Abstract
A drug delivery system (DDS) for combined therapy, based on a short oxidized multiwalled carbon nanotube, is reported. It was prepared exploiting a synthetic approach which allowed loading of two drugs, doxorubicin and metformin, the targeting agent biotin and a radiolabeling tag, to enable labeling with Ga-68 or Cu-64 in order to perform an extensive biodistribution study by PET/CT. The DDS biodistribution profile changes with different administration methods. Once administered at therapeutic doses, the DDS showed a marginal beneficial effect on 4T1 tumor bearing mice, a syngeneic and orthotopic model of triple negative breast cancer, with survival extended by 1 week and 2 days in 20% of the mice. This is encouraging given the aggressiveness of the 4T1 tumor. Furthermore our DDS was well tolerated, ruling out concerns regarding the toxicity of carbon nanotubes.
Keywords: carbon nanotube, combined therapy, metformin, doxorubicin, PET imaging, 4T1 tumor cells
Entry for table contents
A doxorubicin/metformin carrier for based on oxidized MWCNT. Efficacy in vitro and in vivo
1. Introduction
Carbon nanotubes (CNT) have emerged in recent years as promising materials for applications in different fields of nanomedicine ranging from tissue engineering,1 nerve reconstruction2 and drug delivery.3 The latter was the first application to be developed and many examples can be found in the literature showing CNT potentialities, both in vitro and in vivo.4,5 However, together with the interest for these nanomaterials, concerns have been raised on their safety profile6 and their efficiency as drug delivery systems. Several studies on the biodistribution and clearance of various CNT formulations have been reported to assess their pharmacokinetics and to offer a rationale for the production of efficient drug delivery systems (DDS): single- or multiwalled CNT have been compared, they have been modified with polymers and substituted with targeting components,7 or they have been loaded with drugs in efficacy studies.8 The mechanism by which CNT distributes in living organisms is still far from being understood. This is due, mainly, to the heterogeneity of the materials, to the underlying cellular heterogeneity at the target site, and to the great variability of the biological response upon structural variation of the carrier.9 Therefore, rules to predict the biodistribution of CNT are hard to be defined a priori. Analysis is even more complicated when functionalization is introduced on the material because any decoration of the CNT can potentially modify the interaction with living organisms.10
Unlike metallic nanoparticles, oxidized CNT can be decomposed by endogenous peroxidases such as neutrophils myeloperoxidase (MPO) and eosinophils peroxidase (EPO) in vitro.11–13 The degradation process was also documented in vivo for oxidized SWCNT accumulated in mice lungs.14 Similar results were reported for biodegradation of MWCNT by microglia cells after brain injection into mice.15
The clear majority of CNT-based DDS has been produced for applications in oncology with promising results. CNT have been used as carriers for chemotherapy,16,17 gene therapy,18 photodynamic and photothermal therapy.19 Furthermore CNT characteristics, such as the high surface area and the chemical reactivity, simplify the design and synthesis of new DDS able to carry two or more bioactive compounds20. For this reason, CNTs have been largely studied as carriers useful for co-delivery of pairs of cytotoxic drugs, anticancer drugs and siRNA, or other combinations, including different molecules able to interfere with physiology of cancer cells.21 Without a common delivery vehicle, matching pharmacokinetic parameters of such varying structures will be exceptionally difficult.
In this context, we have conducted a research study aimed at the delivery of a combination therapy consisting of a classic cytotoxic drug, doxorubicin (DOXO), and metformin (MET), a diabetes drug known to have anticancer activity. Both drugs were loaded on the same CNT based carrier. We utilized PET/CT to assess the biodistribution of the loaded nanocarrier. PET/CT was also exploited to select the optimal method of administration of the nanomaterial. The efficacy of CNT was tested in an orthotopic preclinical model of triple negative breast cancer. The novelty of the work consists in the unprecedented combination of DOXO and MET, that have already shown a synergetic action, onto a CNT-based DDS and its extensive characterization in vivo by PET. In particular the right route of administration has proven crucial in maximizing tumor accumulation.
2. Results and discussion
MET is used for the treatment of type II diabetes and has recently been highlighted for its anticancer activity, demonstrated by several experimental22,23 and observational24 studies. Although the anticancer mechanism is not completely elucidate, there is evidence that MET acts as an inhibitor of Oxidative Phosphorylation (OxPhos).25 Furthermore, in combination with DOXO, MET demonstrated an interesting synergetic action against cancer stem cells in a mouse model.26 However, the main drawback of MET is the high doses required in vivo. In most in vitro experiments, a millimolar concentration of metformin, toxic for humans, is required. For these reasons, use of a DDS for transport of the DOXO/MET pair may assist in reducing the effective dose of metformin needed for efficacy, minimizing potential side effects. Having the two drugs in close proximity could also prompt a synergistic effect at low doses.
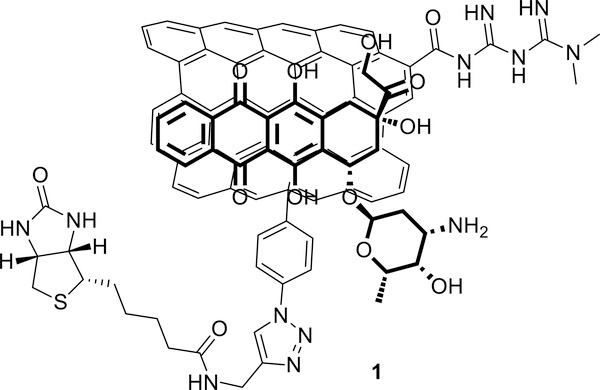
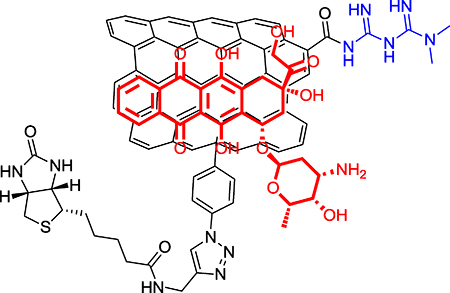
Herein we report assembly, in vitro and in vivo evaluation and biodistribution of a new DDS (compound 1, figure 1) based on ox-MWCNTs loaded with DOXO and MET. A critical point, when preparing a DDS for a combined therapy, is control of the overall drug loading and the ratio of the two drugs. DOXO is loaded onto CNT using the supramolecular interaction between the drug and the CNT walls. The loading process profits from the π-π stacking interaction between the anthracycline moiety of DOXO and the π-system on the CNT, providing high drug loading. The supramolecular interaction is reversible and pH-dependent. The complex is stable at physiologic pH, but release is enhanced in acidic conditions. This behavior provides a release mechanism once the system reaches the tumor compartment. The nanomaterial is thought to be internalized in cancer cells by endocytosis and engulfed in the phago-lysosomes. Lysosomal microenvironments are sufficiently acidic for protonation of the amine group on DOXO and consequent detachment of the drug.27 On the contrary, MET is a small polar molecule that cannot interact easily with CNTs and required covalent binding (Figure 1).
Figure 1:
Structure of the drug delivery system loaded with doxorubicin and metformin
The construct represents a new class of nanomaterial, with only few examples reported of CNT covalently decorated with MET.28,29 Application as a chemotherapeutic agent is uncharted and only Yoo et al. reported an example of a multimodal DDS based on a MWCNT decorated with MET.30 The presence of the MET residues on the CNT increases their polarity, rendering them easily dispersible in water, PBS solution, and blood/serum. Besides bearing the two drugs, compound 1 is decorated with a biotin residue that may act as targeting component to facilitate accumulation in cancer cells overexpressing the biotin receptor.31,32 To study the biodistribution of the nanocarrier, we envisaged using Positron Emission Tomography (PET), a non-invasive imaging technique which allows the detection of a radiolabeled drug in vivo in real time. PET requires the use of a positron-emitting radioisotope, which, if a radiometal, must be chelated to a bifunctional ligand. It was therefore necessary to graft onto the CNT platform a bifunctional chelating moiety, namely DOTA or NOTA, able to complex the radiometal of choice. The presence of a chelator may enable future radiotherapy applications. This concurrence of drugs, targeting selectors and chelators required a complex synthetic strategy that relied on careful evaluation of orthogonal reactions.
2.1. Synthesis
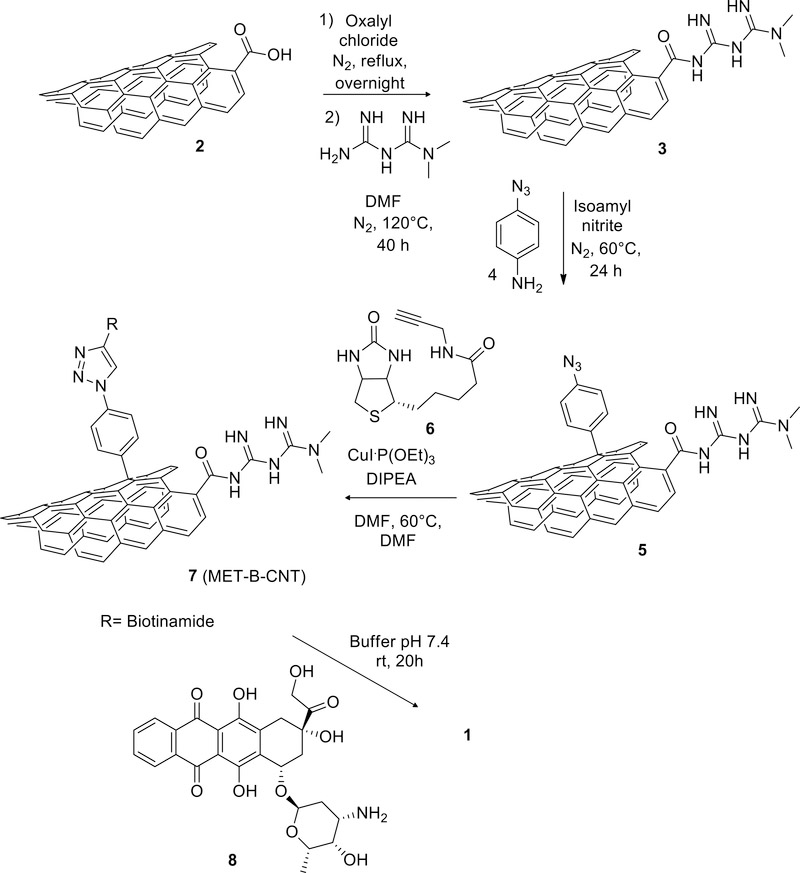
Ox-MWCNT were prepared starting from pristine CoMoCat© MWCNTs by oxidation with nitric and sulfuric acid and obtaining lengths ranging from 50 to 1000 nm. An ultracentrifugation process allowed the isolation of an aliquot of short ox-MWCNT (from now on simply CNT) with an average length of 130 nm (see supporting, figures S1 and S2).17 MET was covalently introduced by amidation, after activation of the carboxylic groups by treatment with oxalyl chloride (Scheme 1). The reaction, repeated three times, showed high reproducibility, with loading of 1.9 ± 0.1 mmol of MET/g of material 3 (MET-CNT), based on elemental analysis of the nitrogen content (see supporting section 2.3). Similar loadings were reported by Yoo et al. that covalently decorated CNT with an analogous approach.30
Scheme 1.
Preparation of DDS 1 (MET-B-DOXO-CNT).
MET-CNT material was then decorated with the targeting component biotin following our “click- approach”.16 The functionalized material was synthesized in two steps: initially, azido groups were introduced on 3 using the Tour reaction33,34 to afford compound 5. Compound 5 was then reacted with biotin derivative 6 to afford compound 7 (MET-B-CNT) (see Scheme 1 and supporting material, section 2.7). FT-IR spectra (see supporting information figure S22 and S23) evidenced the decoration of CNT sidewall with azide groups, confirmed by the appearance of a peak at 2100 cm–1 (azide group stretching) in compound 5 when compared to starting material 3. The same band disappears in compound 7 after the click reaction.
The multimodal DDS 1 (MET-B-DOXO-CNT) was completed with the loading of DOXO, accordingly with a previously published procedure.16 The supramolecular reaction was carried out in phosphate buffer at pH 7.4 (Scheme 1). The amount loaded on the tubes is 41% wt of the material (see supporting material, section 4.2 and 4.3 for the release).
The final composition of MET-B-DOXO-CNT (1) was then 0.75 mmol of DOXO and 1.2 mmol of MET for each gram of material.
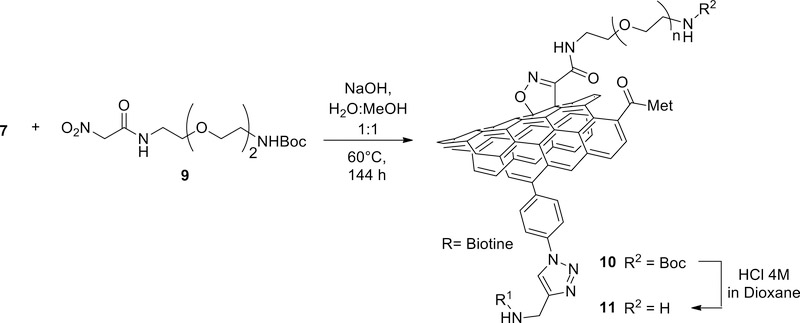
To prepare the radiolabeled DDS necessary for biodistribution studies, a suitable bifunctional chelator was introduced to enable complexation of a radiometal. To achieve this goal, compound 7 was reacted with nitroacetamide 9, via “Machetti-De Sarlo” reaction, 35–39 yielding adduct 10 with a high loading of protected-amine groups (scheme 2).40 Thermogravimetric analysis (TGA) of 10 showed a weight loss of around 48 % between 160 and 260 °C. In compound 7, used as control, in the same conditions the observed loss was only 7 %. The difference in weight loss 41% (1.05 mmol/g based on TGA analysis, see supporting figure S15) is therefore due to the loss of the cycloadduct, confirming that the linker was successfully introduced. Furthermore, FT-IR spectrum of compound 10 showed an increment in the intensity of the 1571 cm–1 band compared to compound 7, due to the N-O stretching of the isoxazoline ring. Then Boc-protecting groups were cleaved under acidic conditions, and the free amine groups in material 11 were titrated via a semiquantitative Kaiser test (0.129 mmol/g) (see supporting material section 4.4).41,42
Scheme 2.
Amino decoration of CNTs via “Machetti-De Sarlo” reaction of nitroacetamide 9.
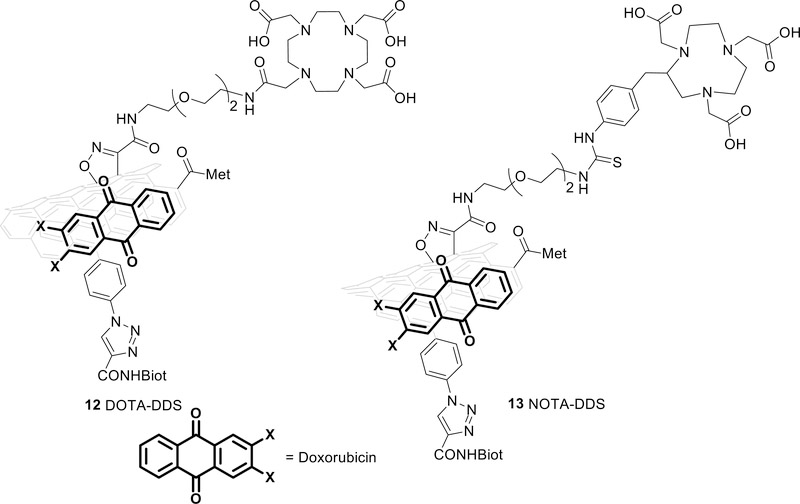
Finally, DOTA or NOTA, ligands commonly used for labeling with Ga-68 and Cu-64,43–45 were bound to CNTs through coupling with the primary amino group. DOTA tri-tert-butyl ester was first activated with N-hydroxysuccinimide (compound 15 in the supporting information) and coupled to compound 11. The loading was quantified using TGA: the thermogravimetric curve of the tri-t-butyl ester DOTA-CNTs and the one of compound 11 were compared and two intervals of temperature were identified for the consumption of organic material. The first one is 160–240 °C with a loss of weight around 2 % and the second is 240–370 °C with a loss of 2.9 %. Based on these data the loading of DOTA was estimated to be 0.1 mmol/g. NOTA-NCS was directly coupled to compound 11 with a loading of 0.01 mmol/g (Kaiser test41). Then, DOXO was loaded and quantified following the protocol described above to obtain products 12 (DOTA-DDS) and 13 (NOTA-DDS), respectively (figure 2) (see supporting material section 2.14 and 4.2). FT-IR of compounds 12 (see supporting info figure S25) evidence the presence of doxorubicin clearly showing the drug fingerprints between 1500 and 800 cm−1
Figure 2:
DOTA-CNT (12) construct on the left and NOTA-CNT construct (13) on the right.
Surprisingly, only a handful of examples focus on the evaluation of in vivo biodistribution of the fully loaded DDS in tumor bearing mice. 9,46–48
2.2. Radiolabeling of the CNTs-adduct
The labeling of the DOTA-CNT with Cu-64 was performed at pH 5.6 in sodium acetate buffer 0.1 M and 37 °C for 30 or 60 minutes, with a labeling efficiency higher than 95%. The reaction afforded a material ([64Cu]12) with a high specific activity (814 GBq/g) and a radiochemical yield (RCY) non-decay corrected of 80% (see supporting material, section 3.4). The stability of the complex in PBS was evaluated at 37 °C and the probe was stable up to 48 h. (For the table of stability see supporting). Since the amount of complexed DOXO can be influenced under the labeling reaction conditions, the decrease of complexed DOXO was assessed by placing DDS-12 in the same reaction condition used for the labeling. Aliquots of volume were then collected after 10, 20, 30 and 60 minutes, diluted and the absorbance at 480–490 nm was measured. De-complexation of DOXO was about 18% after 1 h of incubation which is believed to have a negligible effect on efficacy, considering the high loading of the drug on the nanotube surface. (see supporting material section 4.3)
DOTA-CNT was also labeled with Ga-68. Ga-68 was obtained from a 68Ge/68Ga generator in 4 mL of HCl. 0.1 mL of this solution was buffered at pH 5.6 with sodium acetate 1M and used to radiolabel DOTA-CNT. The reaction was performed at 37 °C with 70% of radiochemical efficiency after 10 minutes. The free metal was removed by centrifugation to obtain [68Ga]12 with 95% radiochemical purity and RCY (not decay corrected) of 27%. (see supporting material, section 3.3) Given the low radiochemical yields obtained, which would have prevented the feasibility of the in vivo studies, NOTA-CNT 13 was used for the labeling with Ga-68. This ligand is able to provide high metal loading even in mild conditions, because the logKML with gallium is ten-fold higher than DOTA.43 Labeling of NOTA-CNT to afford [68Ga]13 complex proceeded at 95–100% at 37 °C and pH 5.6 after 10 minutes (ITLC quality control see supporting). The final RCY (non-decay corrected) was 65% (see supporting material, section 3.2).
2.3. In vitro cytotoxicity
To verify the effective biological activity of the MET-CNT adduct, and to guide the design of in vivo efficacy study, a series of in vitro tests were performed to assess the effect of the nanomaterial in comparison with each component and adduct 1. Test were performed on two different breast cancer cell lines: human MCF-7 and murine 4T1 (Figure 3). Both cell lines are reported as OxPhos dependent 49,50 and overexpress biotin receptor31,51. As expected, cells were sensitive to free MET at high concentrations (ID50 for all cell lines around 450 μg/mL corresponding to a 3.7 mM).
Figure 3.
MTT viability assays of MCF-7 and 4T1 incubated with different concentrations of MET.
The two cancer cell lines were almost non-sensitive to ox-CNTs: MCF7 cells did not show any viability variation with concentrations of ox-CNT up to 40 μg/mL (figure 4, orange), while 4T1 cells showed a modest inhibition of growth at the highest concentration (figure 5, orange). Incubation with MET-CNT was more efficient against MCF7 cells (figure 4, green): an apparent IC50 value was obtained already at 20 μg/mL of MET-CNT, corresponding to 5.16 μg/mL of MET (0.04 mM, almost 100 times more effective with respect to free MET), but with a cytostasis pattern of drug action. On the contrary, no apparent effect was observed incubating 4T1 cells with MET-CNT (figure 5, green). The experiments performed with MET-B-CNT (figure 4 and 5, violet) gave opposite results: the presence of biotin on CNT did not increase efficacy with MCF7 cells (figure 4), but induced enhanced efficacy with 4T1 cells (figure 5). In 4T1 cells these results suggest that the uptake of CNTs is biotin-dependent: the uptake enhancement induced by the presence of biotin induces a higher toxicity. In the case of MCF7 cells, the higher sensitivity, and maybe permeability, shown by these cells towards adduct 3 minimizes the role played by the biotin selector on MET-B-CNT 7
Figure 4.
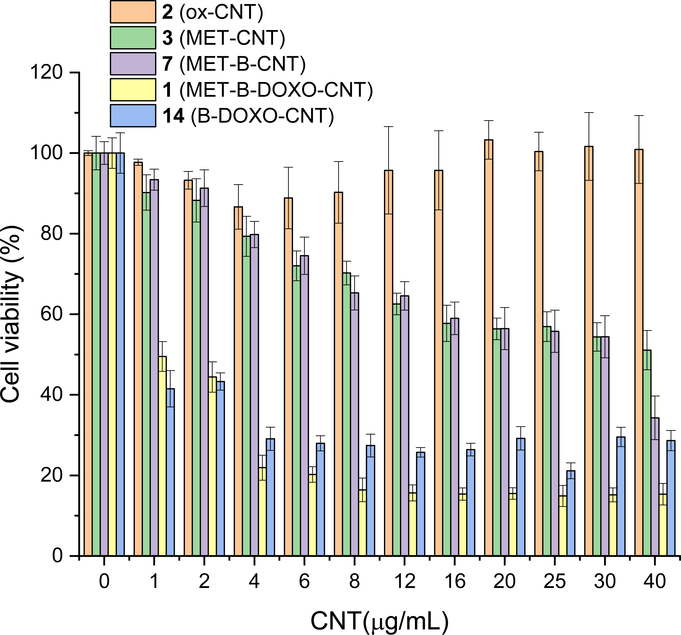
MTT viability assays of MCF-7 incubated with different concentrations of 2 (orange), 3 (green), 7 (violet), 1 (yellow) and B-DOXO-CNT 14 (blue)
Figure 5.
MTT vitality assays of 4T1 incubated with different concentrations of 2 (orange), 3 (green), 7 (violet), 1 (yellow) and B-DOXO-CNT 14 (blue).
It is not possible to say whether the cytotoxic effect of the metformin decorated CNTs is due to the release of metformin or just to the cytotoxicity of the adducts. However, these biological data allowed for the prosecution of the study. Eventually, with complete DDS 1, the effect on viability of MCF7 and 4T1 was remarkable (figure 4 and 5, yellow). With MCF7 cells, the ID50 was 0.7 μg/mL which is in line with the ID50 = 0.66 previously reported for a DDS not conjugated with MET (corresponding to 0.25 μg/mL of DOXO, compound 14 prepared following ref. 17) (see supporting for an expansion). In 4T1 cells (figure 5), the ID50 was higher (ID50 = 4 μg/mL of 1, corresponding to 1.6 μg/mL of DOXO and 0.62 μg/mL of MET). Taken together, results reported in figures 4 and 5 demonstrated that: i) CNTs are well tolerated by cancer cells; ii) MCF7 and 4T1 cells show a different sensitivity against MET-CNTs, with 4T1 cells being almost insensitive to MET-CNTs; iii) biotin increases cytotoxicity of MET-CNTs toward murine 4T1 cells, but did not toward human MCF7 cancer cells, suggesting that uptake of MET-CNTs in 4T1 cells is favored by the presence of biotin linked onto CNTs surface; iv) both 4T1 and MCF7 cell lines show a similar sensitivity toward the complete DDS 1. Based on these results, we chose to perform in vivo biodistribution and efficacy studies on 4T1 tumor bearing mice, which represents a syngeneic and aggressive model for triple negative breast cancer, a challenging benchmark for DDS 1. Importantly, the use of immunocompetent mice would account for the role of the immune system in the biodistribution of the nanomaterial.
2.4. In vivo efficacy evaluation
Fifteen Balb/c mice were inoculated with 10,000 4T1-FLUC cells in the left mammary fat-pad. Two weeks post cell inoculation, tumors were palpable, and the mice were randomly divided into three groups. Tumor sizes were measured via caliper and via bioluminescence imaging (BLI). Given the favorable biodistribution profile (see below), i.p. injections were chosen as method of administration of the DDS. The three groups were treated twice a week i.p. with: A) vehicle (PBS solution), B) DDS 1, 5 mg/kg and C) combination of doxorubicin and metformin (2 mg/kg and 1.29 mg/kg, respectively). Measurements of tumor size with caliper and by BLI suggested that the group treated with compound 1 had an initially slower growth rate (after 2 weeks of treatment) with respect to vehicle and drug combination groups (the overlapping of error bars made these differences not statistically significant). The difference in average tumor size was visible until the third week of treatment when the first mice of vehicle groups reached end point. Between the second and the third week of treatment, an increase of the tumor growth-rate in group B brought the tumor to the size of group C. Within three weeks all mice treated with vehicle reached end-point (figure 6a and 6b). Treatment with the drug combination extended the life by 1 week and 2 days in 20% of the mice. The DDS-treated group showed the longest life expectation with the last mouse reaching end-point after 35 days (Figure 6c). During the treatment period, no side effects were observed in the mice treated with the nanotubes; on the contrary, mice treated with the combination of free drugs showed granulation at the injection site.
Figure 6.
a) Tumor size comparison at different time points based on caliper measurement; b) BLI measurements of tumors after three weeks of treatment; c) survival curve of the three group of mice treated with vehicle, combination of drugs and DDS 1.
Although the benefit of DDS 1 was marginal, DDS 1 was well tolerated by all the animals, lifting some of the concerns about a potential toxic effect of carbon nanomaterials.
2.5. Metabolism
The in vivo stability of [64Cu]12 and [68Ga]13 complexes was measured 1.5 h post injection (see supporting material, section 5.2). Blood and urine samples were analyzed by radio-TLC in order to assess the amount of circulating free metal. Only 11±14 % (n=4) and the 24±6 % (n=3) of free metal were found respectively in blood and urine for [64Cu]12. [68Ga]13 showed a lower stability with 37±17 % (n=3) of free metal in blood. These data indicate a stability in line with other CNT-radiometal complexes reported in literature.42,52 More importantly, the high stability of [64Cu]12 validates the use of PET/CT to assess the biodistribution of the nanomaterial.
2.6. Biodistribution and PET imaging
The in vivo biodistribution of the nano-materials was evaluated by PET/CT at short and long time points, with the short-lived isotope Ga-68 and long-lived isotope Cu-64 respectively. A second study was then performed to elucidate the impact of injection method on the distribution of the materials. For the first study, seven Balb/c mice were inoculated with 4T1 cells in the right mammary fat-pad. Two weeks post inoculation, when tumors were palpable, the cohort of mice was divided into two groups: the first group (n=3) was imaged with [68Ga]13 and the second group (n=4) with [64Cu]12. Both probes were injected intravenously (i.v.).For the first group, the PET/CT protocol comprised a dynamic PET scan of the first 10 min, followed by 15 min of whole body CT scan. The same protocol was applied for 1.5 h and 3 h time points. The second group was imaged following an analogous PET/CT protocol, and adding 24h and 48h time points.
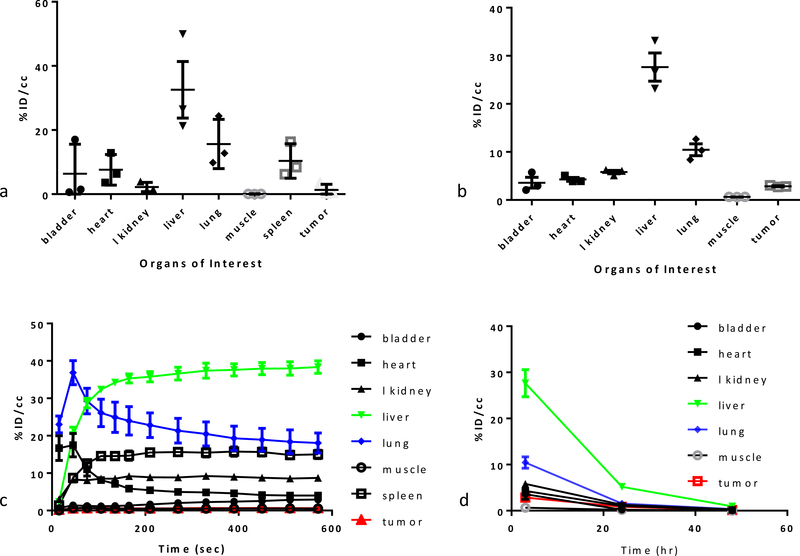
The biodistribution profile at early time points suggests an early renal clearance (5% ID/cc) probably of the free metal. Radioactivity in the heart, used as estimation of the amount of radiopharmaceutical in the blood pool, was less than 10% ID/cc after 3 h, proving the modestly fast blood clearance already observed by others. The higher uptakes are in the liver (30% ID/cc) and lungs (15% ID/cc), confirming a behavior commonly observed for nanoparticles when injected intravenously.9 The uptake in the liver might be caused by Kupffer cells retention but proof lays beyond the scope of this study. In addition, McDavitt et al. demonstrated that liver sinusoidal endothelium plays a crucial role in the CNTs retention and decomposition.48 The spleen (9% ID/cc) also retained significant activity, potentially suggesting a role played by immune cells, especially macrophages, in the uptake of the material. Minimal retention was observed into the muscles. Tumor uptake was around 1.5% ID/cc and might be the result of the enhanced permeability and retention effect (EPR) plus a small contribution of the targeting agent biotin. The net biotin contribution may be studied in the future with CRISPR biotin KO or biotin blocking studies. No radioactivity was observed in the mice cranial cavity, suggesting that the CNTs are not able to cross an intact blood-brain barrier.
The 3h post-injection biodistribution was confirmed by the [64Cu]12 imaging, (see supplementary material section 5.3, figure S13) excluding any metal or ligand dependent effect. The Cu-64 labeled material was taken up mainly in the liver and lungs, similarly to what seen for the Ga-68 labeled CNT; its retention was also similar in the bladder, kidneys and muscles. The blood clearance was fast, with less than 5% in the heart after 3h. The tumor uptake was slightly higher (around 3%). After 24 h, the radioactivity in the tissues dramatically decreased, the activity in all the organs examined was five time lower, with the liver and lungs going from 27% and 10% to 5% and 1.5%, respectively. The tumor uptake remained constant (≈1% ID/cc) for the first 24 hours. At 48h post-injection, the body clearance was almost complete, with activity decreasing to less than 1% in all the main organs. Tumor uptake was also reduced, albeit at a slower rate. Radioactivity was found in the feces, suggesting a hepato-biliary excretion. As an experimental observation, mice looked scruffy after the imaging sessions, but they completely recovered within 2 days, suggesting a good tolerability of the nanomaterial.
2.6.1. Biodistribution with different injections methods
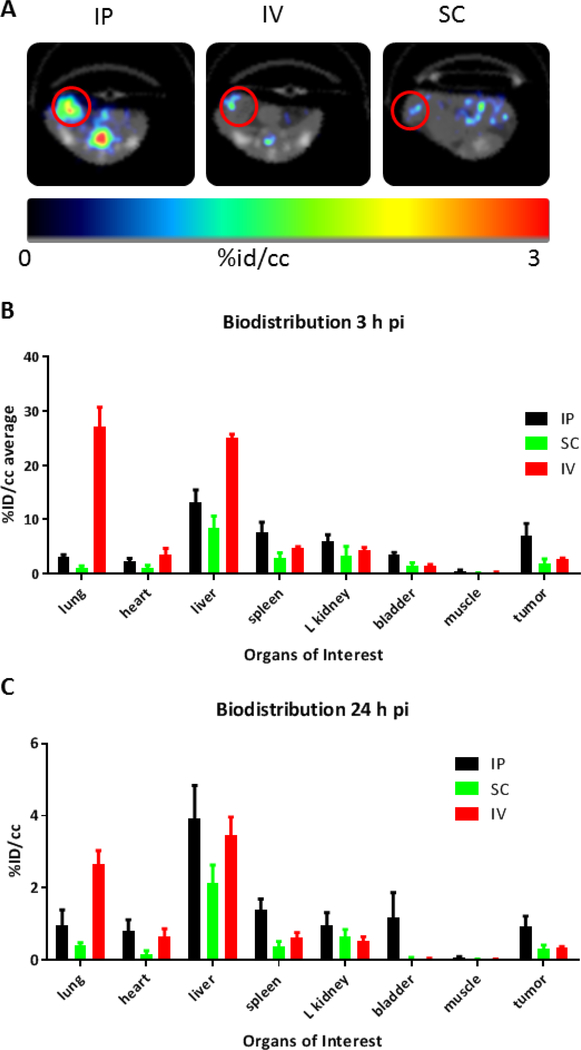
Nine Balb/c mice were inoculated with 4T1 cells in the right mammary fat-pad three weeks prior the imaging session. The cohort was divided in three groups and injected with compound [64Cu]12: intravenously (i.v.), intraperitoneally (i.p.) and subcutaneously (s-c). The PET/CT images were recorded 3 h later by a 10 min PET scan followed by a whole body CT. 24 and 48 hours images were also acquired. The biodistribution profile with the i.v. injection was the same to that shown above, with higher uptakes in liver and lungs, and in tumor the retention of the CNTs was 2.7% ID/cc. These results documented day-to-day and batch-to-batch reproducibility. Administration via subcutaneous injection completely changed the pharmacokinetics: diffusion occurred through the lymphatic system, which was reached after a slow permeation from the site of injection. Our experiment showed that labeled-DDS, s.c. injected, remained at the injection site (see supporting material, figure S13). After 3 hours, only a small amount of the injected material distributed to the organs. The s.c. administered CNTs were mainly sequestered by the liver (8.5% ID/cc), followed by kidney (3.4% ID/cc), spleen (2.8% ID/cc) and bladder (1.5% ID/cc); the uptake in the lungs was only the 1.1% ID/cc. Surprisingly the tumor uptake was around 1.9 % ID/cc suggesting that it was not affected by the reduced circulating material. The extremely low amount in the heart (1.1% ID/cc) and the absence of radioactivity in the muscles confirmed that only a small amount was passed in the blood circulation.
The injection in the peritoneal cavity gave a third biodistribution profile, with a strongly reduced lung uptake (3.1 % ID/cc, around 6 times less than with the i.v. delivery); liver trapping was partially avoided (13.1 ID %/cc) (Figure 8). Slightly higher activity was observed in spleen (7.7 %), kidneys (5.9 %) and bladder (3.4 %), suggesting a concomitant renal excretion of the labeled material. The retention in muscles was, as for the other type of injections, close to zero, the tumor showed the highest uptake among all the injection and Bio-D experiments, with an average ID %/cc close to 7%. However, the variance in the data of the IP series is higher than the i.v. series, as shown by error bars on the tumor uptake, thus reducing its statistical relevance. On the contrary, in the lungs, the difference is undeniable and the i.p. injection strongly limited the off-target uptake.
Figure 8.
A) Transverse PET/CT scans at 24h post i.p., i.v. and s.c. injection of [64Cu]12. B) Biodistribution of [64Cu]12 (n=3) after 3h comparing different type of injection; c) Biodistribution of [64Cu]12 (n=3) after 24h comparing different type of injection.
Activity after 24 hours proportionally decreased in all tissues as a result of probe excretion. Except for the s.c. injection, where most of the radioactivity remained trapped in the injection site, no sign of bioaccumulation was evidenced, neither in the i.v nor in the i.p. administration.
3. Conclusion
In this work, we presented a complete evaluation of a first-generation drug delivery system based on short multi-walled carbon nanotubes. The DDS was designed and synthesized using an orthogonal approach aimed to reduce steric hindrance, maximize solubility and drug loading. Metformin was first introduced via amidation of the carboxylic groups present on the oxidized material, then, with two additional synthetic steps, biotin was covalently appended and doxorubicin was supramolecularly loaded. The DDS was also equipped with NOTA and DOTA ligands to study its biodistribution via PET/CT with two different radiometals. This allowed a preliminary biodistribution profile and characterization of compartmentalization and clearance pathways. The pharmacokinetics of the system was deeply investigated, exploring different methods of administration of the nanomaterial - i.v., i.p. and s.c. - and early and late time points. As a general trend, high uptake was found in the liver, confirming what was observed by others with different types of nanomaterials.8,52,53 Among the three types of injections, the best tumor-targeted biodistribution was observed when the DDS was administered intraperitoneally. This allowed the reduction of the off-target undesired uptake and maximized tumor localization. Independently from the method of administration, hepatobiliary excretion remained the primary route of clearance. Most of the radioactivity cleared within 48h with the activity in the liver reduced by 80% in 24 h. Although, it is not possible to discern if this fast liver clearance is due to the excretion of the entire DDS or to its decomposition without further investigation (above the scopes of the present work), this starts to address some concerns about the nanomaterial’s degradability and behavior in living subjects.
The therapeutic efficacy study provided useful information about in vivo tolerance of the CNT-based DDS: the mice showed no observable effect with an i.p. injection of 5 mg/Kg, twice a week, which points towards low toxicity and compares favorably with unconjugated combinations of the two drugs. Even though an aggressive model of triple negative breast cancer (4T1) was used, the group treated with targeted-MWCNT showed individual survival prolonged by 12 days when compared to the group treated with vehicle alone and 5 days compared to the group treated with the unconjugated combination of the two drugs. Although no long-term survival was observed, the low toxicity and biocompatibility of the system encourages further development of a more focused second generation DDS.
Supplementary Material
Figure 7.
Biodistribution 3h post-injection for [64Cu]12 (a) and [68Ga]13 (b); probe retention in the tissues (calculated from PET/CT of ROI) of [64Cu]12 (c) and its clearance (d).
Table 1.
[64Cu]12 retention indifferent tissues with intraperitoneal injection, mean values and standard deviation.
| Tissues | IP ID%/cc | Standard deviation |
| Lung | 3,07 | 0,80 |
| Heart | 2,33 | 0,88 |
| Liver | 13,12 | 4,03 |
| Spleen | 7,66 | 3,23 |
| L Kidney | 5,91 | 2,26 |
| Bladder | 3,43 | 0,87 |
| Muscle | 0,50 | 0,34 |
| Tumor | 7,00 | 3,98 |
4. Acknowledgments
G. T. and S. C. thank Fondazione CR Firenze for its support to the HORIZON project. G. G. also thanks the “NanoMAX-Encoder” project (Engineering Nanostructures for Cellular Imaging and for Intracellular Delivery of Optically Active Drugs for Cardiac Hypertrophy) for supporting this work. S. C. thank Ministero dell’Istruzione dell’Università e della Ricerca for the FIRB project “Approcci nanotecnologici per la teragnostica dei tumori”. S. C. and P. P. thanks AIRC (Associazione Italiana per la Ricerca sul cancro) for the project “Advanced mass spectrometry tools for cancer research: novel applications in proteomics, metabolomics and nanomedicine” project code 19650 and for project “Assaying tumor metabolic deregulation in live cells”; project code 19515. F.P. and S.T.G. thank the Small Animal Imaging Facility (SAIF) for assistance with PET/CT imaging, a resource supported by the CCSG to MD Anderson Cancer Center (NIH P30CA016672)
5. References
- 1.Dozois MD, Bahlmann LC, Zilberman Y and Tang XS, Carbon N. Y, 2017, 120, 338–349. [Google Scholar]
- 2.Marchesan S, Ballerini L and Prato M, Science, 2017, 356, 1010–1011. [DOI] [PubMed] [Google Scholar]
- 3.Wu D, Si M, Xue H-Y and Wong HL, Int. J. Nanomedicine, 2017, 12, 5879–5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battigelli A, Ménard-Moyon C, Da Ros T, Prato M and Bianco A, Adv. Drug Deliv. Rev, 2013, 65, 1899–1920. [DOI] [PubMed] [Google Scholar]
- 5.Sheikhpour M, Golbabaie A and Kasaeian A, Mater. Sci. Eng. C, 2017, 76, 1289–1304. [DOI] [PubMed] [Google Scholar]
- 6.Francis AP and Devasena T, Toxicol. Ind. Health, 2018, 34, 200–210. [DOI] [PubMed] [Google Scholar]
- 7.Kotagiri N and Kim JW, Int. J. Nanomedicine, 2014, 9, 85–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Pant A, Chin CF, Ang WH, Ménard-Moyon C, Nayak TR, Gibson D, Ramaprabhu S, Panczyk T, Bianco A and Pastorin G, Nanomedicine Nanotechnology, Biol. Med, 2014, 10, 1465–1475. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen NR, Møller P, Clausen PA, Saber AT, Micheletti C, Jensen KA, Wallin H and Vogel U, Basic Clin. Pharmacol. Toxicol, 2017, 121, 30–43. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharya K, Mukherjee SP, Gallud A, Burkert SC, Bistarelli S, Bellucci S, Bottini M, Star A and Fadeel B, Nanomedicine Nanotechnology, Biol. Med, 2016, 12, 333–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kagan VE, Konduru NV, Feng W, Allen BL, Conroy J, Volkov Y, Vlasova II, Belikova NA, Yanamala N, Kapralov A, Tyurina YY, Shi J, Kisin ER, Murray AR, Franks J, Stolz D, Gou P, Klein-Seetharaman J, Fadeel B, Star A and Shvedova AA, Nat. Nanotechnol, 2010, 5, 354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharya K, El-Sayed R, Andón FT, Mukherjee SP, Gregory J, Li H, Zhao Y, Seo W, Fornara A, Brandner B, Toprak MS, Leifer K, Star A and Fadeel B, Carbon N. Y, 2015, 91, 506–517. [Google Scholar]
- 13.Andón FT, Kapralov AA, Yanamala N, Feng W, Baygan A, Chambers BJ, Hultenby K, Ye F, Toprak MS, Brandner BD, Fornara A, Klein-Seetharaman J, Kotchey GP, Star A, Shvedova AA, Fadeel B and Kagan VE, Small, 2013, 9, 2721–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shvedova AA, Kapralov AA, Feng WH, Kisin ER, Murray AR, Mercer RR, St. Croix CM, Lang MA, Watkins SC, Konduru NV, Allen BL, Conroy J, Kotchey GP, Mohamed BM, Meade AD, Volkov Y, Star A, Fadeel B and Kagan VE, PLoS One, 2012, 7, e30923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nunes A, Bussy C, Gherardini L, Meneghetti M, Herrero MA, Bianco A, Prato M, Pizzorusso T, Al-Jamal KT and Kostarelos K, Nanomedicine, 2012, 7, 1485–1494. [DOI] [PubMed] [Google Scholar]
- 16.Fedeli S, Brandi A, Venturini L, Chiarugi P, Giannoni E, Paoli P, Corti D, Giambastiani G, Tuci G and Cicchi S, J. Mater. Chem. B, 2016, 4, 3823–3831. [DOI] [PubMed] [Google Scholar]
- 17.Sciortino N, Fedeli S, Paoli P, Brandi A, Chiarugi P, Severi M and Cicchi S, Int. J. Pharm, 2017, 521, 69–72. [DOI] [PubMed] [Google Scholar]
- 18.Singh A, Trivedi P and Jain NK, Artif. Cells, Nanomedicine, Biotechnol, 2018, 46, 274–283. [DOI] [PubMed] [Google Scholar]
- 19.Denkova AG, de Kruijff RM and Serra-Crespo P, Adv. Healthc. Mater, 2018, 7, 1701211. [DOI] [PubMed] [Google Scholar]
- 20.Dinesh B, Bianco A and Ménard-Moyon C, Nanoscale, 2016, 8, 18596–18611. [DOI] [PubMed] [Google Scholar]
- 21.Biagiotti G, Fedeli S, Tuci G, Luconi L, Giambastiani G, Brandi A, Pisaneschi F, Cicchi S and Paoli P, J. Mater. Chem. B, 2018, 6, 2022–2035. [DOI] [PubMed] [Google Scholar]
- 22.Liu B, Fan Z, Edgerton SM, Deng X-S, Alimova IN, Lind SE and Thor AD, Cell Cycle, 2009, 8, 2031–2040. [DOI] [PubMed] [Google Scholar]
- 23.Alimova IN, Liu B, Fan Z, Edgerton SM, Dillon T, Lind SE and Thor AD, Cell Cycle, 2009, 8, 909–915. [DOI] [PubMed] [Google Scholar]
- 24.DeCensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B and Gandini S, Cancer Prev. Res, 2010, 3, 1451–1461. [DOI] [PubMed] [Google Scholar]
- 25.Bridges HR, Jones AJY, Pollak MN and Hirst J, Biochem. J, 2014, 462, 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch HA, Iliopoulos D, Tsichlis PN and Struhl K, Cancer Res, 2009, 69, 7507–7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mindell JA, Annu. Rev. Physiol, 2012, 74, 69–86. [DOI] [PubMed] [Google Scholar]
- 28.Azizian J, Hekmati M and Dadras OG, Orient. J. Chem, 2014, 30, 667–673. [Google Scholar]
- 29.Mirazi N, Shoaei J, Khazaei A and Hosseini A, Eur. J. Drug Metab. Pharmacokinet, 2015, 40, 343–348. [DOI] [PubMed] [Google Scholar]
- 30.Yoo S, Hou J, Yi W, Li Y, Chen W, Meng L, Si J and Hou X, Sci. Rep, 2017, 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell-Jones G, McTavish K, McEwan J, Rice J and Nowotnik D, J. Inorg. Biochem, 2004, 98, 1625–1633. [DOI] [PubMed] [Google Scholar]
- 32.Ojima I, Acc. Chem. Res, 2008, 41, 108–119. [DOI] [PubMed] [Google Scholar]
- 33.Bahr JL and Tour JM, Chem. Mater, 2001, 13, 3823–3824. [Google Scholar]
- 34.Lomeda JR, Doyle CD, Kosynkin DV, Hwang W-F and Tour JM, J. Am. Chem. Soc, 2008, 130, 16201–16206. [DOI] [PubMed] [Google Scholar]
- 35.Biagiotti G, Cicchi S, De Sarlo F and Machetti F, European J. Org. Chem, 2014, 7906–7915. [Google Scholar]
- 36.De Sarlo F and Machetti F, in Methods and Applications of Cycloaddition Reactions in Organic Syntheses, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, pp. 205–222. [Google Scholar]
- 37.Guideri L, De Sarlo F and Machetti F, Chem. - A Eur. J, 2013, 19, 665–677. [DOI] [PubMed] [Google Scholar]
- 38.Trogu E, Vinattieri C, De Sarlo F and Machetti F, Chem. - A Eur. J, 2012, 18, 2081–2093. [DOI] [PubMed] [Google Scholar]
- 39.Cecchi L, De Sarlo F and Machetti F, Chem. - A Eur. J, 2008, 14, 7903–7912. [DOI] [PubMed] [Google Scholar]
- 40.A complete paper on the use ot this reaction on functionalisation of carbon nanotubes is in preparation and will be published shortly.
- 41.Kaiser E, Colescott RL, Bossinger CD and Cook PI, Anal. Biochem, 1970, 34, 595–598. [DOI] [PubMed] [Google Scholar]
- 42.Lacerda L, Soundararajan A, Singh R, Pastorin G, Al-Jamal KT, Turton J, Frederik P, Herrero M, Li S, Bao A, Emfietzoglou D, Mather S, Phillips WT, Prato M, Bianco A, Goins B and Kostarelos K, Adv. Mater, 2008, 20, 225–230. [Google Scholar]
- 43.Wadas TJ, Wong EH, Weisman GR and Anderson CJ, Chem. Rev, 2010, 110, 2858–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgat C, Hindié E, Mishra AK, Allard M and Fernandez P, Cancer Biother. Radiopharm, 2013, 28, 85–97. [DOI] [PubMed] [Google Scholar]
- 45.Kilian K, Reports Pract. Oncol. Radiother, 2014, 19, S13–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulvey JJ, Villa CH, McDevitt MR, Escorcia FE, Casey E and Scheinberg DA, Nat. Nanotechnol, 2013, 8, 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruggiero A, Villa CH, Holland JP, Sprinkle SR, May C, Lewis JS, Scheinberg DA and McDevitt MR, Int. J. Nanomedicine, 2010, 5, 783–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alidori S, Bowman RL, Yarilin D, Romin Y, Barlas A, Mulvey JJ, Fujisawa S, Xu K, Ruggiero A, Riabov V, Thorek DLJ, Ulmert HDS, Brea EJ, Behling K, Kzhyshkowska J, Manova-Todorova K, Scheinberg DA and McDevitt MR, Nat. Commun, 2016, 7, 12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dennison JB, Molina JR, Mitra S, Gonzalez-Angulo AM, Balko JM, Kuba MG, Sanders ME, Pinto JA, Gomez HL, Arteaga CL, Brown RE and Mills GB, Clin. Cancer Res, 2013, 19, 3703–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simões RV, Serganova IS, Kruchevsky N, Leftin A, Shestov AA, Thaler HT, Sukenick G, Locasale JW, Blasberg RG, Koutcher JA and Ackerstaff E, Neoplasia, 2015, 17, 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vineberg JG, Wang T, Zuniga ES and Ojima I, J. Med. Chem, 2015, 58, 2406–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang JTW, Fabbro C, Venturelli E, Ménard-Moyon C, Chaloin O, Da Ros T, Methven L, Nunes A, Sosabowski JK, Mather SJ, Robinson MK, Amadou J, Prato M, Bianco A, Kostarelos K and Al-Jamal KT, Biomaterials, 2014, 35, 9517–9528. [DOI] [PubMed] [Google Scholar]
- 53.Al-Jamal KT, Nunes A, Methven L, Ali-Boucetta H, Li S, Toma FM, Herrero MA, Al-Jamal WT, Tena Eikelder HMM, Foster J, Mather S, Prato M, Bianco A and Kostarelos K, Angew. Chemie - Int. Ed, 2012, 51, 6389–6393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.