Abstract
BACKGROUND
The current definitions of psychotic illness lack biological validity, motivating alternative biomarker-driven disease entities. Building on experimental constructs—Biotypes—that were previously developed from cognitive and neurophysiologic measures, we contrast brain anatomy characteristics across Biotypes alongside conventional diagnoses, examining gray matter density (GMD) as an independent validator for the Biotypes.
METHODS
Whole brain GMD measures were examined in probands, their relatives, and healthy subjects organized by Biotype and then by DSM-IV-TR diagnosis (n = 1409) using voxel-based morphometry with subsequent subject-level regional characterization and distribution analyses.
RESULTS
Probands grouped by Biotype versus healthy controls showed a stepwise pattern of GMD reductions as follows: Biotype1, extensive and diffusely distributed GMD loss, with the largest effects in frontal, anterior/middle cingulate cortex, and temporal regions; Biotype2, intermediate and more localized reductions, with the largest effects in insula and frontotemporal regions; and Biotype3, small reductions localized to anterior limbic regions. Relatives showed regionally distinct GMD reductions versus healthy controls, with primarily anterior (frontotemporal) effects in Biotype1; posterior (temporo-parieto-cerebellar) in Biotype2; and normal GMD in Biotype3. Schizophrenia and schizoaffective probands versus healthy controls showed overlapping GMD reductions, with the largest effects in frontotemporal and parietal regions; psychotic bipolar probands had small reductions, primarily in frontal regions. GMD changes in relatives followed regional patterns observed in probands, albeit less extensive. Biotypes showed stronger between-group separation based on GMD than the conventional diagnoses and were the strongest predictor of GMD change.
CONCLUSIONS
GMD biomarkers depicted unique brain structure characteristics within Biotypes, consistent with their cognitive and sensorimotor profiles, and provided stronger discrimination for biologically driven biotypes than symptom-based diagnoses.
Keywords: Biological marker, Biotypes, Brain gray matter, Mood disorders-bipolar, Schizophrenia, Voxel-based morphometry
Current nosological constructs in psychiatry are based on phenomenologic criteria. Despite the routine use of biomarkers spanning genetic-, tissue-, organ-, and system-level measures in other fields of medicine, the application of brain biomarkers in psychiatry is lacking. Conventional symptom-based psychiatric diagnoses map poorly onto emerging biomarker-driven constructs (2,3). The absence of biologically based disease definitions hinders progress in identifying mechanistic targets for effective treatment development. High medical need exists in psychiatry for developing disease entities built on brain biology and supported by objective, quantitative, clinically relevant disease biomarkers—the approach recently emphasized by the Research Domain Criteria (RDoC) (4,5).
The Bipolar-Schizophrenia Network for Intermediate Phenotypes has recently developed biomarker criteria based on a large psychosis sample that capture neurobiologically defined groups of cases, Biotypes (1). This approach provided proof of concept that highly heterogeneous symptom-based categories within the psychosis dimension can be reorganized into biologically meaningful constructs (1). In brief, based on multistep multivariate analyses derived from cognitive, electroencephalography-based, and oculomotor paradigms [see Clementz et al. (1) for a detailed description of Biotype development], three such constructs emerged: Biotype1 (B1), capturing cases with poor cognitive and sensorimotor function; Biotype2 (B2), characterized by moderately impaired cognitive function and exaggerated sensorimotor reactivity; and Biotype3 (B3), showing near normal cognitive and sensorimotor functions. The distinctive cognitive and sensorimotor profiles were consistent in probands and their first-degree relatives (1). The conventional diagnoses mapped poorly onto Biotypes, with all targeted DSM-IV-TR groups (i.e., schizophrenia, schizoaffective disorder, and psychotic bipolar disorder) represented in all Biotypes. Brain structure measures were not used in the Biotype development and therefore are available to be examined as independent validators for the Biotype constructs.
Reduced gray matter density (GMD) and cortical thickness are established features of psychotic disorders observed in probands and their biological relatives (6–9). In schizophrenia, studies show broadly distributed neocortical and subcortical GMD reductions, with the most significant loss in frontotemporal regions (10–14). Outcomes vary in bipolar disorder, with psychotic phenotype showing GMD alterations similar to those observed in schizophrenia, while nonpsychotic bipolar is associated with rather preserved GMD (15–19). In the absence of in vivo tissue-level biomarkers, GMD along with other brain imaging measures is the most easily accessible proxy for tissue abnormalities underlying psychotic illness. GMD biomarkers show associations with several functional outcomes, including symptom severity and cognitive and neurophysiologic alterations (20–24). Moreover, measures of brain structure are among the best predictors of “conversion” to psychosis, disease progression, and lifetime cumulative psychosis burden (7,25–29). Finally, GMD biomarkers are sensitive to treatment effects, including both pharmacologic (30–36) and cognitive remediation (37) interventions, making them suitable for testing both “primary” and disease-associated (e.g., medication) effects.
We examined whole brain and regional GMD biomarkers as independent validators for Biotype psychosis constructs, tested alongside conventional diagnoses, asking whether GMD measures depict unique brain structure characteristics across Biotypes, and whether these biomarkers better discriminate biologically based Biotype constructs than the symptom-driven diagnoses. We hypothesized that across the Biotypes, B1 probands will show most extensive and diffusely distributed GMD reductions; B2 probands will have intermediate in magnitude and distribution GMD reductions; and B3 probands will show small, localized GMD reductions, compared to healthy controls (HC); and relatives grouped by their respective probands’ Biotypes (i.e., B1-Rel, B2-Rel, and B3-Rel), compared to HC, will show GMD reductions regionally similar but less extensive than those detected in their respective probands. We also predicted that across conventional diagnoses, probands with schizophrenia (SZ), schizoaffective disorder (SAD), and psychotic bipolar disorder type I (BD) will show regionally overlapping GMD reductions, the largest in magnitude in SZ and SAD and smallest in BD compared to HC; and their relatives (i.e., SZ-Rel, SAD-Rel, and BD-Rel) will show GMD reductions from HC, regionally similar but less extensive than those observed in probands. Finally, we hypothesized that the Biotypes will show stronger between-group separation based on GMD and be able to better predict GMD changes among probands and relatives than the conventional diagnoses.
METHODS AND MATERIALS
Study Sample
GMD characteristics were assessed in 1409 patients (557 probands, 601 first-degree relatives, and 251 HC) who were initially organized by Biotype and then by DSM-IV-TR diagnosis. Descriptive demographic and clinical characteristics are detailed in Table 1 and Supplemental Table S1. The Bipolar-Schizophrenia Network for Intermediate Phenotypes’ logistics and overall sample characteristics are described elsewhere (38); magnetic resonance imaging-pertinent study exclusion criteria are listed in Supplemental Methods. Probands were stable, medicated outpatients. Relatives included both clinically unaffected and those with lifetime psychiatric diagnoses (Supplemental Table S2); the majority of relatives were unmedicated (Table 1). Healthy comparison subjects had no personal history of psychotic or recurrent mood disorders and no family history of SZ/bipolar spectrum disorders in first- or second-degree relatives. Axis I diagnoses were based on the Structured Clinical Interview for DSM-IV-TR (39); Axis II diagnoses in relatives were captured via Structured Interview for DSM-IV Personality Disorders (40). Symptom ratings for psychosis and affective domains and estimates of premorbid intellectual functioning (38) were also gathered.
Table 1.
Sociodemographic and Clinical Characteristics of the Study Sample by Biotypes and DSM-IV-TR Diagnoses
| Sample Characteristics by
Biotype Constructs |
|||||||||
| B1 (n = 150) | B2 (n = 185) | B3 (n = 222) | B1-Rel (n = 159) | B2-Rel (n = 197) | B3-Rel (n =245) | HC (n = 251) | Test Statistic | p Value | |
| Sociodemographic Characteristics | |||||||||
| Age, Years, Mean (SD) | 35.3 (13.1) | 35.4 (11.9) | 35.3 (12.6) | 39.6 (15.3) | 40.7 (16.1) | 41.2 (16.0) | 36.9 (12.1) | F6,1401 = 7.41 | <.001a |
| Sex/Male, n (%) | 80 (53.3) | 87 (47.0) | 116 (52.3) | 59 (37.1) | 65 (33.0) | 82 (33.5) | 109 (43.4) | χ26 = 34.96 | <.001b |
| Handedness, n (%) | χ212 = 11.59 | .48 | |||||||
| Right | 127 (84.7) | 163 (88.1) | 188 (84.7) | 130 (81.8) | 167 (84.8) | 222 (90.6) | 217 (86.5) | ||
| Left | 17 (11.3) | 18 (9.7) | 28 (12.6) | 24 (15.1) | 22 (11.2) | 22 (9.0) | 30 (12.0) | ||
| Ambidextrous | 5 (3.3) | 2(1.1) | 4(1.8) | 3(1.9) | 4 (2.0) | 1 (0.4) | 2 (0.8) | ||
| Ethnicity/Hispanic, n (%) | 18 (12.0) | 20 (9.7) | 12 (5.4) | 23 (14.5) | 22 (11.7) | 17 (6.9) | 26 (10.4) | χ26 = 12.6 | .051 |
| Race, n (%) | χ212 = 88.59 | <.001c | |||||||
| White | 58 (38.7) | 114 (61.6) | 146 (65.8) | 77 (48.2) | 141 (71.6) | 185 (75.5) | 166 (66.1) | ||
| African American | 81 (54.0) | 57 (30.8) | 60 (27.0) | 72 (45.3) | 49 (24.9) | 47 (19.2) | 62 (24.7) | ||
| Other | 11 (7.3) | 14 (7.6) | 16 (7.2) | 10 (6.3) | 7 (3.6) | 14 (5.7) | 23 (9.2) | ||
| Education, Years, Mean (SD) | 12.4 (2.0) | 13.1 (2.3) | 14.1 (2.3) | 13.6 (2.6) | 13.8 (2.5) | 14.7 (2.7) | 15.1 (2.5) | F6,1395 = 28.13 | <.001d |
| Clinical Characteristics, Mean (SD) | |||||||||
| Age of Illness Onset, Years | 20.5 (7.4) | 20.8 (8.9) | 20.3 (8.6) | - | - | - | - | F2,533 = °.14 | .87 |
| Age of First Hospitalization, Years | 22.2 (8.2) | 23.2 (8.4) | 23.3 (8.6) | - | - | - | - | F2,487 = 0.72 | .49 |
| No. of Lifetime Hospitalizations | 5.6 (6.9) | 5.8 (6.6) | 5.0 (8.3) | - | - | - | - | F2,450 = 1.89 | .15 |
| PANSS | |||||||||
| Total | 64.7 (17.6) | 64.0 (17.1) | 60.3 (16.2) | - | - | - | - | F2,544 = 3.85 | <.02e |
| Positive subscale | 16.6 (5.8) | 16.1 (5.9) | 15.4 (5.2) | - | - | - | - | F2,545 = 2.34 | .10 |
| Negative subscale | 16.3 (5.7) | 15.3 (5.4) | 13.9 (5.2) | - | - | - | - | F2,545 = 9.85 | <.001 |
| General symptoms subscale | 31.8 (9.1) | 32.6 (9.0) | 31.1 (8.6) | - | - | - | - | F2,546 = 1.53 | .22 |
| YMRS | 5.7 (5.7) | 6.7 (6.6) | 6.1 (6.2) | - | - | - | - | F2,541 = 0.85 | .43 |
| MADRS | 10.0 (9.7) | 11.1 (9.0) | 10.5 (9.3) | - | - | - | - | F2,541 = 0.61 | .55 |
| GAF | 48.9 (11.8) | 53.1 (13.9) | 55.8 (13.8) | 73.1 (14.3) | 74.7 (14.9) | 77.0 (12.0) | 86.6 (6.5) | F6,1379 = 260.25 | <.0011f |
| WRAT-4 IQ | 89.6 (13.1) | 95.3 (13.6) | 105.9 (14.4) | 93.6 (14.0) | 100.0 (15.2) | 105.3 (13.9) | 103.5 (13.8) | F6,1380 = 37.29 | <.001g |
| Concomitant Medications, | n (%) | ||||||||
| Off Psychotropic Medications | 2 (1.3) | 13 (7.0) | 18 (8.1) | 106 (66.7) | 133 (67.5) | 174 (71.0) | 240 (95.6) | - | - |
| Antipsychotics | 140 (93.3) | 159 (85.9) | 170 (76.6) | 23 (14.5) | 18 (9.1) | 18 (7.3) | 0 (0.0) | - | - |
| CPZ equivalents | 542.5 (436.5) | 502.7 (469.7) | 392.5 (312.8) | 296.8 (472.0) | 447.89 (475.6) | 239.86 (180.09) | - | F5,388 = 2.89 | .01h |
| Mood Stabilizers | |||||||||
| Lithium | 14 (9.3) | 25 (13.5) | 36 (16.2) | 3(1.9) | 7 (3.6) | 4(1.6) | 0 (0.0) | - | - |
| Other | 51 (34.0) | 59 (31.9) | 73 (32.9) | 15 (9.4) | 11 (5.6) | 9 (3.7) | 0 (0.0) | - | - |
| Antidepressants | 57 (38.0) | 83 (44.9) | 102 (45.9) | 27 (17.0) | 43 (21.8) | 46 (18.8) | 3(1.2) | - | - |
| Anxiolytics/Hypnotics | 37 (24.7) | 53 (28.6) | 59 (26.6) | 15 (9.4) | 19 (9.6) | 27 (11.0) | 6 (2.4) | - | - |
| Anticholinergics | 35 (23.3) | 15 (8.1) | 26 (11.7) | 4 (2.5) | 2(1.0) | 1 (0.4) | 0 (0.0) | - | - |
| Stimulants | 10 (6.7) | 9 (4.9) | 19 (8.6) | 8 (5.0) | 6 (3.0) | 5 (2.0) | 2 (0.8) | - | - |
| Other | 2 (1.3) | 6 (3.2) | 6 (2.7) | 1 (0.6) | 4 (2.0) | 0 (0.0) | 0 (0.0) | - | - |
| Combined Medications | 125 (83.3) | 141 (76.2) | 160 (72.1) | 32 (20.1) | 33 (16.8) | 29 (11.8) | 3(1.2) | - | - |
| Sample Characteristics by
Conventional Diagnoses |
|||||||||
| B1 (n = 242) | B2 (n = 138) | B3 (n = 177) | B1-Rel (n = 246) | B2-Rel (n = 159) | B3-Rel (n =196) | HC (n = 251) | Test Statistic | p Value | |
| Sociodemographic
Characteristics | |||||||||
| Age, Years, Mean (SD) | 34.4 (12.3) | 36.0 (11.9) | 36.1 (13.1) | 42.2 (15.4) | 40.3 (15.9) | 38.9 (16.1) | 36.9 (12.1) | F6,1401 = 8.46 | <.001i |
| Sex/Male, n (%) | 165 (68.2) | 58 (42.0) | 60 (33.9) | 84 (34.1) | 53 (33.3) | 69 (35.2) | 109 (43.4) | χ26 = 87.12 | <.001j |
| Handedness, n (%) | χ212 = 19.21 | .08 | |||||||
| Right | 208 (86.0) | 120 (87.0) | 150 (84.7) | 214 (87.0) | 134 (84.3) | 170 (86.7) | 217 (86.5) | ||
| Left | 26 (10.7) | 12 (8.7) | 25 (14.1) | 27 (11.0) | 16 (10.1) | 25 (12.8) | 30 (12.0) | ||
| Ambidextrous | 4(1.7) | 7 (4.3) | 1 (0.6) | 1 (0.4) | 6 (3.8) | 1 (0.5) | 2 (0.8) | ||
| Ethnicity/Hispanic, n (%) | 21 (8.7) | 16 (11.6) | 13 (7.3) | 25 (10.2) | 17 (10.7) | 20 (10.2) | 26 (10.4) | χ26 = 2.38 | .88 |
| Race, n (%) | χ212 = 85.83 | <.001k | |||||||
| White | 112 (46.3) | 75 (54.3) | 131 (74.0) | 139 (56.5) | 102 (64.2) | 161 (82.1) | 166 (66.1) | ||
| African American | 109 (45.0) | 54 (39.1) | 35 (19.8) | 90 (36.6) | 50 (31.4) | 28 (14.3) | 62 (24.7) | ||
| Other | 21 (8.7) | 9 (6.5) | 11 (6.2) | 17 (6.9) | 7 (4.4) | 7 (3.6) | 23 (9.2) | ||
| Education, Years, Mean (SD) | 12.8 (2.2) | 13.1 (2.2) | 14.2 (2.4) | 14.0 (2.4) | 13.9 (2.9) | 14.5 (2.7) | 15.1 (2.5) | F6,1395 = 22.84 | <.001l |
| Clinical Characteristics, Mean (SD) | |||||||||
| Age of Illness Onset, Years | 21.3 (7.5) | 20.2 (9.1) | 19.8 (8.8) | - | - | - | - | F2,533 = 1.71 | .18 |
| Age of First Hospitalization, Years | 22.6 (7.0) | 22.6 (8.7) | 23.8 (9.8) | - | - | - | - | F2,487 = 0.95 | .39 |
| No. of Lifetime Hospitalizations | 5.6 (8.2) | 6.2 (6.1) | 5.5 (7.2) | - | - | - | - | F2,450 = 0.29 | .75 |
| PANSS | |||||||||
| Total | 65.5 (17.2) | 68.5 (16.1) | 54.2 (13.9) | - | - | - | - | F2,544 = 37.3 | <.001m |
| Positive subscale | 16.7 (5.7) | 18.2 (5.2) | 13.1 (4.6) | - | - | - | - | F2,545 = 41.9 | <.001 |
| Negative subscale | 16.7 (6.1) | 15.6 (4.7) | 12.2 (3.9) | - | - | - | - | F2,545 = 40.0 | <.001 |
| General symptoms subscale | 32.1 (8.8) | 34.7 (8.9) | 28.9 (8.2) | - | - | - | - | F2,546 = 17.7 | <.001 |
| YMRS | 5.7 (5.7) | 7.3 (6.3) | 5.9 (6.7) | - | - | - | - | F2,541 = 3.0 | .051 |
| MADRS | 8.5 (8.1) | 14.6 (10.4) | 10.3 (8.9) | - | - | - | - | F2,541 = 20.3 | <.001n |
| GAF | 49.5 (12.6) | 48.8 (11.7) | 61.3 (12.5) | 75.0 (13.7) | 74.2 (14.4) | 76.3 (13.2) | 86.6 (6.5) | F6,1379 = 289.0 | <.001o |
| WRAT-4 IQ | 95.4 (16.0) | 96.4 (14.8) | 102.9 (13.7) | 97.9 (14.7) | 100.3 (16.3) | 103.8 (14.0) | 103.5 (13.8) | F6,1380 = 11.8 | <.001p |
| Concomitant Medications, n (%) | |||||||||
| Off Psychotropic Medications | 13 (5.4) | 7 (5.1) | 13 (7.3) | 186 (75.6) | 102 (64.2) | 125 (63.8) | 240 (95.6) | - | |
| Antipsychotics | 218 (90.1) | 122 (88.4) | 129 (72.9) | 25 (10.2) | 20 (12.6) | 14(7.1) | 0 (0.0) | - | |
| CPZ equivalents | 527.0 (407.1) | 536.8 (463.9) | 328.0 (327.8) | 365.2 (448.6) | 399.4 (477.9) | 164.2 (123.1) | - | F5,388 = 4.94 | <.001q |
| Mood Stabilizers | |||||||||
| Lithium | 14 (5.8) | 16 (11.6) | 45 (25.4) | 2 (0.8) | 5 (3.1) | 7 (3.6) | 0 (0.0) | - | |
| Other | 41 (16.9) | 62 (44.9) | 80 (45.2) | 7 (2.8) | 14 (8.8) | 14(7.1) | 0 (0.0) | - | |
| Antidepressants | 87 (36.0) | 78 (56.5) | 77 (43.5) | 33 (13.4) | 38 (23.9) | 45 (23.0) | 3(1.2) | - | |
| Anxiolytics/Hypnotics | 55 (22.7) | 38 (27.5) | 56 (31.6) | 22 (8.9) | 17 (10.7) | 21 (10.7) | 6 (2.4) | - | |
| Anticholinergics | 40 (16.5) | 20 (14.5) | 16 (9.0) | 5 (2.0) | 2(1.3) | 0 (0.0) | 0 (0.0) | - | |
| Stimulants | 13 (5.4) | 7 (5.1) | 18 (10.2) | 1 (0.4) | 5 (3.1) | 13 (6.6) | 2 (0.8) | - | |
| Other | 5 (2.1) | 5 (3.6) | 4 (2.2) | 1 (0.4) | 4 (2.5) | 0 (0.0) | 0 (0.0) | - | |
| Combined Medications | 164 (67.8) | 121 (87.7) | 141 (79.7) | 32 (13.0) | 42 (26.4) | 31 (15.8) | 3(1.2) | - | |
A one-way analysis of variance with a subsequent post hoc Tukey honestly significant difference test and Yates corrected chi-squared test were used, as appropriate, for demographic and clinical variables; only statistically significant (p < .05) between-group differences are reported.
B1, Biotype 1 probands; B1-Rel, relatives of Biotype 1 probands; B2, Biotype 2 probands; B2-Rel, relatives of Biotype 2 probands; B3, Biotype 3 probands; B3-Rel, relatives of Biotype 3 probands; BD, probands with psychotic bipolar type I disorder; BD-Rel, relatives of probands with psychotic bipolar type I disorder; CPZ equivalents, daily antipsychotic dose chlorpromazine equivalents; GAF, Global Assessment of Functioning; HC, healthy controls; MADRS, Montgomery-Åsberg Depression Rating Scale; PANSS, Positive and Negative Syndrome Scale; SAD, probands with schizoaffective disorder; SAD-Rel, relatives of probands with schizoaffective disorder; SZ, probands with schizophrenia; SZ-Rel, relatives of probands with schizophrenia; WRAT-4 IQ, general intelligence estimate based on Wide Range Achievement Test-4/reading subtest; YMRS, Young Mania Rating Scale.
Age: relatives were older than probands: B1 < B2-Rel (p = .007) and B3-Rel (p < .001), B2 < B2-Rel (p = .004) and B3-Rel (p < .001), B3 < B1-Rel (p = .04), B2-Rel (p = .001) and B3-Rel (p < .001); and B3-Rel were older than HC (p = .01).
Sex: there was a higher proportion of males among B1 compared to all relative groups: B1-Rel (χ21 = 7.57, p = .006), B2-Rel (χ21 = 13.66, p < .001), and B3-Rel (χ21 = 14.60, p < .001). Likewise, B3 had more males than all relative groups: B1-Rel (χ21 = 7.96, p = .005), B2-Rel (χ21 = 15.00, p < .001), and B3-Rel (χ21 = 16.35, p < .001). B2 had a higher proportion of males than their relatives (B2-Rel) (χ21 = 7.27, p = .007) and B3-Rel (χ21 = 7.74, p = .005). HC had a higher proportion of males relative to B2-Rel (χ21 = 4.63, p = .03) and B3-Rel (χ21 = 4.93, p = .03).
Race: B1 had a higher proportion of African Americans than whites relative to B2 (χ21 = 18.31, p < .001), B3 (χ21 = 27.98, p < .001), B2-Rel (χ21 = 34.09, p < .001), B3-Rel (χ21 = 53.92, p < .001), and HC (χ21 = 33.78, p < .001). B2 had a higher proportion of African Americans than whites compared to B1-Rel (χ21 = 6.82, p = .009) and B3-Rel (χ21 = 8.12, p = .004). B3 had a higher proportion of African Americans than whites compared to their respective relatives (B3-Rel) (χ21 = 4.18, p = .04). Likewise, B1-Rel had more African Americans than whites compared to B2-Rel (χ21 = 17.50, p < .001), B3-Rel (χ21 = 31.97, p < .001), and HC (χ21 = 16.65, p < .001).
Education: B1 had fewer years of education than B3 (p < .001), all relative groups (all p < .001), and HC (p < .001). B2 had lower education than B3, B3-Rel, and HC (all p < .001). B3 had fewer years of education than HC (p < .001). Likewise, B1-Rel (p < .001) and B2-Rel (p = .002) had lower education levels than B3-Rel and HC (both p < .001).
PANSS: total score: B1 had a higher score than B3 (p = .04). PANSS negative symptoms subscale: B1 (p < .001) and B2 (p = .02) had higher scores than B3.
GAF: all proband and relative groups scored lower than HC (all p < .001). B1 had lower scores than B2 (p = .046), B3 (p < .001) and all relative groups (all p < .001). Both B2 and B3 had lower scores than all relative groups (all p < .001). B1-Rel had lower scores than B3-Rel (p = .04).
WRAT-4 IQ: B1 had lower IQ estimates than B2 (p = .004) and B3 (p < .001), as well as B2-Rel, B3-Rel, and HC (all p < .001). B2 had lower IQ estimates than B3 (p < .001), B2-Rel (p = .02), B3-Rel (p < .001), and HC (p < .001). B3 had lower scores than B1-Rel and B2-Rel (both p < .001). B1-Rel had lower IQ estimates than B2-Rel, B3-Rel, and HC (all p < .001), and B2-Rel had lower scores than B3-Rel (p = .002).
Daily antipsychotic dose CPZ equivalents by Biotype: B1were treated with higher daily doses of antipsychotic medications than B3 (p = .02).
Age: relatives were older than probands: SZ < SZ-Rel (p < .001), SAD-Rel (p < .001), and BD-Rel (p = .02); SAD < SZ-Rel (p < .001); BD < SZ-Rel (p < .001); and SZ-Rel were older than HC (p < .001).
Sex: there was a higher proportion of males among SZ compared to SAD (χ21 = 23.73, p < .001), BD (χ21 = 46.96, p < .001), SZ-Rel (χ21 = 55.2, p < .001), SAD-Rel (χ21 = 45.58, p < .001), BD-Rel (χ21 = 46.01, p < .001), and HC (χ21 = 29.59, p < .001). HC had a higher proportion of males relative to SZ-Rel (χ21 = 4.12, p = .042).
Race: SZ had a higher proportion of African Americans than whites relative to HC (χ21 = 22.37, p < .001), BD (χ21 = 31.15, p < .001); their own relatives/SZ-Rel (χ21 = 4.18, p = .04), SAD-Rel (χ21 = 9.28, p = .002), and BD-Rel (χ21 = 52.98, p < .001). SAD had a higher proportion of African Americans than whites compared to HC (χ21 = 7.43, p = .006), BD (χ21 = 13.90, p < .001), and BD-Rel (χ21 = 27.91, p < .001). SZ-Rel had a higher proportion of African Americans than whites relative to HC (χ21 = 7.01, p = .008), BD (χ21 = 13.93, p < .001), and BD-Rel (χ21 = 29.45, p < .001). SAD-Rel had more African Americans than whites compared to BD (χ21 = 5.06, p = .02) and BD-Rel (χ21 = 14.60, p < .001). HC had more African Americans (χ21 = 8.64, p = .003) and other races (χ21 = 6.40, p = .01) than whites relative to BD-Rel. SZ had more other races than whites relative to SAD-Rel (χ21 = 4.26, p = .039) and BD-Rel (χ21 = 10.55, p = .001). SZ-Rel had more other races than whites relative to BD-Rel (χ21 = 4.41, p = .039).
Education: SZ had fewer years of education than BD (p < .001), all groups of relatives (all p < .001), and HC (p < .001). SAD had less education than BD (p < .001), SZ-Rel (p = .004), BD-Rel (p < .001), and HC (p < 0.001). BD had less education than HC (p = .005). Both SZ-Rel and SAD-Rel had less education than HC (both p < .001).
PANSS: total score: SZ and SAD had higher scores than BD (both p < .001). PANSS positive symptoms subscale: SAD had higher scores than SZ (p = .02) and BD (p < .001); SZ had higher scores than BD (p < .001). PANSS negative symptoms subscale: SZ and SAD had higher scores than BD (both p < .001). PANSS general symptoms subscale: SAD had higher scores than SZ (p = .01) and BD (p < .001); SZ had higher scores than BD (p < .001).
MADRS: SAD had higher scores than SZ and BD (both p < .001).
GAF: all probands and relatives scored lower than HC (all p < .001). SZ had lower scores than BD (p < .001) and all relative groups (all p < .001). Likewise, SAD had lower scores than BD (p < .001) and all relatives (all p < .001). BD scored lower than all relative groups (all p < .001).
WRAT-4 IQ: SZ had lower IQ estimates than BD (p < .001), SAD-Rel (p = .02), BD-Rel (p < .001), and HC (p < .001). SAD had lower IQ estimates than BD (p = .002), BD-Rel (p < .001), and HC (p < .001). In addition, SZ-Rel had lower IQ estimates than BD (p = .01), BD-Rel (p < .001), and HC (p < .001).
Daily antipsychotic dose CPZ equivalents: both SZ (p = .001) and SAD (p = .004) were treated with higher daily doses of antipsychotic medications compared to BD.
Magnetic Resonance Imaging Acquisition and Processing
T1-weighted structural images were acquired on 3T magnets across five sites; all subjects at each site were scanned on the same magnet for the duration of the study. T1-weighted magnetization prepared rapid acquisition gradient-echo or inversion recovery-prepared spoiled gradient recoil sequences, as appropriate for scanner brands, were administered following the Alzheimer’s Disease Neuroimaging Initiative protocol (available at http://adni.loni.usc.edu/methods/documents/mri-protocols/). The sequence parameters and quality control procedures are detailed in Supplemental Methods.
Whole brain GMD voxelwise analyses with subsequent regional characterization and histogram techniques were used to investigate global and regional GMD biomarkers. The voxelwise analyses were carried out using optimized voxelbased morphometry (41) toolbox (VBM8) for SPM8 (available at http://www.fil.ion.ucl.ac.uk/spm/software/spm8), and incorporated the diffeomorphic anatomical registration through exponentiated lie algebra, a high-dimensional nonlinear intersubject registration tool (42,43) (Supplemental Methods). To capture subject-level GMD characteristics and distribution patterns across the Biotype and DSM-IV-TR constructs, we implemented the histogram analysis (44,45) (detailed in Supplemental Methods).
Statistical Analyses
Group-level imaging analyses were carried out using a series of standard SPM8 full factorial models for each classification approach (i.e., Biotype and DSM-IV-TR), conducted separately in probands versus HC and relatives versus HC. The distinctive group demographic characteristics that are known to affect brain structure (i.e., age, sex, and handedness) (46,47) and site were included as covariates (specified as continuous or categorical regressors, as appropriate) in all primary and exploratory statistical models. Absolute threshold masking was set at 0.1 to retain voxels with low GMD estimates. Cross-site data compatibility analyses are detailed in Supplemental Methods. All primary outcomes in probands and relatives by Biotype and diagnosis were tested at p < .01, using voxelwise familywise error (FWE) correction for multiple comparisons, with clusters of ≥20 contiguous voxels included into resulting t maps (k ≥ 20), consistent across F and t contrasts. To capture subtle structural alterations in relatives, we also performed exploratory analyses at a less stringent threshold (p < .05, voxelwise false discovery rate [FDR] correction, k ≥ 20). In addition, to examine GMD biomarkers unique to relatives unaffected by lifetime psychosis, exploratory analyses in subgroups of relatives without Axis I psychotic disorders or Axis II psychosis spectrum personality disorders (e.g., schizotypal, schizoid, or paranoid) were conducted (n = 487) (Supplemental Table S2).
Regional labeling and statistical outputs were identified using SPM8 and the Group ICA for Functional Magnetic Resonance Imaging Toolbox [GIFT1.3i (48); available at www.sourceforge.net]. Effect sizes for between-group GMD differences were estimated using Cohen’s d derived from t distribution statistics (49). In addition, subject-level regional GMD were sampled from unsmoothed modulated images by defining 5-mm-radius spheres around the peak voxel of significant clusters as identified by GIFT1.3i; descriptive GMD characteristics are reported for the top 10 regions with the highest effect sizes based on the primary (p < .01, FWE, k ≥ 20) threshold.
To further examine predictive features of Biotypes and conventional diagnoses on GMD, we carried out a stepwise regression analysis in IBM SPSS software (version 23; SPSS Inc., Chicago, IL). In this analysis, we first fit Biotype as a predictor for GMD, then added DSM-IV-TR, and finally fit Biotype, DSM-IV-TR, and all covariates used in the primary analyses. We also pursued a reversed stepwise model where DSM-IV-TR was fitted first, followed by Biotype, and, lastly, by DSM-IV-TR, Biotype, and covariates. We conducted these analyses in probands and relatives separately (excluding HC).
Finally, we explored associations between active medication status and GMD in all probands combined contrasting subjects on (n = 469) versus off (n = 82) antipsychotics, antidepressants (on, n = 242; off, n = 309), any mood stabilizers (on, n = 258; off, n = 293), and BD alone on (n = 45) versus off (n = 130) lithium (p < .05, FDR, k ≥ 20). Associations between GMD and daily antipsychotic dose chlorpromazine equivalents (50) were examined in probands with available dose-level medication data (n = 355), using the SPM8 multiple regression model.
RESULTS
Gray Matter Density Biomarkers Across the Biotype Constructs
Probands: Biotypes Versus HC
Probands grouped by the Biotype versus HC model detected a significant between-group effect (F3795 = 10.88, p < .01, FWE, k ≥ 20). Subsequent pairwise comparisons revealed a stepwise pattern of GMD reductions among Biotypes: in B1, broadly distributed cortical and subcortical reductions (t = 5.0–10.3, d = 0.521.06, mean d = 0.77), with the largest effect sizes in bilateral frontal, anterior/middle cingulate cortex, and temporal regions; in B2, intermediate in magnitude GMD reductions (t = 5.1–9.2, d = 0.49–0.89, mean d = 0.66) regionally overlapping with B1 but more sparsely distributed, with the largest effects in insula, frontotemporal, and cingulate regions; and in B3, localized reductions limited to bilateral anterior limbic regions (t = 4.9–6.6, d = 0.45–0.61, mean d = 0.52) (Table 2; Figure 1A and B; Supplemental Table S3). No increases in GMD were detected in any Biotype versus HC.
Table 2.
Gray Matter Structure Biomarkers in Biotypes and Conventional Diagnoses
| Biomarker-Based Biotype
Constructs |
Symptom-Based Conventional
Diagnoses |
|||||
| Biotypes vs. HC | Diagnoses vs. HC | |||||
| Probands | Probands | |||||
| B1 | B2 | B3 | SZ | SAD | BD | |
| HC | ↓↓↓ | ↓↓ | ↓ | ↓↓ | ↓↓ | ↓ |
| Relatives | Relatives | |||||
| B1-Rel | B2-Rel | B3-Rel | SZ-Rel | SAD-Rel | BD-Rel | |
| HC | ↓/Anterior | *↓/Posterior | = | 4 | ↓↓ | = |
| Biotypes Separation |
Biotypes Separation |
|||||
| Probands vs. Probands | Probands vs. Probands | |||||
| B1 | B2 | SZ | SAD | |||
| B3 | ↓↓ | ↓ | BD | ↓ | ↓↓ | |
| Relatives vs. Relatives | Relatives vs. Relatives | |||||
| B1-Rel | B2-Rel | |||||
| B3-Rel | *↓/Anterior | *↓/Posterior | No differences | |||
Arrows indicate gray matter density reductions across study groups; the number of arrows corresponds to the following mean effect sizes, based on the range of effect sizes observed in our analyses: ↓, ≤ 0.55; ↓↓, 0.56–0.74; and ↓↓↓, ≥ 0.75. Asterisks indicate mean effect size derived from the exploratory analyses in relatives (p < .05, voxelwise false discovery rate, k ≥ 20 voxels) where no effects survived the stringent primary statistical threshold; the rest of the mean effect sizes were computed based on the primary threshold (p < .01, voxelwise familywise error, k ≥ 20 voxels).
B1, Biotype 1 probands; B1-Rel, relatives of Biotype 1 probands; B2, Biotype 2 probands; B2-Rel, relatives of Biotype 2 probands; B3, Biotype 3 probands; B3-Rel, relatives of Biotype 3 probands; BD, probands with psychotic bipolar type I disorder; BD-Rel, relatives of probands with psychotic bipolar type I disorder; HC, healthy controls; SAD, probands with schizoaffective disorder; SAD-Rel, relatives of probands with schizoaffective disorder; SZ, probands with schizophrenia; SZ-Rel, relatives of SZ probands.
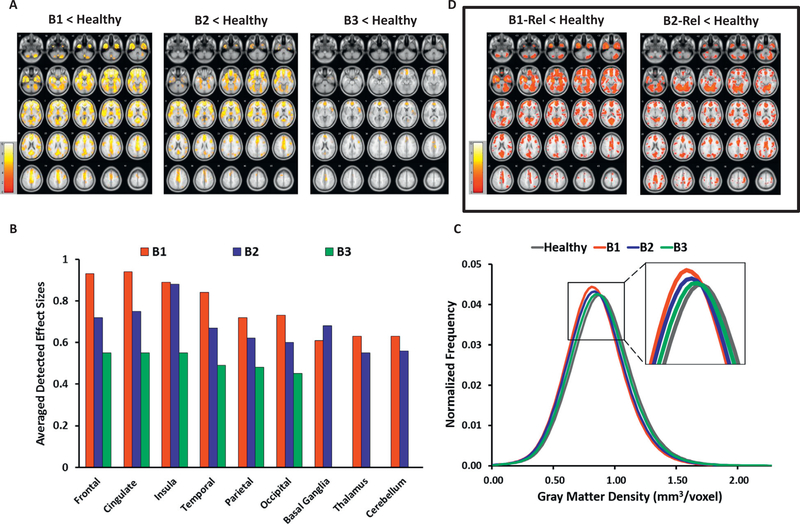
Figure 1.
Gray matter density (GMD) characteristics in probands categorized by Biotype constructs and their relatives, contrasted with healthy subjects. (A) GMD reductions in probands by Biotype vs. healthy controls (HC). The images show voxelwise t maps for GMD reductions in probands grouped by Biotype relative to HC. (B) Regional effect sizes for GMD reductions in probands by Biotype vs. HC. The detected effect sizes are averaged for large brain regions (frontal, temporal, occipital lobes, etc.) across the left and right hemispheres, except for insula (right) and occipital (left) in Biotype 3 probands, where only unilateral effects were depicted. No regional between-group effects in basal ganglia, thalamus, or cerebellum were found in Biotype 3 vs. HC. (C) Whole brain GMD distributions in probands by Biotype and HC. The histogram plots depict within-group distributions of whole brain GMD in probands grouped by Biotype vs. HC. (D) GMD reductions in relatives by Biotype vs. HC (exploratory findings). The images show voxelwise t maps for GMD reductions in first-degree relatives of probands categorized by Biotype, compared with HC. All t maps (A) and regional effect sizes (B) in probands vs. HC are reported at the primary hypotheses-testing threshold (p < .01, familywise error-corrected, k ≥ 20 voxels). All t maps (D) in relatives vs. HC are reported at the exploratory threshold (p < .05, false discovery rate-corrected, k ≥ 20 voxels); the exploratory nature of analyses in relatives is emphasized by the frame in (D). Images (A) and (D) are displayed in neurological convention; color bars indicate the ranges of t values for each between-group contrast. B1, Biotype 1 probands; B1-Rel, relatives of Biotype 1 probands; B2, Biotype 2 probands; B2-Rel, relatives of Biotype 2 probands; B3, Biotype 3 probands.
Subsequent subject-level regional GMD sampling within 5 mm of the peak voxels confirmed the stepwise pattern of GMD reductions among Biotypes (B1 < HC, Δ = 0.05–0.13 mm3/voxel, Δ% = 7.8–14.1%; B2 < HC, Δ = 0.04–0.08 mm3/voxel, Δ% = 5.7–10.0%; and B3 < HC, Δ = 0.01–0.07 mm3/voxel, %Δ = 1.9–8.6%), with the largest effects in frontal, cingulate, and temporal regions in all three Biotypes (Supplemental Table S6).
Consistent with the primary outcomes, the whole brain subject-level GMD distribution analyses revealed a stepwise histogram shift to the left—toward lower GMD—in Biotypes relative to HC, from B3 (the most HC-like), to B2 (intermediate), to B1 (the least HC-like), with all three Biotypes showing reduced GMD (B1, p < .001; B2, p < .001; and B3, p = .004) (Figure 1C). The distribution shape parameters depicted lower full width at half maximum across all Biotypes versus HC (B1, p < .001; B2, p < .001; and B3, p = .004) (Supplemental Table S7).
Relatives: Biotypes Versus HC
Relatives grouped by their respective probands’ Biotype versus HC analysis detected a significant effect of group (F3,839 = 10.95, p < .01, FWE, k ≥ 20), driven by localized GMD reductions in the temporal regions (i.e., bilateral parahippocampal gyri, left middle temporal gyrus, and uncus) in B1-Rel versus HC (t = 5.0–5.8, d = 0.51–0.59, mean d = 0.54; Δ = 0.02–0.07 mm3/voxel, Δ% = 4.4–8.2%) (Table 2; Supplemental Tables S3 and S6). At the exploratory threshold, the significant between-group effect was confirmed (F3,839 = 3.74, p < .05, FDR, k ≥ 20), with pairwise contrasts showing distinctive regional GMD characteristics: reductions in B1-Rel were broadly distributed, with the largest effect sizes in bilateral anterior (frontotemporal and cingulate) regions (t = 2.2–5.0, d = 0.22–0.59, mean d = 0.37); GMD loss in B2-Rel was predominantly posterior (over cerebellum, visual and auditory sensory cortices) (t = 2.3–4.6, d = 0.22–0.45, mean d = 0.33); while GMD in B3-Rel was not different from HC (Table 2; Figure 1D; Supplemental Table S3). When relatives affected by lifetime psychosis spectrum disorders were removed, B1-Rel still showed lower GMD than HC, localized to the left temporal cortex (parahippocampal, middle temporal, and inferior temporal gyri) and posterior cingulate (t = 3.7–4.4; d = 0.41–0.49; mean d = 0.44) (Supplemental Table S3; Supplemental Figure S1A). No increases in GMD were detected in any relative group versus HC.
The histogram analyses revealed a pattern of GMD distributions in relatives similar to their respective probands, with lower GMD and FWHM in B1-Rel (p < .001; p = .002) and B2-Rel (p < .001; p = .003) versus HC, but with a lesser between-group separation (Supplemental Figure S2A; Supplemental Table S7).
Gray Matter Density Biomarkers Across Conventional Diagnoses
Probands: DSM Diagnoses Versus HC
Contrasting probands by conventional diagnoses versus HC detected a significant between-group effect (F3,795 = 10.90, p < .01, FWE, k ≥ 20). Pairwise comparisons revealed regionally overlapping and comparable in magnitude GMD reductions in SZ (t = 5.0–9.8, d = 0.44–0.88, mean d = 0.66) and SAD (t = 5.2–9.1, d = 0.55–0.95, mean d = 0.73) versus HC spanning broad cortical and subcortical regions, with the largest effect sizes in bilateral inferior and middle frontal, cingulate, and insula cortices. BD showed small clusters of GMD reduction primarily localized to frontal and cingulate regions (t = 4.9–6.3, d = 0.48–0.62, mean d = 0.54) (Table 2; Figure 2A and B; Supplemental Table S4). No increases in GMD were detected in any proband group versus HC.
Figure 2.
Gray matter density (GMD) characteristics in probands grouped by DSM-IV-TR diagnoses and their relatives, contrasted with healthy controls (HC). (A) GMD reductions in probands by DSM-IV-TR diagnoses vs. HC. The images show voxelwise t maps for GMD reductions in probands grouped by conventional diagnoses relative to HC. (B) Regional effect sizes for GMD reductions in probands by DSM-IV-TR diagnoses vs. HC. The detected effect sizes are averaged for large brain regions (frontal, temporal, occipital lobes, etc.) across the left and right hemispheres, except for occipital (left) in bipolar probands, where only unilateral effect was depicted. No regional between-group effects in basal ganglia, thalamus, or cerebellum were found in bipolar probands vs. HC. (C) Whole brain GMD distributions in probands by DSM-IV-TR diagnoses and HC. The histogram plots depict within-group distributions of whole brain GMDs in probands grouped by conventional diagnoses vs. HC. (D) GMD reductions in relatives by DSM-IV-TR diagnoses vs. HC (exploratory findings). The images show voxelwise t maps for GMD reductions in first-degree relatives of probands categorized by conventional diagnoses, compared with HC. All t maps (A) and regional effect sizes (B) in probands vs. HC are reported at the primary hypotheses-testing threshold (p < .01, familywise error-corrected, k ≥ 20 voxels). All t maps (D) in relatives vs. HC are reported at the exploratory threshold (p < .05, false discovery rate-corrected, k ≥ 20 voxels); the exploratory nature of analyses in relatives is emphasized by the frame in (D). Images (A) and (D) are displayed in neurological convention; color bars indicate the ranges of t values for each between-group contrast. BD, psychotic bipolar disorder type I probands; SAD, schizoaffective disorder probands; SAD-Rel, relatives of SAD probands; SZ, probands with schizophrenia; SZ-Rel, relatives of SZ probands.
This pattern of GMD changes (i.e., similar reductions in SZ/SAD and rather preserved GMD in BD) was confirmed with the regional GMD sampling (SZ < HC, Δ = 0.03–0.12 mm3/voxel, Δ% = 5.5–12.5%; SAD < HC, Δ = 0.05–0.09 mm3/voxel, Δ% = 7.1–11.3%; and BD < HC, Δ = 0.01–0.07 mm3/voxel, %Δ = 1.7–9.5%), with the largest effects in frontal, cingulate, and insula regions (Supplemental Table S6).
The histogram analysis confirmed highly overlapping GMD distributions in SZ and SAD shifted to the left—toward lower GMD values—while the GMD distribution among bipolar cases was more similar to HC (SZ < HC, p < .001; SAD < HC, p < .001; and BD < HC, p = .003) (Figure 2C; Supplemental Table S7). The distribution shape characteristics depicted lower FWHM in all proband groups versus HC (SZ < HC, p < .001; SAD < HC, p < .001; and BD < HC, p = .009) (Supplemental Table S7).
Relatives: DSM Diagnoses Versus HC
Analyses in relatives by DSM versus HC showed a significant effect of group (F3,839 = 10.94, p < .01, FWE, k ≥ 20), accounted for by a few isolated clusters of lower GMD in the left parahippocampal gyrus and right cerebellum in SZ-Rel (t = 5.0–5.6, d = 0.45–0.50, mean d = 0.47; Δ = 0.02–0.03 mm3/voxel, Δ% = 2.9–4.0%) and in the left parahippocampal gyrus in SAD-Rel (t = 5.5, d = 0.56; Δ = 0.02 mm3/voxel, Δ% = 2.2%) (Table 2; Supplemental Tables S4 and S6). At the exploratory threshold, this between-group effect was confirmed (F3,839 = 4.57, p < .05, FDR, k ≥ 20). Pairwise contrasts captured broader bilateral GMD reductions in SZ-Rel (t = 2.2–5.6, d = 0.2–0.5, mean d = 0.33) and SAD-Rel (t = 2.6–5.5, d = 0.26–0.56, mean d = 0.36) versus HC, regionally similar to the findings in probands, although less extensive (Figure 2D; Supplemental Table S4). No GMD changes were found in BD-Rel versus HC. After removing relatives with lifetime psychotic disorders, SZ-Rel showed a few scattered clusters of GMD reduction (in the left cingulate, parahippocampal gyrus, and the right cerebellum) versus HC (t = 4.2–4.9, d = 0.40–0.46, mean d = 0.42) (Supplemental Table S4; Supplemental Figure S1B); no effects were detected in either SAD-Rel or BD-Rel.
The histogram analyses revealed a similar pattern of GMD distributions among relatives, with the left shift in SZ-Rel (p < .001) and SAD-Rel (p < .001), and lower FWHM in SZ-Rel (p < .001), but no differences in BD-Rel, compared to HC (Supplemental Figure S2B; Supplemental Table S7).
Biotype and DSM-IV-TR Diagnosis Groups Separation Based on GMD
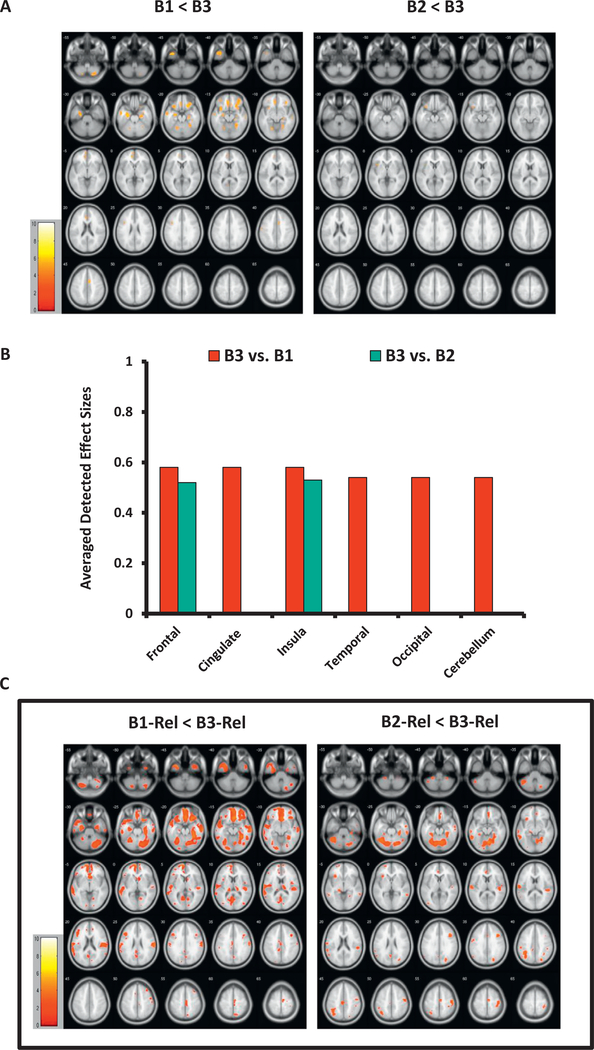
Within-Biotype Proband Comparisons
The Biotype groups showed strong separation from each other among both probands and relatives, with unique regional distribution of GMD differences, especially in Biotype 1 versus Biotype 3 (p < .01, FWE, k ≥ 20). B1 had diminished GMD primarily in anterior (i.e., bilateral frontotemporal and cingulate) regions compared to B3 (t = 4.9–6.3, d = 0.52–0.66, mean d = 0.56; Δ = 0.04–0.09 mm3/voxel, Δ% = 5.6–10.2%). B2 showed reductions in the left inferior frontal gyrus and insula versus B3 (t = 5.2–5.3, d = 0.52–0.53, mean d = 0.53; Δ = 0.04 mm3/voxel, Δ% = 5.4–5.8%), while B1 and B2 did not separate from each other (Table 2; Figure 3A, B; Supplemental Tables S5 and S6).
Figure 3.
The Biotype groups’ separation based on gray matter structure biomarkers. (A) Gray matter density (GMD) differences among probands organized by Biotype. The images show voxelwise t maps for GMD differences among probands within the Biotype approach, with lower densities in B1 and B2 relative to B3. (B) Effect sizes for between-group separation in probands by Biotype. Detected effect sizes for B1 vs. B3 between-group differences are averaged for large brain regions (frontal, temporal, occipital lobe, etc.) across the left and right hemispheres; for B2 vs. B3, unilateral effect sizes are reported: frontal (left), insula (left). (C) GMD differences among relatives grouped by Biotype (exploratory findings). The images show voxelwise t maps for GMD reductions among relatives organized by Biotype, with lower densities in B1-Rel and B2-Rel than in B3-Rel. All t maps (A) and regional effect sizes (B) in probands are reported at the primary hypotheses-testing threshold (p < .01, familywise error-corrected, k ≥ 20 voxels). All t maps (C) in relatives are reported at the exploratory threshold (p < .05, false discovery rate-corrected, k ≥ 20 voxels); the exploratory nature of analyses in relatives is emphasized by the frame in (C). Images (A) and (C) are displayed in neurological convention; color bars indicate ranges of t values for each between-group contrast. B1, Biotype 1 probands; B1-Rel, relatives of Biotype 1 probands; B2, Biotype 2 probands; B2-Rel, relatives of Biotype 2 probands; B3, Biotype 3 probands; B3-Rel, relatives of Biotype 3 probands.
The distribution analyses confirmed lower GMD in both B1 (p < .001) and B2 (p < .001) relative to B3, and lower FWHM in B1 versus B3 (p = .001) (Figure 1C; Supplemental Table S7).
Within-Biotype Relative Comparisons
Among relatives, no significant effect of group was detected at the primary threshold. However, at the exploratory threshold (p < .05, FDR, k ≥ 20), relatives showed regionally distinct patterns of between-group differences by Biotype: in B1-Rel, diffusely distributed GMD loss with the largest clusters in the anterior (i.e., bilateral frontotemporal) regions (t = 2.5–4.8, d = 0.28–0.49, mean d = 0.36) relative to B3-Rel, and in B2-Rel versus B3-Rel, predominantly posterior (i.e., bilateral temporoparietal and cerebellar) GMD reductions (t = 2.8–4.8, d = 0.28–0.46, mean d = 0.34) (Table 2; Figure 3C; Supplemental Table S5).
The histogram analyses confirmed lower GMD in both B1-Rel (p = .007) and B2-Rel (p = .04) versus B3-Rel (Supplemental Figure S2A and Table S7).
No significant differences were detected in unaffected relatives among Biotypes contrasted with each other.
Within-Diagnosis Proband Comparisons
The DSM-IV-TR groups, across both probands and relatives, separated poorly from each other based on GM structure. Among probands (p < .01, FWE, k ≥ 20), a few small unilateral clusters of reduced GMD were found in SZ (right lingual gyrus: t = 5.6; d = 0.55; Δ = 0.01 mm3/voxel, Δ% = 2.3%) and SAD (left precuneus: t = 5.3; d = 0.6; Δ = 0.06 mm3/voxel, Δ% = 8.6%) relative to BD, whereas no differences emerged in SZ versus SAD (Table 2; Supplemental Tables S5 and S6).
The histogram parameters depicted lower GMD in SZ (p = .02) and SAD (p < .001), and lower FWHM in SAD (p = .023) relative to BD, but no separation between SZ and SAD (Figure 2C; Supplemental Table S7).
Within-Diagnosis Relative Comparisons
No differences were detected among DSM-IV-TR-based relative groups at either primary or exploratory thresholds; likewise, no effects were found in nonpsychotic relatives.
The histogram approach depicted reduced GMD estimates in both SZ-Rel (p = .001) and SAD-Rel (p = .009) compared to BD-Rel, as well as lower FWHM in SZ-Rel versus BD-Rel (p = .01) (Supplemental Figure S2B; Supplemental Table S7).
Biotype and DSM-IV-TR Constructs as Predictors for GMD Biomarkers
The results of the stepwise regression analyses (Supplemental Table S8) in probands showed that Biotype was a strong and highly significant predictor for GMD (F1,551 = 24.70, p < .0001, R2 = .043). When DSM-IV-TR was added to the model, Biotype remained a highly significant predictor (p < .0001) while the diagnosis was not (p = .18) (F2,550 = 13.29, p < .0001, R2 = .046). In the third model including Biotypes, DSM-IV-TR, and covariates (i.e., age, sex, handedness, and site), Biotype again was a highly significant predictor for GMD (p < .0001), the diagnosis was not (p = .36), and the only significant predictor among covariates was site (p = .008) (F6,546 = 6.57, p < .0001, R2 = .067). Adding DSM-IV-TR to the model did not predict GMD significantly over and above Biotype alone (ΔR2 = .003, F1,550 = 1.84, p = .18). Although adding covariates did significantly predict GMD over Biotype alone (ΔR2 = .02, F4,546 = 3.11, p = .02), the F and ΔR2 values indicate that Biotype is the strongest predictor for GMD. In relatives, Biotype was a strong and highly significant predictor for GMD (F1,591 = 13.35, p < .0001, R2 = .022). In the second model, both Biotype (p = .004) and diagnosis (p = .007) were significant predictors, albeit with overall lower model parameters (F2,590 = 10.45, p < .0001, R2 = .034). In the third model, Biotype (p = .002), diagnosis (p = .03), and site (p < .0001) were all significant predictors for GMD (F6,586 = 6.17, p < .0001, R2 = .059). Similar to probands, the first (Biotype alone) model in relatives was the strongest of the three based on F values; however, adding diagnosis in model 2 (ΔR2 = .012, F1,590 = 7.41, p = .007) and covariates in model 3 (ΔR2 = .025, F4,586 = 3.92, p = .004) did significantly predict GMD over Biotype alone. A reversed stepwise regression analysis confirmed these results (see footnote in Supplemental Table S8), supporting a stronger predictive function of Biotype than conventional diagnosis, especially among probands.
Associations Between GMD and Concomitant Medications
No associations between GMD and any of the tested concomitant treatments (i.e., antipsychotics, daily antipsychotic dose chlorpromazine equivalents, antidepressants, any mood stabilizers in all probands combined, and lithium in BD alone) were detected at either primary or exploratory thresholds.
DISCUSSION
GMD Biomarkers in Biotypes and Conventional Diagnoses
We examined GM structure biomarkers in novel disease constructs (Biotypes) alongside conventional symptom-based diagnoses in a large psychosis sample. Biotypes proved to capture neurobiologically homogeneous groups based on cognitive and neurophysiologic measures (1), and we predicted that Biotypes would also depict regionally unique brain anatomy characteristics (not used in the development of the Biotypes, therefore tested here as independent measures) and provide stronger between-group separation based on GMD than symptom-driven conventional diagnoses. Our findings showed a stepwise pattern of GMD reductions across probands categorized by Biotype versus HC, with broadly distributed, largest in magnitude reductions in B1 (mean d = 0.77), intermediate in B2 (mean d = 0.66), and small, localized reductions in B3 (mean d = 0.52). Relatives grouped by Biotype contrasted with HC had GMD reductions of a modest magnitude, with distinct regional maps across Biotypes: B1-Rel had diffusely distributed GMD loss, with the largest effects in the anterior (frontotemporal) regions (mean d = 0.37); B2-Rel, by contrast, showed predominantly posterior reductions (in cerebellum; temporal and parietal regions) (mean d = 0.33); and B3-Rel had GMD not different from HC. Notably, when relatives with lifetime psychosis spectrum disorders were removed, B1-Rel still showed GMD reductions localized to the left limbic structures (mean d = 0.44). The three Biotype groups strongly separated from each other based on GMD, and captured unique regional distributions of GMD change, with primarily anterior reductions in B1 probands (mean d = 0.56) and relatives (mean d = 0.36), compared to B3; and predominantly posterior GMD changes in B2-Rel versus B3-Rel (mean d = 0.34). Moreover, Biotype was the strongest predictor for whole brain GMD change in probands, whereas conventional diagnoses lacked the ability to predict such GMD alterations. The biomarker characteristics, including GMD tested here and cognitive and sensorimotor features (1), formed unique biological profiles within each Biotype: poor cognitive and sensorimotor function and the largest GMD reductions in B1; poor cognitive function, exaggerated sensorimotor reactivity, and intermediate GMD reductions in B2; and near normal cognition and sensorimotor reactivity and minimal GMD decrease in B3.
Among conventional diagnoses, all probands showed GMD reductions from controls, regionally overlapping, though smaller in magnitude and more localized in BD (mean d = 0.54) than in SZ (mean d = 0.66) and SAD (mean d = 0.73). Relatives grouped by their probands’ diagnoses, contrasted with healthy, showed GMD changes regionally similar to their respective probands, though less extensive (SZ-Rel [mean d = 0.33] and SAD [mean d = 0.36]), whereas BD-Rel had GMD not different from HC. A subgroup analysis in nonpsychotic relatives captured a few small clusters of GMD reduction in SZ-Rel versus HC scattered over the left cingulate, parahippocampal gyrus, and the right cerebellum (mean d = 0.42), while SAD-Rel and BD-Rel had GMD not different from HC. The symptom-driven diagnostic groups separated poorly from each other based on GM structure characteristics, with a single small cluster of GMD difference in SZ versus BD (right lingual gyrus) and, likewise, in SAD versus BD (left precuneus), and no between-group differences in relatives, even at the exploratory threshold. Diagnosis was not a significant predictor of total GMD among probands, although the results in relatives supported Biotype, conventional diagnosis, and site as all being significant predictors for total GMD, likely reflecting clinical and biological heterogeneity of our sample of relatives.
Neurophysiologic and Tissue-Level Implications
Our findings across the conventional diagnoses are consistent with our previous reports (7,13) and others (9,12,17,25). These structural changes captured with in vivo imaging are in line with postmortem tissue pathology observed in SZ and BD. Histological findings in SZ indicate an overall preservation of cortical neurons, albeit with altered neuronal cell packing, likely caused by reduced interstitial neuropil, generating higher neuronal density and reduced cortical thickness (51,52). Early postmortem studies in BD reported decreased neuronal and glial density but overall normal cortical thickness (52). A recent report by Konopaske et al. (54) showed similar reductions in dendritic spine density and dendrite length in the dorsolateral prefrontal cortex in both SZ and BD (53). These postmortem findings suggest both overlapping (i.e., dendritic changes) and distinct (i.e., overall cortical thickness) anatomical underpinnings for these symptom-driven constructs, possibly reflecting their inherent biological heterogeneity. In addition, long-term medication effects are likely to contribute to resulting tissue- and imaging-based anatomical phenotypes (31,32,34–36). It is possible that a relative GM preservation in BD could be caused by chronic treatment with lithium and that, even if a “primary” disease-associated loss of neocortical volume exists in BD, it could be obscured by a volume-enhancing effect of lithium (31,32,54), despite our negative findings for active lithium use on GMD in this dataset.
To date, no tissue- or molecular-level studies have examined Biotypes or similar biologically driven psychosis constructs. Nevertheless, the unique mapping of GM alterations across Biotypes (i.e., primarily frontotemporal GMD loss in B1 [and their relatives] and B2, and distinct posterior GMD effects in B2-Rel) are largely consistent with cognitive and sensorimotor profiles of the Biotype constructs. Broad, overlapping GMD reductions in the frontotemporal and parietal regions in both B1 and B2 are consistent with the poor cognitive function observed in these probands. Such associations between frontotemporal GM alterations and cognitive dysfunction have been previously reported in the psychosis samples (55–58). In addition, frontotemporal as well as subcortical (e.g., thalamus) and posterior (temporo-occipital) sensory regions have been implicated in sensorimotor processing captured with source localization paradigms in healthy and psychotic populations (59,60). It is possible that GM reductions in these regions in both B2 probands and their relatives can represent an anatomical substrate for exaggerated sensorimotor reactivity characteristic for this Biotype. Altered cell packaging and synaptic changes in regional inhibitory circuitries [e.g., N-methyl-D-aspartate-mediated gamma-aminobutyric acidergic inhibitory interneuron-pyramidal cell assemblies in temporal cortex (61)], along with diminished inhibitory frontal effects onto primary sensory cortices (62,63), may result in exaggerated neural activity captured by electroencephalography-based sensorimotor discriminant function in the B2 construct, similar to previous observations in SZ (64–66). Our finding of GMD loss in unaffected B1-Rel localized to the left limbic structures—the brain system implicated in psychosis formation (67–69)—supports these structural alterations as a potentially heritable biomarker captured by Biotype constructs, paving the way for future genetic studies. Unaffected SZ-Rel also showed a few clusters of GMD reduction, though smaller in magnitude and regionally inconsistent with those in B1-Rel. Therefore, it is unlikely that the structural alterations captured by Biotypes can be simply explained by a slight predominance of SZ cases in B1 in our sample. This finding highlights unexplored subgroups of relatives with unique neurobiological profiles, such as those captured by Biotypes.
Biomarker-Based Approaches in Complex Brain Disorders: Implications for Biotypes
The Biotype constructs provide proof of concept that structural brain characteristics, along with an overall biomarker battery, can reclassify psychotic individuals into groups that are neurobiologically distinctive and appear biologically meaningful. In addition, independent biomarker validators—GM structure as in vivo proxy for tissue-level abnormalities underlying psychotic illness—depict unique brain anatomy characteristics in Biotypes, consistent with their cognitive and sensorimotor profiles. The Biotype constructs are experimental and need to be further tested to show both reproducibility and longitudinal stability. Building on these initial analyses, the brain structure and other independent validators for Biotypes will have to be confirmed in independent samples. Nevertheless, it is likely that Biotypes or similar neurobiological parsing approaches will contribute to defining biological correlates for severe mental illness. Whether these biological constructs become clinically useful depends on their ability to support diagnostic, treatment, and functional disease outcome distinctions. These biological constructs are complementary to DSM definitions and extend clinical diagnoses to underlying neurobiology, providing more targeted, “closer to the brain” disease units. Similar approaches have been successfully applied in other fields of medicine (70–72) and have proven useful in capturing biologically distinct disorders underlying remarkably similar clinical phenotypes. These approaches may be highly advantageous for understanding the neurobiological architecture of complex brain disorders, providing paths for individualized biomarker-based treatments.
Study Limitations and Future Directions
This study has limitations, many of which are beyond the scope of this report, but they are being addressed in our ongoing work. The psychosis subjects included in this sample are by and large chronically ill and medicated. This introduces potential confounds related to medical disease burden, lifetime substance use, and chronic medication effects. Subjects with organic brain disorders, decompensated medical conditions, and active substance use were excluded. Although it was not feasible to account for lifetime chronic treatments in this cross-sectional design, we did test associations between GMD and active medication use, and the results were negative, consistent with our previous report (7). The findings generated by the whole brain (voxel-based morphometry) methodology need to be expanded by region-of-interest approaches looking into finer anatomical characteristics. These data will be complemented by extensive FreeSurfer-based morphometric quantification, the examination of structural connectivity, and brain structure–function interactions. In addition, the cross-sectional nature of the study leaves open the question about longitudinal stability of Biotypes in terms of their disease course, response to treatment, and functional outcomes. This will be addressed in our ongoing and future investigations, along with testing reproducibility of Biotypes in a large independent psychosis sample; expanding Biotype definitions with additional biomarker and environmental and disease-associated (e.g., those pertinent to socioeconomic background or childhood trauma) measures; clinical characterization of Biotypes exploring their unique and overlapping clinical features; genotyping Biotypes; and examining the mediating role of individual biomarkers in the complex gene-environment interactions.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the National Institute of Mental Health Grant Nos. MH077851 (to CAT), MH078113 (to MSK), MH077945 (to GDP), MH077852 (to Gunvant K. Thaker), and MH077862 (to JAS). The National Institute of Mental Health had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
We thank Gunvant K. Thaker, M.D., at the Maryland Psychiatric Research Institute, University of Maryland, who was closely involved with the initial stages of the Bipolar-Schizophrenia Network for Intermediate Phenotypes consortium development and its conceptual and methodological aspects. We also thank Erin Van Enkevort, Ph.D., at the University of Texas Southwestern Medical Center for help with the statistical analyses; and Dorothy Denton, B.A., at the University of Texas Southwestern Medical Center for assistance with the manuscript preparation and formatting. Finally, we thank all clinicians for patients’ referral, the patients themselves, and their families for participation in this study.
JAS has served on advisory boards for Bristol-Myers Squibb, Eli Lilly, Pfizer, Roche, and Takeda, and has received grant support from Janssen. MSK has received research support from Sunovion and GlaxoSmithKline. CAT has served on the advisory board for drug development for IntraCellular Therapies, Inc.; has served as an ad hoc consultant for Eli Lilly, Sunovion, Astellas, Pfizer, and Merck; has been a council member and unpaid volunteer for the National Alliance on Mental Illness; and has served as deputy editor for the American Psychiatric Association.
All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2016.08.030.
Contributor Information
Elena I. Ivleva, University of Texas Southwestern Medical Center, Dallas
Brett A. Clementz, University of Georgia, Athens, Georgia
Anthony M. Dutcher, University of Texas Southwestern Medical Center, Dallas
Sara J.M. Arnold, University of Texas Southwestern Medical Center, Dallas
Haekyung Jeon-Slaughter, University of Texas Southwestern Medical Center, Dallas.
Sina Aslan, Advance MRI, LLC, Frisco; University of Texas at Dallas, Richardson, Texas.
Bradley Witte, University of Texas Southwestern Medical Center, Dallas.
Gaurav Poudyal, University of Texas Southwestern Medical Center, Dallas.
Hanzhang Lu, University of Texas Southwestern Medical Center, Dallas; Johns Hopkins University, Baltimore, Maryland.
Shashwath A. Meda, Institute of Living/Hartford Hospital, Hartford
Godfrey D. Pearlson, Institute of Living/Hartford Hospital, Hartford Yale School of Medicine, New Haven, Connecticut.
John A. Sweeney, University of Texas Southwestern Medical Center, Dallas
Matcheri S. Keshavan, Beth Israel Deaconess Hospital, Harvard Medical School, Boston, Massachusetts
Carol A. Tamminga, University of Texas Southwestern Medical Center, Dallas
REFERENCES
- 1.Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. (2015): Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry 173:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thaker GK (2008): Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull 34:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Insel TR, Cuthbert BN (2015): Medicine. Brain disorders? Precisely. Science 348:499–500. [DOI] [PubMed] [Google Scholar]
- 4.Cuthbert BN (2015): Research Domain Criteria: Toward future psychiatric nosologies. Dialogues Clin Neurosci 17:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Insel TR (2014): The NIMH Research Domain Criteria (RDoC) Project: Precision medicine for psychiatry. Am J Psychiatry 171:395–397. [DOI] [PubMed] [Google Scholar]
- 6.Honea RA, Meyer-Lindenberg A, Hobbs KB, Pezawas L, Mattay VS, Egan MF, et al. (2008): Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry 63:465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivleva EI, Bidesi AS, Keshavan MS, Pearlson GD, Meda SA, Dodig D, et al. (2013): Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry 170:1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulshoff Pol HE, van Baal GC, Schnack HG, Brans RG, van der Schot AC, Brouwer RM, et al. (2012): Overlapping and segregating structural brain abnormalities in twins with schizophrenia or bipolar disorder. Arch Gen Psychiatry 69:349–359. [DOI] [PubMed] [Google Scholar]
- 9.Yu K, Cheung C, Leung M, Li Q, Chua S, McAlonan G (2010): Are bipolar disorder and schizophrenia neuroanatomically distinct? An anatomical likelihood meta-analysis. Front Hum Neurosci 4:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannon TD, van Erp TG, Huttunen M, Lonnqvist J, Salonen O, Valanne L, et al. (1998): Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry 55:1084–1091. [DOI] [PubMed] [Google Scholar]
- 11.Gong Q, Lui S, Sweeney JA (2016): A selective review of cerebral abnormalities in patients with first-episode schizophrenia before and after treatment. Am J Psychiatry 173:232–243. [DOI] [PubMed] [Google Scholar]
- 12.Honea R, Crow TJ, Passingham D, Mackay CE (2005): Regional deficits in brain volume in schizophrenia: A meta-analysis of voxelbased morphometry studies. Am J Psychiatry 162:2233–2245. [DOI] [PubMed] [Google Scholar]
- 13.Ivleva EI, Bidesi AS, Thomas BP, Meda SA, Francis A, Moates AF, et al. (2012): Brain gray matter phenotypes across the psychosis dimension. Psychiatry Res 204:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Deng W, Yao L, Xiao Y, Li F, Liu J, et al. (2015): Brain structural abnormalities in a group of never-medicated patients with long-term schizophrenia. Am J Psychiatry 172:995–1003. [DOI] [PubMed] [Google Scholar]
- 15.McDonald C, Bullmore E, Sham P, Chitnis X, Suckling J, MacCabe J, et al. (2005): Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: Computational morphometry study. Br J Psychiatry 186:369–377. [DOI] [PubMed] [Google Scholar]
- 16.Hallahan B, Newell J, Soares JC, Brambilla P, Strakowski SM, Fleck DE, et al. (2011): Structural magnetic resonance imaging in bipolar disorder: An international collaborative mega-analysis of individual adult patient data. Biol Psychiatry 69:326–335. [DOI] [PubMed] [Google Scholar]
- 17.Selvaraj S, Arnone D, Job D, Stanfield A, Farrow TF, Nugent AC, et al. (2012): Grey matter differences in bipolar disorder: A meta-analysis of voxel-based morphometry studies. Bipolar Disord 14:135–145. [DOI] [PubMed] [Google Scholar]
- 18.Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM (2008): Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry 65:1017–1032. [DOI] [PubMed] [Google Scholar]
- 19.Strasser HC, Lilyestrom J, Ashby ER, Honeycutt NA, Schretlen DJ, Pulver AE, et al. (2005): Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: A pilot study. Biol Psychiatry 57:633–639. [DOI] [PubMed] [Google Scholar]
- 20.Rasser PE, Schall U, Todd J, Michie PT, Ward PB, Johnston P, et al. (2011): Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophr Bull 37:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padmanabhan JL, Tandon N, Haller CS, Mathew IT, Eack SM, Clementz BA, et al. (2015): Correlations between brain structure and symptom dimensions of psychosis in schizophrenia, schizoaffective, and psychotic bipolar I disorders. Schizophr Bull 41:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandt CL, Doan NT, Tonnesen S, Agartz I, Hugdahl K, Melle I, et al. (2015): Assessing brain structural associations with working-memory related brain patterns in schizophrenia and healthy controls using linked independent component analysis. Neuroimage Clin 9:253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubota M, van Haren NE, Haijma SV, Schnack HG, Cahn W, Hulshoff Pol HE, et al. (2015): Association of IQ changes and progressive brain changes in patients with schizophrenia. JAMA Psychiatry 72:803–812. [DOI] [PubMed] [Google Scholar]
- 24.Soh P, Narayanan B, Khadka S, Calhoun VD, Keshavan MS, Tamminga CA, et al. (2015): Joint coupling of awake EEG frequency activity and MRI gray matter volumes in the psychosis dimension: A BSNIP study. Front Psychiatry 6:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E (2008): The anatomy of first-episode and chronic schizophrenia: An anatomical likelihood estimation meta-analysis. Am J Psychiatry 165: 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TG, et al. (2015): Progressive reduction in cortical thickness as psychosis develops: A multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry 77:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, et al. (2009): Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry 66:366–376. [DOI] [PubMed] [Google Scholar]
- 28.Chan RC, Di X, McAlonan GM, Gong QY (2011): Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: An activation likelihood estimation meta-analysis of illness progression. Schizophr Bull 37:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC (2011): Progressive brain change in schizophrenia: A prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry 70: 672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, et al. (2005): Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry 62:361–370. [DOI] [PubMed] [Google Scholar]
- 31.Bearden CE, Thompson PM, Dalwani M, Hayashi KM, Lee AD, Nicoletti M, et al. (2007): Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol Psychiatry 62:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore GJ, Cortese BM, Glitz DA, Zajac-Benitez C, Quiroz JA, Uhde TW, et al. (2009): A longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patients. J Clin Psychiatry 70: 699–705. [DOI] [PubMed] [Google Scholar]
- 33.Lyoo IK, Dager SR, Kim JE, Yoon SJ, Friedman SD, Dunner DL, et al. (2010): Lithium-induced gray matter volume increase as a neural correlate of treatment response in bipolar disorder: A longitudinal brain imaging study. Neuropsychopharmacology 35:1743–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V (2011): Long-term antipsychotic treatment and brain volumes: A longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 68:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S (2013): Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev 37:1680–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vita A, De Peri L, Deste G, Barlati S, Sacchetti E (2015): The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: Does the class matter? A meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol Psychiatry 78:403–412. [DOI] [PubMed] [Google Scholar]
- 37.Eack SM, Hogarty GE, Cho RY, Prasad KM, Greenwald DP, Hogarty SS, et al. (2010): Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: Results from a 2-year randomized controlled trial. Arch Gen Psychiatry 67:674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, et al. (2013): Clinical phenotypes of psychosis in the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry 170:1263–1274. [DOI] [PubMed] [Google Scholar]
- 39.First MB, Spitzer RL, Gibbon M, Williams JBW (1996): Structured Clinical Interview for DSM-IV Axis I Disorders/Patient Edition (SCID-I/ P). New York: New York State Psychiatric Institute, Biometrics Research Department. [Google Scholar]
- 40.Pfohl B, Blum N, Zimmerman M (1997): Structured Interview for DSM-IV Personality (SIDP). Iowa City, IA: Department of Psychiatry, University of Iowa. [Google Scholar]
- 41.Ashburner J, Friston KJ (2000): Voxel-based morphometry—the methods. Neuroimage 11(6 pt 1):805–821. [DOI] [PubMed] [Google Scholar]
- 42.Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- 43.Kurth F, Luders E, Gaser C (2010): VBM8-toolbox manual. Available at: http://dbm.neuro.uni-jena.de/vbm8/VBM8-Manual.pdf; Accessed August 17, 2016.
- 44.Benson RR, Meda SA, Vasudevan S, Kou Z, Govindarajan KA, Hanks RA, et al. (2007): Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. J Neurotrauma 24:446–459. [DOI] [PubMed] [Google Scholar]
- 45.Marquez de la Plata CD, Yang FG, Wang JY, Krishnan K, Bakhadirov K, Paliotta C, et al. (2011): Diffusion tensor imaging biomarkers for traumatic axonal injury: Analysis of three analytic methods. J Int Neuropsychol Soc 17:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W, van Tol MJ, Li M, Miao W, Jiao Y, Heinze HJ, et al. (2012): Regional specificity of sex effects on subcortical volumes across the lifespan in healthy aging. Hum Brain Mapp 35:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renteria ME (2012): Cerebral asymmetry: A quantitative, multifactorial, and plastic brain phenotype. Twin Res Hum Genet 15:401–413. [DOI] [PubMed] [Google Scholar]
- 48.Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001): A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapping 14:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenthal R, Rosnow RL, Rubin DB (2000): Contrasts and Effect Sizes in Behavioral Research: A Correlational Approach. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 50.Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC (2010): Antipsychotic dose equivalents and dose-years: A standardized method for comparing exposure to different drugs. Biol Psychiatry 67: 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Selemon LD, Goldman-Rakic PS (1999): The reduced neuropil hypothesis: A circuit based model of schizophrenia. Biol Psychiatry 45:17–25. [DOI] [PubMed] [Google Scholar]
- 52.Selemon LD, Rajkowska G (2003): Cellular pathology in the dorsolateral prefrontal cortex distinguishes schizophrenia from bipolar disorder. Curr Mol Med 3:427–436. [DOI] [PubMed] [Google Scholar]
- 53.Konopaske GT, Lange N, Coyle JT, Benes FM (2014): Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry 71:1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giakoumatos CI, Nanda P, Mathew IT, Tandon N, Shah J, Bishop JR, et al. (2015): Effects of lithium on cortical thickness and hippocampal subfield volumes in psychotic bipolar disorder. J Psychiatr Res 61: 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hooker CI, Bruce L, Lincoln SH, Fisher M, Vinogradov S (2011): Theory of mind skills are related to gray matter volume in the ventromedial prefrontal cortex in schizophrenia. Biol Psychiatry 70: 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karlsgodt KH, Bachman P, Winkler AM, Bearden CE, Glahn DC (2011): Genetic influence on the working memory circuitry: Behavior, structure, function and extensions to illness. Behav Brain Res 225: 610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wojtalik JA, Eack SM, Pollock BG, Keshavan MS (2012): Prefrontal gray matter morphology mediates the association between serum anticholinergicity and cognitive functioning in early course schizophrenia. Psychiatry Res 204:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo X, Li J, Wang J, Fan X, Hu M, Shen Y, et al. (2014): Hippocampal and orbital inferior frontal gray matter volume abnormalities and cognitive deficit in treatment-naive, first-episode patients with schizophrenia. Schizophr Res 152:339–343. [DOI] [PubMed] [Google Scholar]
- 59.Dien J, Frishkoff GA, Cerbone A, Tucker DM (2003): Parametric analysis of event-related potentials in semantic comprehension: evidence for parallel brain mechanisms. Brain Res Cogn Brain Res 15: 137–153. [DOI] [PubMed] [Google Scholar]
- 60.Williams TJ, Nuechterlein KH, Subotnik KL, Yee CM (2011): Distinct neural generators of sensory gating in schizophrenia. Psychophysiology 48:470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzalez-Burgos G, Lewis DA (2012): NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull 38:950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Homayoun H, Moghaddam B (2007): NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27:11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farzan F, Barr MS, Levinson AJ, Chen R, Wong W, Fitzgerald PB, et al. (2010): Evidence for gamma inhibition deficits in the dorsolateral prefrontal cortex of patients with schizophrenia. Brain 133(pt 5): 1505–1514. [DOI] [PubMed] [Google Scholar]
- 64.Hamm JP, Gilmore CS, Clementz BA (2012): Augmented gamma band auditory steady-state responses: Support for NMDA hypofunction in schizophrenia. Schizophr Res 138:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, et al. (2004): Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A 101: 17288–17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flynn G, Alexander D, Harris A, Whitford T, Wong W, Galletly C, et al. (2008): Increased absolute magnitude of gamma synchrony in firstepisode psychosis. Schizophr Res 105:262–271. [DOI] [PubMed] [Google Scholar]
- 67.Mathew I, Gardin TM, Tandon N, Eack S, Francis AN, Seidman LJ, et al. (2014): Medial temporal lobe structures and hippocampal subfields in psychotic disorders: Findings from the Bipolar- Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. JAMA Psychiatry 71:769–777. [DOI] [PubMed] [Google Scholar]
- 68.Tamminga CA (2013): Psychosis is emerging as a learning and memory disorder. Neuropsychopharmacology 38:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ford JM, Morris SE, Hoffman RE, Sommer I, Waters F, McCarthy-Jones S, et al. (2014): Studying hallucinations within the NIMH RDoC framework. Schizophr Bull 40(suppl 4):S295–S304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waltman P, Pearlman A, Mishra B (2006): Interpreter of maladies: redescription mining applied to biomedical data analysis. Pharmaco-genomics 7:503–509. [DOI] [PubMed] [Google Scholar]
- 71.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. (2012): The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486:346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang Y, Han L, Yuan Y, Li J, Hei N, Liang H (2014): Gene coexpression network analysis reveals common system-level properties of prognostic genes across cancer types. Nat Commun 5: 3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.