Abstract
Today, HIV-infected (HIV+) patients can be treated efficiently with combined antiretroviral therapy (cART), leading to long-term suppression of viral load, in turn increasing life expectancy. While cART reduced the occurrence of HIV-associated dementia, the prevalence of subtle forms of HIV-associated neurocognitive disorders (HAND) is unchanged. This is related to persistent immune activation within the CNS, which is not addressed by cART. Pathologic processes leading to HAND consist of the release of proinflammatory cytokines, chemokines, reactive oxygen metabolites and glutamate, and the release of HIV proteins. Some of those processes can be targeted using medications with immunomodulatory and neuroprotective properties such as dimethyl fumarate, teriflunomide, or minocycline. In this review, we will summarize the knowledge about key pathogenic processes involved in HAND and potential therapeutic avenues to target HAND.
HIV-associated neurocognitive disorders (HAND) are alarming comorbidities in HIV-infected (HIV+) patients over long term. The burden of HAND is more prevalent than is commonly known, with nearly every second HIV+ patient affected by HAND1,e1 (links.lww.com/NXI/A102). In an era of more than 37 million HIV+ patients worldwide,2 the potential impact of HAND on society is alarming. Cognitive decline in HIV+ patients is associated with financial difficulties, depressive and anxiety symptoms, and unemployment.3 The introduction of combined antiretroviral therapy (cART) in the mid-1990s dramatically increased the life expectancy of HIV+ patients and led to a phenotype shift of HAND. Today, the clinical presentation of the most severe phenotype, HIV-associated dementia, is sparse because most patients are under efficient cART; however, there are more patients presenting with subtle forms of the disease such as asymptomatic neurocognitive impairment, associated with depression and insomnia.e2 Astonishingly, the overall prevalence of HAND has not been influenced by the initiation of cART,4 which indicates that the viral load is not the crucial driver for the development of HAND. Moreover, evidence is increasing that self-fueling inflammatory processes within the CNS drive neurodegeneration. Targeting those inflammatory processes—independent of the viral load—with immunomodulatory drugs additionally to cART hence seems to be an interesting approach to reduce chronic inflammation and maintain cognitive function in patients suffering from HAND. Keeping in mind the rising life expectancy of HIV+ patients underlines the importance of understanding the pathogenic mechanisms leading to cognitive impairment and targeting a patient's burdens with new treatment approaches.
In this review, we will discuss key pathogenic mechanisms and potential therapeutic approaches with immunomodulatory drugs, known from the treatment of neuro-inflammatory diseases as potential therapeutic “add-on” to cART.
Pathologic processes driving HAND
Persistent immune activation in the CNS as reason for neuronal loss
Infection of the CNS with HIV occurs through several mechanisms, such as transcytosis and the so called “Trojan Horse Mechanism”, describing the diapedesis of HIV+ monocytes over the blood-brain-barrier (BBB).e3 This process happens during the first 8 days of infection. Histopathologically, HIV leads to encephalitis or leukoencephalopathy. Encephalitis is characterized by the formation of multinucleated giant cells, consisting of infected macrophages, disseminated infiltration of lymphocytes, and macrophages.e4 Leukoencephalopathy leads to loss of myelin in the white matter, astrocytosis, and microglial infiltration.e5 Molecular processes fueling neurodegeneration are manifold and have therefore been described as a dynamic puzzle.5 Besides, epidemiologic co-factors have been connected to HAND. Cardiovascular disease, overweight, diabetes, tobacco, alcohol, and drug abuse seem to promote disease severity6,e6 as well as socio-economic factors, especially educational level of the patientse7 and cART itself due to direct neurotoxic effects.7
Immune reactions and inflammatory changes form the fundament of neuropathologic changes. Experiments with the integrin-inhibitor natalizumab in rhesus macaques in a simian immunodeficiency virus (SIV)-model underline the importance of infected monocytes and T-cells, which carry the virus into the CNS.e8 Infected cells get recognized by glial cells in the CNS and promote a persistent activation mainly of microglia (summarized in Gonzalez-Scarano and Martin Garcia 2005).8 Because of the activation, proinflammatory mediators such as cytokines, chemokines, reactive oxygen metabolites, and glutamate increase in the CNS environment,e9–e11 which ultimately leads to neurodegeneration.9,e12 Recent findings reveal an induction of senescence-like phenotype of microglia by HIV, also associated with increased levels of reactive oxygen species and proinflammatory cytokines.e13
Cytokines/chemokines
A key finding in HAND is the persistent release of chemotactic and proinflammatory cytokines, considered as pathologic correlate of continuous inflammation. Defining specific biomarkers to predict HAND has fueled research efforts. Until now, a broad spectrum of cytokines elevated in the CSF of HIV+ patients has been reported. This research is also driven by the hope to establish biomarkers or a specific cytokine pattern to determine whether patients are susceptible to HAND and whether they might respond to treatment.
In HIV+ patients, pleocytosis leads to higher CSF levels of interferon gamma (IFN-γ), TNF-α, IL-2, IL-6, IL-7, IL-8, and IL-10.10 IFN-γ correlates with the severity of neurologic impairment in HIV+ patients11 but is also elevated in HIV+ patients in the absence of clinical manifestations.e14 This might indicate that IFN-γ is involved in the perpetuation of HAND. Prolonged expression of IFN-γ reduces heme oxygenase-1 expression in human astrocytes,12,e15 which is known for its neuroprotective properties and its role in preventing damage by oxidative stress.e16 This could, in part, explain the role of IFN-γ as one of the key factors in driving HAND.
Polymorphisms in regulatory genes of cytokines and chemokines seem to be involved in the pathogenesis of HAND. A polymorphism in the TNF-α gene is overexpressed in HIV+ patients affected by dementia compared to HIV+ patients without dementia.e17 Similar to this, a mutated CCL2-allel is associated with a 4.5-fold higher risk to develop HIV-associated dementia, whereas the same mutation lowers the risk of getting infected with HIV.e18 Undoubtedly, one of the most prominent mutations in the context of HIV+ and HAND is the CCR5 delta32 mutation, which is the receptor of the chemokine CCL5. A heterozygous genotype of this mutation lowers the prevalence of HAND,e19 supporting the role of chronic CNS inflammation for the development of HAND.
Histopathologic changes during HAND are characterized by infiltrating immune cells. Chemokines play an important role in guiding cells to the site of inflammation. CCL2 (monocyte chemoattractant protein 1) is crucial for the transmigration of HIV+ cells over the BBB.13 Another predominant chemokine in HAND is CXCL10,14,15 which mainly recruits monocytes and T-cells to the location of inflammation.e20,e21 In addition to its chemoattractant properties, CXCL10 is described to induce neurodegeneration via calcium dysregulation in the CNS.e22 The chemokine CCL5, a natural ligand of the HIV entry receptor CCR5 in monocytes, recruits monocytes to the site of inflammation into the CNSe23 and is elevated in neuropsychologically asymptomatic HIV+ patients.14 The chemotactic effects on monocytes might increase the inflammatory state in the CNS. Indeed, the co-culture of HIV+ monocytes amplifies the cytokine secretion of microglia in accordance with enhanced neurotoxicity,14,15 supporting the idea that reducing chemotaxis of monocytes might have positive effects on neurodegeneration. Results from SIV-infected rhesus macaques, focusing on caspase-1 activation by SIV, show that cART is not sufficient to suppress inflammation by immune cells16 in this context.
Similar to the pathogenesis of HAND, neurodegeneration responsible for the progression in multiple sclerosis (MS) is driven mostly by reactive oxygen species and soluble factors instead of infiltrating immune cells. Soluble factors create an environment known as “virtual hypoxia,” which drive neurons into cell death (summarized in: Lassmann, 2018e24). It can be considered as possible that in HAND also soluble factors are the key factors for neurodegeneration or at least show neurodegenerative potential.
In contrast to other neuroinflammatory diseases, the pathology of HAND is also characterized by the involvement of viral proteins, which trigger inflammation or induce neurodegeneration themselves.
HIV proteins
Even in HIV+ patients treated with cART with suppressed systemic viral load, viral reservoirs in the CNS continue to produce inflammatory mediators such as cytokines and chemokines. Viral proteins, which trigger neuroinflammation and neurodegeneration themselvese25 are released by these viral reservoirs as well. The most important viral protein in this context is tat, which acts as a transactivator of transcription.e26 Tat is responsible for manifold processes on several cell types. Tat increases the connexin43 (C ×43) expression by binding to the C ×43 promotor,17 leading to an upregulated gap junctional communication. This could lead to spreading of pathologic signals from infected to uninfected astrocytes, probably inositol trisphosphate 3–related and calcium-related.17 Blocking of HIV-tat release could therefore alter the spreading of pathologic signals via gap-junction upregulation and thus prevent apoptosis of uninfected astrocytes. Recently, it was shown that tat provokes a change of the expression of over 4,000 protein groups within neurons, and among other factors reduces neuronal intrinsic excitability.e27 In line with these findings, tat is able to reduce specific and presumably vulnerable types of interneuronse28 and damages specifically dopamine-rich regions of the CNS.e29 The deleterious effects of tat are supported by the effect on microglial cells. Tat promotes the secretion of various proinflammatory cytokines and reactive oxygen species, which results in an enhanced rate of neurotoxicity.e30,e31 In addition to tat, gp120 is another viral protein, which is released by infected cells and is known to induce an outward current of potassium from microglia. This increase of extracellular potassium is crucial for neurodegeneration.18 Also, the regulatory protein nef generates neuronal cell death, especially in combination with the chemotactic cytokine CXCL10.e32
Those processes are linked to the development of energetic failure, a process known from other neuroinflammatory conditions with degenerative aspects such as MS. Using a model of HIV-1 transgenic rats characterized by the expression of different HIV-proteins revealed changes in mitochondrial proteins involved in electron transport chain, the glycolytic pathways, and mitochondrial trafficking proteins.19 These changes correlate with a reduced function of mitochondria. Mitochondrio-protective strategies could hence provide a potential approach to reduce energetic misbalances and prevent neurodegeneration.
In summary, HIV infection of the CNS creates a proinflammatory and neurotoxic environment, characterized by the reproduction of HIV, the formation of multinucleated giant cells, and the release of cytokines and HIV-proteins, driving ongoing neurodegeneration. Addressing these neuroinflammatory processes in conjunction with antiretroviral approaches might therefore target neurodegeneration.
New therapeutic approaches
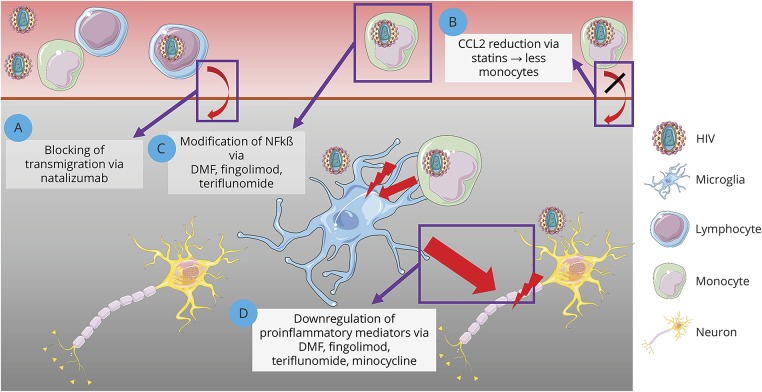
For a long period of time, it was postulated that it might be sufficient to reduce the CNS viral load to efficiently reduce neurodegeneration and the occurrence of HAND. To describe the effectiveness of antiretroviral therapies to penetrate into the CNS, Letendre et al. defined the CNS penetration effectiveness (CPE), consisting of 4 categories of effectiveness to get into the CNS.e33 It was postulated that cART should have high CPE scores in patients with neurocognitive impairment. Until now, it is contradictory whether high CPE might rather induce positive or negative effects. A randomized controlled trial showed slightly positive effects in patients treated with higher CPE.20 In contrast, retrospective analyses done by the Antinori group showed that high CPE is correlated with low performance in neuropsychologic tests.21 Hence, therapeutic approaches focusing only on a modification of cART regimen seem to be incomplete. One aspect, which may confound CPE is the neurotoxic potential of some antiretroviral substances. Efavirenz is known to cause neuropsychiatric side effects such as dysphoric dreams. Thus, new therapeutic approaches are urgently needed to improve cognitive function in HIV-positive individuals. Several promising antiinflammatory candidates are known from the treatment of neuroinflammatory and neurodegenerative conditions and will be reviewed in the context of HAND (therapeutic approaches are summarized in figure).
Figure. Summary of potential treatment approaches for HAND.
Shown are different treatment approaches for HAND targeting key pathogenic processes, involving blocking of lymphocyte trafficking into the CNS (A), reduction of monocyte trafficking (B), modification of NFkß with reduced viral replication (C), and reduction of microglial activation (D). Images were modified from Servier Medical Art, licensed under a creative common license. DMF = Dimethyl fumarate; HAND = HIV-associated neurocognitive disorders; NFkß = nuclear factor ĸß
Dimethyl fumarate
Dimethyl fumarate (DMF) has been licensed as medication for relapsing-remitting MS (RRMS) in 2013 by the Food and Drug Administration. The development of DMF has a history of more than 50 years and was started in the 1950s when Schweckendieck, affected by psoriasis, treated himself with fumarates. In 1994, Fumaderm was licensed in Germany as therapy for psoriasis. Following first reports about efficacy in coincidental MS, a prospective clinical observation showed efficacy in 10 RRMS patients over 72 weeks.e34 The pivotal phase 3 clinical trial DEFINEe35 led to the approval as therapy for RRMS. DMF has pleiotropic effects. DMF modifies the kelch-like ECH-associated protein 1 pathway, which results in upregulation of antioxidative genes.22 The molecule induces a shift towards T-helper 2 cells,e36 decreases the number of mature memory B cells,e37 and modulates microglia to a neuroprotective phenotype.e38. DMF treatment is associated with lymphocytopenia (>30% decline of lymphocytes) because of apoptosis in distinct T-cell subsets.e35,e39,e40 Therefore, it could be argued that DMF therapy is not suitable for the treatment of HIV+ patients, because one of the main treatment goals is to keep the CD4 cell count as high as possible.23 Disregarding the fact that DMF lowers the T-cell count, it holds some properties, which are very promising in the context of HAND.
DMF inhibits the nuclear factor ĸß (NF-kB) pathway.e41 NF-kB activation acts as a starting signal for HIV transcription, which is sensed by viral long-terminal repeats.e42 HIV uses activation of immune cells to reactivate from latency and start transcription and—even with cART—low-level viral protein production. Disturbing this ability of HIV to sense immune cell activation could prevent ongoing and low-level neurodegeneration. In addition to this, tat increases transcriptional activity of NF-kB, which leads to an increased immune cell activation and together with the activity of nef, vpu, and env to a repression of the CD4 receptor in infected cells.e43 In vitro studies underline the putative role of DMF as add-on therapy for patients with HAND. DMF induces antioxidant responses, suppresses HIV replication, and lowers neurotoxin release from infected cells in a model of HIV+ monocyte-derived macrophages.24 Our data indicate that DMF lowers the amount of secreted chemokines in co-culture of microglial cells with HIV-transduced monocytoid cells, which results in significantly decreased neuronal cell death in vitro.15 Summarizing, those data argue for a use of DMF in HAND-affected HIV+ patients. Because of the drawback of lymphopenia and the risk of progressive multifocal leukoencephalopathy (PML), patients should be monitored closely if treated in a clinical trial.
Fingolimod
Fingolimod targets the sphingosin-1 phosphate receptor 1 (S1P1) and is used as escalating therapy in RRMS. Sphingosin-1 phosphate responsiveness is reduced in HIV+ patients, especially in immunologic nonresponders and seems to be one of the driving factors of lymph node enlargement.25,e44 Of interest, S1P1 forms heteromers with the CCR5 receptor, which is mainly responsible for HIV entry into CD4+ T-cells and monocytes. Use of fingolimod as an agonist of S1P1 catalyzes internalization and degradation of the receptor, which also affects the CCR5-and the NF-kB pathway. In the context of HIV infection, fingolimod can inhibit productive infection of systemic monocytes and especially in the immune-privileged CNS it acts as an HIV latency reversing agent.26,e45 Because HIV uses microglia as viral reservoir in the CNS, which produces constantly low levels of viral proteins and proinflammatory factors, addressing these cells with a pharmacologic agent is of great interest.e46 In vitro experiments reveal a decreased release of neurotoxic metalloproteinase proteins after sphingosine-1-phosphate directed treatment, which could also be responsible for reduced neurodegeneration.e47 In addition, fingolimod induces neuroprotective properties in a neuronal progenitor cell line and upregulates glycolysis-related genes in HIV-exposed neurons.e48 One drawback of a therapeutic approach using fingolimod is, similar to other immunomodulatory drugs, that fingolimod can induce lymphocytopenia,e49 arguing for close monitoring in case of a therapeutic trial.
Teriflunomide
Teriflunomide is an oral first-line treatment for RRMS. It mediates an inhibitory effect on the enzyme dihydroorotate dehydrogenase and reduces the proliferation of B and T cells.e50 Like fingolimod and DMF, teriflunomide alters the NF-kB pathway, which results in reduced secretion of proinflammatory and neurotoxic agents (e.g., matrix metalloproteinases, IL-6, and CCL2).15,e51 We showed that teriflunomide reduces microglia-mediated neurotoxicity in vitro.15 The predecessor substance of teriflunomide, leflunomide, shows antiviral properties in polyomavirus BK infection.27 Therefore, teriflunomide was already used in a small trial in HIV+ individuals.28 Treatment decreased inflammation markers and reduced the number of circulating CD4+ T cells, whereas naïve and memory T-cell subsets were not altered. Apoptosis of T cells also was not altered. Notably, teriflunomide was used in cART naïve HIV+ patients and only for a short period of time, which limits the conclusions drawn by this study.28 Moreover, there was no investigation of neuropsychologic outcome after leflunomide therapy.
Natalizumab
Natalizumab is a monoclonal antibody directed against α4-integrin, used for the treatment of RRMS as escalation therapy. To reduce leukocyte trafficking into the CNS—and therefore the infiltration of HIV+ inflammatory cells—natalizumab was used in a SIV-macaque model. Natalizumab treatment 28 days after infection led to stabilization of neuronal injury, reduced numbers of monocytes/macrophages, and reduced productive infection.e8 Early treatment at the time of infection blocked monocyte trafficking and SIV-RNA was not detectable in the CNS.e8 In a model of HIV-associated neuropathy, SIV-infected macaques treated with natalizumab had reduced dorsal root ganglion pathology with decreased inflammation, neuronophagia, and Nageotte nodules, indicating degeneration of ganglion cell bodies and a reduced number of macrophages.29 CD3+ lymphocytes were not affected by natalizumab. Blocking trafficking of monocytes/macrophages into the CNS by natalizumab could be an interesting approach to reduce CNS viral load and deleterious interaction of infected monocytes/macrophages with microglia. However, the occurrence of PML, a feared complication of both HIV-infection and long-term therapy in natalizumab-treated MS patients, could limit the long-term use in HIV-infected individuals. Moreover, a therapeutic approach could be limited to individuals in an early stage of infection because the virus gets into the CNS within 8 days, as reviewed above.
Interferons
Also because of antiviral activity, interferons were studied in MS, leading to approval in the early 1990s. Although until now numerous new therapeutic approaches have been invented, interferons are still commonly used in MS therapy.e52 In HIV infection, interferon mediators (e.g., CXCL10) are upregulated early during disease progression,30 which can also be found in asymptomatic HAND patients.14 In contrast to previously mentioned treatments, interferons should be used with caution in the context of HAND. Experiments in an SIV model reveal pleiotropic effects of interferons with upregulation of antiviral genes on the one side and increased depletion of CD4+ T-cells as well as increased SIV reservoir size on the other side.31 Data from animal experiments indicate a neurotoxic effect of interferons on rat cortical neurons and especially in a HAND-mouse model.32,33
Minocycline
Minocycline is an antibiotic used for the treatment of acne vulgaris and in rheumatoid arthritis. Minocycline has been shown to be effective in several conditions such as clinically isolated syndromee53 and models of progressive MS.34 Minocycline reduces microglial activitye54,e55 and leukocyte trafficking into the CNS.e56 The medication might also induce neuroprotective properties in HAND. Minocycline was investigated in HIV-positive patients with cognitive impairment. Seventy-three patients received either minocycline 100 mg bid or placebo. However, treatment over 24 weeks did not improve cognitive function.35 Although those results were disappointing, minocycline might induce subclinical, protective changes. In a phase 2 randomized placebo-controlled study in 107 HIV+ patients with cognitive impairment therapy with either minocycline 100 mg bid or placebo over a period of 24 weeks led to improvement of lipid and protein markers of oxidative stress such as ceramides.36 Other markers such as neurofilament heavy chain levels did not differ. Hence, it might be worthwhile to test minocycline over a longer period. It could be speculated that the interventional approach to treat over 24 weeks might just be too short to detect positive effects.
Statins
Statins are used for their ability to lower cholesterol levels and prevent cardiovascular diseases. In addition, statins show immunomodulatory properties in the context of autoimmune disease.37 Cardiovascular diseases are more prominent and often connected to higher cholesterol levels in older HIV+ patients.e57,e58 Elevated cholesterol levels are correlated with a faster rate of cognitive decline, which can be reduced by statin use.e59 Statins can alter the proportion of monocytes and additionally lower the expression of the chemokine CCL2, responsible for the accumulation of monocytes in the CNS during HAND progression.38,e60 However, a small study in 7 HIV+ patients revealed no effect of treatment with atorvastatin over 8 weeks on CSF virologic and inflammatory indices.39 Because of their effect on monocytes and the positive effects on the cardiovascular system, statins are nevertheless interesting potential therapeutic agents and should be investigated in a larger clinical trial.
Data availability
Not applicable.
Conclusion
HIV+ can be treated efficiently with cART in the 21st century, leading to nearly normal life expectancy. Associated with this, clinicians face the challenge to optimize long-term complications and help to increase quality of life of infected patients, which is reduced in nearly every second HIV + patient because of the occurrence of mostly subtle cognitive alterations. While cART can alleviate the severity of HAND, it does not affect its prevalence, which indicates the importance of new therapeutic approaches to reduce the burden of HAND (summarized in: Patel, 2018e61). Because of many similarities between immune activation in HAND and MS such as persistent immune activation with release of proinflammatory cytokines, chemokines, and neurotoxic mediators, the translation of MS medications to HAND is obvious. Moreover, other generic medications such as the antibiotic minocycline or statins have properties, which could be advantageous to reduce persistent immune activation and neurotoxicity. Several of the drugs reviewed target cellular pathways, which are important for persistent CNS inflammation and neurodegeneration (e.g., NFĸβ), which makes them ideal candidates for co-treatment with standard cART use.
One drawback of the aforementioned therapeutic approaches is their effect to reduce lymphocyte counts (e.g., DMF, fingolimod, and natalizumab), which is critical in the context of HIV treatment, because one of the main goals is to stabilize the CD4 counts. This should be one great admonisher to carefully check these drugs for their potential use in HAND patients but should not act as a general obviation. Behavior of the immune system within the CNS is not easy to predict, as occurrence of HIV associated immune reconstitution inflammatory syndrome points out. Up to 13% of the HIV+ patients are affected after starting cART, evidencing that also a recovery of the immune system and especially of CD4+ T-cells can cause issues within the CNS.40
In summary, we believe that HAND treatment could be optimized if experience with medications known from other neuroinflammatory conditions would be translated into a clinical trial in patients affected by HAND.
Glossary
- cART
combined antiretroviral therapy
- CPE
CNS penetration effectiveness
- DMF
Dimethyl fumarate
- HAND
HIV-associated neurocognitive disorders
- NF-kB
nuclear factor ĸß
- PML
progressive multifocal leukoencephalopathy
- RRMS
relapsing-remitting MS
- S1P1
sphingosin-1 phosphate receptor 1
- SIV
simian immunodeficiency virus
Appendix. Author contributions
Study funding
The authors acknowledge support by the DFG Open Access Publication Funds of the Ruhr-Universität Bochum.
Disclosure
B. Ambrosius received travel funding from Novartis, has been employed by Celgene GmbH, and received research support from FORUM Anschubfinanzierung, Medizinische, Faktultät Ruhr-Universität Bochum; R. Gold served on the scientific advisory board for TEVA, received speaker honoraria from Biogen, Genzyme, TEVA, Merckserono, BayerSchering, Celgene, Novartis, and served on the editorial board for SAGE Journal, Aktuelle Neurologie, Experimental Neurology; A. Chan served on the scientific advisory board for Almirall, Actelion, Bayer, Biogen, Celgene, Genzyme, Merck, Novartis, Roche, and Teva and received travel funding and/or speaker honoraria for university research funds from Almirall, Actelion, Bayer, Biogen, Celgene, Genzyme, Merck, Novartis, Roche, and Teva; served on the editorial board for Clinical and Translational Neuroscience, topic editor for Journal of International Medical Research, holds a patent for mTOR inhibition as mechanism to increase glucocorticosteroid efficacy, and received research support from Biogen, Genzyme, UCB, Schweizer Nationalfonds, Swiss Multiple Sclerosis Society; S. Faissner received speaker honoraria from Novartis, filed patent applications at the FDA for patents for Treatment for Progressive Multiple Sclerosis, combination therapy with minocycline and hydroxycholorquine for the treatment of Multiple Sclerosis, received research support from Medical Faculty of Ruhr-University Bochum. Disclosures available: Neurology.org/NN.
References
- 1.Heaton RK, Clifford DB, Franklin DR Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010;75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS. UNAIDS Data 2017. Available at: http://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf. Accessed November 1, 2018. [Google Scholar]
- 3.Gilston A. Consent to AIDS testing. Lancet 1988;2:799. [DOI] [PubMed] [Google Scholar]
- 4.Saylor D, Dickens AM, Sacktor N, et al. HIV-associated neurocognitive disorder—pathogenesis and prospects for treatment. Nat Rev Neurol 2016;12:234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zayyad Z, Spudich S. Neuropathogenesis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND). Curr HIV/AIDS Rep 2015;12:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 2012;78:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akay C, Cooper M, Odeleye A, et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol 2014;20:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol 2005;5:69–81. [DOI] [PubMed] [Google Scholar]
- 9.McGuire JL, Gill AJ, Douglas SD, Kolson DL. Central and peripheral markers of neurodegeneration and monocyte activation in HIV-associated neurocognitive disorders. J Neurovirol 2015;21:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Almeida SM, Rotta I, Ribeiro CE, et al. Blood-CSF barrier and compartmentalization of CNS cellular immune response in HIV infection. J Neuroimmunol 2016;301:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrier RD, Hong S, Crescini M, et al. Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND). PLoS One 2015;10:e0116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacsics CE, Gill AJ, Ambegaokar SS, Gelman BB, Kolson DL. Degradation of heme oxygenase-1 by the immunoproteasome in astrocytes: a potential interferon-gamma-dependent mechanism contributing to HIV neuropathogenesis. Glia 2017;65:1264–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci 2006;26:1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faissner S, Ambrosius B, Schanzmann K, et al. Cytoplasmic HIV-RNA in monocytes determines microglial activation and neuronal cell death in HIV-associated neurodegeneration. Exp Neurol 2014;261:685–697. [DOI] [PubMed] [Google Scholar]
- 15.Ambrosius B, Faissner S, Guse K, et al. Teriflunomide and monomethylfumarate target HIV-induced neuroinflammation and neurotoxicity. J Neuroinflammation 2017;14:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kearns AC, Robinson JA, Shekarabi M, Liu F, Qin X, Burdo TH. Caspase-1-associated immune activation in an accelerated SIV-infected rhesus macaque model. J Neurovirol 2018;24:420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berman JW, Carvallo L, Buckner CM, et al. HIV-tat alters Connexin43 expression and trafficking in human astrocytes: role in NeuroAIDS. J Neuroinflammation 2016;13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Xu C, Chen L, Xu P, Xiong H. Involvement of Kv1.3 and p38 MAPK signaling in HIV-1 glycoprotein 120-induced microglia neurotoxicity. Cell Death Dis 2012;3:e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villeneuve LM, Purnell PR, Stauch KL, Callen SE, Buch SJ, Fox HS. HIV-1 transgenic rats display mitochondrial abnormalities consistent with abnormal energy generation and distribution. J Neurovirol 2016;22:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winston A, Duncombe C, Li PC, et al. Does choice of combination antiretroviral therapy (cART) alter changes in cerebral function testing after 48 weeks in treatment-naive, HIV-1-infected individuals commencing cART? A randomized, controlled study. Clin Infect Dis 2010;50:920–929. [DOI] [PubMed] [Google Scholar]
- 21.Libertone R, Lorenzini P, Balestra P, et al. Central nervous system penetration-effectiveness rank does not reliably predict neurocognitive impairment in HIV-infected individuals. J Int AIDS Soc 2014;17:19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011;134:678–692. [DOI] [PubMed] [Google Scholar]
- 23.Turk G, Ghiglione Y, Hormanstorfer M, et al. Biomarkers of progression after HIV acute/early infection: nothing compares to CD4(+) T-cell count? Viruses 2018;10:E34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cross SA, Cook DR, Chi AW, et al. Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and macrophage-mediated neurotoxicity: a novel candidate for HIV neuroprotection. J Immunol 2011;187:5015–5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mudd JC, Murphy P, Manion M, et al. Impaired T-cell responses to sphingosine-1-phosphate in HIV-1 infected lymph nodes. Blood 2013;121:2914–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duquenne C, Gimenez S, Guigues A, et al. Reversing HIV latency via sphingosine-1-phosphate receptor 1 signaling. AIDS 2017;31:2443–2454. [DOI] [PubMed] [Google Scholar]
- 27.Bernhoff E, Tylden GD, Kjerpeseth LJ, Gutteberg TJ, Hirsch HH, Rinaldo CH. Leflunomide inhibition of BK virus replication in renal tubular epithelial cells. J Virol 2010;84:2150–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Read SW, DeGrezia M, Ciccone EJ, et al. The effect of leflunomide on cycling and activation of T-cells in HIV-1-infected participants. PLoS One 2010;5:e11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakritz JR, Thibault DM, Robinson JA, et al. alpha4-Integrin antibody treatment blocks monocyte/macrophage traffic to, vascular cell adhesion molecule-1 expression in, and pathology of the Dorsal root Ganglia in an SIV macaque model of HIV-peripheral neuropathy. Am J Pathol 2016;186:1754–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stacey AR, Norris PJ, Qin L, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol 2009;83:3719–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandler NG, Bosinger SE, Estes JD, et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 2014;511:601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koneru R, Bimonte-Nelson H, Ciavatta V, et al. Reversing Interferon-Alpha Neurotoxicity in a HIV-Associated Neurocognitive Disorders Mouse Model. London, England: AIDS; 2018. [DOI] [PubMed] [Google Scholar]
- 33.Kessing CF, Tyor WR. Interferon-alpha induces neurotoxicity through activation of the type I receptor and the GluN2A subunit of the NMDA receptor. J interferon Cytokine Res 2015;35:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faissner S, Mahjoub Y, Mishra M, et al. Unexpected Additive Effects of Minocycline and Hydroxychloroquine in Models of Multiple Sclerosis: Prospective Combination Treatment for Progressive Disease? Multiple Sclerosis. Basingstoke, England: Houndmills; 2017:1352458517728811. [DOI] [PubMed] [Google Scholar]
- 35.Nakasujja N, Miyahara S, Evans S, et al. Randomized trial of minocycline in the treatment of HIV-associated cognitive impairment. Neurology 2013;80:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacktor N, Miyahara S, Evans S, et al. Impact of minocycline on cerebrospinal fluid markers of oxidative stress, neuronal injury, and inflammation in HIV-seropositive individuals with cognitive impairment. J Neurovirol 2014;20:620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youssef S, Stüve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 2002;420:78–84. [DOI] [PubMed] [Google Scholar]
- 38.Yadav A, Betts MR, Collman RG. Statin modulation of monocyte phenotype and function: implications for HIV-1-associated neurocognitive disorders. J Neurovirol 2016;22:584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Probasco JC, Spudich SS, Critchfield J, et al. Failure of atorvastatin to modulate CSF HIV-1 infection: results of a pilot study. Neurology 2008;71:521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Müller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis 2010;10:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.