Abstract
There are few disease-modifying therapeutics for neurodegenerative diseases, but successes on the development of antisense oligonucleotide (ASO) therapeutics for spinal muscular atrophy and Duchenne muscular dystrophy predict a robust future for ASOs in medicine. Indeed, existing pipelines for the development of ASO therapies for spinocerebellar ataxias, Huntington disease, Alzheimer disease, amyotrophic lateral sclerosis, Parkinson disease, and others, and increased focus by the pharmaceutical industry on ASO development, strengthen the outlook for using ASOs for neurodegenerative diseases. Perhaps the most significant advantage to ASO therapeutics over other small molecule approaches is that acquisition of the target sequence provides immediate knowledge of putative complementary oligonucleotide therapeutics. In this review, we describe the various types of ASOs, how they are used therapeutically, and the present efforts to develop new ASO therapies that will contribute to a forthcoming toolkit for treating multiple neurodegenerative diseases.
The genetic revolution led to the identification of many neurologic disease genes. The initial hope that finding the mutated protein and placing it into a known cellular pathway would lead to rapid development of therapies has remained largely unfulfilled. The ability to target the disease gene or its encoded messenger RNAs (mRNAs) has opened new opportunities for therapy development. Of the many ways to target the expression of RNA, this review will focus on the use of antisense oligonucleotides (ASOs) for therapy of neurologic diseases.
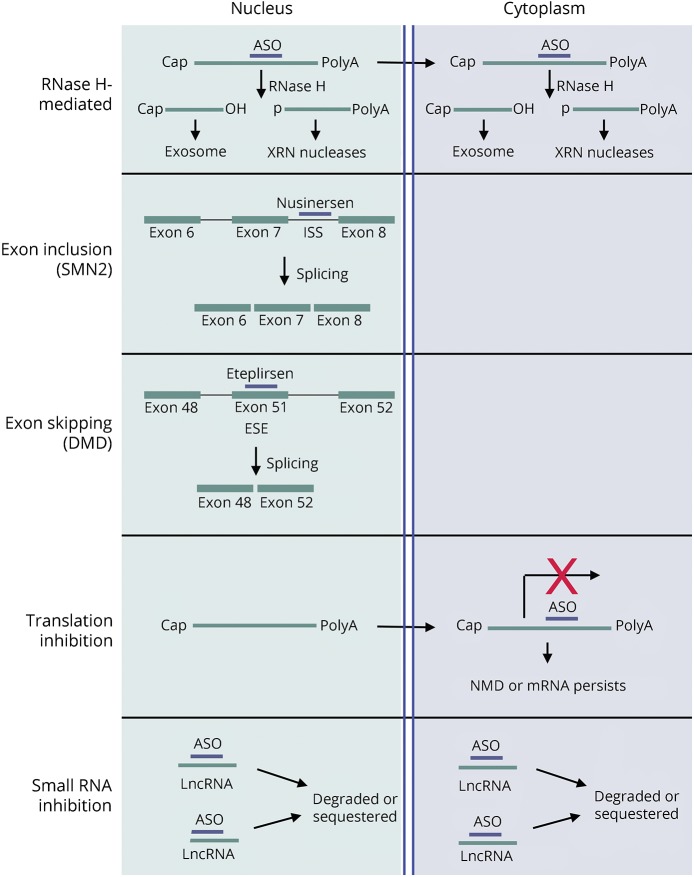
Therapeutic ASOs range from 18 to 30 base pairs (bp) in length. They modify expression of a target mRNA, by either altering splicing or by recruiting RNase H leading to target degradation. RNase H is a ubiquitous cellular enzyme that recognizes DNA:RNA hybrids and cleaves the RNA in the hybrid. The mode of ASO action depends on the target sequence and the precise ASO chemistry used in the design. An illustration of ASO action depending on target type and ASO chemistry is provided in figure 1.
Figure 1. ASO functions.
Target mRNA fates depending on ASO mechanism of action that is determined by where the ASO is targeted and by ASO chemistry. ASO = antisense oligonucleotide; DMD = Duchenne muscular dystrophy; ESE = exonic splicing enhancer; ISS = intronic splicing silencer; LncRNA = long noncoding RNA; SMN = survival motor neuron.
Leading the way currently in medical use is the ASO drug nusinersen that is approved for treating multiple forms of spinal muscular atrophy (SMA).1 Other ASO therapeutics that are Food and Drug Administration (FDA) approved include eteplirsen for Duchene muscular dystrophy (DMD)2 and inotersen for familial amyloid polyneuropathy (FAP).3
ASOs targeting HTT for Huntington disease (HD),4 SOD1 and C9ORF72 for amyotrophic lateral sclerosis (ALS),5,6 and MAPT (TAU) for Alzheimer disease (AD)7 are in early-phase clinical trials. Most current ASO therapeutics do not cross the blood-brain barrier and therefore for targets in the CNS have to be delivered by intraventricular injection in mice or lumbar puncture in humans. Eteplirsen and inotersen have targets that are not in the CNS and are delivered by intravenous or subcutaneous injection, respectively. On the other hand, systemic side effects are limited. Uptake into the CNS is an active process and is not uniform for all cell types or neurons. Chemical modifications can dramatically increase the half-life of ASOs and minimize toxicity.
Oligonucleotide chemistry
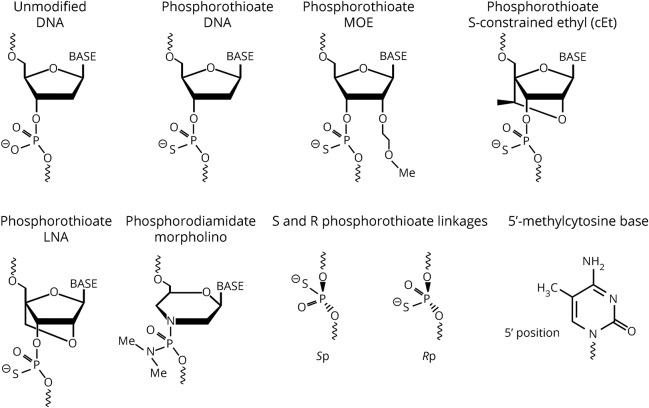
Natural, or unmodified, nucleic acids are susceptible to nuclease degradation and have poor protein binding and thus inefficient tissue uptake precluding their use as drugs.8,9 Multiple types of modifications made to nucleotides, and their linkages can improve various properties increasing ASO suitability as drugs. Most of these modifications alter pharmacokinetics (improved nuclease resistance resulting in a longer half-life), pharmacodynamics (superior affinity for the target RNA), or endocytic uptake, which is controlled by specific sets of cell surface proteins.10,11 But, with the exception of the phosphorothioate (PS) modification to the ASO backbone, most also preclude cleavage by RNase H, which is the desired mechanism of action for many ASOs. Thus, many RNase H ASOs are designed as chimeras, where different bases are a mix of different chemistries, or as gapmers, where some modifications are placed on the “wings” and not the central bases. Yet, for ASOs intended to alter mRNA splicing or translation, chemical compositions that do not support RNase H can be optimal. Thus, ASOs are highly versatile, customizable therapeutic tools. Some of the different ASO compositions and their resultant effects are discussed here, and the relevant structures are presented in figure 2.
Figure 2. Structural elements commonly used in ASOs.
cET = contrained ethyl; MOE = 2′ O-methoxyethyl; LNA = locked nucleic acid. Please see the text for descriptions of the structures.
Oligonucleotide phosphate linkage modifications
Modifications to the oligonucleotide phosphate linkages predominantly assist in nuclease avoidance. In the phosphorodiamidate morpholino (PMO), the phosphodiester (PO) linkages in the oligonucleotide backbone are replaced with nonionic phosphorodiamidate linkages leading to resistance to PO.12 Other ASO types have PS modifications that result in resistance to a broad spectrum of nucleases, support RNase H activity, and increase protein binding, which also improves tissue uptake.10,11
Morpholinos
Morpholinos are oligonucleotides with unique modifications to the ribose sugar that lead to greater target affinity and facilitate nuclease avoidance.12 This modification reduces oligonucleotide-protein interactions.12 Eteplirsen, a 30-bp morpholino-based ASO drug for the treatment of Duchenne muscular dystrophy (DMD), represents the only FDA-approved morpholino therapy for a neurodegenerative (or neuromuscular) disease.
Methoxyethyl oligonucleotides
A chemical modification included in most so-called second-generation ASOs is 2′ O-methoxyethyl (MOE). MOE ASOs have an MOE modification at the 2′-position of the ribose sugar. This change enables enhanced binding affinity to the target mRNA and is considerably less toxic than the 2′-O methyl (OMe) modification. In addition, MOE is sufficiently nuclease resistant that some MOE nucleotides can be synthesized with normal PO linkages so that a mix of PO and PS linkages can be used to fine tune the pharmacokinetics of the ASO. This can facilitate more rapid distribution into tissue while keeping the terminal elimination rate slow.13 MOE modifications also reduce plasma protein binding, which seem to shift ASOs away from hepatic metabolism and toward the kidney for excretion in urine.13 2′-MOE PS ASOs delivered to the CSF can have biological half-lives exceeding 6 months.50,58
Constrained nucleic acids
Nucleotides that are covalently modified to limit conformation are referred to as constrained or locked. Nucleic acids are considered “locked” when they have a methylene bridge connection made between 2′-oxygen and the 4′-carbon of the ribose sugar molecule (figure 2). This bond effectively locks the base into a conformation predominantly characterizing the RNA ribose sugar and prevents the conformation characteristic of the deoxyribose sugar.14 The benefit of locked nucleic acids (LNAs) is that they can produce both increased target specificity and reduced recognition by nucleases. LNAs can hybridize to both DNAs and RNAs forming highly stable double-helix duplexes.14 LNAs can be incorporated into siRNAs15 and gapmer ASOs supporting RNAse H activity.16 LNA ASOs can be more potent in vivo than their 2′-MOE analogs.17
LNAs have been associated with increased liver toxicity18 but can be used in combination with unmodified bases to reduce toxicity while improving ASO efficacy.19,20 To get around hepatotoxicities associated with LNA chemistry, chemists have created a sort of LNA/MOE hybrid by adding a methyl-bridge characteristic of LNAs to MOE oligonucleotides. The result is ASOs with reduced liver toxicity and increased potency.21 There are multiple types of constrained ethyl (cET) and MOE oligonucleotides (S-cEt, R-cEt, S-cMOE, and R-cMOE), where S and R refer to the left and right chiral structures, respectively. ASOs with these structures hybridize to target RNAs with affinities like their corresponding LNAs and have improved liver toxicity and increased resistance to nuclease degradation compared with the LNA chemistry.
Stereopure PS ASOs
PS ASOs are usually stereorandom with regard to chiral PS centers, each of which has 2 distinct stereochemical configurations, making 219 stereoisoforms possible for a 20mer ASO with 19 linkages. Although it is recognized that the 2 stereoisomers (Rp and Sp) differ in their binding affinity and susceptibility to degradation,22–24 there are tradeoffs between the two, and it is still debated whether stereopure isomers can be more potent than stereorandom ASOs. Studies in cell culture have failed to show any potency differences between stereopure and stereorandom ASOs. On the other hand, 1 study reported that ASOs with repeated left-left-right (or SSR) chiral PS centers optimized ASO recognition by RNAse H and resulted in greater potency in vivo.22 Therapeutic development of stereopure ASOs is the strategy used at WAVE Life Sciences.
Peptide nucleic acid
The peptide nucleic acid (PNA) has a peptide in the position of the ribose sugar. PNAs have typically been used for modulating transcription and other applications not supporting RNase H activity.25 But, PNA gapmers have been shown to support RNase H cleavage of target mRNAs.26 However, a PNA ASO targeting exon skipping in the DMD gene was poorly effective compared with an ASO with the 2′-OMe modification.27
5′-methylcytosine modification
Methylation of cytosines at the 5′ position is one way to increase ASO specificity. For example, all cytosines in an ASO against SOD1 included the 5′-methylcytosine modification.5 This inclusion can enhance base pairing by modifying the hydrophobic nature of the ASO. On the other hand, 5′-methylcytosine-thymidine repeats can increase cytotoxicity,28 and CpG motifs can stimulate immunoreactivity that can be at least partially alleviated by including 5′-methylcytosine.29,30
Gapmers, mixed chemistry, and target fate
Gapmer ASOs have “wings” on either side of LNA or MOE modified bases flanking a tract of unmodified bases with PS linkages usually throughout the ASO backbone. Mixed chemistry is intended to maximize resistance to nucleases and minimize toxicity while supporting RNAse H activity.31 The target mRNAs cleaved by RNAse H will be degraded in either the nucleus or the cytoplasm by the exosome complex and XRN exonucleases32 (figure 1). Lead optimization may include a screen for optimal structure activity relationship. For PS ASOs, this may combine cET, LNA, MOE, cMOE, and 5′-methylcytosine chemistries. Tracts of 4 guanosines should be avoided because they can result in complex ASO structures.30 For gapmer ASOs, this may include unmodified bases. Splice-switching oligonucleotides (SSOs) will exclude a gap feature as RNase H activity that could lead to target degradation is unwanted.
Development of ASO therapeutics for neurodegenerative diseases
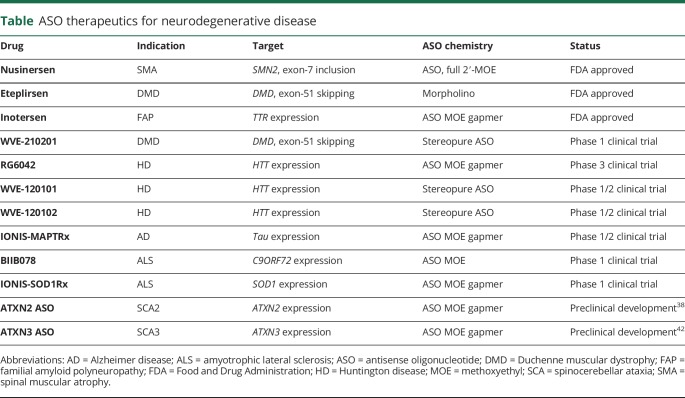
At the time of writing this review, only 3 ASO therapeutics have been approved by the FDA for neurodegenerative or muscular dystrophy diseases. These are nusinersen for SMA, eteplirsen for Duchenne muscular dystrophy (DMD), and inotersen for FAP. A handful of others are in clinical trials or in preclinical development (table).
Table.
ASO therapeutics for neurodegenerative disease
Spinocerebellar ataxias
Three Spinocerebellar ataxias (SCAs), all caused by DNA CAG repeat expansion encoding a polyglutamine (polyQ) repeat, have been investigated as diseases targetable by ASO treatment in mouse models. SCA2 is caused by CAG repeat expansion in exon 1 of the ATXN2 gene.33 The phenotypes in 2 SCA2 mouse models have been well characterized at the morphologic, physiologic, and transcriptome levels.34–36 ATXN2 mutation is also associated with a substantial rise in the expression of the stress granule protein Staufen1 (STAU1) and production of ATXN2/STAU1/TIA1–positive stress granules.37 After screening for ASOs targeting ATXN2 in a human cell line, we took the best ASOs reducing expression to in vivo testing by injection into the lateral ventricle of the mouse. These ASOs had a gapmer design. Several ASOs induced a glial or microglial response, a common off-target effect of some ASOs, and were not further evaluated.
The top lead ASO was designated ASO7 and lowered ATXN2 expression in SCA2 mouse cerebella by >60% for up to 13 weeks, without activating markers of gliosis. When injected into symptomatic mice, ASO7 improved motor function and restored proteomic and physiologic Purkinje cell abnormalities.36,38 The study provided proof of concept of lowering of ATXN2 for treatment of ATXN2-related diseases and the impetus for identifying more efficacious SCA2 ASO for use in humans.
SCA3 (Machado-Joseph disease) is caused by a CAG repeat expansion in exon 10 of the ATXN3 gene. Different strategies have been used to develop SCA3 ASOs. This has included allele-specific ASOs antisense to the expanded CAG repeat that sterically block translation,39 splice-switching MOE ASOs to exclude exon 10 encoding the expanded repeat,40 and MOE gapmer ASOs targeting wild-type and mutant ATXN3 alleles41,42 (figure 1).
SCA7 is characterized by cerebellar ataxia and progressive cone-rod dystrophy and caused by CAG repeat expansion in the ATXN7 gene. ASO therapies are ideally suited for treating the eye, as they can easily be injected intravitreally. Although no longer in use, 2 decades ago, fomivirsen became the first ever FDA-approved ASO drug, injected into the vitreous of the eye for cytomegalovirus retinitis.43 Niu et al.44 developed proof-of-concept data in mice for the use of an ASO therapy for blindness in SCA7. Of note, the ASO therapy was effective in mice with symptomatic eye disease.
Huntington disease
HD is caused by a CAG repeat expansion in an encoded region of the HTT gene. PolyQ expansion in huntingtin causes a gain of toxic function. Development of ASOs targeting HTT has followed different approaches. One targets both mutant and nonmutant alleles, whereas another approach is allele specific whereby ASOs were screened that target single nucleotide polymorphisms in HTT in linkage disequilibrium with expanded CAG repeats.45,46 This strategy may potentially target >75% of HD mutation carriers.47 Currently, 3 ASOs targeting HTT are in clinical trials (table).
Amyotrophic lateral sclerosis
ATXN2 as a target for ALS: Although mutations in several genes cause ALS, a number of studies point to ATXN2 as a therapeutic target for ALS. Over the past decade, it has become well established that intermediate CAG repeat expansions in the ATXN2 gene increase the risk of ALS.48,49 Intertwined with this discovery was the finding that reducing ATXN2 expression improved transactive response DNA-binding protein 43 (TDP-43) toxicity in both yeast and flies.49 When endogenous Atxn2 was reduced in TDP-43 transgenic mice by crossing with Atxn2 knockout mice, survival was significantly improved, TDP-43–positive stress granules were eliminated in motor neurons, and gait scores improved.50 Similarly, the survival of TDP-43 transgenic mice was improved by treating with an ASO targeting the Atxn2 gene.50 The effect of targeting ATXN2 on TDP-43 aggregations might be explained by related effects on STAU1.37
Targeting SOD1 for ALS
Approximately 10% of familial ALS cases are caused by mutations in SOD1 altering its function.51 Phase I clinical testing of the first-in-human SOD1 ASO, intended to lower SOD1 expression, demonstrated that the drug was well tolerated when infused into the CSF, but abundance of the mutant SOD1 protein in CSF was reduced by only ∼12%.52 A reformulated version of the drug designated IONIS-SOD1Rx (BIIB067) is presently undergoing phase 1 clinical trials by Ionis Pharmaceuticals and Biogen.53
Targeting C9ORF72 for ALS
GGGGCC repeat expansions in the C9ORF72 gene are causative of ALS and frontotemporal dementia (FTD).54,55 C9ORF72 repeat expansions result in loss of expression of the normal C9ORF72 gene and gain of C9ORF72 mRNA aggregates and repeat associated non-AUG (RAN) translation products. Mice with expanded C9ORF72 treated by intracerebroventricular injection with ASOs that interfere with translation of GGGGCC expanded C9ORF72 had reduced mRNA foci and RAN translation products, associated with improved anxiety and cognitive function phenotypes.56 With proof of concept established, Ionis Pharmaceuticals and Biogen have undertaken a phase 1 clinical trial of an ASO therapeutic targeting C9ORF72 for ALS.
Alzheimer disease and tauopathies
Targeting Tau for AD and tauopathies
Lowering Tau abundance by targeting expression of the MAPT gene may be therapeutic for AD and FTD. Targeting Mapt in mice with a MOE gapmer ASO reduced Tau expression throughout the CNS and protected against seizures in a mouse seizure model.7 Transgenic mice expressing a human P301S mutant Tau that were treated with Tau ASO had reduced neuronal Tau aggregates and prolonged lifespan from 312 to 348 days.57 Tau was also reduced in the CNS of cynomolgus monkeys following 6 weeks of Tau ASO treatment delivered intrathecally.57 ASOs targeting MAPT splicing to exclude a mutant exon also reduced Tau abundance in neuroblastoma cells and in an MAPT AD mouse model.58 There is another splice-switching ASO strategy for AD that targets the amyloid precursor protein (APP) gene to prevent inclusion of its exon 17 to block APP processing and Aβ production.59 And there is yet another, where an ASO is used to correct splicing of the APOE gene encoding apolipoprotein E.60
Parkinson disease
Reduction of LRRK2 abundance is predicted to be therapeutic for Parkinson disease (PD). This is supported by observations that disease-causing mutations in LRRK2 are associated with elevated α-synuclein expression.61 Targeting Lrrk2 in wild-type mice was well tolerated supporting that PD is not associated with LRRK2 loss of function.62 MOE-gamper ASOs reducing LRRK2 in mice treated with α-synuclein preformed fibirls were also associated with reduced aggregations of phospho(S129)-α-synuclein.63 Some effort has also been made to develop an ASO therapeutic targeting α-synuclein for PD.64
Spinal muscular atrophy and nusinersen
SMA is caused by loss-of-function mutations in the SMN1 gene resulting in the loss of the survival motor neuron (SMN) protein. SMA severity is inversely correlated with copy number of the homologous SMN2 gene. The cDNA encoded by SMN2 is identical to that encoded by SMN1 except for lack of exon 7. The ASO drug nusinersen is an SSO that functions by blocking an intronic splicing silencer element in the SMN2 intron 7 preventing the spliceosome from excluding exon 7 (figure 1). The result of nusinersen action is expression of the functional, full-length SMN protein from the SMN2 gene. In Smn1−/−; SMN2+/+ mice, SSOs that restore SMA2 exon 7 splicing also restored tail and ear necrosis phenotypes.65 Nusinersin was well tolerated in patients with SMA1,66,67 and was approved by the FDA for use in humans for the treatment of SMA in December of 2016.27
Duchenne muscular dystrophy and eteplirsen
DMD is caused by mutation in the DMD gene encoding dystrophin, which is one of the largest gene in the human genome. Most of the DMD mutations causing DMD result in premature dystrophin truncations. The predominant ASO strategy for treating DMD is employment of ASOs to exclude exons resulting in DMD proteins with partially restored functions. Eteplirsen, an ASO that has a PMO oligomer structure, interacts with the DMD pre-mRNA at exon 51 resulting in exon 51 exclusion. As illustrated in figure 1, it is used in DMD patients who have a deletion including exons 49 and 50 resulting in truncated dystrophin; exclusion of exon 51 restores the reading frame from exon 48 to exon 52, partially restoring DMD function.68 Approved by the FDA in 2016 for the treatment of DMD, eteplirsen can be used to treat approximately 14% of DMD cases,69 and efforts are ongoing to develop additional ASOs targeting other exons to treat DMD cases caused by other mutations in the dystrophin gene.68 Wave Life Sciences has also initiated a phase 1 clinical trial for testing a stereopure ASO for DMD exon 51 exon skipping.
Familial amyloid polyneuropathy and inotersen
Missense mutation in the TTR gene encoding transthyretin is the cause of hereditary transthyretin amyloidosis (ATTR). TTR mutations result in transthyretin misfolding and progressive accumulation of amyloid deposition in many tissues resulting in polyneuropathy, multiorgan dysfunction, and cardiomyopathy. Multiorgan failure and cardiac arrest pose a greatest risk of death for ATTR patients. Lowering the total expression of TTR at its source in the liver is an effective strategy for ATTR, which is achieved by systemic treatment using the 2′-MOE ASO drug inotersen,3 now approved by the FDA for FAP. Inotersen is treated systemically with delivery made by weekly subcutaneous injections. In the brain, transthyretin is produced by the choroid plexus, and TTR mutations cause leptomeningeal amyloidosis that potentially could also be treated by ASO therapy targeting TTR expression.70
Controlling off-target effects
Ensuring target specificity is a critical step in ASO therapeutic development. One useful approach is to perform quantitative PCR to assess expression of mRNAs with target sequences including mismatches to the ASO candidate to ensure unchanged expression. Another powerful method is to perform transcriptome analysis (RNA-seq) using tissues from mice that are null for the mRNA target, following a treatment trial with the ASO candidate, where differentially expressed genes would indicate off-targets or perhaps incidental cytotoxicity.
A number of chemical modifications have been developed to improve the pharmacokinetic and pharmacodynamic properties of ASOs. PS or PMO backbones are often used. Modifications at the 2′ position of the sugar can improve affinity, protein binding, and nuclease resistance. Modifications spanning the 2′ and 4′ positions to conformationally constrain the sugar can give dramatic improvements in affinity. All modifications except PS prohibit RNase H cleavage, but this can be rescued by a “gapmer” design where the modification is included only in the “wings” of the oligo, and the central bases are just PS. Although there are a number of new ASO therapies for neurodegenerative diseases in preclinical development and in clinical trials, there are only 2 therapies for neurodegenerative diseases that are FDA approved: nusinersen and eteplirsen. However, the success of nusinersen and eteplirsen and promising data from ongoing clinical trials for other ASO therapies to treat HD and ALS predict a robust outlook for new effective treatments for neurodegenerative diseases.
Acknowledgment
A portion of this work was supported by grants R01NS097903, R21NS081182, R37NS033123, and U01NS103883 from the National Institutes of Neurological Disorders and Stroke (NINDS). EVM is supported by F31 AI22592 (National Institute of Allergy and Infectious Diseases).
Glossary
- AD
Alzheimer disease
- ALS
amyotrophic lateral sclerosis
- ASO
antisense oligonucleotide
- cET
constrained ethyl
- DMD
Duchenne muscular dystrophy
- ESE
exonic splicing enhancer
- FAP
familial amyloid polyneuropathy
- FDA
Food and Drug Administration
- FTD
frontotemporal dementia
- HD
Huntington disease
- LNA
locked nucleic acid
- MOE
methoxyethyl
- OMe
O methyl
- PD
Parkinson disease
- PMO
phosphorodiamidate morpholino
- PNA
peptide nucleic acid
- PO
phosphodiester
- PS
phosphorothioate
- SCA
spinocerebellar ataxia
- SMA
spinal muscular atrophy
- SMN
survival motor neuron
- SSO
splice-switching oligonucleotide
- STAU1
Staufen1
Author contributions
D.R. Scoles wrote the manuscript and produced the figures. E.V. Minikel further contributed to ASO chemistry. S.M. Pulst further contributed to components on movement disorders and neuromuscular diseases.
Study funding
No targeted funding reported.
Disclosure
D.R. Scoles has received research support from the NINDS (U01NS103883 and R01NS097903) and Harrington Discovery Institute. E.V. Minikel has received funding for travel and/or speaker honoraria from Illumina and has received research support from Charles River Laboratories, NIH (F31 AI22952), and Prion Alliance. S.M. Pulst serves on the editorial boards of Journal of Cerebellum, NeuroMolecular Medicine, Experimental Neurology, Neurogenetics, Nature Clinical Practice, and Neurology: Genetics; holds patents for Nucleic acids encoding ataxin-2 binding proteins; Nucleic acid encoding Schwannomin-binding proteins and products related thereto; Transgenic mouse expressing a polynucleotide encoding a human ataxin-2 polypeptide; Methods of detecting SCA-2 nucleic acids; Nucleic acid encoding SCA-2 and products related thereto; Schwannomin-binding-proteins; and Compositions and methods for SCA; has received publishing royalties for The Ataxias (Churchill Livingston, 2007), Genetics in Neurology (ANN Press, 2005), Genetics of Movement Disorders (Academic Press, 2003), Neurogenetics (Oxford University Press, 2000), and Molecular Genetic Testing in Neurology, 2nd to 5th (AAN Press, 1996); serves or has served as a consultant for Ataxion Therapeutics; has received research support from the NIH (RC1NS068897, RC4NS073009, R21NS081182, R21NS079852, and R01NS33123) and the National Ataxia foundation; and has received license fee payments for technology or inventions from the Cedars-Sinai Medical Center. Disclosures available: Neurology.org/NG.
References
- 1.Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy . N Engl J Med 2018;378:625–635. [DOI] [PubMed] [Google Scholar]
- 2.Cirak S, Arechavala-Gomeza V, Guglieri M, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study . Lancet 2011;378:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis . N Engl J Med 2018;379:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Roon-Mom WMC, Roos RAC, de Bot ST. Dose-Dependent lowering of mutant huntingtin using antisense oligonucleotides in Huntington disease patients. Nucleic Acid Ther 2018;28:59–62. [DOI] [PubMed] [Google Scholar]
- 5.McCampbell A, Cole T, Wegener AJ, et al. Antisense oligonucleotides extend survival and reverse decrement in muscle response in ALS models . J Clin Invest 2018;128:3558–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ly CV, Miller TM. Emerging antisense oligonucleotide and viral therapies for amyotrophic lateral sclerosis. Curr Opin Neurol 2018;31:648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeVos SL, Goncharoff DK, Chen G, et al. Antisense reduction of tau in adult mice protects against seizures . J Neurosci 2013;33:12887–12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett CF, Baker BF, Pham N, Swayze E, Geary RS. Pharmacology of antisense drugs. Annu Rev Pharmacol Toxicol 2017;57:81–105. [DOI] [PubMed] [Google Scholar]
- 9.Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol 2010;50:259–293. [DOI] [PubMed] [Google Scholar]
- 10.Crooke ST, Wang S, Vickers TA, Shen W, Liang XH. Cellular uptake and trafficking of antisense oligonucleotides. Nat Biotechnol 2017;35:230–237. [DOI] [PubMed] [Google Scholar]
- 11.Geary RS, Norris D, Yu R, Bennett CF. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev 2015;87:46–51. [DOI] [PubMed] [Google Scholar]
- 12.Summerton J. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim Biophys Acta 1999;1489:141–158. [DOI] [PubMed] [Google Scholar]
- 13.Geary RS, Watanabe TA, Truong L, et al. Pharmacokinetic properties of 2'-O-(2-methoxyethyl)-modified oligonucleotide analogs in rats . J Pharmacol Exp Ther 2001;296:890–897. [PubMed] [Google Scholar]
- 14.Campbell MA, Wengel J. Locked vs. unlocked nucleic acids (LNA vs. UNA): contrasting structures work towards common therapeutic goals. Chem Soc Rev 2011;40:5680–5689. [DOI] [PubMed] [Google Scholar]
- 15.Elmén J, Thonberg H, Ljungberg K, et al. Locked nucleic acid (LNA) mediated improvements in siRNA stability and functionality . Nucleic Acids Res 2005;33:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahlestedt C, Salmi P, Good L, et al. Potent and nontoxic antisense oligonucleotides containing locked nucleic acids . Proc Natl Acad Sci USA 2000;97:5633–5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swayze EE, Siwkowski AM, Wancewicz EV, et al. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals . Nucleic Acids Res 2007;35:687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasuya T, Hori S, Watanabe A, et al. Ribonuclease H1-dependent hepatotoxicity caused by locked nucleic acid-modified gapmer antisense oligonucleotides . Sci Rep 2016;6:30377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamola PJ, Maratou K, Wilson PA, et al. Strategies for in vivo screening and mitigation of hepatotoxicity associated with antisense drugs . Mol Ther Nucleic Acids 2017;8:383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanton R, Sciabola S, Salatto C, et al. Chemical modification study of antisense gapmers . Nucleic Acid Ther 2012;22:344–359. [DOI] [PubMed] [Google Scholar]
- 21.Seth PP, Siwkowski A, Allerson CR, et al. Design, synthesis and evaluation of constrained methoxyethyl (cMOE) and constrained ethyl (cEt) nucleoside analogs . Nucleic Acids Symp Ser (oxf) 2008;553–554. [DOI] [PubMed] [Google Scholar]
- 22.Iwamoto N, Butler DCD, Svrzikapa N, et al. Control of phosphorothioate stereochemistry substantially increases the efficacy of antisense oligonucleotides . Nat Biotechnol 2017;35:845–851. [DOI] [PubMed] [Google Scholar]
- 23.Wan WB, Migawa MT, Vasquez G, et al. Synthesis, biophysical properties and biological activity of second generation antisense oligonucleotides containing chiral phosphorothioate linkages . Nucleic Acids Res 2014;42:13456–13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koziolkiewicz M, Krakowiak A, Kwinkowski M, Boczkowska M, Stec WJ. Stereodifferentiation--the effect of P chirality of oligo(nucleoside phosphorothioates) on the activity of bacterial RNase H. Nucleic Acids Res 1995;23:5000–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaihatsu K, Janowski BA, Corey DR. Recognition of chromosomal DNA by PNAs. Chem Biol 2004;11:749–758. [DOI] [PubMed] [Google Scholar]
- 26.Malchere C, Verheijen J, van der Laan S, et al. A short phosphodiester window is sufficient to direct RNase H-dependent RNA cleavage by antisense peptide nucleic acid. Antisense Nucleic Acid Drug Dev 2000;10:463–468. [DOI] [PubMed] [Google Scholar]
- 27.Aartsma-Rus A. FDA approval of nusinersen for spinal muscular atrophy makes 2016 the year of splice modulating oligonucleotides. Nucleic Acid Ther 2017;27:67–69. [DOI] [PubMed] [Google Scholar]
- 28.Drygin D, Barone S, Bennett CF. Sequence-dependent cytotoxicity of second-generation oligonucleotides. Nucleic Acids Res 2004;32:6585–6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation . Nature 1995;374:546–549. [DOI] [PubMed] [Google Scholar]
- 30.Stein CA. The experimental use of antisense oligonucleotides: a guide for the perplexed. J Clin Invest 2001;108:641–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crooke ST, Lemonidis KM, Neilson L, Griffey R, Lesnik EA,Monia BP. Kinetic characteristics of Escherichia coli RNase H1: cleavage of various antisense oligonucleotide-RNA duplexes . Biochem J 1995;312(pt 2):599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lima WF, De Hoyos CL, Liang XH, Crooke ST. RNA cleavage products generated by antisense oligonucleotides and siRNAs are processed by the RNA surveillance machinery. Nucleic Acids Res 2016;44:3351–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulst SM, Nechiporuk A, Nechiporuk T, et al. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2 . Nat Genet 1996;14:269–276. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Tang TS, Tu H, et al. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2 . J Neurosci 2009;29:9148–9162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meera P, Pulst S, Otis T. A positive feedback loop linking enhanced mGluR function and basal calcium in spinocerebellar ataxia type 2. Elife 2017;6:e26377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dansithong W, Paul S, Figueroa KP, et al. Ataxin-2 regulates RGS8 translation in a new BAC-SCA2 transgenic mouse model . PLoS Genet 2015;11:e1005182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paul S, Dansithong W, Figueroa KP, Scoles DR, Pulst SM. Staufen1 links RNA stress granules and autophagy in a model of neurodegeneration. Nat Commun 2018;9:3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scoles DR, Meera P, Schneider MD, et al. Antisense oligonucleotide therapy for spinocerebellar ataxia type 2 . Nature 2017;544:362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu J, Gagnon KT, Liu J, et al. Allele-selective inhibition of ataxin-3 (ATX3) expression by antisense oligomers and duplex RNAs . Biol Chem 2011;392:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toonen LJA, Rigo F, van Attikum H, van Roon-Mom WMC. Antisense oligonucleotide-mediated removal of the polyglutamine repeat in spinocerebellar ataxia type 3 mice. Mol Ther Nucleic Acids 2017;8:232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLoughlin HS, Moore LR, Chopra R, et al. Oligonucleotide therapy mitigates disease in spinocerebellar ataxia type 3 mice . Ann Neurol 2018;84:64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore LR, Rajpal G, Dillingham IT, et al. Evaluation of antisense oligonucleotides targeting ATXN3 in SCA3 mouse models . Mol Ther Nucleic Acids 2017;7:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marwick C. First “antisense” drug will treat CMV retinitis. JAMA 1998;280:871. [PubMed] [Google Scholar]
- 44.Niu C, Prakash TP, Kim A, et al. Antisense oligonucleotides targeting mutant Ataxin-7 restore visual function in a mouse model of spinocerebellar ataxia type 7. Sci Transl Med 2018;10:eaap8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carroll JB, Warby SC, Southwell AL, et al. Potent and selective antisense oligonucleotides targeting single-nucleotide polymorphisms in the Huntington disease gene/allele-specific silencing of mutant huntingtin. Mol Ther 2011;19:2178–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Southwell AL, Kordasiewicz HB, Langbehn D, et al. Huntingtin suppression restores cognitive function in a mouse model of Huntington's disease. Sci Transl Med 2018;10:eaar3959. [DOI] [PubMed] [Google Scholar]
- 47.Southwell AL, Skotte NH, Bennett CF, Hayden MR. Antisense oligonucleotide therapeutics for inherited neurodegenerative diseases. Trends Mol Med 2012;18:634–643. [DOI] [PubMed] [Google Scholar]
- 48.Neuenschwander AG, Thai KK, Figueroa KP, Pulst SM. Amyotrophic lateral sclerosis risk for spinocerebellar ataxia type 2 ATXN2 CAG repeat alleles: a meta-analysis. JAMA Neurol 2014;71:1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elden AC, Kim HJ, Hart MP, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS . Nature 2010;466:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Becker LA, Huang B, Bieri G, et al. Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice . Nature 2017;544:367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rotunno MS, Bosco DA. An emerging role for misfolded wild-type SOD1 in sporadic ALS pathogenesis. Front Cel Neurosci 2013;7:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller TM, Pestronk A, David W, et al. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study . Lancet Neurol 2013;12:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schoch KM, Miller TM. Antisense oligonucleotides: translation from mouse models to human neurodegenerative diseases. Neuron 2017;94:1056–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011;72:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Renton AE, Majounie E, Waite A, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD . Neuron 2011;72:257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang J, Zhu Q, Gendron TF, et al. Gain of toxicity from ALS/FTD-Linked repeat expansions in C9ORF72 is alleviated by antisense oligonucleotides targeting GGGGCC-containing RNAs . Neuron 2016;90:535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeVos SL, Miller RL, Schoch KM, et al. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy . Sci Transl Med 2017;9:eaag0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sud R, Geller ET, Schellenberg GD. Antisense-mediated exon skipping decreases tau protein expression: a potential therapy for tauopathies. Mol Ther Nucleic Acids 2014;3:e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang JL, Hinrich AJ, Roman B, et al. Targeting amyloid-beta precursor protein, APP, splicing with antisense oligonucleotides reduces toxic amyloid-beta production. Mol Ther 2018;26:1539–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hinrich AJ, Jodelka FM, Chang JL, et al. Therapeutic correction of ApoER2 splicing in Alzheimer's disease mice using antisense oligonucleotides . EMBO Mol Med 2016;8:328–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Volpicelli-Daley LA, Abdelmotilib H, Liu Z, et al. G2019S-LRRK2 expression augments alpha-synuclein sequestration into inclusions in neurons. J Neurosci 2016;36:7415–7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Volta M, Cataldi S, Beccano-Kelly D, et al. Chronic and acute LRRK2 silencing has no long-term behavioral effects, whereas wild-type and mutant LRRK2 overexpression induce motor and cognitive deficits and altered regulation of dopamine release . Parkinson Relat Disord 2015;21:1156–1163. [DOI] [PubMed] [Google Scholar]
- 63.Zhao HT, John N, Delic V, et al. LRRK2 antisense oligonucleotides ameliorate alpha-synuclein inclusion formation in a Parkinson's disease mouse model. Mol Ther Nucleic Acids 2017;8:508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alarcon-Aris D, Recasens A, Galofre M, et al. Selective alpha-synuclein knockdown in monoamine neurons by intranasal oligonucleotide delivery: potential therapy for Parkinson's disease. Mol Ther 2018;26:550–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hua Y, Sahashi K, Hung G, et al. Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev 2010;24:1634–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chiriboga CA, Swoboda KJ, Darras BT, et al. Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy . Neurology 2016;86:890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet 2017;388:3017–3026. [DOI] [PubMed] [Google Scholar]
- 68.Nelson SF, Crosbie RH, Miceli MC, Spencer MJ. Emerging genetic therapies to treat Duchenne muscular dystrophy. Curr Opin Neurol 2009;22:532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim KR, Maruyama R, Yokota T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des Devel Ther 2017;11:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benson MD, Smith RA, Hung G, et al. Suppression of choroid plexus transthyretin levels by antisense oligonucleotide treatment. Amyloid 2010;17:43–49. [DOI] [PubMed] [Google Scholar]