Abstract
Patient: Female, 25
Final Diagnosis: Post-streptococcal glomerulonephritis
Symptoms: Elevated creatinine
Medication: —
Clinical Procedure: —
Specialty: Nephrology
Objective:
Educational purpose
Background:
Post-streptococcal glomerulonephritis (PSGN) is a well-known cause of renal injury. This disease is caused by a prior infection with specific nephritogenic strains of group A beta-hemolytic streptococcus resulting in formation of immune complexes in the glomeruli. Clinical presentation can range from asymptomatic, microscopic hematuria to the nephritic syndrome which is defined by red to brown urine, nephrotic range proteinuria, edema, hypertension, and acute kidney injury. A few reports have described PSGN in kidney transplant recipients in the post-transplantation period. However, biopsy-proven, donor-derived, PSGN in kidney transplant recipients has not been described.
Case Report:
Kidneys were donated from a 25-year-old Caucasian female with no history of hypertension or diabetes who had anoxic brain death in the setting of sepsis due to group A Streptococcus pyogenes bacteremia. The recipients were a 55-year-old male and a 68-year-old female, both of whom had end stage renal disease (ESRD) secondary to hypertensive nephrosclerosis. The recipients had kidney biopsies, one at the time of implantation and the other on post-operative day (POD) 2. Both biopsies showed streptococcal-associated glomerulonephritis. The prompt recognition and treatment of this disease in the immediate post-operative period resulted in histological resolution of the disease as well as good graft outcomes.
Conclusions:
Utilizing kidneys from donors with streptococcal bacteremia is possible while maintaining a high degree of suspicion for possible streptococcal-associated glomerulonephritis.
MeSH Keywords: Glomerulonephritis, Kidney Transplantation, Streptococcal Infections
Background
Infections in organ donors are commonplace. One study demonstrated that 20% of deceased donors have positive peripheral blood cultures [1]. Although certain donor infections are considered high risk and relative contraindicated for transplantation, clinical practice has evolved such that properly selected and treated donors can successfully donate with minimal risk to the potential recipients [1–3]. Acute post-streptococcal glomerulonephritis (PSGN) is a well-known cause of acute kidney injury often related to Streptococcus pyogenes infection. This kidney disease is usually diagnosed about 10 to 14 days after an infection. This is actually an immune complex disease where the immune system is triggered by bacteria to generate glomerular inflammation. The diagnosis is supported by serology, urine analysis, and kidney biopsy, which can be supported by prominent endocapillary proliferation with numerous neutrophils on light microscopy, a granular pattern of C3 deposition on immunofluorescence microscopy, and dome-shaped subepithelial electron-dense deposits, or “humps” which may appear on electron microscopy corresponding to immune complexes [4]. As far as we know, utilization of donor kidneys with streptococcal-associated glomerulonephritides has not been described. We discuss the course and outcome of 2 renal transplant recipients where PSGN was transmitted via the same donor.
Case Report
The donor was a 25-year-old Caucasian female. She had no history of hypertension or diabetes. Brain death was caused by anoxia secondary to sepsis which was determined to be due to group A Streptococcus pyogenes following a hysterectomy for gender reassignment surgery. The terminal creatinine was 1.91 mg/dL.
The donor was treated with metronidazole 500 mg q8h, vancomycin 2g q24 h, and cefepime 1 g q12 h after she was determined to have blood cultures positive for Streptococcus pyogenes. Multi-organ procurement was performed uneventfully. Renal anatomy was unremarkable. The left and right kidneys were both allocated to our transplant institute and utilized for 2 patients.
Recipient 1
Recipient 1 was a 55-year-old African American male with a past medical history of end stage renal disease (ESRD) secondary to hypertensive nephrosclerosis who originally initiated hemodialysis in 1994, then received a deceased donor kidney transplantation in 2001. The kidney lasted 6 years but subsequently failed due to recurrent acute cellular rejection progressing to chronic allograft nephropathy. He had multiple dialysis access revisions and presented with a left thigh arteriovenous graft. Interestingly, he was on outpatient midodrine for low blood pressure when he presented for transplantation.
The transplantation was performed through an intraperito-neal approach but was otherwise uneventful. The kidney was placed in the left lower quadrant above the left thigh graft. The left kidney from the same donor as Recipient 2, was used with a total ischemia time of 14 hours 5 minutes.
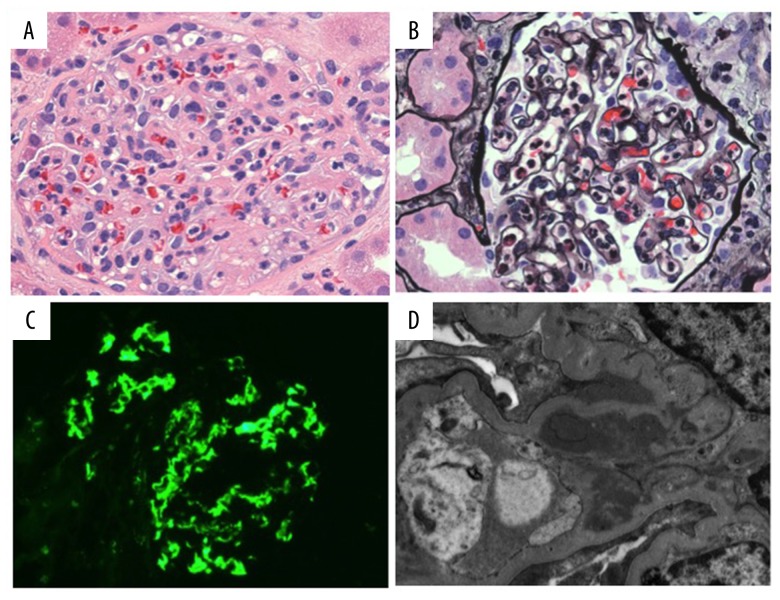
An open kidney biopsy was performed at the time of transplantation due to slow reperfusion of the allograft on visual inspection. Histological examination revealed an acute proliferative glomerulonephritis characterized by mild endocapillary proliferation with neutrophilic exudates (Figure 1A, 1B). No cellular crescents or acute necrotizing lesions were identified. Mesangial proliferation was minimal. Immunofluorescence microscopy revealed mesangial deposition of C3 with partial extension into the capillary loops (Figure 1C). Staining for IgA, IgM, C1q and kappa and lambda light chains was negative within glomeruli. Electron microscopy confirmed mesangial immune deposits (Figure 1D). Rare paramesangial humps were also present and localized to the mesangial reflection (i.e., mesangial waist, Figure 1D). No subendothelial or subepithelial deposits were found along glomerular capillary walls. Tubulointerstitial scarring and inflammation were minimal. There were no signs of rejection. No tubulitis, intimal arteritis, peritubular capillaritis or Cd deposition along peritubular capillaries was identified. Tubules were mildly reactive in appearance, consistent with mild acute tubular injury. The findings were consistent with an infection-associated acute proliferative glomerulonephritis. Correlation with the donor’s antemortem history of Streptococcus pyogenes infection favored PSGN.
Figure 1.
Renal biopsy from Recipient 1. (A) Proliferative glomerulonephritis (hematoxylin and eosin staining, original magnification 400×). (B) Endocapillary hypercellularity with neutrophilic exudates (Jones’ methenamine silver, original magnification 400×). (C) Mesangial C3 (direct immunofluorescence, original magnification 400×). (D) Mesangial immune deposits with small paramesangial hump at the mesangial reflection (arrow) (electron microscopy, original magnification 6000×).
The patient was treated with 1 week of ampicillin-sulbactam and had negative blood cultures. The post-operative course was complicated by an intensive care unit (ICU) admission for hypotension and acidosis which responded to fluid resuscitation and vasopressor support for a 24-hour period. A repeat biopsy was performed on POD 7 due to continued delayed graft function. Pathology revealed acute tubular necrosis but no evidence of glomerulonephritis (not shown). Dialysis was continued until POD 31. Creatinine at 3-months post-transplant was 1.48 mg/dL.
Recipient 2
Recipient 2 was a 68-year-old African American female with chronic kidney disease due to hypertensive nephrosclerosis. She was on hemodialysis for 7 years prior to transplantation. She had a history of cerebrovascular accident and deep vein thrombosis. She presented on warfarin at the time of transplantation. The right kidney (mate kidney to Recipient 1) was used for the transplantation and performed uneventfully with a total ischemia time of 11 hours, 34 minutes.
The patient was started on low dose heparin for the history of deep vein thrombosis (DVT) and she was taken back for surgical exploration on POD 2 due to subcutaneous bleeding likely secondary to heparin. An open kidney biopsy was performed at the time of exploration due to poor urine output. Findings by light, immunofluorescence, and electron microscopy were nearly identical to that described for Recipient 1, and again compatible with PSGN.
The recipient was treated with ampicillin-sulbactam for 7 days and had negative blood cultures. Her creatinine levels remained elevated after hematoma evacuation. Dialysis was initiated due to hyperkalemia and continued until POD 8 at which time urine output improved with a subsequent decrease in creatinine levels. She was discharged with a creatinine of 2.5 mg/dL. Three months post-transplantation, her creatinine level was 0.76 mg/dL.
Discussion
Technological advancements in the diagnosis and treatment of bacterial infections, improvements in sanitation and public hygiene, and as well as higher rates of substance abuse are just some of the factors that have altered the epidemiology and outcomes of bacterial infections worldwide [5]. These global shifts manifest in subtle ways, as evidenced by the increasing incidence of bacterial infection-associated glomerulonephritis in adult patients, especially among the elderly, the debilitated, intravenous drug users, and the immunocompromised (including diabetics and alcoholics) [6,7]. Our case report represents an unusual transmission of a Streptococcus pyogenes associated post-infectious glomerulonephritis from an adult donor to adult recipients.
The pathogenesis of PSGN is complex and was reviewed in Rodriguez-Iturbe and Haas [8]. The clinical spectrum varies from benign, asymptomatic microscopic hematuria to nephritic syndrome with edema, acute kidney injury, and nephrotic range proteinuria. Laboratory findings are significant for a decline in glomerular filtration rate (GFR), but the degree of decline varies. Urinalysis is consistent with microscopic hematuria, proteinuria, and often pyuria. At the time of initial presentation, C3 and CH50 are often significantly depressed [9,10], but will usually return to normal 4 to 8 weeks later. PSGN is self-limited and typically remits with conservative therapy.
On biopsy, the light microscopic findings are variable and may show focal or diffuse proliferative and exudative glomerulonephritis (sometimes with acute necrotizing and crescentic features), mesangial proliferative glomerulonephritis, or rarely, membranoproliferative glomerulonephritis. The mesangial proliferative form is usually seen in the resolving phase of PSGN but may also occur with concomitant or ongoing infection. The alternative complement pathway is activated in PSGN. Accordingly, C3 deposits in glomeruli first, followed by IgG. IgG disappears as the disease wanes, whereas C3 persists for a considerable period of time after clinical resolution. Electron microscopy shows characteristic subepithelial electron dense deposits which appear like dome-shaped “humps.” Subendothelial immune deposits may also be present [11].
Glomerular diseases that develops after kidney transplantation are more often recurrence of native kidney disease. This diagnosis portends a poor prognosis as defined by a decrease in kidney allograft survival. De novo glomerular disease in a renal allograft suggests that the disease was not present in the native kidney prior to transplantation. However, this diagnosis is often difficult to make as only 15% to 20% of patients have native kidney biopsies performed prior to transplantation. The most common de novo glomerular disease diagnosed after transplantation in a Canadian review was focal segmental glomerulosclerosis (FSGS) [12]. Other de novo glomerular diseases include minimal change disease, membranoproliferative glomerulonephritis, membranous nephropathy, collapsing glomerulopathy, pauci-immune glomerulonephritis, IgA nephropathy, anti-GBM nephritis, thrombotic microangiopathy, diabetic nephropathy, and infectious proliferative glomerulonephritis. The frequency, time of onset, treatment, and prognosis of de novo glomerular diseases vary based on the pathological diagnosis [13].
Despite the frequent number of infections observed in the setting of immunosuppression post-transplantation, there are only a few case reports of de novo biopsy-proven post-infectious glomerulonephritis. Moroni et al. reported only 3 out of 827 patients developed post-infectious glomerulonephritis despite 65% of this cohort developing at least one bacterial infection during follow-up [14]. The causes were Escherichia coli bacteremia, cholangitis, and a skin abscess. Two of these 3 patients did eventually lose their allograft function after approximately 9 years and 2 years, respectively, while the third case still had a functional allograft at the time of publication. They also reported 4 prior cases of de novo post-infectious glomerulonephritis. Three cases were due to Staphylococcus aureus and the fourth was due to streptococcus.
Tandon et a. more recently reported a case of de novo biopsy-proven glomerulonephritis in a patient with recurrent VRE UTIs (vancomycin-resistant enterococcus urinary tract infections) refractory of antibiotic therapy who responded to repeated courses of intravenous (IV) antibiotics followed by oral steroids, who eventually underwent an ileal loop diversion and as of publication of the report, had a creatinine level of 1.2 g/dL [15].
These aforementioned case reports illustrate that etiology and presentation varies. This also applies to the histology, some of which revealed endocapillary glomerulonephritis, characterized by exudative and proliferative lesions with immunofluorescence showing granular C3 deposits while other cases revealed necrotizing glomerulonephritis with frequent cellular crescents. The majority of cases presented clinically with acute kidney injury. The prognosis and treatment in these cases also varied and were largely dependent on the clinical-pathologic presentation, as some, but not all of the patients responded to antimicrobial treatment. Most patients also received an initial dose of IV steroids followed by oral steroids that appeared to facilitate therapy in some of the cases [9].
The patients we report on here were unique in that both recipients had biopsy-proven post-infectious glomerulonephritis that was donor-derived as evidenced by histological findings on reperfusion biopsy. Both recipient kidneys were from the same donor, who had group A strep pyogenes bacteremia at the time of anoxic brain injury and death. The pathology on both recipients’ kidneys were similar in that both had glomeruli with neutrophilic exudates and mesangial and endocapillary proliferation as well as immunofluorescence showing mesangial staining positive for C3. Both patients completed a 7-day course of Unasyn with documented follow-up negative blood cultures and both received IV followed by oral steroids with maintenance dose of Prednisone at 5 mg daily by POD #7. In retrospect, the IV and oral steroids served a dual purpose for both immunosuppression and adjunctive treatment for PSGN. Recipient 1 had a prolonged period of delayed graft function compared to Recipient 2, but this was best explained by his complicated immediate post-operative course, specifically his hypotension requiring pressors and need for continuous renal replacement therapy. His hypotension after transplant was the likely cause of his graft developing acute tubular necrosis seen on a repeat biopsy on POD #12. Interestingly, this biopsy revealed no evidence of PSGN. The light microscopic and immunofluorescence findings mentioned in the initial reperfusion biopsy had resolved. Recipient 2 had her last dialysis on POD #8 so we felt there was no clinical indication for a repeat allograft biopsy.
These two patients are the first two case reports we found which show donor-derived acute PSGN that responded to antimicrobial and intravenous steroid therapy. In addition, recipient 1 illustrates, to the best of our knowledge, the first case report showing prompt pathologic resolution of PSGN in a renal allograft. Fortunately, the deceased donor’s group A Streptococcus pyogenes bacteremia created a high enough level of suspicion to lead to a quick diagnosis of PSGN from the implantation biopsy of Recipient 1, which then led to a transplant biopsy on Recipient 2 resulting in early diagnosis and treatment with this recipient as well. Serologies were not sent on either donor or recipients in this case given the early timing of kidney biopsy and prompt pathologic diagnosis. At their 3-month follow-up visits Recipient 1 and Recipient 2 had a serum creatinine of 1.48 and 0.76 mg/dL, respectively.
Conclusions
These 2 case reports showed that accepting an organ from a deceased donor with streptococcal bacteremia is possible as long as there remains a high suspicion for diagnosis and treatment of possible PSGN in the recipients. The transplant provider should have a low threshold for biopsy at re-implantation or within the first week after transplantation, especially if the recipient does not show good allograft function as well as empirically initiate and continue IV antimicrobials for at least 1 week after transplantation. Fortunately, in these cases, immunosuppression can actually facilitate successful treatment of donor-derived PSGN.
References:
- 1.Yuan X, Chen C, Zhou J, et al. Organ donation and transplantation from donors with systemic infection: A single-center experience. Transplant Proc. 2016;48(7):2454–57. doi: 10.1016/j.transproceed.2016.02.092. [DOI] [PubMed] [Google Scholar]
- 2.Simkins J, Muggia V. Favorable outcome in a renal transplant recipient with donor-derived infection due to multidrug-resistant Pseudomonas aeruginosa. J Transpl Infect Dis. 2012;14(3):292–95. doi: 10.1111/j.1399-3062.2011.00674.x. [DOI] [PubMed] [Google Scholar]
- 3.Kovacs CS, Jr, Koval CE, van Duin D, et al. Selecting suitable solid organ transplant donors: Reducing the risk of donor-transmitted infections. World J Transplant. 2014;4(2):43–56. doi: 10.5500/wjt.v4.i2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Iturbe B, Batsford S. Pathogenesis of poststreptococcal glomerulonephritis a century after Clemens von Pirquet. Kidney Int. 2007;71:1094–104. doi: 10.1038/sj.ki.5002169. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–59. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasr SH, Radhakrishnan J, D’Agati VD. Bacterial infection – related glomerulonephritis in adults. Kidney Int. 2013;83:792–803. doi: 10.1038/ki.2012.407. [DOI] [PubMed] [Google Scholar]
- 7.Nasr SH, Markowitz GS, Stokes MB, et al. Acute postinfectious glomerulonephritis in the modern era: Experience with 86 adults and review of the literature. Medicine. 2008;87:21–32. doi: 10.1097/md.0b013e318161b0fc. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Iturbe B, Haas M. Post-streptococcal glomerulonephritis. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: Basic biology to clinical manifestations. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016. [PubMed] [Google Scholar]
- 9.Moudil A, Bagga A, Fredrich R, Jordan S. In: Poststreptococcal and other infection-related glomerulonephritides. 2nd ed. Greenberg A, editor. New York: Academic Press; 1998. [Google Scholar]
- 10.Lewis EJ, Carpenter CB, Schur PH. Serum complement component levels in human glomerulonephritis. Ann Intern Med. 1971;75(4):555–60. doi: 10.7326/0003-4819-75-4-555. [DOI] [PubMed] [Google Scholar]
- 11.Sorger K, Balun J, Hübner FK, et al. The garland type of acute postinfectious glomerulonephritis: Morphological characteristics and follow-up studies. Clin Nephrol. 1983;20(1):17–26. [PubMed] [Google Scholar]
- 12.Chailimpamontree W, Dmitrienko S, Li G, et al. Probability, predictors, and prognosis of posttransplantation glomerulonephritis. J Am Soc Nephrol. 2009;20:843–51. doi: 10.1681/ASN.2008050454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponticelli C, Moroni G, Glassock RJ. De novo glomerular diseases after renal transplantation. Clin J Am Soc Nephrol. 2014;9:1479–87. doi: 10.2215/CJN.12571213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moroni G, Papaccioli D, Banfi G, et al. Acute post-bacterial glomerulonephritis in renal transplant patients: description of three cases and review of the literature. Am J Transplant. 2004;4:132–36. doi: 10.1046/j.1600-6135.2003.00283.x. [DOI] [PubMed] [Google Scholar]
- 15.Tandon T, Mujtaba M, Mishler D, et al. Early Enterococcus-associated acute postinfectious glomerulonephritis after kidney transplant. Clin Kidney J. 2014;7:426–27. doi: 10.1093/ckj/sfu069. [DOI] [PMC free article] [PubMed] [Google Scholar]