Abstract
Purpose
Our previous study proved that FOXM1 regulates colorectal cancer (CRC) cell metastasis through epithelial–mesenchymal transition program. The aim of this study is to further explore the underlying mechanism of FOXM1 in CRC.
Materials and methods
In this study, we detected the mRNA and protein expressions of FOXM1 and β-catenin in CRC tissues and their corresponding normal-appearing tissues (NATs) by quantitative reverse transcription-PCR and western blot analysis, respectively. Then the potential link between FOXM1 and β-catenin in CRC tissues was analyzed. Furthermore, we systematically analyzed the biological functions of FOXM1 in CRC cells after reconstitution of FOXM1 expression in vitro. Moreover, the mechanism of FOXM1-promoted CRC progression by improving β-catenin nuclear translocation was also discussed.
Results
Our data demonstrated that FOXM1 and β-catenin were upregulated in CRC tissues compared with the corresponding NATs (P<0.05). Clinicopathologic analysis revealed that increased FOXM1 (or β-catenin) expression positively correlated with some clinicopathologic features, such as tumor size, TNM stage, lymphatic metastasis, and distant metastasis (P<0.05). Meanwhile, the possible relationships between FOXM1 and β-catenin in CRC samples were evaluated using SPSS software, and a significant positive correlation was found (P<0.05). In vitro data demonstrate that elevated FOXM1 expression exerted oncogenic effects on CRC via activation of β-catenin signaling pathway. The inhibition of β-catenin by siRNAs significantly attenuates FOXM1-induced malignant activities.
Conclusion
The data suggested that FOXM1/β-catenin is critical for malignancy of CRC, which may constitute a potential therapeutic strategy for CRC.
Keywords: FOXM1, β-catenin, colorectal cancer, signaling pathway
Introduction
Colorectal cancer (CRC) is the third most common cancer and the fourth leading cause of cancer-related deaths in broad areas of the world, accounting for nearly 1.2 million new patients and 600,000 deaths/year.1 Despite many improvements in early detection, neoadjuvant therapy, and surgical treatment, metastatic CRC still has a poor prognosis, and 5-year survival rate is not exceeding 12%.2 Therefore, identification of new prognostic factors to improve therapeutic approaches of CRC is imperative.
FOXM1, which belongs to a winged-helix transcription factor family, is a typical proliferation-associated transcription factor. FOXM1 has been proved to control both G1/S and G2/M phase progression by transcriptional regulation of a set of genes that are essential for cell cycle progression, such as p21Cip1, p27Kip1 proteins, Cdc25A, and Cdc25B.3,4 Emerging evidence demonstrated that FOXM1 was aberrantly overexpressed in multiple types of malignancies, which suggested that FOXM1 might play a critical role in tumor initiation.5–8 Additionally, FOXM1 promoted tumor metastasis in various types of tumors, such as pancreatic cancer, CRC, lung cancer, ovarian cancer, and glioma.7–11 For example, in 2007, Dai et al reported that FOXM1 contributed to glioma progression by enhancing MMP-2 gene transcription.12 Li et al and Zhang et al found that FOXM1 directly and significantly correlated with transactivation of vascular endothelial growth factor (VEGF) expression and promoted the angiogenic ability of gastric cancer and glioma cells.13,14 A study by Yang et al in 2015 showed that knockdown of FOXM1 regulated CRC metastasis through reversing the acquisition of epithelial– mesenchymal transition phenotype.15 Furthermore, FOXM1 overexpression was considered as a molecular marker that predicted increased invasive/metastatic potential of CRC and a poorer prognosis.16
Wnt signaling pathway is commonly considered to play an important role in the carcinogenic process, especially in CRC. Approximately 90% of CRCs have an activating mutation of the canonical Wnt signaling pathway, ultimately leading to the nuclear accumulation of β-catenin through inactivating APC mutations or activating β-catenin mutations, which subsequently combines with T-cell factor (TCF) and lymphoid enhancing factor transcription factors to activate downstream gene transcription, such as c-Myc and cyclin D1.17,18 Previously, many studies revealed that the expression of β-catenin was regulated by FOXM1.33,34 FOXM1 was identified as a new downstream target of Wnt signaling, which was essential for β-catenin/TCF4 transactivation. In glioma, FOXM1 interacted with β-catenin and promoted the nuclear accumulation of β-catenin to induce glioma stem cell self-renewal and tumorigenesis through controlling Wnt target-genes expression. Yoshida et al used FOXM1B transgenic mice and conditional FOXM1 knockout mice to examine the role of FOXM1B in colon cancer development and proliferation, and found that specific deletion of FOXM1 inhibited colorectal tumorigenesis and potential regulation of β-catenin/TCF4 signaling.19 Though there have been many studies to investigate the role of FOXM1 and β-catenin independently, the interaction of FOXM1 and β-catenin on CRC progression in vitro has still not been studied.
In this study, we first detected the mRNA and protein expressions of FOXM1 and β-catenin in 124 CRC tissues and their corresponding normal-appearing tissues (NATs) by quantitative reverse transcription-PCR (qRT-PCR) and western blot analysis, respectively. Then the potential link between FOXM1 and β-catenin in CRC tissues was analyzed. Furthermore, we systematically analyzed the biological functions of FOXM1 in CRC cells after reconstitution of FOXM1 expression in vitro. Moreover, the mechanism of FOXM1-promoted CRC progression by improving β-catenin nuclear translocation was also discussed. Our new findings advanced our understanding of FOXM1-mediated oncogenic mechanisms of CRC.
Materials and methods
Cell lines and tumor samples
Human CRC cell lines (HCT116 and DLD-1) were purchased from the Cell Resource Center, Chinese Academy of Sciences Committee (Shanghai, China) and maintained in PRIM-1640 supplemented with 10% fetal bovine serum (Sijiqing Biological Engineering Materials Co. Ltd., Hangzhou, China) at 37°C in a humidified atmosphere containing 5% CO2. Human CRC tissues and their corresponding NATs (at least 2 cm distant from the tumor site) were obtained from 124 patients at the Department of General Surgery, the First Affiliated Hospital of Soochow University from October 2008 to October 2016. All patients underwent surgery and had a clear histological diagnosis. None of the patients had received radiotherapy or chemotherapy before surgery. The study was approved by Institute Research Ethics Committee of the First Affiliated Hospital of Soochow University and written informed consent was obtained from all patients prior to the study.
Cell transfection
To obtain FOXM1 overexpressing cells, we transfected pcDNA3.1-FOXM1 or control vector pcDNA3.1 plasmids into DLD-1 cells with the Lipofectamine® 2000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions.11 To silence FOXM1 expression, FOXM1 siRNA oligonucleotides (sense: UGGUUAAUAAUCUUGAUCCCA and antisense: GGAUCAAGAUUAUUAACCACC) were designed, syn-thesized, and transfected into HCT116 cells. Additionally, RNA interference targeting β-catenin (sense: UUACA-ACUGCAUGUUUCAGCA and antisense: CUGAAACAU-GCAGUUGUAAAC) were also synthesized by Shanghai Gene Pharma Co., Ltd. (Shanghai, China) and transfected into FOXM1 overexpressing DLD-1 cells. After 48 hours transfection, cells were collected and the transfection efficiencies of overexpressing plasmids or siRNAs were detected by western blot and qRT-PCR.
qRT-PCR
Total RNA from cultured cells or CRC tissues was extracted using TRIzol™ Reagent (Thermo Fisher Scientific). cDNA was synthesized from 2 µg of RNA using the the RevertAid™ First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). The PCR analysis was performed using PowerUp™ SYBR® Green PCR Master Mix (Thermo Fisher Scientific) on the 7,500 real-time PCR system (Thermo Fisher Scientific). The sequences of the PCR primers were listed as follows: FOXM1 5′-GGAGGAAATGCCACACTTAGCG-3′ (sense) and 5′-TAGGACTTCTTGGGTCTTGGGGTG-3′ (antisense); β-actin 5′-CCACACTGTGCCCATCTACG-3′ (sense) and 5′-AGGATCTTCATGAGGTAGTCAGTCAG-3′ (antisense); β-catenin 5′-AAA GCGGCTGTTAGTCACTGG-3′ (sense) and 5′-GACTTG GGAGGTATCCACATCC-3′ (antisense); cyclin D1 5′-TGAAGCCAGCTCACAGTGCT-3′ (sense) and 5′-AGCCAGGATGGTTGAGGTAA-3′ (antisense); c-Myc 5′-CTTGAACAGCTACGGAACTC-3′ (sense) and 5′-GAGGCAGTTTACATTATGGC-3′ (antisense); TCF-4 5′-GCTGAGCTGCCCAGGAATAT-3′ (sense) and 5′-GCAGAGGCCTGAGTAATTATCAGAA-3′ (antisense). β-actin was used as a reference to obtain the relative fold change for the targets using the 2−ΔΔCt method. Each sample was tested in triplicate and each experiment was performed at least three times.
Western blot analysis
Tumor tissues or cells were washed with ice-cold PBS and lysed in ice-cold radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Nantong, China) to obtain whole protein extracts. The nuclear protein lysates were prepared with a NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific) according to the manufacturer’s instructions. Proteins in the lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Membranes were incubated with primary antibodies: polyclonal rabbit antibody against human FOXM1 (1:1000; Abcam, Cambridge, UK), rabbit anti-Cyclin D1 (1:1000; Sigma-Aldrich Co., St Louis, MO, USA), rabbit antic-Myc (1:1000; Sigma-Aldrich Co.), rabbit anti-TCF-4 (1:500; Sigma-Aldrich Co.), rabbit anti-Lamin B1 (1:1000; Abcam), and rabbit anti-β-actin (1:1000; Beyotime Institute of Biotechnology). The signals from the primary antibody were amplified by incubating with horse radish peroxidase conjugated anti-rabbit IgG (1:1000; Beyotime Institute of Biotechnology) and detected with Amersham™ ECL™ Western Blotting Detection Reagents (GE Healthcare, Boston, MA, USA).
Cell proliferation and colony formation assay
Cell proliferation was determined by MTT assay. After transfection with overexpression plasmids or siRNAs for 24 hours, cells were digested, resuspended, and seeded at a density of 4×103 cells/well in 96-well culture plates at 37°C in 5% CO2. Then cells were incubated with 20 µL MTT solution (5 mg/mL) at 37°C for 4 hours. Formazan crystals were dissolved with dimethylsulfoxide and the absorbance was measured at 450 nm using a microplate reader at 24, 48, and 72 hours. Each sample was four replicate wells and the experiment was repeated at least three times. For colony formation assay, cells were digested to a single-cell suspension by trypsin. Then cells were plated into 6-well plates at a density of 2×103 cells/well. After 2 weeks of incubation in complete culture medium at 37°C in 5% CO2, the colonies were fixed and stained with 0.1% crystal violet. Finally, the colonies containing at least 50 cells were counted.
Migration and invasion assays
Transwell assays were used to detect the cell migration and invasion ability. This experiment was performed by using the transwell chambers that equipped with a pore size of 8μm (Corning Systems, Inc., San Jose,CA, USA) which were pre-coated with or without Matrigel (BD Biosciences, San Jose, CA, USA). Briefly, cells were digested, resuspended, and seeded into the upper wells at a density of 2×104 cells/well, while medium containing 10% FBS was placed in the lower wells as a chemoattractant. After 24 hours incubation at 37°C, cells on the upper side of the insert filter were completely removed by wiping with a cotton swab and cells on the bottom surface of filters were fixed in methanol, then stained with 0.1% crystal violet. The migrated or invaded cells were counted microscopically in five randomly selected fields at 200× magnification.
Statistical analyses
All statistical analyses were performed with SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). The association between FOXM1 (or β-catenin) expression and clinicopathologic characteristics were analyzed using χ2-test or Fisher’s exact test. Spearman test was used for analyzing the correlation. Significant differences between the groups were determined using the student’s t-test. P<0.05 was considered statistically significant. Values for all measurements were expressed as the mean ± SD with at least three independent experiments.
Results
FOXM1 and β-catenin expressions levels were upregulated in CRC tissues and associated with clinicopathologic parameters of CRC
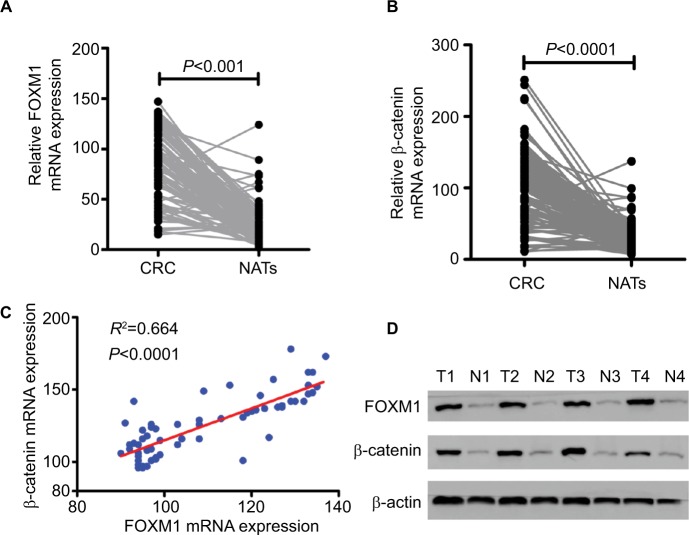
First, the mRNA expressions levels of FOXM1 and β-catenin in 124 CRC tissues and their corresponding NATs were determined by qRT-PCR. As shown in Table 1, our results showed that FOXM1 or β-catenin mRNA levels were overexpressed in 62.9% (78/124) or 67.7% (84/124) of CRC tissues, respectively, compared to those in adjacent nontumorous tissues (FOXM1 37.9% [47/124] or β-catenin 34.7% [43/124]) (P<0.05). Meanwhile, the results of Figure 1A and 1B also demonstrated that the mRNA expressions levels of FOXM1 or β-catenin in CRC tissues were significantly higher than those in corresponding NATs (P<0.05). Furthermore, we chose four CRC tissues and detected the protein expressions levels of FOXM1 and β-catenin. Our western blot analysis revealed that protein levels of FOXM1 and β-catenin were markedly upregulated in tumor samples compared to normal tissues (Figure 1D). These results indicate that FOXM1 and β-catenin might be involved in human colorectal carcinogenesis.
Table 1.
The expression of FOXM1 and β-catenin in 124 CRCs and NATs
| FOXM1 expression
|
χ2 | P-value | β-catenin expression
|
χ2 | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Low (%) | High (%) | Low (%) | High (%) | |||||
|
| ||||||||
| CRCs | 46 (37.1) | 78 (62.9) | 15.51 | <0.001 | 40 (32.3) | 84 (67.7) | 27.89 | <0.001 |
| NATs | 77 (62.1) | 47 (37.9) | 81 (65.3) | 43 (34.7) | ||||
Abbreviations: CRCs, colorectal cancers; NATs, normal-appearing tissues.
Figure 1.
FOXM1 mRNA expression is upregulated in human CRC tissues and positively correlated with β-catenin expression.
Notes: (A and B) The relative mRNA expression levels of FOXM1and β-catenin were determined by qRT-PCR and normalized against β-actin control. Paired two-sample t-tests were used to compare the mean value for each gene between the tumor tissues and corresponding NATs. P<0.001 or P<0.0001 was considered significant. (C) A significant positive correlation was found between FOXM1 and β-catenin mRNA expression in colorectal tumor tissues (R2=0.664, P<0.0001). (D) Protein expression of FOXM1 and β-catenin was determined by western blot analysis in colorectal tumor (T) and corresponding NATs (N) from four individual patients. β-actin served as an internal control.
Abbreviations: CRC, colorectal cancer; NATs, normal-appearing tissues; qRT-PCR, quantitative RT-PCR.
To further explore the clinical roles of FOXM1 and β-catenin in CRC, we analyzed the potential relationships between FOXM1 (or β-catenin) expression and clinicopathologic parameters in CRC patients. As shown in Table 2, we observed that increased FOXM1 expression positively correlated with the tumor size (P=0.015), lymphatic metastasis (P=0.004), and distant metastasis (P=0.012). However, FOXM1 expression did not correlate to the age, gender, tumor location, tumor differentiation, and TNM stage of CRC patients (all P>0.05). Similar phenomena were also observed in β-catenin expression and our data implied that the high mRNA level of β-catenin was significantly associated with these clinicopathologic parameters, including the tumor size (P=0.011), TNM stage (P=0.003), lymphatic metastasis (P=0.006), and distant metastasis (P<0.0001).
Table 2.
Correlation between the expression of FOXM1 and β-catenin with clinicopathological parameters in colorectal carcinomas
| Parameters | Total | FOXM1 expression
|
P-value | β-catenin expression
|
P-value | ||
|---|---|---|---|---|---|---|---|
| Low | High | Low | High | ||||
|
|
|
||||||
| n=124 | n=46 | n=78 | n=40 | n=84 | |||
|
| |||||||
| Age (years) | 0.535 | 0.320 | |||||
| <60 | 42 | 14 | 28 | 16 | 26 | ||
| ≥60 | 82 | 32 | 50 | 24 | 58 | ||
| Sex | 0.432 | 0.339 | |||||
| Male | 51 | 21 | 30 | 14 | 37 | ||
| Female | 73 | 25 | 48 | 26 | 47 | ||
| Tumor location | 0.632 | 0.239 | |||||
| Proximal colon | 32 | 13 | 19 | 13 | 19 | ||
| Distal colon and rectum | 92 | 33 | 59 | 27 | 65 | ||
| Tumor size | 0.015* | 0.011* | |||||
| <5 cm | 26 | 15 | 11 | 13 | 11 | ||
| ≥5 cm | 98 | 31 | 67 | 27 | 73 | ||
| Differentiation | 0.356 | 0.198 | |||||
| Well, moderately | 87 | 30 | 57 | 25 | 62 | ||
| Poorly | 37 | 16 | 21 | 15 | 22 | ||
| TNM stage | 0.223 | 0.003* | |||||
| I/II | 48 | 21 | 27 | 23 | 25 | ||
| III/IV | 76 | 25 | 51 | 17 | 59 | ||
| Lymphatic metastasis | 0.004* | 0.006* | |||||
| Yes | 56 | 13 | 43 | 11 | 45 | ||
| No | 68 | 33 | 35 | 29 | 39 | ||
| Distant metastasis | 0.012* | <0.0001* | |||||
| Yes | 64 | 17 | 47 | 3 | 61 | ||
| No | 60 | 29 | 31 | 37 | 23 | ||
Note:
P<0.05.
FOXM1 positively correlated with β-catenin in CRC tissues
The possible relationships between FOXM1 and β-catenin in 124 CRC samples were evaluated using SPSS software and a significant positive correlation between FOXM1 and β-catenin mRNA expressions was found (R2=0.664, P<0.0001) in CRC patients (Figure 1C, Table 3).
Table 3.
The relationship of FOXM1 and β-catenin in colorectal carcinomas
| FOXM1 expression
|
R | P-value | ||
|---|---|---|---|---|
| Low | High | |||
|
| ||||
| β-catenin expression | ||||
| Low | 25 | 15 | 0.664 | P<0.0001 |
| High | 21 | 63 | ||
FOXM1 promoted the tumorigenesis of CRC in vitro
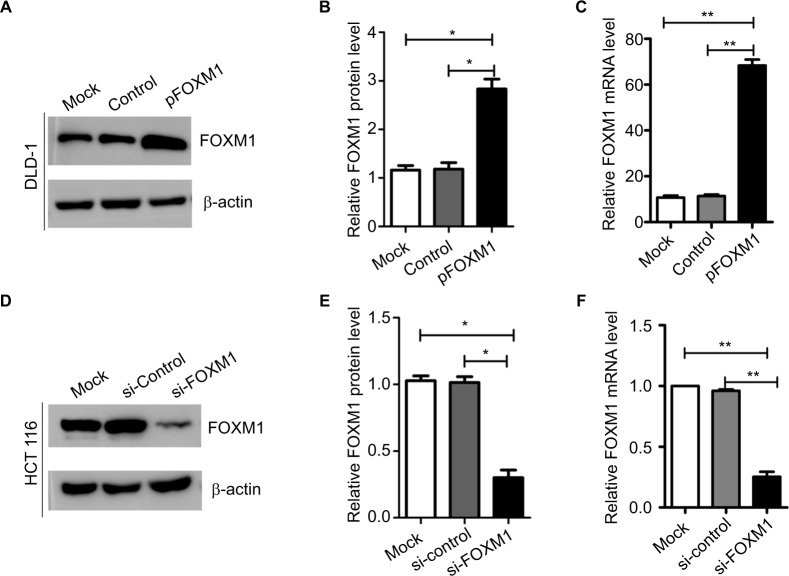
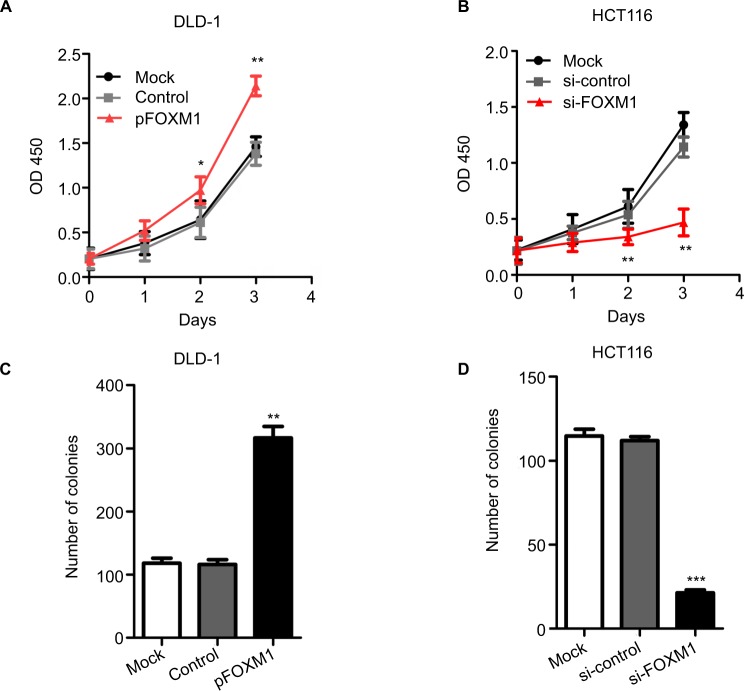
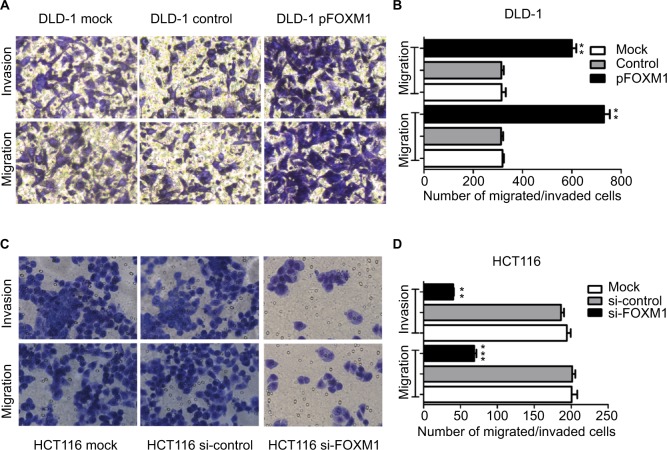
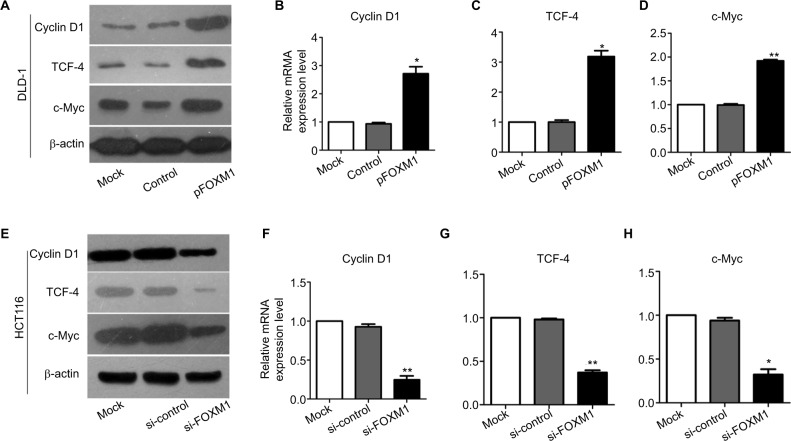
To explore the effects of FOXM1 on CRC progression in vitro, we obtained FOXM1 upregulating and downregulating cells for our subsequent experiments in vitro. When pcDNA3.1-FOXM1 plasmid or FOXM1-siRNA oligonucleotides were successfully transfected into DLD-1 or HCT116 cells, respectively, our results from western blot and PCR analysis confirmed the transfection efficiencies of FOXM1 plasmids or siRNAs (P<0.05, Figure 2A–F). The results of MTT and colony formation assay showed that FOXM1 enhanced the abilities of cell proliferation and colony formation in DLD-1 cells, whereas silencing FOXM1 expression in HCT116 cells significantly inhibited the cell proliferation and colony formation compared to the control group (P<0.05, Figure 3A–D). Meanwhile, the abilities of metastasis were evaluated by cell migration and invasion assay. Our data demonstrated that upregulation of FOXM1 significantly increased the abilities of cell migration and invasion in DLD-1 cells, whereas FOXM1 knocking down attenuated the migration and invasion capacities of HCT116 cells (P<0.05, Figure 4A–D). Taken together, these results suggested that FOXM1 could promote CRC growth and progression in vitro.
Figure 2.
Expression of FOXM1 protein and mRNA in colon cancer cells after transfection with pFOXM1 or si-FOXM1.
Notes: (A) DLD-1 cells were transfected with either untransfected (mock), pcDNA3.1 (control), or pFOXM1 plasmids. Western blots demonstrated that FOXM1 protein levels were significantly increased after transfection with pFOXM1. (B) The relative protein folds in DLD-1 after transfection with pFOXM1, *P<0.05. (C) Relative FOXM1 mRNA expression levels of DLD-1 cells were upregulated markedly after transfection with pFOXM1, **P<0.01. (D) HCT116 cells were transfected with either untransfected (mock), FOXM1 si-control, or si-FOXM1. Western blots demonstrated that FOXM1 protein levels were significantly reduced after transfection with si-FOXM1. (E) The relative protein folds in HCT116 after transfection with si-FOXM1. (F) Relative FOXM1 mRNA expression levels of HCT116 cells were decreased after transfection with FOXM1 siRNA which were quantified by real-time PCR (**P<0.01). β-actin was evaluated as an internal control. Data are presented as mean ± SD for at least three independent experiments. Statistical analysis was done using Student’s t-tests.
Abbreviations: pFOXM1, pcDNA3.1-FOXM1; si-control, control-siRNA; si-FOXM1, FOXM1 siRNA.
Figure 3.
The proliferation and clonogencity of colon cancer cells after transfection with pFOXM1 or si-FOXM1.
Notes: (A and C) The results from MTT and colony formation assays show that upregulated FOXM1 expression by transfection with pFOXM1 significantly increased the cell proliferation and clonogencity of DLD-1 cells compared to those of control-transfected cells (*P<0.05, **P<0.01). (B and D) Conversely, downregulation of FOXM1 attenuated the proliferation and clonogencity of HCT116 cells after transfection with si-FOXM1 (**P<0.01, ***P<0.001). Data are shown as mean ± SD. Experiments were repeated three times.
Abbreviations: pFOXM1, pcDNA3.1-FOXM1; si-control, control-siRNA; si-FOXM1, FOXM1 siRNA.
Figure 4.
Impact of altered FOXM1 expression on colon cancer cell migration and invasion in vitro. DLD-1 and HCT116 cells were transfected with pFOXM1 and si-FOXM1, respectively.
Notes: (A) DLD-1 and (C) HCT116 cells migration and invasion were detected by the Transwell assays and were determined as described in Materials and Methods. Representative migrated or invaded colon cancer cells were photographed. (B and D) Statistical graphs show the relative number of migrated and invaded cells. Upregulated FOXM1 expression significantly promoted migration and invasion of DLD-1 cells. However, downregulated FOXM1 expression markedly suppressed the migration and invasion capacity of HCT116 cells. Data are shown as the mean ± SD. **P<0.01, ***P<0.001, compared with controls. Scale bar 200μm.
Abbreviations: pFOXM1, pcDNA3.1-FOXM1; si-control, control-siRNA; si-FOXM1, FOXM1 siRNA.
FOXM1 provoked the β-catenin signaling pathway to promote CRC progression
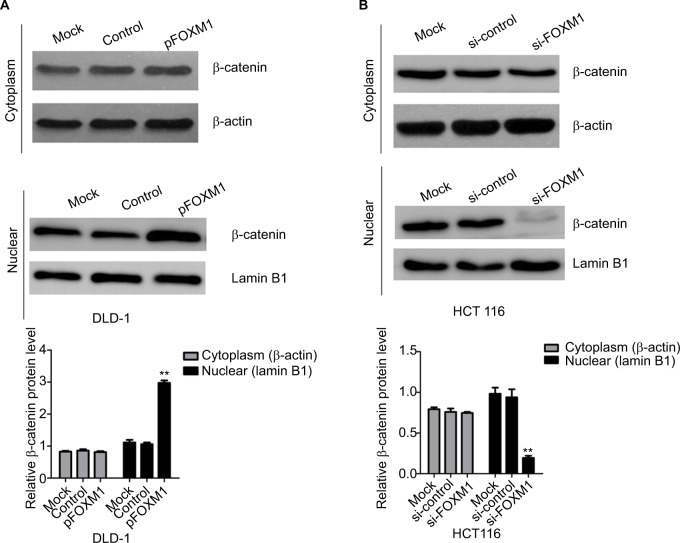
Our previous clinical data implied that FOXM1 positively correlated with β-catenin in CRC. To uncover the underlying mechanism of FOXM1-mediated oncogenic activity, we examined the effect of FOXM1 on the activation of β-catenin signaling. First, we examined the protein and mRNA levels of cyclin D1, c-Myc, and TCF-4, which are critical downstream targets of β-catenin signaling pathway when FOXM1 was restored or silenced in CRC cells. Our results showed that FOXM1 overexpression gave rise to an increase of cyclin D1, TCF-4, and c-Myc in DLD-1 cells in both protein and mRNA levels (Figure 5A–D) but were inhibited by FOXM1 siRNAs in HCT116 cells (Figure 5E–H). We detected the expression of FOXM1 and β-catenin in both the cytoplasm and the nucleus by western blots. Interestingly, the results showed that the nuclear protein level of β-catenin was increased by FOXM1 overexpression in DLD-1 cells but decreased in HCT116 cells with FOXM1 knockdown, whereas the cytoplasmic level was not changed (Figure 6A, B). These data indicate that FOXM1 triggers β-catenin signaling pathway potential by improving nuclear translocation of β-catenin in colon cancer cells.
Figure 5.
Effects of FOXM1 on the expressions of β-catenin target genes in CRC cells.
Notes: The protein and mRNA levels of β-catenin target genes, such as cyclin D1, TCF-4 and c-Myc, were measured using western blots and qRT-PCR analysis. (A–D) The mRNA expression of cyclin D1, TCF-4, and c-Myc was found to be increased significantly in FOXM1-overexpression DLD-1 cells compared to mock and control transfectants. *P<0.05, **P<0.01. (E–H) FOXM1 knocking down obviously downregulated the cyclin D1, TCF-4, and c-Myc mRNA expression levels in HCT116 cells after tranfection with si-FOXM1. **P<0.01, *P<0.05. All the results were measured at least three times.
Abbreviations: qRT-PCR, quantitative RT-PCR; si-control, control-siRNA; si-FOXM1, FOXM1 siRNA; TCF-4, T-cell factor-4; pFOXM1, pcDNA3.1-FOXM1.
Figure 6.
Influence of FOXM1 on β-catenin expression in nuclear and cytoplasm.
Notes: DLD-1 and HCT116 cells were transfected with FOXM1 plasmids or siRNAs for 48 hours. The expressions of β-catenin in nuclear and cytoplasm were examined by western blots. (A) Cytoplasmic and nuclear levels of β-catenin were examined in DLD-1 after transfection with FOXM1 plasmids. (B) Cytoplasmic and nuclear levels of β-catenin were examined in HCT116 after transfection with FOXM1 siRNAs. β-actin was used as the loading control for cytoplasmic protein. Lamin B1 was used as the loading control for nuclear proteins. Each experiment was repeated at least three times. **P<0.01.
Abbreviations: si-control, control-siRNA; si-FOXM1, FOXM1 siRNA.
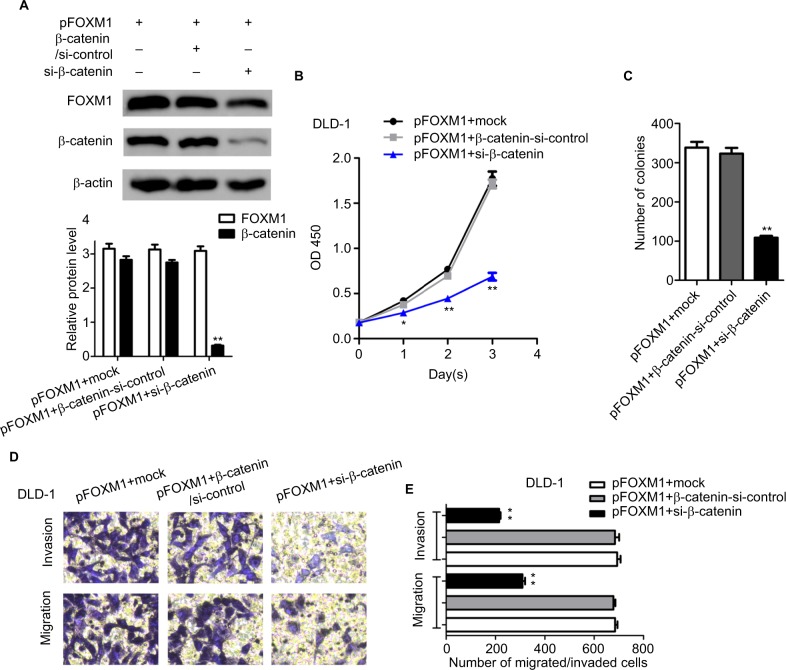
Silencing β-catenin rescued the FOXM1-induced tumorigenicity in CRC cells
The previous study of FOXM1 on activation of β-catenin signaling prompted us to investigate the effect of β-catenin inhibition on FOXM1-mediated malignant activities. Cells with FOXM1 overexpression were transfected with β-catenin siRNA. Multiple biological experiments in vitro were performed, such as MTT, colony formation, and transwell assays. The data demonstrated that silencing β-catenin expression did not change the levels of FOXM1 in FOXM1-overexpression cells, compared to the control cells which were transfected with mock or β-catenin-si-control plasmid (Figure 7A, P>0.05). Additionally, FOXM1-promoted colon cancer growth and formation were eliminated by β-catenin siRNA (Figure 7B, 7C). Similarly, increased cell migration and invasion by FOXM1 overexpression was also eliminated (Figure 7D, E). Taken together, our data suggest that FOXM1 and β-catenin are important for CRC cell progression and inhibition of their expression is a promising strategy for CRC therapy.
Figure 7.
Silencing β-catenin rescued the FOXM1-induced tumorigenicity in CRC cells.
Notes: (A) DLD-1 overexpressing cells were subjected to transfection with mock or β-catenin-si-control or si-β-catenin. The protein expression levels of FOXM1 and β-catenin were detected by western blots. β-actin was an internal control. (B) MTT assays show that silencing of β-catenin in DLD-1 FOXM1-overexpressing cells could lead to significant inhibition of cell proliferation. (C) Downexpression of β-catenin significantly reduced the cell clonogencity of DLD-1 FOXM1-overexpressing cells compared with those of control-transfected cells. **P<0.01. (D and E) Transwell assays indicated that a significant decrease of migrated or invaded capability was also observed in DLD-1 FOXM1-overexpressing cells with β-catenin silencing. Each experiment was repeated at least three times. *P<0.05, **P<0.01, **P<0.001, compared to control-siRNA and untreated group. Scale bar 100μm.
Abbreviations: CRC, colorectal cancer; pFOXM1, pcDNA3.1-FOXM1; si-control, control-siRNA; si-FOXM1, FOXM1 siRNA.
Discussion
There is growing evidence that FOXM1 serves as a key oncogene and novel prognostic biomarker in the initiation and progression of many cancers.9,20–23 However, the underlying mechanism of FOXM1 in CRC is still not fully elucidated. To explore the significance of altered FOXM1 in CRC, we detected the mRNA expressions of FOXM1 in 124 CRC tissues by qRT-PCR and found that FOXM1 was significantly upregulated in CRC tissues. As described in previous study, β-catenin is a key molecule in the canonical Wnt signaling pathway in many human cancers.24 Our results from PCR analysis also showed that β-catenin mRNA expression was markedly enhanced in CRC tissues, whereas lower expression of β-catenin was found in adjacent normal tissues. We consistently chose four CRC tissues and detected the protein expressions of FOXM1 and β-catenin. Our western blot analysis revealed that protein levels of FOXM1 and β-catenin were also markedly upregulated in tumor samples compared to normal tissues. Besides, our clinicopathologic analysis revealed that increased FOXM1 (or β-catenin) expression positively correlated with some clinical features, such as the tumor size, TNM stage, lymphatic metastasis, or distant metastasis. Interestingly, the possible relationships between FOXM1 and β-catenin in CRC samples were evaluated using SPSS software and a significant positive correlation was found. Hence, these results indicated that FOXM1 and β-catenin might work together in human colorectal carcinogenesis.
The long-term survival of CRC patients is extremely unsatisfactory because of recurrences and distant metastases. It has been reported that liver metastasis after curative colectomy occurs in nearly 50% of colon cancer patients.25 Cell growth, invasion, and metastasis are now regarded as important characteristics of cancers. To further explore the effects of FOXM1 on CRC progression, we obtained FOXM1 upregulating and downregulating cells in vitro. Our experiments demonstrated that upregulation of FOXM1 significantly increased the abilities of cell proliferation, colony formation, migration, and invasion in DLD-1 cells, whereas silencing FOXM1 expression exerted an obvious inhibition of tumor cell growth and metastasis in HCT116 cells. Thus, there was strong evidence that FOXM1 plays an important role in the development of CRCs.
The diverse behavior of FOXM1 depends on its various target genes in different cancer types. It has been reported that a variety of FOXM1 downstream target molecules are involved in regulating tumor progression and invasive processes, such as MMP-2, MMP-9, uPAR, VEGF, PLAUR, Snail,10,26–28 or some microRNAs.29–31 Furthermore, altera tions in FOXM1 signaling pathway have been reported to be associated with tumor initiation and progression. For instance, the aberrant activation of Hedgehog (Hh) signaling promoted CRC cell proliferation by directly binding to the promoter of FOXM1 and transactivating the activity of FOXM1, and the dysregulation of the Hh-Gli1-FOXM1 axis was essential for human CRC tumorigenesis.32 In 2007, Yoshida et al constructed FOXM1 transgenic and knockout mice models, and initially found that FOXM1 was critical for the proliferation and growth of CRC with decreased expression of cyclin A2, cyclin B1, survivin, and TCF4 genes. β-catenin has proved to be a subunit of the cadherin protein complex, which mediates the Wnt transcriptional response.24 Our current data demonstrated that overexpression of FOXM1 resulted in the accumulation of nuclear β-catenin and facilitated the expression of β-catenin downstream target genes, including TCF-4, c-Myc, and cyclin D1, which indicates that FOXM1 might manifest its protumor activity by enhancing the transactivation of β-catenin signaling pathway. These results were partly consistent with previous studies in other cancers. For example, FOXM1 was proved to be a downstream component of Wnt signaling and was critical for β-catenin transcriptional function in glioma.33 A similar study was also reported by Chen in 2016. They found that FOXM1 accumulation in the nucleus promoted recruitment of β-catenin to Wnt target-gene promoter and activated the Wnt signaling pathway by protecting the β-catenin/TCF4 complex in glioma.34
Conclusion
In summary, our study demonstrated that FOXM1 and β-catenin were both upregulated in CRC tissues and enhanced FOXM1 expression positively correlated with β-catenin in CRC. In vitro, restoring FOXM1 expression significantly promoted the CRC growth and metastasis by activating the β-catenin downstream effectors, whereas silencing β-catenin could diminish the FOXM1-induced tumorigenicity. Our results provided convincing evidence that elevated FOXM1 expression exerted oncogenic activities towards CRC via activation of β-catenin signaling, which suggests that FOXM1 and β-catenin are important for CRC cell progression and that inhibition of their expression is a promising strategy for CRC therapy.
Acknowledgments
This study was supported by grants from the Special Subject of Diagnosis Treatment of Key Clinical Diseases of Suzhou City Science and Technology Bureau (LCZX201401) and Jiangsu Province’s Youth Provincial Talents Program (QNRC2016723).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Kalin TV, Ustiyan V, Kalinichenko VV. Multiple faces of FoxM1 transcription factor: lessons from transgenic mouse models. Cell Cycle. 2011;10(3):396–405. doi: 10.4161/cc.10.3.14709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teh MT. FoxM1 coming of age: time for translation into clinical benefits? Front Oncol. 2012;2:146. doi: 10.3389/fonc.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad A, Wang Z, Kong D, et al. FoxM1 down-regulation leads to inhibition of proliferation, migration and invasion of breast cancer cells through the modulation of extra-cellular matrix degrading factors. Breast Cancer Res Treat. 2010;122(2):337–346. doi: 10.1007/s10549-009-0572-1. [DOI] [PubMed] [Google Scholar]
- 6.Kim IM, Ackerson T, Ramakrishna S, et al. The forkhead box M1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res. 2006;66(4):2153–2161. doi: 10.1158/0008-5472.CAN-05-3003. [DOI] [PubMed] [Google Scholar]
- 7.Kong X, Li L, Li Z, et al. Dysregulated expression of FoxM1 isoforms drives progression of pancreatic cancer. Cancer Res. 2013;73(13):3987–3996. doi: 10.1158/0008-5472.CAN-12-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang HG, Xu XW, Shi XP, et al. Overexpression of forkhead box protein M1 (FoxM1) plays a critical role in colorectal cancer. Clin Transl Oncol. 2016;18(5):527–532. doi: 10.1007/s12094-015-1400-1. [DOI] [PubMed] [Google Scholar]
- 9.Xu N, Jia D, Chen W, et al. FoxM1 is associated with poor prognosis of non-small cell lung cancer patients through promoting tumor metastasis. PLoS One. 2013;8(3):e59412. doi: 10.1371/journal.pone.0059412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen N, Wang Y, Wen L, et al. Overexpression of FoxM1 predicts poor prognosis and promotes cancer cell proliferation, migration and invasion in epithelial ovarian cancer. J Transl Med. 2014;12(1):134. doi: 10.1186/1479-5876-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu M, Dai B, Kang SH, et al. FoxM1B is overexpressed in human glioblastomas and critically regulates the tumorigenicity of glioma cells. Cancer Res. 2006;66(7):3593–3602. doi: 10.1158/0008-5472.CAN-05-2912. [DOI] [PubMed] [Google Scholar]
- 12.Dai B, Kang SH, Gong W, et al. Aberrant FoxM1B expression increases matrix metalloproteinase-2 transcription and enhances the invasion of glioma cells. Oncogene. 2007;26(42):6212–6219. doi: 10.1038/sj.onc.1210443. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Zhang N, Jia Z, et al. Critical role and regulation of transcription factor FoxM1 in human gastric cancer angiogenesis and progression. Cancer Res. 2009;69(8):3501–3509. doi: 10.1158/0008-5472.CAN-08-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Zhang N, Dai B, et al. FoxM1B transcriptionally regulates vascular endothelial growth factor expression and promotes the angiogenesis and growth of glioma cells. Cancer Res. 2008;68(21):8733–8742. doi: 10.1158/0008-5472.CAN-08-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang K, Jiang L, Hu Y, et al. Short hairpin RNA-mediated gene knockdown of FOXM1 inhibits the proliferation and metastasis of human colon cancer cells through reversal of epithelial-to-mesenchymal transformation. J Exp Clin Cancer Res. 2015;34(1):40. doi: 10.1186/s13046-015-0158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu XY, Zhu ZM, Chen LB, et al. FOXM1 expression correlates with tumor invasion and a poor prognosis of colorectal cancer. Acta Histo-chem. 2012;114(8):755–762. doi: 10.1016/j.acthis.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Giles RH, Van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653(1):1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 18.Behrens J, Lustig B. The Wnt connection to tumorigenesis. Int J Dev Biol. 2004;48(5-6):477–487. doi: 10.1387/ijdb.041815jb. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida Y, Wang IC, Yoder HM, Davidson NO, Costa RH. The forkhead box M1 transcription factor contributes to the development and growth of mouse colorectal cancer. Gastroenterology. 2007;132(4):1420–1431. doi: 10.1053/j.gastro.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 20.Pilarsky C, Wenzig M, Specht T, Saeger HD, Grützmann R. Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia. 2004;6(6):744–750. doi: 10.1593/neo.04277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uddin S, Ahmed M, Hussain A, et al. Genome-wide expression analysis of middle Eastern colorectal cancer reveals FoxM1 as a novel target for cancer therapy. Am J Pathol. 2011;178(2):537–547. doi: 10.1016/j.ajpath.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia L, Huang W, Tian D, et al. Upregulated FoxM1 expression induced by hepatitis B virus X protein promotes tumor metastasis and indicates poor prognosis in hepatitis B virus-related hepatocellular carcinoma. J Hepatol. 2012;57(3):600–612. doi: 10.1016/j.jhep.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Xia JT, Wang H, Liang LJ, et al. Overexpression of FoxM1 is associated with poor prognosis and clinicopathologic stage of pancreatic ductal adenocarcinoma. Pancreas. 2012;41(4):629–635. doi: 10.1097/MPA.0b013e31823bcef2. [DOI] [PubMed] [Google Scholar]
- 24.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20(1):781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 25.van Cutsem E, Nordlinger B, Adam R, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42(14):2212–2221. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Banerjee S, Kong D, Li Y, Sarkar FH. Down-regulation of forkhead box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res. 2007;67(17):8293–8300. doi: 10.1158/0008-5472.CAN-07-1265. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Wei P, Peng Z, et al. The critical role of dysregulated FOXM1-PLAUR signaling in human colon cancer progression and metastasis. Clin Cancer Res. 2013;19(1):62–72. doi: 10.1158/1078-0432.CCR-12-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei P, Zhang N, Wang Y, et al. FoxM1 promotes lung adenocarcinoma invasion and metastasis by upregulating SNAIL. Int J Biol Sci. 2015;11(2):186–198. doi: 10.7150/ijbs.10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma N, Zhang W, Qiao C, et al. The tumor suppressive role of MiRNA-509-5p by targeting FOXM1 in non-small cell lung cancer. Cell Physiol Biochem. 2016;38(4):1435–1446. doi: 10.1159/000443086. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Yu X, Bai Q. miR-204 inhibits invasion and epithelial-mesenchymal transition by targeting FOXM1 in esophageal cancer. Int J Clin Exp Pathol. 2015;8(10):12775–12783. [PMC free article] [PubMed] [Google Scholar]
- 31.Duan N, Hu X, Yang X, Cheng H, Zhang W. MicroRNA-370 directly targets FoxM1 to inhibit cell growth and metastasis in osteosarcoma cells. Int J Clin Exp Pathol. 2015;8(9):10250–10260. [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Hu G, Du Y, et al. Aberrant activation of hedgehog signaling promotes cell proliferation via the transcriptional activation of forkhead Box M1 in colorectal cancer cells. J Exp Clin Cancer Res. 2017;36(1):23. doi: 10.1186/s13046-017-0491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang N, Wei P, Gong A, et al. FoxM1 promotes β-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell. 2011;20(4):427–442. doi: 10.1016/j.ccr.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Li Y, Xue J, et al. Wnt-induced deubiquitination FoxM1 ensures nucleus β-catenin transactivation. EMBO J. 2016;35(6):668–684. doi: 10.15252/embj.201592810. [DOI] [PMC free article] [PubMed] [Google Scholar]