Abstract
ADP-ribosylation (ADPr) is a reversible post-translational modification of proteins, which controls major cellular and biological processes, including DNA damage repair, cell proliferation and differentiation, metabolism, stress and immune responses. In order to maintain the cellular homeostasis, diverse ADP-ribosyl transferases and hydrolases are involved in the fine-tuning of ADPr systems. The control of ADPr network is vital, and dysregulation of enzymes involved in the regulation of ADPr signalling has been linked to a number of inherited and acquired human diseases, such as several neurological disorders and in cancer. Conversely, the therapeutic manipulation of ADPr has been shown to ameliorate several disorders in both human and animal models. These include cardiovascular, inflammatory, autoimmune and neurological disorders. Herein, we summarize the recent findings in the field of ADPr, which support the impact of this modification in human pathophysiology and highlight the curative potential of targeting ADPr for translational and molecular medicine.
Keywords: ADP-ribosylation, signalling, translational medicine
1. Introduction
Uni- and multicellular organisms rely on multiple dynamic molecular processes dictating cellular growth, cell division, prompt adaptation to environmental changes and survival. A functional and dynamic communication between cellular macromolecules is essential to control these fundamental biological processes. At the molecular level, proteins and small molecules are responsible for orchestrating these cellular responses. Cells have evolved mechanisms able to regulate dynamically proteins' functions through chemical modifications. In this regard, post-translational modifications (PTMs) can efficiently and very rapidly control a multitude of cellular processes in a time-dependent fashion by affecting the conformation, activity, stability, interactions and the sequestration of proteins to cellular compartments and organelles [1].
So far, more than 300 PTMs have been described; each one is involved in a range of fundamental cellular and biological processes. Functional alterations in the proteins governing PTM systems are frequently dysregulated in human disease [1].
Among the PTMs, there is ADP-ribosylation (ADPr). ADPr is the transfer of a single or multiple ADP-ribose unit(s) from nicotinamide adenine dinucleotide (NAD+) onto target protein substrates. Importantly, ADP-ribose nucleotide units can be also transferred onto nucleic acids and small molecules, such as on acetyl chemical groups to produce O-acetyl-ADP-ribose (OADPR) during de-acetylation reactions [2–4].
Although ADPr of proteins was first described in the early 1960s, our understanding of the cellular processes regulated by ADPr is still in its infancy [5–8]. Indeed, strikingly little is known about most of the proteins involved in ADPr and the governed signalling pathways. Such a gap in the knowledge also translates into a lack of understanding of many potentially related pathogenic mechanisms. Yet the therapeutic modulation of ADPr is emerging as a strategy with high potential in the clinic of certain human cancer types [9,10].
However, an in-depth understanding of molecular networks controlled by ADPr can not only further potentiate current clinical strategies, but also impact on the treatment of many other human diseases with no available therapy identified so far. Herein we discuss the most recent discoveries available in the scientific community supporting the central role of ADPr in the pathophysiology of many acquired and hereditary human diseases (summarized in table 1) and highlight the outcomes of the pharmacological modulation of ADPr for the clinical treatment of these disorders.
Table 1.
Alterations of ADPr genes associated with human inherited pathologies.
| gene | gene alteration | disease/disorder | references |
|---|---|---|---|
| transferases | |||
| PARP9 | overexpression | B-aggressive lymphoma | [11,12] |
| breast cancer | [13] | ||
| PARP14 | overexpression | B-aggressive lymphoma | [11,14] |
| sarcoma | [15] | ||
| asthma | [16] | ||
| hepatocellular carcinoma | [17] | ||
| PARP15 | overexpression | B-aggressive lymphoma | [11] |
| readers/erasers | |||
| ALC1 (CHD1L) | overexpression | hepatocellular carcinoma | [18] |
| breast cancer | [19] | ||
| colorectal carcinoma | [20] | ||
| ARH1 | missense mutations | lung, breast and colon cancers | [21] |
| ARH3 | truncations/mutations | neurodegenerative diseases | [22,23] |
| GDAP2 (MacroD3) | point mutations | ataxia, progressive spasticity and dementia | [24] |
| MacroD1 | overexpression | endometrial carcinoma | [25] |
| gastric carcinoma | [26] | ||
| colorectal carcinoma | [27,28] | ||
| breast carcinoma | [29,30] | ||
| MacroD2 | single-nucleotide polymorphisms | autism | [31–33] |
| microdeletion Int 5 | kabuki syndrome | [34,35] | |
| locus deletions | various cancers | [36,37] | |
| deletions, missense mutations | colorectal cancer | [38] | |
| TARG1 | premature stop codon | neurodegeneration | [39] |
2. ADP-ribosyl transferases
ADPr is carried out by transferase enzymes that, based on the homology of their catalytic domain with bacterial toxins, are classified in two enzyme superfamilies: the cholera toxin-like ADP-ribosyl transferases (ARTCs) and the diphtheria toxin-like ADP-ribosyl transferases (ARTDs) [2,40,41]. These two classes of enzymes share an evolutionarily conserved protein fold, called ADP-ribosyl transferase (ART) domain [40,41]. The ART protein fold is characterized by two central β-sheets, one anti-parallel sheet containing three to five β strands, and one sheet composed of four to five β strands [40–42].
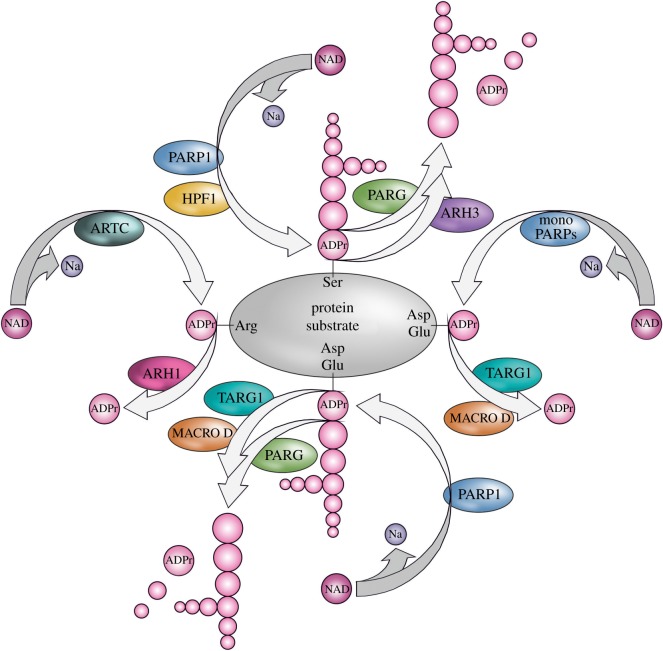
Three crucial amino acids within the ART domain define the affiliation to cholera or diphtheria toxin-like superfamilies, the R-S-E and H-Y-E triads, respectively. The first two amino acids in the triad are important for the NAD+ binding, while the common glutamate functions in catalysis [40–42]. ARTCs and ARTDs also differ for their specificity to target distinct amino acids. Most of the characterized ARTCs target protein substrates on arginine residues in proteins through an N-glycosidic bond producing arginine-ADPr (Arg-ADPr; figure 1). The founding member of ARTC family is the cholera toxin from Vibrio cholerae. Cholera toxin modifies arginine 187 of the stimulatory Gsα subunit of heterotrimeric G protein. ADPr of Gsα leads to constitutive activation of cyclic AMP-signalling pathway and, in turn, a dramatic efflux of ions and water from infected enterocytes, leading to watery diarrhoea [43,44].
Figure 1.
Enzymes and mechanisms of protein ADP-ribosylation. NAD, nicotinamide adenine dinucleotide; Na, nicotinamide; ADPr, ADP-ribose.
ARTD group of transferases most commonly modify acidic groups [45] (figure 1). The founding member of ARTD family is the diphtheria toxin, an exotoxin secreted by Corynebacterium diphtheriae, which catalyses the modification of the eukaryotic elongation factor-2 (EF-2) at a modified amino acid called diphthamide, thus inhibiting the translation machinery of the host [44,46]. For further details about bacterial ADP-ribosyl transferase toxins (bARTTs), refer to §9.
Four members of ARTC superfamily are expressed in humans (ARTC1, ARTC3, ARTC4, and ARTC5) and six in mice (Artc1, Artc2.1, Artc2.2, Artc3, Artc4 and Artc5). ARTC1, ARTC2, ARTC3 and ARTC4 enzymes are bound to the cellular plasma membrane by a glycosyl-phosphatidyl-inositol (GPI) anchor, while ARTC5 is an extracellular secreted enzyme. ARTC1, ARTC2 and ARTC5 show mono-ADP-ribosyl transferase activity and modify arginine side chains of protein substrates (figure 1). On the contrary, ARTC3 and ARTC4 lack the R-S-E motif in the active centre and therefore are probably inactive enzymes [47,48].
Mainly, extracellular or plasma membrane-residing proteins are substrates of Arg-ADPr, such as P2X7 and haemopexin [47,48]. However, several studies revealed Arg-ADPr of intracellular proteins as well (e.g. BIP, GAPDH and tubulin) [49–52], though the ARTs responsible for intracellular Arg-ADPr remain largely unknown.
Seventeen members of the ARTD superfamily have been identified in mammals and are known as poly(ADP-ribose) polymerases (PARPs) [3,41,53]. PARPs most commonly transfer ADP-ribose onto aspartic/glutamic acids (Asp/Glu-ADPr), through ester linkages, and on serine (Ser-ADPr) residues through O-glycosylation [54,55] (figure 1). Several PARPs can produce chains of ADP-ribose polymers (also called poly(ADP-ribose), thus abbreviated as PAR), where repeating single ADP-ribose units (up to 200 in length) are linked via unique O-glycosidic ribose-ribose bonds [45,56–58]. This type of modification is generally named poly(ADP-ribosyl)ation (PARylation). Well-characterized PARPs able to generate PARylation are PARP1, PARP2, Tankyrase-1 and Tankyrase-2 [45]. However, the remaining human PARP members are instead only capable of transferring a single ADP-ribose group to their target proteins, thus producing mono(ADP-ribosyl)ation (also abbreviated as MARylation) [45,59,60] (figure 1).
3. ADP-ribosyl hydrolases
ADPr is a fully reversible PTM. Two unrelated protein families show hydrolytic activity against proteins modified by ADPr, with diverse target specificity; the ADP-ribosyl-acceptor hydrolases (ARHs) and the macrodomain-containing enzymes.
Mg2+-dependent ADP-ribosyl-acceptor hydrolases (ARHs) are classified as DraG-like fold-containing proteins, based on the homology encountered with the bacterial dinitrogenase reductase-activating glycohydrolase (DraG). Bacterial DraG homologues have been described as mono(ADP-ribosyl) hydrolases that control nitrogen fixation by counteracting the arginine modifying ART activity of DraT [61]. Three ARH members are found in mammals: ARH1 (also named ADPRH), ARH2 (also named ADPRHL1) and ARH3 (also named ADPRHL2). While an enzymatic activity has not been identified for ARH2, ARH1 and ARH3 have distinct substrate specificities [62–64]. As seen for the bacterial DraG proteins, ARH1 reverses Arg-ADPr synthesized by both mammal endogenous ARTCs and bacterial toxins [64] (figure 1). Indeed, Arh1-deficient mice show enhanced sensitivity to cholera toxin infection [65]. By contrast, ARH3 shows high activity on O-glycosidic bonds and is the only known enzyme possessing hydrolytic activity against Ser-ADPr [66] (figure 1). Interestingly, ARH3 is inhibited by the metabolite ADP-ribosyl arginine, suggesting a cross talk between ADPr systems [67].
Macrodomain-containing proteins share a common ADP-ribose recognition domain, which is called macrodomain. The macrodomain is an ADP-ribose binding unit that plays crucial roles in the sensing and hydrolysis of ADPr in different cellular contexts [68]. Macrodomains are found in vertebrates as well as in many bacteria, archaea, viruses and plants, suggesting their evolutionary conservation and wide utility [69]. Depending on the type, macrodomains can exhibit the ability to bind ADP-ribose or PAR or OADPR (a metabolite released from the sirtuin-mediated NAD+-dependent deacetylation reaction). In addition, some macrodomains also act as ADP-ribosyl hydrolases [42,68–71]. Nevertheless, several macrodomain-containing proteins have been suggested to bind RNA intermediates instead of ADP-ribose [69,72].
Among the 12 macrodomain-containing proteins encoded by the human genome, only four exhibit catalytic activity [2,3,68,69]. The poly(ADP-ribosyl)glycohydrolase (PARG) is the only macrodomain-containing protein that efficiently cleaves PAR chains, though, it is unable to remove the terminal ADP-ribose linked to protein substrates [73]. Conversely, MacroD1 and MacroD2 of the MacroD subfamily of proteins as well as Terminal ADP-ribose glycosylhydrolase 1 (TARG1/C6orf130 or OARD1) specifically hydrolyse protein MARylation on acidic residues [39,74,75] (figure 1).
4. PARP1
PARP1 is the best-studied PARP enzyme, which is also the most ubiquitous and abundant PARP protein [57]. Together with PARP2 and PARP3, PARP1 belongs to the DNA-dependent nuclear PARPs group whose catalytic activity is potently stimulated by DNA breaks [10,76,77]. However, over the years, PARP1 functions have been expanded with roles in DNA damage repair as well as transcription, chromatin structure and metabolism [76,78–80]. Thus, PARP1 appears to be involved in both basal processes and response to cellular stresses with implications in human disease, particularly in cancer. For instance, PARP1 functions in DNA damage repair are the most attractive strategy to induce selective cell death in DNA damage repair-deficient cancers. Novel and specific structure-based chemicals acting as inhibitors of DNA damage PARPs (most notably PARP1) have been developed and under experimentation for treatment of pathological conditions [9,10,81,82]. The topic of PARP1 inhibitors in cancer will not be discussed in detail in this review.
The regulation of PARP1 activity is essential. A distinct PARP1 interacting protein milieu may play a crucial role in the fine-tuning of PARP1 when it functions in specific physiological processes or stress conditions. One of the best-characterized PARP1 accessory proteins is Histone PARylation Factor 1 (HPF1), which is required during the switch from basal conditions to stress response [83]. HPF1 has a central role in triggering PARP1-dependent ADPr of histone proteins as well as of many other DNA damage-related proteins following genotoxic stresses [83,84]. In the presence of DNA damage, HPF1 directs PARP1 to modify target proteins on serine residues within conserved motifs usually preceded by lysine residues (KS motifs) [54,84,85] (figure 1). Notably, most of the DNA damage-inducible ADPr is lost in the absence of HPF1. Importantly, PARP1 can still modify itself and other proteins on acidic residues in both DNA-damaged and undamaged cells in the absence of HPF1 [85].
In addition to the modification of DNA repair proteins, PARP1/HPF1-dependent Ser-ADPr targets include many other proteins involved in the maintenance of the genome stability. Indeed, in response to oxidative DNA damage, Ser-ADPr has been found linked to RNA processing, chromatin modification, splicing, transcription factors and mitotic proteins [84,86,87]. Interestingly, Ser-ADPr often overlaps with phosphorylation sites on proteins; such as Ser-ADPr of Ser10 on Histone H3, a well-known mitotic marker [54,85,88]. Proteome-wide studies have further expanded this observation, showing that Ser-ADPr occupies serine phosphorylation sites of many proteins that are also target of the mitotic regulators Aurora A and Aurora B kinases [86]. In addition, Ser-ADPr has been shown to compete with other PTMs by steric hindrance, in particular with modifications targeting the histone tails [88,89].
4.1. Role of PARP1 in human diseases
Although PARP1 genetic alterations are not associated with any known inherited disease, PARP1 is involved in the pathogenesis of many human disorders. For instance, depletion of NAD+ induced by PARP1 over-activation as well as excessive synthesis of PAR associates with ischaemia reperfusion injury, myocardial infarction and neurodegenerative disorders [90–94]. These disorders as well as many other acute or chronic pathological processes share a common pathogenic mechanism, which involves the production of reactive oxygen (ROS) or nitrogen species (NOS) followed by DNA damage and PARP1 activation. For instance, PARP1 was found activated in myocardial sections of patients with circulatory shock, with a degree of PARP activation correlating with the degree of myocardial dysfunction. Similar observations were made in circulating leucocytes in patients affected by myocardial infarction and therapeutic revascularization [95–97]. Moreover, PARP1 activation was shown in brain specimens of patients who died of stroke or brain ischaemia attributable to cardiac arrest, as well as in patients affected by brain trauma [98,99]. Finally, there is evidence for a boost of PARylation mediated by PARP1 in autoimmune (e.g. systemic lupus erythematosus) and inflammatory diseases (e.g. colitis), as well as in human atherosclerotic plaques, microvessels and lymphocytes of type 2 diabetic patients [100–108].
This body of information suggests a central role for PARP1 in human disorders. Indeed, the chemical modulation of PARP1 can be proposed to ameliorate or treat many pathological conditions, from cardiovascular, inflammatory and autoimmune diseases to neurological disorders. We next describe the role of PARP1 activation and the effects of its inhibition in the pathogenesis of neurological disorders, such as in a rare cerebellar ataxia caused by biallelic loss of function mutations of XRCC1, Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS) and Alzheimer's disease (AD) [94,109,110].
4.2. PARP1 in neurological disorders
The base excision repair (BER) X-ray repair cross-complementing 1 (XRCC1) protein is a molecular scaffold protein that is recruited by PAR and PARP1 on DNA damage foci. The BRCT domain of XRCC1 mediates its recruitment on DNA damage sites, and it is vital for the assembly of DNA single-strand break repair (SSBR) protein factors [111–115]. Importantly, several DNA-end processing enzymes recruited by XRCC1 are mutated in human ataxias, such as the spinocerebellar ataxia with axonal neuropathy-1 (SCAN1; mutated in TDP1), ataxia oculomotor apraxia-1 (AOA1; mutated in aprataxin) and ataxia oculomotor apraxia-4 (AOA4; mutated in PNKP) [116–121]. Furthermore, compound heterozygous mutations in human XRCC1 gene were shown to be responsible for ocular motor apraxia, axonal neuropathy and progressive cerebellar ataxia [109]. Mechanistically, in the presence of DNA damage, XRCC1 depletion results in severe delays in DNA SSBR repair and hyper-recombination phenotypes, which are accompanied by PARP1 hyper-activation followed by elevated levels of nuclear ADPr. The hyper-recombination as well as the cerebellar ataxia phenotype in Xrcc1 knockout mice is rescued by Parp1 gene deletion but not by enzymatic inhibition of PARP1. Thus, preventing the binding of PARP1 to DNA but not its enzymatic inhibition can be exploited for the therapeutic treatment of clinical cerebellar ataxias associated with unrepaired SSBs [109].
The genetic or enzymatic modulation of PARP1 has been also proposed for other common neurodegenerative diseases, such as PD, ALS and AD. These neurological disorders have a common pathogenic mechanism, which is characterized by aggregation of cytotoxic proteins, elevated levels of oxidative stress followed by DNA damage, PARP1 activation and excess of cellular levels of PAR.
In PD, intracellular monomeric α-synuclein assembles into higher-ordered protein aggregates that can spread from cell to cell [122]. Aggregates of α-synuclein activate nitric oxide synthase followed by production of NOS, which in turn cause DNA damage and activation of PARP1, and nuclear production of PAR. In a pathogenic loop, PAR is transported into the cytosol where it binds α-synuclein and further accelerates fibrillization and misfolding of the cytotoxic protein. Accumulation of pathologic α-synuclein ultimately leads to cell death via parthanatos and neuronal dysfunction. Inhibition of PARP activity or Parp1 gene deletion fully mitigates neuron-to-neuron transmission of pathologic α-synuclein and neurotoxicity; thus, PARP inhibitors (PARPi) can be exploited as therapeutic intervention for PD [110].
The liaison between protein aggregations, ROS formation, DNA damage and PARP1 activation has been also largely shown in AD. A peptide of 39–42 amino acids (Aβ) is the major component of protein aggregates present in AD senile plaques. Aβ is produced by the sequential proteolytic processing of the amyloid precursor protein (APP) by β- and γ-secretases [123]. Genetic and/or environmental factors are responsible for an imbalance between production and clearance of A β, which in turn leads to Aβ oligomerization and production of higher-order soluble assemblies and protofibrils and fibrils [123]. Through the impairment of the mitochondrial electron transport and the interaction with metal ions (Cu2+, Zn2+ and Fe2+), the aggregation of Aβ leads to ROS production and PARP1 activation [124–131]. The chemical inhibition of PARP1 blocks the accumulation of PAR and the morphological transformation in microglia-induced Aβ [130].
In ALS, the normally nuclear RNA/DNA-binding protein TDP-43 redistributes in the cytoplasm of affected neurons and glial cells, and forms phosphorylated protein aggregates [132,133]. TDP-43 and other proteins mutated in ALS (e.g. Ataxin-2) are a component of stress granules (SGs). SGs are cytoplasmic membraneless structures composed of RNAs and associated proteins structures, which form well cellular-defined zones of stalled translation complexes in response to a variety of environmental stresses that interfere with mRNA translation [134,135]. Among the cellular stresses inducing SGs there are heat shock, glucose deprivation, oxidative stress and viral infection [136,137]. Importantly, several PARPs and PAR itself have been shown to localize and regulate SGs formation; for instance, PARP1, Tankyrase-1 (also known as PARP5a), PARP12, the two PARP13 splice variants (PARP13.1 and PARP13.2) and PARP15 [138–142]. In ALS, motor neurons in the spinal cord show high levels of nuclear staining of PAR, suggesting massive PARP1 activation. In turn, PARP1 activity facilitates the nuclear export and the cytoplasmic aggregation of TDP-43 by an unknown mechanism. Indeed, the specific inhibition of PARP1 by Veliparib mitigates the formation of stress-induced aggregates of TDP-43 in the cytoplasm [141]. Altogether, these data suggest that PARP1 plays a central role in the formation of stress granules and, therefore, in the pathogenesis of TDP-43-dependent ALS [140,141]. Interestingly, other PARPs have a clear role in the pathophysiology of TDP-43-associated ALS in addition to PARP1; Drosophila melanogaster Tankyrase-1 and Tankyrase-2 (also known as PARP-5a and PARP-5b; refer to §6 for details) regulate the specific cytoplasmic aggregation of TDP-43. Importantly, contrary to PARP1, the inhibition of tankyrases does not alter the overall formation of SGs [142]. Thus, the differential impact of PARPs' inhibition on formation of SGs suggests that PARP1 is deputed to the overall control of SG formation, while tankyrase activity is specifically required for TDP-43 nuclear-SG translocation. Through a PAR-binding motif in its N-terminal nuclear localization sequence, TDP-43 non-covalently binds PAR. The binding of TDP-43 to PAR leads to liquid–liquid phase separation of protein, which is required for its accumulation in stress granules. Downregulation of tankyrases and inhibition of PARP catalytic activity by using small-molecules reduces the accumulation of TDP-43 in the cytoplasm and potently mitigates neurodegeneration [142].
Altogether, all studies summarized above uncover a common role for PAR in the regulation of the subcellular re-distribution of proteins in response to cellular stresses and, eventually, in their cytosolic aggregation. Thus, the inhibition of PARPs’ functions can be considered a therapeutic strategy for neurological disorders that are characterized by PAR-dependent protein aggregation. Inhibitors of PARP1 activity possessing significant brain penetration are already commercially available, such as Pamiparib (BeiGene/Merck Serono) [10]. Nevertheless, new drugs may be required to specifically treat certain disorders that show different pathogenic mechanisms (e.g. XRCC1-dependent ataxias) [109].
4.3. PARP1 in inflammation-induced colorectal cancer
It is worth mentioning the contribution of PARP1 as well as of other ADPr players (see §§7 and 8) to the pathogenesis of inflammatory bowel disease. Colitis refers to inflammation of the inner lining of the colon. There are numerous causes of colitis including infection, ischaemia and allergic reactions. The inflammatory bowel diseases, Crohn's disease (CD) and ulcerative colitis (UC), are chronic inflammatory disorders of the gastrointestinal tract of unknown aetiology. The diseases are thought to be the result of a dysregulated mucosal immune response to commensal gut flora in genetically susceptible individuals [143–147]. Importantly, the association between long-standing and extensive colitis and an increased risk of colorectal cancer (CRC) is well established [148–150].
PARP1 plays crucial roles in both colon inflammation and CRC. By using protocols of carcinogenesis in animal models, it has been shown that PARP1 is required to protect against DNA alkylation and oxidation damage during the initial steps of CRC carcinogenesis. Consistent with this, PARP1-deficient mice challenged with alkylating drugs show high levels of DNA strand breaks compared with control animals, thus, confirming that PARP1 works as a caretaker tumour suppressor gene [151]. In addition, PARP1 promotes tumour growth by supporting the focal inflammation during the tumour progression. Indeed, PARP1-deficient mice show an attenuated innate immune response. The pro-inflammatory functions of PARP1 pass through the modulation of NF-κB activity and following activation of the IL6-STAT3-Cyclin D1 axis. Importantly, tissue microarray analyses reveal that PARP1 is overexpressed in human CRC and its expression levels correlate with disease progression [151].
5. Macrodomain-containing PARPs in human disease
Twelve macrodomain-containing proteins are encoded in the human genome, including the previously mentioned hydrolase enzymes [3,42,68,69]. Three understudied PARP members are equipped with a number of macrodomains in addition to the PARP catalytic protein fold; thus, they are named Macro-PARPs [3,11,68]. Macro-PARPs were originally identified as members of a B-aggressive lymphoma protein family, which includes PARP9 (B-aggressive lymphoma 1; BAL1, also called ARTD9), PARP14 (BAL2, also called ARTD8) and PARP15 (BAL3, also called ARTD7) [11].
PARP9 (BAL1) was identified in a genome-wide search for risk-related genes in chemo-resistant diffuse large B-cell lymphoma (DLBCL), the most common non-Hodgkin lymphoma. PARP9 is largely overexpressed in DLBCL and promotes cell migration [11,12]. PARP9 is also overexpressed in breast cancer [13]. At the molecular level, PARP9 plays roles in DNA damage repair. In response to DNA damaging agents, PARP9 localizes at the DNA damage foci via its macrodomain, which drives PARP9 at the PARP1-generated PAR foci. There, PARP9 interacts with the E3 ligase DTX3L (also known as B lymphoma- and BAL-associated protein; BBAP) and promotes DNA damage repair via the ubiquitination-dependent recruitment of BRCA1 (Breast Cancer Type 1 susceptibility protein), 53BP1 (p53-binding protein 1) and RAP80 (receptor-associated protein 80) [152]. PARP9 activity negatively regulates the function of PARP9/DTX3 L heterodimer complex by transferring single units of ADP-ribose specifically on the carboxyl terminal of glycine 76 of ubiquitin molecules, thus interfering with the canonical protein ubiquitylation system [153]. The oncogenic potential of PARP9 has been described to be dependent on its transcriptional functions, particularly required for IFNγ-mediated host inflammatory response. PARP9, whose expression is activated by IFNγ, interacts with the IFNγ receptor complex and STAT1 acting as a transcriptional co-repressor of anti-proliferative and pro-apoptotic genes, and as a co-activator for the transcription of responsive proto-oncogenes, such as IRF2 and B-cell CLL/lymphoma 6 (BCL6) [154,155].
PARP14 (BAL2) was initially identified as an interactor and transcriptional collaborator for Signal Transducer and Activator of Transcription 6 (STAT6) and, therefore, named Co-activator of STAT6 (CoaSt6) [156]. PARP14 plays roles mainly in transcription of interleukin-4 (IL4)-responsive genes, which control cell survival, metabolism and proliferation [14]. Under non-stimulating conditions, PARP14 binds the transcriptional repressors histone deacetylase 2 (HDAC2) and HDAC3 at IL4-responsive promoters [16]. Under IL4 stimulation, PARP14 ADP-ribosylates HDAC 2 and 3 leading to their dissociation and the recruitment of transcriptional co-activators including the p100 cofactor, which is also a substrate of PARP14 [16,157]. This process leads to the transcription of IL4-responsive genes, which are vital for both B and T cells. In B cells, PARP14-dependent transcription of IL4-responsive genes transduces pro-survival and anti-apoptotic signals [14]. In addition, by regulating the binding of STAT6 to the Gata3 promoter, PARP14 and its enzyme activity are required for differentiation of T cells towards a T helper type-2 (Th2) lineage [158]. Th2 cells and Th2 cytokines (e.g. IL4, 5 and 13) associate with the promotion of IgE and eosinophilic responses and play a central role in the response to allergens, therefore, Th2 are the initiators of the allergic asthmatic condition [159]. Inhibition of PARP14 attenuates allergic airway disease, and it has been proposed as therapeutic strategy for asthma [158].
In addition to the transcriptional functions, PARP14 plays crucial roles in the metabolic control of cancer cells. PARP14 is indeed involved in the control of the cytokine-regulated glycolysis and glucose oxidation, thus, aiding the B-lymphoid oncogenesis [160]. Studies on solid tumours, such as sarcoma and hepatocarcinoma, further corroborate the link between PARP14 and cellular metabolism. In sarcoma cancer cells, PARP14 was shown to stabilize the glycolytic enzymes phosphoglucose isomerase (PGI) [15]. When secreted into the extracellular environment, PGI acts as a cytokine eliciting motogenic and differentiation cellular responses and, in addition, facilitates angiogenesis, metastasis and vessel leakiness [161–163]. In hepatocellular carcinoma, PARP14 inhibits JNK1-dependent phosphorylation and activation of the pyruvate kinase M2 isoform (PKM2), thus, promoting the aerobic glycolysis (Warburg effect) of cancer cells [17].
Lastly, PARP14 is involved in PARP10-dependent intracellular signalling. PARP14 binds MARylated proteins with high-affinity through its macrodomains. Among the ADPr proteins, PARP14 binds very efficiently automodified PARP10 and MARylated substrates of PARP10, such as the small GTPase RAN and the component of the NF-κB signal transduction pathway NEMO [164].
6. Role of tankyrases in the pathogenesis of cherubism
Tankyrase-1 and Tankyrase-2 are PARP enzymes characterized by large ankyrin repeating domains. Tankyrases play roles in telomere length maintenance which is particularly relevant for ageing, homologous recombination-mediated DNA damage response, mitosis, pexophagy, and Wnt- and Notch-mediated signal transduction [165–174].
Differently from other PARPs, tankyrases engage their protein substrates through the ankyrin domains within their protein sequence that bind very efficiently a well-defined octapeptide consensus within protein substrates [175]. The consensus for binding to tankyrase proteins consists of arginine in position 1, small and hydrophobic residue in position 4, aspartate in position 5, however, glutamic acid, valine, glutamine, tyrosine, isoleucine and cysteine are equally tolerated, and glycine in position 6 [175]. Protein–protein interaction is therefore the prerequisite for tankyrase-dependent PARylation. A large number of binders/substrates of tankyrase proteins have been proposed by interaction studies (608 proteins) [173,175]. Among tankyrase-interacting proteins there are AXIN1/2, the telomeric-repeat binding factor-1 (TRF1), the insulin-responsive amino-peptidase (IRAP), the 182-kDa tankyrase-binding protein (TAB182), the nuclear mitotic apparatus protein-1 (NuMA1), 3BP2, Notch2, HectD1, NKD1 and NKD2, the CBP80/CBP20-dependent translation initiation factor (CTIF), BLZF1 (basic leucine zipper factor 1), CASC3 (cancer susceptibility factor 3), the component of the HIPPO signalling pathways AMOT (Angiomotin) and PTEN, although it is not clear whether the latter is a direct tankyrase-binding protein or substrate [165,168,170,173,176–180]. Following the interaction and PARylation, a large portion of tankyrase-binding proteins become targets of proteasome degradation [175]. Indeed, tankyrase-mediated PARylation of protein substrates acts as a scaffold for recruitment of the PAR binding motif-containing protein RNF146, an E3 ubiquitin ligase. Thus, RNF146 binds tankyrases' PARylated substrates and ubiquitinates them, leading to their proteasome degradation. The liaison between tankyrase-mediated ADPr and proteasome degradation was initially observed in the regulation of the proliferative WNT pathway in CRC cells through the modification and following degradation of AXIN1/2, then expanded to many other cellular processes regulated by tankyrase substrates [10,170,181]. Thereby, the interest in targeting tankyrase proteins for the pharmacological modulation of pathological conditions has increased in recent times. In particular, the involvement of tankyrases in Wnt signalling (related to tumourigenesis) and glucose homeostasis (related to diabetes) promises advances for targeting tankyrases for therapeutic interventions, as demonstrated by the pre-clinical experimentation of tankyrase inhibitors for treatment of CRC [10,170,181–186]. In addition, the chemical inhibition of tankyrase proteins has been proposed for treatment of brain injuries of the newborn. Indeed, tankyrase small inhibitors stabilize Axin2 levels in oligodendrocyte progenitor cells from brain and spinal cord, thus accelerating differentiation and myelination after hypoxic and demyelinating injury [187].
Dysregulation of tankyrase-mediated binding and degradation of protein substrates has been recognized as the pathogenic mechanism of cherubism, a dominantly inherited human disorder. Cherubism is a bone inflammatory destructive disease characterized by deformities of the facial bones [188]. Cherubism is caused by single missense mutations in Sh3bp2, the gene that encodes the adaptor protein 3BP2 [189]. Most 3BP2 mutations associated with cherubism cluster within the peptide sequence RSPPDG, such as R413Q, P416H, or G418R mutations, which serve as a tankyrase-interacting motif. Similar to all known targets of tankyrase, PARylation of 3BP2 leads to its proteasome degradation, which is required for controlling 3BP2 protein levels within the cells. In cherubism, 3BP2 mutations in the RSPPDG hexapeptide impair tankyrase-mediated protein degradation, which in turn translates into elevated steady-state protein levels of 3BP2 in primary cells deputed to maintain the bone homeostasis, namely osteoclasts. As a result of this dysregulation, the signalling pathway including SRC, SYK and VAV proteins is up-regulated leading to uncontrolled activation of osteoclasts’ functions and peculiar interosseous fibrocystic lesions in cherubism-affected patients [175,190].
7. Role of ADP-ribosyl hydrolases in human disease
7.1. Macrod1 and MacroD2
MacroD1 and MacroD2 are related mono(ADP-ribosyl) hydrolases belonging to a subfamily of proteins present in both eukaryotes and prokaryotes [191]. MacroD1 and D2 contain nearly identical catalytic macrodomains that, by using substrate-assisted catalysis, hydrolyse the ester bond joining the ADP-ribose to the acidic residues of acceptor proteins or cleaving OADPR [74,192]. However, MacroD1 and D2 cannot hydrolyse the O-glycosidic bond of Ser-ADPr [66] (figure 1).
MacroD1 (also named Leukaemia-Related Protein 16; LRP16) contains a leading sequence localizing the protein at the mitochondria; nevertheless, its roles in transcription as a cofactor for androgen and oestrogen receptors and in NF-κB signal transduction cascade have been largely established [193–196].
MacroD1 appears overexpressed in several human cancers (such as endometrial carcinoma, gastric carcinoma, CRC and breast carcinoma) and its expression levels correlate with poor prognostic outcomes [25–30]. It is worth mentioning that the oncogenic potential of MacroD1 in CRC depends on its ability to activate the pro-survival NF-κB-dependent signalling in the presence of DNA damage. When stimulated by DNA-damaging agents, MacroD1 enriches in the cytosol of CRC cells where it interacts with double-stranded RNA-dependent kinase (PKR), thus facilitating the kinase activation, and promoting the binding with IKKβ. The formation of MacroD1/PKR/IKKβ ternary complex triggers the activation of anti-apoptotic signals mediated by NF-κB. Importantly, a screening of molecules targeting MacroD1 macrodomain led to the identification of a small molecule (MRS2578) that, both in vitro and in vivo, abrogates MacroD1- and NF-κB-dependent pro-survival signals synergistically with DNA-damaging chemotherapies [28].
The other member of the MacroD subfamily, MacroD2, also shows connections with the NF-κB pathway. MacroD2 was shown to play a central role in reverting PARP10-dependent MARylation of protein substrates, as in the case of GSK3β kinase, a kinase involved in the WNT pathway. Additionally, MacroD2 may revert PARP10-dependent MARylation of NEMO (NF-κB essential modulator), modification of which results in reduced NEMO polyubiquitylation and decreased NF-κB signalling [68,75,197,198].
In disease, MacroD2 shows association with neurological disorders, such as autism and kabuki syndrome (KS), as well as cancer.
Autism is a heterogeneous neurodevelopmental disorder defined by deficits in language and social behaviour, as well as patterns of repetitive behaviours of high heritability [199,200]. However, a simpler genetic basis for autistic or autistic-like traits is recognizable in around 5% of autism individuals with diseases [201]. Single-nucleotide polymorphisms (SNPs) associated with autism are found in few candidate genes, among them the MacroD2 gene [31–33]. kabuki (or Niikawa-Kuroki) syndrome is a genetically heterogeneous dominant mental retardation with a described autosomal transmission. It is characterized by postnatal growth retardation, typical facial defects, fetal pads, cleft palate and major malformations of the heart, kidneys and vertebra [202]. A mutation screening revealed a 250 kilobase de novo microdeletion at 20p12.1, which hits intron 5 of MACROD2 gene and associates in one patient with kabuki syndrome [34]. An intron 5 deletion of the MACROD2 gene was also reported in a patient displaying a kabuki-like phenotype [35]. It is worth mentioning that the association between an intron deletion of MACROD2 gene and kabuki syndrome is reported in a small number of clinical cases. Thus, more research is needed to clarify the specific link between MACROD2 and KS [203]. Genome-wide DNA copy-number analyses across human cancers have indeed revealed that common focal deletions of MACROD2 genomic locus happen in multiple malignancies, such as in stomach adenocarcinoma, cervical squamous cell carcinoma and endocervical adenocarcinoma, esophageal carcinoma, uterine corpus endometrial carcinoma, uterine carcinosarcoma, lung adenocarcinoma, liver hepatocellular carcinoma and thyroid carcinoma [36,37]. Importantly, some MACROD2 somatic mutations are found in CRC. Loss of variable size of the genomic locus containing MACROD2 as well as missense mutations is quite frequent in CRC. Importantly, some MACROD2 somatic mutations observed in cancer are predicted to interfere with binding of ADP-ribose to the catalytic pocket of MacroD2, therefore leading to increased sensitivity to genotoxic stress, and chromosomal instability in CRC [38].
7.2. Terminal ADP-ribose glycosylhydrolase 1 (TARG1)
TARG1 (c6orf130) is a macrodomain-containing protein with similar substrate specificities as seen for MacroD1 and MacroD2—it can cleave glutamate-linked protein ADPr, OADPR and phosphate-linked ADPr on nucleic acids [73,75,204,205] (figure 1). Nevertheless, the macrodomain of TARG1 is very diverged from those in PARG and both MacroD1 and MacroD2 proteins, and adopts a distinct catalytic mechanism [39].
A distinct homozygous sequence variant of the TARG1 gene was found in a family with a number of members affected by a severe and progressive neurodegeneration and seizure disorders. The sequence variant associated with disease is characterized by a premature stop codon within the exon 4 of TARG1 locus and predicts the formation of a truncated and not functional TARG1 enzyme. Importantly, TARG1 knockdown in human cells leads to significant proliferation defects and sensitivity to DNA damage [39].
Interestingly, the phenotype of TARG1-mutated patients somewhat resembles a clinical case described in the early 1980s of an 8-year-old male who died after a 6-year course of progressive neurologic degeneration and renal failure. Biochemical studies performed on bioptic specimens obtained from this patient showed the lysosomal accumulation of glutamyl ribose 5-phosphate (a glutamate amino acid linked to a phosphoribose group), which was proposed to arise from the inability to cleave the glutamate-linked ADPr on proteins. However, the identity of the deficient gene remained uncovered [206,207]. The accumulation of peptides linked to a phosphoribose group (phosphoribosylated peptides) suggests the presence of alternative hydrolytic mechanisms in cells that allow cleavage of the phosphodiester bond within MAR or PAR attached to a protein. Such pathways could intervene both under physiological and pathological conditions; for instance, when not functional hydrolytic enzymes (e.g. in the case of TARG1) lead to an excess and toxic accumulation of MARylated and PARylated proteins. Interestingly, specific members of two unrelated classes of phosphodiesterases were shown to possess ability to produce protein phosphoribosylation in vitro, the nucleoside diphosphates linked to X (any moiety) (NUDIX) and ectonucleotide pyrophosphatase/phosphodiesterase (ENPP) [208–210].
7.3. Poly(ADP-ribosyl)glycohydrolase (PARG)
PARG contains a highly diverged macrodomain fold and its structure has been extensively studied [73,211–214]. Distinctly from TARG1, MacroD1 and MacroD2, PARG has an insertion of a unique catalytic loop in the conserved globular macrodomain fold, which contains the catalytic residues and is essential for degradation of PAR chains. PARG preferably binds PAR at the chain termini and sequentially degrades ADP-ribose units (exo-glycohydrolase activity; figure 1). The endo-glycohydrolytic cleavage of PAR chains is also catalysed by PARG, but this activity is less efficient [213,215,216]. Yet PARG endo-glycohydrolase activity may become significant in the presence of excessive PAR production, for instance in cells or tissues exposed to abundant oxidative stress as observed in neurological disorders caused by aggregation of cytotoxic proteins (e.g. in PD) [110]. Indeed, the release of free long PAR fragments was shown to trigger apoptotic signalling [217].
Although genetic mutations have not been directly linked to human diseases, PARG cellular functions may significantly contribute to the pathogenesis of hereditary and acquired disorders. Contrary to PARP1 inhibition or deletion, which is not lethal for cells and mice (although it increases radiosensitivity), PARG knockout results in embryonic lethality in mouse model as a result of PAR accumulation and cellular apoptosis. However, Parg null mouse trophoblast-derived stem cells can be successfully cultivated in the presence of PARP inhibitors, suggesting that PARP1 inactivation can rescue PARG deletion [218]. Similarly, Parg null Drosophila melanogaster flies die at the embryonic stage; however, when grown at a permissive temperature, survival is increased. The surviving flies display PAR accumulation, neurodegeneration, reduced locomotion and premature death [219]. In line with data obtained in the D. melanogaster model, depletion of nuclear PARG isoforms in mice results in PAR accumulation in the brain [220]. Altogether, the body of information provided by PARG-deficient models further supports the essential role of PAR in the regulation of cellular homeostasis, especially in neuronal cells.
PARG functions have also been linked with the pathogenesis of inflammatory and neoplastic disorders. As for PARP1, a murine experimental model of colitis shows the contribution of PARG in sustaining the inflammatory response in the colon. Mice harbouring a deletion of the 110-kDa isoform of PARG protein, which are viable and fertile, are resistant to colon injury when challenged by dinitrobenzene sulfonic acid (DNBS) and show an attenuated inflammatory response [221].
According to experimental models of colitis, the serum titre of antibodies against PARG is a marker of mucosal damage caused by refractory ulcerative colitis [222].
Database analysis of sequencing data from The Cancer Genome Atlas (TCGA) revealed that PARG is overexpressed in many tumour types, in particular in breast tumour tissues, where it appears to be approximately fivefold more expressed than in normal epithelium. Approximately 15% of all invasive ductal breast tumours showed elevated PARG mRNA level, with the frequency reaching the 20% in HER2-positive and triple-negative subtypes. Thus, PARG levels are associated with a poor prognosis in breast cancers. Depletion of PARG significantly impairs the growth and metastasis of triple-negative breast tumours, in both in vitro and in vivo models, thus highlighting the therapeutic potential of PARG inhibition in breast cancer [223]. Importantly, the inhibition of PARG has already been proposed as a therapeutic treatment of human cancers [10,224]. This would be particularly appropriate for the treatment of aggressive breast cancers. It is worth mentioning that PARG inactivation often occurs as a resistance mechanism to PARP inhibitors in human serous ovarian and triple-negative breast cancers. Indeed, the genetic loss of PARG restores PAR formation and partially rescues PARP1 signalling [225].
7.4. ADP-ribosyl-acceptor hydrolase 1 (ARH1)
ARH1 is a cytosolic and ubiquitously expressed protein. Although the structure and mechanism are highly similar to ARH3 [63], ARH1 possesses a robust mono-ADP-ribosyl hydrolytic activity towards N-glycosidic bonds of arginine-modified proteins, and no activity against Ser-ADPr [48,62,63,226,227] (figure 1). By using both in vitro and in vivo models, it was shown that ARH1 plays a role in tumour genesis and progression. Indeed, Arh1-deficient mice spontaneously develop multiple malignancies, including lymphoma, hepatocellular carcinoma and hemangio-/rhabdomyosarcoma [228]. Studies performed in Arh1 heterozygous mice and in nude mice injected with Arh1-null MEFs showed the loss of heterozygosity (LOH) of the remaining Arh1 allele or loss of Arh1 gene activity due to spontaneous mutagenesis. Genome sequencing of mice revealed that Arh1 gene mutations were located in exons encoding the catalytic site. Analysis of human cancer COSMIC database revealed 32 ARH1 mutations found in human lung, breast and colon cancers; 70% of those mutations were missense mutations with single-base substitution, which surprisingly overlap with the mutations that spontaneously generate in Arh1 heterozygous mice [21]. Among those mutations, the D56N hits one of the two conserved aspartates (positions 60 and 61 in mouse Arh1) that are required for Mg2+ coordination and hydrolase activity [63,229].
7.5. ADP-ribosyl-acceptor hydrolase 3 (ARH3)
ARH3 was initially identified as a back-up PAR-degrading enzyme. Similar to PARG, ARH3 primarily cleaves the chains as exo-glycohydrolase, however, its specific activity against long PAR chains is nearly two levels of magnitude lower than for PARG [62,63,66,230]. Later on, ARH3 was shown to be the main hydrolase responsible for cleaving the ADPr from modified serine residues [66,231] (figure 1). The catalytic fold of ARH3 is completely different compared with PARG, which is instead a macrodomain-containing protein. In turn, the structural divergence reflects in a different conformation of ADP-ribose within the catalytic pocket as well as in a different catalytic mechanism [63,232–234]. As for ARH1, the presence of conserved aspartates (D77 and D78 in human ARH3), are essential for coordination of Mg2+ within ARH hydrolase [229].
At the cellular level, most of the ARH3 is found in cytoplasm, nucleus and mitochondria. The mitochondrial localization of ARH3 is determined by the presence of a mitochondrial-targeting sequence at the N-terminus, which suggests a role for ARH3 for ADPr degradation in mitochondria [230,235]. Nevertheless, all the ARH3 cellular functions described so far seem to converge on safeguarding genome stability. As discussed above, Ser-ADPr is the most abundant type of ADPr modification in response to genotoxic stress and it can be reversed only by ARH3, as far as we know. ARH3 is indeed able to cleave the terminal O-glycosidic bond joining the ADP-ribose and the serine of modified protein substrates [66,85]. ARH3 was also shown to act on free oligomers of PAR in cells released upon PARG endoglycohydrolase activity [236,237]. By doing so, ARH3 may control a mechanism of PARP1/PAR/AIF-mediated cell death (also known as Parthanatos) [236]. Altogether these data support the hypothesis that ARH3 could be involved in the pathogenesis of human disorders characterized by the cytotoxic and pro-apoptotic accumulation of PAR, such as in neurological disorders (e.g. in PD and AD).
Importantly, autosomal-recessive inherited genetic variants of ARH3 are directly linked with neurodegenerative disorders. Two independent studies have described 28 individuals belonging to fourteen families, which associate recessive and inactivating ARH3 gene mutations with paediatric-onset neurodegenerative disorder characterized by brain atrophy, developmental delay or regression, seizures, infection-associated episodes of ataxia, and axonal sensori-motor neuropathy [22,23]. It should be noted that most of the detected truncations/mutations predictably affect protein stability. As expected, ARH3 deficiencies associate with the accumulation of cellular ADPr, which drastically affects cell viability. Both the cellular accumulation of ADPr and the following cell death are prevented by treatment with PARP inhibitors [22,23]. Thus, these results propose once again the inhibition of PARP1 as a therapeutic strategy for the treatment of neurodegenerative diseases.
It is worth mentioning that, although all ARH3 patients show overall overlapping clinical features, both studies have not established an obvious genotype–phenotype correlation, for instance, regarding the onset and additional complications of the disorder. This observation suggests that additional factors, such as the genetic background or the exposure to environmental challenges, may contribute to the phenotypic variability among individuals. Considering the crucial roles of ARH3 in response to cellular stresses (e.g. oxidative and DNA damage insults), the exposure to stress conditions may be particularly important to anticipate the onset or worsen the neurodegenerative traits of disease.
8. Additional macrodomain-containing proteins in human disease
8.1. GDAP2 (MacroD3)
GDAP2 gene (found in metazoans and plants) encodes an uncharacterized additional macrodomain-containing protein, which has been recently linked to a human hereditary disorder characterized by ataxia, progressive spasticity and dementia [24]. Although GDAP2 macrodomain is similar to the one of MacroD1/2 proteins, it does not seem to bind derivatives of ADP-ribose, but instead, it appears to possess some affinity for poly(A) [69,72]. Yet the pathogenic mechanisms underlying GDAP2 deficiency remain unclear.
8.2. ALC1 (CHD1L)
The human ALC1 (amplified in liver cancer 1; also known as CHD1L (chromodomain-helicase-DNA-binding protein 1-like) gene encodes a member of the SNF2 (sucrose non-fermenter 2) superfamily of ATPases. Among SNF2 family members, ALC1 is unique because it includes a macrodomain that is capable of binding PAR. The binding of ALC1 to activated and PARylated PARP1 is crucial, but not sufficient, for DNA-dependent ATPase and ATP-dependent nucleosome remodelling activities [238–243].
ALC1 was originally identified as a gene amplified in hepatocellular carcinomas [18]. Overexpression of the ALC1 protein was found to transform human cells and to be oncogenic in mice [18,244,245]. A role for the oncogene ALC1 has also been demonstrated in breast and CRC [19,20].
In addition, gene mutations in human ALC1 were found in patients affected by congenital anomalies of the kidney and urinary tract [246].
9. ADPr and infectious disease
From the perspective of human pathologies bacterial ADPr systems can roughly be divided into two groups: the secreted exotoxins, which participate directly in promoting bacterial infection and associated symptoms; and those that have an internal role in bacterial stress-response. The latter potentially have major functions in persistence and have been proposed as potential therapeutic targets.
The exotoxins group encompasses a variety of bacterial ADP-ribosyl transferase toxins (bARTTs). MARylation of eukaryotic targets by bARTT is usually irreversible and aims at nucleotide-binding proteins, prevalently GTP- and, in some cases, ATP-binding proteins [44]. Interestingly, the deficiency of human ARH1 hydrolase leads to an increased sensitivity to cholera toxin, suggesting that bacterial ADPr can be reversed by the host hydrolases [65].
As outlined in §2 of this review, two subfamilies of bARTTs can be distinguished based on their structure and target proteins: diphtheria-like and cholera-like toxins, the latter encompassing an additional two subgroups, C2-like binary and C3-like toxins.
Diphtheria toxin ADP-ribosylates the eukaryotic elongation factor 2 (EF2), a GTP-binding protein essential for protein synthesis in the cell. The modification halts the entire protein synthesis and, in turn, leads to cell death [44]. The same mechanism of action is shared by the Exotoxin A from Pseudomonas aeruginosa, a ubiquitous multidrug-resistant pathogen [247].
Cholera and cholera-toxin-like proteins (e.g. the heat-labile enterotoxin from Escherichia coli and the pertussis toxin from Bordetella pertussis) transfer ADP-ribose onto heterotrimeric G proteins. The modification locks the subunit α of G proteins in a GTP-bound state, which constitutively stimulates host adenylate cyclase. In the case of cholera and enterotoxin, constitutive activation of G proteins results in opening and efflux of the chloride ions together with water [44]. Pertussis toxin acts towards virtually all mammalian cell types and has a broad array of effects on host cell activities [248]. ADP-ribosyl transferase subunit of typhoid toxin from Salmonella typhi (exclusively human pathogen) is structurally similar to pertussis toxin; however, the pathogenic mechanisms as well as the proteins substrate(s) of this toxin remain unknown [249].
The C2-like toxins from Clostridium sp. [44,250] and the newly characterized SpvB from Salmonella sp. [251] are examples of toxins ADP-ribosylating non-polymerized form of actin. The MARylated G-actin, upon incorporation into filaments, inhibits further integrations resulting in serious impairments of cellular cytoskeleton.
The C3-like toxins expressed by Clostridium botulinum, Bacillus cereus, Staphylococcus aureus and others target small Rho GTPase enzymes, which modulate actin polymerization. The MARylation of Rho GTPase alters the interaction with protein partners, thus locking themselves in a deactivated state. The consequences are similar to that of the C2-like toxins—the disintegration of the cytoskeleton. The recently described SpyA from Streptococcus pyogenes targets another cytoskeletal protein-vimentin, and actin to a lesser degree [252].
In addition, a very intriguing class of bARTTs has been described in Legionella pneumophila. The Legionella protein SdeA modifies ubiquitin molecules of the host by transferring ADPr on arginine 42, thus impairing the physiological ubiquitination processes. Through a process of phosphoribosyl-ubiquitination, MARylated ubiquitin is in turn transferred onto serine residues of protein substrates, therefore modulating the endogenous functions of modified proteins, such as Rab33 [253–255]. This ADPr system is reversible, as it can be counteracted by another bacterial protein, SidJ, acting as a hydrolase [256].
One of the best-studied stress-response systems in bacteria is the toxin-antitoxin (TA) module. There are more than 1000 TA modules known [257]. Among them, the only known module to exploit the ADPr system is the DarT/DarG module, which is found in various bacteria, including the global pathogen Mycobacterium tuberculosis [258]. DarT is an ART able to MARylate the single-stranded DNA on specific thymidine residues, which impairs cellular processes essential for bacterial growth and activates SOS response. The macrodomain protein DarG, which hydrolyses the ADP-ribosylated DNA, counteracts DarT activity [258].
Another example of ADPr system in bacterial stress response is operated by sirtuins. While the mammalian sirtuins seem to act primarily as NAD-dependent deacetylases, a diverged class of sirtuins present in pathogenic bacteria and fungi (called SirTMs) exhibits a robust protein ADPr activity that is regulated by another protein modification: lipoylation. This mechanism was shown to modulate the microbial oxidative stress response [259].
10. ADPr in viral infections
Viruses from the Coronaviridae, Togaviridae and Hepeviridae families all contain genes encoding macrodomain-containing proteins, suggesting a role for ADPr during infection diseases [260–265]. Notably, several human PARPs have been shown to be activated and function in the host antiviral response. For instance, PARPs 9, 12, 13 and 14 are among the 62 Interferon-stimulated genes and overexpression of PARPs 7, 10 or 12 inhibits alphavirus replication [266]. In addition, PARPs 5a, 12, 13, 14 and 15 localize at the stress granules, well-known cytoplasmic structures with antiviral functions; interestingly, the integrity of stress granules is inhibited by the alphaviral macrodomain-containing nsP3 [138,267]. Thus, it appears that ADPr is required for a proper host antiviral response and that viruses have evolved systems (mainly consisting of macrodomain-containing proteins) able to modulate defensive host ADPr systems. Not surprisingly, PARPs 4, 9, 13, 14 and 15 show a rapid evolution as a result of a strong recurrent positive selection in the attempt to escape the modulation operated by viral proteins [268,269].
11. Concluding remarks
Numerous pioneering findings have shown the impact of ADPr on many vital cellular processes, the dysregulation of which is known to lead to human disorders. Many genes involved in ADPr are now known to be mutated or dysregulated in various acquired and hereditary diseases, such as neurological disorders and cancer. By contrast, the pharmacological modulation of ADPr by small-molecule inhibitors can be a potent tool to treat human diseases. Research within the ADPr field has been progressing particularly fast in recent years, and it is hoped that this will provide new avenues for the therapeutic interventions.
Supplementary Material
Acknowledgements
The authors thank Kerryanne Crawford (University of Oxford) and Giuliana Catara (Institute of Protein Biochemistry) for helpful comments on the manuscript.
Data accessibility
This article has no additional data.
Competing interests
The authors declare no competing interests.
Funding
L.P. was supported by a fellowship from the Italian Foundation for Cancer Research (FIRC, Milan, Italy; grant no. 14895). P.M. and A.M. are supported by the Croatian Science Foundation (IP-2016-06-4242). Work in I.A.'s laboratory is supported by Wellcome Trust (grant nos. 101794 and 210634) and Cancer Research United Kingdom (grant no. C35050/A22284).
References
- 1.Jensen ON. 2006. Interpreting the protein language using proteomics. Nat. Rev. Mol. Cell Biol. 7, 391–403. ( 10.1038/nrm1939) [DOI] [PubMed] [Google Scholar]
- 2.Palazzo L, Mikoč A, Ahel I. 2017. ADP-ribosylation: new facets of an ancient modification. FEBS J. 284, 2932–2946. ( 10.1111/febs.14078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lüscher B, Bütepage M, Eckei L, Krieg S, Verheugd P, Shilton BH. 2018. ADP-ribosylation, a multifaceted posttranslational modification involved in the control of cell physiology in health and disease. Chem. Rev. 118, 1092–1136. ( 10.1021/acs.chemrev.7b00122) [DOI] [PubMed] [Google Scholar]
- 4.Ringel AE, Tucker SA, Haigis MC. 2018. Chemical and physiological features of mitochondrial acylation. Mol. Cell. 72, 610–624. ( 10.1016/j.molcel.2018.10.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambon P, Weill JD, Mandel P. 1963. Nicotinamide mononucleotide activation of new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochem. Biophys. Res. Commun. 11, 39–43. ( 10.1016/0006-291X(63)90024-X) [DOI] [PubMed] [Google Scholar]
- 6.Chambon P, Weill JD, Strosser MT, Mandel P, Doly J. 1966. On the formation of a novel adenylic compound by enzymatic extracts of liver nuclei. Biochem. Biophys. Res. Commun. 25, 638–643. ( 10.1016/0006-291X(66)90502-X) [DOI] [Google Scholar]
- 7.Nishizuka Y, Ueda K, Nakazawa K, Hayaishi O. 1967. Studies on the polymer of adenosine diphosphate ribose. I. Enzymic formation from nicotinamide adenine dinuclotide in mammalian nuclei. J. Biol. Chem. 242, 3164–3171. [PubMed] [Google Scholar]
- 8.Sugimura T, Fujimura S, Hasegawa S, Kawamura Y. 1967. Polymerization of the adenosine 5′-diphosphate ribose moiety of nad by rat liver nuclear enzyme. Biochim. Biophys. Acta Nucleic Acids Protein Synth. 138, 438–441. ( 10.1016/0005-2787(67)90507-2) [DOI] [PubMed] [Google Scholar]
- 9.Lord CJ, Ashworth A. 2017. PARP inhibitors: Synthetic lethality in the clinic. Science 355, 1152–1158. ( 10.1126/science.aam7344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palazzo L, Ahel I. 2018. PARPs in genome stability and signal transduction: implications for cancer therapy. Biochem. Soc. Trans. 46, 1681–1695. ( 10.1042/BST20180418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguiar RC, Takeyama K, He C, Kreinbrink K, Shipp MA. 2005. B-aggressive lymphoma family proteins have unique domains that modulate transcription and exhibit poly(ADP-ribose) polymerase activity. J. Biol. Chem. 280, 33 756–33 765. ( 10.1074/jbc.M505408200) [DOI] [PubMed] [Google Scholar]
- 12.Aguiar RC, Yakushijin Y, Kharbanda S, Salgia R, Fletcher JA, Shipp MA. 2000. BAL is a novel risk-related gene in diffuse large B-cell lymphomas that enhances cellular migration. Blood 96, 4328–4334. [PubMed] [Google Scholar]
- 13.Tang X, Zhang H, Long Y, Hua H, Jiang Y, Jing J. 2018. PARP9 is overexpressed in human breast cancer and promotes cancer cell migration. Oncol. Lett. 16, 4073–4077. ( 10.3892/ol.2018.9124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho SH, Goenka S, Henttinen T, Gudapati P, Reinikainen A, Eischen CM, Lahesmaa R, Boothby M. 2009. PARP-14, a member of the B aggressive lymphoma family, transduces survival signals in primary B cells. Blood 113, 2416–2425. ( 10.1182/blood-2008-03-144121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanagawa T, Funasaka T, Tsutsumi S, Hu H, Watanabe H, Raz A. 2007. Regulation of phosphoglucose isomerase/autocrine motility factor activities by the poly(ADP-ribose) polymerase family-14. Cancer Res. 67, 8682–8689. ( 10.1158/0008-5472.CAN-07-1586) [DOI] [PubMed] [Google Scholar]
- 16.Mehrotra P, Riley JP, Patel R, Li F, Voss L, Goenka S. 2011. PARP-14 functions as a transcriptional switch for Stat6-dependent gene activation. J. Biol. Chem. 286, 1767–1776. ( 10.1074/jbc.M110.157768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iansante V, et al. 2015. PARP14 promotes the Warburg effect in hepatocellular carcinoma by inhibiting JNK1-dependent PKM2 phosphorylation and activation. Nat. Commun. 6, 7882 ( 10.1038/ncomms8882) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma NF, et al. 2008. Isolation and characterization of a novel oncogene, amplified in liver cancer 1, within a commonly amplified region at 1q21 in hepatocellular carcinoma. Hepatology 47, 503–510. ( 10.1002/hep.22072) [DOI] [PubMed] [Google Scholar]
- 19.Mu QJ, Li HL, Yao Y, Liu SC, Yin CG, Ma XZ. 2015. Chromodomain helicase/ATPase DNA-binding protein 1-like gene (CHD1 L) expression and implications for invasion and metastasis of breast cancer. PLoS ONE 10, e0143030 ( 10.1371/journal.pone.0143030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji X, et al. 2013. CHD1 L promotes tumor progression and predicts survival in colorectal carcinoma. J. Surg. Res. 185, 84–91. ( 10.1016/j.jss.2013.05.008) [DOI] [PubMed] [Google Scholar]
- 21.Kato J, Vekhter D, Heath J, Zhu J, Barbieri JT, Moss J. 2015. Mutations of the functional ARH1 allele in tumors from ARH1 heterozygous mice and cells affect ARH1 catalytic activity, cell proliferation and tumorigenesis. Oncogenesis 4, e151 ( 10.1038/oncsis.2015.5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danhauser K, et al. 2018. Bi-allelic ADPRHL2 mutations cause neurodegeneration with developmental delay, ataxia, and axonal neuropathy. Am. J. Hum. Genet. 103, 817–825. ( 10.1016/j.ajhg.2018.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh SG, et al. 2018. Biallelic mutations in ADPRHL2, encoding ADP-ribosylhydrolase 3, lead to a degenerative pediatric stress-induced epileptic ataxia syndrome. Am. J. Hum. Genet. 103, 431–439. ( 10.1016/j.ajhg.2018.07.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eidhof I, et al. 2018. GDAP2 mutations implicate susceptibility to cellular stress in a new form of cerebellar ataxia. Brain 141, 2592–2604. ( 10.1093/brain/awy198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng YG, Han WD, Zhao YL, Huang K, Si YL, Wu ZQ, Mu YM. 2007. Induction of the LRP16 gene by estrogen promotes the invasive growth of Ishikawa human endometrial cancer cells through the downregulation of E-cadherin. Cell Res. 17, 869–880. ( 10.1038/cr.2007.79) [DOI] [PubMed] [Google Scholar]
- 26.Li YZ, Zhao P, Han WD. 2009. Clinicopathological significance of LRP16 protein in 336 gastric carcinoma patients. World J. Gastroenterol. 15, 4833–4837. ( 10.3748/wjg.15.4833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xi HQ, Zhao P, Han WD. 2010. Clinicopathological significance and prognostic value of LRP16 expression in colorectal carcinoma. World J. Gastroenterol. 16, 1644–1648. ( 10.3748/wjg.v16.i13.1644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Wu Z, An X, Mei Q, Bai M, Hanski L, Li X, Ahola T, Han W. 2017. Blockade of the LRP16-PKR-NF-κB signaling axis sensitizes colorectal carcinoma cells to DNA-damaging cytotoxic therapy. Elife 6, e27301 ( 10.7554/eLife.27301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao DX, Han WD, Zhao YL, Pu YD, Mu YM, Luo CH, Li XH. 2006. Expression and clinical significance of LRP16 gene in human breast cancer. Ai Zheng. 25, 866–870. [PubMed] [Google Scholar]
- 30.Zhao P, Lu Y, Han W. 2010. Clinicopathological significance and prognostic value of leukemia-related protein 16 expression in invasive ductal breast carcinoma. Cancer Sci. 101, 2262–2268. ( 10.1111/j.1349-7006.2010.01658.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anney R, et al. 2010. A genome-wide scan for common alleles affecting risk for autism. Hum. Mol. Genet. 19, 4072–4082. ( 10.1093/hmg/ddq307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsang KM, Croen LA, Torres AR, Kharrazi M, Delorenze GN, Windham GC, Yoshida CK, Zerbo O, Weiss LA. 2013. A genome-wide survey of transgenerational genetic effects in autism. PLoS ONE 8, e76978 ( 10.1371/journal.pone.0076978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones RM, Cadby G, Blangero J, Abraham LJ, Whitehouse AJO, Moses EK. 2014. MACROD2 gene associated with autistic-like traits in a general population sample. Psychiatr. Genet. 24, 241–248. ( 10.1097/YPG.0000000000000052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maas NM, et al. 2007. The C20orf133 gene is disrupted in a patient with Kabuki syndrome. J. Med. Genet. 44, 562–569. ( 10.1136/jmg.2007.049510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuniba H, et al. 2009. Molecular karyotyping in 17 patients and mutation screening in 41 patients with Kabuki syndrome. J. Hum. Genet. 54, 304–309. ( 10.1038/jhg.2009.30) [DOI] [PubMed] [Google Scholar]
- 36.Beroukhim R, et al. 2010. The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905. ( 10.1038/nature08822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dulak AM, et al. 2012. Gastrointestinal adenocarcinomas of the esophagus, stomach, and colon exhibit distinct patterns of genome instability and oncogenesis. Cancer Res. 72, 4383–4393. ( 10.1158/0008-5472.CAN-11-3893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakthianandeswaren A, et al. 2018. MACROD2 haploinsufficiency impairs catalytic activity of PARP1 and promotes chromosome instability and growth of intestinal tumors. Cancer Discov. 8, 988–1005. ( 10.1158/2159-8290.CD-17-0909) [DOI] [PubMed] [Google Scholar]
- 39.Sharifi R, et al. 2013. Deficiency of terminal ADP-ribose protein glycohydrolase TARG1/C6orf130 in neurodegenerative disease. EMBO J. 32, 1225–1237. ( 10.1038/emboj.2013.51) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hottiger MO, Hassa PO, Luscher B, Schuler H, Koch-Nolte F. 2010. Toward a unified nomenclature for mammalian Adp-ribosyltransferases. Trends Biochem. Sci. 35, 208–219. ( 10.1016/j.tibs.2009.12.003) [DOI] [PubMed] [Google Scholar]
- 41.Cohen MS, Chang P. 2018. Insights into the biogenesis, function, and regulation of ADP-ribosylation. Nat. Chem. Biol. 14, 236–243. ( 10.1038/nchembio.2568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barkauskaite E, Jankevicius G, Ahel I. 2015. Structures and mechanisms of enzymes employed in the synthesis and degradation of PARP-dependent protein ADP-ribosylation. Mol. Cell. 58, 935–946. ( 10.1016/j.molcel.2015.05.007) [DOI] [PubMed] [Google Scholar]
- 43.Cassel D, Pfeuffer T. 1978. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc. Natl Acad. Sci. USA 75, 2669–2673. ( 10.1073/pnas.75.6.2669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon NC, Aktories K, Barbieri JT. 2014. Novel bacterial ADP-ribosylating toxins: structure and function. Nat. Rev. Microbiol. 12, 599–611. ( 10.1038/nrmicro3310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vyas S, Matic I, Uchima L, Rood J, Zaja R, Hay RT, Ahel I, Chang P. 2014. Family-wide analysis of poly(ADP-ribose) polymerase activity. Nat Commun. 5, 4426 ( 10.1038/ncomms5426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Ness BG, Howard JB, Bodley JW. 1980. ADP-ribosylation of elongation factor 2 by diphtheria toxin. Isolation and properties of the novel ribosyl-amino acid and its hydrolysis products. J. Biol. Chem. 255, 10 717–10 720. [PubMed] [Google Scholar]
- 47.Koch-Nolte F, Kernstock S, Mueller-Dieckmann C, Weiss MS, Haag F. 2008. Mammalian ADP-ribosyltransferases and ADP-ribosylhydrolases. Front. Biosci. 13, 6716–6729. ( 10.2741/3184) [DOI] [PubMed] [Google Scholar]
- 48.Laing S, Unger M, Koch-Nolte F, Haag F. 2011. Adp-ribosylation of arginine. Amino Acids 41, 257–269. ( 10.1007/s00726-010-0676-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matic I, Ahel I, Hay RT. 2012. Reanalysis of phosphoproteomics data uncovers ADP-ribosylation sites. Nat. Methods 9, 771–772. ( 10.1038/nmeth.2106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fabrizio G, Di Paola S, Stilla A, Giannotta M, Ruggiero C, Menzel S, Koch-Nolte F, Sallese M, Di Girolamo M. 2015. ARTC1-mediated ADP-ribosylation of GRP78/BiP: a new player in endoplasmic-reticulum stress responses. Cell Mol. Life Sci. 72, 1209–1225. ( 10.1007/s00018-014-1745-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martello R, Leutert M, Jungmichel S, Bilan V, Larsen SC, Young C, Hottiger MO, Nielsen ML. 2016. Proteome-wide identification of the endogenous ADP-ribosylome of mammalian cells and tissue. Nat. Commun. 7, 12917 ( 10.1038/ncomms12917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leutert M, et al. 2018. Proteomic characterization of the heart and skeletal muscle reveals widespread arginine ADP-ribosylation by the ARTC1 ectoenzyme. Cell Rep. 24, 1916–1929. ( 10.1016/j.celrep.2018.07.048) [DOI] [PubMed] [Google Scholar]
- 53.Gibson BA, Kraus WL. 2012. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat. Rev. Mol. Cell Biol. 13, 411–424. ( 10.1038/nrm3376) [DOI] [PubMed] [Google Scholar]
- 54.Leidecker O, et al. 2016. Serine is a new target residue for endogenous ADP-ribosylation on histones. Nat. Chem. Biol. 12, 998–1000. ( 10.1038/nchembio.2180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crawford K, Bonfiglio JJ, Mikoč A, Matic I, Ahel I. 2018. Specificity of reversible ADP-ribosylation and regulation of cellular processes. Crit. Rev. Biochem. Mol. Biol. 53, 64–82. ( 10.1080/10409238.2017.1394265) [DOI] [PubMed] [Google Scholar]
- 56.de Murcia G, Ménissier de Murcia J. 1994. Poly(ADP-ribose) polymerase: a molecular nick-sensor. Trends Biochem. Sci. 19, 172–176. ( 10.1016/0968-0004(94)90280-1) [DOI] [PubMed] [Google Scholar]
- 57.D'Amours D, Desnoyers S, D'Silva I, Poirier GG. 1999. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem. J. 342, 249–268. ( 10.1042/bj3420249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rippmann JF, Damm K, Schnapp A. 2002. Functional characterization of the poly(ADP-ribose) polymerase activity of tankyrase 1, a potential regulator of telomere length. J. Mol. Biol. 323, 217–224. ( 10.1016/S0022-2836(02)00946-4) [DOI] [PubMed] [Google Scholar]
- 59.Kleine H, Poreba E, Lesniewicz K, Hassa PO, Hottiger MO, Litchfield DW, Shilton BH, Lüscher B. 2008. Substrate-assisted catalysis by PARP10 limits its activity to mono-ADP-ribosylation. Mol. Cell. 32, 57–69. ( 10.1016/j.molcel.2008.08.009) [DOI] [PubMed] [Google Scholar]
- 60.Bütepage M, Eckei L, Verheugd P, Lüscher B. 2015. Intracellular mono-ADP-ribosylation in signaling and disease. Cells 4, 569–595. ( 10.3390/cells4040569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berthold CL, Wang H, Nordlund S, Högbom M. 2009. Mechanism of ADP-ribosylation removal revealed by the structure and ligand complexes of the dimanganese mono-ADP-ribosylhydrolase DraG. Proc. Natl Acad. Sci. USA 106, 14 247–14 252. ( 10.1073/pnas.0905906106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oka S, Kato J, Moss J. 2006. Identification and characterization of a mammalian 39-kDa poly(ADP-ribose) glycohydrolase. J. Biol. Chem. 281, 705–713. ( 10.1074/jbc.M510290200) [DOI] [PubMed] [Google Scholar]
- 63.Rack JGM, Ariza A, Drown BS, Henfrey C, Bartlett E, Shirai T, Hergenrother PJ, Ahel I. 2018. (ADP-ribosyl)hydrolases: structural basis for differential substrate recognition and inhibition. Cell Chem. Biol. 25, 1533–1546. ( 10.1016/j.chembiol.2018.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bu X, Kato J, Moss J. 2018. Emerging roles of ADP-ribosyl-acceptor hydrolases (ARHs) in tumorigenesis and cell death pathways. Biochem. Pharmacol. 2952, 30 411–30 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watanabe K, Kato J, Zhu J, Oda H, Ishiwata-Endo H, Moss J. 2018. Enhanced sensitivity to cholera toxin in female ADP-ribosylarginine hydrolase (ARH1)-deficient mice. PLoS ONE 13, e0207693 ( 10.1371/journal.pone.0207693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fontana P, Bonfiglio JJ, Palazzo L, Bartlett E, Matic I, Ahel I. 2017. Serine ADP-ribosylation reversal by the hydrolase ARH3. Elife 6, e28533 ( 10.7554/eLife.28533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Drown BS, Shirai T, Rack JGM, Ahel I, Hergenrother PJ. 2018. Monitoring poly(ADP-ribosyl)glycohydrolase activity with a continuous fluorescent substrate. Cell Chem. Biol. 25, 1562–1570. ( 10.1016/j.chembiol.2018.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feijs KL, Forst AH, Verheugd P, Lüscher B. 2013. Macrodomain-containing proteins: regulating new intracellular functions of mono(ADP-ribosyl)ation. Nat. Rev. Mol. Cell Biol. 14, 443–451. ( 10.1038/nrm3601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rack JG, Perina D, Ahel I. 2016. Macrodomains: structure, function, evolution, and catalytic activities. Annu. Rev. Biochem. 85, 431–454. ( 10.1146/annurev-biochem-060815-014935) [DOI] [PubMed] [Google Scholar]
- 70.Karras G, Kustatscher G, Buhecha HR, Allen MD, Pugieux C, Sait F, Bycroft M, Ladurner AG. 2005. The macro domain is an ADP-ribose binding module. EMBO J. 24, 1911–1920. ( 10.1038/sj.emboj.7600664) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tong L, Denu JM. 2010. Function and metabolism of sirtuin metabolite O-acetyl-ADP-ribose. Biochim. Biophys. Acta 1804, 1617–1625. ( 10.1016/j.bbapap.2010.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neuvonen M, Ahola T. 2009. Differential activities of cellular and viral macro domain proteins in binding of ADP-ribose metabolites. J. Mol. Biol. 385, 212–225. ( 10.1016/j.jmb.2008.10.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Slade D, Dunstan MS, Barkauskaite E, Weston R, Lafite P, Dixon N, Ahel M, Leys D, Ahel I. 2011. The structure and catalytic mechanism of a poly(ADP-ribose) glycohydrolase. Nature. 477, 616–620. ( 10.1038/nature10404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jankevicius G, Hassler M, Golia B, Rybin V, Zacharias M, Timinszky G, Ladurner AG. 2013. A family of macrodomain proteins reverses cellular mono-ADP-ribosylation. Nat. Struct. Mol. Biol. 20, 508–514. ( 10.1038/nsmb.2523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosenthal F, et al. 2013. Macrodomain-containing proteins are new mono-ADP-ribosylhydrolases. Nat. Struct. Mol. Biol. 20, 502–507. ( 10.1038/nsmb.2521) [DOI] [PubMed] [Google Scholar]
- 76.Gupte R, Liu Z, Kraus WL. 2017. PARPs and ADP-ribosylation: recent advances linking molecular functions to biological outcomes. Genes Dev. 31, 101–126. ( 10.1101/gad.291518.116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Langelier MF, Eisemann T, Riccio AA, Pascal JM. 2018. PARP family enzymes: regulation and catalysis of the poly(ADP-ribose) posttranslational modification. Curr. Opin. Struct. Biol. 53, 187–198. ( 10.1016/j.sbi.2018.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bai P, Cantó C. 2012. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab. 16, 290–295. ( 10.1016/j.cmet.2012.06.016) [DOI] [PubMed] [Google Scholar]
- 79.Luo X, Kraus WL. 2012. On PAR with PARP: cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev. 26, 417–432. ( 10.1101/gad.183509.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Posavec MM, Crawford K, Ahel I. 2017. PARP, transcription and chromatin modeling. Semin. Cell Dev. Biol. 63, 102–113. ( 10.1016/j.semcdb.2016.09.014) [DOI] [PubMed] [Google Scholar]
- 81.D'Andrea AD. 2018. Mechanisms of PARP inhibitor sensitivity and resistance. DNA Repair (Amst). 71, 172–176. ( 10.1016/j.dnarep.2018.08.021) [DOI] [PubMed] [Google Scholar]
- 82.Pilié PG, Tang C, Mills GB, Yap TA. 2019. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 16, 81–104. ( 10.1038/s41571-018-0114-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gibbs-Seymour I, Fontana P, Rack JGM, Ahel I. 2016. HPF1/C4orf27 Is a PARP-1-interacting protein that regulates PARP-1 ADP-ribosylation activity. Mol. Cell 62, 432–442. ( 10.1016/j.molcel.2016.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonfiglio JJ, et al. 2017. Serine ADP-ribosylation depends on HPF1. Mol. Cell 65, 932–940. ( 10.1016/j.molcel.2017.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Palazzo L, Leidecker O, Prokhorova E, Dauben H, Matic I, Ahel I. 2018. Serine is the major residue for ADP-ribosylation upon DNA damage. Elife 7, e34334 ( 10.7554/eLife.34334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Larsen SC, Hendriks IA, Lyon D, Jensen LJ, Nielsen ML. 2018. Systems-wide analysis of serine ADP-ribosylation reveals widespread occurrence and site-specific overlap with phosphorylation. Cell. Rep. 24, 2493–2505. ( 10.1016/j.celrep.2018.07.083) [DOI] [PubMed] [Google Scholar]
- 87.Ando Y, et al. 2019. ELTA: enzymatic labeling of terminal ADP-ribose. Mol. Cell. 73, 845–856. ( 10.1016/j.molcel.2018.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bartlett E, Bonfiglio JJ, Prokhorova E, Colby T, Zobel F, Ahel I, Matic I. 2018. Interplay of histone marks with serine ADP-ribosylation. Cell Rep. 24, 3488–3502. ( 10.1016/j.celrep.2018.08.092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liszczak G, Diehl KL, Dann GP, Muir TW. 2018. Acetylation blocks DNA damage-induced chromatin ADP-ribosylation. Nat. Chem. Biol. 14, 837–840. ( 10.1038/s41589-018-0097-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eliasson MJ, et al. 1997. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat. Med. 3, 1089–1095. ( 10.1038/nm1097-1089) [DOI] [PubMed] [Google Scholar]
- 91.Chiarugi A. 2002. Poly(ADP-ribose) polymerase: killer or conspirator? The 'suicide hypothesis' revisited. Trends Pharmacol. Sci. 23, 122–129. ( 10.1016/S0165-6147(00)01902-7) [DOI] [PubMed] [Google Scholar]
- 92.Jagtap PG, Baloglu E, Southan GJ, Mabley JG, Li H, Zhou J, van Duzer J, Salzman AL, Szabó C. 2005. Discovery of potent poly(ADP-ribose) polymerase-1 inhibitors from the modification of indeno[1,2-c]isoquinolinone. J. Med. Chem. 48, 5100–5103. ( 10.1021/jm0502891) [DOI] [PubMed] [Google Scholar]
- 93.Pacher P, Szabo C. 2008. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am. J. Pathol. 173, 2–13. ( 10.2353/ajpath.2008.080019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martire S, Mosca L, d'Erme M. 2015. PARP-1 involvement in neurodegeneration: a focus on Alzheimer's and Parkinson's diseases. Mech. Ageing Dev. 146–148, 53–64. ( 10.1016/j.mad.2015.04.001) [DOI] [PubMed] [Google Scholar]
- 95.Tóth-Zsámboki E, et al. 2006. Activation of poly(ADP-ribose) polymerase by myocardial ischemia and coronary reperfusion in human circulating leukocytes. Mol. Med. 12, 221–228. ( 10.2119/2006-00055.Toth-Zsamboki) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Soriano FG, et al. 2006. Potential role of poly(adenosine 5'-diphosphate-ribose) polymerase activation in the pathogenesis of myocardial contractile dysfunction associated with human septic shock. Crit. Care Med. 34, 1073–1079. ( 10.1097/01.CCM.0000206470.47721.8D) [DOI] [PubMed] [Google Scholar]
- 97.Yao L, Huang K, Huang D, Wang J, Guo H, Liao Y. 2008. Acute myocardial infarction induced increases in plasma tumor necrosis factor-alpha and interleukin-10 are associated with the activation of poly(ADP-ribose) polymerase of circulating mononuclear cell. Int. J. Cardiol. 123, 366–368. ( 10.1016/j.ijcard.2007.06.069) [DOI] [PubMed] [Google Scholar]
- 98.Love S, Barber R, Wilcock GK. 1999. Neuronal accumulation of poly(ADP-ribose) after brain ischaemia. Neuropathol. Appl. Neurobiol. 25, 98–103. ( 10.1046/j.1365-2990.1999.00179.x) [DOI] [PubMed] [Google Scholar]
- 99.Love S, Barber R, Wilcock GK. 2000. Neuronal death in brain infarcts in man. Neuropathol. Appl. Neurobiol. 26, 55–66. ( 10.1046/j.1365-2990.2000.00218.x) [DOI] [PubMed] [Google Scholar]
- 100.Okolie EE, Shall S. 1979. The significance of antibodies to poly(adenosine diphosphate-ribose) in systemic lupus erythematosus. Clin. Exp. Immunol. 36, 151–164. [PMC free article] [PubMed] [Google Scholar]
- 101.Negri C, et al. 1990. Autoantibodies to poly(ADP-ribose)polymerase in autoimmune diseases. Autoimmunity 6, 203–209. ( 10.3109/08916939009041040) [DOI] [PubMed] [Google Scholar]
- 102.Jeoung D, Lim Y, Lee EB, Lee S, Kim HY, Lee H, Song YW. 2004. Identification of autoantibody against poly (ADP-ribose) polymerase (PARP) fragment as a serological marker in systemic lupus erythematosus. J. Autoimmun. 22, 87–94. ( 10.1016/j.jaut.2003.10.009) [DOI] [PubMed] [Google Scholar]
- 103.Reumaux D, Mézière C, Colombel JF, Duthilleul P, Mueller S. 1995. Distinct production of autoantibodies to nuclear components in ulcerative colitis and in Crohn's disease. Clin. Immunol. Immunopathol. 77, 349–357. ( 10.1006/clin.1995.1162) [DOI] [PubMed] [Google Scholar]
- 104.Pacher P, Szabó C. 2005. Role of poly(ADP-ribose) polymerase-1 activation in the pathogenesis of diabetic complications: endothelial dysfunction, as a common underlying theme. Antioxid. Redox Signal. 7, 1568–1580. ( 10.1089/ars.2005.7.1568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pacher P, Szabó C. 2007. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc. Drug Rev. 25, 235–260. ( 10.1111/j.1527-3466.2007.00018.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Szabó C, Zanchi A, Komjáti K, Pacher P, Krolewski AS, Quist WC, LoGerfo FW, Horton ES, Veves A. 2002. Poly(ADP-Ribose) polymerase is activated in subjects at risk of developing type 2 diabetes and is associated with impaired vascular reactivity. Circulation 106, 2680–2686. ( 10.1161/01.CIR.0000038365.78031.9C) [DOI] [PubMed] [Google Scholar]
- 107.Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. 2002. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation 106, 927–932. ( 10.1161/01.CIR.0000026393.47805.21) [DOI] [PubMed] [Google Scholar]
- 108.Adaikalakoteswari A, Rema M, Mohan V, Balasubramanyam M. 2007. Oxidative DNA damage and augmentation of poly(ADP-ribose) polymerase/nuclear factor-kappa B signaling in patients with type 2 diabetes and microangiopathy. Int. J. Biochem. Cell Biol. 39, 1673–1684. ( 10.1016/j.biocel.2007.04.013) [DOI] [PubMed] [Google Scholar]
- 109.Hoch NC, et al. 2017. XRCC1 mutation is associated with PARP1 hyperactivation and cerebellar ataxia. Nature 541, 87–91. ( 10.1038/nature20790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kam TI, et al. 2018. Poly(ADP-ribose) drives pathologic α-synuclein neurodegeneration in Parkinson's disease. Science 362, eaat8407 ( 10.1126/science.aat8407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Masson M, Niedergang C, Schreiber V, Muller S, Menissier-de Murcia J, de Murcia G. 1998. XRCC1 is specifically associated with poly(ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Mol. Cell Biol. 18, 3563–3571. ( 10.1128/MCB.18.6.3563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.El-Khamisy SF, Masutani M, Suzuki H, Caldecott KW. 2003. A requirement for PARP-1 for the assembly or stability of XRCC1 nuclear foci at sites of oxidative DNA damage. Nucleic Acids Res. 31, 5526–5533. ( 10.1093/nar/gkg761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Okano S, Lan L, Caldecott KW, Mori T, Yasui A. 2003. Spatial and temporal cellular responses to single-strand breaks in human cells. Mol. Cell Biol. 23, 3974–3981. ( 10.1128/MCB.23.11.3974-3981.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lan L, Nakajima S, Oohata Y, Takao M, Okano S, Masutani M, Wilson SH, Yasui A. 2004. In situ analysis of repair processes for oxidative DNA damage in mammalian cells. Proc. Natl Acad. Sci. USA 101, 13 738–13 743. ( 10.1073/pnas.0406048101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Breslin C, Hornyak RA, Rulten SL, Hanzlikova H, Oliver AW, Caldecott KW. 2015. The XRCC1 phosphate-binding pocket binds poly (ADP-ribose) and is required for XRCC1 function. Nucleic Acids Res. 43, 6934–6944. ( 10.1093/nar/gkv623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moreira MC, et al. 2001. The gene mutated in ataxia-ocular apraxia 1 encodes the new HIT/Zn-finger protein aprataxin. Nat. Genet. 29, 189–193. ( 10.1038/ng1001-189) [DOI] [PubMed] [Google Scholar]
- 117.Takashima H, et al. 2002. Mutation of TDP1, encoding a topoisomerase I-dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nat. Genet. 32, 267–272. ( 10.1038/ng987) [DOI] [PubMed] [Google Scholar]
- 118.El-Khamisy SF, Saifi GM, Weinfeld M, Johansson F, Helleday T, Lupski JR, Caldecott KW. 2005. Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. Nature 434, 108–113. ( 10.1038/nature03314) [DOI] [PubMed] [Google Scholar]
- 119.Ahel I, Rass U, El-Khamisy SF, Katyal S, Clements PM, McKinnon PJ, Caldecott KW, West SC. 2006. The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates. Nature 443, 713–716. ( 10.1038/nature05164) [DOI] [PubMed] [Google Scholar]
- 120.Rass U, Ahel I, West SC. 2007. Defective DNA repair and neurodegenerative disease. Cell 130, 991–1004. ( 10.1016/j.cell.2007.08.043) [DOI] [PubMed] [Google Scholar]
- 121.Bras J, et al. 2015. Mutations in PNKP cause recessive ataxia with oculomotor apraxia type 4. Am. J. Hum. Genet. 96, 474–479. ( 10.1016/j.ajhg.2015.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kalia LV, Kalia SK, McLean PJ, Lozano AM, Lang AE. 2013. α-Synuclein oligomers and clinical implications for Parkinson disease. Ann. Neurol. 73, 155–169. ( 10.1002/ana.23746) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Larson ME, Lesné SE. 2012. Soluble Aβ oligomer production and toxicity. J. Neurochem. 120(Suppl. 1), 125–139. ( 10.1111/j.1471-4159.2011.07478.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tillement L, Lecanu L, Papadopoulos V. 2011. Alzheimer's disease: effects of β-amyloid on mitochondria. Mitochondrion 11, 13–21. ( 10.1016/j.mito.2010.08.009) [DOI] [PubMed] [Google Scholar]
- 125.Cha MY, Han SH, Son SM, Hong HS, Choi YJ, Byun J, Mook-Jung I. 2012. Mitochondria-specific accumulation of amyloid β induces mitochondrial dysfunction leading to apoptotic cell death. PLoS ONE 7, e34929 ( 10.1371/journal.pone.0034929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pithadia AS, Lim MH. 2012. Metal-associated amyloid-β species in Alzheimer's disease. Curr. Opin. Chem. Biol. 16, 67–73. ( 10.1016/j.cbpa.2012.01.016) [DOI] [PubMed] [Google Scholar]
- 127.Ahmad W, Ijaz B, Shabbiri K, Ahmed F, Rehman S. 2017. Oxidative toxicity in diabetes and Alzheimer's disease: mechanisms behind ROS/ RNS generation. J. Biomed. Sci. 24, 76 ( 10.1186/s12929-017-0379-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abeti R, Abramov AY, Duchen MR. 2011. Beta-amyloid activates PARP causing astrocytic metabolic failure and neuronal death. Brain 134, 1658–1672. ( 10.1093/brain/awr104) [DOI] [PubMed] [Google Scholar]
- 129.Angelova PR, Abramov AY. 2014. Interaction of neurons and astrocytes underlies the mechanism of Aβ-induced neurotoxicity. Biochem. Soc. Trans. 42, 1286–1290. ( 10.1042/BST20140153) [DOI] [PubMed] [Google Scholar]
- 130.Kauppinen TM, et al. 2011. Poly(ADP-ribose)polymerase-1 modulates microglial responses to amyloid β. J. Neuroinflammation 8, 152 ( 10.1186/1742-2094-8-152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martire S, et al. 2013. PARP-1 modulates amyloid beta peptide-induced neuronal damage. PLoS ONE 8, e72169 ( 10.1371/journal.pone.0072169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mackenzie IR, et al. 2007. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann. Neurol. 61, 427–434. ( 10.1002/ana.21147) [DOI] [PubMed] [Google Scholar]
- 133.Hasegawa M, et al. 2008. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann. Neurol. 64, 60–70. ( 10.1002/ana.21425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Anderson P, Kedersha N. 2008. Stress granules: the Tao of RNA triage. Trends Biochem. Sci. 33, 141–150. ( 10.1016/j.tibs.2007.12.003) [DOI] [PubMed] [Google Scholar]
- 135.Li YR, King OD, Shorter J, Gitler AD. 2013. Stress granules as crucibles of ALS pathogenesis. J. Cell Biol. 201, 361–372. ( 10.1083/jcb.201302044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kedersha N, et al. 2005. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 169, 871–884. ( 10.1083/jcb.200502088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kwon S, Zhang Y, Matthias P. 2007. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes Dev. 21, 3381–3394. ( 10.1101/gad.461107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leung AK, Vyas S, Rood JE, Bhutkar A, Sharp PA, Chang P. 2011. Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm. Mol. Cell 42, 489–499. ( 10.1016/j.molcel.2011.04.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bock FJ, Todorova TT, Chang P. 2015. RNA regulation by poly(adp-ribose) polymerases. Mol. Cell 58, 959–969. ( 10.1016/j.molcel.2015.01.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Catara G, Grimaldi G, Schembri L, Spano D, Turacchio G, Lo Monte M, Beccari AR, Valente C, Corda D. 2017. PARP1-produced poly-ADP-ribose causes the PARP12 translocation to stress granules and impairment of Golgi complex functions. Sci. Rep. 7, 14035 ( 10.1038/s41598-017-14156-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McGurk L, et al. 2018. Nuclear poly(ADP-ribose) activity is a therapeutic target in amyotrophic lateral sclerosis. Acta Neuropathol. Commun. 6, 84 ( 10.1186/s40478-018-0586-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McGurk L, Gomes E, Guo L, Mojsilovic-Petrovic J, Tran V, Kalb RG, Shorter J, Bonini NM. 2018. Poly(ADP-ribose) prevents pathological phase separation of TDP-43 by promoting liquid demixing and stress granule localization. Mol. Cell 71, 703–717. ( 10.1016/j.molcel.2018.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Danese S, Fiocchi C. 2011. Ulcerative colitis. N. Engl. J. Med. 365, 1713–1725. ( 10.1056/NEJMra1102942) [DOI] [PubMed] [Google Scholar]
- 144.Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. 2014. Immunopathology of inflammatory bowel disease. World J. Gastroenterol. 20, 6–21. ( 10.3748/wjg.v20.i1.6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. 2017. Ulcerative colitis. Lancet 389, 1756–1770. ( 10.1016/S0140-6736(16)32126-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Harrison OJ, Maloy KJ. 2011. Innate immune activation in intestinal homeostasis. J. Innate Immun . 3, 585–593. ( 10.1159/000330913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lei-Leston AC, Murphy AG, Maloy KJ. 2017. Epithelial cell inflammasomes in intestinal immunity and inflammation. Front. Immunol. 8, 1168 ( 10.3389/fimmu.2017.01168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rogler G. 2014. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 345, 235–241. ( 10.1016/j.canlet.2013.07.032) [DOI] [PubMed] [Google Scholar]
- 149.Van Der Kraak L, Gros P, Beauchemin N. 2015. Colitis-associated colon cancer: is it in your genes? World J. Gastroenterol. 21, 11 688–11 699. ( 10.3748/wjg.v21.i41.11688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Al Bakir I, Curtius K, Graham TA. 2018. From colitis to cancer: an evolutionary trajectory that merges maths and biology. Front. Immunol. 9, 2368 ( 10.3389/fimmu.2018.02368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dörsam B, et al. 2018. PARP-1 protects against colorectal tumor induction, but promotes inflammation-driven colorectal tumor progression. Proc. Natl Acad. Sci. USA 115, E4061–E4070. ( 10.1073/pnas.1712345115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yan Q, Xu R, Zhu L, Cheng X, Wang Z, Manis J, Shipp MA. 2013. BAL1 and its partner E3 ligase, BBAP, link Poly(ADP-ribose) activation, ubiquitylation, and double-strand DNA repair independent of ATM, MDC1, and RNF8. Mol. Cell Biol. 33, 845–857. ( 10.1128/MCB.00990-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang CS, et al. 2017. Ubiquitin modification by the E3 ligase/ADP-ribosyltransferase Dtx3 L/Parp9. Mol Cell. 66, 503–516. ( 10.1016/j.molcel.2017.04.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Juszczynski P, Kutok JL, Li C, Mitra J, Aguiar RC, Shipp MA. 2006. BAL1 and BBAP are regulated by a gamma interferon-responsive bidirectional promoter and are overexpressed in diffuse large B-cell lymphomas with a prominent inflammatory infiltrate. Mol. Cell Biol. 26, 5348–5359. ( 10.1128/MCB.02351-05) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Camicia R, Bachmann SB, Winkler HC, Beer M, Tinguely M, Haralambieva E, Hassa PO. 2013. BAL1/ARTD9 represses the anti-proliferative and pro-apoptotic IFNγ-STAT1-IRF1-p53 axis in diffuse large B-cell lymphoma. J. Cell Sci. 126, 1969–1980. ( 10.1242/jcs.118174) [DOI] [PubMed] [Google Scholar]
- 156.Goenka S, Boothby M. 2006. Selective potentiation of Stat-dependent gene expression by collaborator of Stat6 (CoaSt6), a transcriptional cofactor. Proc. Natl Acad. Sci. USA 103, 4210–4215. ( 10.1073/pnas.0506981103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Goenka S, Cho SH, Boothby M. 2007. Collaborator of Stat6 (CoaSt6)-associated poly(ADP-ribose) polymerase activity modulates Stat6-dependent gene transcription. J. Biol. Chem. 282, 18 732–18 739. ( 10.1074/jbc.M611283200) [DOI] [PubMed] [Google Scholar]
- 158.Mehrotra P, Hollenbeck A, Riley JP, Li F, Patel RJ, Akhtar N, Goenka S. 2013. Poly (ADP-ribose) polymerase 14 and its enzyme activity regulates T(H)2 differentiation and allergic airway disease. J. Allergy Clin. Immunol. 131, 521–531. ( 10.1016/j.jaci.2012.06.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Berger A. 2000. Th1 and Th2 responses: what are they? Br. Med. J. 321, 424 ( 10.1136/bmj.321.7258.424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cho SH, Ahn AK, Bhargava P, Lee CH, Eischen CM, McGuinness O, Boothby M. 2011. Glycolytic rate and lymphomagenesis depend on PARP14, an ADP ribosyltransferase of the B aggressive lymphoma (BAL) family. Proc. Natl Acad. Sci. USA 108, 15 972–15 977. ( 10.1073/pnas.1017082108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Watanabe H, Takehana K, Date M, Shinozaki T, Raz A. 1996. Tumor cell autocrine motility factor is the neuroleukin/phosphohexose isomerase polypeptide. Cancer Res. 56, 2960–2963. [PubMed] [Google Scholar]
- 162.Funasaka T, Haga A, Raz A, Nagase H. 2001. Tumor autocrine motility factor is an angiogenic factor that stimulates endothelial cell motility. Biochem. Biophys. Res. Commun. 285, 118–128. ( 10.1006/bbrc.2001.5135) [DOI] [PubMed] [Google Scholar]
- 163.Funasaka T, Haga A, Raz A, Nagase H. 2002. Tumor autocrine motility factor induces hyperpermeability of endothelial and mesothelial cells leading to accumulation of ascites fluid. Biochem. Biophys. Res. Commun. 293, 192–200. ( 10.1016/S0006-291X(02)00202-4) [DOI] [PubMed] [Google Scholar]
- 164.Forst AH, et al. 2013. Recognition of mono-ADP-ribosylated ARTD10 substrates by ARTD8 macrodomains. Structure 21, 462–475. ( 10.1016/j.str.2012.12.019) [DOI] [PubMed] [Google Scholar]
- 165.Smith S, Giriat I, Schmitt A, de Lange T. 1998. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science 282, 1484–1487. ( 10.1126/science.282.5393.1484) [DOI] [PubMed] [Google Scholar]
- 166.Smith S, de Lange T. 1999. Cell cycle dependent localization of the telomeric PARP, tankyrase, to nuclear pore complexes and centrosomes. J. Cell Sci. 112, 3649–3656. [DOI] [PubMed] [Google Scholar]
- 167.Dynek JN, Smith S. 2004. Resolution of sister telomere association is required for progression through mitosis. Science 304, 97–100. ( 10.1126/science.1094754) [DOI] [PubMed] [Google Scholar]
- 168.Chang P, Coughlin M, Mitchison TJ. 2005. Tankyrase-1 polymerization of poly(ADP-ribose) is required for spindle structure and function. Nat. Cell Biol. 7, 1133–1139. ( 10.1038/ncb1322) [DOI] [PubMed] [Google Scholar]
- 169.Hsiao SJ, Smith S. 2008. Tankyrase function at telomeres, spindle poles, and beyond. Biochimie 90, 83–92. ( 10.1016/j.biochi.2007.07.012) [DOI] [PubMed] [Google Scholar]
- 170.Huang SM, et al. 2009. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614–620. ( 10.1038/nature08356) [DOI] [PubMed] [Google Scholar]
- 171.Palazzo L, Della Monica R, Visconti R, Costanzo V, Grieco D. 2014. ATM controls proper mitotic spindle structure. Cell Cycle 13, 1091–1100. ( 10.4161/cc.27945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nagy Z, Kalousi A, Furst A, Koch M, Fischer B, Soutoglou E. 2016. Tankyrases promote homologous recombination and check point activation in response to DSBs. PLoS Genet. 12, e1005791 ( 10.1371/journal.pgen.1005791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bhardwaj A, Yang Y, Ueberheide B, Smith S. 2017. Whole proteome analysis of human tankyrase knockout cells reveals targets of tankyrase-mediated degradation. Nat. Commun. 8, 2214 ( 10.1038/s41467-017-02363-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li X, Han H, Zhou MT, Yang B, Ta AP, Li N, Chen J, Wang W. 2017. Proteomic analysis of the human tankyrase protein interaction network reveals its role in pexophagy. Cell Rep. 20, 737–749. ( 10.1016/j.celrep.2017.06.077) [DOI] [PubMed] [Google Scholar]
- 175.Guettler S, LaRose J, Petsalaki E, Gish G, Scotter A, Pawson T, Rottapel R, Sicheri F. 2011. Structural basis and sequence rules for substrate recognition by Tankyrase explain the basis for cherubism disease. Cell 147, 1340–1354. ( 10.1016/j.cell.2011.10.046) [DOI] [PubMed] [Google Scholar]
- 176.Sbodio JI, Chi NW. 2002. Identification of a tankyrase-binding motif shared by IRAP, TAB182, and human TRF1 but not mouse TRF1. NuMA contains this RXXPDG motif and is a novel tankyrase partner. J. Biol. Chem. 277, 31 887–31 892. ( 10.1074/jbc.M203916200) [DOI] [PubMed] [Google Scholar]
- 177.Zhang Y, et al. 2011. RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling. Nat Cell Biol. 13, 623–629. ( 10.1038/ncb2222) [DOI] [PubMed] [Google Scholar]
- 178.Li N, et al. 2015. Poly-ADP ribosylation of PTEN by tankyrases promotes PTEN degradation and tumor growth. Genes Dev. 29, 157–170. ( 10.1101/gad.251785.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang W, Li N, Li X, Tran MK, Han X, Chen J. 2015. Tankyrase inhibitors target YAP by stabilizing angiomotin family proteins. Cell Rep. 13, 524–532. ( 10.1016/j.celrep.2015.09.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Krastev DB, Pettitt SJ, Campbell J, Song F, Tanos BE, Stoynov SS, Ashworth A, Lord CJ. 2018. Coupling bimolecular PARylation biosensors with genetic screens to identify PARylation targets. Nat. Commun. 9, 2016 ( 10.1038/s41467-018-04466-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Riffell JL, Lord CJ, Ashworth A. 2012. Tankyrase-targeted therapeutics: expanding opportunities in the PARP family. Nat. Rev. Drug Discov. 11, 923–936. ( 10.1038/nrd3868) [DOI] [PubMed] [Google Scholar]
- 182.Chi NW, Lodish HF. 2000. Tankyrase is a golgi-associated mitogen-activated protein kinase substrate that interacts with IRAP in GLUT4 vesicles. J. Biol. Chem. 275, 38 437–38 444. ( 10.1074/jbc.M007635200) [DOI] [PubMed] [Google Scholar]
- 183.Yeh TY, Beiswenger KK, Li P, Bolin KE, Lee RM, Tsao TS, Murphy AN, Hevener AL, Chi NW. 2009. Hypermetabolism, hyperphagia, and reduced adiposity in tankyrase-deficient mice. Diabetes 58, 2476–2485. ( 10.2337/db08-1781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Guo HL, et al. 2012. The Axin/TNKS complex interacts with KIF3A and is required for insulin-stimulated GLUT4 translocation. Cell Res. 22, 1246–1257. ( 10.1038/cr.2012.52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Su Z, Deshpande V, James DE, Stöckli J. 2018. Tankyrase modulates insulin sensitivity in skeletal muscle cells by regulating the stability of GLUT4 vesicle proteins. J. Biol. Chem. 293, 8578–8587. ( 10.1074/jbc.RA117.001058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mizutani A, et al. 2018. RK-287107, a potent and specific tankyrase inhibitor, blocks colorectal cancer cell growth in a preclinical model. Cancer Sci. 109, 4003–4014. ( 10.1111/cas.13805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fancy SP, et al. 2011. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat. Neurosci. 14, 1009–1016. ( 10.1038/nn.2855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jones WA, Gerrie J, Pritchard J. 1950. Cherubism--familial fibrous dysplasia of the jaws. J. Bone Joint Surg. Br. 32-B, 334–347. ( 10.1302/0301-620X.32B3.334) [DOI] [PubMed] [Google Scholar]
- 189.Ueki Y, et al. 2001. Mutations in the gene encoding c-Abl-binding protein SH3BP2 cause cherubism. Nat. Genet. 28, 125–126. ( 10.1038/88832) [DOI] [PubMed] [Google Scholar]
- 190.Levaot N, et al. 2011. Loss of Tankyrase-mediated destruction of 3BP2 is the underlying pathogenic mechanism of cherubism. Cell 147, 1324–1339. ( 10.1016/j.cell.2011.10.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Perina D, Mikoč A, Ahel J, Ćetković H, Žaja R, Ahel I. 2014. Distribution of protein poly(ADP-ribosyl)ation systems across all domains of life. DNA Repair (Amst). 23, 4–16. ( 10.1016/j.dnarep.2014.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chen D, et al. 2011. Identification of macrodomain proteins as novel O-acetyl-ADP-ribose deacetylases. J. Biol. Chem. 286, 13 261–13 271. ( 10.1074/jbc.M110.206771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang J, Zhao YL, Wu ZQ, Si YL, Meng YG, Fu XB, Mu YM, Han WD. 2009. The single-macro domain protein LRP16 is an essential cofactor of androgen receptor. Endocr. Relat. Cancer 16, 139–153. ( 10.1677/ERC-08-0150) [DOI] [PubMed] [Google Scholar]
- 194.Wu Z, et al. 2015. An LRP16-containing preassembly complex contributes to NF-κB activation induced by DNA double-strand breaks. Nucleic Acids Res. 43, 3167–3179. ( 10.1093/nar/gkv161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wu Z, et al. 2011. LRP16 integrates into NF-κB transcriptional complex and is required for its functional activation. PLoS ONE 6, e18157 ( 10.1371/journal.pone.0018157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Agnew T, Munnur D, Crawford K, Palazzo L, Mikoč A, Ahel I. 2018. MacroD1 is a promiscuous ADP-ribosyl hydrolase localized to mitochondria. Front. Microbiol. 9, 20 ( 10.3389/fmicb.2018.00020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Verheugd P, Forst AH, Milke L, Herzog N, Feijs KL, Kremmer E, Kleine H, Lüscher B. 2013. Regulation of NF-κB signalling by the mono-ADP-ribosyltransferase ARTD10. Nat. Commun. 4, 1683 ( 10.1038/ncomms2672) [DOI] [PubMed] [Google Scholar]
- 198.Feijs KL, Kleine H, Braczynski A, Forst AH, Herzog N, Verheugd P, Linzen U, Kremmer E, Lüscher B. 2013. ARTD10 substrate identification on protein microarrays: regulation of GSK3β by mono-ADP-ribosylation. Cell Commun. Signal. 11, 5 ( 10.1186/1478-811X-11-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Folstein S, Rutter M. 1977. Infantile autism: a genetic study of 21 twin pairs. J. Child Psychol. Psychiatry 18, 297–321. ( 10.1111/j.1469-7610.1977.tb00443.x) [DOI] [PubMed] [Google Scholar]
- 200.Ritvo ER, Freeman BJ, Mason-Brothers A, Mo A, Ritvo AM. 1985. Concordance for the syndrome of autism in 40 pairs of afflicted twins. Am. J. Psychiatry 142, 74–77. ( 10.1176/ajp.142.1.74) [DOI] [PubMed] [Google Scholar]
- 201.Miles JH. 2011. Autism spectrum disorders—a genetics review. Genet. Med. 13, 278–294. ( 10.1097/GIM.0b013e3181ff67ba) [DOI] [PubMed] [Google Scholar]
- 202.Wessels MW, Brooks AS, Hoogeboom J, Niermeijer MF, Willems PJ. 2002. Kabuki syndrome: a review study of three hundred patients. Clin. Dysmorphol. 11, 95–102. ( 10.1097/00019605-200204000-00004) [DOI] [PubMed] [Google Scholar]
- 203.Lintas C, Persico AM. 2018. Unraveling molecular pathways shared by Kabuki and Kabuki-like syndromes. Clin. Genet. 94, 283–295. ( 10.1111/cge.12983) [DOI] [PubMed] [Google Scholar]
- 204.Peterson FC, Chen D, Lytle BL, Rossi MN, Ahel I, Denu JM, Volkman BF. 2011. Orphan macrodomain protein (human C6orf130) is an O-acyl-ADP-ribose deacylase: solution structure and catalytic properties. J. Biol. Chem. 286, 35 955–35 965. ( 10.1074/jbc.M111.276238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Munnur D, Ahel I. 2017. Reversible mono-ADP-ribosylation of DNA breaks. FEBS J. 284, 4002–4016. ( 10.1111/febs.14297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Williams JC, Chambers JP, Liehr JG. 1984. Glutamyl ribose 5-phosphate storage disease: a hereditary defect in the degradation of poly(ADP-ribosylated) proteins. J. Biol. Chem. 259, 1037–1042. [PubMed] [Google Scholar]
- 207.Williams JC, Verani R, Alcala H, Butler IJ, Rosenberg HS. 1986. Glutamyl ribose-5-phosphate storage disease: nephrotic syndrome and cerebral atrophy. Pediatr. Pathol. 5, 277–294. ( 10.3109/15513818609068855) [DOI] [PubMed] [Google Scholar]
- 208.Palazzo L, et al. 2015. Processing of protein ADP-ribosylation by Nudix hydrolases. Biochem. J. 468, 293–301. ( 10.1042/BJ20141554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Daniels CM, Thirawatananond P, Ong S, Gabelli SB, Leung AK. 2015. Nudix hydrolases degrade protein-conjugated ADP-ribose. Sci. Rep. 5, 18271 ( 10.1038/srep18271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Palazzo L, et al. 2016. ENPP1 processes protein ADP-ribosylation in vitro. FEBS J. 283, 3371–3388. ( 10.1111/febs.13811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dunstan MS, Barkauskaite E, Lafite P, Knezevic CE, Brassington A, Ahel M, Hergenrother PJ, Leys D, Ahel I. 2012. Structure and mechanism of a canonical poly(ADP-ribose) glycohydrolase. Nat. Commun. 3, 878 ( 10.1038/ncomms1889) [DOI] [PubMed] [Google Scholar]
- 212.Kim IK, Kiefer JR, Ho CM, Stegeman RA, Classen S, Tainer JA, Ellenberger T. 2012. Structure of mammalian poly(ADP-ribose) glycohydrolase reveals a flexible tyrosine clasp as a substrate-binding element. Nat. Struct. Mol. Biol. 19, 653–656. ( 10.1038/nsmb.2305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lambrecht MJ, Brichacek M, Barkauskaite E, Ariza A, Ahel I, Hergenrother PJ. 2015. Synthesis of dimeric ADP-ribose and its structure with human poly(ADP-ribose) glycohydrolase. J. Am. Chem. Soc. 137, 3558–3564. ( 10.1021/ja512528p) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tucker JA, et al. 2012. Structures of the human poly (ADP-ribose) glycohydrolase catalytic domain confirm catalytic mechanism and explain inhibition by ADP-HPD derivatives. PLoS ONE 7, e50889 ( 10.1371/journal.pone.0050889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Braun SA, Panzeter PL, Collinge MA, Althaus FR. 1994. Endoglycosidic cleavage of branched polymers by poly(ADP-ribose) glycohydrolase. Eur. J. Biochem. 220, 369–375. ( 10.1111/j.1432-1033.1994.tb18633.x) [DOI] [PubMed] [Google Scholar]
- 216.Barkauskaite E, et al. 2013. Visualization of poly(ADP-ribose) bound to PARG reveals inherent balance between exo- and endo-glycohydrolase activities. Nat. Commun. 4, 2164 ( 10.1038/ncomms3164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Andrabi SA, et al. 2006. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc. Natl Acad. Sci. USA 103, 18 308–18 313. ( 10.1073/pnas.0606526103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Koh DW, et al. 2004. Failure to degrade poly(ADP-ribose) causes increased sensitivity to cytotoxicity and early embryonic lethality. Proc. Natl Acad. Sci. USA 101, 17 699–17 704. ( 10.1073/pnas.0406182101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hanai S, Kanai M, Ohashi S, Okamoto K, Yamada M, Takahashi H, Miwa M. 2004. Loss of poly(ADP-ribose) glycohydrolase causes progressive neurodegeneration in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 101, 82–86. ( 10.1073/pnas.2237114100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cozzi A, et al. 2006. Poly(ADP-ribose) accumulation and enhancement of postischemic brain damage in 110-kDa poly(ADP-ribose) glycohydrolase null mice. J. Cereb. Blood Flow Metab. 26, 684–695. ( 10.1038/sj.jcbfm.9600222) [DOI] [PubMed] [Google Scholar]
- 221.Cuzzocrea S, et al. 2007. Role of poly(ADP-ribose) glycohydrolase in the development of inflammatory bowel disease in mice. Free Radic. Biol. Med. 42, 90–105. ( 10.1016/j.freeradbiomed.2006.09.025) [DOI] [PubMed] [Google Scholar]
- 222.Hamanaka S. et al. 2018. Investigation of novel biomarkers for predicting the clinical course in patients with ulcerative colitis. J. Gastroenterol. Hepatol. 33, 1975–1983. ( 10.1111/jgh.14297) [DOI] [PubMed] [Google Scholar]
- 223.Marques M, et al. 2018. Oncogenic activity of poly (ADP-ribose) glycohydrolase. Oncogene 38, 2177–2191. ( 10.1038/s41388-018-0568-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gravells P, Neale J, Grant E, Nathubhai A, Smith KM, James DI, Bryant HE. 2018. Radiosensitization with an inhibitor of poly(ADP-ribose) glycohydrolase: a comparison with the PARP1/2/3 inhibitor olaparib. DNA Repair (Amst). 61, 25–36. ( 10.1016/j.dnarep.2017.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gogola E, et al. 2018. Selective loss of PARG restores PARylation and counteracts PARP inhibitor-mediated synthetic lethality. Cancer Cell 33, 1078–1093. ( 10.1016/j.ccell.2018.05.008) [DOI] [PubMed] [Google Scholar]
- 226.Moss J, Tsai SC, Adamik R, Chen HC, Stanley SJ. 1988. Purification and characterization of ADP-ribosylarginine hydrolase from turkey erythrocytes. Biochemistry 27, 5819–5823. ( 10.1021/bi00415a063) [DOI] [PubMed] [Google Scholar]
- 227.Kato J, Zhu J, Liu C, Moss J. 2007. Enhanced sensitivity to cholera toxin in ADP-ribosylarginine hydrolase-deficient mice. Mol. Cell Biol. 27, 5534–5543. ( 10.1128/MCB.00302-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kato J, Zhu J, Liu C, Stylianou M, Hoffmann V, Lizak MJ, Glasgow CG, Moss J. 2011. ADP-ribosylarginine hydrolase regulates cell proliferation and tumorigenesis. Cancer Res. 71, 5327–5335. ( 10.1158/0008-5472.CAN-10-0733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Konczalik P, Moss J. 1999. Identification of critical, conserved vicinal aspartate residues in mammalian and bacterial ADP-ribosylarginine hydrolases. J. Biol. Chem. 274, 16 736–16 740. ( 10.1074/jbc.274.24.16736) [DOI] [PubMed] [Google Scholar]
- 230.Mueller-Dieckmann C, Kernstock S, Lisurek M, von Kries JP, Haag F, Weiss MS, Koch-Nolte F. 2006. The structure of human ADP-ribosylhydrolase 3 (ARH3) provides insights into the reversibility of protein ADP-ribosylation. Proc. Natl Acad. Sci. USA 103, 15 026–15 031. ( 10.1073/pnas.0606762103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Abplanalp J, et al. 2017. Proteomic analyses identify ARH3 as a serine mono-ADP-ribosylhydrolase. Nat. Commun. 8, 2055 ( 10.1038/s41467-017-02253-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Pourfarjam Y, Ventura J, Kurinov I, Cho A, Moss J, Kim IK. 2018. Structure of human ADP-ribosyl-acceptor hydrolase 3 bound to ADP-ribose reveals a conformational switch that enables specific substrate recognition. J. Biol. Chem. 293, 12 350–12 359. ( 10.1074/jbc.RA118.003586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Voorneveld J, Rack JGM, Ahel I, Overkleeft HS, van der Marel GA, Filippov DV. 2018. Synthetic α- and β-Ser-ADP-ribosylated peptides reveal α-Ser-ADPr as the native epimer. Org. Lett. 20, 4140–4143. ( 10.1021/acs.orglett.8b01742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wang M, Yuan Z, Xie R, Ma Y, Liu X, Yu X. 2018. Structure-function analyses reveal the mechanism of the ARH3-dependent hydrolysis of ADP-ribosylation. J. Biol. Chem. 293, 14 470–14 480. ( 10.1074/jbc.RA118.004284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Niere M, Kernstock S, Koch-Nolte F, Ziegler M. 2008. Functional localization of two poly(ADP-ribose)-degrading enzymes to the mitochondrial matrix. Mol. Cell Biol. 28, 814–824. ( 10.1128/MCB.01766-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mashimo M, Kato J, Moss J. 2013. ADP-ribosyl-acceptor hydrolase 3 regulates poly (ADP-ribose) degradation and cell death during oxidative stress. Proc. Natl Acad. Sci. USA 110, 18 964–18 969. ( 10.1073/pnas.1312783110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tanuma S, Sato A, Oyama T, Yoshimori A, Abe H, Uchiumi F. 2016. New insights into the roles of NAD+-poly(ADP-ribose) metabolism and poly(ADP-ribose) glycohydrolase. Curr. Protein Pept. Sci. 17, 668–682. ( 10.2174/1389203717666160419150014) [DOI] [PubMed] [Google Scholar]
- 238.Gottschalk AJ, et al. 2009. Poly(ADP-ribosyl)ation directs recruitment and activation of an ATP-dependent chromatin remodeler. Proc. Natl Acad. Sci. USA 106, 13 770–13 774. ( 10.1073/pnas.0906920106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ahel D, et al. 2009. Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science 325, 1240–1243. ( 10.1126/science.1177321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gottschalk AJ, Trivedi RD, Conaway JW, Conaway RC. 2012. Activation of the SNF2 family ATPase ALC1 by poly(ADP-ribose) in a stable ALC1·PARP1·nucleosome intermediate. J. Biol. Chem. 287, 43 527–43 532. ( 10.1074/jbc.M112.401141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sellou H, et al. 2016. The poly(ADP-ribose)-dependent chromatin remodeler Alc1 induces local chromatin relaxation upon DNA damage. Mol. Biol. Cell. 27, 3791–3799. ( 10.1091/mbc.e16-05-0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lehmann LC, et al. 2017. Mechanistic insights into autoinhibition of the oncogenic chromatin remodeler ALC1. Mol. Cell. 68, 847–859. ( 10.1016/j.molcel.2017.10.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Singh HR, et al. 2017. A poly-ADP-ribose trigger releases the auto-inhibition of a chromatin remodeling oncogene. Mol. Cell. 68, 860–871. ( 10.1016/j.molcel.2017.11.019) [DOI] [PubMed] [Google Scholar]
- 244.Chen M, Huang JD, Hu L, Zheng BJ, Chen L, Tsang SL, Guan XY. 2009. Transgenic CHD1 L expression in mouse induces spontaneous tumors. PLoS ONE 4, e6727 ( 10.1371/journal.pone.0006727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Cheng W, Su Y, Xu F. 2013. CHD1 L: a novel oncogene. Mol. Cancer 12, 170 ( 10.1186/1476-4598-12-170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Brockschmidt A, et al. 2012. CHD1 L: a new candidate gene for congenital anomalies of the kidneys and urinary tract (CAKUT). Nephrol. Dial. Transplant. 27, 2355–2364. ( 10.1093/ndt/gfr649) [DOI] [PubMed] [Google Scholar]
- 247.Michalska M, Wolf P. 2015. Pseudomonas Exotoxin A: optimized by evolution for effective killing. Front. Microbiol. 6, 963 ( 10.3389/fmicb.2015.00963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Carbonetti NH. 2015. Contribution of pertussis toxin to the pathogenesis of pertussis disease. Pathog. Dis. 73, ftv073 ( 10.1093/femspd/ftv073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chong A, Lee S, Yang YA, Song J. 2017. The role of typhoid toxin in Salmonella typhi virulence. Yale J. Biol. Med. 90, 283–290. [PMC free article] [PubMed] [Google Scholar]
- 250.Schwan C, Stecher B, Tzivelekidis T, van Ham M, Rohde M, Hardt WD, Wehland J, Aktories K. 2009. Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 5, e1000626 ( 10.1371/journal.ppat.1000626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chu Y, Gao S, Wang T, Yan J, Xu G, Li Y, Niu H, Huang R, Wu S. 2016. A novel contribution of spvB to pathogenesis of Salmonella Typhimurium by inhibiting autophagy in host cells. Oncotarget 7, 8295–8309. ( 10.18632/oncotarget.6989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Coye LH, Collins CM. 2004. Identification of SpyA, a novel ADP-ribosyltransferase of Streptococcus pyogenes. Mol. Microbiol. 54, 89–98. ( 10.1111/j.1365-2958.2004.04262.x) [DOI] [PubMed] [Google Scholar]
- 253.Sherwood RK, Roy CR. 2016. Autophagy evasion and endoplasmic reticulum subversion: the Yin and Yang of legionella intracellular infection. Annu. Rev. Microbiol. 70, 413–433. ( 10.1146/annurev-micro-102215-095557) [DOI] [PubMed] [Google Scholar]
- 254.Kotewicz KM, et al. 2017. A single legionella effector catalyzes a multistep ubiquitination pathway to rearrange tubular endoplasmic reticulum for replication. Cell Host Microbe 21, 169–181. ( 10.1016/j.chom.2016.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bhogaraju S, Kalayil S, Liu Y, Bonn F, Colby T, Matic I, Dikic I. 2016. Phosphoribosylation of ubiquitin promotes serine ubiquitination and impairs conventional ubiquitination. Cell. 167, 1636–1649. ( 10.1016/j.cell.2016.11.019) [DOI] [PubMed] [Google Scholar]
- 256.Qiu J, et al. 2017. A unique deubiquitinase that deconjugates phosphoribosyl-linked protein ubiquitination. Cell Res. 27, 865–881. ( 10.1038/cr.2017.66) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pandey DP, Gerdes K. 2005. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 33, 966–976. ( 10.1093/nar/gki201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jankevicius G, Ariza A, Ahel M, Ahel I. 2016. The toxin-antitoxin system DarTG catalyzes reversible ADP-ribosylation of DNA. Mol. Cell. 64, 1109–1116. ( 10.1016/j.molcel.2016.11.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rack JG, et al. 2015. Identification of a class of protein ADP-ribosylating sirtuins in microbial pathogens. Mol Cell. 59, 309–320. ( 10.1016/j.molcel.2015.06.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Li C, Debing Y, Jankevicius G, Neyts J, Ahel I, Coutard B, Canard B. 2016. Viral macro domains reverse protein ADP-ribosylation. J Virol. 90, 8478–8486. ( 10.1128/JVI.00705-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.McPherson RL, et al. 2017. ADP-ribosylhydrolase activity of Chikungunya virus macrodomain is critical for virus replication and virulence. Proc. Natl Acad. Sci. USA 114, 1666–1671. ( 10.1073/pnas.1621485114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Eckei L, et al. 2017. The conserved macrodomains of the non-structural proteins of Chikungunya virus and other pathogenic positive strand RNA viruses function as mono-ADP-ribosylhydrolases. Sci. Rep. 7, 41746 ( 10.1038/srep41746) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Abraham R, Hauer D, McPherson RL, Utt A, Kirby IT, Cohen MS, Merits A, Leung AKL, Griffin DE. 2018. ADP-ribosyl-binding and hydrolase activities of the alphavirus nsP3 macrodomain are critical for initiation of virus replication. Proc. Natl Acad. Sci. USA 115, E10 457–E10 466. ( 10.1073/pnas.1812130115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Fehr AR, Jankevicius G, Ahel I, Perlman S. 2018. Viral macrodomains: unique mediators of viral replication and pathogenesis. Trends Microbiol. 26, 598–610. ( 10.1016/j.tim.2017.11.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Leung AKL, McPherson RL, Griffin DE. 2018. Macrodomain ADP-ribosylhydrolase and the pathogenesis of infectious diseases. PLoS Pathog. 14, e1006864 ( 10.1371/journal.ppat.1006864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Atasheva S, Frolova EI, Frolov I. 2014. Interferon-stimulated poly(ADP-Ribose) polymerases are potent inhibitors of cellular translation and virus replication. J. Virol. 88, 2116–2130. ( 10.1128/JVI.03443-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.McInerney GM, Kedersha NL, Kaufman RJ, Anderson P, Liljeström P. 2005. Importance of eIF2alpha phosphorylation and stress granule assembly in alphavirus translation regulation. Mol. Biol. Cell. 16, 3753–3763. ( 10.1091/mbc.e05-02-0124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Daugherty MD, Young JM, Kerns JA, Malik HS. 2014. Rapid evolution of PARP genes suggests a broad role for ADP-ribosylation in host-virus conflicts. PLoS Genet. 10, e1004403 ( 10.1371/journal.pgen.1004403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nikiforov A, Kulikova V, Ziegler M. 2015. The human NAD metabolome: functions, metabolism and compartmentalization. Crit. Rev. Biochem. Mol. Biol. 50, 284–297. ( 10.3109/10409238.2015.1028612) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.