Abstract
Communication signals often comprise an array of colours, lines, spots, notes or odours that are arranged in complex patterns, melodies or blends. Receiver perception is assumed to influence preference and thus the evolution of signal design, but evolutionary biologists still struggle to understand how perception, preference and signal design are mechanistically linked. In parallel, the field of empirical aesthetics aims to understand why people like some designs more than others. The model of processing bias discussed here is rooted in empirical aesthetics, which posits that preferences are influenced by the emotional system as it monitors the dynamics of information processing and that attractive signals have effective designs that maximize information transmission, efficient designs that allow information processing at low metabolic cost, or both. We refer to the causal link between preference and the emotionally rewarding experience of effective and efficient information processing as the processing bias, and we apply it to the evolutionary model of sensory drive. A sensory drive model that incorporates processing bias hypothesizes a causal chain of relationships between the environment, perception, pleasure, preference and ultimately the evolution of signal design, both simple and complex.
Keywords: evolution of aesthetics and beauty, cognition, emotion, information theory, sensory exploitation and pre-existing bias, sexual signals
1. The complexity and diversity of communication signals
Evolutionary biologists continue to puzzle over the evolution of elaborate communication signals (figure 1). Explanations are dominated by three hypotheses that are not necessarily mutually exclusive. One describes communication signals as quality indicators, whereby some feature (Glossary) of the signal correlates with the fitness of the receiver (e.g. [4,5]); for example, when males expressing the most extreme sexual ornaments sire the healthiest offspring. Another derives from the verbal models of Fisher [6], in which signals become exaggerated and diversified simply as a result of genetic covariation with receiver preferences (e.g. [2,7]). The third highlights the role of sensory perception and cognition, as in models of pre-existing bias and sensory drive [8–10]. Models of pre-existing bias assume that preferences evolve in a context other than mating, and communication signals that subsequently match those preferences are favoured. Sensory drive emphasizes the importance of the environment in shaping perception, and thus preferences. For example, if animal visual systems are tuned to local light conditions, the most effective visual signals will be those that maximally stimulate that particular tuning (e.g. [11]).
Figure 1.
Open questions about visual signals. The design of visual communication signals has puzzled biologists since Darwin, who agreed with his contemporaries that (a) feathers of the great argus pheasant Argusianus argus (credit: Bernard Dupont) were ‘more like a work of art than of nature’ [1, p. 258]. The design of a signal—the arrangement of its features—can be complex, as in (b) the flower of Passiflora maliformis (credit: Nick Hobgood), or simple, as in (c) the clownfish Amphiprion melanopus (credit: Richard Ling). Regardless, in most cases, there is no clear answer as to why the features of a signal are arranged as they are. Design diversification is just as enigmatic, as in (d–g) the Maratus genus of jumping spider (credits: Jurgen Otto). Why should the abdomen of one species feature red vertical bars surrounded by yellow patches transected by thin, curved blue lines (d), while its close congener sports dark eyespots and thick horizontal red bars on a background of turquoise blue surrounded by a yellow margin (e)? Extreme signal diversification is commonly attributed to the model of Lande [2], which hypothesizes that the trajectory of signal evolution changes with drift, or chance changes in female preferences; however, drift alone would ultimately produce random noise. It is generally accepted that receiver psychology influences the selection of signal design, and in some cases, selected designs are more effective in transmitting information than non-preferred designs [3]. Why this occurs is still unknown. For two decades, cognitive scientists have studied the efficacy and the efficiency (§3) of artistic paintings in stimulating the perceptual, cognitive and emotional systems of humans. We propose that their results can shed light on the evolution of nature's designs.
Several authors have proposed that quality indicators and Fisherian models refer to the strategic component of signals, whereas perception models refer to signal efficacy [3,8]. The strategic component is the actual content, the information being conveyed; efficacy describes the ability of a signal to reliably transmit the strategic component and thus refers to its form, or design. Here, we leverage a growing body of literature in the cognitive science of human aesthetics to argue that, as well as efficacy, an additional component of signal design—efficiency, the ability to process information at low metabolic cost—is likely to play a major role in shaping signal design. Current perception models focus primarily on efficacy and explain signal features that are easily detected, like colour or contrast; we suggest that extending these models to further account for efficiency can explain some of the most enigmatic and complex signal patterns in nature (figure 1). Moreover, cognitive scientists have proposed that both effective and efficient information processing can influence preference via monitoring of information processing by the emotional system (box 1). We refer to this incidental effect of information processing on preference as a ‘processing bias’. We suggest that these two ideas—the expansion of perception models to incorporate efficiency, and a direct link between information processing and preference (i.e. processing bias)—offer evolutionary biologists a novel interdisciplinary framework for interpreting the evolution of signal design.
2. Efficacy and efficiency: two aspects of information processing and signal design
Claude Shannon's information theory undoubtedly resides in the pantheon of scientific theories that have dramatically impacted civilization [25]. Information theory addresses two fundamental aspects of information processing: transmission and compression (reviewed in [26]). During transmission, random errors can be introduced in a signal, which make information noisy. Efficacy defines the ability of an information-processing system to minimize noise, and thus to maximize information transmission. Compression occurs because communication channels (i.e. the physical transmission medium) are often limited in their carrying capacity, or limited energetically. Compression is allowed by the presence of redundant information in a signal and thus is often referred to as ‘redundancy reduction’. The term efficiency is classically used to describe the ability of an information-processing system to maximize information compression [26,27].
Optimal information processing should simultaneously maximize efficacy and efficiency, and this dual maximization is at the heart of all modern information-processing technologies. For example, many pixels in a digital visual image are redundant—they are the same as or easily predicted by the value of adjacent pixels. JPEG compression takes advantage of that redundancy; instead of reproducing the precise value of every pixel of a raw digital image, JPEG essentially smoothes values across adjacent pixels, transmitting one bit of information instead of many. But because an image still needs to be informative after decompression, the loss of information in a JPEG is barely detectable to a human eye. Importantly, however, maximizing efficacy and efficiency are two competing goals. Increasing compression (efficiency) adds noise, which degrades information transmission (efficacy). Consequently, there is a trade-off between efficacy and efficiency, and information processing has no absolute optimum. The optimal solution depends on the relative importance of either efficacy or efficiency relative to the goal pursued. JPEG processing software, for example, often permits users to manually set a compression level, depending on whether one wants to store a few heavy but high-quality, artistic pictures, or many vacation photos using minimum space.
Box 1. How animals process information.
Information processing describes the mechanisms that produce a behavioural output from a stimulus input. For most behavioural outputs, animals do not simply reflexively respond to external events, or stimuli; rather, they build meaning by extracting and transforming information from these stimuli. Information processing requires three brain systems: perception, cognition and emotion (figure 2) [12,13].
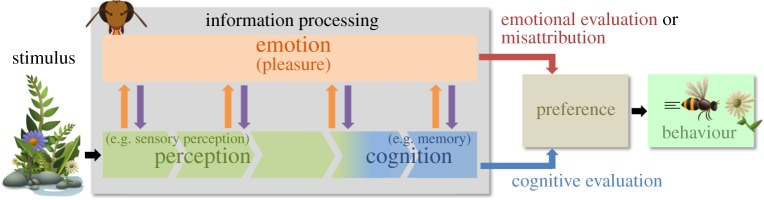
Figure 2.
Information processing in animal brains. The information conveyed by a stimulus (e.g. a flower) is processed by perceptual and then cognitive neurons of the receiver (e.g. a bee), leading to a cognitive evaluation of the costs and benefits of the interaction outcome (e.g. quantity of nectar; blue arrow). Along the processing pathway, pleasure is triggered when processing is effective or efficient (e.g. conspicuous flower; orange arrows). This pleasure could contribute to a fast emotional evaluation of the costs and benefits of interacting with the signaller or of the direct energetic benefits of processing an efficient stimulus (red arrow). Alternatively, or in addition, pleasure can result from evaluating progress in information processing and thereby help regulate the process of information gathering [18] (violet arrows). Because the receiver is not aware that pleasure is triggered by efficient processing, by default s/he misattributes it to the stimulus, which may bias preference towards this stimulus (red arrow).
Perception is the foundational system of information processing and its function is to build an internal representation of the external world. This is achieved by first converting a stimulus into a neural code, and then by hierarchically extracting information from this code. The extracted information is increasingly complex (e.g. simple line segments in early visual stages and entire objects in higher stages) and global (e.g. neurons respond to stimuli spanning the whole visual field only in higher stages [14]). Cognition is the brain system where highly integrated processes occur. It helps build a meaningful representation from perception by providing knowledge about the environment, which notably requires memory. Information processed by perception and then by cognition gives rise to a cognitive evaluation of a stimulus (along a continuum of negative to positive) that indicates the costs or benefits of the stimulus for the receiver.
The third brain system, emotion, also gives rise to an evaluation, consciously experienced or not, along a continuum of negative to positive, reflecting the receiver's interaction with the environment [15]. For example, fear of predators is a negative emotional evaluation that reflects a highly costly interaction. Like its cognitive counterpart, the emotional evaluation influences preference, and the behaviour [16]. The emotional and cognitive evaluations have nevertheless distinct neurochemical bases, and most importantly they differ in the timing of their effects, the emotional evaluation developing earlier during information processing than the cognitive evaluation [17].
Emotions are determined by affects, which play an important role in informing the receiver about the rate of progress towards a goal, and reward it for successful progress [18]. The core rewarding affect is pleasure [19]. In addition to mediating the emotional evaluation, affects also have a meta-informative function: they evaluate progress in information processing [18] and thereby help regulate the process of information gathering. Depending on how pleasurable information processing is, the receiver will continue the same processing strategy, change its strategy, or stop processing information [18].
The cognitive and emotional evaluations, and a misattributed meta-informative evaluation (see §4), are judgements that influence preference at varying degrees depending on the behavioural task. For example, when facing a predator, the emotional evaluation can override other judgements to enforce a fast and adaptive response (‘emotional behaviour’ [15]). However, in most communication systems, including in animal courtship, the relative contribution of these different judgements is an unexplored research area.
The tripartite model of information processing is a highly simplified description of how animal brains process information. Yet it has two main advantages that make it useful for evolutionary biology. First, it excludes brain processes that are still hotly debated among cognitive scientists, such as the relative importance of feedback interactions between cognition and perception [20]. Second, the model likely applies to most if not all brained animals. Even tiny brains such as those of insects are capable of complex cognitive operations (reviewed in [21]) and emotions. Compared with cognition, non-human emotions have been historically more controversial, but interest in their study has increased in recent years, with the development of experimental frameworks for their analysis [15,22]. For example, using an experimental approach similar to those used in humans to study pessimism and optimism (a ‘half-full versus half-empty glass’ approach), a recent study found that bees who experienced a punishing or a rewarding event were more likely to subsequently respond negatively or positively, respectively, to an ambiguous task [23]. As in humans, these animal emotions are modulated by affects [23], which also monitor the dynamics of information processing [24].
Perceptual and cognitive systems appear to have been selected for optimal information processing. Selection for efficacy is supported by numerous adaptations that increase signal intensity (e.g. the summation of signals conveyed by multiple neurons [28]) or decrease noise (e.g. the averaging of signals conveyed by multiple neurons [29]). For example, in a variety of terrestrial and aquatic animals, photoreceptors are tuned to the lighting environment [11,30,31]. This tuning increases the signal-to-noise ratio, and thus the ability to detect or discriminate among stimuli. The tuning of photoreceptors to ambient light and the adaptation of communication signals to maximize conspicuousness or detectability (i.e. efficacy) are some of the strongest evidence in support of sensory drive [32].
Selection for efficiency has been well documented in neuroscience. Attneave [33] and Barlow [34] were the first to apply the information theoretical definition of efficiency to animal perception, hypothesizing that animal brains reduce redundancies to provide an ‘economical description’ of the world. Information processing is a heavy metabolic cost: in humans, neuronal activity in the visual system alone accounts for 2.5–3.5% of a resting body's overall energy requirements [35]. Reducing the amount of neuronal activity required to process information should thus increase both efficiency and evolutionary fitness.
Brain adaptations to reduce redundancies have been studied mainly in visual communication, because visual stimuli naturally present a high level of spatial redundancy. Spatial redundancy can be characterized by lower- and higher-order statistics, both of which are processed in early stages of visual perception (in mammals: from the retina to the first visual cortical area [36–39]). Two examples of lower-order statistics are the spatial auto-correlation function and degree of scale-invariance; neurons in early stages of visual perception have adapted to these lower-order statistics of natural stimuli [40–45]. Higher-order statistics include sparseness, a measure of the neural activity required to encode a scene [45,46]. Visual modelling has shown that the neural code of natural stimuli is particularly sparse and thus suggests that a critical function of early visual perception is to efficiently process natural stimuli [45].
3. Efficacy and efficiency influence preference
Research to date therefore indicates that perception and cognition use multiple strategies to both effectively and efficiently process information. In parallel, a growing body of literature on aesthetics demonstrates that effectively and efficiently processed stimuli are attractive to both humans and other animals. A link between information processing and preference has been documented in two subfields of aesthetics research: experimental psychology, which analyses the effects of putatively aesthetic stimuli on behaviour; and computational aesthetics, which addresses the spatial redundancy of aesthetic stimuli [47]. Over the last two decades, experimental psychologists have uncovered the ‘fluency effect’, by which people are attracted to stimuli that are fluently processed in the brain [48–52]. Fluency can be defined as the subjective experience of ease or difficulty in completing a mental task [53]. Fluency does not refer to the information processing itself but rather to a feeling triggered by certain aspects of information processing [53]. To our knowledge, these aspects have not yet been defined functionally; however, the nature of stimulus features that increase fluency strongly suggests that the aspects of efficacy and efficiency adequately predict the feelings associated with fluency.
(a). Effective stimuli are attractive
The most well-known examples of attractive stimulus features are conspicuousness and symmetry, which are associated with fluency in the psychological literature [48,50], and also clearly with efficacy in information processing. Given the choice between conspicuous versus low-contrasting circles, people tend to prefer conspicuous circles [48]. Conspicuousness affects preference even when the design of the signal should play no role; for example, people are more trusting and more willing to follow instructions of a text and find it more pleasant when written in highly contrasting font [53]. Preference for conspicuous colour stimuli occurs not only in humans, but also insects [54] and birds [55].
Symmetry is attractive to humans [56] and to many other animals [57]. In evolutionary biology, symmetry is often thought to be attractive because it indicates developmental quality [58]. However, symmetry also facilitates object detection and recognition, and thus increases efficacy [57,59]. For example, in a study of newly hatched chicks, naive individuals innately preferred asymmetric geometric forms. Preference for symmetry appeared only in chicks that were allowed to forage independently; chicks that were hand-fed by researchers never developed a preference for symmetry [60]. This suggests that a key factor in symmetry preference was the improvement of sensorimotor skills during active food manipulation [60], rather than an innate preference typically assumed by indicator models. Thus, the efficacy of symmetrical features itself probably influences preference, independently of the role of these features in signalling quality.
Another universal attraction exists for prototypes, the most representative stimuli of a perceptual category. Prototypes are processed fluently [48,50], probably because they are effective. Indeed, prototype-like stimuli are most quickly and precisely categorized and stored the longest in memory [61]. Prototypes are also the most attractive. The attractiveness of prototypes has been shown in humans, using various biological, inanimate or abstract visual stimuli [61–63], and in other animals, notably in studies of the ‘peak shift effect’, when animals prefer an exaggerated version of the feature that distinguishes two perceptual categories [64]. For example, if a rat is trained to choose a rectangle with a 4 : 3 aspect ratio over a square, in subsequent testing trials, the rat will choose a 3 : 2 rectangle over a 4 : 3 rectangle. The 3 : 2 rectangle is preferred because, in this example, it prototypifies rectangularity—the rule that the rat learned in order to differentiate a rectangle from a square—more than the 4 : 3 rectangle does [65].
In evolutionary biology, prototypes are often thought to be attractive because they exemplify features that define a fitness-related (i.e. quality) category (e.g. men should prefer the most feminine women because femininity indicates fertility [66]). However, a study in chickens analysed preference for human faces: birds that were trained to choose the average female face from a range of female and male faces were found to respond maximally to female faces that were more feminine than average during testing trials, and specifically to the face that was also rated as being most beautiful by human subjects [67]. This and other results (e.g. [61]) suggest that prototypes influence preference in part because they increase processing efficacy independently of the quality of the stimulus.
Repetition over time is another feature that increases both preference and fluency [48]. Repeated stimuli are effective because they provide prior knowledge about the structure of the signal, which allows neurons to anticipate and compensate for noise [29]. Known in psychology as the ‘mere exposure effect’ [68], people tend to prefer repeated stimuli over stimuli to which they have never been exposed. For example, the mere repetition of a melody is sufficient to increase preference for it, at least in initial stages [69].
(b). Efficient stimuli are attractive
Several features promoting attractiveness, revealed by psychological studies on fluency, are also associated with efficiency. Prototypicality and repetition over time are efficiently processed in addition to being effective. Prototypes are sparsely encoded, and thus economical, because they only need to stimulate a few highly selective neurons to be recognized [70]. Repetition also increases sparseness in the neural code because the selectivity of neurons tends to be tuned to features to which they are frequently exposed [71]. Surface and line continuity also promote efficiency. Continuous surfaces/lines are redundant, and thus highly predictable, and are preferred over discontinuous lines and heterogeneous surfaces [72]. Preference for continuous shapes also has been shown in non-human primates [73] and birds [74].
Computational aesthetics provides more direct evidence that sparseness elicits preference. A common way to measure the sparseness of an image representation is to first train artificial neurons to process images of natural scenes while minimizing the number of simultaneously active neurons (a sparseness constraint) [45]. This creates a proxy set of neurons for the primary visual cortex of mammals (V1) that is ‘adapted’ to sparsely encode terrestrial environments, which is then used to estimate the sparseness of images: sparse images will activate fewer trained neurons. Renoult et al. [75] used this approach to model the sparseness of images of female faces. Sparseness was positively correlated with face attractiveness as rated by men, and explained up to 17% of the variance in attractiveness. Using the same approach, Holzleitner et al. [76] found that sparseness was the highest predictor of face attractiveness when compared with body mass index, sexual dimorphism, averageness and asymmetry. It is worth noting that features unrelated to efficiency could drive sparse coding in V1 (e.g. a smooth skin texture that indicates youth and health); these results therefore would benefit from analysing sparseness in higher levels of information processing. Nevertheless, in another study that directly modelled V1 from neurophysiological data, image sparseness was negatively correlated with aversiveness: images of abstract patterns with a lower degree of sparseness were more highly aversive to human subjects [77]. Thus, images that are sparsely processed by environmentally tuned visual systems (i.e. trained neurons) are attractive.
Additional support for a link between efficiency and preference comes from computational studies of artwork. Natural terrestrial scenes are characterized by an elevated and characteristic degree of scale-invariance, often measured as the fractal dimension D or as the slope of the Fourier power spectrum 1/f [26,61,78]. Several studies demonstrate that people prefer both abstract and representational images with fractal dimensions that mimic those of an average natural terrestrial scene [44,79,80]. Other studies demonstrate that artistic paintings have a degree of scale-invariance similar to that of natural scenes; for example, faces painted by portrait artists across time and cultures exhibit natural values of scale-invariance even though real faces typically do not [81]. It has been suggested that artists increase the attractiveness of their work by unconsciously mimicking the spatial statistics of natural scenes to which our brain has adapted to efficiently process, thus creating art that is ‘easy on the eye’ [43,82,83].
4. The processing bias
Cognitive research therefore strongly suggests that people and other animals prefer effective and efficient visual stimuli, and moreover that effective and efficient processing is a pleasant experience [84]. One explanation for this observation, rooted in the quality indicator model of sexual selection, is if neurons are tuned to stimuli with the greatest impact on receivers' fitness. This explanation is related to the psychological hypothesis that perceptual and cognitive fluency may be pleasant because it indicates that a stimulus is familiar and thus less likely to cause harm [68,85]. In this case, effective and efficient processing would indicate the value, or quality, of the signaller; signallers of the highest quality will display the most optimally processed traits. Receivers that are rewarded for attending to those traits are assumed to prefer them and seek them out; thus, selection should favour a pleasurable response to effective and efficient processing. A quality indicator explanation for pleasure in information processing therefore implicates the stimulus as the selective agent shaping perceptual systems, rather than vice versa, or at least playing a lead role in a coevolutionary process. It furthermore presupposes that a receiver evaluates the stimulus positively, and responds to it appetitively, thus establishing a link between pleasure and preference. Importantly, however, not all effective and efficient stimuli that affect receiver fitness trigger a positive evaluation. Aposematic traits, for example, facilitate the recognition and memorization of dangerous species [86], and are judged to be beautiful, but they trigger fear, a negative or aversive emotional evaluation (e.g. in snakes; see [87]; box 1). Animals can form positive (aesthetic) judgements about stimuli that trigger an aversive emotional response or even about stimuli that are probably neutral with respect to fitness, like artwork, suggesting a typical quality indicator model may not be a sufficient explanation.
An alternative explanation refers to the ‘meta-informative function’ of pleasure, which helps monitor progress in information processing and is triggered when processing is effective and efficient [18,84] (box 1). A meta-informative function of pleasure is supported by a corpus of studies showing that information seeking and problem solving are experienced as intrinsically pleasurable activities [88] (see also [89]). This pleasure is probably adaptive—effective and efficient processing can yield direct benefits like reducing response time [90] and decreasing metabolic costs. Unlike a standard quality indicator hypothesis, however, the meta-informative function of pleasure does not necessarily associate pleasure with desire, which is consistent with psychological research in liking and wanting. Though we generally ‘like what we want, and want what we like’ [91], pleasure and desire are mediated by different neurotransmitters that can be released separately [12,92]. Cognitive scientists have investigated whether and why people (or other animals) might find stimuli pleasant solely due to information processing itself and not from an evaluation of stimulus benefits. The answer appears to be a misattribution, which describes the fact that people are usually unaware of the source of pleasure and, by default, tend to attribute it to the stimulus rather than to information processing itself [53,61]. Misattribution of pleasure therefore implies that the link between efficient processing and preference is a sensory or cognitive ‘bias’, a judgement that is a by-product of the adaptive function of pleasure to monitor progress in information processing. We call this the processing bias (for a discussion on how to test processing bias, see electronic supplementary material, box S1).
Like other pre-existing biases, processing bias implicates perceptual systems as the driver of signal evolution, specifically as signals evolve to leverage or exploit a pre-existing bias for effective and efficient information processing. That bias will be shaped by the environment not only to maximize signal detection and discrimination but also to minimize cost, for example, by minimizing habitat-specific redundancies, and this tuning for efficient processing can be exploited by the patterns and textures of animal signals. Importantly, a leading role for perceptual biases in signal evolution does not rule out signals as quality indicators. If highly effective or efficient signals are costly, then signallers can indicate their fitness with easily processed displays. Indeed, cognitive or emotional evaluations and quality indicator models may be more relevant for understanding why some traits evolve as signals in the first place, whereas processing and other pre-existing biases might explain the preference for particular designs. Finally, signals themselves are a component of the environment to which perceptual systems can adapt, especially in later (higher) stages of information processing (see below); thus, although we hypothesize processing biases as driving signal evolution, they also contribute to a coevolutionary process between signaller and receiver.
5. Importance of efficiency for the evolution of communication
(a). Extending sensory drive to efficiency
Sensory drive describes the influence of the external environment on the design of communication signals through its effects on perception and cognition [8,10]. To date, sensory drive has been framed primarily to explain the efficacy of signals, where signal evolution is driven by neurons that are tuned to maximize detection, discrimination or recognition in a particular set of environmental conditions. Here, we extend the model to include efficiency, because neurons are also tuned to efficiently process the characteristic redundant features of their habitats. Just as artists mimic spatial features of natural scenes to make their artwork more attractive, a sensory drive model that incorporates efficiency predicts that organisms have evolved communication signals that match the lower-order (e.g. the degree of scale-invariance) and higher-order (e.g. sparseness) statistics of their environments.
Recognizing the importance of efficiency in signal design will probably increase our estimate of the role of sensory drive in evolution. In visual communication, canonical examples of sensory drive come from aquatic habitats, which vary in the colour of ambient light (e.g. [11,91]). By contrast, terrestrial habitats vary little in ambient light, such that the role of sensory drive in terrestrial species has remained contentious (e.g. [93]). Studying spatially redundant features could provide broader support for a role of sensory drive in signal evolution, because these features differ strongly across both terrestrial [42] and aquatic habitats.
Historically, sensory drive has focused on how signal detection (efficacy) is shaped by the transmission channel, for example, the colour of ambient light or the acoustic characteristics of background noise. However, perception and cognition can adapt to other aspects of the environment as well (e.g. food items [30] or sexual displays [94]). Neuronal tuning thus probably reflects adaptation to many biotic and abiotic environmental stimuli, creating multiple efficacy ‘niches’ to which signals can adapt (i.e. exploit), such as by evolving conspicuous colours, symmetrical and prototypical patterns or combinations of these features [9]. The same reasoning holds for efficiency. In the visual system of primates, for example, neurons in the primary cortex are tuned to simple, redundant features of habitats (i.e. simple oriented line segments). Later in the processing pathway, neurons are tuned to efficiently process more complex and specific features (e.g. familiar faces; see box 1). Multiple stages of information processing thus also create multiple efficiency ‘niches’ to which signals can adapt, as local environments vary not only in spatial statistics but also community composition (which can affect the features used to efficiently classify categories like mate, competitor or predator). Which components of the environment play the dominant role in shaping the relationship between processing and preference thus will co-depend on the biological task (foraging, mating, etc.), stage of processing (peripheral versus integrative), the relative importance of efficacy and efficiency and the relative weights of emotional and cognitive evaluations (box 1). These multiple parameters create a highly dimensional landscape to which signals can adapt in a coevolutionary process.
(b). Studying efficiency in evolution
A variety of empirical approaches can test whether communication signals have evolved to be efficiently processed (for a discussion on how to estimate processing efficiency, see electronic supplementary material, box S2), and furthermore, whether the adaptation of neural systems to efficiently process local habitats drives the evolution of signal design (i.e. sensory drive). These approaches will mirror those that test the efficacy component of sensory drive, which focus mainly on signal detection and discrimination. Assuming that efficiency-driven preference originates from redundant features of the habitat, the sensory drive model predicts that redundant features of signals should match redundant features of habitats. The model thus predicts interspecific variation in patterns and texture will be correlated with variation in habitat, and convergence will occur between unrelated species living in similar habitats.
A sensory drive model of efficiency can also be tested intraspecifically. Here, predictions of sensory drive might differ from those of quality indicator models. Whereas indicator models predict that sexual signals will exhibit the most regular patterns (e.g. the highest fractal dimension), sensory drive based on processing bias predicts that signals should evolve towards environmental-like statistics (e.g. natural values of auto-correlation, scale-invariance, sparseness) rather than towards maxima. For example, the black bib of the red-legged partridge (Alectoris rufa) is a male sexual ornament whose spatial statistics (fractal dimension D) are associated with the higher individual condition [78,95], consistent with predictions of indicator models. But whether the fractal dimension of the bib closely matches that of its habitat, or whether the female preference is predicted by the fractal dimension, remains to be tested.
The strongest support for sensory drive is a complete sequence whereby habitat features match neuronal tuning, neuronal tuning is correlated with preference, and preference is correlated with the signal design. Such a sequence appears to have been established for efficacy in the African cichlid fish of the genus Pundamilia, where sister species live in blue- or redshifted light. Pundamilia nyererei lives in redshifted light, which increases the signal-to-noise ratio (conspicuousness) of red stimuli against a dark background. Pundamilia nyererei exhibit higher expression of long wavelength (red) sensitive photoreceptors [96] and are more sensitive to red light [97]. Female P. nyererei prefer red stimuli, and males have evolved reddish coloration [11]. As for efficiency, each of these correlations has been shown in one system or another (see above), but to our knowledge, they remain to be shown altogether in a single system.
Camouflage patterns pose an interesting problem with respect to efficacy and efficiency. Because they match the spatial features of environments and are thus efficiently processed, camouflage patterns should be attractive to predators; yet, such designs are selected to be undetectable. Camouflage patterns are therefore at the same time efficient but almost completely ineffective (from an information-processing perspective), so the pleasure triggered by efficient processing of camouflage is unlikely to have played a role in the evolution of the pattern or of the predator's decision. However, if predation pressure is relaxed or lost, for example, as observed during island colonization, signallers are free to evolve conspicuous socio-sexual colour signals on top of their ancestral camouflage. Pleasure derived from processing efficient camouflage patterns would then compound the pleasure of effectively processing conspicuous features, and signallers that have retained their ancestral camouflage should be preferred. Camouflage patterns could thus easily be co-opted for sexual signalling. Although a link between camouflage and sexual signalling has been ignored in the recent literature, the idea is not new. Renowned nineteenth-century artist and naturalist Abbott Thayer was once mocked for suggesting that all animal patterns, even the peacock's tail, are cryptic [98]. Investigating the links between efficacy and efficiency, and between sexual selection and camouflage, could partially vindicate that perspective.
6. A broader outlook
Historically in evolutionary biology, the mechanisms that link stimulus and behaviour have been modelled using simplified frameworks that ignore the complexity of brain processes [99]. This simplified approach has possibly been motivated by a fear of anthropomorphism, but also because brain processes were once considered elusive and unpredictable (but see [100]). Advances in comparative cognition [101,102], however, are allowing evolutionary biologists to investigate the ubiquitous role of cognitive processes in social, sexual and natural selection, and in speciation [100,103–105]. We suggest that further accounting for affects and emotions, and for efficiency in perceptual and cognitive processes, can address persistent outstanding questions in animal communication and beyond. In humans, for example, pleasure mediated by effective and efficient processing is also known to bias judgement of truth (review in [106]); for example, reading an identical email three times rather than once increases the perceived veracity of its content [107]. The influence of efficacy and efficiency on truth assessment stresses that design and the strategic component of signals are interconnected, and suggests that pre-existing bias and sensory drive could play a more widespread role in the evolution of honest signalling than is currently assumed.
A central point is that behaviours can be motivated by rewards arising from the monitoring of neural processes independent of the evaluation of benefits provided by a signaller. Here, we examined the link between the environment, information processing, pleasure and preferences. Other types of intrinsically rewarding processes include curiosity, an adaptive behaviour aimed at filling a gap in knowledge, for which reward also originates from information seeking itself and not from the good or bad use of that information [108]. Psychologists and philosophers have suggested that such rewards could be major determinants of aesthetic preferences, and thus could help explain the diversity of works of art [109] (box 2). Studying intrinsically rewarding processes could similarly reveal how elaborate communication signals evolve in other species (figure 1), and models of sensory drive and pre-existing bias offer especially appropriate evolutionary frameworks for integrating hypotheses and results from the humanities, cognitive psychology, neurophysiology, computer science and behavioural ecology to understand the evolution of signal design.
Box 2. Evolutionary aesthetics.
Unravelling the functional bases of aesthetics has been a major research aim in cognitive sciences over the last two decades. Although no unique definition of aesthetics has yet emerged, results overall agree that aesthetic experiences are affective, independent of the sensory modality and the perceptual domain, and rooted in the interaction between a stimulus and a receiver (i.e. any object, organism or even landscape could operate aesthetically [12,110]). The German philosopher Immanuel Kant and others further suggested that the aesthetic experience is a state of ‘disinterested interest’, an engagement with objects without the desire to acquire, control or manipulate them [12]. This idea has been supported recently by brain imaging studies (e.g. [111]).
A large body of work suggests that the pleasure experienced by effective and efficient information processing fulfils all criteria of an aesthetic experience. Studies show that artists select, consciously or not, formal (e.g. colours and patterning) or conceptual features to manipulate the efficacy and efficiency of perceptual or cognitive processing, respectively [43,81–83]. Artists also develop complex strategies to delay the acquisition of information (e.g. suspense), or initially confuse the receiver in order to amplify the aesthetic pleasure of a sudden increase in processing efficacy (the ‘Aha’ effect [112]).
The processing bias model discussed here is an evolutionarily framed extension of the models of fluency [50,56], efficient coding [52], and the pleasure-interest model of aesthetic liking [51], proposed by cognitive scientists to explain the results described above. The popularity of these models has grown among biologists, social scientists and philosophers due to their unique ability to simultaneously account for both the universal and subjective dimensions of aesthetics and to explain complex aesthetic experience beyond controlled, laboratory conditions [110].
Extending the scope of aesthetics from the arts to natural communication, some authors have proposed that many signals in plants and animals could be aesthetic [54,55], and that beautiful animal signals manipulate processing fluency [56,57]. For example, the peacock tail trapping peahens' gaze into a back-and-forth motion [58] or the visual illusions of bowerbirds [59] could be strategies to exploit pleasure caused by effective or efficient information processing. As a cautionary note, however, we stress that the similarity between artwork and natural aesthetic signals is homologous in process (i.e. they have similar biological bases), not in function [55]. The processing bias model thus does not predict that works of art function as sexual signals.
One aspect of aesthetic communication in plants and animals that remains to be determined is whether receivers actively search for an aesthetic experience. The human parallels are museum visitors, moviegoers and book readers who spend time and money to be rewarded by nothing more than the pure pleasure of an information-processing experience [55]. In other words, mates or pollinators may select partners or plants for their aesthetic reward in addition to the benefits of these resources.
Supplementary Material
Acknowledgements
The authors thank M. Burns, S. Hulse, B. Lohr, K. Omland and other members of the UMBC ecology and evolution journal club, B. Godelle, D. Gomez, and other members of the CEFE and ISEM journal clubs in Montpellier. They are also grateful to I. Cuthill, G. Rosenthal and two anonymous referees for constructive feedback on an earlier version of the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
The two authors contributed equally to this review.
Competing interests
We declare we have no competing interests.
Funding
The collaboration between authors is funded by National Science Foundation grant no. IOS-1708543, and by PICS-CNRS.
Glossary
- efficacy
information processing with a limited loss of information
- efficiency
information processing with an economical use of resources
- feature
measurable property of a stimulus
- information
property of a stimulus that reduces uncertainty about the environment
- information processing
describes how information is detected, internally transmitted, coded, stored and retrieved in the animal brain and sensory systems
- neuronal selectivity
the range of stimulus features that activate a neuron
- processing bias
judgement modulated by affect, which is influenced by the level of efficacy and efficiency in information processing; in cognitive sciences, processing bias is often referred to as an aesthetic judgement
- sensory drive
the hypothesis that the tuning of perceptual and cognitive systems to effectively and efficiently process information in environmental stimuli generates selection on communication signals due to a direct effect of effective and efficient processing on receiver preference
- stimulus
component of the external environment causing a physiological response (e.g. a landscape, an individual or a communication signal)
References
- 1.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray. [Google Scholar]
- 2.Lande R. 1981. Models of speciation by sexual selection on polygenic traits. Proc. Natl Acad. Sci. USA 78, 3721–3725. ( 10.1073/pnas.78.6.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilford T, Dawkins MS. 1991. Receiver psychology and the evolution of animal signals. Anim. Behav. 42, 1–14. ( 10.1016/S0003-3472(05)80600-1) [DOI] [Google Scholar]
- 4.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Kokko H. 2001. Fisherian and ‘good genes’ benefits of mate choice: how (not) to distinguish between them. Ecol. Lett. 4, 322–326. ( 10.1046/j.1461-0248.2001.00224.x) [DOI] [Google Scholar]
- 6.Fisher RA. 1930. The genetical theory of natural selection. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.Prum RO. 2010. The Land-Kirkpatrick mechanism is the null model of evolution by intersexual selection: implications for meaning, honesty, and design in intersexual signals. Evolution 64, 3085–3100. ( 10.1111/j.1558-5646.2010.01054.x) [DOI] [PubMed] [Google Scholar]
- 8.Endler JA, Basolo AL. 1998. Sensory ecology, receiver biases and sexual selection. Trends Ecol. Evol. 13, 415–420. ( 10.1016/S0169-5347(98)01471-2) [DOI] [PubMed] [Google Scholar]
- 9.Ryan MJ, Cummings ME. 2013. Perceptual biases and mate choice. Ann. Rev. Ecol. Evol. 44, 437–459. ( 10.1146/annurev-ecolsys-110512-135901) [DOI] [Google Scholar]
- 10.Endler JA. 1993. Some general comments on the evolution and design of animal communication systems. Phil. Trans. R. Soc. Lond. B 340, 215–225. ( 10.1098/rstb.1993.0060) [DOI] [PubMed] [Google Scholar]
- 11.Seehausen O, et al. 2008. Speciation through sensory drive in cichlid fish. Nature 455, 620–626. ( 10.1038/nature07285) [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee A, Vartanian O. 2016. Neuroscience of aesthetics. Ann. N. Y. Acad. Sci. 1369, 172–194. ( 10.1111/nyas.13035) [DOI] [PubMed] [Google Scholar]
- 13.Wyer RS Jr, Clore GL, Isbell LM. 1999. Affect and information processing. Adv. Exp. Soc. Psychol. 31, 1–77. ( 10.1016/S0065-2601(08)60271-3) [DOI] [Google Scholar]
- 14.Hubel DH, Wiesel TN. 1962. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J. Physiol. 160, 106–154. ( 10.1113/jphysiol.1962.sp006837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson DJ, Adolphs R. 2014. A framework for studying emotions across species. Cell 157, 187–200. ( 10.1016/j.cell.2014.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pessoa L. 2008. On the relationship between emotion and cognition. Nature Rev. Neurosci. 9, 148–148. ( 10.1038/nrn2317) [DOI] [PubMed] [Google Scholar]
- 17.Finucane ML, Alhakami A, Slovic P, Johnson SM. 2000. The affect heuristic in judgments of risks and benefits. J. Behav. Decis. Mak. 13, 1–17. () [DOI] [Google Scholar]
- 18.Schwarz N. 2011. Feelings-as-information theory. In Handbook of theories of social psychology, vol. 1 (eds PAM van Lange, AW Kruglanski, ET Higgins), pp. 289–308. London, UK: Sage [Google Scholar]
- 19.Berridge KC, Kringelbach ML. 2008. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology 199, 457–480. ( 10.1007/s00213-008-1099-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Firestone C, Scholl BJ. 2016. Cognition does not affect perception: evaluating the evidence for ‘top-down’ effects. Behav. Brain Sci. 39, 1–77. ( 10.1017/S0140525X15000965) [DOI] [PubMed] [Google Scholar]
- 21.Giurfa M. 2013. Cognition with few neurons: higher-order learning in insects. Trends Neurosci. 36, 285–294. ( 10.1016/j.tins.2012.12.011) [DOI] [PubMed] [Google Scholar]
- 22.Paul ES, Mendl MT. 2018. Animal emotion: descriptive and prescriptive definitions and their implications for a comparative perspective. Appl. Anim. Behav. Sci. 205, 202–209. ( 10.1016/j.applanim.2018.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry CJ, Baciadonna L, Chittka L. 2016. Unexpected rewards induce dopamine-dependent positive emotion-like state changes in bumblebees. Science 353, 1529–1531. ( 10.1126/science.aaf4454) [DOI] [PubMed] [Google Scholar]
- 24.Bromberg-Martin ES, Hikosaka O. 2009. Midbrain dopamine neurons signal preference for advance information about upcoming rewards. Neuron 63, 119–126. ( 10.1016/j.neuron.2009.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shannon CE. 1948. A mathematical theory of communication. Bell Syst. Technic. J. 27, 379–423. ( 10.1002/j.1538-7305.1948.tb01338.x) [DOI] [Google Scholar]
- 26.Hyvärinen A, Hurri J, Hoyer PO. 2009. Natural image statistics: a probability approach to early computational vision. London, UK: Springer-Verlag. [Google Scholar]
- 27.MacKay DJC, Mac Kay DJC. 2003. Information theory, inference and learning algorithms. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 28.Stöckl AL, O'Carroll DC, Warrant EJ. 2016. Neural summation in the hawkmoth visual system extends the limits of vision in dim light. Curr. Biol. 26, 821–826. ( 10.1016/j.cub.2016.01.030) [DOI] [PubMed] [Google Scholar]
- 29.Faisal AA, Selen L.PJ, Wolpert DM. 2008. Noise in the nervous system. Nat. Rev. Neurosci. 9, 292–303. ( 10.1038/nrn2258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osorio D, Vorobyev M. 1996. Colour vision as an adaptation to frugivory in primates. Proc. R. Soc. B 263, 593–599. ( 10.1098/rspb.1996.0089) [DOI] [PubMed] [Google Scholar]
- 31.Osorio D, Vorobyev M. 2005. Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc. R. Soc. B 272, 1745–1752. ( 10.1098/rspb.2005.3156). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuller RC, Endler JA. 2018. A perspective on sensory drive. Curr. Zool. 64, 465–470. ( 10.1093/cz/zoy052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Attneave F. 1954. Some informational aspects of visual perception. Psychol. Rev. 61, 183 ( 10.1037/h0054663) [DOI] [PubMed] [Google Scholar]
- 34.Barlow H. 1961. Possible principles underlying the transformations of sensory messages. In Sensory communication (ed. Rosenblith W.), pp. 217–234. Cambridge, MA: MIT Press. [Google Scholar]
- 35.Attwell D, Laughlin SB. 2001. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 21, 1133–1145. ( 10.1097/00004647-200110000-00001) [DOI] [PubMed] [Google Scholar]
- 36.Dan Y, Atick JJ, Reid RC. 1996. Efficient coding of natural scenes in the lateral geniculate nucleus: experimental test of a computational theory. J. Neurosci. 16, 3351–3362. ( 10.1523/JNEUROSCI.16-10-03351.1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitkow X, Meister M. 2012. Decorrelation and efficient coding by retinal ganglion cells. Nat. Neurosci. 15, 628–635. ( 10.1038/nn.3064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simoncelli EP, Olshausen BA. 2001. Natural image statistics and neural representation. Annu. Rev. Neurosci. 24, 1193–1216. ( 10.1146/annurev.neuro.24.1.1193) [DOI] [PubMed] [Google Scholar]
- 39.Simoncelli EP. 2003. Vision and the statistics of the visual environment. Curr. Opin. Neurobiol. 13, 144–149. ( 10.1016/S0959-4388(03)00047-3) [DOI] [PubMed] [Google Scholar]
- 40.Srinivasan MV, Laughlin SB, Dubs A. 1982. Predictive coding: a fresh view of inhibition in the retina. Proc. R. Soc. Lond B 22, 427–459. [DOI] [PubMed] [Google Scholar]
- 41.Atick JJ. 1992. Could information theory provide an ecological theory of sensory processing? Netw. Comput. Neural Syst. 3, 213–251. ( 10.1088/0954-898X_3_2_009) [DOI] [PubMed] [Google Scholar]
- 42.Pouli T, Reinhard E, Cunningham DW. 2014. Image statistics in visual computing. Boca Raton, FL: Taylor & Francis Group. [Google Scholar]
- 43.Taylor R, Spehar B, Hagerhall C, Van Donkelaar P.. 2011. Perceptual and physiological responses to Jackson Pollock's fractals. Front. Hum. Neurosci. 5, 60 ( 10.3389/fnhum.2011.00060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor RP, Spehar B. 2016. Fractal fluency: an intimate relationship between the brain and processing of fractal stimuli. In The fractal geometry of the brain (ed. Di Leva A.), pp. 485–496. Berlin, Germany: Springer. [Google Scholar]
- 45.Olshausen BA, Field D. 1996. Emergence of simple-cell receptive field properties by learning a sparse code for natural images. Nature 381, 607–609. ( 10.1038/381607a0) [DOI] [PubMed] [Google Scholar]
- 46.Field DJ. 1987. Relations between the statistics of natural images and the response properties of cortical cells. J. Opt. Soc. Am. A 4, 2379–2394. ( 10.1364/JOSAA.4.002379) [DOI] [PubMed] [Google Scholar]
- 47.Brachmann A, Redies C. 2017. Computational and experimental approaches to visual aesthetics. Front. Comp. Neurosci. 11, 102 ( 10.3389/fncom.2017.00102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reber R, Winkielman P, Schwarz N. 1998. Effects of perceptual fluency on affective judgments. Psychol. Sci. 9, 45–48. ( 10.1111/1467-9280.00008) [DOI] [Google Scholar]
- 49.Reber R. 2012. Processing fluency, aesthetic pleasure, and culturally shared taste. In Aesthetic science, connecting minds, brains, and experience (eds Shimamura AP, Palmer SE), pp. 223–249. Oxford, UK: Oxford University Press. [Google Scholar]
- 50.Winkielman P, Schwarz N, Fazendeiro T, Reber R. 2003. The hedonic marking of processing fluency: implications for evaluative judgment. In The psychology of evaluation: affective processes in cognition and emotion (eds Musch J, Klauer KC), pp. 189–217. Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- 51.Graf LKM, Landwehr JR. 2015. A dual-process perspective on fluency-based aesthetics: the pleasure-interest model of aesthetic liking. Person. Soc. Psychol. Rev. 19, 395–410. ( 10.1177/1088868315574978) [DOI] [PubMed] [Google Scholar]
- 52.Redies C. 2007. A universal model of esthetic perception based on the sensory coding of natural stimuli. Spat. Vis. 21, 97–117. ( 10.1163/156856807782753886) [DOI] [PubMed] [Google Scholar]
- 53.Oppenheimer DM. 2008. The secret life of fluency. Trends Cogn. Sci. 12, 237–241. ( 10.1016/j.tics.2008.02.014) [DOI] [PubMed] [Google Scholar]
- 54.Naug D, Arathi HS. 2007. Receiver bias for exaggerated signals in honeybees and its implications for the evolution of floral displays. Biol. Lett. 3, 635–637. ( 10.1098/rsbl.2007.0436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cazetta E, Schaefer HM, Galetti M. 2009. Why are fruits colorful? The relative importance of achromatic and chromatic contrasts for detection by birds. Evol. Ecol. 23, 233–244. ( 10.1007/s10682-007-9217-1) [DOI] [Google Scholar]
- 56.Reber R, Schwarz N. 2006. Perceptual fluency, preference, and evolution. Polish Psychol. Bull. 1, 16–22. [Google Scholar]
- 57.Enquist M, Arak A. 1994. Symmetry, beauty and evolution. Nature 372, 169–172. ( 10.1038/372169a0) [DOI] [PubMed] [Google Scholar]
- 58.Grammer K, Thornhill R. 1994. Human (Homo sapiens) facial attractiveness and sexual selection: the role of symmetry and averageness. J. Comp. Psychol. 108, 233–242. ( 10.1037/0735-7036.108.3.233) [DOI] [PubMed] [Google Scholar]
- 59.Garner WR. 1974. The processing of information structure. Potomac, MD: Lawrence Erlbaum Associates. [Google Scholar]
- 60.Clara E, Regolin L, Vallortigara G. 2007. Preference for symmetry is experience dependent in newborn chicks (Gallus gallus). J. Exp. Psychol. 33, 12–20. [DOI] [PubMed] [Google Scholar]
- 61.Winkielman P, Halberstadt J, Fazendeiro T, Catty S. 2006. Prototypes are attractive because they are easy on the mind. Psychol. Sci. 17, 799–806. ( 10.1111/j.1467-9280.2006.01785.x) [DOI] [PubMed] [Google Scholar]
- 62.Farkas A. 2002. Prototypicality-effect in surrealist paintings. Empir. Stud. Arts 20, 127–136. ( 10.2190/UD7Y-GN8P-Q0EV-Q13J) [DOI] [Google Scholar]
- 63.Whitfield TWA, Slatter PE. 1979. The effects of categorization and prototypicality on aesthetic choice in a furniture selection task. Brit. J. Psychol. 70, 65–75. ( 10.1111/j.2044-8295.1979.tb02144.x) [DOI] [Google Scholar]
- 64.ten Cate C, Rowe C. 2007. Biases in signal evolution: learning makes a difference. Trends Ecol. Evol. 22, 380–387. ( 10.1016/j.tree.2007.03.006) [DOI] [PubMed] [Google Scholar]
- 65.Ramachandran VS, Hirstein W. 1999. The science of art: a neurological theory of aesthetic experience. J. Conscious. Stud. 6, 15–51. [Google Scholar]
- 66.Perrett DI, Lee KJ, Penton-Voak I, Rowland D, Yoshikawa S, Burt DM, Henzi SP, Castles DL, Akamatsu S. 1998. Effects of sexual dimorphism on facial attractiveness. Nature 394, 884 ( 10.1038/29772) [DOI] [PubMed] [Google Scholar]
- 67.Ghirlanda S, Jansson L, Enquist M. 2002. Chickens prefer beautiful humans. Hum. Nat. 13, 383–389. ( 10.1007/s12110-002-1021-6) [DOI] [PubMed] [Google Scholar]
- 68.Zajonc RB. 1968. Attitudinal effects of mere exposure. J. Person. Social Psychol. 9, 1–27. ( 10.1037/h0025848) [DOI] [Google Scholar]
- 69.Pereira CS, Teixeira J, Figueiredo P, Xavier J, Castro SL, Brattico E. 2011. Music and emotions in the brain: familiarity matters. PLoS ONE 6, e27241 ( 10.1371/journal.pone.0027241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quiroga RQ, Kreiman G, Koch C, Fried I. 2008. Sparse but not ‘grandmother-cell'coding in the medial temporal lobe. Trends Cogn. Sci. 12, 87–91. ( 10.1016/j.tics.2007.12.003) [DOI] [PubMed] [Google Scholar]
- 71.Sengpiel F, Stawinski P, Bonhoeffer T. 1999. Influence of experience on orientation maps in cat visual cortex. Nat. Neurosci. 2, 727–732. ( 10.1038/11192) [DOI] [PubMed] [Google Scholar]
- 72.Silvia PJ, Barona CM. 2009. Do people prefer curved objects? Angularity, expertise, and aesthetic preference. Empir. Stud. Arts 27, 25–42. ( 10.2190/EM.27.1.b) [DOI] [Google Scholar]
- 73.Munar E, Gómez-Puerto G, Call J, Nadal M. 2015. Common visual preference for curved contours in humans and great apes. PLoS ONE 10, e0141106 ( 10.1371/journal.pone.0141106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fantz RL. 1957. Form preferences in newly hatched chicks. J Comp. Physiol. Psychol. 50, 422–430. ( 10.1037/h0044973) [DOI] [PubMed] [Google Scholar]
- 75.Renoult JP, Bovet J, Raymond M. 2016. Beauty is in the efficient coding of the perceiver. R. Soc. open sci. 3, 160027.. ( 10.1098/rsos.160027). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holzleitner IJ, et al. In press. A data-driven model of women's facial attractiveness reliably outperforms theory-driven models. PsyArXiv Preprints ( 10.31234/osf.io/vhc5k [DOI] [Google Scholar]
- 77.Hibbard PB, O'Hare L. 2015. Uncomfortable images produce non-sparse responses in a model of primary visual cortex. R. Soc. open sci. 2, 140535 ( 10.1098/rsos.140535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pérez-Rodríguez L, Jovani R, Stevens M. 2017. Shape matters: animal colour patterns as signals of individual quality. Proc. R. Soc. B 284, 20162446 ( 10.1098/rspb.2016.2446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spehar B, Wong S, van de Klundert S, Lui J, Clifford CWG, Taylor R.. 2015. Beauty and the beholder: the role of visual sensitivity in visual preference. Front. Hum. Neurosci. 9, 514 ( 10.3389/fnhum.2015.00514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Juricevic I, Land L, Wilkins A, Webster MA. 2010. Visual discomfort and natural image statistics. Perception 39, 884–899. ( 10.1068/p6656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Redies C, Hänisch J, Blickhan M, Denzler J. 2007. Artists portray human faces with the Fourier statistics of complex natural scenes. Netw. Comput. Neural. Syst. 18, 235–248. ( 10.1080/09548980701574496) [DOI] [PubMed] [Google Scholar]
- 82.Graham DJ, Field DJ. 2007. Statistical regularities of art images and natural scenes: spectra, sparseness and nonlinearities. Spat. Vis. 21, 149–164. ( 10.1163/156856807782753877) [DOI] [PubMed] [Google Scholar]
- 83.Graham DJ, Redies C. 2010. Statistical regularities in art: relations with visual coding and perception. Vision Res. 50, 1503–1509. ( 10.1016/j.visres.2010.05.002) [DOI] [PubMed] [Google Scholar]
- 84.Winkielman P, Cacioppo JT. 2001. Mind at ease puts a smile on the face: psychophysiological evidence that processing facilitation elicits positive affect. J. Person. Social Psychol. 81, 989 ( 10.1037/0022-3514.81.6.989) [DOI] [PubMed] [Google Scholar]
- 85.Reber R, Schwarz N, Winkielman P. 2004. Processing fluency and aesthetic pleasure: is beauty in the perceiver's processing experience? Person. Social Psychol. Rev. 8, 364–382. ( 10.1207/s15327957pspr0804_3) [DOI] [PubMed] [Google Scholar]
- 86.Green NF, Urquhart HH, van den Berg CP, Marshall NJ, Cheney KL. 2018. Pattern edges improve predator learning of aposematic signals. Behav. Ecol. 29, 1481–1486. ( 10.1093/beheco/ary089) [DOI] [Google Scholar]
- 87.Landová E, Bakhshaliyeva N, Janovcová M, Peléšková Š, Suleymanova M, Polák J, Guliev A, Frynta D. 2018. Association between fear and beauty evaluation of snakes: cross-cultural findings. Front. Psychol. 9, 333 ( 10.3389/fpsyg.2018.00333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gottlieb J, Oudeyer P.-Y, Lopes M, Baranes A. 2013. Information-seeking, curiosity, and attention: computational and neural mechanisms. Trends Cogn. Sci. 17, 585–593. ( 10.1016/j.tics.2013.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fang X, Singh S, Ahluwalia R. 2007. An examination of different explanations for the mere exposure effect. J. Consumer Res. 34, 97–103. ( 10.1086/513050) [DOI] [Google Scholar]
- 90.Luce RD. 1986. Response times: their role in inferring elementary mental organization. Oxford, UK: Oxford University Press. [Google Scholar]
- 91.Cummings ME, Endler JA. 2018. 25 Years of sensory drive: the evidence and its watery bias. Current Zool. 64, 471–484. ( 10.1093/cz/zoy043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berridge KC, Robinson TE, Aldridge JW. 2009. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr. Opin. Pharm. 9, 65–73. ( 10.1016/j.coph.2008.12.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stoddard MC, Prum RO. 2008. Evolution of avian plumage color in a tetrahedral color space: a phylogenetic analysis of new world buntings. Am. Nat. 171, 755–776. ( 10.1086/587526). [DOI] [PubMed] [Google Scholar]
- 94.Bybee SM, Yuan F, Ramstetter MD, Llorente-Bousquets J, Reed RD, Osorio D, Briscoe AD. 2012. UV photoreceptors and UV-yellow wing pigments in Heliconius butterflies allow a color signal to serve both mimicry and intraspecific communication. Am. Nat. 179, 38–51. ( 10.1086/663192) [DOI] [PubMed] [Google Scholar]
- 95.Pérez-Rodríguez L, Jovani R, Mougeot F. 2013. Fractal geometry of a complex plumage trait reveals bird's quality. Proc. R. Soc. B 280, 20122783 ( 10.1098/rspb.2012.2783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carleton KL, Spady TC, Streelman JT, Kidd MR, McFarland WN, Loew ER. 2008. Visual sensitivities tuned by heterochronic shifts in opsin gene expression. BMC Biol. 6, 22 ( 10.1186/1741-7007-6-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maan ME, Hofker KD, van Alphen JJM, Seehausen O.. 2006. Sensory drive in cichlid speciation. Am. Nat. 167, 947–954. ( 10.1086/503532) [DOI] [PubMed] [Google Scholar]
- 98.Thayer GH. 1918. Concealing-coloration in the animal kingdom: an exposition of the laws of disguise through color and pattern: being a summary of Abbott H. Thayer's discoveries. New York, NY: Macmillan. [Google Scholar]
- 99.Kreutzer M, Aebischer V. 2015. The riddle of attractiveness: looking for an ‘aesthetic sense’ within the hedonic mind of the beholders. In Current perspectives on sexual selection (ed. Hoquet T.), pp. 263–287. Berlin, Germany: Springer. [Google Scholar]
- 100.Mendelson TC, Fitzpatrick CL, Hauber ME, Pence CH, Rodríguez RL, Safran RJ, Stern CA, Stevens JR. 2016. Cognitive phenotypes and the evolution of animal decisions. Trends Ecol. Evol. 31, 850–859. ( 10.1016/j.tree.2016.08.008) [DOI] [PubMed] [Google Scholar]
- 101.Perry CJ, Barron AB, Chittka L. 2017. The frontiers of insect cognition. Curr. Op. Behav. Sci. 16, 111–118. ( 10.1016/j.cobeha.2017.05.011) [DOI] [Google Scholar]
- 102.Wasserman EA, Zentall TR. 2006. Comparative cognition: experimental explorations animal intelligence. Oxford, UK: Oxford University Press. [Google Scholar]
- 103.Skelhorn J, Rowe C. 2016. Cognition and the evolution of camouflage. Proc. R. Soc. B 283, 20152890 ( 10.1098/rspb.2015.2890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cummings ME. 2015. The mate choice mind: studying mate preference, aversion and social cognition in the female poeciliid brain. Anim. Behav. 103, 249–258. ( 10.1016/j.anbehav.2015.02.021) [DOI] [Google Scholar]
- 105.Rosenthal GG. 2018. Evaluation and hedonic value in mate choice. Curr. Zool. 64, 485–492. ( 10.1093/cz/zoy054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schwarz N. 2017. Of fluency, beauty, and truth: inferences from metacognitive experiences. In Metacognitive diversity: an interdisciplinary approach (eds Proust J, Fortier M), pp. 25–46. New York, NY: Oxford University Press. [Google Scholar]
- 107.Weaver K, Garcia SM, Schwarz N, Miller DT. 2007. Inferring the popularity of an opinion from its familiarity: a repetitive voice can sound like a chorus. J. Person. Social Psychol. 92, 821–833. ( 10.1037/0022-3514.92.5.821) [DOI] [PubMed] [Google Scholar]
- 108.Loewenstein G. 1994. The psychology of curiosity: a review and reinterpretation. Psychol. Bull. 116, 75–98. ( 10.1037/0033-2909.116.1.75) [DOI] [Google Scholar]
- 109.Schaeffer J-M. 2015. L'expérience esthétique. Paris, France: Editions Gallimard. [Google Scholar]
- 110.Shimamura AP, Palmer SE. 2012. Aesthetic science: connecting minds, brains, and experience. New York, NY: Oxford University Press. [Google Scholar]
- 111.Leder H, Gerger G, Brieber D, Schwarz N. 2014. What makes an art expert? Emotion and evaluation in art appreciation. Cogn. Emot. 28, 1137–1147. ( 10.1080/02699931.2013.870132) [DOI] [PubMed] [Google Scholar]
- 112.Muth C, Carbon C-C. 2013. The Aesthetic Aha: on the pleasure of having insights into Gestalt. Acta Psychol. 144, 25–30. ( 10.1016/j.actpsy.2013.05.001) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.