Abstract
Theory suggests that symbionts can readily evolve more parasitic or mutualistic strategies with respect to hosts. However, many symbionts have stable interactions with hosts that improve nutrient assimilation or confer protection from pathogens. We explored the potential for evolution of increased parasitism or decreased parasitism and mutualism in a natural gut symbiosis between larvae of Plutella xylostella and the microbe Enterobacter cloacae. We focused on interactions with the pathogen, Bacillus thuringiensis: selecting for parasitism in terms of facilitating pathogen infection, or increased mutualism in terms of host protection. Selection for parasitism led to symbionts increasing pathogen-induced mortality but reduced their competitive ability with pathogens and their in vitro growth rates. Symbionts did not evolve to confer protection from pathogens. However, several lineages evolved reduced parasitism, primarily in terms of moderating impacts on host growth, potentially because prudence pays dividends through increased host size. Overall, the evolution of increased parasitism was achievable but was opposed by trade-offs likely to reduce fitness. The evolution of protection may not have occurred because suppressing growth of B. thuringiensis in the gut might provide only weak protection or because evolution towards protective interactions was opposed by the loss of competitive fitness in symbionts.
Keywords: evolution of virulence, host–microbe interactions, mutualism, pathogen protection, prudence, symbiosis
1. Background
Symbiosis describes a continuum of interactions. These interactions range from parasitic, in which the fitness gains of one partner impose costs on the other partner; to mutualistic, in which both partners gain fitness benefits from their interaction. Of these, mutualistic interactions pose the most challenging evolutionary problem [1–3], predominantly because opportunities for defection, i.e. switching from mutualistic to exploitative behaviours, can offer substantial short-term fitness benefits [1,4]. The ready transitions across this parasite–mutualism continuum in experimental evolution and in clinical microbes suggest that evolutionary conflict between hosts and symbionts is real [5]. For example, change in ecological factors, such as the nature of symbiont transmission in jellyfish can have dramatic effects on parasitism [6]. Nevertheless, stable mutualistic interactions are widespread, particularly between metazoans and their respective microbiota [7–11].
Early theoretical frameworks addressing the evolution of mutualism are based on extensions of Triver's original Prisoner's Dilemma [4]. For microbe–host interactions, the fidelity of partner interactions, such as that maintained by vertical transmission, is known to be important for the evolution of mutualism [3,6]. In the absence of vertical transmission, the prolonged spatial and temporal association can promote mutualistic symbioses [1,12], while imposing punishment or sanctions on defectors can also maintain cooperative interactions between microbial symbionts and metazoan hosts [13,14]. Nevertheless, many microbial symbionts do not have strict vertical transmission [5,10,15] and this lack of fidelity creates the potential for defection or increased parasitism.
Protection from parasites or pathogens represents one of the major axes of the parasite–mutualist continuum [16] and there has been increasing interest in these tripartite relationships [17]. However, there have been few experimental evolution studies of symbionts in this context, and theory in this area is still developing [17,18]. For example, experimental evolution has shown that parasitoids can evolve to overcome microbial-based defenses in aphid symbioses [19]. Additionally, the evolution of protection can rapidly evolve de novo in microbial assemblages in model systems via selection on increased antagonism between bacteria [20] and as a consequence of adaptation to a novel niche [21]. An important next step is to understand how selection on symbionts along this continuum operates in natural assemblages.
In this study, we used a lepidopteran larval host and a symbiotic bacterium, Enterobacter cloacae (Ec), which is commonly isolated from insect intestines and can show commensal, mutualistic or parasitic interactions with hosts [22–25]. Although recent studies have suggested that Lepidoptera have a transient plant-derived microbiome [26], Ec forms persistent infections in the midgut [24]. Our experimental pathogen, Bacillus thuringiensis kurstaki (Bt) is a widespread and commonly occurring lepidopteran pathogen [27]. Effective replication of Bt in vivo requires host death and invasion of the haemocoel [28]; this pathogen disintegrates the gut with pore-forming Cry toxins and quorum-regulated lytic enzymes [29,30]. Importantly, this disintegration of the gut epithelium facilitates co-invasion of the cadaver by both gut microbes and Bt. The rich fat/protein resources in the cadaver present a short-term fitness gain for other microbes inhabiting the gut. Enterobacter isolates can have a protective effect on Bt infections, reducing mortality by approximately 10% during co-infection in caterpillars [25] and have been shown to have dramatic antagonistic effects on pathogens in mosquito midguts [31]. In contrast to the mutualistic and/or protective role of several gut symbionts [32], the ancestral Ec in this study slightly increases the risk of mortality from pathogen infections and is mildly parasitic in this experimental system (see below), emphasizing that a parasite/symbiont continuum exists both for pathogen protection and for general impacts on host fitness, otherwise known as virulence.
The prevalence of protective symbioses [16,32] suggests that there may be barriers or trade-offs that prevent symbionts defecting, or becoming increasingly parasitic. We hypothesized that different pathogen infection outcomes would determine distinct selective pressures on symbioses and reveal these trade-offs. Here, we imposed direct selection on the outcome of a three-way host–symbiont–pathogen interaction. We predicted that symbiont transmission from healthy hosts would select for symbionts that protected hosts from infection, or at least for less parasitic symbionts. Conversely, we predicted that transmission from dead, infected hosts would select for increased parasitism in terms of pathogen facilitation. We explored the life-history consequences of symbionts evolved under these divergent infection outcomes, testing the fundamental theoretical expectation that increased parasitism should evolve readily if there is a short-term fitness gain for the symbiont.
2. Material and methods
(a). Study system
Experimental hosts were larvae of a diet-adapted population (Geneva) of the diamondback moth, Plutella xylostella. These were reared gnotobiotically on an autoclaved diet, as described previously [25] and infected with the focal symbiont, Ec str. jjbc, isolated from P. xylostella in an insectary at the University of Oxford. Selection experiments used a spontaneous rifampicin resistant mutant of Ec (Anc3 rifR), routinely cultured in Lysogeny Broth (LB) containing 100 µg ml−1 rifampicin at 30°C. Larvae readily acquire persistent intestinal infections after feeding on inoculated food.
We characterized the impact of inoculation with the Ec ancestor on the growth of larvae on a typical host plant (Brassica oleracea var. Hispi) as well as on artificial diet in order to assess whether these symbionts were mutualistic or parasitic in terms of host growth. Larvae emerging from surface-sterilized eggs [25] were inoculated with Ec on an artificial diet using 1000-fold dilutions of overnight culture (5 ml LB, 150 rpm), or with blank saline (0.45% w/v NaCl) and reared at 24°C for 3 days. Thereafter, groups of 10 larvae were weighed, transferred to Petri dishes containing diet (N = 14) or leaf discs (N = 20). All cabbage leaf discs were surfaced sterilized with 2% sodium hypochlorite solution (immersion 40 min, followed by four rinses in sterile de-ionized water) before use. Survival and larval mass were recorded after another 3 days. These assays were repeated (on artificial diet only) after experimental evolution to assess for changes in symbiont parasitism in the absence of pathogens. Here larvae were inoculated with one of two evolved clones per lineage.
Bacterial fitness was measured in competition experiments with a standard Ec mutant (11.1B strepR nalR) with spontaneous resistance to both streptomycin (50 µg ml−1) and nalidixic acid (15 µg ml−1), derived by sequential selection for resistance to these antibiotics. The pathogen used in this study, Bt nalRG was derived from the biopesticide product DiPel WP, as described previously [33] and was cultivated on LB with 12 µg ml−1 nalidixic acid. Pathogen inocula were prepared from aliquoted frozen spore preparations, produced in a single batch, and did not evolve in this experiment.
(b). Selection regime
Our selection regime used the outcome of a pathogen challenge to manipulate selection pressures on symbionts (figure 1a). We imposed two treatments in sequential passage: Ec were either recovered from melanized cadavers killed by Bt—‘dead passage’; or from the survivors of this pathogen challenge—‘live passage’ (figure 1a). Thus, we imposed selection on gut symbionts to either increase or decrease infection risk. Treatments were replicated six times; independent lineages are denoted by abbreviations of their passage treatment (live = L, dead = D) and a unique replicate number. The design incorporated between-host competition by pooling cadavers or live larvae in each round of selection, which is expected to favour large microbial population size (figure 1) [34].
Figure 1.
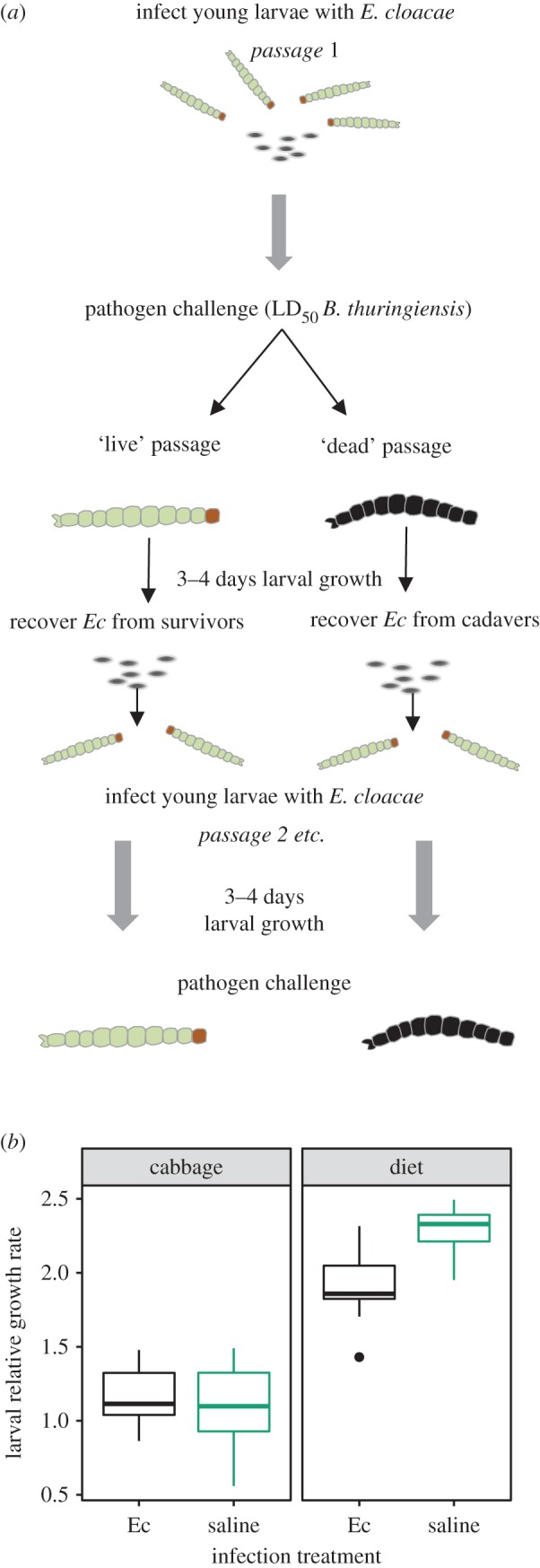
Background to the biology of the system and design of selection experiment. (a) Design of experimental evolution of E. cloacae symbionts for protection from infection (live passage) or pathogen facilitation (dead passage). Selection proceeded by infecting insects with the focal E. cloacae symbiont then presenting these insects with a pathogen challenge of Bt at a dose sufficient to kill approximately 50% of larvae. After 5 days of this pathogen challenge, symbionts were recovered from all cadavers in each dish (dead passage) or two to three large healthy fourth instar larvae (live passage) and populations quantified by dilution plating. The experiment ran for 12 rounds of infection (approx. 180 generations in vivo). Each independent replicate comprised seven sub-populations—in both treatments, the three subpopulations with the largest Ec populations were pooled and used to prepare inocula for the next round of infection. (b) The effect of E. cloacae inoculation on growth of diamondback moth larvae on a typical host plant and on artificial diet. Larval relative growth rate was measured as ln(final mass/initial mass) over 3 days. (Online version in colour.)
Larvae were inoculated with Ec as above. The pathogen challenge consisted of exposing early third instar larvae to a fresh diet inoculated with 100 µl of 50–60 CFU µl−1 Bt. Each selection lineage comprised 70 larvae in seven subpopulations of 10 larvae in a 50 mm Petri dish. Ec were recovered by homogenizing larvae/cadavers in 500 µl saline with 4 mm ball bearings using a Qiagen tissue Lyzer and were enumerated by dilution plating. Changes in Ec fitness and life-history traits were assessed after 12 rounds of passage.
(c). Assessing trade-offs and population level pay-offs after selection for pathogen facilitation or protection
(i). Pathogen bioassays
We tested whether evolved Ec lines conferred increased or decreased mortality risk under pathogen challenge relative to their ancestor. Bioassays used three doses of Bt nalRG (20, 60, 180 CFU µl−1), with 60 larvae per dose per lineage. Assays included a saline control (no Ec) and a double blank control (neither Bt nor Ec). Mortality was recorded for 5 days. No substantial mortality (greater than 2/60 larvae) was recorded in the double blank controls.
(ii). Growth in cadavers in the presence of the pathogen
We quantified the ability of evolved Ec lineages to replicate in cadavers in the presence of the focal pathogen. Evolved lineages and their ancestor were grown overnight and mixed in 50 : 50 ratios with Bt. This mixture was diluted by a factor of 2 × 10−4 and 2 µl injected into body cavities of fourth instar P. xylostella larvae (15 larvae per treatment, weighing ≅ 7 mg each), using 10 µl Hamilton syringes. Initial abundance of competitors was confirmed by plating on selective antibiotics. Twenty-four hours after injection, Ec density was measured in 12 cadavers per mixture as above and used to calculate relative growth rates of evolved Ec lineages, the ‘Malthusian parameter’, i.e. ln (final count)/(initial count).
(iii). Relative fitness in insects
Injection into the haemocoel was used to assess how selection had affected the intra-specific competitive ability of evolved Ec lineages with respect to a common ancestor clone (11.1B strepR nalR). Here, cadavers were homogenized 36 h after injection, and bacterial densities were quantified using discriminating antibiotics (rifampicin and streptomycin). Relative fitness was calculated using the change in the proportion of evolved lineages with respect to the ancestors, Vevolved = x2(1 − x1)/x1(1 − x2) where x1 is the initial proportion of evolved lineages and x2 is their final proportion [35].
(iv). Growth and population size in the larval gut
We measured symbiont population sizes in the larval gut, in insects reared in even-aged larval cohorts that emerged from eggs within a 24 h period. Larvae were inoculated with evolved lineages and reared in 55 mm Petri dishes as per experiments above. Three days after transfer to a fresh diet, insects were weighed and homogenized in order to score Ec population size per unit insect mass.
(v). Exponential growth
In addition to quantifying fitness and replication in vivo, we also tested for trade-offs in terms of in vitro growth. Cultures in 96 well plates (excluding outer wells) were used to calculate exponential growth rates (Vmax), using a 2 × 10−7 dilution of an overnight culture as initial inocula. Experiments measured absorbance (OD600) using a Spectra Max plate reader with read and shake intervals every 5 min for 10 h incubation at 25°C. Softmax Pro proprietary software was used to estimate Vmax. Lineages were replicated 10 times in two blocks.
(d). Statistical analysis
Mortality bioassays were analysed with Cox proportional hazards models using the cluster function to account for random effects associated with the selection line. Analysis was carried out in R v. 3.3.2 with the package survival v. 2.4. Analysis of treatment effects on Ec virulence or life-history traits used mixed model ANOVAs with lineage replicate as a random factor in package nlme and treatment (live or dead passage) as a fixed factor. Comparison of models with different fixed effect structures used likelihood ratio tests after re-fitting models with maximum likelihood. In exploring life-history trade-offs, we used linear models and regression deletion diagnostics (influence.measures and lm.influence in the base R statistics package) to identify outliers with a large effect on model outcomes.
In order to test for differences between evolved lineages and their ancestor (which often occurred in the absence of overall treatment effects) models were re-fitted as one-way ANOVAs (with lineage as factor) using generalized linear modelling, with ancestor as the intercept. When lineage explained significant variation, planned post hoc comparisons of evolved strains and the ancestor used default treatment contrasts to compare parameter estimates with respect to the common baseline of the Ec ancestor. Analogous procedures were used to compare mortality rates in survivorship analysis, using post hoc z tests to compare evolved lineages with the ancestor. All analyses were supported by appropriate model checking (homoscedasticity, constant proportional hazards for Cox models).
3. Results
(a). Characterization of study system
Ec is commensal for diamondback moth larvae on a typical host plant but slightly parasitic on artificial diet (diet * infection interaction F1,63 = 15.1, p < 0.001, figure 1b). However, experimental evolution on an artificial diet is preferable since this allows for better control of contamination. Throughout the selection experiment, we monitored Ec density in live insects and cadavers harvested during the live and dead selection regimes respectively. Reproductive rate, and therefore the number of generations under selection, was similar in both treatments (electronic supplementary material, figure S1, mixed model with lineage within passage as a random effect, d.f. = 1,11, χ2 = 3.46, p = 0.063).
(b). Responses to selection for pathogen facilitation and protection
We predicted that selecting for the invasion of dead hosts would lead to pathogen facilitation in our focal symbiont Ec, while selection in surviving hosts would result in increased protection from pathogen attack, or moderation of parasitism. As predicted, lineages in the dead passage treatment substantially increased pathogen-associated mortality relative to the ancestor (figure 2a, hazard 0.70, s.e. 0.13, z = 4.18, p < 0.0001). However, lineages from the live passage regime did not, overall, differ from the ancestral strain (figure 2a, hazard 0.32, s.e. 0.13, z = 1.28, p = 0.2). All Ec strains increased pathogen-induced mortality substantially with respect to saline controls without gut bacteria (hazard −0.927, s.e. 0.19, z = −155.8, p < 0.0001, figure 2a) and there was a significant effect of Bt dose (hazard 1.426, s.e. 0.05, z = 17.9, p < 0.0001). Lineage level variation showed that 4/6 dead passage lines increased pathogen mortality rates while only one live passage lineage (L2) evolved in the predicted direction i.e. towards reduced mortality, if not actual protection (figure 2a).
Figure 2.
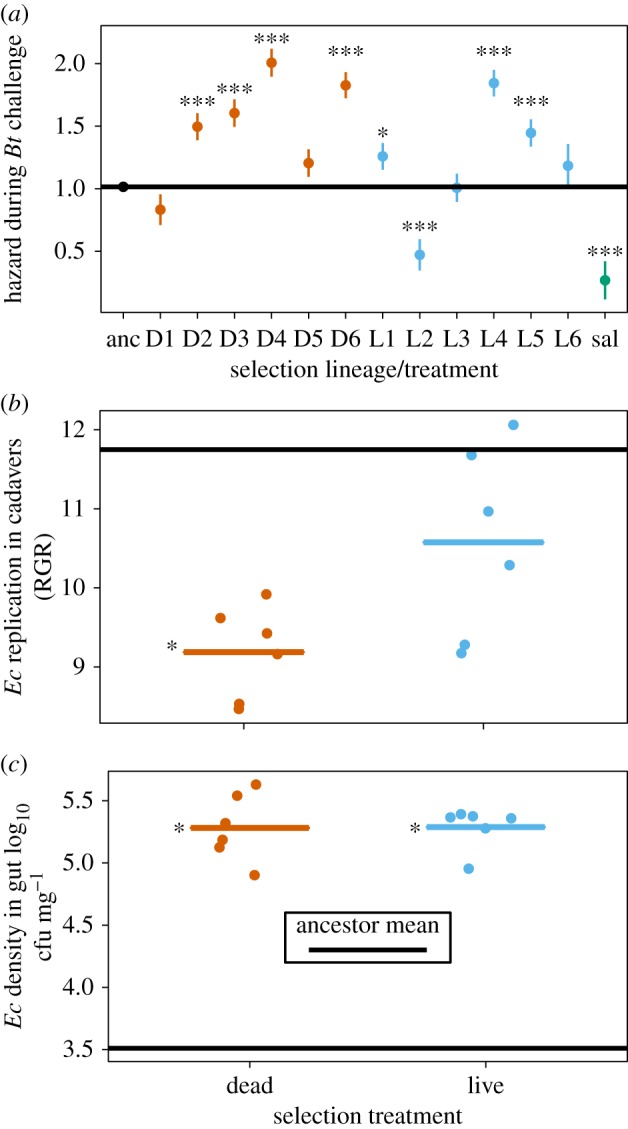
Population level pay-offs after selection for increased parasitism or increased protection in a gut symbiont, E. cloacae (Ec). (a) Passage in dead, infected hosts results in symbionts increasing the risk of death following pathogen challenge. Data show effects of inoculation with evolved and ancestral Ec on mortality risk during a pathogen challenge with B. thuringiensis. Points are parameter estimates of instantaneous hazard from survivorship analysis of bioassay data. Selection lines in red (prefixed with D) are the dead passage lineages; selection lines in blue (prefixed with L) are the live passage lineages. Black horizontal lines represent mean values for the ancestor. Saline refers to controls that were not inoculated with Ec. (b) Passage in dead hosts consistently reduced the ability to replicate in Bt-killed cadavers across all evolved lineages. (c) In both selection treatments, evolved lineages increase their ability to colonize the insect gut. In all panels, horizontal lines indicate mean values for ancestor E. cloacae. Asterisks indicate lineages (or treatments) that are significantly different from the ancestor: *p < 0.05, **p < 0.01, *** p < 0.001. For (b,c) data are means for each evolved lineage, with treatment means plotted as bars. (Online version in colour.)
A reasonable expectation was that symbionts selected to produce pathogen-killed cadavers would replicate more effectively in this habitat. However, Ec lineages in the dead passage regime consistently reproduced less effectively than their ancestors in the presence of the Bt pathogen (figure 2b; Likelihood ratio = 8.85, d.f. = 1, p = 0.012). In contrast, the replication of live passage lineages in the presence of Bt appeared unchanged after 12 rounds of selection (figure 2b). This was confirmed statistically through model simplification: pooling ancestor and live passage treatments had no significant effect on explanatory of the statistical model (likelihood ratio = 1.23, d.f. = 1, p = 0.267). Another expected trade-off was that selection for reproduction in the parasitic niche of the insect haemocoel would reduce the ability to replicate in live hosts. However, we observed that both live and dead passaged lineages increased population sizes in the larval gut to a similar extent (test for treatment main effect likelihood ratio = 0.12, d.f. = 1, p = 0.73—figure 2c; effect sizes relative to ancestor—dead treatment = 0.56, s.e. = 0.21; live treatment = 0.60, s.e. = 0.21). Over two independent experiments, 5/6 live passage lineages and three dead passage lineages reached higher population sizes in larval guts than the ancestor (electronic supplementary material, figure S2, lineage effect F12,265 = 2.43, p < 0.01). This increase in population size was partly, but not entirely, driven by an increase in Ec density per unit mass of insect in five lineages (lineage effect F12,265 = 6.69, p < 0.0001).
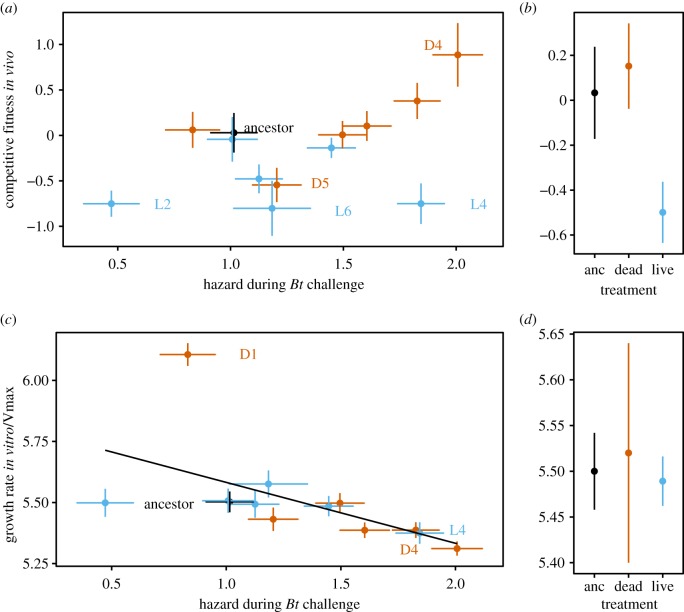
While dead passage strains counterintuitively reduced their ability to replicate in cadavers containing Bt, the competitive fitness of these Ec lineages remained high in the absence of the pathogen (figure 3a,b). By contrast, live passaged lineages decreased their ability to grow in the parasitic compartment of the insect haemocoel after 12 passages of being restricted to the gut (linear mixed model, likelihood ratio = 6.64, p < 0.01, figure 3a,b).
Figure 3.
Potential trade-offs with pathogen facilitation and protection in symbiont fitness and replication. (a) Covariation between competitive fitness of symbionts in haemocoel (without pathogen) and mortality risk during pathogen challenge. As above, mortality risk is represented as the instantaneous hazard parameter estimates from survivorship analyses. Labels indicate lineages that have evolved significantly different fitness from the ancestor; lineage D4 was the only strain to increase competitive fitness (post hoc comparison t = 2.836, p = 0.0051). (b) Treatment level means for competitive fitness relative to the ancestor. (c) Pathogen facilitation (mortality risk) trades off against an exponential growth rate of E. cloacae in vitro. Labels indicate lineages that have evolved growth rates significantly different than the ancestor (post hoc tests: L4 t = −2.03, p = 0.044; D4 t = −3.04, p = 0.003, D1 t = 9.58, p < 0.001). (d) Treatment level means for live and dead passage lineages (N = 6) relative to the ancestor. Ancestor data points are coloured black, dead passage lineages are orange/red and live passaged lineages light blue throughout. Centroids are means (±s.e.) for both x- and y-axes.
(c). Covariation and potential trade-offs between life-history traits in evolved symbionts
We explored the relationship between how symbionts affected pathogen-induced mortality and their ability to replicate in vivo and in vitro (figure 3). There was some evidence for an association for both these life-history traits. Variation in replication rate in vivo, as measured by competitive fitness, could explain nearly 25% of the variation in pathogen-induced mortality risk (R2 = 0.24), although this was only a near-significant trend (F1,11 = 3.39, p = 0.093, figure 3a). This analysis, however, was strongly affected by lineage L4. Regression deletion diagnostics confirmed that these data point had the greatest influence on the estimation of slope and the highest Cook's distance. Biologically, this lineage was unusual in having slow replication and high virulence (figures 3c and 4) and is an aggressive early colonizer of larvae (electronic supplementary material, figure S3). Without the formally influential L4 lineage, competitive fitness was a strong predictor of mortality during pathogen challenge, explaining around 50% of the variation between selected lineages (F1,10 = 9.90, p = 0.011, R2 = 0.49, figure 3a).
Figure 4.
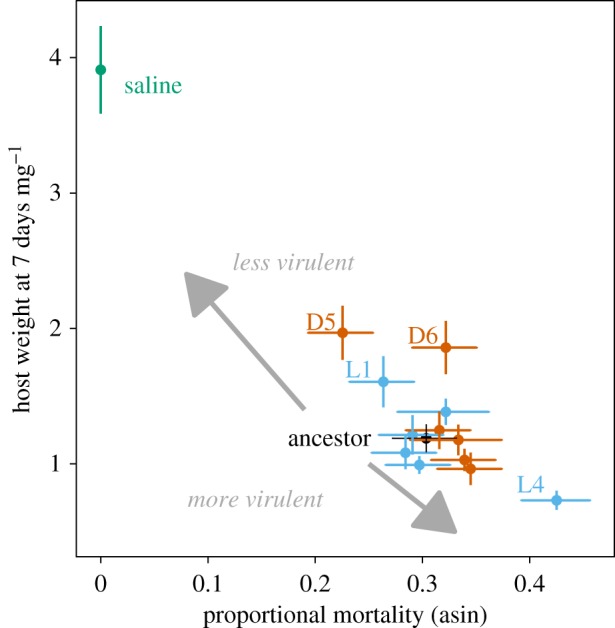
Changes in direct impact on host fitness (virulence) in experimental evolution of gut symbionts. Effects of symbiont inoculation on growth and mortality were assessed in the absence of the Bt pathogen. Final larval mass correlates with mortality rates imposed directly by gut bacteria infection (p < 0.01). Labelled lineages are significantly different from the ancestor: lineages D5 and L4 impose significantly lower and higher mortality, respectively, than the ancestor (z = −0.22, p = 0.027, z = 3.36, p = 0.00079); while insects inoculated with lineages D5, D6 and L1 reached increased final weight relative to insects infected with ancestors (t = 4.085, p < 0.001; t = 3.51, p < 0.001; t = 2.19, p < 0.05, respectively, while L4 produced larvae with lower mass (t = −2.42, p < 0.05). Data on the y-axis are final weight after a defined period of growth since egg hatch and inoculation with symbiont, data on the x-axis are arcsine (sqrt) transformed larvae mortality observed over this period of growth. Data are means of 28 replicates per lineage in two independent experiments. (Online version in colour.)
Exponential growth rate in vitro, by contrast, was associated with decreased risk of mortality during pathogen challenge (figure 3c, F1,11 = 5.2, p = 0.044). This relationship was not dependent on the highly influential lineage with particularly high growth (after excluding lineage D1—F1,10 = 12.9, p < 0.01). Although there was no association between competitive fitness in vivo and exponential growth in vitro (R2 = 0.007), strong phenotypic divergence in either of these traits was associated with altered pathogen mortality risk.
We also explored the consequence of selection treatments on symbiont virulence, i.e. the direct impact of Ec on host weight gain and mortality in the absence of the pathogen since virulence summarizes another major axis of the parasite–mutualist continuum. Notably, several lineages evolved in the direction of increased mutualism. Here, there was no significant effect of treatment on virulence (figure 4, likelihood ratio 2.58, d.f. = 2, p = 0.274), although there was substantial variation between lineages (figure 4, one-way ANOVA F12,331 = 7.7, p < 0.001). Final insect weight was negatively correlated with the level of mortality imposed by Ec infection (F1,11 = 10.1, p < 0.01, figure 4) showing evidence of consistent variation in two measures of virulence. Since host weight was strongly correlated with Ec population size (electronic supplementary material, figure S4), the fitness gains associated with moderated virulence are likely to arise via group size (e.g. for D5). Increased competitive ability did not appear to be a consistent driver of altered virulence since increased (L4) and decreased virulence (D5) were both associated with reduced competitive fitness.
4. Discussion
The focal symbiont Ec clearly responded to the selection for increased parasitism: lineages passaged in hosts that repeatedly succumbed to infection increased the risks of subsequent infections and pathogen-induced mortality. However, there were several phenotypic changes accompanying increased parasitism that could act as a constraint. Foremost of these was that facilitating pathogen invasion led to symbionts being less able to grow in the cadaver produced by that pathogen. Since reduced ability to exploit cadavers was associated with the presence of the pathogen and not the loss of ability to grow in insects generally, this reduced growth can be attributed to a reduction in ability to compete interspecifically with Bt.
Although counterintuitive, this result fits well with earlier studies, i.e. if blocking pathogen invasion is associated with increased antagonistic competition between symbiont and pathogen [20], then it should not be surprising that facilitating pathogen invasion is associated with decreased competitive ability. In others words, there is a fundamental trade-off between aiding pathogens and competing with pathogens, it was not possible to do both well. In addition, facilitating pathogen invasion imposed an additional trade-off in terms of reduced in vitro growth rate of Enterobacter. Many intestinal microbes of insects also need to persist and grow in other niches, such as those on plants [36,37]. A reduced replication rate outside the host is therefore likely to have some negative fitness consequences. In short, in this symbiosis, it was possible to force the evolution of a symbiont toward increased parasitism along the axis of pathogen facilitation/protection. However, increasing parasitism along this axis might not happen as readily as theory predicts [3,6], as helping pathogens was associated with two important negative consequences that are likely to constrain phenotypic change in natural systems.
While we saw the predicted increase in pathogen facilitation, attempts to select for increased mutualism did not produce improved pathogen protection, in contrast to previous work [20,21,38]. Other Enterobacter isolates have also shown some protection of larvae from Bt infections, suggesting that the evolution of protection is biologically plausible [25]. In addition, the number of generations of selection experienced in the live and dead treatments was very similar. In contrast to other experimental evolution studies, adaptation to the host in terms of increased group size or competitive fitness did not correlate with increased pathogen protection [21]. In fact, increased competitive fitness tended to be associated with pathogen facilitation rather than protection, making the evolution of protection potentially very costly, although evidence for the relationship is weaker than for other trade-offs seen in this study.
One additional explanation arises from the biology of the main virulence factors (the Cry toxins) in B. thuringiensis. The production of Cry toxins occurs at sporulation after Bt has killed and invaded hosts. Cry toxin production is therefore important for infecting the next host, rather than in the current infection [39]. Importantly, host mortality is largely determined by how much Cry toxin is ingested [40]. Germination and growth of the pathogen in the gut does affect mortality, and this can be shown experimentally by giving insects antibiotics [25]. However, the quantity of spores is less important than the quantity of Cry toxin [40]. Therefore, even if symbionts are able to suppress the growth of Bt in the gut, this may not substantially affect infectivity. While Bt is unusual in the extent to which it can pre-package virulence factors, a number of gut pathogens have evolved mechanisms, such as the use of prophage, which enable them to be resilient to competition [41,42]. We would predict that antagonistic interactions between symbionts and pathogens are more likely to protect hosts [20,43] when replication of the pathogens is important in establishing infections.
Both live and dead passaged symbionts increased their population sizes in the gut of host larvae in both selection treatments. This consistent response in group size was no doubt facilitated by imposing group-level competition during selection [34], although it is important to note that here increases in group size were not typically associated with altruistic or individually costly phenotypes. Different selection treatments, however, responded to selection very differently in terms of competitive fitness in the haemocoel. Here, live passaged symbionts, free of selection in the haemocoel for 12 rounds of infection significantly reduced their ability to exploit this compartment. Specialization to exploit different host compartments can evolve rapidly in commensals and pathogens. For example, the forced passage in a novel gut environment led to a specialized phenotypic change in fungi [21]. Similarly, an evolutionary change associated with a switch to bacteraemia (virulent replication in the blood) has occurred via few genetic changes in Staphylococcus aureus and Bacillus anthracis [44,45]. In B. anthracis, this is associated with reduced expression of toxins or quorum-regulated virulence factors known to be important for infections arising from the gut [29]. In this study, loss of ability to grow in the insect haemocoel suggests that the ancestral Ec isolate is a genuine opportunist pathogen, implying that regular exposure to the parasitic niche of the haemocoel may have been a feature of its evolutionary history.
Experimental evolution also led to phenotypic shifts along a second axis of the parasite–mutualist continuum (virulence or mortality and host growth). The change along the directly selected axis of pathogen protection was largely predicted. However, effects on symbiont virulence were not under direct selection, and mutations affecting host mortality or growth could have been favoured in either treatment. Therefore, it was unsurprising that virulence evolved in ways that were independent of our selection regime. Nevertheless, the variation that evolved in this study reflects variation in the field [23] and presented several informative patterns relating to shifts in virulence. For example, the evolution of moderated virulence occurred in several lineages, while increased virulence occurred in one lineage only. As with the pathogen protection axis, increased parasitism in terms of virulence could have negative consequences, here most obviously in terms of reduced symbiont population size in smaller hosts, a factor expected to limit production of infectious propagules and ongoing transmission [46–48].
In conclusion, then, the evolution of increased exploitation is possible over short timescales in naturally occurring insect symbionts. However, the biological details are important here. There appear to be fundamental dichotomies in terms of both facilitating pathogens and also competing with them; while it is also difficult to increase host mortality without impacting host growth rate. These fundamental trade-offs may constrain parasitic strategies in insect symbionts and favour long-term commensal or mutualistic associations. Thus, additional features such as partner choice, vertical transmission or policing are not necessarily required to enforce prudent or mutualistic behaviour, especially if traits such as host protection arise indirectly as a consequence of niche occupation or via antagonistic interactions with invading microbes.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Thanks to Angus Buckling and Tatiana Dimitriu and two anonymous referees for helpful comments.
Data accessibility
All data are available from the Dryad Digital Repository at: https://doi.org/10.5061/dryad.bf144m6 [49].
Authors' contributions
B.R. conceived the study, while A.C.M. and L.M. contributed to experimental design. B.R., A.C.M. and L.M. conducted the experiments; B.R. analysed the data, B.R. wrote the first draft, all authors contributed to revisions.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by a NERC fellowship to B.R. (NE/E012671/1) and by an MRC innovation award (MR/N013824/1).
References
- 1.Sachs JL, Mueller UG, Wilcox TP, Bull JJ. 2004. The evolution of cooperation. Q. Rev. Biol. 79, 135–160. ( 10.1086/383541) [DOI] [PubMed] [Google Scholar]
- 2.West SA, Griffin AS, Gardner A. 2007. Evolutionary explanations for cooperation. Curr. Biol. 17, R661–R672. ( 10.1016/j.cub.2007.06.004) [DOI] [PubMed] [Google Scholar]
- 3.Foster KR, Wenseleers T. 2006. A general model for the evolution of mutualisms. J. Evol. Biol. 19, 1283–1293. ( 10.1111/j.1420-9101.2005.01073.x) [DOI] [PubMed] [Google Scholar]
- 4.Trivers RL. 1971. Evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57. ( 10.1086/406755) [DOI] [Google Scholar]
- 5.Sachs JL, Essenberg CJ, Turcotte MM. 2011. New paradigms for the evolution of beneficial infections. Trends Ecol. Evol. 26, 202–209. ( 10.1016/j.tree.2011.01.010) [DOI] [PubMed] [Google Scholar]
- 6.Sachs JL, Wilcox TP. 2006. A shift to parasitism in the jellyfish symbiont Symbiodinium microadriaticum. Proc. R. Soc. B 273, 425–429. ( 10.1098/rspb.2005.3346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West SA, Kiers ET, Simms EL, Denison RF. 2002. Sanctions and mutualism stability: why do rhizobia fix nitrogen? Proc. R. Soc. Lond. B 269, 685–694. ( 10.1098/rspb.2001.1878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaltenpoth M, Roeser-Mueller K, Koehler S, Peterson A, Nechitaylo TY, Stubblefield JW, Herzner G, Seger J, Strohm E. 2014. Partner choice and fidelity stabilize coevolution in a Cretaceous-age defensive symbiosis. Proc. Natl Acad. Sci. USA 111, 6359–6364. ( 10.1073/pnas.1400457111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamdi C, et al. 2011. Gut microbiome dysbiosis and honeybee health. J. Appl. Entomol. 135, 524–533. ( 10.1111/j.1439-0418.2010.01609.x) [DOI] [Google Scholar]
- 10.Kiers ET, van der Heijden MGA. 2006. Mutualistic stability in the arbuscular mycorrhizal symbiosis: exploring hypotheses of evolutionary cooperation. Ecology 87, 1627–1636. ( 10.1890/0012-9658(2006)87[1627:MSITAM]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 11.Scarborough CL, Ferrari J, Godfray HCJ. 2005. Aphid protected from pathogen by endosymbiont. Science 310, 1781 ( 10.1126/science.1120180) [DOI] [PubMed] [Google Scholar]
- 12.Bull JJ, Rice WR. 1991. Distinguishing mechanisms for the evolution of cooperation. J. Theor. Biol. 149, 63–74. ( 10.1016/S0022-5193(05)80072-4) [DOI] [PubMed] [Google Scholar]
- 13.Úbeda F, Duéñez-Guzmán EA. 2010. Power and corruption. Evolution 65, 1127–1139. ( 10.1111/j.1558-5646.2010.01194.x) [DOI] [PubMed] [Google Scholar]
- 14.West SA, Kiers ET, Pen I, Denison RF. 2002. Sanctions and mutualism stability: when should less beneficial mutualists be tolerated? J. Evol. Biol. 15, 830–837. ( 10.1046/j.1420-9101.2002.00441.x) [DOI] [Google Scholar]
- 15.Ebert D. 2013. The epidemiology and evolution of symbionts with mixed-mode transmission. Ann. Rev. Ecol. Evol. Syst. 44, 623–643. ( 10.1146/annurev-ecolsys-032513-100555) [DOI] [Google Scholar]
- 16.Engel P, Moran NA. 2013. The gut microbiota of insects—diversity in structure and function. FEMS Microbiol. Rev. 37, 699–735. ( 10.1111/1574-6976.12025) [DOI] [PubMed] [Google Scholar]
- 17.Vorburger C, Perlman SJ. 2018. The role of defensive symbionts in host–parasite coevolution. Biol. Rev. 93, 1747–1764. ( 10.1111/brv.12417) [DOI] [PubMed] [Google Scholar]
- 18.Rafaluk-Mohr C, Ashby B, Dahan DA, King KC. 2018. Mutual fitness benefits arise during coevolution in a nematode-defensive microbe model. Evol. Lett. 2, 246–256. ( 10.1002/evl3.58) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dion E, Zele F, Simon JC, Outreman Y. 2011. Rapid evolution of parasitoids when faced with the symbiont-mediated resistance of their hosts. J. Evol. Biol. 24, 741–750. ( 10.1111/j.1420-9101.2010.02207.x) [DOI] [PubMed] [Google Scholar]
- 20.King KC, et al. 2016. Rapid evolution of microbe-mediated protection against pathogens in a worm host. ISME J. 10, 1915–1924. ( 10.1038/ismej.2015.259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tso GHW, et al. 2018. Experimental evolution of a fungal pathogen into a gut symbiont. Science 362, 589–595. ( 10.1126/science.aat0537) [DOI] [PubMed] [Google Scholar]
- 22.Lauzon CR, Sjogren RE, Wright SE, Prokopy RJ. 1998. Attraction of Rhagoletis pomonella (Diptera: Tephritidae) flies to odor of bacteria: apparent confinement to specialized members of Enterobacteriaceae. Environ. Entomol. 27, 853–857. ( 10.1093/ee/27.4.853) [DOI] [Google Scholar]
- 23.Thakur A, Dhammi P, Saini HS, Kaur S. 2015. Pathogenicity of bacteria isolated from gut of Spodoptera litura (Lepidoptera: Noctuidae) and fitness costs of insect associated with consumption of bacteria. J. Invertebr. Pathol. 127, 38–46. ( 10.1016/j.jip.2015.02.007) [DOI] [PubMed] [Google Scholar]
- 24.Watanabe K, Abe K, Sato M. 2000. Biological control of an insect pest by gut-colonizing Enterobacter cloacae transformed with ice nucleation gene. J. Appl. Microbiol. 88, 90–97. ( 10.1046/j.1365-2672.2000.00904.x) [DOI] [PubMed] [Google Scholar]
- 25.Raymond B, Johnston PR, Wright DJ, Ellis RJ, Crickmore N, Bonsall MB. 2009. A mid-gut microbiota is not required for the pathogenicity of Bacillus thuringiensis to diamondback moth larvae. Environ. Microbiol. 11, 2556–2563. ( 10.1111/j.1462-2920.2009.01980.x) [DOI] [PubMed] [Google Scholar]
- 26.Hammer TJ, Janzen DH, Hallwachs W, Jaffe SP, Fierer N. 2017. Caterpillars lack a resident gut microbiome. Proc. Natl Acad. Sci. USA 114, 9641–9646. ( 10.1073/pnas.1707186114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raymond B. 2017. The biology, ecology and taxonomy of Bacillus thuringiensis and related bacteria. In Bacillus thuringiensis and Lysinibacillus sphaericus: characterization and use in the field of biocontrol (eds Fiuza L, Polanczyk R, Crickmore N), pp. 19–39. Cham, Switzerland: Springer. [Google Scholar]
- 28.Raymond B, Elliot SL, Ellis RJ. 2008. Quantifying the reproduction of Bacillus thuringiensis HD-1 in cadavers and live larvae of Plutella xylostella. J. Invertebr. Pathol. 98, 307–313. ( 10.1016/j.jip.2008.01.005) [DOI] [PubMed] [Google Scholar]
- 29.Zhou L, Slamti L, Nielsen-Leroux C, Lereclus D, Raymond B. 2014. The social biology of quorum-sensing in a naturalistic host pathogen system. Curr. Biol. 24, 2417–2422. ( 10.1016/j.cub.2014.08.049) [DOI] [PubMed] [Google Scholar]
- 30.Raymond B, Johnston PR, Nielsen-Leroux C, Lereclus D, Crickmore N. 2010. Bacillus thuringiensis: an impotent pathogen? Trends Microbiol. 18, 189–194. ( 10.1016/j.tim.2010.02.006) [DOI] [PubMed] [Google Scholar]
- 31.Cirimotich CM, Dong Y, Clayton AM, Sandiford SL, Souza-Neto JA, Mulenga M, Dimopoulos G. 2011. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science (New York, NY) 332, 855–858. ( 10.1126/science.1201618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dillon RJ, Dillon VM. 2004. The gut bacteria of insects: nonpathogenic interactions. Annu. Rev. Entomol. 49, 71–92. ( 10.1146/annurev.ento.49.061802.123416) [DOI] [PubMed] [Google Scholar]
- 33.van Leeuwen E, Neill SOA, Matthews A, Raymond B. 2015. Making pathogens sociable: the emergence of high relatedness through limited host invasibility. ISME J. 9, 2328 ( 10.1038/ismej.2015.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffin AS, West SA, Buckling A. 2004. Cooperation and competition in pathogenic bacteria. Nature 430, 1024–1027. ( 10.1038/nature02744) [DOI] [PubMed] [Google Scholar]
- 35.Ross-Gillespie A, Gardner A, West SA, Griffin AS. 2007. Frequency dependence and cooperation: theory and a test with bacteria. Am. Nat. 170, 331–342. ( 10.1086/519860) [DOI] [PubMed] [Google Scholar]
- 36.Chrostek E, Pelz-Stelinski K, Hurst GDD, Hughes GL. 2017. Horizontal transmission of intracellular insect symbionts via plants. Front. Microbiol. 8, 628–629. ( 10.3389/fmicb.2017.02237z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basset A, Khush RS, Braun A, Gardan L, Boccard F, Hoffmann JA, Lemaitre B. 2000. The phytopathogenic bacteria Erwinia carotovora infects Drosophila and activates an immune response. Proc. Natl Acad. Sci. USA 97, 3376–3381. ( 10.1073/pnas.97.7.3376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashby B, King KC. 2017. Friendly foes: the evolution of host protection by a parasite. Evol. Lett. 1, 211–221. ( 10.1002/evl3.19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62, 775–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cornforth DM, Matthews A, Brown SP, Raymond B. 2015. Bacterial cooperation causes systematic errors in pathogen risk assessment due to the failure of the independent action hypothesis. PLoS Pathog. 11, e1004775 ( 10.1371/journal.ppat.1004775.s002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown SP, Inglis RF, Taddei F. 2009. Evolutionary ecology of microbial wars: within-host competition and (incidental) virulence. Evol. Appl. 2, 32–39. ( 10.1111/j.1752-4571.2008.00059.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown SP, Le Chat L, De Paepe M, Taddei F. 2006. Ecology of microbial invasions: amplification allows virus carriers to invade more rapidly when rare. Curr. Biol. 16, 2048–2052. ( 10.1016/j.cub.2006.08.089) [DOI] [PubMed] [Google Scholar]
- 43.Dillon RJ, Charnley AK. 1988. Inhibition of Metarhizium anisopliae by the gut bacterial-flora of the desert locust: characterization of antifungal toxins. Can. J. Microbiol. 34, 1075–1082. ( 10.1139/m88-189) [DOI] [Google Scholar]
- 44.Recker M, et al. 2017. Clonal differences in Staphylococcus aureus bacteraemia-associated mortality. Nat. Microbiol. 2, 1381–1388. ( 10.1038/s41564-017-0001-x) [DOI] [PubMed] [Google Scholar]
- 45.Slamti L, et al. 2004. Distinct mutations in PlcR explain why some strains of the Bacillus cereus group are nonhemolytic. J. Bacteriol. 186, 3531–3538. ( 10.1128/JB.186.11.3531-3538.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ebert D, Weisser WW. 1997. Optimal killing for obligate killers: the evolution of life histories and virulence of semelparous parasites. Proc. R. Soc. Lond. B 264, 985–991. ( 10.1098/rspb.1997.0136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raymond B, Vanbergen A, Pearce I, Hartley SE, Cory JS, Hails RS. 2002. Host plant species can influence the fitness of herbivore pathogens: the winter moth and its nucleopolyhedrovirus. Oecologia 131, 533–541. ( 10.1007/s00442-002-0926-4) [DOI] [PubMed] [Google Scholar]
- 48.Ben-Ami F, Mouton L, Ebert D. 2008. The effects of multiple infections on the expression and evolution of virulence in a Daphnia–endoparasite system. Evolution 62, 1700–1711. ( 10.1111/j.1558-5646.2008.00391.x) [DOI] [PubMed] [Google Scholar]
- 49.Matthews AC, Mikonranta L, Raymond B. 2019. Data from: Shifts along the parasite–mutualist continuum are opposed by fundamental trade-offs Dryad Digital Repository. ( 10.5061/dryad.bf144m6) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Matthews AC, Mikonranta L, Raymond B. 2019. Data from: Shifts along the parasite–mutualist continuum are opposed by fundamental trade-offs Dryad Digital Repository. ( 10.5061/dryad.bf144m6) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data are available from the Dryad Digital Repository at: https://doi.org/10.5061/dryad.bf144m6 [49].