Abstract
Several independent experiments suggest that cell walls of Bacillus subtilis are protonated during growth. When cells were grown in the presence of fluorescein-labeled dextran to saturate the cell walls, centrifuged, and suspended in PBS, fluorescence-activated cell sorter analyses revealed the bacteria were only poorly fluorescent. In contrast, when the bacteria were purged with N2 to dissipate protonmotive force (pmf) fluorescence became intense. Upon reconstitution of the pmf with phenazine methosulfate, glucose, and oxygen, fluorescence declined. Another approach used pH-dependent chemical modification of cell walls. The walls of respiring B. subtilis cells were amenable to carboxylate modification by [14C]ethanolamine and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide. The carbodiimide activation of carboxylate groups occurs only in acidic conditions. Upon dissipation of pmf the walls were refractory to chemical modification. Ammonium groups can be condensed with FITC in alkaline medium, but the condensation is very slow in acidic buffers. It was found that the derivatization of the walls with FITC could occur in the absence of pmf. The use of pH-dependent fluorophores and pH-dependent chemical modification reactions suggest that cell walls of respiring B. subtilis cells have a relatively low pH environment. This study shows a bacterium has a protonated compartment. Acidification of cell walls during growth may be one means of regulating autolytic enzymes.
The bacterium Bacillus subtilis is a Gram-positive spore-forming rod that has been used extensively in molecular biology and biotechnology. The bacterium has a relatively simple cell wall consisting of approximately equal amounts of peptidoglycan and teichoic acid. During a normal doubling, 50% of the wall is turned over into the culture medium each generation and is not reused for growth (1, 2). Turnover is restricted to the outermost cylindrical peptidoglycan, or wall that is at least one generation old (3, 4). The excision of the peripheral wall is brought about by the action of the autolysin N-acetyl-muramoyl-l-alanine amidase. All bacteria are thought to possess autolysins (5), yet surprisingly very little is known about the regulation of the enzymes.
It was observed by Jolliffe et al. (6) that any condition imposed on B. subtilis leading to dissipation of the protonmotive force (pmf) caused the bacteria to autolyze, suggesting the energized membrane was coupled to autolysin regulation. Agents, such as azide and carbonylcyanide-m-chlorophenylhydrazone, initiated autolysis of B. subtilis. In fact, any condition that prohibited carbon metabolism, including anaerobiosis and starvation, seemed to promote autolysis. Kemper et al. (7) speculated that during carbon utilization, secreted protons could neutralize the negative charges in the cell wall, resulting in a relatively low pH surrounding the protoplast. This acidic pH would prevent autolysin activity, thus providing a means for autolysin inhibition. By use of pH-sensitive probes and selective chemical modification of functional groups it is now shown that the cell wall of a bacterium is acidified during metabolism.
Materials and Methods
Bacterial Strains.
B. subtilis 168 (trp) and SR 22 (trp) were obtained from the Bacillus Genetic Stock Center, Ohio State University, Columbus, and maintained by regular transfer on Penassay agar.
Chemicals.
All salts were of reagent grade. FITC-labeled dextran (molecular weight 10,000) and FITC were purchased from Sigma.
Labeling Cells with FITC-Dextran.
B. subtilis 168 cells were grown overnight in Penassay broth with shaking at 37°C. Prewarmed medium (20 ml) containing 1.8 mg/ml of filter sterilized FITC-dextran was inoculated with 1.0 ml of overnight culture. The cells were grown to midexponential phase at 37°C in a gyratory shaker (200 rpm).
Fluorescence of FITC-Dextran-Labeled Cell Walls.
Two 5-ml samples of cells in the medium were placed in two glass tubes (13 × 100 mm) and maintained on ice. One tube was covered with parafilm, and nitrogen gas was purged through the suspension by using a Pasteur pipette. After 20 s, the pipette was removed, and the tube was sealed and placed on ice. For examination by flow cytometry, 1 ml of cells in growth medium was placed in a microfuge, washed and suspended in 0.5 ml of PBS (20 mM phosphate/150 mM NaCl/2 mg/ml glucose, pH 7.3) containing 1.8 mg/ml of unlabeled dextran (molecular weight 10,000) while all of the time preventing the introduction of oxygen. The cells were immediately subjected to fluorescence-activated cell sorter (FACS) analysis (Becton-Dickinson FACScan with a 15-mW argon laser tuned to 488 nm). To re-energize the cells, suspensions were washed one time and suspended in PBS-glucose containing 1.8 mg/ml unlabeled dextran and 400 μM phenazine methosulfate (6).
Covalent Coupling of FITC to the Cell Wall.
Cells grown in Penassay broth were harvested in exponential phase and washed once in PBS-glucose (pH 8) or sodium acetate-glucose (pH 5). The cells were suspended in PBS (pH 8) or acetate (pH 5) to an absorbance of 0.5. Cells were then treated for 10 min with either N2 gas or 1,3-dicyclohexylcarbodiimide (DCCD) (100 μM final concentration) to cause loss of pmf, after which FITC was added. The control, oxygenated cell suspensions were treated with FITC only. All cell suspensions were incubated for 30 min at 37°C, boiled for 2.5 min, and washed four times in PBS (pH 8.0) or acetate (pH 5). The cells were finally suspended in PBS (pH 8.0) or acetate (pH 5) and analyzed by FACScan. Samples in PBS at pH 8 were also viewed with direct fluorescence microscopy.
Modification of the Cell Wall by [14C]Ethanolamine Coupling.
Cells grown in Penassay broth were harvested at exponential phase and washed two times with PBS-glucose. The cells were suspended in PBS-glucose to an absorbance of 0.4 and divided into two portions. Sodium azide (40 mM final concentration) was added to one, whereas the same volume of PBS was added to the other. After 5 min, 1-ethyl-3-(3-dimethylamino-propyl) carbodiimide (EDAC) (50 mM final concentration) was added to each set of cells. [14C]ethanolamine (2.5 μ Ci) was added after EDAC activation for 5 min. Samples of 0.2 ml were taken every 30 s up to 5 min. The samples were filtered, washed, placed in scintillation vials, and counted. In some experiments the carbodiimide-nucleophile reaction was used to introduce nonradioactive glycine into the cell walls.
Incorporation of Glycinamide in the Cell Wall.
Cells were grown in Penassay broth in 3-liter flasks. The cells were harvested at midexponential phase and washed two times with PBS-glucose. The cells were suspended in PBS-glucose and divided into three samples. Under constant stirring, sodium azide (40 mM final concentration) was added to one sample, whereas the second and third sets remained untreated. After 5 min, EDAC was added to the azide-treated cells and one set of untreated cells. Glycinamide (50 mM final concentration) was then added to each of cells treated with the EDAC and samples were removed at 15 min. The cell walls were then prepared as described by Birdsell and Doyle (8) and freed of contaminants by hot SDS and hot distilled water. The cell wall samples were then subjected to amino acid analysis (Biomedical Research Service Laboratory, University of Kansas, Lawrence).
Results
FACs Analysis of FITC-Dextran-Labeled B. subtilis Cells.
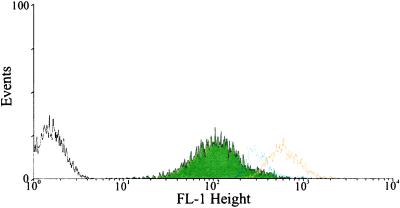
Demchick and Koch (9) determined that the rate of penetration of dextrans into the cell wall matrix of B. subtilis was a function of dextran molecular weight. Our strategy was to use a FITC-dextran as a reporter for acidic or alkaline conditions. Cells were thus grown in the presence of FITC-dextran (10,000 molecular weight), a size that could readily enter into the cell wall matrix. It was reasoned that the FITC-dextran would exhibit little fluorescence during cellular metabolism if the walls were acting as a reservoir for secreted protons. In contrast, cell walls saturated with FITC-dextran in cells lacking protonic potential should fluoresce because the wall pH will be that of the surrounding buffer. B. subtilis cells in PBS-glucose were labeled with FITC-dextran and then purged with N2 gas to dissipate the protonic potential. The resulting fluorescence intensities were then determined by using FACs. Cells with de-energized membranes exhibited significantly greater fluorescence compared with control cells (Fig. 1). Cell membranes can be re-energized with the addition of an artificial electron donor and a carbon source (6). pmf was restored with phenazine methosulfate and glucose in de-energized cells. Upon restoration of pmf the fluorescence intensity returned to the level of metabolizing cells (Fig. 1). In separate control experiments, when walls were removed from cells with lysozyme in 0.5 M mannitol, the resulting protoplasts lacked fluorescence, showing that the reporter group signals were restricted to the wall domain.
Figure 1.
Histograms of B. subtilis 168 cells using FACS. B. subtilis cells were labeled with FITC-dextran and purged with N2 gas, and the pmf was reconstituted with phenazine methosulfate, glucose, and oxygen. Black line, unlabeled cells; green fill, cells labeled with FITC-dextran; orange line, cells labeled with FITC-dextran and purged with N2 gas; blue line, cells labeled with FITC-dextran, purged with N2 gas with the pmf reconstituted.
Covalent Coupling of FITC to the Cell Wall.
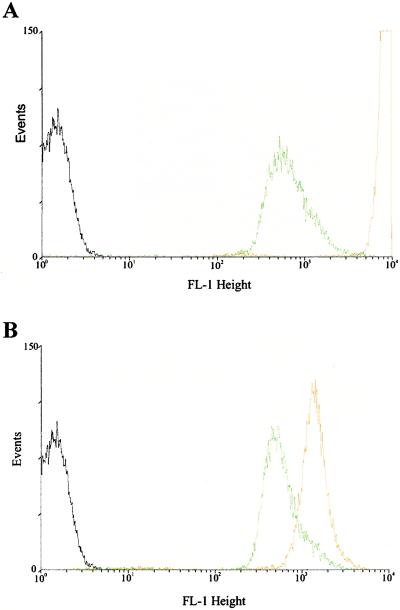
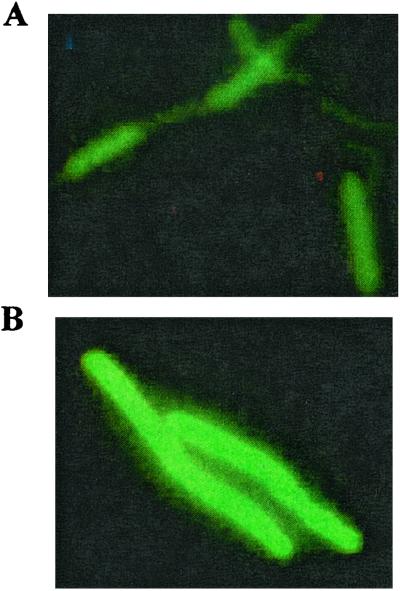
Derivatization of FITC to proteins occurs more readily at an alkaline pH, such as pH 8 or 9. In the cell wall of B. subtilis, a free amino group provides the only site for derivatization with FITC; this reaction will not proceed if the wall is protonated. B. subtilis cells were treated with DCCD, a reagent that dissipates pmf. Both de-energized and energized cells in PBS (pH 8) were derivatized with FITC, washed, and analyzed with FACs (Fig. 2). The fluorescence intensity of the de-energized cells was markedly greater than the fluorescence intensity of energized cells. This finding suggests that upon treatment with DCCD the cell wall protons are titrated away and the wall takes on the pH of its environment. FITC more readily coupled to the de-energized cells, a finding expected if the walls of the energized cells possessed a low pH. The differences in intensity between metabolizing and de-energized cells were not as pronounced when the cells were in PBS at pH 5. Direct fluorescence microscopy of FITC-coupled B. subtilis cells subjected to N2 gas exhibited significantly greater fluorescence intensities than did the corresponding control cells (Fig. 3).
Figure 2.
Coupling of FITC to B. subtilis 168 in the presence and absence of an energy poison. Cells in PBS-glucose buffer at pH 8.0 (A). Cells in acetate-glucose buffer at pH 5.0 (B). Energy poison was 100 μM DCCD. Black line, untreated control cells; green line, cells treated with FITC; orange line, cells subjected to DCCD and treated with FITC.
Figure 3.
Fluorescence micrographs of B. subtilis 168 cells derivatized with FITC. Cells PBS (pH 8) treated with FITC (50 μg/3 ml of cells) (A). Cells in PBS purged with N2 gas and then treated with FITC (50 μg/3 ml of cells) (B).
Chemical Modification of the Cell Wall.
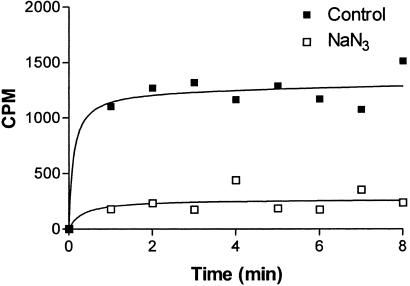
The primary functional groups of the B. subtilis cell wall are the carboxylate, ammonium and phosphate (teichoic acid). Chemical modification of the cell wall is possible at the carboxylate and free amino groups (10). EDAC, a water-soluble carbodiimide, activates the carboxylate groups in the wall and will promote covalent bond formation between nucleophiles and the activated carboxylate function. This reaction will proceed only in an acidic environment as it requires protonation of the carbodiimide (11, 12). B. subtilis cells were de-energized with sodium azide. Both de-energized and energized cells in PBS at pH 7.3 were activated with EDAC and then treated with the nucleophile [14C]ethanolamine. In some experiments nonradioactive glycine was introduced into the cell walls by use of EDAC plus glycinamide. Cells with an intact pmf (control cells) consistently exhibited more radioactivity or higher amounts of glycine than the de-energized cells (Fig. 4, Table 1).
Figure 4.
Modification of B. subtilis 168 by ethanolamine and EDAC. Cells in PBS-glucose buffer (pH 7.3) were mixed with EDAC and [14C]ethanolamine at room temperature in the presence and absence of 40 mM sodium azide. At intervals, samples were removed, rapidly filtered, washed, and counted by liquid scintillation. The results shown represent the average of three separate trials. The curves were generated by graphpad software.
Table 1.
Incorporation of glycine in the cell wall of B. subtilis 168
| Wall composition | Untreated wall | EDAC + glycinamide | Azide + EDAC + glycinamide |
|---|---|---|---|
| Glutamic acid | 0.18 | 0.19 | 0.16 |
| Alanine | 0.52 | 0.50 | 0.43 |
| Diaminopimelic acid | 0.38 | 0.58 | 0.47 |
| Glycine | 0.007 | 0.12 | 0.06 |
Suspensions of cells in PBS-glucose (pH 7.3) were activated with EDAC, and 100 mM glycinamide was added. After 30 min the cells were centrifuged, washed two times in water, and broken by sonication. Walls were obtained by differential centrifugation and extracted in boiling 0.1% SDS. The insoluble walls were then extracted several times in hot water and freeze-dried. The walls were then hydrolyzed in acid and subjected to amino acid analysis. Azide concentration was 40 mM. The numbers shown above are in μmol/mg of wall. Glutamic acid, alanine, and diaminopimelic acid are shown for comparison purposes.
Discussion
B. subtilis has a typical Gram-positive cell surface. The peptidoglycan-teichoic acid wall has a diameter of ≈35 nm (13). The very periphery of the cell wall of B. subtilis is slightly serrated, whereas the wall facing the cytoplasmic membrane is smooth (14). The wall can be made to expand or contract depending on the pH and ionic composition of the growth media (15). Results are presented suggesting that actively metabolizing cells have a wall environment distinct from a wall environment of cells lacking metabolism. Metabolizing B. subtilis cells labeled with FITC-dextran consistently exhibited a lower fluorescence intensity than de-energized B. subtilis cells. Furthermore, fluorescence of de-energized B. subtilis cells approached that of metabolizing cells upon the addition of phenazine methosulfate, an agent capable of restoring the pmf. The consistently low fluorescence intensity of FITC-dextran observed for metabolizing B. subtilis cells strongly suggests the wall environment is acidic.
Chemical modification was used as an alternative approach of probing the cell wall pH. Coupling of FITC to ammonium groups was used to indirectly probe cell surface acidity. The fluorescence intensity of B. subtilis cells derivatized with FITC was always greater when the cells were incapable of metabolism. The covalent coupling of FITC to the cell surface is therefore a function of the metabolic state of the bacteria. The coupling would be expected to be minimal for cells actively metabolizing, whereas cells with a dissipated pmf would have a pH equivalent to that of the buffer and should be readily amenable to coupling with FITC. These results were observed with not only N2-purged bacteria but also DCCD-treated cells, indicating the method of pmf dissipation is unimportant. Coupling of [14C]ethanolamine or glycinamide to cell wall carboxylate groups occurred to a greater extent when the cells were metabolizing before the addition of carbodiimide. Cells that were metabolically poisoned contained significantly less radioactivity and glycine (Fig. 4, Table 1), findings expected if the cell surface pH was relatively low.
The composite interpretation of this study strongly suggests that dissipation of the pmf of B. subtilis cells results in loss of a local low pH in the cell wall. The loss of a local low pH in the wall may permit enzymes such as autolysins to become active. Prior studies in B. subtilis have linked modulation of the cell wall enzymes to pmf (6, 7). Recently, Gründling et al. (16) have provided a mechanism to account for holin-induced lysis in bacteria. Holins are bacteriophage-encoded proteins, which have the ability to induce lysis in bacteria. Gründling et al. suggested holins become integral membrane components, reaching a critical threshold needed to initiate lysis. Once this threshold concentration of holins has been achieved at discrete sites in the cytoplasmic membrane pmf is dissipated and lysis ensues.
In B. subtilis, cell surface elongation occurs by an inside-to-outside growth mechanism. As cell wall facing the cytoplasmic membrane is added to the inner wall face older, pre-existing wall is “pushed” to the cell periphery (3, 17). The older peripheral wall is susceptible to autolysins and is turned over at the rate of 50% per generation (17). The wall near the cytoplasmic membrane is resistant to turnover during growth, but becomes susceptible to autolysis when pmf is dissipated. Actively metabolizing cells therefore may regulate their autolysins by creating a compartment (wall) with a pH low enough to prevent enzyme function. This work shows a bacterium may generate a compartment with a pH distinct from any other part of the cell. Although this research has been restricted to B. subtilis, it is likely that other microorganisms, including yeasts, may have cell wall compartments with relatively low pHs.
Acknowledgments
We thank Dr. Sam Wellhausen for performing the FACs analyses. The work was supported in part by the RJD Research Fund.
Abbreviations
- FACS
fluorescence-activated cell sorter
- DCCD
1,3-dicyclohexylcarbodiimide
- EDAC
1-ethyl-3-(3-dimethylamino-propyl) carbodiimide
- pmf
protonmotive force
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Koch A L. J Theor Biol. 1986;120:73–84. doi: 10.1016/s0022-5193(86)80018-2. [DOI] [PubMed] [Google Scholar]
- 2.Doyle R J, Chaloupka J, Vinter V. Microbiol Rev. 1988;52:554–567. doi: 10.1128/mr.52.4.554-567.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mobley H L, Koch A L, Doyle R J, Streips U N. J Bacteriol. 1984;158:169–179. doi: 10.1128/jb.158.1.169-179.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pooley H M. J Bacteriol. 1976;125:1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shockman G D, Holtje J-V. In: Bacterial Cell Wall. Ghuysen J-M, Hakenbeck R, editors. Amsterdam: Elsevier; 1994. pp. 131–166. [Google Scholar]
- 6.Jolliffe L K, Doyle R J, Streips U N. Cell. 1981;25:753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- 7.Kemper M A, Urrutia M M, Beveridge T J, Koch A L, Doyle R J. J Bacteriol. 1993;175:5690–5696. doi: 10.1128/jb.175.17.5690-5696.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birdsell D C, Doyle R J. J Bacteriol. 1973;113:198–202. doi: 10.1128/jb.113.1.198-202.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demchick P, Koch A L. J Bacteriol. 1996;178:768–773. doi: 10.1128/jb.178.3.768-773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle R J, Matthews T H, Streips U N. J Bacteriol. 1980;143:471–480. doi: 10.1128/jb.143.1.471-480.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunkel G R, Mehrabian M, Martinson H G. Mol Cell Biochem. 1981;34:3–13. doi: 10.1007/BF02354846. [DOI] [PubMed] [Google Scholar]
- 12.Hoare D G, Koshland D E., Jr J Biol Chem. 1967;242:2447–2453. [PubMed] [Google Scholar]
- 13.Graham L L, Beveridge T J. J Bacteriol. 1994;176:1413–1421. doi: 10.1128/jb.176.5.1413-1421.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonnenfeld E M, Beveridge T J, Koch A L, Doyle R J. J Bacteriol. 1985;163:1167–1171. doi: 10.1128/jb.163.3.1167-1171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle R J, Marquis R E. Trends Microbiol. 1994;2:57–60. doi: 10.1016/0966-842x(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 16.Gründling A, Manson M D, Young R. Proc Natl Acad Sci USA. 2001;98:9348–9352. doi: 10.1073/pnas.151247598. . (First Published July 17, 2001; 10.1073/pnas.151247598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch A L, Doyle R J. J Theor Biol. 1985;117:137–157. doi: 10.1016/s0022-5193(85)80169-7. [DOI] [PubMed] [Google Scholar]