure 1(b)).18,20,23,98 Several mediators seem to prompt both angiogenesis and axonal outgrowth and communicate with the cells within affected neurovascular units after cerebral ischemia. These include VEGF19,20,69–70,126 (Figure 2(a) and (b)), TGF-β,20,150 Ang-1151,152 (Figure 2(b)), platelet-derived growth factor-B (PDGF-B),153 and brain-derived neurotrophic factor (BDNF).154 In other words, each cell in the neurovascular unit could communicate with the other cells by several mediators.28 This is very similar to the situation displayed by innate inflammatory responses within the neurovascular unit.28,155–157
Other processes
A process termed endothelial-mesenchymal transition (EndMT) has received attention in recent years. During EndMT, the stable interactions of endothelial cells and mesenchymal cells (e.g. pericytes, smooth muscle cells, fibroblasts) can be disturbed when claudin-5, occludin, and VE-cadherin within the junctional complex disaggregate or are lost thereby increasing endothelial permeability.158–160 These events are similar to the changes accompanying increased microvessel permeability during focal ischemia52 and are seen during the formation of cerebral cavernous malformation. EndMT involves β1-integrins, oxygen-free radicals, angiopoietin-2, TGF-β, and VEGF.161–163 Although contributions of EndMT to microvascular changes after cerebral ischemia have not yet been investigated, elements of EndMT may be seen in post-ischemic angiogenesis as currently described.
Some questions that remain
A number of issues involving the development, presence, and contributions of angiogenesis in the CNS following focal cerebral ischemia, in a number of models and in humans, for (i) injury evolution, (ii) clearance of tissue debris, and (iii) processes leading to functional improvement, have not been rigorously explored or described yet. These include
(1) the location of the core versus peripheral regions, and the timing of changes in these locations/regions for each model and for the ischemic stroke patient,
(2) differences in mechanism and outcome between the ischemic core and the penumbra when angiogenesis has been augmented,
(3) the sequence and interaction of molecular and cellular mechanisms of angiogenesis after cerebral ischemia,
(4) how angiogenesis might work for functional recovery after cerebral ischemia,
(5) constructive interactions between angiogenesis and axonal outgrowth after cerebral ischemia,
(6) how cell–cell interactions that modulate angiogenesis, axonal outgrowth, and functional outcomes might proceed in response to injury or be inhibited in the normoxic condition,
(7) vascular cell-extracellular matrix and neuron interactions that modulate these processes,
(8) how the components of the neurovascular unit contribute to angiogenesis, and how they are altered, and
(9) whether the new cerebral vessels and axons are functional.
Conclusion
Angiogenesis appears to be stimulated in the border of the ischemic core in rodent and non-human primate models acutely following the onset of focal ischemia. New capillaries are observed 4 to 14 days after cerebral ischemia in these model systems. Furthermore, axonal outgrowth is observed in the ischemic periphery surrounding the angiogenesis. Several interventions appear to enhance angiogenesis and axonal outgrowth and might result in functional recovery; however, causality and specific mechanisms have not yet been described. The regions of angiogenesis in the ischemic core and its surrounding tissue (ischemic periphery) may be novel targets for treatment. However, further discrete focused work to develop clear mechanisms for functional recovery based on angiogenesis is required.
Supplemental Material
Supplemental Material for Angiogenesis in the ischemic core: A potential treatment target? by Masato Kanazawa, Tetsuya Takahashi, Masanori Ishikawa, Osamu Onodera, Takayoshi Shimohata and Gregory J del Zoppo in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a Grant-in-Aid for Scientific Research (Research Project Number: 15K19478 and 18K07493), a grant from Takeda Science Foundation, the Bayer Scholarship for Cardiovascular Research, Japan Cardiovascular Research Foundation, Astellas Foundation for Research on Metabolic Disorders, and Young Investigator Okamoto Award, and Medical Research Encouragement Prize of the Japan Medical Association (MK).This work has been supported by parts of the grants NS 053716, NS 038710, and NS 036945 from the National Institutes of Health (GJdZ).
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:
M Kanazawa: None.
T Takahashi: None.
M Ishikawa: None.
O Onodera: None.
T Shimohata: An academic adviser of the ShimoJani LLC biotech company.
G J del Zoppo: None.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Ito U, Spatz M, Walker JT, Jr, et al. Experimental cerebral ischemia in mongolian gerbils. I. Light microscopic observations. Acta Neuropathol 1975; 32: 209–223. [DOI] [PubMed] [Google Scholar]
- 2.Ito U, Kuroiwa T, Nagasao J, et al. Temporal profiles of axon terminals, synapses and spines in the ischemic penumbra of the cerebral cortex: ultrastructure of neuronal remodeling. Stroke 2006; 37: 2134–2139. [DOI] [PubMed] [Google Scholar]
- 3.Etchevers HC, Vincent C, Le Douarin NM, et al. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 2001; 128: 1059–1068. [DOI] [PubMed] [Google Scholar]
- 4.Eichmann A, Thomas JL. Molecular parallels between neural and vascular development. Cold Spring Harb Perspect Med 2013; 3: a006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia – the ischemic penumbra. Stroke 1981; 12: 723–725. [DOI] [PubMed] [Google Scholar]
- 6.Astrup J, Symon L, Branston NM, et al. Cortical evoked potential and extracellular K + and H + at critical levels of brain ischemia. Stroke 1977; 8: 51–57. [DOI] [PubMed] [Google Scholar]
- 7.Heiss WD, Kracht L, Grond M, et al. Early [(11)C] Flumazenil/H(2)O positron emission tomography predicts irreversible ischemic cortical damage in stroke patients receiving acute thrombolytic therapy. Stroke 2000; 31: 366–369. [DOI] [PubMed] [Google Scholar]
- 8.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 2006; 60: 508–517. [DOI] [PubMed] [Google Scholar]
- 9.Albers GW, Marks MP, Kemp S, et al. DEFUSE 3 investigators thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del Zoppo GJ, Sharp FR, Heiss WD, et al. Heterogeneity in the penumbra. J Cereb Blood Flow Metab 2011; 31: 1836–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hossmann KA. Disturbances of cerebral protein synthesis and ischemic cell death. Prog Brain Res 1993; 96: 161–177. [DOI] [PubMed] [Google Scholar]
- 12.Sharp FR, Lu A, Tang Y, et al. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab 2000; 20: 1011–1032. [DOI] [PubMed] [Google Scholar]
- 13.Wang YQ, Cui HR, Yang SZ, et al. VEGF enhance cortical newborn neurons and their neurite development in adult rat brain after cerebral ischemia. Neurochem Int 2009; 55: 629–636. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Zhang Z, Wang Y, et al. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke 2004; 35: 1732–1737. [DOI] [PubMed] [Google Scholar]
- 15.Dawson DA, Hallenbeck JM. Acute focal ischemia-induced alterations in MAP-2 immunostaining: description of temporal changes and utilization as a marker for volumetric assessment of acute brain injury. J Cereb Blood Flow Metab 1996; 16: 170–174. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H, Shimohata T, Wang JQ, et al. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci 2005; 25: 9794–9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tagaya M, Liu KF, Copeland B, et al. DNA scission after focal brain ischemia. Temporal differences in two species. Stroke 1997; 28: 1245–1254. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Liu F, Welser-Alves JV, et al. Up-regulation of fibronectin and the α5β1 and αvβ3 integrins on blood vessels within the cerebral ischemic penumbra. Exp Neurol 2012; 233: 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abumiya T, Lucero J, Heo JH, et al. Activated microvessels express vascular endothelial growth factor and integrin alpha(v)beta3 during focal cerebral ischemia. J Cereb Blood Flow Metab 1999; 19: 1038–1050. [DOI] [PubMed] [Google Scholar]
- 20.Kanazawa M, Miura M, Toriyabe M, et al. Microglia preconditioned by oxygen-glucose deprivation promote functional recovery in ischemic rats. Sci Rep 2017; 7: 42582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marti HJ, Bernaudin M, Bellail A, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol 2000; 156: 965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill WD, Hess DC, Martin-Studdard A, et al. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol 2004; 63: 84–96. [DOI] [PubMed] [Google Scholar]
- 23.Ishizaka S, Horie N, Satoh K, et al. Intra-arterial cell transplantation provides timing-dependent cell distribution and functional recovery after stroke. Stroke 2013; 44: 720–726. [DOI] [PubMed] [Google Scholar]
- 24.Jiang MQ, Zhao YY, Cao W, et al. Long-term survival and regeneration of neuronal and vasculature cells inside the core region after ischemic stroke in adult mice. Brain Pathol 2017; 27: 480–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res 1998; 53: 637–644. [DOI] [PubMed] [Google Scholar]
- 26.del Zoppo GJ, Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol 2006; 26: 1966–1975. [DOI] [PubMed] [Google Scholar]
- 27.del Zoppo GJ, Milner R, Mabuchi T, et al. Vascular matrix adhesion and the blood-brain barrier. Biochem Soc Transac 2006; 34: 1261–1266. [DOI] [PubMed] [Google Scholar]
- 28.del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience 2009; 158: 972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.del Zoppo GJ. Relationship of neurovascular elements to neuron injury during ischemia. Cerebrovasc Dis 2009; 27(Suppl 1): 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.del Zoppo GJ. The neurovascular unit in the setting of stroke. J Intern Med 2010; 267: 156–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.del Zoppo GJ. The neurovascular unit, matrix proteases, and innate inflammation. Ann N Y Acad Sci 2010; 1207: 46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daneman R, Zhou L, Kebede AA, et al. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010; 468: 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010; 68: 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonkowski D, Katyshev V, Balabanov RD, et al. The CNS microvascular pericyte: pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids Barriers CNS 2011; 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 2016; 19: 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 2017; 96: 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab 2003; 23: 137–149. [DOI] [PubMed] [Google Scholar]
- 38.Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med 2005; 39: 51–70. [DOI] [PubMed] [Google Scholar]
- 39.del Zoppo GJ, Schmid-Schönbein GW, Mori E, et al. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke 1991; 22: 1276–1283. [DOI] [PubMed] [Google Scholar]
- 40.Tsutsumi K. Experimental cerebral infarction in the dog: scanning electron microscopy with vascular endocasts of the microvessels in the ichemic brain. Neurol Med Chir 1986; 26: 595–600. [DOI] [PubMed] [Google Scholar]
- 41.Tsutsumi K, Shibata S, Inoue M, et al. Experimental cerebral infarction in the dog: ultrastructural study of microvessels in subacute cerebral infarction. Neurol Med Chir 1986; 27: 73–77. [DOI] [PubMed] [Google Scholar]
- 42.Mettouchi A, Meneguzzi G. Distinct roles of beta1 integrins during angiogenesis. Eur J Cell Biol 2006; 85: 243–247. [DOI] [PubMed] [Google Scholar]
- 43.Risau W. Mechanisms of angiogenesis. Nature 1997; 386: 671–674. [DOI] [PubMed] [Google Scholar]
- 44.Cai W, Schaper W. Mechanisms of arteriogenesis. Acta Biochim Biophys Sin 2008; 40: 681–692. [PubMed] [Google Scholar]
- 45.Risau W, Esser S, Engelhardt B. Differentiation of blood-brain barrier endothelial cells. Pathologie et Biologie 1998; 46: 171–175. [PubMed] [Google Scholar]
- 46.Greenberg DA. Cerebral angiogenesis: a realistic therapy for ischemic disease?. Methods Mol Biol 2014; 1135: 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugiyama Y, Yagita Y, Oyama N, et al. Granulocyte colony-stimulating factor enhances arteriogenesis and ameliorates cerebral damage in a mouse model of ischemic stroke. Stroke 2011; 42: 770–775. [DOI] [PubMed] [Google Scholar]
- 48.Liebeskind DS. Anatomic considerations in therapeutic arteriogenesis for cerebral ischemia. Circulation 2004; 109: e4. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, Prabhakar P, Sealock R, et al. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab 2010; 30: 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manoonkitiwongsa PS, Jackson-Friedman C, McMillan PJ, et al. Angiogenesis after stroke is correlated with increased numbers of macrophages: the clean-up hypothesis. J Cereb Blood Flow Metab 2001; 21: 1223–1231. [DOI] [PubMed] [Google Scholar]
- 51.Yu SW, Friedman B, Cheng Q, et al. Stroke-evoked angiogenesis results in a transient population of microvessels. J Cereb Blood Flow Metab 2007; 27: 755–763. [DOI] [PubMed] [Google Scholar]
- 52.Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 2000; 106: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayashi T, Noshita N, Sugawara T, et al. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab 2003; 23: 166–180. [DOI] [PubMed] [Google Scholar]
- 54.Krupinski J, Kałuza J, Kumar P, et al. Some remarks on the growth-rate and angiogenesis of microvessels in ischemic stroke. Morphometric and immunocytochemical studies. Patol Pol 1993; 44: 203–209. [PubMed] [Google Scholar]
- 55.Krupinski J, Kaluza J, Kumar P, et al. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994; 25: 1794–1798. [DOI] [PubMed] [Google Scholar]
- 56.Slevin M, Kumar P, Gaffney J, et al. Can angiogenesis be exploited to improve stroke outcome? Mechanisms and therapeutic potential. Clin Sci 2006; 111: 171–183. [DOI] [PubMed] [Google Scholar]
- 57.Chen D, Lee J, Gu X, et al. Intranasal delivery of apelin-13 is neuroprotective and promotes angiogenesis after ischemic stroke in mice. ASN Neuro 2015; 7(5): 1759091415605114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarrazin S, Lyon M, Deakin JA, et al. Characterization and binding activity of the chondroitin/dermatan sulfate chain from endocan, a soluble endothelial proteoglycan. Glycobiology 2010; 20: 1380–1388. [DOI] [PubMed] [Google Scholar]
- 59.Zhang RL, Chopp M, Roberts C, et al. Stroke increases neural stem cells and angiogenesis in the neurogenic niche of the adult mouse. PLoS One 2014; 9: e113972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, Chen J, Zhang CL, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia 2005; 49: 407–17. [DOI] [PubMed] [Google Scholar]
- 61.Kanazawa M, Igarashi H, Kawamura K, et al. Inhibition of VEGF signaling pathway attenuates hemorrhage after tPA treatment. J Cereb Blood Flow Metab 2011; 31: 1461–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernández-Klett F, Potas JR, Hilpert D, et al. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J Cereb Blood Flow Metab 2013; 33: 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li X and Carmeliet P Targeting angiogenic metabolism in disease. Science 2018; 59: 1335–1336. [DOI] [PubMed] [Google Scholar]
- 64.Dvorak HF, Brown LF, Detmar M, et al. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995; 146: 1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 65.Senger DR. Molecular framework for angiogenesis: a complex web of interactions between extravasated plasma proteins and endothelial cell proteins induced by angiogenic cytokines. Am J Pathol 1996; 149: 1–7. [PMC free article] [PubMed] [Google Scholar]
- 66.Adams RH and Alitalo K Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 2007; 8: 464–478. [DOI] [PubMed] [Google Scholar]
- 67.Hayashi T, Abe K, Suzuki H, et al. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke 1997; 28: 2039–2044. [DOI] [PubMed] [Google Scholar]
- 68.Namiki A, Brogi E, Kearney M, et al. Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J Biol Chem 1995; 270: 31189–31195. [DOI] [PubMed] [Google Scholar]
- 69.Ogunshola OO, Antic A, Donoghue MJ, et al. Paracrine and autocrine functions of neuronal vascular endothelial growth factor (VEGF) in the central nervous system. J Biol Chem 2002; 277: 11410–11415. [DOI] [PubMed] [Google Scholar]
- 70.Ijichi A, Sakuma S, Tofilon PJ. Hypoxia-induced vascular endothelial growth factor expression in normal rat astrocyte cultures. Glia 1995; 14: 87–93. [DOI] [PubMed] [Google Scholar]
- 71.Kovács Z, Ikezaki K, Samoto K, et al. VEGF and flt. Expression time kinetics in rat brain infarct. Stroke 1996; 27: 1865–1872. [DOI] [PubMed] [Google Scholar]
- 72.Forstreuter F, Lucius R, Mentlein R. Vascular endothelial growth factor induces chemotaxis and proliferation of microglial cells. J Neuroimmunol 2002; 132: 93–98. [DOI] [PubMed] [Google Scholar]
- 73.Gille H, Kowalski J, Li B, et al. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem 2001; 276: 3222–3230. [DOI] [PubMed] [Google Scholar]
- 74.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003; 9: 669–676. [DOI] [PubMed] [Google Scholar]
- 75.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A 1993; 90: 10705–10709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerhardt H, Golding M, Fruttiger M, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 2003; 161: 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soldi R, Mitola S, Strasly M, et al. F. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J 1999; 18: 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bates DO, Curry FE. Vascular endothelial growth factor increases microvascular permeability via a Ca(2+)-dependent pathway. Am J Physiol 1997; 273: H687–H694. [DOI] [PubMed] [Google Scholar]
- 79.Eliceiri BP, Paul R, Schwartzberg PL, et al. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell 1999; 4: 915–924. [DOI] [PubMed] [Google Scholar]
- 80.Sun Y, Jin K, Xie L, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 2003; 111: 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gale NW, Thurston G, Hackett SF, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell 2002; 3: 411–423. [DOI] [PubMed] [Google Scholar]
- 82.Tsiamis AC, Morris PN, Marron MB, et al. Vascular endothelial growth factor modulates the Tie-2: Tie-1 receptor complex. Microvasc Res 2002; 63: 149–158. [DOI] [PubMed] [Google Scholar]
- 83.Xu K, Lamanna JC. Chronic hypoxia and the cerebral circulation. J Appl Physiol 2006; 100: 725–730. [DOI] [PubMed] [Google Scholar]
- 84.Lidahl P, Hellstrom M, Kalen M, et al. Endothelial-perivascular cell signaling in vascular development: lessons from knockout mice. Curr Opin Lipidol 1998; 9: 407–411. [DOI] [PubMed] [Google Scholar]
- 85.Sato TN, Tozawa Y, Deutsch U, et al. Distinct roles of the receptor tyrosie kinases Tie-1 and Tie-2 in blood vessel formation. Nature 1995; 376: 70–74. [DOI] [PubMed] [Google Scholar]
- 86.Hayes AJ, Huang WQ, Mallah J, et al. Angiopoietin-1 and its receptor Tie-2 participate in the regulation of capillary-like tubule formation and survival of endothelial cells. Microvasc Res 1999; 58: 224–237. [DOI] [PubMed] [Google Scholar]
- 87.Lennmyr F, Ata KA, Funa K, et al. Expression of vascular endothelial growth factor (VEGF) and its receptors (Flt-1 and Flk-1) following permanent and transient occlusion of the middle cerebral artery in the rat. J Neuropathol Exp Neurol 1998; 57: 874–882. [DOI] [PubMed] [Google Scholar]
- 88.Zhang ZG, Zhang L, Tsang W, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab 2002; 22: 379–392. [DOI] [PubMed] [Google Scholar]
- 89.Gurnik S, Devraj K, Macas J, et al. Angiopoietin-2-induced blood-brain barrier compromise and increased stroke size are rescued by VE-PTP-dependent restoration of Tie2 signaling. Acta Neuropathol 2016; 131: 753–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin TN, Nian GM, Chen SF, et al. Induction of Tie-1 and Tie-2 receptor protein expression after cerebral ischemia-reperfusion. J Cereb Blood Flow Metab 2001; 21: 690–701. [DOI] [PubMed] [Google Scholar]
- 91.Gautam J, Yao Y. Roles of pericytes in stroke pathogenesis. Cell Transplant 2018; 27: 1798–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dore-Duffy P, Owen C, Balabanov R, et al. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc Res 2000; 60: 55–69. [DOI] [PubMed] [Google Scholar]
- 93.Dore-Duffy P, Katychev A, Wang X, et al. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab 2006; 26: 613–624. [DOI] [PubMed] [Google Scholar]
- 94.Kokovay E, Li L, Cunningham LA. Angiogenic recruitment of pericytes from bone marrow after stroke. J Cereb Blood Flow Metab 2006; 26: 545–555. [DOI] [PubMed] [Google Scholar]
- 95.Tagaya M, Haring HP, Stuiver I, et al. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab 2001; 21: 835–846. [DOI] [PubMed] [Google Scholar]
- 96.Haring HP, Akamine BS, Habermann R, et al. Distribution of integrin-like immunoreactivity on primate brain microvasculature. J Neuropathol Exp Neurol 1996; 55: 236–245. [DOI] [PubMed] [Google Scholar]
- 97.Milner R, Hung S, Erokwu B, et al. Increased expression of fibronectin and the alpha 5 beta 1 integrin in angiogenic cerebral blood vessels of mice subject to hypobaric hypoxia. Mol Cell Neurosci 2008; 38: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang H, Huang Q, Wang F, et al. Cerebral ischemia-induced angiogenesis is dependent on tumor necrosis factor receptor 1-mediated up-regulation of α5β1 and αVβ3 integrins. J Neuroinflammation 2016; 13: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li L, Welser-Alves J, van der Flier A, Boroujerdi A, et al. An angiogenic role for the alpha5beta1 integrin in promoting endothelial cell proliferation during cerebral hypoxia. Exp Neurol 2012; 237: 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tanjore H, Zeisberg EM, Gerami-Naini B, et al. β1 integrin expression on endothelial cells is required for angiogenesis but not for vasculogenesis. Dev Dyn 2008; 237: 75–82. [DOI] [PubMed] [Google Scholar]
- 101.Felcht M, Luck R, Schering A, et al. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest 2012; 122: 1991–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun J, Yu L, Huang S, et al. Vascular expression of angiopoietin1, α5β1 integrin and tight junction proteins is tightly regulated during vascular remodeling in the post-ischemic brain. Neuroscience 2017; 362: 248–256. [DOI] [PubMed] [Google Scholar]
- 103.Kim S, Bell K, Mousa SA, et al. Regulation of angiogenesis in vivo by ligation of integrin α5β1 with the central cell-binding domain of fibronectin. Am J Pathol 2000; 156: 1345–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zechariah A, ElAli A, Doeppner TR, et al. Vascular endothelial growth factor promotes pericyte coverage of brain capillaries, improves cerebral blood flow during subsequent focal cerebral ischemia, and preserves the metabolic penumbra. Stroke 2013; 44: 1690–1697. [DOI] [PubMed] [Google Scholar]
- 105.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shindo A, Liang AC, Maki T, et al. Subcortical ischemic vascular disease: roles of oligodendrocyte function in experimental models of subcortical white-matter injury. J Cereb Blood Flow Metab 2016; 36: 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takase H, Washida K, Hayakawa K, et al. Oligodendrogenesis after traumatic brain injury. Behav Brain Res 2018; 340: 205–211. [DOI] [PubMed] [Google Scholar]
- 108.Tsai HH, Niu J, Munji R, et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 2016; 351: 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tuszynski MH, Steward O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron 2012; 74: 777–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Geoffroy CG, Zheng B. Myelin-associated inhibitors in axonal growth after CNS injury. Curr Opin Neurobiol 2014; 27: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bradbury EJ, Moon LD, Popat RJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002; 416: 636–640. [DOI] [PubMed] [Google Scholar]
- 112.Pearse DD, Pereira FC, Marcillo AE, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med 2004; 10: 610–616. [DOI] [PubMed] [Google Scholar]
- 113.Rosenzweig ES, Brock JH, Lu P, et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat Med 2018; 24: 484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weidner N, Ner A, Salimi N, et al. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A 2001; 98: 3513–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bareyre FM, Kerschensteiner M, Raineteau O, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci 2004; 7: 269–277. [DOI] [PubMed] [Google Scholar]
- 116.David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 1981; 214: 931–933. [DOI] [PubMed] [Google Scholar]
- 117.Benfey M, Aguayo AJ. Extensive elongation of axons from rat brain into peripheral nerve grafts. Nature 1982; 296: 150–152. [DOI] [PubMed] [Google Scholar]
- 118.Izumi Y, Wakita S, Kanbara C, et al. Integrin α5β1 expression on dopaminergic neurons is involved in dopaminergic neurite outgrowth on striatal neurons. Sci Rep 2017; 7: 42111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Y, Jiang N, Powers C, et al. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke 1998; 29: 1972–1980. [DOI] [PubMed] [Google Scholar]
- 120.del Zoppo GJ, Abumiya T, Okada Y, et al. , Angiogenesis in the setting of focal cerebral ischemia. In: Krieglstein J, Klumpp S. (eds). Pharmacology of cerebral ischemia, Stuttgart: MedPharm Scientific Publishers, 2002, pp. 119–130. [Google Scholar]
- 121.Arai K, Jin G, Navaratna D, et al. Brain angiogenesis in developmental and pathological processes: neurovascular injury and angiogenic recovery after stroke. FEBS J 2009; 276: 4644–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Font MA, Arboix A, Krupinski J. Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr Cardiol Rev 2010; 6: 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Teng H, Zhang ZG, Wang L, et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab 2008; 28: 764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Uwamori H, Higuchi T, Arai K, et al. Integration of neurogenesis and angiogenesis models for constructing a neurovascular tissue. Sci Rep 2017; 7: 17349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jin K, Zhu Y, Sun Y, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A 2002; 99: 11946–11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol 2006; 66: 236–242. [DOI] [PubMed] [Google Scholar]
- 127.Lei WL, Xing SG, Deng CY, et al. Laminin/β1 integrin signal triggers axon formation by promoting microtubule assembly and stabilization. Cell Res 2012; 22: 954–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tan CL, Kwok JC, Patani R, et al. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J Neurosci 2011; 31: 6289–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Osada T, Gu YH, Kanazawa M, et al. Interendothelial claudin-5 expression depends on cerebral endothelial cell-matrix adhesion by β(1)-integrins. J Cereb Blood Flow Metab 2011; 31: 1972–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Izawa Y, Gu YH, Osada T, et al. β1-integrin-matrix interactions modulate cerebral microvessel endothelial cell tight junction expression and permeability. J Cereb Blood Flow Metab 2018; 38: 641–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mabuchi T, Kitagawa K, Ohtsuki T, et al. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke 2000; 31: 1735–1743. [DOI] [PubMed] [Google Scholar]
- 132.Kanazawa M, Kawamura K, Takahashi T, et al. Multiple therapeutic effects of progranulin on experimental acute ischaemic stroke. Brain 2015; 138: 1932–1948. [DOI] [PubMed] [Google Scholar]
- 133.Zarruk JG, Greenhalgh AD, David S. Microglia and macrophages differ in their inflammatory profile after permanent brain ischemia. Exp Neurol 2018; 301: 120–132. [DOI] [PubMed] [Google Scholar]
- 134.Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012; 43: 3063–3070. [DOI] [PubMed] [Google Scholar]
- 135.Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation 2011; 8: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kanazawa M, Ninomiya I, Hatakeyama M, et al. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int J Mol Sci 2017; 18: E2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jin Q, Cheng J, Liu Y, et al. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav Immunity 2014; 40: 131–142. [DOI] [PubMed] [Google Scholar]
- 138.Losordo DW, Vale PR, Symes JF, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation 1998; 98: 2800–2804. [DOI] [PubMed] [Google Scholar]
- 139.Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet 2002; 360: 427–435. [DOI] [PubMed] [Google Scholar]
- 140.Hoksbergen AW, Legemate DA, Csiba L, et al. Absent collateral function of the circle of Willis as risk factor for ischemic stroke. Cerebrovasc Dis 2003; 16: 191–198. [DOI] [PubMed] [Google Scholar]
- 141.Hossmann KA. Pathophysiology and therapy of experimental stroke. Cell Mol Neurobiol 2006; 26: 1057–1083. [DOI] [PubMed] [Google Scholar]
- 142.Faber JE, Chilian WM, Deindl E, et al. A brief etymology of the collateral circulation. Arterioscler Thromb Vasc Biol 2014; 34: 1854–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Artru AA. Relationship between cerebral blood volume and CSF pressure during anesthesia with isoflurane or fentanyl in dogs. Anesthesiology 1984; 60: 575–579. [DOI] [PubMed] [Google Scholar]
- 144.Archer DP, Labrecque P, Tyler JL, et al. Cerebral blood volume is increased in dogs during administration of nitrous oxide or isoflurane. Anesthesiology 1987; 67: 642–648. [DOI] [PubMed] [Google Scholar]
- 145.Wei L, Erinjeri JP, Rovainen CM, et al. Collateral growth and angiogenesis around cortical stroke. Stroke 2001; 32: 2179–2184. [DOI] [PubMed] [Google Scholar]
- 146.Wang Z, Tsai LK, Munasinghe J, et al. Chronic valproate treatment enhances postischemic angiogenesis and promotes functional recovery in a rat model of ischemic stroke. Stroke 2012; 43: 2430–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Taguchi A, Soma T, Tanaka H, et al. Administration of CD34 + cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest 2004; 114: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moriyama Y, Takagi N, Hashimura K, et al. Intravenous injection of neural progenitor cells facilitates angiogenesis after cerebral ischemia. Brain Behav 2013; 3: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jin Y, Barnett A, Zhang Y, et al. Poststroke Sonic Hedgehog agonist treatment improves functional recovery by enhancing neurogenesis and angiogenesis. Stroke 2017; 48: 1636–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yi JJ, Barnes AP, Hand R, et al. TGF-beta signaling specifies axons during brain development. Cell 2010; 142: 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hori S, Ohtsuki S, Hosoya K, et al. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem 2004; 89: 503–513. [DOI] [PubMed] [Google Scholar]
- 152.Kawamura K, Takahashi T, Kanazawa M, et al. Effects of angiopoietin-1 on hemorrhagic transformation and cerebral edema after tissue plasminogen activator treatment for ischemic stroke in rats. PLoS One 2014; 9: e98639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Renner O, Tsimpas A, Kostin S, et al. Time- and cell type-specific induction of platelet-derived growth factor receptor-beta during cerebral ischemia. Brain Res Mol Brain Res 2003; 113: 44–51. [DOI] [PubMed] [Google Scholar]
- 154.Kokaia Z, Andsberg G, Yan Q, et al. Rapid alterations of BDNF protein levels in the rat brain after focal ischemia: evidence for increased synthesis and anterograde axonal transport. Exp Neurol 1998; 154: 289–301. [DOI] [PubMed] [Google Scholar]
- 155.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab 1999; 19: 819–834. [DOI] [PubMed] [Google Scholar]
- 156.Rius J, Guma M, Schachtrup C, et al. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature 2008; 453: 807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011; 17: 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen PY, Simons M. When endothelial cells go rogue. EMBO Mol Med 2016; 8: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gertz K, Kronenberg G, Uhlemann R, et al. Partial loss of VE-cadherin improves long-term outcome and cerebral blood flow after transient brain ischemia in mice. BMC Neurol 2016; 16: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gasparics Á, Rosivall L, Krizbai IA, et al. When the endothelium scores an own goal: endothelial cells actively augment metastatic extravasation through endothelial-mesenchymal transition. Am J Physiol Heart Circ Physiol 2016; 310: H1055–H1063. [DOI] [PubMed] [Google Scholar]
- 161.Maddaluno L, Rudini N, Cuttano R, et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature 2013; 498: 492–496. [DOI] [PubMed] [Google Scholar]
- 162.Dejana E, Hirschi KK and Simons M. The molecular basis of endothelial cell plasticity. Nat Commun 2017; 8: 14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bravi L, Malinverno M, Pisati F, et al. Endothelial cells lining sporadic cerebral cavernous malformation cavernomas undergo endothelial-to-mesenchymal transition. Stroke 2016; 47: 886–890. [DOI] [PubMed] [Google Scholar]
- 164.Li L, Welser JV, Dore-Duffy P, et al. In the hypoxic central nervous system, endothelial cell proliferation is followed by astrocyte activation, proliferation, and increased expression of the α6β4 integrin and dystroglycan. Glia 2010; 58: 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Angiogenesis in the ischemic core: A potential treatment target? by Masato Kanazawa, Tetsuya Takahashi, Masanori Ishikawa, Osamu Onodera, Takayoshi Shimohata and Gregory J del Zoppo in Journal of Cerebral Blood Flow & Metabolism


