Abstract
Triple X syndrome (47, XXX) occurs in approximately 1:1,000 female births and has a variable phenotype of physical and psychological features. Prenatal diagnosis rates of 47, XXX are increasing due to non-invasive prenatal genetic testing. Previous studies suggest that prenatal diagnosed females have better neurodevelopmental outcomes. This cross-sectional study describes diagnosis, physical features, medical problems, and neurodevelopmental features in a large cohort of females with 47, XXX. Evaluation included review of medical and developmental history, physical exam, cognitive, and adaptive testing. Medical and developmental features were compared between the prenatal and postnatal diagnosis groups using rate calculations and Fisher’s exact test. Cognitive and adaptive tests scores were compared using t-tests. Seventy-four females age 6 months–24 years (mean 8.3 years) participated. Forty-four (59.5%) females were in the prenatal diagnosis group. Mean age of postnatal diagnosis was 5.9 years; developmental delay was the most common indication for postnatal genetic testing. Common physical features included hypertelorism, epicanthal folds, clinodactyly, and hypotonia. Medical problems included dental disorders (44.4%), seizure disorders (16.2%), genitourinary malformations (12.2%). The prenatal diagnosis group had higher verbal (P < 0.001), general ability index (P = 0.004), and adaptive functioning scores (P < 0.001). Rates of ADHD (52.2% vs. 45.5%, P = 0.77) and learning disabilities (39.1% vs. 36.3%, P = 1.00) were similar between the two groups. These findings expand on the phenotypic features in females with Triple X syndrome and support that prenatally ascertained females have better cognitive and functional outcomes. However, prenatally diagnosed females are still at risk for neurodevelopmental disorders. Genetic counseling and treatment recommendations are summarized.
Keywords: 47, XXX, triple X syndrome, triplo-X, trisomy X, sex chromosome aneuploidy (SCA)
INTRODUCTION
Triple X Syndrome or 47, XXX is a sex chromosome aneuploidy (SCA) in which affected females may have a variety of physical, medical, and psychological features. The incidence of 47, XXX is approximately one in 1,000 live born females [Jacobs, 1979]. In contrast to other trisomies, Triple X syndrome does not have a characteristic physical appearance at birth. Thus, affected females are identified via prenatal testing (amniocentesis, chorionic villus sampling, or non-invasive prenatal testing) or via postnatal diagnostic evaluation on the basis of an identified medical diagnosis or neurodevelopmental condition such as developmental delays or learning disabilities. Triple X syndrome remains a relatively under diagnosed condition as only an estimated 10% of cases are clinically ascertained [Nielsen, 1990]. This low rate of diagnosis likely results from a combination of factors, including decreased awareness of features among medical providers, the lack of distinct physical findings in Triple X beyond a tendency for tall stature, and the variability in the presentation of cognitive or behavioral differences, which often do not reach current thresholds of severity for genetic testing based on current genetic testing guidelines [Manning et al., 2010; Moeschler et al., 2014].
Prenatal ascertainment of females with 47, XXX is increasing with the advent of fetal DNA analysis through non-invasive prenatal screening of maternal blood (NIPT) for common chromosomal aneuploidies such as Trisomies 13, 18, and 21. A diagnosis of Triple X syndrome is commonly an unexpected finding, and practitioners must then counsel expectant families regarding this condition with broad phenotypic variability [Lalatta and Tint, 2013]. Data comparing medical features and neurodevelopmental outcomes of prenatal versus postnatally ascertained females is limited. Prior studies suggest that females with Triple X syndrome diagnosed prenatally tend to have better neurodevelopmental outcomes with respect to IQ and frequency of academic difficulties or psychiatric conditions [Robinson et al., 1992; Linden and Bender, 2002]. However, this study was limited by its small sample size (n = 17) and its descriptive design. Elucidation of the presence and extent of differences between prenatally and postnatally ascertained females would equip practitioners with more accurate information to counsel families in the relevant clinical context.
Medical, physical, and psychological features observed in females with 47, XXX have been described by various case reports and case series. These studies have described features including tall stature [Robinson et al., 1979; Ratcliffe et al., 1994; Liebezeit et al., 2003], genitourinary malformations [Lin et al., 1993], seizures [Olanders and Sellden, 1975; Grosso et al., 2004; Roubertie et al., 2006], premature ovarian failure [Villanueva and Rebar, 1983; Holland, 2001; Goswami et al., 2003]. Psychological disorders including anxiety and depression, psychotic disorders, and ADHD have been described [Kusumi and Prange, 1973; Linden et al., 1988; Woodhouse et al., 1992; Bender et al., 1995; Tartaglia et al., 2012]. Full reviews of the literature of Triple X are available [Otter et al., 2010; Tartaglia et al., 2010].
The most comprehensive studies on Triple X syndrome involved girls who were identified prospectively by newborn screening and followed longitudinally as part of a series multi-center studies of sex chromosome aneuploidies [Stewart et al., 1982; Nielsen, 1990; Ratcliffe et al., 1990; Robinson et al., 1990]. Participants across sites were found to be tall for their age and often have other physical features such as epicanthal folds or clinodactyly. Importantly, these studies highlighted that in contrast to autosomal trisomies where intellectual disability is common, females with Triple X often had intelligence within the normal range, although mean IQ scores were 10–15 points lower than sibling controls. A systematic review compiling results of these longitudinal studies showed that affected females were at high risk for speech and language disorders, motor deficits, learning/academic problems, and psychosocial difficulties, although there was broad variability in outcomes [Legett et al., 2010]. The major limitation of these studies, as is common with most previous studies of Triple X syndrome, was small sample size.
In this project, we aimed to describe medical, physical, psychological, and developmental features of a larger cohort of females with Triple X syndrome. We also aimed to compare medical and neurodevelopmental features by timing of ascertainment (i.e., prenatal vs. postnatal diagnosis) in the subset of participants age 5 years through young adulthood. Finally, on the basis of our findings, we provide recommendations for genetic counseling and medical care.
MATERIALS AND METHODS
Recruitment
Participants were recruited to participate in IRB-approved studies on health and development of children and adults with sex chromosome aneuploidies (SCA) from 2005 to 2014. Participants were recruited from multiple sources including national advocacy and support organizations for SCAs and an interdisciplinary clinic for children and adolescents with SCA [Tartaglia et al., 2015]. Additional participants were identified by referral through genetics and pediatrics clinics at each site. The study was carried out at UC-Davis Medical Center MIND Institute and Children’s Hospital Colorado, eXtraordinarY Kids Clinic. All participants or their parents signed an IRB-approved consent form developed for each site prior to participation.
Participants
Inclusion criteria included females with 47, XXX with less than 20% mosaicism on cytogenetic testing and physical examination by a single physician (N.T.) with experience in examining children with SCA. The study cohort included participants seen only for research and participants seen for clinical care who consented to share clinical data for the research database. Clinical care included developmental monitoring, general consultation regarding Triple X, and/or evaluation due to neurodevelopmental or psychological concerns. Initial visit type was designated for each participant as research-only or clinical care to help with later analysis of recruitment bias. If a participant had been seen more than once for clinical care, data were taken from the most recent assessment with the most comprehensive data. Seventy-four participants met inclusion criteria and are further described below and in Table I.
TABLE I.
Demographic Features
| Total (N = 74) | Prenatal (N = 44) | Postnatal (N = 30) | P | |
|---|---|---|---|---|
| Mean age at evaluation (SD) | 8.8 years (sd 6.3) | 7.7 years (sd 5.9) | 10.3 years (sd 6.6) | 0.07 |
| Age range | 6 months–24 years | 6 months–20 years | 9 months–24 years | |
| Age group at evaluation | ||||
| 6 months–5 years | n = 34 | n = 24 | n = 10 | |
| 6–12 years | n = 18 | n = 10 | n = 8 | |
| 13–24 years | n = 22 | n = 10 | 12 | |
| School age to adult subseta mean age | 12.3 years (sd 5.1) n = 45 | 11.5 years (sd 5.3) n = 23 | 13.0 years (sd 5.1) n = 22 | 0.33 |
| Mean age cognitive testing | 12.1 years (sd 4.3) n = 37 | 11.9 years (sd 4.9) n = 18 | 12.3 years (sd 3.8) n = 19 | 0.83 |
| Mean age adaptive testing | 9.6 years (sd 6.2) n = 46 | 8.7 years (sd 6.2) n = 26 | 10.5 years (sd 6.1) n = 20 | 0.31 |
| Race | ||||
| %White | 87.8% | 86.4% | 93.4% | 0.46 |
| %Asian | 8.1% | 9.1% | 3.3% | |
| %Black or African American | 4.1% | 4.5% | 3.3% | |
| Ethnicity | ||||
| %Hispanic or Latino | 6.8% | 4.5% | 10% | 0.39 |
| %Not Hispanic or Latino | 93.2% | 95.5% | 90% | |
| Mean SES scoreb | 51.0 (sd 10.1) | 52.5 (sd 9.8) | 48.4 (sd 10.2) | 0.14 |
| Recruitment source | ||||
| %Research-only | 54.1% (n = 40) | 59.1% | 46.7% | 0.35 |
| %Clinical | 45.9% (n = 34) | 40.1% | 53.3% | |
School age to adult subset defined as participants age 5 years or older.
SES score defined as score on 2 Factor Hollingshead Score.
Evaluation
The parent or primary caregiver of each participant first completed a comprehensive questionnaire detailing birth, medical, developmental, and psychological history. These features were then reviewed in a semi-structured direct interview. Medical and educational records were reviewed, including genetic testing results, to confirm a 47, XXX karyotype with less than 20% mosaicism. Height, weight, and occipitofrontal head circumference were measured directly and converted to Z-scores (standard deviation scores) based on US CDC growth chart data. The presence or absence of common dysmorphic features and other specific features known to be associated with Triple X syndrome and other SCAs were assessed by physical examination. Poor dentition was classified as four or more caries/fillings, significant malocclusion, current or previous orthodontia, the need for previous dental surgery, or taurodontism. Developmental assessment of children less than 5 years of age was completed using the Bayley Scales of Infant Development Second or Third Edition [Bayley, 1993, 2006] or Mullen Scales of Early Learning—AGS Edition [Mullen, 1989]. Scores were converted to standard scores with a mean of 100 and standard deviation of 15 to allow for comparison between tests. Cognitive evaluation of participants 5 years of age or older was performed using the Wechsler Scales of Intelligence including WASI [Wechsler, 1999], WISC-IV [Wechsler, 2004], WAIS-III [Wechsler, 1997], or WAIS-IV [Wechsler, 2008]. General Abilities Index (GAI) was calculated for all scales to allow for comparison between tests [Tulsky et al., 2001]. For four participants, cognitive scores were obtained by review of results of comparable standardized assessments administered by a licensed psychologist obtained within the previous 12 months. Adaptive functioning was assessed using either the Vineland-2 Adaptive Functioning Interview [Sparrow et al., 2005] or the Adaptive Behavior Assessment System—2nd Edition [Harrison and Oakland, 2003]. Learning disability (LD) was classified as identification of a learning disability by a previous psychological evaluation by a school psychologist, neuropsychologist, or developmental-behavioral pediatrician. Language-based LD including those identified with a diagnosis of dyslexia, and math specific LD were included in this category. ADHD was defined as a clinical diagnosis in which they met DSM-IV Criteria. Neurodevelopmental diagnoses were reviewed by a single clinician (N.T.) with experience in diagnosis and classification of these disorders. Socio-economic status was calculated using a Two Factor Hollingshead Score [Hollingshead, 1965].
Analysis of Results
Subjects were grouped according to age at evaluation for analysis of physical features or by time of ascertainment (i.e., prenatal vs. postnatal diagnosis) for comparisons of medical, neurodevelopmental features, and cognitive and adaptive testing. The subset of participants from school age (age 5) through adulthood were identified, and only this subset was used for calculation of frequencies of intellectual disability (ID), learning disabilities, ADHD, psychiatric conditions, or special education supports given that many of these diagnoses are usually not made until a child reaches school age. Further, only this school age to adult subset was used for prenatal versus postnatal comparisons of psychological diagnoses, cognitive, and adaptive functioning. Incomplete data are noted within tables, with data missing due to caregivers being unsure or unable to recollect the information. Percentages reported included only data that was definitively absent or present, thus the total n included in calculations varies between fields but is noted in the tables. Comparison of prenatal versus postnatal rates were calculated using the Fisher’s exact test in SPSS. Comparison of mean scores on cognitive testing was performed using student’s t-test. Correction for multiple comparisons was made based on the number of comparisons. Statistical significance was set at P < 0.05.
RESULTS
Demographic Features
Demographic features are reported in Table I. Seventy-four participants with Triple X were included in the study. Forty-four (59.5%) participants were in the prenatal diagnosis group and 30 (41%) participants were in the postnatal diagnosis group. Mean age at evaluation was 8.8 years (standard deviation 6.3 years, range 6 months–24.6 years). Overall, the mean age of the prenatal diagnosis group was slightly younger compared to the postnatal diagnosis group (P = 0.07). However, among the subset of school aged to adult (5 years or older) females included for comparison of neurodevelopmental diagnoses (n = 46) and analyses of cognitive and adaptive testing (n = 37), there were no significant differences in age between the prenatal and postnatal groups (neurodevelopmental diagnoses: P = 0.33, cognitive tests P = 0.83, adaptive tests P = 0.31). There was no significant difference in SES between the prenatal and postnatal diagnosis groups (P = 0.14). Most (89.9%) of the participants were Caucasian and non-Hispanic, and there was no difference in proportions of race or ethnicity between prenatal and postnatal groups. There was an equal distribution of participants recruited for research versus clinical care between the prenatal and postnatal groups (P = 0.35). Preliminary comparisons between the research-only and clinical recruitment groups showed no significant differences on Fisher’s Exact tests in age (P = 0.24), proportion with a prenatal diagnosis (P = 0.34), frequency of speech delay (P = 0.79), rates of special education (P = 0.48), or adaptive composite (82 vs. 81, P = 0.78). The research group had a significantly lower FSIQ compared to the clinic group (85.6 ± 16 vs. 95.4 ± 14, P = 0.04), which is opposite to what would be expected if the clinical group was more affected. Thus, it was felt that including data from the clinical population provided the most comprehensive data as long as biases were considered.
Diagnosis of 47, XXX
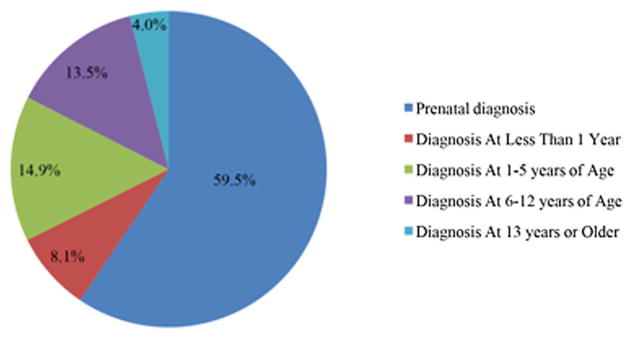
The age at diagnosis and the indication for genetic testing are presented in Figure 1 and Table II. Common indications for prenatal testing included advanced maternal age (n = 36, 81.8%) and abnormal serum alpha fetoprotein (AFP) screen (n = 3, 6.8%). For three subjects, genetic testing was performed after anatomic abnormalities were identified on prenatal ultrasound including ventriculoseptal defects and clubfoot (n = 1), prominent nuchal fold (n = 1) and a two-vessel umbilical cord (n = 1).
FIG. 1.
Age at diagnosis of Triple X Syndrome. [Color figure can be viewed at wileyonlinelibrary.com].
TABLE II.
Primary Indications for Genetic Testing in Triple X Syndrome
| Prenatal diagnosis, n = 44 (%) | Postnatal diagnosis, n = 30 (%) | |
|---|---|---|
| Prenatal | ||
| Advanced maternal age | 38 (86.4) | |
| Abnormal AFPa screen | 3 (6.8) | |
| Abnormal ultrasound | 3 (6.8) | |
| Postnatal | ||
| Dysmorphic features | 1 (3.3) | |
| Hypotonia | 1 (3.3) | |
| Developmental delay | 19 (63.3) | |
| Learning disability | 4 (13. 3) | |
| Behavioral difficulties | 2 (6.7) | |
| Otherb | 3 (10. 0) | |
AFP refers to alpha feto protein.
Other included: failure to thrive, short stature, and a history of neuropathy and developmental delays.
Among participants with postnatal ascertainment, the most common primary indication for genetic testing included developmental delay (n = 19, 63%) and learning disability (n = 4, 13%). Less common primary indications included: behavioral difficulties (n = 2, 7%), hypotonia (n = 1, 3.5%), dysmorphic features (n = 1, 3.5%), and other (n = 3, 10%). For the three subjects with “other” reasons, concerns included: failure to thrive (n = 1), short stature (n = 1), and a history of neuropathy and developmental delays (n = 1). Twelve (40%) participants had more than one indication for genetic testing including a combination of developmental and physical features.
Eight percent (n = 6) in the postnatal diagnosis group were diagnosed at less than 1 year of age, and these cases were associated with more significant medical problems and dysmorphic features noted at the time of birth (i.e., cleft palate/Pierre Robin sequence, congenital heart malformation, or hemihypertrophy), or significant early motor delays. Approximately 37% (n = 11) in the postnatal diagnosis group were diagnosed between 1 and 5 years of age. The majority in this age group underwent genetic testing due to the presence of speech or motor delays. Four subjects in this age group received genetic testing following the diagnosis of an autism spectrum disorder. Approximately 33% (n = 10) of patients were diagnosed between ages 6 and 12 years, primarily as part of the evaluation for learning and/or behavioral problems, although all individuals in this group frequently had a history of developmental delays that had not previously prompted genetic testing. Four percent (n = 3) were diagnosed after 13 years of age when evaluated for a longstanding history of learning disabilities and/or mental health problems.
Physical Examination Findings
Growth parameters and physical exam features are presented in Table III. Mean heights in the 6–12 years and 13+ year age groups were approximately one standard deviation above the mean height for age. Mean adult height (defined as height at age 18 years or older) was 171 cm or 5 ft 7.3 in (n = 6, SD 7.7 cm, range 157–179 cm). Common physical features across all age groups include hypertelorism, epicanthal folds, 5th digit clinodactyly, pes planus, and hypotonia. Several features varied with age. Epicanthal folds, hypertelorism, pes planus, and hypotonia were more commonly noted in females in the 0–5 year age group. Conversely kyphoscoliosis and tremor tended to increase with age. Figure 2 shows common facial and physical features of girls with Triple X syndrome.
TABLE III.
Physical Examination Results*
| Age 0–5 years, n = 34 (%) | Age 6–12 years, n = 18 (%) | Age 13 years +, n = 22 (%) | Total, n = 74 (%) | |
|---|---|---|---|---|
| Median height (Z-score) | 0.30 | 1.10 | 1.08 | 0.72 |
| Height range (Z-score) | −2.6 to 2.0 | −0.62 to 2.26 | −2.95 to 2.73 | −2.95 to 2.73 |
| Median weight (Z-score) | 0.13 | 0.09 | 0.73 | 0.42 |
| Weight range (Z-score) | −1.81 to 2.44 | −1.63 to 1.57 | −1.52 to 2.60 | −1.81 to 2.60 |
| Body mass index (Z-score) | 0.25 | −0.18 | 0.33 | 0.09 |
| Body mass index range (Z-score) | −2.18 to 2.20 | −2.25 to 0.84 | −2.58 to 2.31 | −2.58 to 2.31 |
| Median head circumference (Z-score) | 0.19 | −0.22 | 0.54 | 0.21 |
| Head circumference range | −1.76 to 2.78 | −1.47 to 1.25 | −2.1 to 3.20 | −2.16 to 3.20 |
| Facial asymmetry | 1/32 (3.1) | 1/17 (5.9) | 4/22 (18.2) | 6/71 (8.5) |
| Epicanthal folds | 23/32 (71.8) | 3/17 (17.6) | 5/22 (22.7) | 31/71 (43.7) |
| Hypertelorism | 21/32 (65.6) | 4/17 (23.5) | 12/22 (54.5) | 37/71 (43.7) |
| Upslanting palpebral fissures | 5/32 (15.6) | 4/17 (23.5) | 2/22 (9.1) | 11/71 (15.5) |
| High arch palate | 2/32 (6.3) | 2/17 (11.8) | 2/22 (9.1) | 6/71 (8.5) |
| Poor dentitiona | 5/32 (15.6) | 4/17 (23.5) | 8/22 (36.4) | 17/71 (23.9) |
| 5th digit clinodactyly | 12/33 (36.3) | 6/17 (35.3) | 8/22 (36.4) | 26/72 (36.1) |
| Pes planus | 16/32 (50.0) | 5/16 (31.3) | 5/22 (22.7) | 26/71 (36.6) |
| Ankle pronation | 8/32 (25.0) | 1/17 (5.9) | 2/22 (9.1) | 11/71 (15.5) |
| Scoliosis or Kyphosis | 7/22 (31.8) | |||
| Hypotonia | 22/33 (66.7) | 7/17 (41.2) | 7/22 (31.8) | 36/72 (50.0) |
| Tremorb | 3/33 (9.1) | 5/17 (29.4) | 9/22 (40.9) | 17/72 (23.6) |
Number of subjects at top of column unless otherwise noted.
Poor dentition classified as four or more caries/fillings, significant malocclusion, current or previous orthodontia, or edentulous.
Tremor included intention and/or postural tremor.
FIG. 2.

(A and B) Two female with 47, XXX with mild hypertelorism and lack of dysmorphic facial features.
Medical Features and Medications
A summary of common medical features is presented in Table IV. Eighty-eight percent (64/72) of infants were born at term, with the average birth weight of 3.086 kg (6 Lbs 12 oz). Twenty-three percent (17/72) had a history of a congenital anomaly including cleft lip or palate or velopharyngeal insufficiency (n = 3), cardiac defect (n = 6), renal or genitourinary abnormality (n = 9), and club foot (n = 2). Early feeding problems including poor latch or suck and slow feeding were present in 34% (25/73) of infants.
TABLE IV.
Medical Features in Triple X Syndrome
| Medical feature | Prior reportsd (%) | Total number (%) | Prenatal diagnosis (%) | Postnatal diagnosis (%) | P |
|---|---|---|---|---|---|
| Ophthalmologic disorders | |||||
| Congenital cataracts | – | 2/74 (2.7) | 0/44 (0) | 2/30 (6.7) | |
| Strabismus | – | 11/74 (14.9) | 6/44 (13.6) | 5/30 (16.7) | 0.749 |
| ENT disorders | |||||
| Recurrent otitis media | – | 15/72 (21.1) | 12/44 (27.3) | 3/28 (10.7) | 0.137 |
| Cleft palate/velopharyngeal insufficiency | – | 3/73 (4.1) | 2/43 (4.7) | 1/30 (3.3) | |
| Dental disordersa | – | 32/72 (44.4) | 19/42 (45.2) | 13/30 (43.3) | 1.000 |
| Respiratory disorders | |||||
| Asthma | – | 16/71 (22.5) | 9/43 (20.9) | 7/28 (25.0) | 0.774 |
| Hospitalization for respiratory infection or asthma | – | 12/74 (16.2) | 3/44 (6.8) | 9/30 (30.0) | 0.011* |
| Cardiac disorders | |||||
| Congenital heart defectsb | – | 6/74 (8.1) | 4/44 (9.1) | 3/30 (10.0) | 1.000 |
| Gastrointestinal disorders | |||||
| Gastroesophageal reflux | – | 6/74 (8.1) | 2/44 (4.5) | 4/30 (13.3) | 0.215 |
| Constipation | – | 34/74 (45.9) | 15/44 (34.1) | 19/30 (63.3) | 0.018* |
| Functional abdominal pain | 12–45% | 14/74 (18.9) | 9/44 (20.5) | 5/30 (16.7) | 0.769 |
| Genitourinary disorders | |||||
| Genitourinary abnormalitiesc | 5–16% | 9/74 (12.2) | 7/44 (15.9) | 2/30 (6.7) | 0.296 |
| Musculoskeletal disorders | |||||
| Congenital hip dysplasia | 2–12% | 2/74 (2.7) | 2/44 (4.5) | 0/30 (0) | |
| Clubfoot | – | 2/74 (2.7) | 2/44 (4.5) | 0/30 (0) | |
| Neurologic disorders | |||||
| Seizure disorder | 11–15% | 12/74 (16.2) | 1/44 (2.3) | 11/30 (36.7) | <0.001*,** |
| Headaches | – | 13/73 (17.8) | 4/43 (9.3) | 9/30 (30.0) | 0.031* |
| Tremor | 6–20% | 12/74 (16.2) | 5/44 (11.4) | 7/30 (23.3) | 0.208 |
| Other | |||||
| Environmental or food allergies | – | 21/74 (28.4) | 12/44 (27.3) | 9/30 (30.0) | 0.799 |
Dental disorders included: poor dentition classified as four or more caries/fillings, significant malocclusion, current, or previous orthodontia.
Congenital heart defects included: atrial septal defect (n = 3) and ventricular septal defect (n = 5).
Genitourinary abnormalities included: cystic dysplastic kidneys (n = 4), unilateral kidney (n = 1), ureter malformation (n = 1), bilateral hydronephrosis (n = 3).
P = 0.05 or less.
Result remained statistically significant after correction for multiple comparisons.
Overall, seizures were present in 16.2% of participants. Seizures remained significantly more frequent in the postnatal group after corrections for multiple comparisons. Seizure types included partial complex seizures or generalized tonic clonic seizures. Of females with a history of seizures, six had abnormalities on EEG including spike wave epileptiform discharges and diffuse slowing. All participants with seizures and EEG abnormalities were on anticonvulsants at the time of evaluation (n = 6, 8.1%). The other most common medical features included dental disorders (44.4%), constipation (45.9%), environmental and food allergies (28.4%), and functional abdominal pain (18.9%). Thirty percent (22/73) reported frequent respiratory infections and this was the most common reason for hospitalization among all subjects. Median age of menarche was 12 years (n = 18, range 9–14 years) which corresponds to national data on age of menarche [Chumlea et al., 2003]. There were no girls who had experienced premature ovarian failure, although the maximum age in our cohort was 24.
Approximately 20% (n = 15) of all participants were on psychotropic medications at the time of evaluation and 33.7% (n = 25) were on other medications as presented in Table V. Other common medications included treatments for constipation, asthma, or allergies.
TABLE V.
Current Medications
| Medication class | Number (%) |
|---|---|
| Psychopharmacologic medications | 15/74 (20.3) |
| ADHD medicationsa | 6/74 (8.1) |
| Serotonin specific reuptake inhibitors | 10/74 (13.5) |
| Atypical antipsychoticsb | 5/74 (6.8) |
| Mood stabilizersc | 2/74 (2.7) |
| Otherd | 2/74 (2.7) |
| Anticonvulsants | 6/74 (8.1) |
| Respiratory medicationse | 12/74 (16.2) |
| GI medications for constipationf | 7/74 (9.5) |
ADHD medications included: methylphenidate, amphetamine/dextroamphetamine, atomoxetine, guanfacine.
Atypical antipsychotics included: aripiprazole, quietapine, risperidone.
Mood stabilizers included: gabapentin, lithium, topiramate.
Other included: non-SSRI antidepressants such as buproprion.
Respiratory medications included: albuterol, fluticasone, levalbuterol, montelukast.
GI medications included: lactulose, polyethylene glycol.
Neuroimaging
Thirty five percent (26/74) of participants had previously obtained neuroimaging as part of an evaluation for seizures, hypotonia, or developmental delays. Review of MRI reports for subjects with identified abnormalities included findings of frontal or subcortical white matter abnormalities (n = 4) and generalized volume loss (n = 1). Neuroimaging revealed incidental findings in two subjects including a pineal gland cyst (n = 1) and a colloid cyst within the 3rd ventricle (n = 1).
Neurodevelopmental and Psychiatric Conditions
Among all participants, average age of milestones including first steps was 15.3 months (n = 57, SD = 3.2 months, range 10–25 months) and first word was 12.9 months (n = 37, SD 4.3 months, range 7–24 months). Rates of neurodevelopmental and psychiatric diagnoses and results of cognitive and adaptive testing are presented in Table VI. Speech delays were common in both groups (69.0% vs. 76.7%, P = 0.438). Rates of autism spectrum disorders were higher in the postnatal group (4.5% vs. 20.0%, P = 0.055). Both groups scored in the average range on tests measuring early cognitive and developmental skills. There was a trend toward higher fine motor scores among the prenatal group (P = 0.054). The discrepancy between the higher frequency of reported developmental delays among the postnatal group and average test scores on direct assessment is likely related to the small number of participants in the postnatal group with early developmental testing available. These participants with early developmental testing were diagnosed on the basis of medical features (e.g., hemihypertrophy and cleft palate) that may not be associated with developmental problems. Among participants with early developmental testing, receptive language scores were higher than expressive language scores (P < 0.001) and fine motor scores were greater than gross motor scores (P < 0.001). About half (39/74) of participants received early intervention services.
TABLE VI.
Neurodevelopmental Diagnoses and Cognitive Testinga
| Diagnosis | Total number (%) | Prenatal diagnosis (%) | Postnatal diagnosis (%) | P | |
|---|---|---|---|---|---|
| Any developmental Delay | 57/72 (79.2) | 29/42 (69.0) | 28/30 (93.3) | 0.010* | |
| Speech delay | 52/72 (72.2) | 29/42 (69.0) | 23/30 (76.7) | 0.438 | |
| Motor delay | 36/72 (50.0) | 16/42 (38.1) | 20/30 (66.7) | 0.017* | |
| Learning disability | 17/45 (37.8) | 9/23 (39.1) | 8/22 (36.3) | 1.000 | |
| Intellectual disability | 6/45 (13.3) | 1/23 (4.3) | 5/22 (22.7) | 0.096 | |
| Current language disorders | 14/45 (31.1) | 6/23 (26.1) | 8/22 (36.4) | 0.530 | |
| Current or previous special education supports | 29/45 (64.4) | 12/23 (52.2) | 17/22 (77.3) | 0.120 | |
| ADHD | 22/45 (48.9) | 12/23 (52.2) | 10/22 (45.5) | 0.768 | |
| Autism spectrum disorder | 8/74 (10.8) | 2/44 (4.5) | 6/30 (20.0) | 0.055 | |
| Sensory integration disorder | 10/74 (13.5) | 4/44 (9.1) | 6/30 (20.0) | 0.299 | |
| Any psychiatric mood disorderb | 17/45 (37.8) | 8/23 (34.8) | 9/22 (40.9) | 0.763 | |
| Anxiety disorderc | 9/45 (19.5) | 3/23 (13.0) | 6/22 (27.3) | 0.284 | |
| Depression/dysthymic | 8/45 (17.8) | 4/23 (17.4) | 4/22 (18.2) | 1.000 | |
| Bipolar or psychotic | 6/45 (13.3) | 4/23 (17.4) | 2/22 (9.1) | 0.665 | |
| Cognitive and adaptive testing | Total mean (SD), n | Prenatal diagnosis mean (SD) | Postnatal diagnosis mean (SD) | P | |
| Early development | |||||
| Cognitive | 99.9 (14.1), n = 27 | 99.7 (15.6), n = 20 | 100.3 (9.6), n = 7 | 0.927 | |
| Expressive language | 93.2 (14.5), n = 19 | 92.5 (15.1), n = 17 | 99.5 (7.8), n = 2 | 0.532 | |
| Receptive language | 104.5 (15.9), n = 19 | 105.4 (16.6), n = 17 | 97.0 (2.8), n = 2 | 0.497 | |
| Gross motor | 86.3 (12.9), n = 17 | 87.4 (11.6), n = 14 | 81.0 (20.1), n = 3 | 0.451 | |
| Fine motor | 95.4 (14.4), n = 25 | 98.2 (11.9), n = 20 | 84.4 (19.3), n = 5 | 0.054 | |
| School aged or adult | |||||
| Verbal IQ (VIQ) | 86.0 (13.1), n = 37 | 93.3 (11.3), n = 18 | 79.1 (10.8), n = 19 | <0.001*,** | |
| Performance IQ (PIQ) | 90.6 (15.7) | 95.1 (14.9) | 86.4 (15.5) | 0.090 | |
| General abilities index | 87.7 (14.4) | 94.4 (12.5) | 81.3 (13.4) | 0.004*,** | |
| Working memory | 87.4 (16.2), n = 20 | 96.8 (12.2), n = 10 | 77.9 (14.5), n = 10 | 0.005* | |
| Processing speed | 86.5 (11.9) | 89.3 (10.4) | 83.6 (13.2) | 0.297 | |
| Adaptive | |||||
| Adaptive composite | 84.8 (20.0), n = 46 | 95.3 (15.6), n = 26 | 71.2 (16.8), n = 20 | <0.001*,** | |
Intellectual disability, learning disability, ADHD, and psychiatric mood disorders included children 5 years or older at time of evaluation.
Several participants had more than one psychiatric disorder.
Anxiety disorder includes generalized anxiety disorder, separation anxiety, or obsessive compulsive disorder with anxiety.
P = 0.05 or less.
Remained statistically significant after correction for multiple comparisons (P < 0.0045).
Learning disabilities (LD) such as dyslexia, language based LD and math specific LD (39.1% vs. 36.3%, P = 1.00) and ADHD (52.2% vs. 45.5%, P = 0.768) were common among our participants with Triple X and were similar between the prenatal and postnatal diagnosis groups. Overall rates of ID were low (13.3%), and 5 of the 6 females with ID were postnatally diagnosed. Among all participants cognitive scores were in the low average range (see Table VI). There was not a significant difference between Verbal and Performance IQ (P = 0.175). Participants in the prenatal diagnosis group had higher mean verbal IQ (P < 0.001), GAI (P = 0.004), and working memory (P = 0.005). The prenatal group also had higher composite scores on adaptive testing (P < 0.001). In the postnatal group, composite scores of adaptive functioning were lower than GAI (P = 0.045). After adjusting for multiple comparisons, the postnatal group still had lower VIQ, GAI, and Adaptive Composite scores. Among females in the postnatal diagnosis group, there was not a significant difference in GAI scores between females with a seizure disorder and those without a seizure disorder (Mann Whitney test, P = 0.34). Among all participants age 5 years or greater, 64.4% (n = 29) received special education supports in school.
Approximately 38% of participants met DSM-IV criteria for a psychiatric disorder including anxiety (19.5%), depression or dysthymic disorders (17.8%), or Bipolar Disorder or Psychotic Disorder (13.3%). Rates of these disorders were not significantly different between the prenatal diagnosis group and the postnatal diagnosis group.
DISCUSSION
Prior to discussion of study results, it is important to note that the results and percentages reported are those found in the group of participants in this study. Given the high incidence of Triple X in the general population (estimated at 1:1,000 females) and the low rate of diagnosis, it is likely that the results identified in this cohort are not representative of the entire population of individuals with Triple X. These limitations apply to both ascertainment groups (prenatal and postnatal) as many older females in the prenatal diagnosis group have sought clinical care for ongoing health, developmental, or behavioral concerns. However, to our knowledge this is the largest cohort of females with Triple X syndrome described to date, and thus include valuable data and comparisons as long as limitations of ascertainment and recruitment biases are considered.
Overall, our findings are in concordance with features described in previous prospective studies of females with Triple X syndrome including tall stature, language delays, and anxiety [Linden et al., 1988; Nielsen, 1990; Ratcliffe et al., 1990; Robinson et al., 1990]. Our results build on previous studies by expanding associated medical features and incorporating current neurodevelopmental diagnoses. We also directly compared features by time of ascertainment (prenatal vs. postnatal) to highlight key similarities and differences and to help guide counseling for families with either a prenatal or postnatal diagnosis.
Nearly 60% of participants in our study were ascertained prenatally and the vast majority of those (86.4%) were identified after screening based on advanced maternal age. The incidence of Triple X syndrome is weakly associated with increased maternal age [Ferguson-Smith and Yates, 1984]. More widespread use of prenatal diagnostic testing and development of non-invasive prenatal testing strategies such as cell free fetal DNA will likely increase the number of prenatally diagnosed cases [Lalatta and Tint, 2013; Hooks et al., 2014]. Indeed, the diagnosis of SCAs is usually not the primary diagnosis of interest in prenatal genetic testing. Nevertheless, once a SCA is identified, practitioners are faced with counseling parents of a Triple X syndrome fetus regarding the prognosis and treatment of affected females. Given these factors, it is crucial to equip practitioners and genetic counselors with comprehensive information regarding associated features and outcomes. See further genetic counseling recommendations in Box 1 and clinical treatment recommendations in Box 2.
Box 1. Genetic Counseling for Triple X syndrome.
How does Triple X occur?
It arises from a non-disjunction event resulting in an additional X chromosome, most commonly maternal in origin (90% maternal vs. 10% paternal). Of the maternal non-disjunction events, approximately 60% occur during meiosis I (associated with advanced maternal age), 20% during meiosis II, and 20% after fertilization as a post-zygotic mitotic non-disjunction event [Jacobs et al., 1988].
Can Triple X be mosaic?
Because mitotic non-disjunction events can occur after fertilization, these cases may be commonly associated with mosaicism, such as 47, XXX/45, X or 47, XXX/46, XX. Further medical evaluation and genetic counseling may be indicated, depending on which type of mosaicism is present, especially if there is a 45, X cell line.
Why do the features associated with Triple X occur?
In typical 46, XX females, one X chromosome is randomly inactivated in all cells within the body, however an estimated 5–10% of X chromosome genes escape inactivation and are expressed from both X’s. Females with 47, XXX have two inactive X chromosomes and one active X chromosome, however genes that escape X-inactivation are expressed from all 3 X chromosomes. It is hypothesized that this over expression of genes leads to the phenotypic features of Triple X. For example, the SHOX gene on the X escapes X-inactivation and over expression is related to tall stature in Triple X [Ottensen et al., 2010]. Many X chromosome genes are involved in neurodevelopment.
What should a genetic counseling session for Triple X syndrome include?
Genetic counseling should address the possible medical, developmental, and psychological features as outlined in this study and other published reviews [Legett et al., 2010; Otter et al., 2010; Tartaglia et al., 2010], relevant to the age of the child at the time of diagnosis and evaluation. The low ascertainment rates (approx. 10%) in comparison to incidence (1:1,000 female births) and the variability of the phenotype between females with Triple X should be discussed. If a prenatal diagnosis, couples should be informed that: (i) there is no known increased risk of miscarriage of baby with Triple X; (ii) facial and physical appearance of girls and women with Triple X is usually normal or very subtle; and (iii) children diagnosed prenatally may have higher IQ and adaptive skills compared to postnatal cases, however the risk for speech delays or learning disabilities are still present. Evaluation and treatment recommendations as detailed in Box 2 should be presented. The genetic counselor should also discuss considerations when disclosing the diagnosis to their daughter, family members, and other important parties (such as their school team), with further details and resources described in Dennis et al. [2015]. Resources should be provided.
What are some common struggles of receiving a Triple X syndrome diagnosis?
Couples presented with a prenatal diagnosis of Triple X may not have been prepared for this under-recognized diagnosis. Couples commonly struggle with understanding the significant variability in the phenotype, and the uncertainty of whether their daughter will exhibit any or all of the associated features. It is important for parents to appreciate the significant role of other family genes inherited, stressing that the child’s prognosis is relative to their entire genetic makeup, as well as the impact of factors such as home environment, educational supports, etc. Parents often find relief in knowing how to find help for associated problems if they do occur. Parents presented with a postnatal diagnosis may struggle with understanding their daughter in the context of the new diagnosis, although many find relief in understanding many of her symptoms.
What supports and resources are available?
The main parent advocacy organization for Triple X in the United States is AXYS (www.genetic.org), which offers many educational materials, family conferences, social media sites, and regional support groups. Many families find it helpful to talk with other parents of girls with Triple X. In prenatal cases it is helpful to show real-life photographs (Fig. 1) rather than those in outdated medical publications.
Box 2. Evaluation and Treatment Recommendations for Females With Triple X syndrome.
| Medical or psychological feature | Recommendation for follow-up and further evaluation |
|---|---|
| Developmental delay (Age 0–3) | Comprehensive developmental assessments should be performed for all children, with evaluation of cognitive, speech-language, motor, social, and adaptive functioning domains using standardized measures. If indicated, initiation of early interventions including developmental, speech, occupational, or physical therapies. If prenatal diagnosis: Evaluations at 6–9 months, 12–15 months, 18–24 months, and 30–36 months. Sooner or more frequent if any developmental concerns. If postnatal diagnosis: Evaluation at diagnosis, and then at ages recommended above. |
| Learning disabilities | Monitor learning and academic performance from preschool throughout education. Psychological evaluations to assess cognitive functioning, learning disabilities at key times during education: early elementary, late elementary, middle school, high school, transition to post-secondary programming. Special education supports (504 plans or Individual Education Plans) as needed. Evidence-based interventions for learning disabilities if identified. |
| ADHD/executive functioning problems | Education of parents/caretakers about executive functioning (EF) and symptoms of EF deficits. Screening of ADHD symptoms by school system and primary care provider with input from family and school as presentation may vary in different environments. Formal evaluation of executive functioning by psychologist or neuropsychologist beginning at 7–8 years of age, and at key times during education: late elementary, middle school, high school, transition to post-secondary programming. Implementation of educational strategies and supports for EF and ADHD symptoms at school and home if present. Consideration of medication treatment for attentional disorders/ADHD if present. |
| Speech–language disorders | Assessment with an experienced pediatric speech and language pathologist with evaluation of expressive–receptive language abilities, higher-order language skills, pragmatic/social use of language, and disorders of speech production (developmental dyspraxia/apraxia) or hypernasality due to possible VPI. Recommended yearly evaluation of speech from birth to 4 years, then every 2–3 years depending on presence or severity of impairment. Speech–language therapy through early intervention, school system and/or privately if indicated. |
| Motor skills | After age 3 years, monitor fine and gross motor skills, balance, coordination, motor planning. Occupational and/or physical therapy interventions if motor deficits causing difficulties with handwriting, play or recreational activities, dressing, eating or other self-care skills. |
| Social/emotional problems | Evaluation by developmental pediatrician, child psychiatrist and/or psychologist related to behavioral difficulties, anxiety, social functioning, autism spectrum disorder, and other behavioral or emotional concerns. Therapy/counseling, school supports and/or medication treatment if indicated. |
| Adaptive functioning problems | Evaluation of adaptive functioning using standardized measures including domains of self-care, communication, social, community use, safety, and self-direction should be included as part of the psychological or educational evaluations recommended above. Occupational therapy to address self-care and other adaptive domains as needed. |
| Cardiac anomalies | Cardiology consultation or Echocardiogram/EKG for all new diagnoses or after birth in a prenatal diagnosis. |
| Abdominal pain or constipation | Evaluation and treatment with primary care provider if present. Referral to gastroenterology if needed. |
| Seizures | Neurologic history, including questions about staring spells or atypical movements. Neurology consultation and/or EEG may be indicated. Anticonvulsant medication(s) if indicated. |
| Ovarian function/fertility | Evaluation by endocrinologist or gynecologist for abnormal pubertal development, irregular menses, or fertility concerns. |
| Autoimmune problems | Thyroid screening every 1–2 years starting around age 10, or sooner if concerning symptoms. Discussion and monitoring of other autoimmune symptoms with primary care provider. |
| Renal anomalies | Renal ultrasound should be performed for all new diagnoses or by 6 months after birth in a prenatal diagnosis. |
| Genetics | In a prenatal diagnosis, postnatal confirmatory genetic testing is recommended, including FISH testing for mosaicism. Consultation with genetic counselor and/or clinical genetics. |
Comparisons between the prenatal and the postnatal groups demonstrate a general finding of improved outcomes and fewer medical features in the prenatal group. This affirms previous reports from a smaller sample [Linden et al., 1988]. Reasons for this finding include that females in the prenatal diagnosis group are likely more representative of the phenotypic spectrum including more mildly affected females, compared to the postnatal group who are ascertained due to some developmental or medical concerns. Because prenatally diagnosed females are identified before birth, they are also more likely to receive early developmental screening and interventions, and to benefit from educational supports initiated due to knowledge of their genetic condition. Comparisons of outcomes in participants who had and had not received early intervention therapies were not performed in this study due to the variability of criteria for qualification for early intervention service between States in the United States, the retrospective nature of this type of data, and also because some participants may not have needed early intervention which complicates data interpretation.
While previous studies have attributed improved outcomes to higher socioeconomic status in those with a prenatal diagnosis [Linden and Bender, 2002], differences in SES were not significant between the prenatal and postnatal samples in this study. This finding emphasizes the importance of a supportive environment and close developmental follow-up from birth. Further research is needed to better analyze the factors associated with SES that may contribute to outcomes, such as parental cognitive abilities or increased access to services. Finally, the increased rate of intellectual disability and seizures in the postnatal group is important as these are likely an indicator of more significant abnormalities in brain connectivity.
Ascertainment and recruitment biases are always considerations in SCA research and limit our ability to generalize our findings to an unselected population of all females with Triple X syndrome. Our study participants were recruited primarily through postings on support sites for Triple X syndrome and through an interdisciplinary clinic for SCA. In general, it is assumed that patients recruited from a neurodevelopmental clinic are likely to have more significant neurodevelopmental involvement and, thus, represent more severely affected individuals. However, given the paucity of SCA clinics specializing in medical or psychological differences in Triple X, parents often seek evaluation (whether clinical or research) for the opportunity to talk with professionals experienced with Triple X regardless of the severity of their child’s symptoms. Another subgroup of clinical patients are infants or young children with a prenatal diagnosis who have not developed clinical concerns but who are seeking clinical care for general developmental monitoring. Further, while a “pure” research sample may be considered less biased, those who choose to participate in research may be families with children who are having more difficulties and are more involved with support organizations. Additionally, in the absence of cytogenetic newborn screening for SCAs, currently practitioners encounter females with Triple X syndrome in either prenatal or postnatal diagnosis scenarios. Hence, describing females in these two clinical groups is relevant as it can equip practitioners to evaluate and treat affected females and effectively counsel families in common clinical situations.
Another consideration is that approximately 50% of the study group was less than 5 years of age at the time of evaluation and, thus, were not included in comparisons of neurodevelopmental and psychiatric diagnoses. These participants received age appropriate measures of developmental functioning (Bayley Scales of Infant Development or Mullen Scales of Early Learning) which were not significantly different between prenatal and postnatal groups with the exception of fine motor skills. These younger participants also provided important information regarding clinical ascertainment, anthropometrics, and physical exam features which are often age-dependent, and medical features present in early childhood. Longitudinal and prospective research in Triple X is needed throughout the lifespan, and the increasing population of prenatally identified infants through non-invasive prenatal screening provides an opportunity for these studies.
The low percentage of minorities in this study is likely due to many factors, including under-ascertainment in minority populations, and recruitment through support groups that are largely Internet-based and, thus, may be less accessible to minorities of lower socioeconomic status. Efforts and strategies to improve inclusion and participation of underrepresented minorities is greatly needed in SCA research.
The phenotype of Triple X syndrome is likely explained in part by over expression of genes that escape X inactivation. However, the specific genes involved have not yet been identified. Neuroimaging studies have demonstrated females with 47, XXX have reduced whole brain and frontal-temporal volumes, decreased white matter, and changes in cortical density [Warwick et al., 1999; Lenroot et al., 2014]. Many of these involved regions are important in language, executive function, social behavior, and anxiety, which are commonly affected in 47, XXX [Lenroot et al., 2014]. The specific mechanisms by which genes from the supernumerary chromosome influence brain development to yield such neuroanatomical differences remain poorly understood.
As previous researchers have suggested [Linden et al., 1988] and our results demonstrate there is considerable individual variability among females with Triple X syndrome. Variability is not an inherently unique feature of Triple X syndrome. Rather, due to increased use of genetic testing more individuals with mild or atypical phenotypes of many genetic conditions have been identified. The factors contributing to this phenotypic variability is of great interest to providers and to families with a prenatal diagnosis, yet is also multifactorial and involves a complex interplay of genes and environmental influences. A multitude of chromosomal regions and genes have been implicated in learning disabilities [Haworth and Plomin, 2010] and psychiatric disorders [Sullivan et al., 2012]. Background genetic and familial influences also play a role. In a study of males with the sex chromosome trisomy XXY, those with a family history of learning disabilities tended to have poorer neurodevelopmental outcomes [Samango-Sprouse et al., 2013]. The addition of supernumerary X chromosome in individuals already at risk for cognitive and mental health problems due to other affected genes may result in poorer outcomes. Environmental factors also modulate outcomes as Bender et al. [1987] demonstrated that children with SCAs reared in an unstable home environment had worse psychosocial adaptation and more developmental disorders. Positive environmental factors such as family support and increased developmental surveillance may help build resiliency and increase intervention for emerging developmental problems at an earlier age, potentially leading to improved outcomes. Overall, while prenatally diagnosed cases generally have better cognitive and adaptive functioning, all girls with Triple X are at risk for neurodevelopmental and social-emotional difficulties that require ongoing screening and various supports throughout into adulthood.
Acknowledgments
Grant sponsor: MIND Institute at University of California-Davis and the Madigan Foundation; Grant sponsor: NIH/NCATS Colorado CTSI; Grant number: UL1TR001082; Grant sponsor: NIH/NINDS; Grant number: K23NS070337.
We would like to acknowledge all families and participants in the study, and appreciate support from the MIND Institute at University of California-Davis and the Madigan Foundation. This work was also supported by NIH/NCATS Colorado CTSI Grant UL1TR001082 and NIH/NINDS K23NS070337. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
References
- Bayley N. Bayley scales of infant development. 2. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- Bayley N. Bayley scales of infant and toddler development. 3. San Antonio, TX: Harcourt Assessment Inc; 2006. [Google Scholar]
- Bender BG, Linden MG, Robinson A. Environmental and developmental risk in children with sex chromosome abnormalities. J Am Acad Child Adolesc Psychiatry. 1987;26:499–503. doi: 10.1097/00004583-198707000-00006. [DOI] [PubMed] [Google Scholar]
- Bender BG, Harmon RJ, Linden MG, Robinson A. Psychosocial adaptation of 39 adolescents with sex chromosome abnormalities. Pediatrics. 1995;96:302–308. [PubMed] [Google Scholar]
- Chumlea WC, Schubert CM, Roche AF, Kunlin HE, Lee PA, Himes JH, Sun SS. Age at menarche and racial comparisons in U.S. girls. Pediatrics. 2003;111:110–113. doi: 10.1542/peds.111.1.110. [DOI] [PubMed] [Google Scholar]
- Dennis A, Howell S, Cordeiro L, Tartaglia N. “How should I tell my child?” Disclosing the diagnosis of sex chromosome aneuploidies. J Genet Couns. 2015;24:88–103. doi: 10.1007/s10897-014-9741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson-Smith MA, Yates JR. Maternal age specific rates for chromosome aberrations and factors influencing them: Report of a collaborative European study on 52 965 amniocenteses. Prenat Diagn. 1984;4:5–44. doi: 10.1002/pd.1970040704. [DOI] [PubMed] [Google Scholar]
- Goswami R, Goswami D, Kabra M, Gupta N, Dubey S, Dadhwal V. Prevalence of the triple, 1052 triple X syndrome in phenotypically normal women with premature ovarian failure and its association with autoimmune thyroid disorders. Fertil Steril. 2003;80:1052–1054. doi: 10.1016/s0015-0282(03)01121-x. [DOI] [PubMed] [Google Scholar]
- Grosso S, Farnetani MA, Di Bartolo RM, Berardi R, Pucci L, Mostardini R, Anichini C, Bartalini G, Galimberti D, Morgese G, Balestri P. Electroencephalographic and epileptic patterns in X chromosome anomalies. J Clin Neurophysiol. 2004;21:249–253. doi: 10.1097/00004691-200407000-00003. [DOI] [PubMed] [Google Scholar]
- Harrison PL, Oakland T. Adaptive behavior assessment system. 2. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Haworth CM, Plomin R. Quantitative genetics in the era of molecular genetics: Learning abilities and disabilities as an example. J Am Acad Child Adolesc Psychiatr. 2010;49:783–793. doi: 10.1016/j.jaac.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland CM. 47, XXX in an adolescent with premature ovarian failure and autoimmune disease. J Pediatr Adolesc Gynecol. 2001;14:77–80. doi: 10.1016/s1083-3188(01)00075-4. [DOI] [PubMed] [Google Scholar]
- Hollingshead AdB. Two-factor index of social position. New Haven, Connecticut: Hollingshead; 1965. [Google Scholar]
- Hooks J, Wolfberg AJ, Wang ET, Struble CA, Zahn J, Juneau K, Mohseni M, Huang S, Bogard P, Song K, Oliphant A, Musci TJ. Non-invasive risk assessment of fetal sex chromosome aneuploidy through directed analysis and incorporation of fetal fraction. Prenat Diagn. 2014;34:496–499. doi: 10.1002/pd.4338. [DOI] [PubMed] [Google Scholar]
- Jacobs PA. The incidence and etiology of sex chromosome abnormalities in man. Birth Defects Orig Artic Ser. 1979;15:3–14. [PubMed] [Google Scholar]
- Jacobs PA, Hassold TJ, Whittington E, Butler G, Collyer G, Keston M, Lee M. Klinefelter’s syndrome: An analysis of the origin of the additional sex chromosome using molecular probes. Ann Hum Genet. 1988;52:93–109. doi: 10.1111/j.1469-1809.1988.tb01084.x. [DOI] [PubMed] [Google Scholar]
- Kusumi Y, Prange AJ., Jr Triple mosaicism of X chromosomes with bipolar affective psychosis. Dis Nerv Syst. 1973;34:94–97. [PubMed] [Google Scholar]
- Lalatta F, Tint GS. Counseling parents before prenatal diagnosis: Do we need to say more about the sex chromosome aneuploidies? Am J Med Genet Part A. 2013;161A:2873–2879. doi: 10.1002/ajmg.a.36226. [DOI] [PubMed] [Google Scholar]
- Legett V, Jacobs P, Nation K, Scerif G, Bishop D. Neurocognitive outcomes of individuals with a sex chromosome trisomy: XXX, XYY, or XXY: A systematic review. Dev Med Child Neurol. 2010;52:119–129. doi: 10.1111/j.1469-8749.2009.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Blumenthal JD, Wallace GL, Clasen LS, Lee NR, Giedd JN. A case-control study of brain structure and behavioral characteristics in 47, XXX syndrome. Genes Brain Behav. 2014;13:841–849. doi: 10.1111/gbb.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebezeit BU, Rohrer TR, Singer H, Doerr HG. Tall stature as a presenting symptom in a girl with triple X syndrome. J Pediatr Endocrinol Metab. 2003;16:233–235. doi: 10.1515/jpem.2003.16.2.233. [DOI] [PubMed] [Google Scholar]
- Lin HJ, Ndiforchu F, Patell S. Exstrophy of the cloaca in a 47, XXX child: Review of genitourinary malformations in triple-X patients. Am J Med Genet. 1993;15:761–763. doi: 10.1002/ajmg.1320450619. [DOI] [PubMed] [Google Scholar]
- Linden MG, Bender BG, Harmon RJ, Mrazek DA, Robinson A. 47, XXX: What is the prognosis? Pediatrics. 1988;82:619–630. [PubMed] [Google Scholar]
- Linden MG, Bender BG. Fifty-one prenatally diagnosed children and adolescents with sex chromosome abnormalities. Am J Med Genet. 2002;110:11–18. doi: 10.1002/ajmg.10394. [DOI] [PubMed] [Google Scholar]
- Manning M, Hudgins L ACMG Professional Practice and Guidelines Committee. Array-based technology and recommendations for utilization in medical genetics practice for detection of chromosomal abnormalities. Genet Med. 2010;12:742–745. doi: 10.1097/GIM.0b013e3181f8baad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeschler J, Shevell M AAP Committee on Genetics. Clinical report: Comprehensive evaluation of the child with intellectual disability or global developmental delays. Pediatrics. 2014;134:e903–e918. doi: 10.1542/peds.2014-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E. Mullen scales of early learning. Cranston, RI: TOTAL Child Inc; 1989. [Google Scholar]
- Nielsen J. Sex chromosome abnormalities found among 34,910 newborn children: Results from a 13-year incidence study in Arhus, Denmark. Birth Defects Orig Artic Ser. 1990;26:209–223. [PubMed] [Google Scholar]
- Olanders S, Sellden U. Electroencephalographic investigation. In: Olanders S, editor. Females with supernumerary X chromosomes: A study of 39 psychiatric cases. Goteborg, Sweden: University of Goteborg; 1975. pp. 77–85. [Google Scholar]
- Ottensen AM, Aksglaede L, Garn I, Tartaglia N, Tassone F, Gravholt CH, Bojesen A, Sørensen K, Jørgensen N, Raipert-De Meyts E, Gerdes T, Lind AM, Kjaeraad S, Juul A. Increased number of sex chromosomes affects height in a non-linear fashion: A study of 305 patients with sex chromosome aneuploidies. Am J Med Genet A. 2010;152A:1206–1212. doi: 10.1002/ajmg.a.33334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter M, Schrander-Stumpel CT, Curfs LM. Triple X syndrome: A review of the literature. Eur J Hum Genet. 2010;18:265–271. doi: 10.1038/ejhg.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe S, Butler G, Jones M. Edinburgh study of growth and development of children with sex chromosome abnormalities. Birth Defects Orig Artic Ser. 1990;26:1–44. [PubMed] [Google Scholar]
- Ratcliffe SG, Pan H, McKie M. The growth of XXX females: Population based studies. Ann Hum Biol. 1994;21:57–66. doi: 10.1080/03014469400003072. [DOI] [PubMed] [Google Scholar]
- Roubertie A, Humbertclaude V, Leydet J, Lefort G, Echenne B. Partial epilepsy and 47, XXX karyotype: Repot of four cases. Pediatr Neurol. 2006;3:69–74. doi: 10.1016/j.pediatrneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Robinson A, Puck M, Pennington B, Borelli J, Hudson M. Abnormalities of the sex chromosomes: A prospective study on randomly identified newborns. Birth Defects Orig Artic Ser. 1979;15:203–241. [PubMed] [Google Scholar]
- Robinson A, Bender BG, Linden MG. Summary of clinical findings in children and young adults with sex chromosome anomalies. Birth Defects Orig Artic Ser. 1990;26:225–228. [PubMed] [Google Scholar]
- Robinson A, Bender BG, Linden MG. Prognosis of prenatally diagnosed children with sex chromosome aneuploidy. Am J Med Genet. 1992;44:365–368. doi: 10.1002/ajmg.1320440319. [DOI] [PubMed] [Google Scholar]
- Samango-Sprouse CA, Stapleton E, Sadeghin T, Gropman AL. Is it all the X: Familial learning dysfunction and the impact of behavioral aspects of the phenotypic presentation of XXY? Am J Med Gen C Semin Med Genet. 2013;163C:27–34. doi: 10.1002/ajmg.c.31353. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti VD, Balla AD. Vineland adaptive behavior scales. 2. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- Stewart DA, Netley CT, Park E. Summary of clinical findings of children with 47, XXY 47, XYY, and 46, XXX karyotypes. Birth Defects Orig Artic Ser. 1982;18:1–5. [PubMed] [Google Scholar]
- Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: The emerging picture and its implications. Nat Rev Genet. 2012;13:537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia N, Borodyanskya M, Davis S, Ono M, Hansen R, Hagerman R. Tremor in sex chromosome aneuploidy. J Invest Med. 2007;55:322. [Google Scholar]
- Tartaglia NR, Howell S, Sutherland A, Wilson R, Wilson L. A review of trisomy X (47, XXX) Orphanet J Rare Dis. 2010;5:8. doi: 10.1186/1750-1172-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia NR, Ayari N, Hutaff-Lee C, Boada R. Attention-deficit hyperactivity disorder symptoms in children and adolescents with sex chromosome aneuploidy: XXY, XXX, XYY, and XXYY. J Dev Behav Pediatr. 2012;33:309–318. doi: 10.1097/DBP.0b013e31824501c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia N, Howell S, Wilson R, Janusz J, Boada R, Frazier J, Pfeiffer M, Martin S, Regan K, McSwegin S, Zeitler P. The eXtraordinarY kids clinic: A model of interdisciplinary care for children and adolescents with sex chromosome aneuploidy. J Multidiscip Healthc. 2015;8:323–334. doi: 10.2147/JMDH.S80242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsky DS, Saklofske D, Wilkins C, Weiss LG. Development of a general ability index for the Weschler adult intelligence scale-third edition. Psychol Assess. 2001;13:566–571. doi: 10.1037//1040-3590.13.4.566. [DOI] [PubMed] [Google Scholar]
- Villanueva AL, Rebar RW. Triple-X syndrome and premature ovarian failure. Obstet Gynecol. 1983;62:70s–73s. [PubMed] [Google Scholar]
- Warwick MM, Doody GA, Lawrie SM, Kestelman JN, Best JJ, Johnstone EC. Volumetric magnetic resonance imaging study of the brain in subjects with sex chromosome aneuploidies. J Neurol Neurosurg Psychiatry. 1999;66:628–632. doi: 10.1136/jnnp.66.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. New York, NY: The Psychological Corporation; 1999. [Google Scholar]
- Wechsler D. The Wechsler intelligence scale for children. 4. London, United Kingdom: Pearson Assessment; 2004. [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 4. San Antonio, TX: Pearson Assessment; 2008. [Google Scholar]
- Woodhouse W, Holland A, McLean G, Reveley A. The association between triple X and psychosis. Br J Psychiatry. 1992;160:554–557. doi: 10.1192/bjp.160.4.554. [DOI] [PubMed] [Google Scholar]