Abstract
Background
Questions remain regarding both the safety and efficacy of bariatric surgery in patients with inflammatory bowel diseases (IBD), including the effects of bariatric surgery on the course of disease. We report a case series from a tertiary care IBD referral center and review the existing literature regarding the safety and efficacy of bariatric surgery in IBD patients.
Objectives
Examine the safety and efficacy of bariatric surgery in IBD patients. Explore possible effects of weight loss on postoperative IBD course.
Method
We performed a retrospective review of patients at our center undergoing bariatric surgery with a concurrent IBD diagnosis, collecting baseline characteristics, surgery type, and postoperative course (including IBD outcomes and weight loss). Data from these patients were combined with available data from the existing literature to calculate standardized means with standard error, variance, and confidence intervals (CI).
Results
Data from 13 patients who had undergone bariatric surgery at our facility were combined with data from 8 other studies to create a study population of 101 patients. Of these, 61 had Crohn's disease, 37 ulcerative colitis, and 3 IBD-unspecified, with a mean preoperative BMI of 44.2 (95% CI 42.9–45.7). Following surgery, a mean excess weight loss of 68.4% was demonstrated (95% CI, 65.7–71.2). Of the 101 patients, 22 experienced early and 20 experienced late postoperative complications. Postoperatively, 10 patients experienced a flare of IBD, 20 remained in remission, and 7 patients were able to discontinue immunosuppressive therapy.
Conclusions
Based on available studies, bariatric surgery appears to be both an effective and safe option for weight loss in patients with IBD.
Keywords: Obesity, Crohn's disease, Ulcerative colitis, Roux-en-Y gastric bypass, Sleeve gastrectomy
Introduction
The incidence and prevalence of obesity in the United States continue to rise, with recent data from a national survey estimating that nearly 38% of adults over age 20 are obese and 71% of adults over age 20 are overweight or obese [1]. In the inflammatory bowel diseases (IBD) population, a recent review has cited that 15–40% of IBD patients are obese and an additional 20–40% of IBD patients are overweight [2]. The impact of obesity on the disease course of patients with IBD is unclear, with some reports suggesting a worsened IBD disease course for obese patients [3, 4].
Bariatric surgery is the most effective long-term therapy for morbid obesity and obesity-related comorbidities. However, the safety and efficacy of bariatric surgery in the IBD patient population is not well understood. There is concern that bariatric surgery may cause disease flares or may lead to the development of fistulas or other complications in patients with IBD, particularly those with Crohn's disease (CD). The most recent bariatric surgery guidelines from the Society of American Gastrointestinal and Endoscopic Surgeons suggest that the most common bariatric surgery methods are contraindicated in patients with CD [5, 6, 7, 8].
Several small studies have explored the effects of bariatric surgery in the IBD patient population. These studies have suggested that bariatric surgery is safe, efficacious, and may even have disease-modifying effects such as reduction in symptoms, clinical/endoscopic remission, and reduction in need for therapeutic immunomodulatory agents [9, 10, 11, 12]. The objectives of this study were to examine safety and efficacy of bariatric surgery in patients with IBD, as well as to explore any impact on IBD disease course after bariatric surgery.
Materials and Methods
Patient Population
We performed a review of all patients with an existing diagnosis of CD or ulcerative colitis (UC) who underwent bariatric surgery at the University of North Carolina (UNC) Hospitals between January 1, 2008 and December 31, 2017. Patients were identified for inclusion into the study using International Classification of Diseases 9th and 10th Clinical Modification (ICD-9-CM and ICD-10-CM) coding (555.xx and K50.x for CD, 556.xx and K51.x for UC, full bariatric surgery coding available in online suppl. Table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000496925). The Informatics for Integrating Biology and the Bedside (i2b2) platform through the Carolina Data Warehouse for Health was used to identify patients with these codes [13, 14].
Patients were eligible for inclusion, provided they met each of the following criteria: (i) age > 18 years, (ii) history of CD or UC, and (iii) history of bariatric surgery performed at UNC Hospitals between January 1, 2000 and December 31, 2017. Eligible bariatric surgery procedures included sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), or laparoscopic adjustable gastric banding (LAGB). Patients were excluded if a diagnosis of IBD was established after bariatric surgery or if a bariatric surgery was performed in another hospital system. In addition to the ICD-9-CM and ICD-10-CM coding, IBD diagnosis was verified by manual chart review performed by a licensed gastroenterologist.
Definition of Covariates
To identify potential predictors of complications, we analyzed both demographic and clinical factors, including age, sex, preoperative body mass index (BMI), phenotype, prior IBD therapies, and prior IBD-related surgeries. Additionally, outcomes were compared with respect to the type of bariatric surgery utilized. Patient data was followed up up to 24 months postoperatively, if available.
Systematic Review of the Literature
Following the identification of the UNC cohort, we conducted a systematic review of the existing literature. We conducted a search through May 1, 2018 using PubMed/MEDLINE, Web of Science, and Scopus. We used the following search phrase: “inflammatory bowel disease” OR “Crohn's disease” OR “ulcerative colitis” OR “indeterminate colitis” OR “IBD” AND “bariatric surgery” OR “Roux-en-Y” OR “gastric bypass” OR “sleeve gastrectomy” OR “RYGB” OR “laparoscopic adjustable gastric banding” OR “LAGB.” Results were limited to English language articles. Results were manually reviewed for relevant case reports, case series, or cohort studies. All primary studies which reported on IBD patients undergoing any form of bariatric surgery were included. Review articles were excluded. Data from the individual studies was combined with our current case series to yield summary data on postoperative bariatric course, safety, and postoperative IBD course.
Statistical Analysis
In the initial evaluation of the UNC cohort, continuous variables were summarized using means and standard deviations (SD). Cumulative statistics were then calculated. Where applicable, data from the UNC cohort was combined with available data from the existing literature to calculate standardized means with standard error, variance, and confidence intervals (CI). All analyses were performed using SAS (version 9.4) statistical software (SAS Institute, Cary, NC, USA) and Comprehensive Meta-Analysis (version 3) (Biostat, Englewood, NJ, USA). The study was approved by the Institutional Review Board at UNC Chapel Hill School of Medicine.
Results
Case Series
In our case series, we identified 13 patients with IBD (9 patients with CD and 4 with UC) who underwent bariatric surgery at UNC Hospitals. Baseline characteristics included mean age of 48.1 years (SD 10.6), 82% female, and baseline BMI of 44.6 (SD 7.7). IBD phenotype data was available for 8 of the patients (4 with CD, 4 with UC), as shown in Table 1. Three of the patients had a prior history of IBD-related fistulas. At the time of surgery, most patients were not on any IBD-related medical therapy (n = 7). Of those on therapy, 3 patients with CD (thiopurine n = 1, anti-TNF n = 1, anti-TNF + thiopurine n = 1) and 1 patient with UC (thiopurine) were on immunosuppressive medications. Mean follow-up after bariatric surgery was 52 weeks (SD 28).
Table 1.
Individual patient characteristics, bariatric surgery-related and IBD-related outcomes from UNC cohort
| Patient | Age, years | Sex | IBD type | IBDphenotype | Prior IBD surgery | Preop. IBD medications | Preop. BMI |
Surgery type |
6 monthspostop. BMI |
12 monthspostop. BMI | Early postop. complications | Late postop. complications | Postop. IBD medications | Postop. IBD flare |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39 | F | CD | A2L1B1 | None | Adalimumab | 43.91 | SG | 31.28 | 28.94 | None | None | No changes | No |
| 2 | 52 | M | CD | A3L2B1 | None | None | 42.16 | SG | 31.65 | 31.78 | None | None | No medications | No |
| 3 | 56 | F | CD | N/A | SBR | Thiopurine | 36.77 | SG | 30.15 | 31.20 | None | None | No changes | No |
| 4 | 49 | F | CD | A3L1B3 | SBR | 5-ASA | 44.39 | SG | 30.91 | 26.41 | None | None | No changes | No |
| 5 | 40 | F | CD | A2L2B3 | IBD-related Surgery |
Adalimumab, Thiopurine |
36.58 | SG | 32.38 | 31.14 | None | None | Stopped thiopurine | No |
| 6 | 36 | F | CD | N/A | None | None | 61.54 | SG | 50.36 | 42.60 | N&V | None | No medications | No |
| 7 | 45 | F | CD | N/A | SBR | 5-ASA | 40.91 | SG | 28.90 | 25.38 | None | None | Medications discontinued | No |
| 8 | 69 | F | CD | N/A | SBR | None | 60.41 | RYGB | 44.95 | 34.02 | N&V | N&V | No medications | No |
| 9 | 54 | F | CD | N/A | None | None | 42.75 | SG | 32.54 | 31.24 | None | None | No medications | No |
| 10 | 41 | F | UC | A3E2 | TAC w/IPAA | None | 42.78 | RYGB | 32.75 | N/A | SBO, N&V | Incisional abscess | No medications | No |
| 11 | 37 | F | UC | A2E3 | TAC w/EI | None | 42.51 | LAGB | 36.84 | 33.93 | N&V, stenosis | Band slippage | No medications | No |
| 12 | 42 | F | UC | A3E3 | None | 5-ASA, Thiopurine |
45.58 | RYGB | 35.17 | N/A | N&V | None | N/A | No |
| 13 | 65 | M | UC | A3E1 | None | None | 39.77 | SG | 33.90 | N/A | None | None | No medications | No |
| Mean (SE) | 48.1 (2.9) | 44.6 (2.1) | 34.8 (1.7) | 31.7 (1.5) |
EI, end ileostomy; IPAA, ileo-anal pouch anastomosis; N&V, nausea and vomiting; SBO, small bowel obstruction; SBR, small bowel resection; TAC, total abdominal colectomy.
Bariatric surgery procedures in our case series included SG (n = 9), RYGB (n = 3), and LAGB (n = 1). Of the 9 patients with CD, 8 (89%) underwent SG while only 1 (11%) underwent RYGB. Of the 4 patients with UC, 1 (25%) underwent SG, 2 (50%) underwent RYGB, and 1 (25%) underwent LAGB. Average BMI at 6 months postoperatively was 34.8 ± 6.2 and at 12 months postoperatively it was 31.7 ± 4.8. Postoperative complications were divided into early (< 30 days from surgery date) and late (> 30 days from surgery date). Early postoperative complications (n = 5) included stenosis (n = 1), small bowel obstruction (n = 1), and persistent nausea and vomiting (n = 3). Late postoperative complications included band slippage (n = 1) that ultimately led to a revisional operation with RYGB, persistent nausea and vomiting (n = 1), and incisional-site abscess (n = 1). Returns to the operating room were noted in both the early (n = 1) and late (n = 1) periods. At 3, 6, and 12 months postoperatively, no patients (n = 13) were noted to have a flare of their underlying IBD, based on routine clinical follow-up. Following surgical intervention, 2 patients were noted to have IBD-related medical therapy discontinued (1 aminosalicylate, 1 thiopurine) with the remainder (n = 11) having no changes in their medical therapy.
Literature Review with Case Series
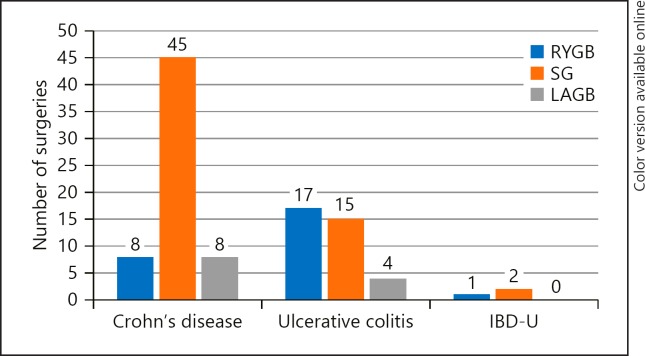
Review of the literature identified 8 case series or case reports with 88 total patients, which were published between 2006 and 2018. Data from these 88 patients were combined with the current 13-patient series to bring the total sample size to 101 patients. Of these 101 patients, 61 had CD, 37 had UC, and 3 patients had a diagnosis of IBD-unspecified (Table 2). Baseline characteristics for the overall cohort include a standardized mean age of 47 years (95% CI 44.8–49.3), 79% female patients, and a baseline BMI of 44.2 (95% CI 42.9–45.7). The average duration of IBD diagnosis at the time of surgery was 11.7 years (95% CI 9.7–13.7). Phenotype data was available for 30 patients and included ileal disease in 6, ileocecal disease in 5, small bowel disease in 2, ileocolonic disease in 3, colonic disease in 5, left-sided colonic disease in 2, sigmoiditis in 1, proctitis in 1, and pancolitis in 4 of the patients. Twenty-four patients had a history of prior IBD-related surgery that included both small and large bowel resections (see details in Table 3). Seventy patients were on medications in the preoperative period including 21 on thiopurines, 10 on biologics, and 10 on steroids. Among the 101 patients in our population, 63 underwent SG, 26 underwent RYGB, 11 underwent LAGB, and 1 patient underwent vertical banded gastroplasty. See Figure 1 for details on type of bariatric surgery by underlying IBD diagnosis.
Table 2.
Comparison of essential characteristics of included studies
| Year | Study | Type | Sample | e Age, years | Percent female |
CD | UC | IBD-U | BMI |
|---|---|---|---|---|---|---|---|---|---|
| 2018 | UNC | Retrospective review | 13 | 48.1±10.6 | 85% | 9 | 4 | 0 | 44.6±7.7 |
| 2017 | Aelferset al.[11] | Retrospective review | 45 | 44.1±12.1 | 84% | 27 | 15 | 3 | 44.6±7.0 |
| 2016 | Aminianet al.[9] | Retrospective review | 20 | 54±10.5 | 70% | 7 | 13 | 0 | 50.1±9.0 |
| 2015 | Colomboet al. [10] | Prospective | 6 | 47.2±9.5 | 67% | 5 | 1 | 0 | 40.6±3.7 |
| 2015 | Keidar et al. [12] | Prospective | 10 | 39.7±11.9 | 90% | 8 | 2 | 0 | 42.6±5.6 |
| 2014 | DelPradoet al. [23] | Retrospective review | 1 | N/A | N/A | 0 | 1 | 0 | N/A |
| 2013 | Ungaret al. [5] | Retrospective case-control | 4 | 50.8±15.6 | 75% | 4 | 0 | 0 | 45±5.3 |
| 2010 | Moum and Jahnsen [24] | Case report | 1 | 40 | 100% | 1 | 0 | 0 | 45 |
| 2006 | Lascano et al. [25] | Case report | 1 | 39 | 0% | 0 | 1 | 0 | 57 |
| Total / standardized mean | 101 | 47.1 (44.8–49.3) | 78% | 61 | 37 | 3 | 44.17 (42.9–45.7) | ||
CD, Crohn's disease; IBD-U, inflammatory bowel disease-unspecified; UC, ulcerative colitis; UNC, University of North Carolina.
Table 3.
Bariatric Surgery-related Outcomes for Individual Studies
| Study | History of IBD-related surgery | RYGB | SG | LAGB | VG | 6 months EWL% | Early complications | Late complications |
|---|---|---|---|---|---|---|---|---|
| UNC | 4 small bowel resections, 1 | 3 | 9 | 1 | 0 | 63.5±17.9 | 1 stenosis, 1 SBO, | 1 band slippage, 1 N&V, |
| TPC w/EI, 1 TAC w/EI, 1 other | 3 N&V | 1 WI | ||||||
| Aelferset al.[11] | 9 prior surgery | 13 | 26 | 6 | 0 | 62.9±27.1 | 1 GE bleeding, 1 | 1 pyelonephritis w/PNC, |
| AKI on CKD, 3 | 2 passage complaints, | |||||||
| passage complaints, | 1 hypokalemia, 1 N&D, | |||||||
| 1 WI, 1 anemia, 1 N&V |
1 dehydration, 1 urolithiasis | |||||||
| Aminianet al.[9] | 5 TPC | 8 | 9 | 3 | 0 | 58.9±21.1 | 5 dehydration, 1 PE, | 2 PNC, 2 ventral hernia, |
| 1 WI | 1 marginal ulcer | |||||||
| Colomboet al. [10] | 2 small bowel resections | 0 | 5 | 0 | 1 | 74.5±11.2 | 1 N&V | |
| Keidar et al. [12] | None | 0 | 9 | 1 | 0 | 71.4±5.9 | 1 staple line leak | 4 VitD deficiency |
| Del Prado et al. [23] | N/A | 0 | 0 | 1 | 0 | N/A | N/A | N/A |
| Ungar et al. [5] | 1 terminal ileocectomy and hemicolectomy | 0 | 4 | 0 | 0 | 60.3±13.7 | 1 staple line bleeding | N/A |
| Moum and Jahnsen [24] | None | 1 | 0 | 0 | 0 | 60 | N/A | N/A |
| Lascano et al. [25] | None | 1 | 0 | 0 | 0 | 80 | N/A | N/A |
| Totals/standardized mean | 26 | 63 | 11 | 1 | 68.43 (65.7–71.2) | |||
VG, vertical banded gastroplasty; CKD, chronic kidney disease; EWL, excess weight loss; GE, gastroenterostomy; IBD, inflammatory bowel disease; N&D, nausea and diarrhea; N&V, nausea and vomiting; TPC, total proctocolectomy; PE, pulmonary embolism; PNC, pancreatitis; SBO, small bowel obstruction; UNC, University of North Carolina; VitD, vitamin D; WI, wound infection.
Fig. 1.
Type of bariatric surgery by underlying IBD diagnosis. RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy; LAGB, laparoscopic adjustable gastric banding; IBD-U, inflammatory bowel disease-unspecified.
In terms of bariatric outcomes, the average percent excess weight loss across all patients included was 68.4% (95% CI 65.7–71.2), at the longest available follow-up time. In the postoperative period, early complications were reported in 22% of patients, including 5 episodes of dehydration, 2 wound infections, 2 staple line issues, and 1 pulmonary embolism (Table 3). Additionally, 20% of patients experienced late complications including 3 episodes of pancreatitis, 2 ventral hernias, 1 marginal ulcer, and 1 episode of lap-band slippage that resulted in a return to the operating room for revision of the LAGB to a RYGB. Despite these complications, only 4 patients required repeat operation in the 12 months following their initial bariatric surgery.
Regarding the postoperative IBD course, patients were generally noted to have favorable outcomes in the postoperative period as well. Seven patients were able to de-escalate their immunosuppressive therapy (6 discontinued steroids, 1 discontinued thiopurine; additionally, 3 patients decreased thiopurine dosages > 50% of baseline dose, though the reason for the decrease in dosage was not specified). Only 2 patients required escalation in their IBD-related immunosuppressive therapy. Twenty patients were reported to be in clinical remission following their surgeries. However, not all patients had a favorable postoperative IBD course, with 10 patients noted to have an IBD flare.
Discussion/Conclusion
With the progressive rise of obesity in the IBD population, a better understanding of obesity-directed therapies in the context of IBD is becoming increasingly relevant to clinical practice. In the present analysis, we review our single institution experience as well as the existing literature on bariatric surgery amongst patients with IBD. In this cohort, bariatric surgery appeared to be effective at producing weight loss and safe in terms of surgical outcomes. Moreover, there was no clear signal for worsening of IBD after bariatric surgery.
Bariatric surgery remains the most effective modality for producing weight loss amongst patients with morbid obesity and obesity-related complications. Bariatric surgery also reverses metabolic complications of obesity, including insulin resistance, hyperlipidemia and sleep apnea, and these effects appear durable up to 10 years [15]. The current literature suggests approximate excess weight loss of 50–80% for RYGB, 40–80% for SG, and 30–60% for LAGB [15, 16, 17, 18, 19]. Results from our current study showed average weight loss of 68%, which is congruent with published estimates of postbariatric surgery weight loss in non-IBD patients. Moreover, postoperative complications were relatively low in this cohort, with early complications in 22%, late complications in 20%, and only 4% of patients requiring a return to the operating room due to a complication. This is also consistent with published estimates of bariatric surgery complications in non-IBD patients, with a recent meta-analysis reporting a rate of 13–21% of any postoperative complication and reoperation rates of 3–12% [19]. Hence, bariatric surgery in patients with IBD appears to have similar efficacy and safety profiles as bariatric surgery in the general population.
Another clinically important question is the safety of bariatric surgery in terms of the underlying IBD, including potential for IBD flares or fistulizing complications after bariatric surgery. In our current study, a small proportion of patients (7%) were able to discontinue steroids or thiopurines and approximately 20% were noted to be in clinical remission after bariatric surgery. A small subset of patients required escalation of IBD medications (2%) or were reported to have a flare of IBD (10%). Clinical severity of IBD flare was not specified in these cases, but there were no reports of fistulizing complications after bariatric surgery. While postoperative IBD course was not available for all patients and follow-up was relatively short, the available data suggest that a small percentage of patients will have an IBD flare after bariatric surgery and a small percentage will be able to de-escalate medications or achieve remission. As previously suggested, there remains an open question whether weight loss after bariatric surgery may mitigate the underlying IBD pathology [20, 21]. However, our data cannot clearly answer this question.
These data also raise the question of patient selection when considering bariatric surgery. Because all the available studies are retrospective in nature, we cannot account for patient selection. It is possible that clinicians preferentially selected patients with milder IBD severity when referring patients for bariatric surgery. There is some indication of this in the data, as 30% of patients were on no medications for IBD and only 10% were on biologics, which is considerably lower than the market share of biologics in the US in the last 10 years [22]. Moreover, type of surgery may be an important consideration. Most CD patients in this cohort had SG (74%). By contrast, UC patients were more evenly divided between RYGB (47%) and SG (42%). While we cannot draw strong conclusions about type of surgery, there does appear to be considerably more experience with SG in patients with CD.
Taken together, the data from our review may provide some guidance for clinicians considering bariatric surgery in their patients with IBD. Given the apparently good weight loss outcomes in this population, it would seem reasonable to use traditional bariatric criteria when considering IBD patients for referral to bariatric surgery (e.g., BMI > 40 or BMI ≥35 with weight-related comorbidities [6]). Since many of the patients in our dataset may have had milder IBD phenotype, it would seem prudent to carefully evaluate IBD disease severity prior to referral for bariatric surgery, favoring patients with milder IBD severity. In terms of type of surgery, there is more experience with SG for patients with CD but a more even mix of experience with SG and RYGB for patients with UC.
The current study reports on a large cohort of patients with IBD undergoing bariatric surgery, with information on postoperative bariatric outcomes, surgical outcomes and underlying IBD course. Nevertheless, there are some important limitations. Despite using data from multiple settings and centers, all included data is retrospective in nature. Given that we combined data from multiple cohorts, outcome measures were not consistently reported across all patients. Additionally, there was missing data for certain outcomes of interest; most notably data regarding postoperative IBD course was not reported for multiple patients. Follow-up was also relatively short, on the order of 1–2 years depending on the study, so we cannot draw long-term conclusions about IBD complications many years or decades after bariatric surgery. Finally, in each of the included studies, the data come largely from academic medical centers, which may limit the generalizability of these results to a wider population. However, it is also likely that patients with IBD considering bariatric surgery may be more likely to undergo these procedures at a referral center.
In conclusion, data from our case series and review of the literature suggest that bariatric surgery has a similar efficacy and safety profile in patients with IBD as in the general population. In terms of IBD disease course, a small percentage of patients experienced an IBD flare after bariatric surgery while a small number were noted to be in remission or were able to stop IBD medications. Hence, bariatric surgery appears to be a safe and effective option for carefully selected patients with IBD.
Statement of Ethics
The study protocol was approved by the University of North Carolina School of Medicine's institutional review board.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research was supported, in part, by grants from the Crohn's and Colitis Foundation (E.L.B.).
Author Contributions
J.L.H. was involved in the study concept and design, acquisition of the data, analysis and interpretation of the data, and drafting and critical revision of the manuscript. E.L.B., H.H.H., K.L.I., and A.J. were involved in study concept and design, acquisition of the data, analysis and interpretation of the data, and critical revision of the manuscript. All authors reviewed and approved the final draft of the manuscript submitted.
Supplementary Material
Supplementary data
Acknowledgement
i2b2 software was used in conducting this study. i2b2 is the flagship tool developed by the i2b2 (Informatics for Integrating Biology and the Bedside) Center, a National Institutes of Health (NIH) funded National Center for Biomedical Computing based at Partners HealthCare System. The i2b2 instance at the University of North Carolina is supported by the National Center for Advancing Translational Sciences (NCATS), NIH, through Grant Award Number UL1TR002489. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.National Center for Health Statistics (US). Health, United States With chartbook on long-term trends in health. Cent Dis Control. 2016;2017:314–7. [PubMed] [Google Scholar]
- 2.Singh S, Dulai PS, Zarrinpar A, Ramamoorthy S, Sandborn WJ. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb;14((2)):110–21. doi: 10.1038/nrgastro.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurnool S, Nguyen NH, Proudfoot J, Dulai PS, Boland BS, Vande Casteele N, et al. High body mass index is associated with increased risk of treatment failure and surgery in biologic-treated patients with ulcerative colitis. Aliment Pharmacol Ther. 2018 Jun;47((11)):1472–9. doi: 10.1111/apt.14665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hass DJ, Brensinger CM, Lewis JD, Lichtenstein GR. The impact of increased body mass index on the clinical course of Crohn's disease. Clin Gastroenterol Hepatol. 2006 Apr;4((4)):482–8. doi: 10.1016/j.cgh.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Ungaro R, Fausel R, Chang HL, Chang S, Chen LA, Nakad A, et al. Bariatric surgery is associated with increased risk of new-onset inflammatory bowel disease: case series and national database study. Aliment Pharmacol Ther. 2018 Apr;47((8)):1126–34. doi: 10.1111/apt.14569. [DOI] [PubMed] [Google Scholar]
- 6.SAGES Guidelines Committee SAGES guideline for clinical application of laparoscopic bariatric surgery. Surg Obes Relat Dis. 2009 May-Jun;5((3)):387–405. doi: 10.1016/j.soard.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Boutros M, Maron D. Inflammatory bowel disease in the obese patient. Clin Colon Rectal Surg. 2011 Dec;24((4)):244–52. doi: 10.1055/s-0031-1295687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frolkis AD, Dykeman J, Negrón ME, Debruyn J, Jette N, Fiest KM, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013 Nov;145((5)):996–1006. doi: 10.1053/j.gastro.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 9.Aminian A, Andalib A, Ver MR, Corcelles R, Schauer PR, Brethauer SA. Outcomes of Bariatric Surgery in Patients with Inflammatory Bowel Disease. Obes Surg. 2016 Jun;26((6)):1186–90. doi: 10.1007/s11695-015-1909-y. [DOI] [PubMed] [Google Scholar]
- 10.Colombo F, Rizzi A, Ferrari C, Frontali A, Casiraghi S, Corsi F, et al. Bariatric surgery in patients with inflammatory bowel disease: an accessible path? Report of a case series and review of the literature. J Crohn's Colitis. 2015 Feb;9((2)):185–90. doi: 10.1093/ecco-jcc/jju011. [DOI] [PubMed] [Google Scholar]
- 11.Aelfers S, Janssen IM, Aarts EO, Smids C, Groenen MJ, Berends FJ. Inflammatory Bowel Disease Is Not a Contraindication for Bariatric Surgery. Obes Surg. 2018 Jun;28((6)):1681–7. doi: 10.1007/s11695-017-3076-9. [DOI] [PubMed] [Google Scholar]
- 12.Keidar A, Hazan D, Sadot E, Kashtan H, Wasserberg N. The role of bariatric surgery in morbidly obese patients with inflammatory bowel disease. Surg Obes Relat Dis. 2015 Jan-Feb;11((1)):132–6. doi: 10.1016/j.soard.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Murphy SN, Weber G, Mendis M, Gainer V, Chueh HC, Churchill S, et al. Serving the enterprise and beyond with informatics for integrating biology and the bedside (i2b2) J Am Med Inform Assoc. 2010 Mar-Apr;17((2)):124–30. doi: 10.1136/jamia.2009.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy SN, Wilcox A. Mission and Sustainability of Informatics for Integrating Biology and the Bedside (i2b2) eGEMs (Generating Evid Methods to Improv patient outcomes) 2014;2((2)):1–6. doi: 10.13063/2327-9214.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duvoisin C, Favre L, Allemann P, Fournier P, Demartines N, Suter M. Roux-en-Y Gastric Bypass: Ten-year Results in a Cohort of 658 Patients. Ann Surg. 2018 Dec;268((6)):1019–25. doi: 10.1097/SLA.0000000000002538. [DOI] [PubMed] [Google Scholar]
- 16.Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis. 2009 Jul-Aug;5((4)):469–75. doi: 10.1016/j.soard.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Chang D, Lee W, Chen JC, Ser KH, Tsai PL, Lee YC. Thirteen-Year Experience of Laparoscopic Sleeve Gastrectomy : Surgical Risk, Weight Loss, and Revision Procedures. 2018;28:2991–7. doi: 10.1007/s11695-018-3344-3. [DOI] [PubMed] [Google Scholar]
- 18.Garb J, Welch G, Zagarins S, Kuhn J, Romanelli J. Bariatric surgery for the treatment of morbid obesity: a meta-analysis of weight loss outcomes for laparoscopic adjustable gastric banding and laparoscopic gastric bypass. Obes Surg. 2009 Oct;19((10)):1447–55. doi: 10.1007/s11695-009-9927-2. [DOI] [PubMed] [Google Scholar]
- 19.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014 Mar;149((3)):275–87. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonçalves P, Magro F, Martel F. Metabolic inflammation in inflammatory bowel disease: crosstalk between adipose tissue and bowel. Inflamm Bowel Dis. 2015 Feb;21((2)):453–67. doi: 10.1097/MIB.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 21.Morshedzadeh N, Rahimlou M, Asadzadeh Aghdaei H, Shahrokh S, Reza Zali M, Mirmiran P. Association Between Adipokines Levels with Inflammatory Bowel Disease (IBD): systematic Reviews. Dig Dis Sci. 2017 Dec;62((12)):3280–6. doi: 10.1007/s10620-017-4806-5. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, MacIsaac D, Wong JJ, Sellers ZM, Wren AA, Bensen R, et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther. 2018 Feb;47((3)):364–70. doi: 10.1111/apt.14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Prado P, Papasavas PK, Tishler DS, Stone AM, Ng JS, Orenstein SB. Laparoscopic placement of adjustable gastric band in patients with autoimmune disease or chronic steroid use. Obes Surg. 2014 Apr;24((4)):584–7. doi: 10.1007/s11695-013-1122-9. [DOI] [PubMed] [Google Scholar]
- 24.Moum B, Jahnsen J. [Obesity surgery in inflammatory bowel disease] Tidsskr Nor Laegeforen. 2010 Mar;130((6)):638–9. doi: 10.4045/tidsskr.09.1281. [DOI] [PubMed] [Google Scholar]
- 25.Lascano CA, Soto F, Carrodeguas L, Szomstein S, Rosenthal RJ, Wexner SD. Management of ulcerative colitis in the morbidly obese patient: is bariatric surgery indicated? Obes Surg. 2006 Jun;16((6)):783–6. doi: 10.1381/096089206777346718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data