Abstract
Background
Therapeutic drug monitoring (TDM) of antiepileptic drugs (AEDs) is often necessary to prevent associated destructive toxicities. Tandem mass spectrometry (MS/MS) with stable-isotope-labeled (SIL) internal standards (ISs) is considered the gold standard for the measurement of AEDs. This study presents the development and validation of a clinical ultra-performance liquid chromatography (U-HPLC)-MS/MS method for the concurrent measurement of gabapentin, lamotrigine, levetiracetam, monohydroxy derivative (MHD) of oxcarbazepine, and zonisamide in human serum.
Methods
To determine the optimal assay analyte range, one year of AED TDM results (n = 1,825) were evaluated. Simple protein precipitation with acetonitrile containing isotopically labeled ISs was employed. Reverse-phase U-HPLC chromatographic separation was used having a total run time of three minutes. Quantification of analytes was accomplished using electrospray ionization in positive ion mode and collision-induced dissociation MS. Assay parameters were evaluated per FDA bioanalytical guidelines.
Results
After evaluating internal patient data, the analytical measuring range (AMR) of the assay was established as 0.1–100 μg/mL. All AEDs were linear across the AMR, with R2 values ranging from 0.9988 to 0.9999. Imprecision (% coefficient of variation(CV)) and inaccuracy (% difference (DIF)) were calculated to be <20% for the lower limit of quantitation (LLOQ) and <15% for the Low, Mid, and High levels of quality controls (QC) across the AMR. All AEDs demonstrated acceptable assay parameters for carryover, stability under relevant storage conditions, matrix effects, recovery, and extraction and processing efficiency. Additionally, the assay displayed acceptable concordance to results obtained from a national reference laboratory, with Deming regression R2 of 0.99 and slope values ranging from 0.89 to 1.17.
Conclusions
A simple, cost-effective, and robust U-HPLC-MS/MS method for monitoring multiple antiepileptic drugs was developed and validated to address the clinical needs of patients at our institution.
Keywords: anti-epileptic drugs (AEDs), therapeutic drug monitoring (TDM), mass spectrometry, LC-MS/MS
Introduction
Worldwide, approximately 50 million people live with epilepsy and about one-quarter of this population is treated with antiepileptic drugs (AEDs)1. Even though newer generation AEDs, such as gabapentin (GBP), lamotrigine (LTG), levetiracetam (LEV), oxcarbazepine (OXC), and zonisamide (ZNS) may offer better safety profiles compared to older ones, they can still result in rare but destructive toxicities associated with use, including hematopoietic dysfunction, pruritus, rash, Stevens-Johnson syndrome, anxiety, agitation, suicidal ideation, hepatic failure, gastrointestinal issues, and neurologic dysfunction.2 Dosing these medications in the absence of therapeutic drug monitoring (TDM) can be challenging due to the narrow therapeutic ranges associated with these drugs, namely: GBP, 2–20 μg/mL; LTG, 3–14 μg/mL; LEV, 12–46 μg/mL; OXC, 3–35 μg/mL; and ZNS, 10–40 μg/mL.3 Compounding this difficult task is the significant variability between individuals in rates of excretion for LEV and rates of metabolism for ZNS, OXC, and LTG.4 When treatment is suboptimal, especially in adolescent groups, therapeutic compliance may need to be assessed. Additionally, some epileptic patients may have complex medical issues requiring several medications; therefore, drug-drug interactions are a common source of altered serum concentrations of AEDs. Although significant research has been devoted to AED biomarkers that predict therapeutic response, determination of AED serum levels is the first-line testing upon medication complications.5 Therefore, TDM of AEDs is often necessary to adequately and safely treat these patients.
Numerous methodologies have been developed for the measurement of AED levels in patient samples (Supplementary Table 1). The preferred method for AED TDM is liquid chromatography-tandem mass spectrometry (LC-MS/MS) using stable-isotope-labeled (SIL) internal standards (ISs). There are several considerations and competing factors to consider for implementation of a clinical TDM assay, including sensitivity, analytical measuring range (AMR), turn-around-time (TAT), specimen volume (especially in pediatric populations), technical complexity, cost effectiveness, and clinical laboratory operations. We reviewed over 50 published methods developed to measure AEDs, and only 11 utilize ultra-performance liquid chromatography-tandem mass spectrometry (U-HPLC-MS/MS), which can dramatically improve analytical run times without sacrificing peak resolution (Supplemental Table 2).6–13 Additionally, none of these reported U-HPLC methods are able to measure GBP, LTG, LEV, OXC, and ZNS simultaneously.14, 15 These five drugs/metabolites are the most frequently ordered AEDs for TDM at our institution. A multiplexed approach to measuring them would not only reduce costs by in-sourcing tests but would also offer operational efficiencies by being able to batch orders for any drug level into one run. Therefore, we sought to develop a U-HPLC-MS/MS method that could be easily implemented in a clinical laboratory at a tertiary academic medical center, with an emphasis on maintaining simplicity, low costs, and operational efficiency. Here, we present the validation of a rapid U-HPLC-MS/MS method for the simultaneous measurement of GBP, LTG, LEV, monohydroxy derivative (MHD) of OXC (the main, active OXC metabolite, also known as licarbazepine), and ZNS in serum.
Materials and Methods
Materials
Water was obtained using a Milli-Q ultrafiltration system (>18.2 megohm-cm resistivity), MilliporeSigma (Burlington, MA). Optima grade isopropanol (IPA), acetonitrile (ACN), formic acid, and methanol (MeOH) were purchased from Fisher Chemical (Pittsburgh, PA). GBP, GBP-D10, LTG, LTG-13C,15N4, LEV, LEV-D6, (±)-10,11-dihydro-10-hydroxycarbamazepine (MHD), (±)-10,11-dihydro-10-hydroxycarbamazepine (MHD)-13C6, ZNS, ZNS-13C6 were acquired from Cerilliant Corporation (Round Rock, TX) (Supplementary Figure 1). All other reagents were used without further purification and from commercial suppliers.
Sample preparation
Upon receipt, standards were stored at −80 °C as recommended by the manufacturer. IS protein precipitation stock solution was prepared in ACN at the following final concentrations and stored at −80 °C until used, undergoing only one freeze-thaw cycle: GBP-D10 (1 μg/mL), LTG-13C,15N4 (1 μg/mL), LEV-D6 (1 μg/mL), (±)-10,11-MHD-13C6 (1 μg/mL), and ZNS-13C6 (1 μg/mL).
Discarded serum samples were obtained according to an approved Institutional Review Board protocol. AED-negative serum was identified by U-HPLC-MS/MS analysis, pooled, and used to prepare calibrator and quality control (QC) samples. Calibration standards were prepared at the following concentrations: 0 μg/mL, 0.1 μg/mL, 0.5 μg/mL, 1 μg/mL, 5 μg/mL, 20 μg/mL, 50 μg/mL, and 100 μg/mL. QC standards were prepared at the following concentrations: 0.1 μg/mL (lower limit of quantification, LLOQ), 0.3 μg/mL (low), 30 μg/mL (mid), and 85 μg/mL (high). Calibration, QC serum samples, and IS protein precipitation solution were stored at −80 °C and then thawed at room temperature for use, undergoing only one freeze-thaw cycle unless otherwise noted. Twenty microliters of the serum sample was pipetted into 100 μL of protein precipitation solution (ACN with 1 μg/mL of each IS), vortex mixed for 10 s, then clarified by centrifugation (20,800 g) for 10 min. In a PFTE vial, 10 μL of the supernatant was then diluted into 90 μL of Milli-Q filtered water, vortexed for 10 s, and then placed into the autosampler, which was maintained at 15 °C. Samples were injected in 10 μL volumes per run for analyte quantification.
Analyte separation and quantification
The U-HPLC system consisted of a Waters (Milford, MA) ACQUITY UPLC I-Class System with a high-pressure binary pump, autosampler, column oven, and a flow-through needle for injections. Chromatography was performed using a reverse-phase Waters ACQUITY UPLC BEH C18 Column (2.1 × 30 mm, 1.7 μm particle size), which was maintained at 45 °C. The two mobile phases consisted of solvent A (2 mmol/L ammonium acetate in Milli-Q filtered water with 0.1% formic acid) and solvent B (2 mmol/L ammonium acetate in MeOH with 0.1% formic acid). The flow rate was 0.5 mL/min. Solvent B gradient conditions were as follows: initially set to 2% for 0.10 min, linear gradient from 2% to 12% for 0.05 min, sustained at 12% for 0.35 min, linear gradient from 12% to 50% for 1 min, linearly raised to 99% over 0.1 min, continued at 99% for 0.6 min, lowered linearly to 2% over 0.1 min, and continued at 2% for 0.7 min, for a total run time of 3.0 min. The syringe and flow through needle were cleaned between runs using a strong and a weak wash (1:1:1:1 Milli-Q filtered water/MeOH/IPA/ACN v/v/v/v and 9:1 Milli-Q filtered water:ACN v/v, respectively).
MS analysis was performed in the positive electrospray ionization mode (ESI) and collision-induced dissociation (CID) MS/MS on a Waters Xevo TQD tandem quadrupole mass spectrometer. Precursor-product ion transitions, retention time, cone voltage, and collision energy were optimized for each analyte and IS (Table 1). The following flow-dependent parameters were used: desolvation temperature, 650 °C; desolvation gas flow, 1000 L/h; capillary voltage, 3.5 kV; source temperature, 150 °C; cone gas flow, 0 L/h; extractor, 3.0 V; and RF lens, 2.5 V. Drug levels were quantified from the most abundant and reliable product ion for each analyte using Mass Lynx v4.1 (version SCN855, Waters, Milford, MA).
Table 1.
U-HPLC-MS/MS characteristics per analyte
| Analyte | Elution time (min) | Monitored transitions (m/z) | Cone (V) | Collision (V) |
|---|---|---|---|---|
|
| ||||
| Gabapentin | 0.94 | 172 → 154 | 29 | 15 |
| Gabapentin-D10 | 0.92 | 182 → 147 | 29 | 15 |
| Lamotrigine | 1.36 | 256 → 43 | 55 | 33 |
| Lamotrigine-13C,15N4 | 1.36 | 261 → 48 | 55 | 33 |
| Levetiracetam | 0.94 | 171 → 69 | 17 | 31 |
| Levetiracetam-D6 | 0.92 | 177 → 69 | 17 | 31 |
| MHD | 1.68 | 255 → 194 | 25 | 20 |
| MHD-13C6 | 1.68 | 261 → 200 | 25 | 20 |
| Zonisamide | 1.2 | 213 → 132 | 27 | 15 |
| Zonisamide-13C6 | 1.2 | 219 → 138 | 27 | 15 |
Method Validation
This assay was validated in accordance with the guidelines published by the Food and Drug Administration (FDA): Guidance for Industry, Bioanalytical Method Validation.16
Calibration curve analysis and LLOQ
Analyte calibrators, as previously described, spanning 0.1–100 μg/mL were assayed at the beginning and end of each experimental run and calibration curves were created using a 1/x2-weighted linear regression. The linearity of the calibration curve was assessed by averaging three independent runs. The LLOQ was established by meeting the FDA suggested attributes of a coefficient of variation (CV) ≤ 20% and an inaccuracy of ≤ +/−20% during the validation process.
Imprecision, inaccuracy, and carryover
Imprecision was assessed by calculating the percent coefficient of variation (% CV) and inaccuracy was determined by calculating the percent deviation (% DEV) from the theoretical concentration. Measurements for both parameters were performed at four QC levels (LLOQ, low, mid, and high) for each analyte, assessing intra-day (n = 6) and inter-day characteristics (three independent runs, total n = 18). Carryover was evaluated according to guidelines in CLSI document EP10-A2 by injecting high (85 μg/mL) and low (0.1 and 0.3 μg/mL) analyte samples in the following order: L1, L2, L3, H1, H2, L4, H3, H4, L5, L6, L7, L8, H5, H6, L9, H7, H8, L10, H9, H10, H11.17 Potential increases in the low sample values were analyzed by EP Evaluator release 9 (Data Innovations, South Burlington, VT).
Stability
Stability was performed at four QC levels (LLOQ, low, mid, and high) for each analyte using four technical replicates, assessing % difference (% DIF) between freshly made QCs and the following conditions: QC samples left at RT for 24 h prior to processing, QC samples that underwent three freeze-thaw cycles between RT (20 °C) and −80 °C prior to processing, QC samples left at −80 °C for 32 days prior to processing, and processed QC samples left in the autosampler for 24 h at 15 °C. Analytes were considered stable when the % DIF was ≤ +/−15%.
Matrix effects (ME), recovery efficiency (RE), and processing efficiency (PE)
ME, RE, and PE were assessed at three QC levels (low, mid, and high) by spiking each analyte into six specimens of drug-free pooled human serum before and after protein precipitation and comparing to neat specimens, in which drugs were spiked into 30% ACN/H2O (v/v) prior to protein precipitation. Percent results were determined by comparing the ratio of analyte to IS raw peak areas between the following groups: ME, post-precipitated spiked serum to spiked ACN/H2O; RE, pre-precipitated spiked serum to post-precipitated spiked serum; and PE, pre-precipitated spiked serum to spiked ACN/H2O.18
Method Comparison
Ten patient samples for each drug were obtained from Mayo Medical Laboratories (Rochester, MN), which utilized a LC-MS/MS method for analyte quantification. Comparison between methodologies was accomplished using Deming regression using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA)
Results
Empirical determination of assay range
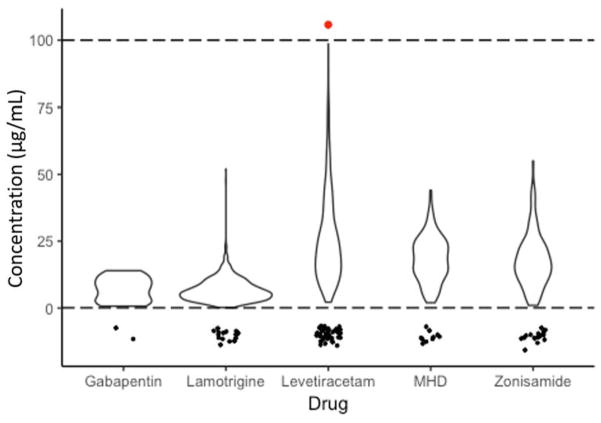
To guide our assay parameters, we analyzed antiepileptic drug level results in our patient population measured at a national reference laboratory for a period of one year (Figure 1). The average GBP serum level was 7.0 ± 4.4 μg/mL (n = 23), with two samples falling below the LLOQ of 0.5 μg/mL. For LTG, the average serum level was 6.9 ± 4.5 μg/mL (n = 902), with 17 samples falling below the LLOQ of 0.2 μg/mL. The average LEV serum level was 26.2 ± 17.0 μg/mL (n = 538), with 43 samples falling below the LLOQ of 2.0 μg/mL. MHD had an average serum level of 18.3 ± 8.5 μg/mL (n = 162), with 10 samples falling below the LLOQ of 1.0 μg/mL, and the average ZNS serum level was 17.9 ± 10.1 μg/mL (n = 111), with 17 samples falling below the LLOQ of 1.0 μg/mL. Therefore, an assay range of 0.1 μg/mL to 100 μg/mL was subsequently chosen for all analytes.
Figure 1. Violin plot of one year of AED TDM values of our patient population determined by a reference laboratory.
Based upon this analysis, lower and upper limit cutoffs of 0.1 and 100 μg/mL, represented by the dotted lines, were subsequently chosen for method development. Values below the reportable ranges of the reference lab assays are represented as points below the dashed line at 0.1 μg/mL to illustrate how many values fell below the reportable range. Note: the x-axis width of the plot at any given value is proportional to the frequency of that value.
Optimization of U-HPLC-MS/MS method
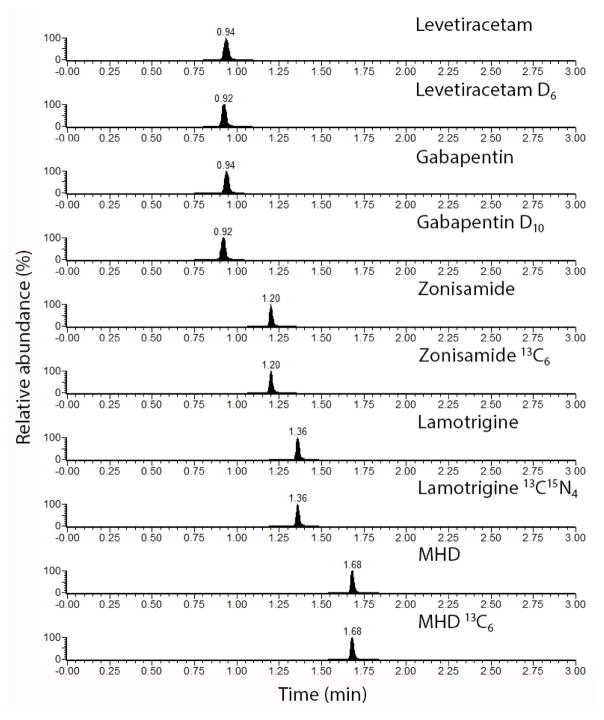
The C18 U-HPLC method was optimized to give maximum separation between analytes, in order to increase the per analyte dwell time, while simultaneously achieving a 3 min total analytical run time, including equilibration of the column (Figure 2). All analytes eluted between 0.92 and 1.68 min. MS parameters that achieved the greatest sensitivity of the AEDs were determined (Table 1).
Figure 2.
Representative U-HPLC-MRM chromatogram of all analytes and corresponding internal standards.
Imprecision, inaccuracy, and linearity
Intra and inter-day inaccuracy were within the acceptable ranges per FDA bioanalytical guidelines for all levels of QC (% DEV ≤ ±20 for the LLOQ and % DEV ≤ ±15 for low, mid, and high QC, Table 2). Likewise, intra and inter-day imprecision were also within the acceptable ranges per the FDA bioanalytical guidelines for all levels of QC (% CV ≤ 20 for the LLOQ and % CV ≤ 15 for low, mid, and high QC, Table 2). All analytes measured were linear within the AMR with R2 values ranging from 0.9988 to 0.9999 (Table 2).
Table 2.
Linearity, inaccuracy, and imprecision characteristics.
| Intra-assay (n = 6) | Inter-assay (n = 18) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Assay range (μg/mL) | Linearity (R2) | QC level | Mean (μg/mL) | Imprecision (% CV) | Inaccuracy (% Dev) | Mean (μg/mL) | Imprecision (% CV) | Inaccuracy (% Dev) | |
|
| |||||||||
| GBP | 0.1–100 | 0.9988 | LLOQ | 0.110 | 8.22 | 14.3 | 0.120 | 8.91 | 17.8 |
| Low | 0.280 | 7.30 | −7.89 | 0.310 | 14.9 | 3.65 | |||
| Mid | 34.0 | 2.16 | 13.2 | 33.4 | 3.77 | 11.4 | |||
| High | 91.1 | 5.63 | 7.20 | 89.9 | 4.35 | 5.72 | |||
|
| |||||||||
| LTG | 0.1–100 | 0.9998 | LLOQ | 0.120 | 7.96 | 16.0 | 0.120 | 7.92 | 19.7 |
| Low | 0.280 | 4.54 | −8.06 | 0.310 | 9.88 | 2.91 | |||
| Mid | 33.2 | 4.71 | 10.6 | 32.8 | 6.17 | 9.19 | |||
| High | 97.0 | 7.40 | 14.2 | 94.7 | 6.01 | 11.4 | |||
|
| |||||||||
| LEV | 0.1–100 | 0.9992 | LLOQ | 0.100 | 13.2 | 0.00 | 0.110 | 17.3 | 13.6 |
| Low | 0.330 | 8.56 | 9.67 | 0.340 | 6.48 | 15.0 | |||
| Mid | 31.2 | 2.18 | 3.95 | 31.3 | 2.02 | 4.37 | |||
| High | 85.2 | 1.14 | 0.210 | 83.0 | 2.49 | −2.40 | |||
|
| |||||||||
| MHD | 0.1–100 | 0.9999 | LLOQ | 0.120 | 4.73 | 15.5 | 0.120 | 4.33 | 16.2 |
| Low | 0.330 | 3.75 | 10.2 | 0.330 | 2.82 | 10.7 | |||
| Mid | 31.2 | 1.44 | 4.07 | 31.2 | 1.66 | 3.95 | |||
| High | 89.2 | 2.07 | 4.99 | 89.0 | 1.87 | 4.70 | |||
|
| |||||||||
| ZNS | 0.1–100 | 0.9998 | LLOQ | 0.120 | 7.50 | 18.3 | 0.120 | 11.1 | 18.4 |
| Low | 0.320 | 4.17 | 5.28 | 0.330 | 6.83 | 10.4 | |||
| Mid | 33.7 | 4.36 | 12.5 | 33.1 | 4.40 | 10.5 | |||
| High | 91.3 | 2.83 | 7.46 | 94.2 | 3.54 | 10.8 | |||
Stability and carryover
Sample stability was assessed by comparing fresh QC samples and samples that were exposed to various temperatures. All analytes were stable at RT for 24 h (% DIF ≤ 15), except for the LLOQ concentration (0.1 μg/mL) for ZNS (Table 3). All analytes were also stable for three freeze-thaw cycles (−80 °C to 20 °C defining one cycle), except for MHD at the mid level (15.27%) and ZNS at mid and high levels (16.33%, 15.33%, Table 3). Lastly, all analytes were stable at −80 °C for 32 days and for 24 h at 15 °C in the autosampler (Table 3).
Table 3.
Stability evaluation for antiepileptic drugs (AEDs).
| QC level | RT, 24 h (% DIF) | Freeze-thaw (% DIF) | Long term, 32 days (% DIF) | 15 °C for 24 h (% DIF) | |
|---|---|---|---|---|---|
|
| |||||
| GBP | LLOQ | −0.440 | 1.28 | −1.35 | 0.850 |
| Low | 7.44 | −1.25 | −6.45 | 4.06 | |
| Mid | 1.74 | 13.9 | −2.43 | 0.400 | |
| High | 2.32 | 9.77 | −8.67 | 7.32 | |
| LTG | LLOQ | −11.1 | 4.86 | 11.9 | 8.17 |
| Low | −3.63 | 2.03 | 2.28 | −2.90 | |
| Mid | 9.37 | 9.07 | 10.4 | −0.300 | |
| High | −1.13 | 9.24 | 8.96 | 0.390 | |
| LEV | LLOQ | 1.58 | −9.68 | −14.1 | −3.23 |
| Low | 6.48 | −0.920 | −7.39 | −2.89 | |
| Mid | −0.130 | 10.6 | 0.410 | −1.68 | |
| High | 2.09 | 11.8 | −0.630 | −4.05 | |
| MHD | LLOQ | −7.98 | −1.89 | −1.91 | −1.26 |
| Low | 0.460 | 2.96 | −3.04 | 0.000 | |
| Mid | 2.26 | 15.3* | 2.42 | 0.910 | |
| High | −0.610 | 11.6 | −0.140 | 2.32 | |
| ZNS | LLOQ | −18.7* | −10.23 | 0.900 | −5.60 |
| Low | 3.84 | −0.420 | −8.59 | −0.070 | |
| Mid | 2.52 | 16.3* | −2.09 | 0.330 | |
| High | 1.66 | 15.3* | −1.97 | 5.10 | |
Did not meet stability criteria
GBP, LEV, and MHD demonstrated acceptable levels of carryover between a high and low sample (85 μg/mL to 0.1 μg/mL), whereas ZNS and LTG exhibited carryover at the 85 μg/mL to 0.1 μg/mL levels, but did not at the 85 μg/mL to 0.3 μg/mL levels (data not shown). Therefore, when this method is being used to measure unknown samples, ZNS and LTG samples that have concentrations <0.3 μg/mL should be repeated if they were preceded by a sample with a high drug level.
Selectivity and matrix effects
Selectivity was assessed by analyzing pooled human serum for endogenous metabolites corresponding to the same retention times and ion mass transitions as the AEDs measured in this study. The chromatogram of the pooled serum did not demonstrate any corresponding peaks; therefore, this assay is highly selective for the measured analytes (data not shown).
Matrix effects were calculated and are summarized in Table 4. While all of the analytes demonstrate varying degrees of ion suppression, the difference between the IS and analytes is ≤ 10% and therefore the ISs behave in a similar fashion. This assay functions well for accurately quantifying AEDs.
Table 4.
Evaluation of matrix effects, recovery efficiency, and processing efficiency for antiepileptic drugs (AEDs) measured along with corresponding internal standards (IS).
| % Matrix effects(n = 6) | % Recovery efficiency(n = 6) | % Processing efficiency(n = 6) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Analyte | IS | Analyte | IS | Analyte | IS | |
|
| ||||||
| GBP | 106 | 98 | 95 | 105 | 100 | 103 |
| LTG | 105 | 106 | 91 | 97 | 95 | 104 |
| LEV | 100 | 106 | 100 | 96 | 100 | 102 |
| MHD | 104 | 104 | 97 | 98 | 101 | 102 |
| ZNS | 104 | 103 | 101 | 99 | 106 | 102 |
Method comparison
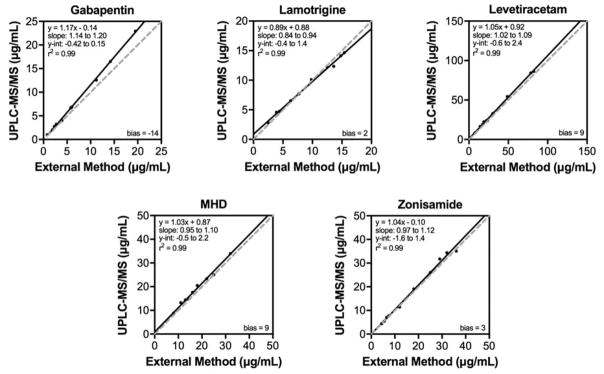
Ten samples per drug (50 in total) were obtained from a national reference laboratory, which measured the drug levels by LC-MS/MS. These values were compared to the drug levels measured by the described U-HPLC-MS/MS method (Figure 3). Using Deming regression for analysis, R2 (and slopes) of 0.99 (1.17), 0.99 (0.89), 0.99 (1.05), 0.99 (1.03), and 0.99 (1.04) were calculated for GBP, LTG, LEV, MHD, and ZNS, respectively. These results demonstrate that the two methods show an acceptable concordance.
Figure 3. Method comparison.
Drug levels measured by the described U-HPLC-MS/MS TDM assay (y-axis) and levels measured by an LC-MS/MS method at a reference laboratory (x-axis). The equation of the line, slope, y-intercept, and R2 value is displayed for each comparison. Solid line — Deming regression; dashed line — 1:1 line.
Discussion
The validated U-HPLC-MS/MS method described herein simultaneously measures human serum drug levels for GBP, LTG, LEV, MHD, and ZNS over the range of 0.1–100 μg/mL. It improves upon previously reported methodologies as this is the first report of a multiplex AED TDM assay of these drugs that uses isotopic standards for each analyte (see Supplementary Tables 1 & 2). Additionally, we simultaneously achieved shorter run times, smaller sample volumes, and a wider AMR than the vast majority of methodologies published. Most importantly, we purposely optimized this assay for qualities desired by a typical hospital laboratory, balancing speed, workflow simplicity, and operational throughput while minimizing reagent and labor costs.19 We established a suitable AMR for our patient population by analyzing one year of AED TDM results. Taking into account all analytes in the panel, the lowest measured therapeutic range value was 2 μg/mL (GBP) and our highest was 46 μg/mL (LEV). From our analysis of one year of AED TDM values, 4.9% (89/1825) of AED serum values were below the reference lab’s reportable range. To maximize information to clinicians for dosing adjustment changes and minimize retesting required for diluting samples above our reported range, we chose to develop an assay with an AMR from 0.1 to 100 μg/mL.
We also recognized that clinicians at our institution would benefit tremendously from a fast turn-around time (TAT) to rapidly manage patients with toxic and sub-therapeutic drug levels. We were able to minimize assay time by implementing a one-step protein precipitation with ACN and designing a U-HPLC method that allows for a 3 min total run time, which includes column re-equilibration. An additional advantage of this assay is that it only requires 20 μL of sample volume; this enables testing on neonatal and pediatric populations and reduces reagent costs.
As a clinical laboratory at a tertiary academic medical center, one of our main goals was to develop a cost-effective assay. Therefore, we used the same liquid chromatography system as the assays already implemented in the laboratory to maximize use of common reagents and standard operating procedures. We also chose to prepare our samples using a rapid protein precipitation protocol to decrease the complexity and hands-on time for this assay. By multiplexing five analytes into one assay, staffing requirements are reduced to one station, which minimizes labor expenses. Lastly, by establishing an in-house assay, we are now able to save on reference laboratory send out costs.
Although our method has many advantages, it has a few limitations as well. U-HPLC-MS/MS instrumentation has a relatively high capital cost; however, with the significant improvements on throughput due to shortened run times, clinical laboratories can establish more TDM assays on the same equipment compared to LC systems. Additionally, this methodology is adaptable to LC-MS/MS systems. To balance need with reagent costs, we specifically chose the five highest-volume AED analytes for our patient population that were amenable to ESI positive ion mode MS. Therefore, other AED TDM requests would have to be sent to a reference laboratories. Finally, although we used isotopes of all the analytes as ISs to compensate for the minimally observed matrix effects, the deuterated drug analogs had a slightly shorter retention time compared to their non-deuterated counterparts, which is a well-known phenomenon.20 Upon commercial availability, use of 13C and 15N isotopes of LEV and GBP as ISs could further minimize potential matrix effects.
Conclusion
We have developed and validated a cost-effective, robust, simple, and rapid U-HPLC-MS/MS method for TDM of common AEDs in human serum. We expect this method can be implemented in clinical laboratories performing mass spectrometry.
Supplementary Material
Acknowledgments
We would like to thank Waters Corporation for supporting this study. Additionally, we would like to acknowledge funding support for MJ Palte and SS Basu from the NHLBI of the National Institutes of Health under award number T32HL007627.
References
- 1.World Health Organization. [Accessed March 6, 2017];Epilepsy: Fact Sheet [WHO Web site] 2017 Feb; Available at: http://www.who.int/mediacentre/factsheets/fs999/en/
- 2.French J, Gazzola D. New generation antiepileptic drugs: what do they offer in terms of improved tolerability and safety? Ther Adv Drug Saf. 2011;2:141–158. doi: 10.1177/2042098611411127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krasowski M. Therapeutic drug monitoring of the newer anti-epilepsy medications. Pharmaceuticals (Basel) 2010;3:1909–1935. doi: 10.3390/ph3061908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Micromedex Solutions. [Accessed March 6, 2017];Clinical Knowledge Solutions [Micromedex Solutions Web site] 2017 Available at: http://www.micromedexsolutions.com.
- 5.Pitkanen A, Loscher W, Vezzani A, et al. Advances in the development of biomarkers for epilepsy. Lancet Neurol. 2016;15:843–856. doi: 10.1016/S1474-4422(16)00112-5. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt M, Shah S, Shivprakash Rapid ultraperformance liquid chromatography-tandem mass spectrometry method for quantification of OCB and its metabolite in human plasma. Biomed Chromatogr. 2011;25:751–759. doi: 10.1002/bmc.1510. [DOI] [PubMed] [Google Scholar]
- 7.Chahbouni A, Sinjewel A, den Burger JC, et al. Rapid quantification of GBP, pregabalin, and vigabatrin in human serum by ultraperformance liquid chromatography with mass-spectrometric detection. Ther Drug Monit. 2013;35:48–53. doi: 10.1097/FTD.0b013e31827788c0. [DOI] [PubMed] [Google Scholar]
- 8.Domingues DS, Pinto MA, de Souza ID, et al. Determination of drugs in plasma samples by high-performance liquid chromatography-tandem mass spectrometry for therapeutic drug monitoring of schizophrenic patients. J Anal Toxicol. 2016;40:28–36. doi: 10.1093/jat/bkv107. [DOI] [PubMed] [Google Scholar]
- 9.Juenke JM, Brown PI, Johnson-Davis KL, et al. Simultaneous quantification of LEV and GBP in plasma by ultra-pressure liquid chromatography coupled with tandem mass spectrometry detection. Ther Drug Monit. 2011;33:209–213. doi: 10.1097/FTD.0b013e31820b1fce. [DOI] [PubMed] [Google Scholar]
- 10.Juenke JM, McGraw JP, McMillin GA, et al. Performance characteristics and patient comparison of the ARK Diagnostics levetiracetam immunoassay with an ultra-high performance liquid chromatography with tandem mass spectrometry detection method. Clin Chim Acta. 2012;413:529–531. doi: 10.1016/j.cca.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Juenke JM, Miller KA, Ford MA, et al. A comparison of two FDA approved LTG immunoassays with ultra-high performance liquid chromatography tandem mass spectrometry. Clin Chim Acta. 2011;412:1879–1882. doi: 10.1016/j.cca.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Karinen R, Vindenes V, Hasvold I, et al. Determination of a selection of anti-epileptic drugs and two active metabolites in whole blood by reversed phase UPLC-MS/MS and some examples of application of the method in forensic toxicology cases. Drug Test Anal. 2015;7:634–644. doi: 10.1002/dta.1733. [DOI] [PubMed] [Google Scholar]
- 13.Shibata M, Hashi S, Nakanishi H, et al. Detection of 22 antiepileptic drugs by ultra-performance liquid chromatography coupled with tandem mass spectrometry applicable to routine therapeutic drug monitoring. Biomed Chromatogr. 2012;26:1519–1528. doi: 10.1002/bmc.2726. [DOI] [PubMed] [Google Scholar]
- 14.Dupouey J, Doudka N, Belo S, et al. Simultaneous determination of four antiepileptic drugs in human plasma samples using an ultra-high-performance liquid chromatography tandem mass spectrometry method and its application in therapeutic drug monitoring. Biomed Chromatogr. 2016;30:2053–2060. doi: 10.1002/bmc.3789. [DOI] [PubMed] [Google Scholar]
- 15.Farouk F, ElKady EF, Azzazy HME. Simultaneous UPLC-MS/MS determination of antiepileptic agents for dose adjustment. Biomed Chromatogr. 2017;31:e3921. doi: 10.1002/bmc.3921. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM) [Accessed September 5, 2016];Guidance for industry: bioanalytical method validation. 2001 May; Available at: https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf.
- 17.Krouwer JS, Cenbrowski GS, Tholen DW. Preliminary evaluation of quantitative clinical laboratory methods; approved guideline. 3. Pennsylvania: Clinical and Laboratory Standards Institutes; 2014. [Google Scholar]
- 18.Matuszewski B, Constanzer M, Chavez-Eng C. Matrix effect in quantitative LC/MS/MS analyses of biological fluids: a method for determination of finasteride in human plasma at picogram per milliliter concentrations. Anal Chem. 1998;70:882–889. doi: 10.1021/ac971078+. [DOI] [PubMed] [Google Scholar]
- 19.Basu S, Petrides A, Mason D, et al. A rapid UPLC-MS/MS assay for the simultaneous measurement of fluconazole, voriconazole, posaconazole, itraconazole, and hydroxyitraconazole concentrations in serum. Clin Chem Lab Med. 2016;6:836–844. doi: 10.1515/cclm-2016-0418. [DOI] [PubMed] [Google Scholar]
- 20.Iyer SS, Zhang ZP, Kellogg GE, et al. Evaluation of deuterium isotope effects in normal-phase LC-MS-MS separations using a molecular modeling approach. J Chromatogr Sci. 2004;42:383–387. doi: 10.1093/chromsci/42.7.383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.