Abstract
Individual variation in parasite defences, such as resistance and tolerance, can underlie heterogeneity in fitness and could influence disease transmission dynamics. Glucocorticoid hormone concentrations often change in response to fluctuating environmental conditions and mediate changes in immune function, resource allocation and tissue repair. Thus, changes in glucocorticoid hormone concentrations might mediate individual variation in investment in resistance versus tolerance. In this study, we experimentally increased glucocorticoid concentrations in red-winged blackbirds (Agelaius phoeniceus) that were naturally infected with haemosporidian parasites, and assessed changes in resistance and tolerance of infection. Glucocorticoid treatment increased burdens of Plasmodium, the parasite causing avian malaria, but only in the absence of co-infection with another Haemosporidian, Haemoproteus. Thus, glucocorticoids might reduce resistance to infection, but co-infection can mitigate the negative consequences of increased hormone concentrations. Glucocorticoid treatment also decreased tolerance of infection. We found no evidence that the inflammatory immune response or rate of red blood cell production underlie the effects of glucocorticoids on resistance and tolerance. Our findings suggest that exogenous glucocorticoids can increase the costs of haemosporidian infections by both increasing parasite numbers and reducing an individual's ability to cope with infection. These effects could scale up to impact populations of both host and parasite.
Keywords: co-infection, corticosterone, tolerance, resistance, malaria, Plasmodium
1. Introduction
Parasite infections are universal, but host responses to infection can vary widely across individuals, populations and species. When faced with infection, hosts can invest in two defence strategies: resistance and tolerance [1]. Resistance entails killing parasites and/or limiting their reproduction to reduce the total parasite burden. Tolerance involves minimizing the costs of infection for a given parasite burden by limiting or repairing damage. Importantly, tolerance allows a host to cope with infection without reducing the parasites' fitness [1,2]. Investment in resistance and/or tolerance could change parasite burdens and the costs of infection for the host, and as a result influence not only host fitness, but also parasite transmission [3,4].
Resistance and tolerance of infection can change when organisms encounter challenging environmental or social conditions, such as resource limitation or over-crowding [5–7]. Furthermore, glucocorticoid hormones, which are key mediators of responses to such challenges in vertebrates, also can directly influence resistance and tolerance [8,9]. Experimental increases in glucocorticoids tend to reduce resistance, which is primarily attributed to immunosuppression caused by prolonged elevation of glucocorticoids [9,10]. Glucocorticoids influence immunity through diverse mechanisms, such as limiting inflammation through their influence on immune signalling pathways, regulating adaptive immunity via effects on lymphocyte activation and apoptosis, and influencing cytokine activity (reviewed in [11]). Glucocorticoid-driven decreases in resistance can cause elevations in parasite burden and/or the length of time organisms are infectious [8]. As a result, hosts with elevated glucocorticoids might be more likely to transmit infection, which ultimately could change disease transmission dynamics within a population [8,12].
The effects of glucocorticoids on tolerance are less clear. In an observational field study, we found that red-winged blackbirds (Agelaius phoeniceus) with naturally higher circulating concentrations of corticosterone, the primary avian glucocorticoid, were more tolerant of haemosporidian parasites, including avian malaria [13]. Birds with higher corticosterone maintained higher haematocrit for a given parasite burden than birds with lower corticosterone, suggesting corticosterone might increase tolerance. We found no evidence that corticosterone increased tolerance by elevating red blood cell production rates [13], but corticosterone could suppress pro-inflammatory Th1 activity and thereby reduce the number of cells destroyed by the host's immune system [11,14]. Experimental elevation of corticosterone in other host–parasite systems have been shown to decrease [8] or have no effect [15] on tolerance. However, to our knowledge, these laboratory-based experiments are not associated with similar field-based observational or experimental studies. Furthermore, these studies do not assess other factors that are likely to influence tolerance in free-living animals, which could result in differences between laboratory and field studies. For example, co-infection with multiple parasite species is common among wildlife and can alter the intensity of infections, the immune response and cost of infection [16–18]. Infection with one parasite species can stimulate an immune response that allows the host to better defend against other infections, or alternatively, one infection can increase host susceptibility to other infections through mechanisms such as immunosuppression [19]. Competition for host resources among parasite species or lineages might also alter parasite burdens within hosts such that the more competitive (and often more virulent) parasite will outnumber the less competitive parasite within the host [20]. Thus, co-infection could also impact the effects of glucocorticoids on resistance and tolerance.
In this study, we experimentally increased circulating corticosterone concentrations in male red-winged blackbirds naturally infected with one to three genera of haemosporidian parasites: malaria (Plasmodium) and malaria-like parasites (Haemoproteus and Leucocytozoon). Avian haemosporidians are vector-transmitted parasites with complex life cycles, and are frequently used as a model system to evaluate the ecological and evolutionary consequences of infection [21]. Although observational studies report positive, negative and non-significant relationships between infection status or parasite burden and both health and fitness metrics, experimental treatments of chronic infections consistently demonstrate negative consequences of infection in terms of biomarkers (e.g. haematocrit, oxidative stress), survival and reproductive success [22–25]. Here, we investigate if (i) corticosterone treatment affects resistance and tolerance to infection, (ii) effects of corticosterone on resistance and tolerance vary with co-infection status with multiple haemosporidian genera, and (iii) effects of corticosterone were mediated through mechanisms of immunosuppression and/or tissue repair. We predicted that exogenous corticosterone would increase parasite burden, indicating a reduction in resistance. Based on our findings in free-living birds, we also predicted that corticosterone would reduce the rate at which measures of host health decline as parasite burden increases, indicating an increase in tolerance [1,2]. Co-infection could modulate corticosterone's effects on resistance and/or tolerance to haemosporidians, and these effects could vary with genus of the infecting parasites. We also predicted that corticosterone would potentially increase tolerance and reduce resistance by suppressing immune function and/or increase tolerance by upregulating tissue repair.
2. Material and methods
(a). Study population
Red-winged blackbirds breeding in the marshes at the Queen's University Biological Station (QUBS; 44°34′02.3″ N, 76°19′28.4″ W) and in the surrounding Rideau Canal region of southeastern Ontario, Canada, have high haemosporidian infection prevalence. In the late 1980s to early 1990s, blood smear analyses revealed that 30–71% of birds in the QUBS population tested positive for the parasites [26–28]. In 2013–2015, PCR-based screening of breeding adults indicated that haemosporidian prevalence was 97.97 ± 4.55% (mean ± s.e.) and 64.73 ± .43% (mean ± s.e.) were infected with parasites from at least two genera of haemosporidians [13].
(b). Experimental design and data collection
In April–May 2015, we captured 89 adult males either using V-top Troyer traps baited with seeds and conspecific decoys or mist nets paired with conspecific song and call playback. We housed birds in groups of three in large outdoor flight aviaries (2.4 × 6.1 × 2.4 m, 30 aviaries in the complex; see the electronic supplementary material, Methods and Results, for detailed information about bird capture and husbandry).
We randomly assigned birds to one of three treatment groups, such that each aviary contained one bird from each treatment group, with the exception of one aviary containing only two birds. Treatment groups were low-dose corticosterone (0.1 mg implant, n = 30), high-dose corticosterone (0.5 mg implant, n = 30) and control (vehicle-only implant, n = 29) (Innovative Research of America, Sarasota, FL, USA). We sampled birds once pre-treatment, then immediately implanted the pellets subcutaneously on the birds' backs. We sampled each bird again 7, 14 and 21 days post-treatment. Corticosterone doses and sampling periods were selected based on a pilot study conducted in 2014 (methods and results for the pilot study are located in the electronic supplementary material). At each sampling period we collected 500 µl of blood and measured body mass. We used blood samples to assess each individual's health, immune function, and tissue repair, and parasite presence and burdens. We evaluated host health using four metrics that can be impaired by haemosporidian infection: haematocrit [22], haemoglobin [22], body mass [29] and oxidative balance [30,31]. We also assessed an indicator of genomic stability, the percentage of red blood cells containing micronuclei [32,33]. Micronuclei are formed when chromosomes break or are not incorporated into the nucleus during cell division [32]. Birds infected with haemosporidian parasites upregulate red blood cell production to compensate for the cells destroyed during infection [22], and this increase in cell production might result in higher error rates during cell division. We also assessed two immune metrics that can increase in response to haemosporidian infection: (i) nitric oxide, an inflammatory signalling molecule and anti-parasitic defence [34–36]; and (ii) PIT54 (avian analogue of haptoglobin), an acute-phase, anti-inflammatory protein and haemoglobin scavenger [37–39]. Because both haemosporidian parasites and the immune response to malarial parasites damage red blood cells [40], we assessed the level of red blood cell production as an indicator of tissue repair rate. Parasite presence/absence and burden were assessed pre-treatment and 7 days post-treatment, and all other measures were assessed at every sampling point.
After blood sample collection, we immediately measured haemoglobin in approximately 5 µl of fresh blood using a haemoglobin meter (HemoCue HB 201+, HemoCue AB, Ängelholm, Sweden) and we created two replicate blood smears. We stained the blood smears with Giemsa stain and used the blood smears to assess haemosporidian parasite burdens (number of infected cells per approx. 10 000 red blood cells), and the level of red blood cell production (estimated with polychromasia, the relative number of young red blood cells which appear polychromatic, or intensely-stained with Giemsa). We centrifuged the remaining blood samples in capillary tubes and measured haematocrit using the average from two capillary tubes of blood. We then separated the plasma from the red blood cells and froze both at −20°C. We used the blood cells to assess the presence/absence of Plasmodium, Haemoproteus and Leucocytozoon via a PCR-restriction enzyme assay (modified from [41]). We used the plasma to measure corticosterone concentration via radioimmunoassay [42] and PIT54, nitric oxide, reactive oxygen species, and total antioxidant capacity via commercial colorimetric assay kits. See electronic supplementary material, Methods and Results, for details about blood sampling, blood processing and laboratory assays.
(c). Statistical analyses
We performed all data analyses in R v. 3.3.2 [43]. Before conducting any analyses we transformed plasma corticosterone concentration values with a natural log function and parasite burdens as ln(burden + 1). Log transformations of corticosterone is common practice in field endocrinology because hormone concentrations are rarely normally distributed. Because parasite burdens within hosts are often overdispersed, parasite burden is frequently modelled using negative binomial or overdispersed Poisson error distributions. Neither of these distributions fitted our data as well as linear regression models with transformed parasite data (see electronic supplementary material, Analysis for resistance), and thus we used this transformation for our parasite data throughout our analyses. For all regression analyses, we initially summarized model results using Type III sums of squares using the R package ‘car’ [44]. However, if there were no significant interaction terms, we used Type II sums of squares, which is more statistically powerful in the absence of significant interactions [45,46]. However, if the switch to Type II sums of squares altered the statistical significance of interaction terms (the interaction terms change from non-significant to significant), we used Type III sums of squares, which is the more conservative approach. We compared the effects among levels within categorical factors (e.g. levels of corticosterone treatment) with pairwise contrasts adjusted with Tukey's multiple comparisons using the R package ‘lsmeans’ [47]. All mixed models were run with the R package ‘lme4’ [48] and all graphs were created using the R package ‘ggplot2’ [49].
(i). Effectiveness of corticosterone treatment
We determined whether or not the implants effectively elevated corticosterone by performing a linear mixed effect model predicting change in corticosterone from pre-treatment. As predictor variables, we included individual identity as a random effect, and pre-treatment corticosterone, treatment, day of the experiment and the interaction between treatment and day as fixed effects.
(ii). Effect of glucocorticoids and co-infection on resistance
We assessed the effects of corticosterone on resistance and tolerance at 7 days post-implantation because (i) our pilot study indicated that parasite burden peaked at this day (electronic supplementary material, Pilot Study, figure S2) and (ii) this timing allowed us to avoid confusing corticosterone driven changes in parasite burden with the high burdens associated with the acute stage of a newly acquired infection, as it generally takes at least 8–15 days for a natural infection to reach the acute stage [39,50]. We assessed resistance as the change in parasite burden after treatment. Using a separate analysis for each parasite genus, we tested the effect of corticosterone treatment on change in parasite burden, including only individuals that were PCR-positive for the focal genus. We performed linear models including change in parasite burden as the response variable and corticosterone treatment, pre-treatment parasite burden, the presence/absence of each non-focal parasite genus, and the interactions between treatment and the presence/absence of each non-focal parasite genus as explanatory variables. Because we found an effect of the high-dose implant and co-infection status on Plasmodium burden (see Results below) we conducted two post hoc analyses aimed at determining if the effects of corticosterone on Plasmodium burden varied with co-infection status. We used an analysis of variance and Tukey's multiple comparisons to assess how the change in Plasmodium burden varied with overall co-infection status within the high-dose treatment. We also tested if there was a relationship between co-infection status and Plasmodium burden pre-treatment using a simple linear regression with Plasmodium burden as the response variable and the presence/absence of both Haemoproteus and Leucocytozoon, and their interaction, as explanatory variables.
Because the Haemoproteus and Leucocytozoon parasites detected in blood cells can transmit infection to insect vectors, host resistance is directly linked to probability of disease transmission. However, Plasmodium has multiple parasite life stages within red blood cells, and only the gametocyte stage can infect vectors [40]. Therefore, to investigate specific effects of corticosterone on Plasmodium disease transmission, we also tested the effect of treatment on the change in Plasmodium gametocyte number. We measured gametocyte number rather than a ratio of gametocytes to other life stages because the raw numbers determine the likelihood of infecting a vector [51]. We performed a linear model analysis including change in gametocytes as the response variable and corticosterone treatment, pre-treatment parasite burden, the presence/absence of Haemoproteus and Leucocytozoon, and the interactions between treatment and the presence/absence of both Haemoproteus and Leucocytozoon as explanatory variables.
(iii). Effect of glucocorticoids and co-infection on tolerance
We measured tolerance as the slope of the relationship between parasite burden and proxies for host fitness or health among individuals within the same treatment group [2]. A sharper decline in fitness or health as parasite burden increases (a steeper slope) indicates lower tolerance. To test the effect of corticosterone on tolerance, we performed linear regressions predicting measures of health (haematocrit, haemoglobin, body mass, oxidative balance and micronuclei) including the following predictor variables: treatment group, total haemosporidian parasite burden, and the interaction between treatment group and parasite burden. A significant interaction between corticosterone treatment and parasite burden indicates an effect of corticosterone on tolerance. Because we evaluated multiple, potentially non-independent metrics of tolerance, we adjusted the p-values for false discovery rate to account for multiple comparisons for analyses indicating significant corticosterone by parasite burden interaction terms [52,53]. We included all birds that were PCR-positive for haemosporidians and used total parasite burden across genera in the analyses because all three genera have the potential to influence health [23,54,55].
We performed separate analyses to determine if co-infection was associated with tolerance or modulated any of the effects of corticosterone on tolerance. Only a subset of all possible combinations of co-infections were observed in our sample (electronic supplementary material, Methods and Results, table S3), and, as a result, we did not have the statistical power to include the presence/absence of each genus and their interactions in a regression model. Instead, we included infection status as a categorical variable where the levels were the combinations of infecting genera. We excluded birds with any infection status where n < 5 within any of the three treatment groups, which allowed us to test for effects of co-infection among three infection statuses: infection by Plasmodium only, by Plasmodium and Haemoproteus, and by Plasmodium and Leucocytozoon. The models testing the effect of co-infection and corticosterone treatment on tolerance included the measures of health as the response variables, and treatment, total haemosporidian parasite burden, infection status, and all two-way interactions as predictor variables. We did not perform this analysis for micronuclei because our sample was insufficient for a model with the predictors of interest.
(iv). The effects of corticosterone and co-infection on tissue repair and immune function
To investigate potential mechanisms for effects of corticosterone on resistance and tolerance, we assessed whether corticosterone treatment affected immune metrics and/or red blood cell production using linear mixed models. If we found any significant effects of corticosterone, we adjusted the p-values for false discovery rate to account for multiple comparisons [52,53]. We included individual identity as a random effect, and pre-treatment value of the response variable, day, treatment, change in parasite burden, and pairwise-interactions of day, treatment, and change in parasite burden as fixed effects. As in the analyses of tolerance, we tested for the additional effect of co-infection using a subset of the data including birds infected with Plasmodium, Plasmodium and Haemoproteus, or Plasmodium and Leucocytozoon, and added the following fixed effects to the model: co-infection status and the pairwise interactions between co-infection status and day, treatment, and parasite burden.
3. Results
(a). Effectiveness of corticosterone treatment
We measured glucocorticoid concentrations in 257 blood samples collected from 88 individuals. The high corticosterone treatment (0.5 mg) elevated plasma corticosterone concentrations throughout the experiment above both the control implant (t = 2.58, p = 0.03) and the low corticosterone implant (0.1 mg; t = 3.18, p = 0.006). At the times of sampling, the corticosterone concentrations of birds in the low dose treatment did not differ from birds receiving the sham implant (t = -0.62, p = 0.81) (electronic supplementary material, Methods and Results, figure S3). Seven days after implantation, birds in the high-dose implant group had corticosterone concentrations of (mean ± s.e.) 8.79 ± 1.89 ng ml−1, which is similar to concentrations in free-living, breeding males, which we previously measured as 12.31 ± 1.86 ng ml−1 [13]. Across all treatments, the change in corticosterone from the initial value decreased over time, indicating a waning effect of the implants or upregulated negative feedback and clearance mechanisms (F = 14.66, p = 0.0002).
(b). Effect of glucocorticoids and co-infection on resistance
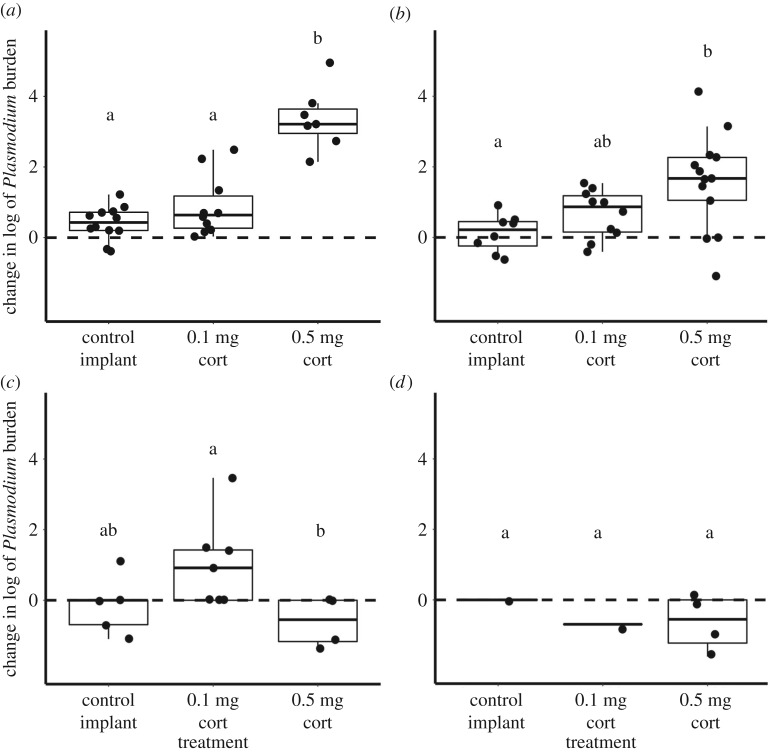
The high-dose corticosterone treatment affected resistance, as indicated by an increased Plasmodium burden, but the effect depended on co-infection status. There were significant interactions between treatment and both Haemoproteus (F = 16.74, p < 0.0001, n = 82) and Leucocytozoon (F = 4.15, p = 0.02, n = 82) infection status, and the three-way interaction between treatment, Haemoproteus, and Leucocytozoon approached significance (F = 2.93, p = 0.06, n = 82). If birds were infected with Plasmodium but not Haemoproteus, the high-dose implant increased Plasmodium burdens more than the control implant (figure 1a,b), but the effect disappears in the presence of a Haemoproteus co-infection (figure 1c,d). The high-dose implant also increased Plasmodium burden more than the low-dose implant if a bird was only infected with Plasmodium (figure 1a), but not when co-infected with Leucocytozoon and/or Haemoproteus (figure 1b–d). In birds co-infected with Plasmodium and Haemoproteus (figure 1c), the low-dose implant increased Plasmodium burden more than the high-dose implant, but that effect is partially driven by a single bird with a large increase in parasite burden, and if that individual is removed from the analysis, the effect is no longer significant.
Figure 1.
The effect of treatment on Plasmodium burden by co-infection status with (a) Plasmodium only, (b) Plasmodium and Leucocytozoon, (c) Plasmodium and Haemoproteus, and (d) Plasmodium, Haemoproteus, and Leucocytozoon. Letters (a, b) represent significant differences (p < 0.05) among treatments within the same panel. In the absence of Haemoproteus, (a,b), high-dose corticosterone causes an increase in Plasmodium burden, but the effect disappears with Haemoproteus co-infection (c,d).
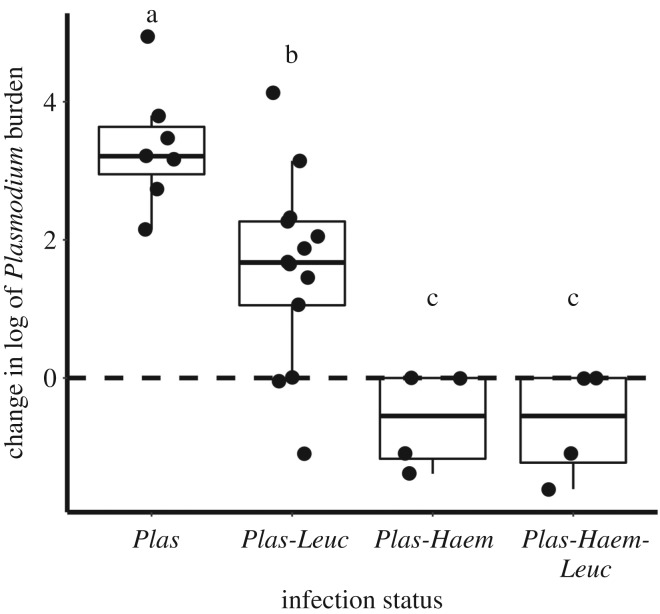
Within the high-dose treatment, change in parasite burden was associated with co-infection status (F = 15.62, p < 0.0001, n = 28). Birds infected with Plasmodium alone experienced greater increases in Plasmodium burden than those with co-infections, and none of the birds co-infected with Haemoproteus displayed increased Plasmodium burden (figure 2). The relationship between Haemoproteus co-infection and Plasmodium burden also existed pre-treatment. Pre-treatment, lower Plasmodium burdens were associated with Haemoproteus co-infection (F = 54.9, p < 0.0001, n = 81), but there was no relationship with Leucocytozoon co-infection (F = 0.84, p = 0.36, n = 81) or an interaction between Haemoproteus and Leucocytozoon (F = 1.84, p = 0.18, n = 81) (electronic supplementary material, Methods and Results, figure S4).
Figure 2.
The relationship between co-infection status and change in parasite burden in burdens receiving the high-dose corticosterone treatment. Letters (a, b, c) represent significant differences (p < 0.05) among co-infection statuses. Co-infection with either or both Haemoproteus and Leucocytozoon limits the effect of treatment on Plasmodium burden.
Corticosterone treatment increased the overall number of Plasmodium gametocytes. As with total Plasmodium burden, the effect of corticosterone treatment on the number of Plasmodium gametocytes depended on co-infection. The high-dose implants caused an increase in gametocytes relative to birds receiving both the control and low-dose implants in the absence of Haemoproteus (versus control, t = 4.00, p = 0.0005, n = 28; versus low, t = 3.35, p = 0.004, n = 28) and Leucocytozoon (versus control, t = 2.92, p = 0.01, n = 28; versus low, t = 3.06, p = 0.009, n = 28; additional details in the electronic supplementary material, Methods and Results).
Corticosterone treatment had no effect on resistance to Haemoproteus (F = 1.95, p = 0.17, n = 23) or Leucocytozoon (F = 0.92, p = 0.41, n = 37), as measured by change in burdens of these parasites. Because fewer individuals were infected with Haemoproteus and Leucocytozoon than with Plasmodium, and there were limited sample sizes of some co-infection statuses in our study (electronic supplementary material, Methods and Results, table S3), we could not test for an effect of co-infection on change in burdens of these genera.
(c). Effects of glucocorticoids and co-infection on tolerance
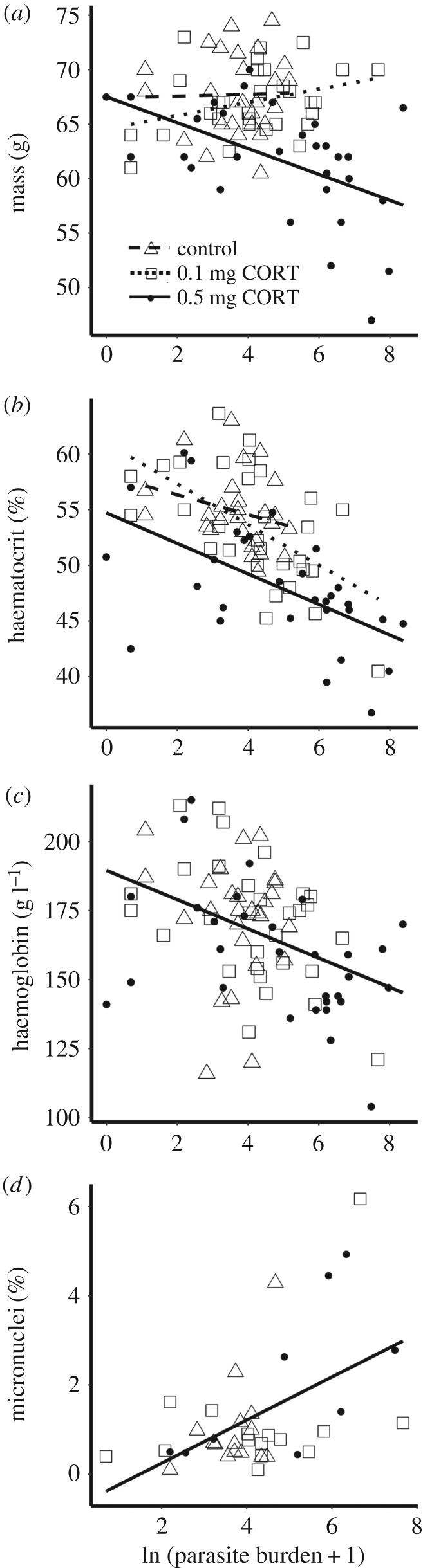
Corticosterone treatment reduced tolerance to infection with haemosporidian parasites as estimated through body mass (interaction between treatment and parasite burden; F = 5.98, p = 0.004, n = 87, false discovery rate adjusted p = 0.02). Body mass of birds in the high-dose group declined more steeply as parasite burden increased compared with birds in the low-dose group (t = −3.35, p = 0.004, n = 87) (figure 3a). However, the slope of the mass–parasite burden relationship did not differ between the high-dose and control groups (t = −1.60, p = 0.25, n = 87) or the low-dose and control groups (t = 0.62, p = 0.81, n = 87; figure 3a). The range of parasite burden in the control group (2–174 parasites per 10 000 red blood cells) is much smaller than the range in the low-dose (1–2143 parasites per 10 000 red blood cells) or high-dose (0–4346 parasites per 10 000 red blood cells) groups, and thus we might not have been able to detect a difference between the high-dose and control groups, if it were present. However, when we performed the same analysis combining the control and low-dose groups, which had similar corticosterone concentrations at the time of sampling, birds in the high-dose group demonstrated a steeper decline in body mass with increasing parasite burden than the other groups combined (t = −3.35, p = 0.0012, n = 87).
Figure 3.
The effect of corticosterone treatment on tolerance to infection estimated using (a) body mass, (b) haematocrit and (c) haemoglobin. Open triangles and dashed line represent birds in the control group, open squares and dotted line represent the low-dose group, and solid circles and solid line represent the high-dose group. (a) Among birds implanted with the high dose, body mass was lower in birds with higher parasite burdens, and the slope was significantly different from the low-dose group or the low and control groups combined, indicating lower tolerance. (b) Birds in the high corticosterone group had lower mean haematocrit than birds in the other treatment groups. There was no difference in the slope of the relationship between haematocrit and parasite burden among treatment groups, indicating no difference in tolerance. (c) There was no difference in mean haemoglobin concentrations or the slope of the haemoglobin–parasite burden relationship among treatment groups.
Corticosterone treatment had no effect on tolerance when estimated using haematocrit, haemoglobin or micronuclei data. Although both corticosterone treatment (F = 11.78, p < 0.0001, n = 87) and parasite burden (F = 31.40, p < 0.0001, n = 87) were associated with haematocrit, the slope of the relationship between haematocrit and parasite burden was similar across corticosterone treatments (F = 0.59, p = 0.55, n = 87; figure 3b). Haemoglobin declined with increasing parasite burden (F = 13.19, p = 0.0005, n = 87), but did not differ with treatment (F = 1.40, p = 0.25, n = 87) or the interaction of treatment and parasite burden (F = 0.37, p = 0.69, n = 87; figure 3c). Micronuclei increased with increasing parasite burden (F = 10.11, p = 0.0032, n = 39), indicating that higher infection intensities are associated with greater chromosomal damage, but micronuclei were unrelated to corticosterone treatment (F = 0.69, p = 0.51, n = 39) and the interaction of treatment and parasite burden (F = 0.73, p = 0.49, n = 39; figure 3d). Oxidative balance was not associated with parasite burden (F = 0.46, p = 0.50, n = 84), and there was no interaction of treatment and parasite burden (F = 0.40, p = 0.96, n = 84).
We assessed whether or not co-infection was correlated with tolerance using a subset of the full dataset, including individuals with the most common combinations of infecting genera (more than five birds per group): Plasmodium only, Plasmodium and Haemoproteus, and Plasmodium and Leucocytozoon. Within this subset, the interaction between co-infection status and parasite burden was not significant in any of the models predicting costs of infection (all p > 0.07), suggesting that co-infection does not influence tolerance.
(d). The effects of corticosterone and co-infection on tissue repair and immune function
Corticosterone treatment did not change rates of red blood cell production (Wald χ2 = 2.01, p = 0.37, 240 observations of 81 individuals). However, red blood cell production was correlated with change in parasite burden, but the effect varied across days of the study (Wald χ2 = 3.62, p ≤ 0.0001, 240 observations of 81 individuals). Co-infection status had no effect on red blood cell production (Wald χ2 = 0.29, p = 0.86, 210 observations of 71 individuals).
Corticosterone treatment did not change birds' plasma haptoglobin (Wald χ2 = 1.20, p = 0.55, 237 observations of 82 individuals) or nitric oxide concentrations (Wald χ2 = 0.14, p = 0.93, 139 observations of 56 individuals). Neither immune measure was associated with change in parasite burden (haptoglobin, Wald χ2 = 0.10, p = 0.75, 237 observations of 82 individuals; nitric oxide, Wald χ2 = 0.08, p = 0.78, 139 observations of 56 individuals). Co-infection was not associated with either immune measure (haptoglobin, Wald χ2 = 0.29, p = 0.86, 210 observations of 73 individuals; nitric oxide, Wald χ2 = 0.98, p = 0.61, 123 observations of 50 individuals).
4. Discussion
Our results suggest that treatment with exogenous glucocorticoid hormones can amplify the costs of haemosporidian infection for a host by reducing both resistance and tolerance to infection. In addition, we found evidence indicating that exogenous glucocorticoids might increase the risk of disease transmission. The higher of two biologically conservative exogenous corticosterone doses caused an increase in avian malaria (Plasmodium) burden and in the total number of gametocytes, the malaria life stage responsible for transmission to the insect vector. Interestingly, we only found this effect of exogenous hormone on malaria burden in the absence of co-infection with a related parasite, Haemoproteus. Corticosterone treatment also reduced infection tolerance such that birds in the high-dose treatment experienced steeper declines in body mass with increasing parasite burden. We found no evidence supporting an immunosuppressive role for either corticosterone or co-infection, suggesting that suppression of the inflammatory response might not underlie glucocorticoids' effects on resistance and tolerance and/or other components of immunity might be more important for responses to malaria infection in this species. Our findings suggest that individual variation in both glucocorticoid concentration and co-infection status can influence parasite dynamics within hosts and, as a result, might also alter transmission dynamics in a population.
Our findings are similar to those of other studies also reporting that increases in corticosterone can cause increases in parasite burden [9,56]. Additionally, we find that corticosterone treatment increases the total number of malaria gametocytes. Glucocorticoids' effects on parasite burden have often been interpreted as the result of immunosuppression (e.g. [9,11]); however, rather than reducing host resistance, the treatment might have directly caused the parasites to increase their rate of replication within the host. Haemosporidian parasites can respond to subtle changes in their hosts, including detecting and responding to the host's response to a mosquito bite [57], and in vitro studies of malaria and related protozoans have shown that glucocorticoids can increase parasite replication in the absence of a host immune system [58–60]. Therefore, we cannot determine if changes in parasite burden were caused by responses to corticosterone by the host or the parasites, or both. In addition, elevated glucocorticoids can increase host attractiveness to mosquito vectors [61], and increasing the total number of gametocytes could further improve transmission success [51].
If malaria-infected birds were co-infected with Haemoproteus, the higher-dose treatment did not increase malaria parasite burden or the number of gametocytes. Similarly, another study of co-infection demonstrated that the presence of one avian haemosporidian species can reduce the probability of infection with another species [62]. Competitive interactions among parasites can yield negative relationships between parasite infection intensities [63]. For example, competition for shared resources, like blood cells or haemoglobin in the case of haemosporidians, could result in higher burdens of the more competitive parasite [64]. Alternatively, infection with a parasite could result in cross immunity, in which a host has increased resistance to a different parasite [65,66]. However, our study found no support for differences in immune function with co-infection using the immune metrics we measured. Regardless of the mechanism, co-infections with multiple haemosporidian parasites could contribute to parasite dynamics within bird populations.
In our previous field study of red-winged blackbirds, higher endogenous plasma corticosterone concentrations were associated with maintaining higher haematocrit for a given parasite burden [13]. In the present study, exogenous corticosterone treatment had no effect on tolerance when measured using haematocrit or haemoglobin and instead reduced tolerance as estimated with body mass. Our results also differ from a study of zebra finches (Taeniopygia guttata), where corticosterone manipulation had no effect on tolerance of West Nile infection when estimated with body mass, although there was a tendency for the birds receiving a high-dose implant to have lower tolerance in terms of flight ability [8]. Energy regulation is a central role of glucocorticoids [67], and corticosterone-driven changes in metabolism coupled with the challenge of parasite infection might lead to the decline in tolerance as estimated with body mass. As a measure of energy regulation, change in body mass could reflect the cumulative energetic costs of infection, such as the resources required to upregulate some immune defences or repair damaged tissue. Thus, we might be better able to detect changes in this more integrative measure of tolerance than with those metrics that reflect more limited costs of infection, such as haematocrit or micronuclei, which are indicative of red blood cell damage. In addition, the discrepancy between our observational and experimental studies could be the result of an unmeasured physiological factor driving the observational correlation.
Dissimilarities between natural and captive environments, or the effects of manipulated, exogenous hormone concentrations and endogenous hormone concentrations, could contribute to the contrasting outcomes observed here and in the prior field study [13]. Physiological responses to stimuli can vary in captive and wild settings [68,69]. In our captive study, birds are freed from food limitation and the energetic and physical demands of breeding, but experience a different physical and social environment as well as more frequent encounters (visual and physical contact) with humans, which could be perceived as increased predation risk. Any of these differences could alter hypothalamic–pituitary–adrenal (HPA) axis activity at various levels (e.g. receptors, hormone concentrations, negative feedback) or change various components of the immune response [69,70]. Further, exogenous corticosterone manipulations might not always effectively simulate the effects of individual variation in hormones. Even if hormone implants increase corticosterone within a natural physiological range, as they did in our study, they do not match the pulsatile, cyclic release of endogenous hormones [70,71]. As a result, experimental elevations of corticosterone could affect the HPA axis differently than endogenous corticosterone concentrations even if plasma levels of the hormone appear similar (e.g. binding to different receptor types or altering negative feedback), which could alter the downstream effects of corticosterone. In addition, individuals' regulation of hormone concentrations has been shaped by selection; any hormone manipulation that shifts individuals away from endogenous levels could have negative effects, such as the reduction in tolerance seen here. Moving forward, investigators need to address whether factors underlying variation in glucocorticoids, including life-history strategy or the physical and social environment, are also associated with changes in resistance and tolerance to parasites.
In summary, experimentally augmented glucocorticoid concentrations and co-infection status can contribute to among individual differences in the two key host defence strategies, resistance and tolerance. To our knowledge, this is the first study to demonstrate a significant glucocorticoid-driven change in parasite burden that varies with co-infection status. Elevated glucocorticoids had twofold costs: birds suffered reduced resistance, as indicated by higher parasite burdens and associated reductions in haemoglobin and haematocrit, and reduced tolerance, reflected in higher costs of infection, in terms of reduced body mass. The increase in Plasmodium burden could also increase malaria transmission rates, suggesting a mechanism by which individual glucocorticoid levels could link to population-level processes. Thus, through its effects on an individual's endocrine physiology, environmental variation could lead to variation in host–parasite dynamics at the individual and population level.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
For assistance in the field and laboratory, we thank S. Burns, C. Dale, S. Gong, T. Griffiths, T. Kelly, H. Kenyon, M. Kernbach, S. Kim, C. Moser-Purdy, V. Rohwer, P. St. John, G. Tricola, and A. Van Tol, as well as QUBS staff for logistical support. We are grateful to D. Hawley and the Hawley lab for discussions about experimental design and analysis as well as assistance with parasite PCRs and immune assays. We thank M. Vitousek for use of laboratory space and equipment. We thank the Vitousek lab, L. B. Martin lab, W. Hopkins and J. Walters for discussion and feedback on the manuscript, and E. Mudrak at the Cornell Statistical Consulting Unit for statistical advice.
Ethics
All procedures were approved by the Virginia Tech Institutional Animal Care and Use Committee (13-030 BIOL) and the Queen's University Animal Care Committee (2013-027).
Data accessibility
The datasets supporting this article have been uploaded as part of the supplementary material and are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.g9n8g17 [72].
Authors' contributions
L.A.S., I.T.M. and F.B. designed the experiment. L.A.S. and A.M.D. conducted the experiment. L.A.S., M.F.H., I.T.M., E.B.G., M.M., A.M.D. and D.C. contributed to laboratory assays. L.A.S. performed statistical analyses and drafted the manuscript. All authors edited and approved the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Funding for this research came from a National Science Foundation grant no. IOS-1145625 (F.B., M.F.H. and I.T.M.), an EPA STAR Fellowship (FP-917686) (L.A.S.), a Sigma Xi GIAR (L.A.S.), the Society of Canadian Ornithologists' Taverner Award (L.A.S.), Virginia Tech Sigma Xi (L.A.S.), the Virginia Tech Global Change Center (L.A.S.) and the Virginia Tech Graduate School Interdisciplinary Graduate Education Program (L.A.S.). L.A.S. was supported by a fellowship from the Virginia Tech Institute for Critical Technology and Applied Science. The aviaries were funded by a Canadian Foundation for Innovation grant to P. R. Martin. The EPA has not formally reviewed this publication, the EPA does not endorse products or services described here, and any views expressed here belong to the authors alone.
References
- 1.Råberg L, Graham AL, Read AF. 2009. Decomposing health: tolerance and resistance to parasites in animals. Phil. Trans. R. Soc. B 364, 37–49. ( 10.1098/rstb.2008.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Råberg L, Sim D, Read AF. 2007. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318, 812–814. ( 10.1126/science.1148526) [DOI] [PubMed] [Google Scholar]
- 3.Gopinath S, Lichtman JS, Bouley DM, Elias JE, Monack DM. 2014. Role of disease-associated tolerance in infectious superspreaders. Proc. Natl Acad. Sci. USA 111, 15 780–15 785. ( 10.1073/pnas.1409968111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller MR, White A, Boots M. 2006. The evolution of parasites in response to tolerance in their hosts: the good, the bad, and apparent commensalism. Evolution 60, 945–956. ( 10.1111/j.0014-3820.2006.tb01173.x) [DOI] [PubMed] [Google Scholar]
- 5.Ould P, Welch HE. 1980. The effect of stress on the parasitism of mallard ducklings by Echinuria uncinata (Nematoda: Spirurida). Can. J. Zool. 58, 228–234. ( 10.1139/z80-026) [DOI] [Google Scholar]
- 6.Kutzer MAM, Armitage SAO. 2016. The effect of diet and time after bacterial infection on fecundity, resistance, and tolerance in Drosophila melanogaster. Ecol. Evol. 6, 4229–4242. ( 10.1002/ece3.2185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clough D, Prykhodko O, Råberg L. 2016. Effects of protein malnutrition on tolerance to helminth infection. Biol. Lett. 12, 20160189 ( 10.1098/rsbl.2016.0189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gervasi SS, Burgan SC, Hofmeister E, Unnasch TR, Martin LB. 2017. Stress hormones predict a host superspreader phenotype in the West Nile virus system. Proc. R. Soc. B 284, 20171090 ( 10.1098/rspb.2017.1090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Applegate JE. 1970. Population changes in latent avian malaria infections associated with season and corticosterone treatment. J. Parasitol. 56, 439–443. ( 10.2307/3277599) [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Prieto I, Fernandez-Juricic E, Martin J, Regis Y. 2009. Antipredator behavior in blackbirds: habituation complements risk allocation. Behav. Ecol. 20, 371–377. ( 10.1093/beheco/arn151) [DOI] [Google Scholar]
- 11.Cain DW, Cidlowski JA. 2017. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 17, 233–247. ( 10.1038/nri.2017.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin LB, Burgan SC, Adelman JS, Gervasi SS. 2016. Host competence: an organismal trait to integrate immunology and epidemiology. Integr. Comp. Biol. 56, 1225–1237. ( 10.1093/ICB/ICW064) [DOI] [PubMed] [Google Scholar]
- 13.Schoenle LA, Schoepf I, Weinstein NM, Moore IT, Bonier F. 2018. Higher plasma corticosterone is associated with reduced costs of infection in red-winged blackbirds. Gen. Comp. Endocrinol. 256, 89–98. ( 10.1016/j.ygcen.2017.07.006) [DOI] [PubMed] [Google Scholar]
- 14.Elenkov IJ. 2004. Glucocorticoids and the Th1/Th2 balance. Ann. NY Acad. Sci. 1024, 138–146. ( 10.1196/annals.1321.010) [DOI] [PubMed] [Google Scholar]
- 15.Murone J, DeMarchi JA, Venesky MD. 2016. Exposure to corticosterone affects host resistance, but not tolerance, to an emerging fungal pathogen. PLoS ONE 11, e0163736 ( 10.1371/journal.pone.0163736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, Begon M. 2010. Species interactions in a parasite community drive infection risk in a wildlife population. Science 330, 243–246. ( 10.1126/science.1190333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Roode JC, Helinski MEH, Anwar MA, Read AF. 2005. Dynamics of multiple infection and within-host competition in genetically diverse malaria infections. Am. Nat. 166, 531–542. ( 10.1086/491659) [DOI] [PubMed] [Google Scholar]
- 18.Dhondt A, Dhondt KV, Nazeri S. 2017. Apparent effect of chronic Plasmodium infections on disease severity caused by experimental infections with Mycoplasma gallisepticum in house finches. Int. J. Parasitol. 6, 49–53. ( 10.1016/j.ijppaw.2017.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bordes F, Morand S. 2011. The impact of multiple infections on wild animal hosts: a review. Infect. Ecol. Epidemiol. 1, 1–10. ( 10.3402/iee.v1i0.7346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell AS, de Roode JC, Sim D, Read AF. 2006. Within-host competition in genetically diverse malaria infections: parasite virulence and competitive success. Evolution 60, 1358–1371. ( 10.1111/j.0014-3820.2006.tb01215.x) [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Longoria L, Magallanes S, González-Blázquez M, Refollo Y, de Lope F, Marzal A. 2016. New approaches for an old disease: studies on avian malaria parasites for the twenty-first century challenges. In Current topics in malaria (ed Rodriguez-Morales AJ.), pp. 163–183. London, UK: IntechOpen. [Google Scholar]
- 22.Schoenle LA, Kernbach M, Haussmann MF, Bonier F, Moore IT. 2017. An experimental test of the physiological consequences of avian malaria infection. J. Anim. Ecol. 86, 1483–1496. ( 10.1111/1365-2656.12753) [DOI] [PubMed] [Google Scholar]
- 23.Asghar M, Hasselquist D, Zehtindjiev P, Westerdahl H, Bensch S. 2015. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347, 436–438. ( 10.1126/science.1261121) [DOI] [PubMed] [Google Scholar]
- 24.Knowles SCL, Palinauskas V, Sheldon BC. 2010. Chronic malaria infections increase family inequalities and reduce parental fitness: experimental evidence from a wild bird population. J. Evol. Biol. 23, 557–569. ( 10.1111/j.1420-9101.2009.01920.x) [DOI] [PubMed] [Google Scholar]
- 25.Martínez-de la Puente J, Merino S, Tomás G, Moreno J, Morales J, Lobato E, García-Fraile S, Belda EJ. 2010. The blood parasite Haemoproteus reduces survival in a wild bird: a medication experiment. Biol. Lett. 6, 663–665. ( 10.1098/rsbl.2010.0046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weatherhead PJ. 1990. Secondary sexual traits, parasites, and polygyny in red-winged blackbirds, Agelaius phoeniceus. Behav. Ecol. 1, 125–130. ( 10.1093/beheco/1.2.125) [DOI] [Google Scholar]
- 27.Weatherhead PJ, Bennett GF. 1991. Ecology of red-winged blackbird parasitism by haematozoa. Can. J. Zool. 69, 2352–2359. ( 10.1139/z91-331) [DOI] [Google Scholar]
- 28.Weatherhead PJ, Metz KJ, Bennett GF, Irwin RE. 1993. Parasite faunas, testosterone and secondary sexual traits in male red-winged blackbirds. Behav. Ecol. Sociobiol. 33, 13–23. ( 10.1007/BF00164342) [DOI] [Google Scholar]
- 29.Cellier-Holzem E, Esparza-Salas R, Garnier S, Sorci G. 2010. Effect of repeated exposure to Plasmodium relictum (lineage SGS1) on infection dynamics in domestic canaries. Int. J. Parasitol. 40, 1447–1453. ( 10.1016/j.ijpara.2010.04.014) [DOI] [PubMed] [Google Scholar]
- 30.Isaksson C, Sepil I, Baramidze V, Sheldon BC. 2013. Explaining variance of avian malaria infection in the wild: the importance of host density, habitat, individual life-history and oxidative stress. BMC Ecol. 13, 15 ( 10.1186/1472-6785-13-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delhaye J, Jenkins T, Christe P. 2016. Plasmodium infection and oxidative status in breeding great tits, Parus major. Malar. J. 15, 531 ( 10.1186/s12936-016-1579-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishna G, Hayashi M. 2000. In vivo rodent micronucleus assay: protocol, conduct and data interpretation. Mutat. Res. 455, 155–166. ( 10.1016/S0027-5107(00)00117-2) [DOI] [PubMed] [Google Scholar]
- 33.Zúñiåga-González G, et al. 2000. Spontaneous micronuclei in peripheral blood erythrocytes from 54 animal species (mammals, reptiles and birds): part two. Mutat. Res. 467, 99–103. ( 10.1016/S1383-5718(00)00021-8) [DOI] [PubMed] [Google Scholar]
- 34.Sild E, Hõrak P. 2009. Nitric oxide production: an easily measurable condition index for vertebrates. Behav. Ecol. Sociobiol. 63, 959–966. ( 10.1007/s00265-009-0710-0) [DOI] [Google Scholar]
- 35.Bichet C, Cornet S, Larcombe S, Sorci G. 2012. Experimental inhibition of nitric oxide increases Plasmodium relictum (lineage SGS1) parasitaemia. Exp. Parasitol. 132, 417–423. ( 10.1016/j.exppara.2012.09.008) [DOI] [PubMed] [Google Scholar]
- 36.de Macchi BM, Miranda FJB, de Souza FS, de Carvalho ECQ, Albernaz AP, do Nascimento JLM, Damatta RA. 2013. Chickens treated with a nitric oxide inhibitor became more resistant to Plasmodium gallinaceum infection due to reduced anemia, thrombocytopenia and inflammation. Vet. Res. 44, 8 ( 10.1186/1297-9716-44-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wicher KB, Fries E. 2006. Haptoglobin, a hemoglobin-binding plasma protein is present in bony fish and mammals but not in frog and chicken. Proc. Natl Acad. Sci. USA 103, 4168–4173. ( 10.1073/pnas.0508723103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quaye IK. 2008. Haptoglobin, inflammation and disease. Trans. R. Soc. Trop. Med. Hyg. 102, 735–742. ( 10.1016/j.trstmh.2008.04.010) [DOI] [PubMed] [Google Scholar]
- 39.Ellis VA, Cornet S, Merrill L, Kunkel MR, Tsunekage T, Ricklefs RE. 2015. Host immune responses to experimental infection of Plasmodium relictum (lineage SGS1) in domestic canaries (Serinus canaria). Parasitol. Res. 114, 3627–3636. ( 10.1007/s00436-015-4588-7) [DOI] [PubMed] [Google Scholar]
- 40.Valkiūnas G. 2005. Avian malaria parasites and other Haemosporidia. Boca Raton, FL: CRC Press. [Google Scholar]
- 41.Beadell JS, Fleischer R. 2005. A restriction enzyme-based assay to distinguish between avian hemosporidians. J. Parasitol. 91, 683–685. ( 10.1645/GE-3412RN) [DOI] [PubMed] [Google Scholar]
- 42.Schoenle LA, Dudek AM, Moore IT, Bonier F. 2017. Red-winged blackbirds (Agelaius phoeniceus) with higher baseline glucocorticoids also invest less in incubation and clutch mass. Horm. Behav. 90, 1–7. ( 10.1016/j.yhbeh.2017.02.002) [DOI] [PubMed] [Google Scholar]
- 43.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 44.Fox J, Weisberg S. 2011. An R companion to applied regression, 2nd edn Thousand Oaks, CA: Sage. [Google Scholar]
- 45.Smith CE, Cribbie R. 2014. Factorial ANOVA with unbalanced data: a fresh look at the types of sums of squares. J. Data Sci. 12, 385–404. [Google Scholar]
- 46.Hector A, von Felten S, Schmid B. 2010. Analysis of variance with unbalanced data: an update for ecology and evolution. J. Anim. Ecol. 79, 308–316. ( 10.1111/j.1365-2656.2009.01634.x) [DOI] [PubMed] [Google Scholar]
- 47.Lenth RV. 2016. Least-squares means: the R package lsmeans. J. Stat. Softw. 69, 1–33. ( 10.18637/jss.v069.i01) [DOI] [Google Scholar]
- 48.Bates D, Malchar M, Bolker BM, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 49.Wickham H. 2009. ggplot2: elegant graphics for data analysis. New York, NY: Springer; See http://ggplot2.org. [Google Scholar]
- 50.Palinauskas V, Valkiūnas G, Bolshakov CV, Bensch S. 2008. Plasmodium relictum (lineage P-SGS1): effects on experimentally infected passerine birds. Exp. Parasitol. 120, 372–380. ( 10.1016/j.exppara.2008.09.001) [DOI] [PubMed] [Google Scholar]
- 51.Drakeley CJ, Secka I, Correa S, Greenwood BM, Targett GAT. 1999. Host haematological factors influencing the transmission of Plasmodium falciparum gametocytes to Anopheles gambiae s.s. mosquitoes. Trop. Med. Int. Health 4, 131–138. ( 10.1046/j.1365-3156.1999.00361.x) [DOI] [PubMed] [Google Scholar]
- 52.Pike N. 2011. Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol. Evol. 2, 278–282. ( 10.1111/j.2041-210X.2010.00061.x) [DOI] [Google Scholar]
- 53.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300. [Google Scholar]
- 54.Marzal A, de Lope F, Navarro C, Møller AP. 2005. Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142, 541–545. ( 10.1007/s00442-004-1757-2) [DOI] [PubMed] [Google Scholar]
- 55.Karell P, Bensch S, Ahola K, Asghar M. 2017. Pale and dark morphs of tawny owls show different patterns of telomere dynamics in relation to disease status. Proc. R. Soc. B 284, 20171127 ( 10.1098/rspb.2017.1127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belden LK, Kiesecker JM. 2005. Glucocorticosteroid hormone treatment of larval treefrogs increases infection by Alaria sp. trematode cercariae. J. Parasitol. 91, 686–688. ( 10.1645/GE-397R) [DOI] [PubMed] [Google Scholar]
- 57.Cornet S, Nicot A, Rivero A, Gandon S. 2014. Evolution of plastic transmission strategies in avian malaria. PLoS Pathog. 10, e1004308 ( 10.1371/journal.ppat.1004308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maswoswe SM, Peters W, Warhurst DC. 1985. Corticosteroid stimulation of the growth of Plasmodium falciparum gametocytes in vitro. Ann. Trop. Med. Parasitol. 79, 607–616. ( 10.1080/00034983.1985.11811968) [DOI] [PubMed] [Google Scholar]
- 59.Escobedo G, Roberts CW, Carrero JC, Morales-Montor J. 2005. Parasite regulation by host hormones: an old mechanism of host exploitation? Trends Parasitol. 21, 588–593. ( 10.1016/j.pt.2005.09.013) [DOI] [PubMed] [Google Scholar]
- 60.Li M, Leatherland JF, Woo PTK. 2013. Cortisol and dexamethasone increase the in vitro multiplication of the haemoflagellate, Cryptobia salmositica, possibly by interaction with a glucocorticoid receptor-like protein. Int. J. Parasitol. 43, 353–360. ( 10.1016/j.ijpara.2012.11.009) [DOI] [PubMed] [Google Scholar]
- 61.Gervasi S, Burkett-Cadena N, Burgan SC, Schrey AW, Hassan HK, Unnasch TR, Martin LB. 2016. Host stress hormones alter vector feeding preferences, success and productivity. Proc. R. Soc. B 283, 20161278 ( 10.1098/rspb.2016.1278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clark NJ, Wells K, Dimitrov D, Clegg SM, Hoye B. 2016. Co-infections and environmental conditions drive the distributions of blood parasites in wild birds. J. Anim. Ecol. 85, 1461–1470. ( 10.1111/1365-2656.12578) [DOI] [PubMed] [Google Scholar]
- 63.Lafferty KD. 2010. Interacting parasites. Science 330, 187–188. ( 10.1126/science.1196915) [DOI] [PubMed] [Google Scholar]
- 64.Graham AL. 2008. Ecological rules governing helminth microparasite coinfection. Proc. Natl Acad. Sci. USA 105, 566–570. ( 10.1073/pnas.0707221105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fenton A, Perkins SE. 2010. Applying predator-prey theory to modelling immune-mediated, within-host interspecific parasite interactions. Parasitology 137, 1027–1038. ( 10.1017/S0031182009991788) [DOI] [PubMed] [Google Scholar]
- 66.Cox FE. 2001. Concomitant infections, parasites and immune responses. Parasitology 122.S, S23–S38. ( 10.1017/S003118200001698X) [DOI] [PubMed] [Google Scholar]
- 67.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89. ( 10.1210/edrv.21.1.0389) [DOI] [PubMed] [Google Scholar]
- 68.Martin LB, Kidd L, Liebl AL, Coon CAC. 2011. Captivity induces hyper-inflammation in the house sparrow (Passer domesticus). J. Exp. Biol. 214, 2579–2585. ( 10.1242/jeb.057216) [DOI] [PubMed] [Google Scholar]
- 69.Calisi RM, Bentley GE. 2009. Lab and field experiments: are they the same animal? Horm. Behav. 56, 1–10. ( 10.1016/j.yhbeh.2009.02.010) [DOI] [PubMed] [Google Scholar]
- 70.Sopinka NM, Patterson LD, Redfern JC, Pleizier NK, Belanger CB, Midwood JD, Crossin GT, Cooke SJ. 2015. Manipulating glucocorticoids in wild animals: basic and applied perspectives. Conserv. Physiol. 3, 1–16. ( 10.1093/conphys/cov031.Introduction) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fusani L. 2008. Endocrinology in field studies: problems and solutions for the experimental design. Gen. Comp. Endocrinol. 157, 249–253. ( 10.1016/j.ygcen.2008.04.016) [DOI] [PubMed] [Google Scholar]
- 72.Schoenle LA, Moore IT, Dudek AM, Garcia EB, Mays M, Haussmann MF, Cimini D, Bonier F. 2019. Data from: Exogenous glucocorticoids amplify the costs of infection by reducing resistance and tolerance, but effects are mitigated by co-infection Dryad Digital Repository. (doi:10.5061.dryad.g9n8g17) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Schoenle LA, Moore IT, Dudek AM, Garcia EB, Mays M, Haussmann MF, Cimini D, Bonier F. 2019. Data from: Exogenous glucocorticoids amplify the costs of infection by reducing resistance and tolerance, but effects are mitigated by co-infection Dryad Digital Repository. (doi:10.5061.dryad.g9n8g17) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the supplementary material and are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.g9n8g17 [72].