Abstract
Background:
Insulin is one of the highest risk medications used in hospitalized patients. Multiple complex factors must be considered in determining a safe and effective insulin regimen. We sought to develop a computerized clinical decision support (CDS) tool to assist hospital-based clinicians in insulin management.
Methods:
Adapting existing clinical practice guidelines for inpatient glucose management, a design team selected, configured, and implemented a CDS tool to guide subcutaneous insulin dosing in non–critically ill hospitalized patients at two academic medical centers that use the EpicCare® electronic medical record (EMR). The Agency for Healthcare Research and Quality (AHRQ) best practices in CDS design and implementation were followed.
Results:
A CDS tool was developed in the form of an EpicCare SmartForm, which generates an insulin regimen by integrating information about the patient’s body weight, diabetes type, home and hospital insulin requirements, and nutritional status. Total daily recommended insulin doses are distributed into respective basal and nutritional doses with a tailored correctional insulin scale. Preimplementation, several approaches were used to communicate this new tool to clinicians, including emails, lectures, and videos. Postimplementation, a support team was available to address user technical issues. Feedback from stakeholders has been used to continuously refine the tool. Inclusion of the programming in the EMR vendor’s community library has allowed dissemination of the tool outside our institution.
Conclusions:
We have developed an EMR-based tool to guide SQ insulin dosing in non–critically ill hospitalized patients. Further studies are needed to evaluate adoption and clinical effectiveness of this intervention.
Keywords: clinical decision support systems, diabetes mellitus, hospital management insulin, subcutaneous (SQ)
Background and Significance
Insulin is one of the highest risk medications used in hospitalized patients.1 Given its relatively narrow therapeutic index, inappropriate insulin dosing may cause hypoglycemia or severe hyperglycemia. Iatrogenic hypoglycemia has been associated with cardiac ischemia, arrhythmias, and neurological impairment.2 Inpatient hyperglycemia has been associated with increased short-term mortality, length of hospital stay, and readmissions in a variety of conditions and patient populations.3-7 As 20-40% of hospitalized patients in the United States have diabetes or hyperglycemia8 and insulin is the standard of care for inpatient glucose management,9 identifying safe and effective insulin management approaches for these patients is paramount.
There are multiple barriers to safely and effectively managing insulin in the complex hospital environment.8 At admission, clinicians must take into account the patient’s preadmission insulin regimen and level of glycemic control, current comorbidities, newly prescribed medications that may affect glucose homeostasis (eg, steroids), and planned nutritional status. Throughout hospitalization, clinicians must also appraise trends in glycemic control to safely adjust the initial insulin regimen. Many factors, such as acute kidney injury, can develop rapidly during hospitalization and significantly alter insulin requirements. Clinicians must maintain a high degree of situational awareness for proactive insulin dose adjustments, which may be burdensome for providers who are not diabetes specialists and who are managing busy inpatient services.
Clinical decision support (CDS) systems have been adopted in health care settings to improve processes of care and/or clinical outcomes for various clinical situations.10 Some commonly recognized examples of CDS systems in the inpatient setting include drug-drug interaction alerts and duplicate medication prescribing alerts. To minimize the potential for inappropriate prescribing leading to iatrogenic hypoglycemia, several components of subcutaneous (SQ) insulin order sets are recommended, which include weight-based insulin dosing with guidance, specific insulin regimens based on nutritional status, built-in indication and holding parameters, and prompts.8 A SQ insulin order set in our legacy electronic medical record (EMR) contained all of these components. While use of this order set was associated with significant reductions in rates of hypoglycemia over a several-year period, hyperglycemia rates were not changed.11
In December 2016, our health system’s academic medical centers transitioned to a new inpatient EMR, EpicCare®. With this planned change, we recognized an opportunity to enhance our previous SQ insulin order set by increasing automation to improve clinician efficiency and the safety and accuracy of insulin prescribing. Thus, a CDS design team was assembled to develop a new SQ insulin CDS system in parallel with a comprehensive SQ insulin order set. This paper outlines the methods used in selecting, configuring, and implementing our SQ insulin CDS system and describes its components in detail. Our approach may be of use to clinicians interested in formulating and implementing a CDS system in the hospital setting and is applicable to other clinical conditions and workflows.
Methods
Setting
This quality improvement project was conducted at two academic medical centers: The Johns Hopkins Hospital (JHH), a 1154-bed hospital, and Johns Hopkins Bayview Medical Center (JHBMC), a 445-bed hospital, both located in Baltimore, Maryland. Prior to December 2016, each of these hospitals had its own legacy inpatient EMR system but shared the same outpatient EMR (Epic). Both hospitals transitioned to the same inpatient EMR (EpicCare) over a phased rollout period (JHBMC on December 1, 2015; JHH on July 1, 2016) with a jointly planned configuration. The SQ insulin order set in the legacy EMR at JHH had basic CDS capabilities, whereas the order set at JHBMC did not.
Design Methodology
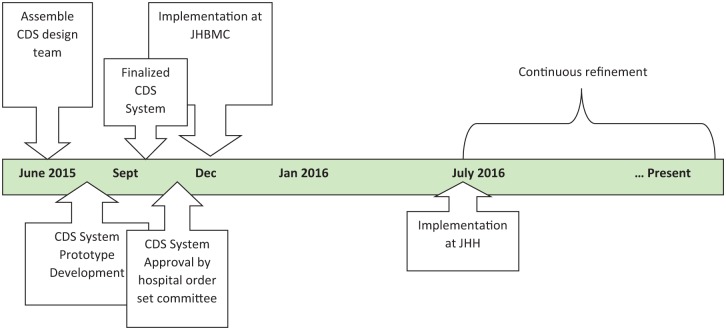
For this project, we used a development and implementation framework endorsed by the Agency for Healthcare Research and Quality (AHRQ).10 As this was considered a quality improvement project, formal institutional review board approval was not required. The project was conducted between May 1, 2015, and March 26, 2018. A timeline of the development and implementation process is shown in Figure 1. In addition to prospective data collection, email communications between CDS design team members and other organizational collaborators were archived and analyzed retrospectively after completion of the project.
Figure 1.
CDS system development and implementation process and timeline.
Table 1 outlines the steps followed in the development and implementation of this CDS system. Since its development occurred as part of a transition to a new EMR, there was strong institutional support from the chief medical information officers (CMIOs) to develop and refine existing insulin-related CDS systems. The physician champion for this project is an endocrinologist who served as the chair of the hospital’s glucose steering committee. A three-member clinical decision support (CDS) design team consisting of the physician champion, a nurse informatics leader, and an Epic technical consultant was formed. The team met weekly, either in-person or remotely typically in 60- to 90-minute sessions, to plan and develop the new CDS.
Table 1.
Design and Implementation Process Highlighting Relevant Decisions and Findings Within the AHRQ Framework.
| Process | Description |
|---|---|
| Assemble CDS team with organizational buy-in | ● CDS team members with appropriate expertise
identified ● Strong organizational buy-in from IT leadership to reproduce and refine existing CDS interventions. |
| Understand foundational considerations and range of intervention types | ● Planned transition to Epic inpatient EMR, with goal to
minimize disruption to existing workflow. ● CDS Five Rights: ∘ Right Information: Guideline-supported insulin dosing recommendations ∘ To the right person: Prescribing clinicians ∘ In the right place in the workflow: Admission Navigator ∘ In the right format: Dynamic SmartForm with recommendations tied to order set ∘ Through the right channel: EMR ● Range of Available intervention types considered |
| Use core action model to select CDS intervention type and workflow opportunity | ● Core action plan: ∘ Recognize patterns: Need to identify patients requiring insulin ∘ Formulate plan: Determine insulin total daily dose and distribute into basal-bolus-correctional components ∘ Execute plan: Need seamless integration of CDS tool with insulin order set ∘ Respond to events: Existing glucose/insulin display helps providers identify hypoglycemic and hyperglycemic events that may require adjustment of insulin doses ∘ Communicate: Consultation to the inpatient diabetes service is part of order set; documentation of last person and time that CDS tool is used for intrateam communication ● SmartForm linked to the SQ insulin order set addresses 3 of the 5 core action components (recognition of patterns, formulation, and execution of plan), while built-in glucose/management display facilitates responding to events and built-in consultation order enhances communication |
| Configure CDS intervention with stakeholder input | ● Branching medical logic developed by physician
champion ● Software engineer developed prototype of SmartForm ● Software engineer built SQ insulin CDS tool accessible within SQ insulin navigator ● Independent review by two physicians to validate SmartForm recommendations ● Application of interface design principles (perceptual grouping, consistent terminology, working, color, density of information, etc) ● SQ CDS tool made optional in admission workflow ● Feedback by group interview of house staff and presentation of tool to various CDS committees |
| Develop and implement intervention rollout plan | ● Tip sheet for providers ● Physician champion presented tool at academic conferences ● Demonstration video developed and shared with house staff |
| Implementation | ● Phased rollout over 2 hospitals ∘ BMC: 12/2015 ∘ JHH: 7/2016 ● Software engineer available to provide technical support during “go-live” ● Annual orientation for physicians |
| Establish knowledge management governance structure and processes to maintain CDS system up to date | ● SQ insulin order set and CDS tool overseen by the hospital’s glucose steering committee, which has authority to make changes to both so as to align with hospital policies and evolving clinical evidence |
| Update CDS tool based on user feedback as needs arise | ● Stakeholders can provide feedback by placing Epic help
ticket or by attending quarterly glucose steering committee
meetings ● Monitoring of patient safety adverse event reporting system to look for patterns of glucose management that might require adjustment of the CDS tool |
The first objective of the CDS team was to understand institutional practices in inpatient glucose management and EMR CDS capabilities. A primary goal was to minimize disruption to prescribers’ existing workflow as much as possible while improving features of the legacy SQ insulin order sets. To select the CDS intervention, the core action model was used in conjunction with the “Five Rights of CDS.” According to the core action model, CDS systems should be selected based on appraisal of their ability to achieve one or more of these desired core actions: (1) recognition of patterns, (2) formulation of plan, (3) execution of plan, (4) responding to events, and (5) communication. The CDS design team narrowed the focus of this intervention to the first three core actions. The CDS system would need to allow providers to (1) identify patients requiring insulin (recognize patterns), (2) determine the insulin total daily dose (TDD) and distribute into the basal-bolus-correctional insulin components (formulate plan), and (3) link to actual insulin orders (execute plan). To address the core action of communication, the SQ order set maintained a built-in consultation to the inpatient diabetes service to allow admitting clinicians to obtain support if needed; in addition, it was important that the use of the CDS system be trackable within the EMR for intrateam monitoring and communication.
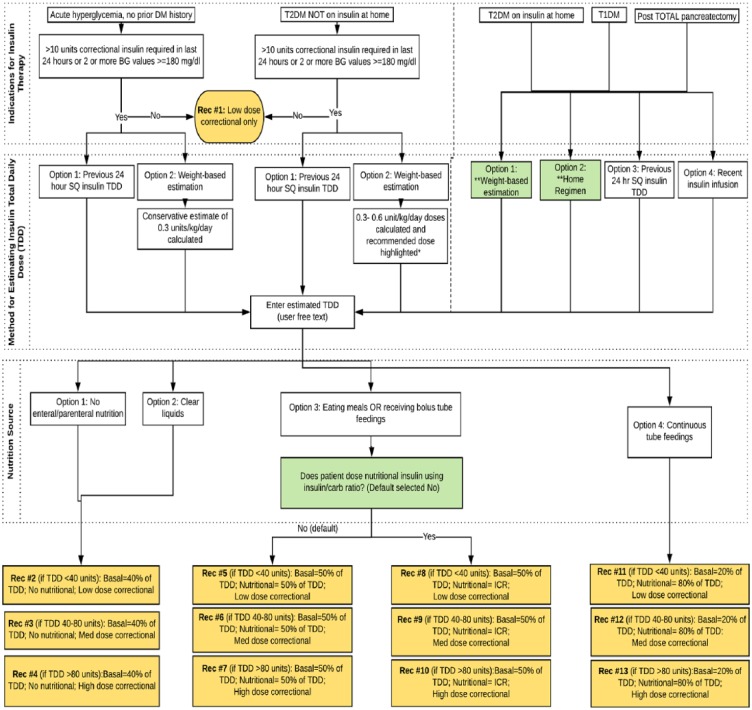
The EpicCare EMR offered a range of CDS formats, including SmartForms, SmartSets, order sets, grouped orders, navigators, best practice advisories/alerts (BPAs), required documentation, and medication warnings. The CDS design team selected the EpicCare SmartForm as the intervention format. SmartForms are customizable and dynamic forms that can integrate user-entered and EMR data usually used for documentation purposes. We were able to further adapt this format to provide tailored recommendations based on user-entered and EMR-gathered data. Using LucidChart® diagramming software, the physician champion developed a branching medical logic algorithm with 13 discrete insulin dosing recommendations (Figure 2).
Figure 2.
Branching medical Logic for SQ Decision Support Tool. A total of 13 discrete recommendation types are provided based on user responses. ICR, patient will report his or her insulin to carbohydrate ratio; Med, medium; TDD, total daily dose (eg, 1 unit for each 10 grams of carbohydrates consumed).
The IT consultant then used this medical logic to develop a prototype SmartForm that underwent a series of iterations over approximately 8 weeks. The recommendations for insulin dosing were generally adapted from clinical practice guidelines;12 however, the recommendation for weight-based dosing for patients with acute hyperglycemia and no prior history of diabetes (0.3 units/kg/day) was determined based on expert opinion. The physician champion solicited feedback on these recommendations via a teleconference of a national working group of clinicians interested in the use of EMRs for diabetes care.
Development and Approval Process
Prototype development of the SmartForm consisted of regular email exchange between the software engineer and physician champion. There were two objectives at this stage of development: (1) validation of correct programming of medical logic and (2) application of best practices in interface design. For the validation step, the CDS system was added to the EMR training environment and two physicians (physician champion and senior endocrinology fellow) independently checked that all the branching pathways in the CDS system arrived at the correct recommendation within the SmartForm and that the recommendations linked appropriately to the relevant insulin orders. With respect to the interface design, the same two physicians evaluated the CDS system on appearance, perceptual grouping of data relationships (eg, similar colors, fonts, and bolding for categories), consistency in terminology, density of information on screen, workflow integration, and editing (spelling/ grammar).
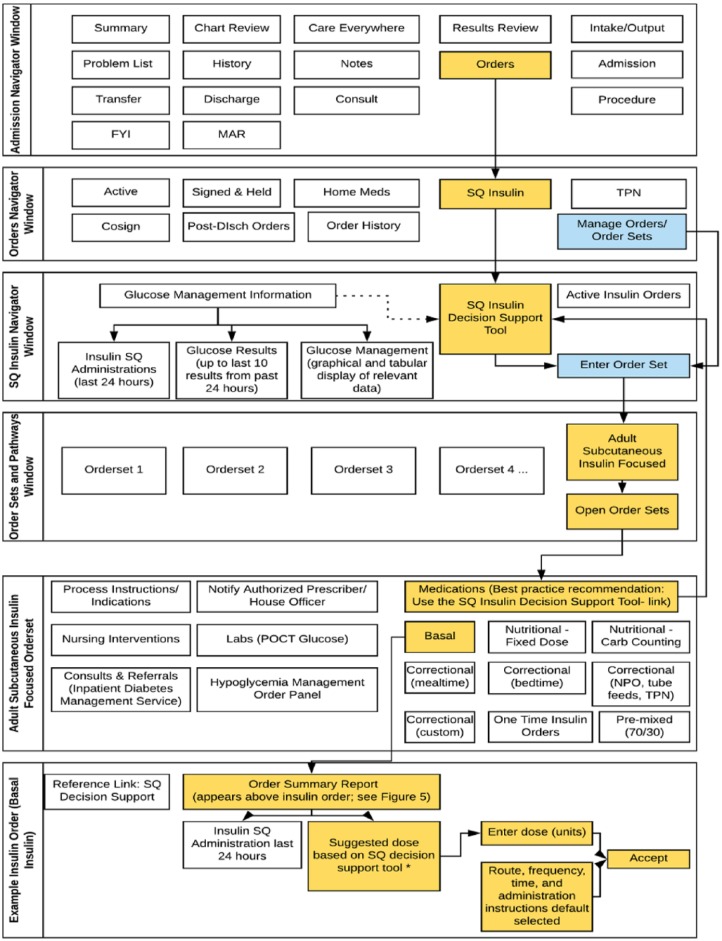
Figure 3 summarizes the insulin prescribing workflow within the EMR. The SQ insulin CDS tool was made a part of an admission navigator tab devoted to SQ insulin, providing quick access to relevant data (glucose values and insulin doses) and links to the SQ insulin order set. Responses expected to have low frequency of selection (eg, carbohydrate counting as method of nutritional insulin dosing) were default selected to “no.” An “advice rather than command” principle was followed: the insulin CDS tool was made optional within the insulin order set; however, a requirement was made to discontinue all home insulins before accessing the SQ insulin order set to avoid duplication of medications and use of nonformulary insulins in the hospital. Since the SmartForm is dynamic (ie, recommendations change based on responses), providers can navigate backward to update the SmartForm or quickly restart using a “Clear All” button if needed. A log documents the last estimated total daily dose (in units) without the need to open the CDS tool, as well as the name of the provider who completed the tool last and the date/timestamp.
Figure 3.
Epic workflow for insulin management. This workflow is shown for only one insulin type (basal insulin), but the same workflow would apply to nutritional insulin. Orange boxes indicate best practice approach (recommended) in which clinicians complete the SQ insulin decision support tool prior to entering insulin doses. Blue boxes indicate alternative approach which bypasses the SQ insulin decision support tool. Disch, discharge; FYI, for your information; MAR, Medication Administration Record; POCT, point of care testing; SQ, subcutaneous; TPN, total parenteral nutrition. *If SQ decision support tool not completed, this section remains blank in the order “summary report” (see Figure 5).
Stakeholder feedback on the prototype CDS tool was elicited in-person and electronically by demonstration to a multidisciplinary Department of Medicine Epic Workgroup and to hospitalists and internal medicine residents at regularly scheduled academic conferences and from a focus group session of seven internal medicine residents. Some of the key feedback received from the Department of Medicine Epic Workgroup were identification of an incongruence between indication for insulin therapy (based on blood glucose criteria and/or correctional insulin doses), need to default the units for insulin orders to “units” rather than “units/kg,” and a request that the results of the SQ insulin CDS tool prepopulate the appropriate component of the insulin order. The main feedback received from hospitalists and residents included reducing amount of text and making the tool optional to provide flexibility in workflow.
The SQ insulin CDS tool received final approval by the hospitals’ EMR order set review committee. This process consisted of a 30-minute presentation of the CDS tool and insulin order set by the physician champion. The committee requested a demonstration of initial insulin prescribing and change in insulin dosing both with and without the use of the CDS tool. Following final approval, the insulin order set and SQ CDS tool were implemented at one hospital (JHBMC). The CDS tool was intended for use in the nonobstetrical adult population; however, within a few days of go-live, obstetrics leadership in the hospital indicated that they wished to remove language from the insulin order set that excluded pregnant women. Otherwise, there were no technical issues reported by users related to the order set or CDS tool. The tool was implemented six months later at the second hospital (JHH). Since release of the tool, there have been several modifications to the insulin order set, prompting minor changes to be made for consistency in the CDS tool. However, no fundamental changes in the logic behind the insulin dosing recommendations have been made since initial release.
Results
The SQ Insulin CDS Tool was designed for routine SQ insulin management of adult patients on medical and surgical wards. Exclusion criteria include patients on insulin pumps, pregnant women, patients with diabetic ketoacidosis or hyperglycemic hyperosmolar syndrome, hyperkalemia, cystic fibrosis, or patients receiving total parenteral nutrition. The CDS tool guides the provider in selection of an appropriate basal-bolus insulin (BBI) regimen through a series of questions. The first question asks indications for insulin therapy: acute hyperglycemia (no prior history of diabetes), type 2 diabetes patients treated with noninsulin antihyperglycemic agents at home, and insulin-requiring patients (type 2 diabetes on home insulin, type 1 diabetes, and post-pancreatectomy diabetes). For acutely hyperglycemic patients and patients with T2DM not on home insulin, the need for BBI therapy is guided by the total amount of correctional insulin and/or evidence of persistent hyperglycemia (2 or more blood glucose values ⩾180 mg/dl). For these patients, two methods of estimating the total daily insulin requirement are provided: (1) previous 24 hours SQ insulin TDD and (2) weight-based estimation (0.3 units/kg/day for acute hyperglycemia without prior history of diabetes or 0.3-0.6 unit/kg/day for non-insulin-requiring T2DM patients). For insulin-requiring patients, four methods are provided for estimating the insulin TDD: (1) weight-based estimation, (2) home insulin TDD, (3) previous 24-hour SQ insulin requirements, or (4) recent IV insulin infusion doses.
For all weight-based estimations, the patient’s body weight is automatically pulled into the tool and insulin dosages are calculated accordingly. The recommended dose of insulin is based on the patient’s BMI, diabetes type, and/or steroid use. Multiple weight-based estimates are provided so that the clinician can select the appropriate dose based on the clinical scenario (Figure 4). The CDS tool highlights in green the weight-based estimate purely on the basis of the patient’s BMI (0.4 units/kg/day for BMI 18.5-24.9 kg/m2; 0.5 units/kg for BMI 25-30 kg/m2; 0.6 units/kg for BMI >30 kg/m2). Providers can also free text a different weight-based multiplier with automatic calculation of the TDD, which may be useful for patients with insulin resistance who may require doses of 0.8-1.0 units/kg or greater. The clinician then enters an estimated TDD based on the information provided by the tool.
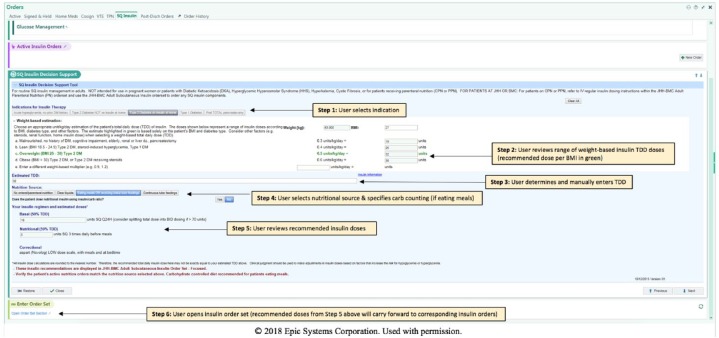
Figure 4.
Screenshots of the subcutaneous (SQ) Insulin clinical decision support (CDS) tool recommendations (see Figure 2 for branching logic). SQ Insulin CDS tool is accessed from a “SQ Insulin” tab under Orders shown at top of screen. SQ CST tool window can be minimized. Example shown here is for a patient with type 2 diabetes not on insulin at home who is eating regular meals. Step 1: User selects indication for insulin therapy. Step 2: Weight-based insulin total daily dose (TDD) is automatically calculated from weight (in this case 63 kg), with recommended dose shown in green based on the BMI (in this case 27 kg/m2). Step 3: User reviews TDD recommendations from Step 2 to determine an insulin TDD, which is manually entered. Step 4: User selects nutritional source (in this case, “eating meals or receiving bolus tube feedings”) and specifies whether patient uses carbohydrate counting at home (default selected to “no”). Step 5: Insulin recommendations are provided and may be reviewed by user here on in the order summary report in the corresponding insulin order (ie, recommendations carry forward). Step 6: User opens insulin order set (see Figure 5 for remaining steps).
Next, a nutrition source is selected, which is used to distribute the insulin TDD into basal, bolus, and correctional components. Patients who are NPO or receiving clear liquids receive 40% basal, 60% nutritional. Patients who are eating meals or receiving bolus tube feeds receive 50% basal and 50% nutritional. Patients who are on continuous tube feedings receive 20% basal and 80% nutritional given the increased requirement for nutritional coverage with a continuous source of carbohydrate exposure. In all cases, a correctional scale (low, medium, or high) is recommended based on the patient’s estimated TDD, which assumes the patient’s total requirement when receiving nutrition. Specifically, low, medium, and high dose scales are recommended for insulin TDD of <40, 40-80, and >80 units, respectively.
Since some patients receive nutritional insulin at home using an insulin-to-carbohydrate ratio (ICR), an additional question is asked for patients who are eating meals or receiving bolus tube feeds: “Does patient dose nutritional insulin using insulin/carb ratio?” The response to this question is default selected to “no” given the low expected prevalence of carbohydrate counting patients in the hospital. If the answer to this question is “yes,” the basal dose is calculated as 50% of the TDD and the patient’s reported ICR is manually entered into the nutritional insulin order by the clinician (ie, CDS tool does not provide mealtime dosage recommendations when using this method).
Following completion of the CDS tool, the clinician selects a link to open the order set which displays only the relevant components of the SQ insulin order set (basal, nutritional, and correctional dose) selected based on the results of the CDS tool. Unfortunately, due to limitations in Epic programming, prepopulation of insulin doses in the orders was not possible; however, the results of the insulin CDS tool are displayed immediately above the insulin order for provider reference (Figure 5). To modify insulin orders, providers can either directly adjust medication doses (bypassing the CDS tool) or reuse the CDS tool (with previous results saved). Alternatively, they can enter a new TDD or modify nutrition categories, resulting in automatic changes to the insulin regimen and recommended doses.
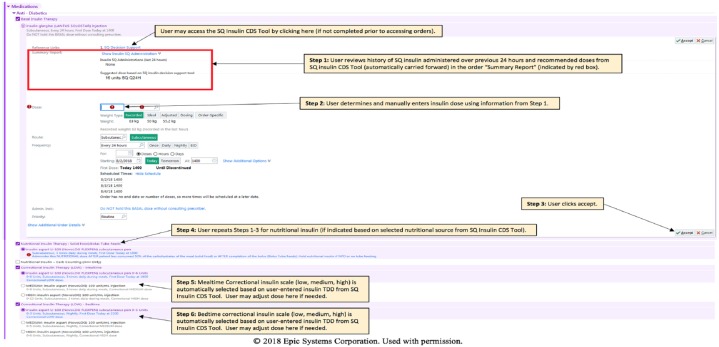
Figure 5.
Screenshot of a subcutaneous (SQ) insulin order showing how recommendations from SQ insulin clinical decision support (CDS) tool are displayed above where the provider is to enter the desired insulin dose. Red box indicates order “Summary Report,” which captures recommended doses from the SQ insulin CDS tool and all administered SQ insulin doses in previous 24 hours. User can adjust dose of all insulin orders at this step. User can also directly access the SQ Insulin CDS Tool with reference link at top of the order.
Discussion
We have described the development of a SQ insulin CDS tool for use in non–critically ill inpatients using the EpicCare SmartForm format linked to a comprehensive SQ insulin order set. This tool is fully integrated into the standard provider workflow of admission order entry and can be accessed throughout the admission to provide recommendations for insulin dosing for a variety of clinical scenarios as discussed above. We anticipate that use of this tool will increase the use of BBI regimen for hospitalized patients, which may improve glycemic outcomes.9
CDS tools of varying levels of sophistication have been developed for management of insulin in different care settings, ranging from reference protocols/algorithms to comprehensive computerized order sets13,14 to even more complicated models of physiologic blood glucose trends that can provide insulin dosing recommendations.15,16 There are currently two commercial FDA-approved software platforms, Endotool® and Glucommander®, and one in clinical trials, GlucoTab®, that are available to inpatient care sites that provide SQ insulin dosage recommendations based on patient-specific input that can be imported from the institution’s EMR; however, a limitation of these products is that their workflow requires exiting and reentering the EMR, unlike our tool which is embedded in our EMR.
The development process of our SQ insulin CDS tool illustrates key aspects for institutions seeking to develop their own. We sought to optimize usability by using established frameworks for CDS development. As has been frequently reported, institutional buy-in and support were fundamental to the development and implementation process. It was important to have a team comprised of both medical informatics experts as well as a content expert to translate the clinical recommendations into the branching logic of the CDS tool. Another key component was demonstrations of the tool for the end users who provided immediate feedback during an iterative design process. A challenge of implementation was generating awareness given the large number of providers across different departments and the annual turnover of house staff.
In our design process, a standard tool available in the EMR that is generally used just for customizable data collection and documentation was adapted to provide an output tailored specifically to inputs drawn automatically from the EMR and separately from the ordering provider. A significant benefit of building a tool like this is the ability to share it without any additional cost across health systems through the EMR vendor’s community library, allowing for easy dissemination to thousands of hospitals using one of the most widely used EMRs in this country.17 Clearly, the relevance and utility of our SQ insulin CDS tool will depend largely on demonstration of its effectiveness on glycemic control and/or process measures, such as use of basal-bolus insulin. We are actively evaluating adoption of the tool, concordance between prescribed and recommended insulin regimens, and glycemic outcomes through a retrospective analysis of patient admissions of adults with diagnoses of diabetes, evidence of inpatient hyperglycemia, or inpatient administration of SQ insulin.
Conclusion
We have developed a CDS system to assist providers with SQ insulin dosing in non–critically ill hospitalized patients. Leveraging an existing tool within our institution’s EMR allowed for seamless integration into provider work flow and can be a model for development of CDS systems for other clinical conditions that would benefit from standardization of prescriber practice in response to multifaceted clinical data.
Supplementary Material
Footnotes
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; BBI, basal bolus insulin; BPAs, best practice advisories/alerts; CDS, clinical decision support; CMIO, chief medical information officer; EMR, electronic medical record; ICR, insulin-to-carbohydrate ratio; JHBMC, Johns Hopkins Bayview Medical Center; JHH, Johns Hopkins Hospital; SQ, subcutaneous; TDD, total daily dose.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NM was supported by K23DK111986-01 by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Supplementary Material: Supplemental Video Demonstration is available at https://www.dropbox.com/s/62yfqcr6zmlyp7b/SQ%20Insulin%20decision%20support%20tool%20demo.mov?dl = 0.
References
- 1. Wang Y, Eldridge N, Metersky ML, et al. National trends in patient safety for four common conditions, 2005-2011. N Engl J Med. 2014;370(4):341-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brutsaert E, Carey M, Zonszein J. The clinical impact of inpatient hypoglycemia. J Diabetes Complications. 2014;28(4):565-572. [DOI] [PubMed] [Google Scholar]
- 3. Evans NR, Dhatariya KK. Assessing the relationship between admission glucose levels, subsequent length of hospital stay, readmission and mortality. Clin Med (Lond). 2012;12(2):137-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. [DOI] [PubMed] [Google Scholar]
- 5. Martin WG, Galligan J, Simpson S, Greenaway T, Burgess J. Admission blood glucose predicts mortality and length of stay in patients admitted through the emergency department. Intern Med J. 2015;45(9):916-924. [DOI] [PubMed] [Google Scholar]
- 6. Eurich DT, Gamble JM, Marrie TJ, Majumdar SR. Dysglycaemia and 90 day and 1 year risks of death or readmission in patients hospitalised for community-acquired pneumonia. Diabetologia. 2010;53(3):497-503. [DOI] [PubMed] [Google Scholar]
- 7. Foo K, Cooper J, Deaner A, et al. A single serum glucose measurement predicts adverse outcomes across the whole range of acute coronary syndromes. Heart. 2003;89(5):512-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kulasa K, Juang P. How low can you go? Reducing rates of hypoglycemia in the non-critical care hospital setting. Curr Diab Rep. 2017;17(9):74. [DOI] [PubMed] [Google Scholar]
- 9. Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16-38. [DOI] [PubMed] [Google Scholar]
- 10. Osheroff JA, Teich JM, Levick D, et al. Improving Outcomes With Clinical Decision Support: An Implementer’s Guide. 2nd ed. Boca Raton, FL: CRC Press; 2012. [Google Scholar]
- 11. Munoz M, Pronovost P, Dintzis J, et al. Implementing and evaluating a multicomponent inpatient diabetes management program: putting research into practice. Jt Comm J Qual Patient Saf. 2012;38(5):195-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Handelsman Y, Mechanick JI, Blonde L, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract. 2011;17(suppl 2):1-53. [DOI] [PubMed] [Google Scholar]
- 13. Schnipper JL, Liang CL, Ndumele CD, Pendergrass ML. Effects of a computerized order set on the inpatient management of hyperglycemia: a cluster-randomized controlled trial. Endocr Pract. 2010;16(2):209-218. [DOI] [PubMed] [Google Scholar]
- 14. Guerra YS, Das K, Antonopoulos P, et al. Computerized physician order entry-based hyperglycemia inpatient protocol and glycemic outcomes: the CPOE-HIP study. Endocr Pract. 2010;16(3):389-397. [DOI] [PubMed] [Google Scholar]
- 15. Montani S, Magni P, Bellazzi R, Larizza C, Roudsari AV, Carson ER. Integrating model-based decision support in a multi-modal reasoning system for managing type 1 diabetic patients. Artif Intell Med. 2003;29(1-2):131-151. [DOI] [PubMed] [Google Scholar]
- 16. Hernando ME, Gómez EJ, Corcoy R, del Pozo F. Evaluation of DIABNET, a decision support system for therapy planning in gestational diabetes. Comput Methods Programs Biomed. 2000;62(3):235-248. [DOI] [PubMed] [Google Scholar]
- 17. Bresnick J. Epic Systems Leads in Hospital EHR, Business Intelligence Adoption; 2017. https://healthitanalytics.com/news/epic-systems-leads-in-hospital-ehr-business-intelligence-adoption. Accessed April 6, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.