Abstract
Patient: Female, 24
Final Diagnosis: Myeloid sarcoma of the breast
Symptoms: Breast lump
Medication: —
Clinical Procedure: Core needle biopsy
Specialty: Radiology
Objective:
Rare disease
Background:
Myeloid sarcoma is a rarely observed extramedullary presentation of myeloid leukemia that seldom manifests in the breast. Myeloid sarcoma can occur before, concurrently with, or following acute myeloid leukemia presentation. Few reports have focused on the imaging findings in cases of myeloid sarcoma of the breast, and the existing findings are variable and nonspecific; the present case report aimed to bridge this gap.
Case Report:
A 24-year-old female presented with a palpable lump at the upper outer quadrant of her right breast. She had noticed the mass 2 days prior to presentation. She was first diagnosed with acute myelogenous leukemia 18 months before the lump presentation and had undergone haploidentical stem cell transplantation 6 months prior. At the time of the breast lump presentation, she was undergoing chemotherapy for relapsed acute myeloid leukemia. Ultrasonography of her right breast revealed a circumscribed, oval mass corresponding to the palpable lump. Ultrasonography-guided 14-gauge core needle biopsy was performed on the breast mass, leading to a pathological diagnosis of myeloid sarcoma.
Conclusions:
We reported a case of myeloid sarcoma involving the breast. On sonography, although the internal echotexture resembled that of breast hamartoma, the observed hard elasticity and high vascularity raised suspicions of malignancy.
MeSH Keywords: Breast; Elasticity Imaging Techniques; Leukemia, Myeloid, Acute; Sarcoma, Myeloid; Ultrasonography, Mammary
Background
Myeloid sarcoma is a rarely observed hematologic malignancy occurring as a soft tissue mass at extramedullary sites [1,2]. Myeloid sarcomas comprise myeloid cells with variable degrees of maturation and usually manifest simultaneously with or subsequently to the diagnosis of myeloid leukemia [3]. Premature cells that are differentiated from hematopoietic precursor cells interact with fibroblasts to induce myeloid metaplasia in nonhematopoietic tissues [4]. Breasts are rarely sites of myeloid sarcoma involvement [4]. Few studies have reported on the radiologic features of breast myeloid sarcoma [4].
This report describes a case of myeloid sarcoma that presented 6 months after stem-cell transplantation in a woman with known acute myeloid leukemia (AML). The clinical, gray-scale, color-Doppler sonographic, and sonoelastographic features of the case are presented in detail herein.
Case Report
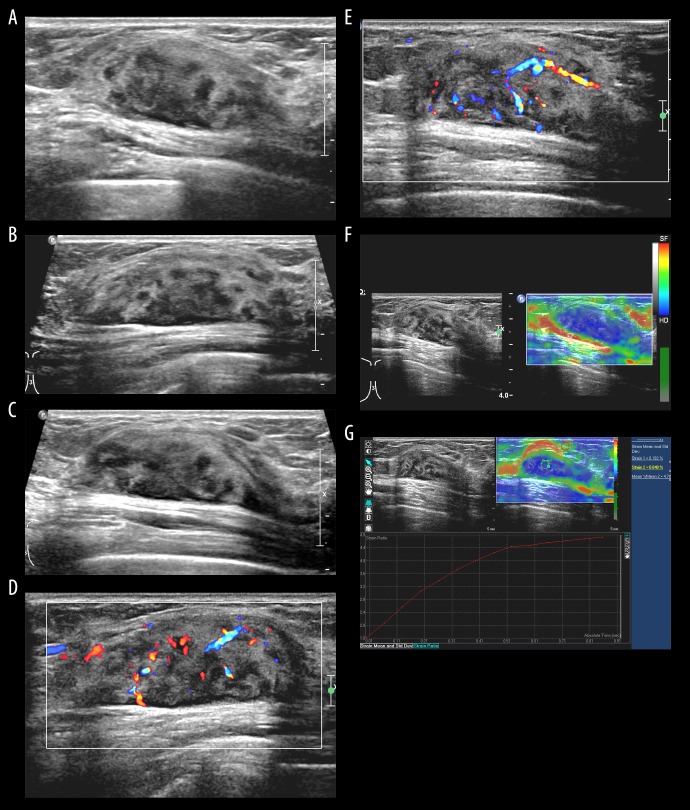
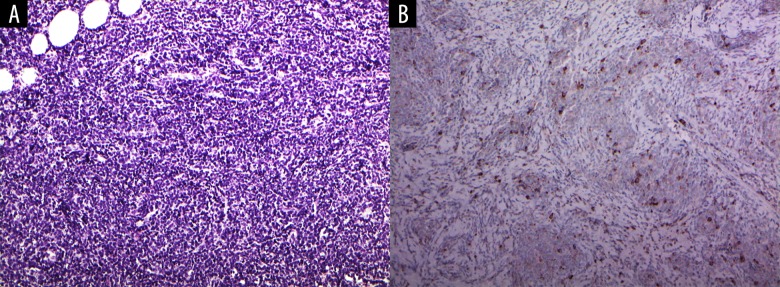
A 24-year-old female presented with a complaint of a hard, painless lump palpated in the upper outer quadrant of her right breast. She had noticed the mass 2 days prior to presentation. She had a notable medical history of AML that was first diagnosed 18 months before the lump presentation and was accompanied by extramedullary involvement of multiple lymph nodes. Following multiple cycles of induction and consolidation chemotherapy, she experienced repeated complete remission and relapse. Six months before the presentation of the breast lump, she had undergone haploidentical stem cell transplantation. At the time at which she noticed the breast lump, she was undergoing chemotherapy for relapsed AML involving the bone marrow and extramedullary sites of multiple lymph nodes at both the inguinal and neck areas, in the axillae, pelvic, and retroperitoneal spaces. Conventional ultrasonography of her right breast led to the detection of a 4.3-cm-sized, indistinct, oval, heterogeneous-echoic mass with posterior enhancement in the upper outer quadrant, corresponding to the palpable lump (Figure 1A–1C). The heterogenous echotexture comprised multiple nodular hypoechoic areas and intervening hyperechoic areas. On color-Doppler sonography, the mass showed markedly increased interval vascularity in a disordered and branching pattern (Figure 1D, 1E). On strain elastography, the lesion appeared stiff (blue color in the entire lesion) (Figure 1F) and the strain ratio was 4.78 (Figure 1G). The strain ratio was calculated by dividing the average strain of the reference (adjacent normal breast tissue) by the average strain of the mass; the best cutoff strain ratio value for malignancy ranges from 2.27 to 3.06 [5–9]. Mammography was not performed. The sonographic features – characterized by an oval shape and mixed internal echogenicity – resembled those of a hamartoma. However, along with the clinical history of AML, based on an indistinct margin status, strong internal vascularity and hard elasticity, the mass was assessed as Bi-RADS 4. Under sonography guidance, 14 gauge-core needle biopsy was performed on the mass. Microscopically, the biopsied breast lesion was shown to be a hypercellular lesion with compact sheets of immature mononuclear cells with entrapped fat (Figure 2A). Immunohistochemical staining of myeloperoxidase showed focal positivity, suggesting the presence of myeloid blasts (Figure 2B). The histomorphology, cytologic features, and immunoprofile of the lump were consistent with those of myeloid sarcoma.
Figure 1.
The transverse (A), longitudinal (B), and radial (C) grayscale ultrasonograms of the right breast taken at the upper outer quadrant show an indistinct, oval, heterogeneous-echoic mass with posterior enhancement. Longitudinal (D) and radial (E) color-Doppler sonogram show disordered and branching vessels within the mass. (F) Strain elastogram shows homogeneous blue color coding in the mass, indicating the hard, elastic nature of the mass (red depicted as soft, green as intermediate, and blue as hard). (G) The strain ratio of the mass relative to the adjacent fat tissue was 4.78, also implying the apparent hard elasticity.
Figure 2.
(A) Microscopic finding of the breast biopsy shows a hypercellular lesion with compact sheets of immature mononuclear cells with entrapped fat (hematoxylin and eosin 200×). (B) Immunohistochemical (IHC) staining of myeloperoxidase (MPO) shows focal positivity, suggesting the presence of myeloid blasts (MPO 200×).
Discussion
Leukemic involvement is observed extremely rarely in the breast [10,11]. Of all breast leukemias, acute myelogenous leukemia is the most commonly occurring and it presents as myeloid sarcoma [11]. Myeloid sarcoma is an extramedullary hematological malignant tumor of myeloid cells showing varying degrees of maturation, and is most commonly observed in patients with previously diagnosed myeloid leukemia [3,12]. According to the World Health Organization, myeloid sarcomas can be classified into 3 categories: blastic (composed mainly of myeloblasts), immature (composed of myeloblast and promyelocytes), and differentiated (composed of promyelocytes and more mature neutrophils) [13].
Myeloid sarcoma usually manifests concurrently or following the diagnosis of AML, chronic myeloid leukemia, myeloproliferative diseases, or myelodysplastic syndrome [13], and the presence of AML commonly and typically induces or predisposes a person to the development of myeloid sarcoma [4]. However, under rare circumstances, myeloid sarcoma can occur as an isolated extramedullary mass without a previous or concurrent diagnosis of AML (no evidence of peripheral blood or bone marrow involvement at the time of diagnosis) [1,13]. Many of these isolated cases subsequently develop AML [1]. Myeloid sarcoma is also known as granulocytic sarcoma, monocytic sarcoma, extramedullary myeloid cell tumor, myeloblastoma, and chloroma [1]. The term “chloroma” was initially adopted owing to the greenish color assumed by the tumor cells on myeloperoxidase staining [1]; the term is currently not in use because not all tumor cells assumed a greenish color on staining [14].
Myeloid sarcoma can affect any part of the body, and the most commonly affected sites are the lymph nodes, skin, bones, central nervous system, and soft tissue [14]. Breast involvement is rarely observed in myeloid sarcoma [2,13]. In 2005, up to 67 cases of breast myeloid sarcoma were listed in the literature [1]; thereafter, additional cases were sporadically reported until recently [4,15,16]. Breasts are the most commonly observed sites of first extramedullary relapse after stem-cell transplantation [13]. Cunningham et al. reported 20 cases of breast myeloid sarcoma as the first extramedullary relapse of AML after stem-cell transplantation [17]. The time interval between stem-cell transplantation and breast relapse ranged from 2 to 73 months (mean 17 months). In our case, the leukemic relapse in the breast occurred 6 months after stem-cell transplantation and extramedullary involvement was already present before the breast relapse.
The gold standard for the final diagnosis of myeloid sarcoma is histopathological examination combined with immunohistochemical testing [4]. Myeloperoxidase staining is often strongly positive in myeloid sarcoma [3].
Clinically, myeloid sarcoma presents with non-specific symptoms that are usually painless or painful palpable breast lumps involving one or both breasts [4]. Breast skin involvement and axillary lymph node enlargement may accompany such cases, while nipple discharge and retraction are not commonly observed [4].
The existing knowledge on the radiologic features of breast leukemia was obtained from sporadic case reports [18], and these features tended to vary across individual cases [12]. While a few recent articles attempted to define the representative radiologic features of intramammary hematologic malignancies [18,19], the radiologic features were non-specific, reflecting a broad spectrum of hematologic malignancies encompassing lymphoma, leukemia, and plasmacytoma [19] or both myeloid and lymphoid leukemia [18] as a whole. According to a comprehensive review of published case reports on breast leukemia, on sonography, breast leukemia typically presents as solitary or multiple masses that are mostly homogeneously hypoechoic with microlobulated or indistinct margins [18]. Breast leukemia rarely presents as architectural distortions on sonography [18]. Twenty-seven reports have presented the sonographic findings of breast myeloid sarcoma (Table 1); of the cases presented in those reports, the sonographic findings were presented with real images in 15 and were only text-described in 12 (Table 1).
Table 1.
Summary of published reports that present sonographic findings of myeloid sarcoma of the breast.
| Case | Authors | Published year | Age, years | Sex | Laterality | Multiplicity | Sonographic findings | |
|---|---|---|---|---|---|---|---|---|
| Reports that provide real sonographic images | Vascularity | |||||||
| 1 | Hiorns et al. [20] | 1997 | 32 | F | L | Single | Lobulated mass moderately well-defined, with slightly uneven echogenicity and acoustic shadowing | Not assessed |
| 2 | Ahrar et al. [21] | 1998 | 15 | F | R | Single | Nonhomogeneous mass with spiculated and angular margins | Increased |
| 3 | Son et al. [22] | 1998 | 42 | F | B | Multiple |
|
Not assessed |
| 4 | Fitoz et al. [23] | 2002 | 13 | F | L | Single | Large mass with lobulated contours and heterogeneous echotexture | Increased |
| 5 | Guermaziet al. [24] | 2002 | 41 | F | L | Single | Irregular, non-homogeneous hypoechoic mass with ill-defined margin and posterior shadow | Not assessed |
| 6 | Kinishita et al. [25] | 2006 | 53 | F | B | Multiple | Low-echoic masses with irregular margin and posterior shadowing | Not assessed |
| 7 | Lim et al. [2] | 2008 | 58 | F | R | Single | Oval, heterogeneous (mixed iso- and hypo-echogenicity) mass surrounded by a pseudocapsule | None |
| 8 | Toumeh et al. [13] | 2012 | 47 | F | B | Multiple | Multiple masses | Not assessed |
| 9 | Kim et al. [14] | 2013 | 39 | F | R | Multiple | Heterogeneous hypoechoic masses with multifocal cystic portion | Increased |
| 10 | Kim et al. [14] | 2013 | 48 | F | B | Multiple | Heterogeneous hypoechoic masses with echogenic boundary | Increased |
| 11 | Kim et al. [26] | 2013 | 20 | F | B | Multiple |
|
Not assessed |
| 12 | Ozsoy et al. [12] | 2016 | 21 | F | B | Multiple | Lobulated, hypoechoic solid masses with circumscribed contours (some of them with microlobulated margins) | Increased |
| 13 | Edison et al. [27] | 2017 | 8 | F | B | Multiple | Round/oval, parallel masses with partially indistinct and lobulated margins, heterogeneous echotexture | Increased |
| 14 | Cheung et al. [15] | 2018 | 46 | F | B | Multiple | Ill-defined conglomerated masses of heterogenous hyperechogenicity and hypoechogenicity | Increased |
| 15 | Zhai et al. [4] | 2018 | 34 | F | R | Multiple |
|
Increased |
| 16 | Current | 2019 | 24 | F | R | Single | Partially indistinct, oval, heterogeneous mass with posterior acoustic enhancement | Increased |
| Reports that only provide text-description of sonographic features | ||||||||
| 17 | Khoury et al. [28] | 2000 | 51 | F | B | Multiple | Thickened and coarse breast parenchyma bilaterally with distorted echotexture, a small irregular hypoechoic lesion in the left breast | Not assessed |
| 18 | Ngu et al. [29] | 2001 | 40 | F | B | Multiple | Bilateral breast cysts | Not assessed |
| 19 | Dutta Roy et al. [30] | 2004 | 72 | F | L | Single | Ill-defined, non-homogeneous, irregular mass with areas of increases echogenicity | Not assessed |
| 20 | Shea et al. [31] | 2004 | 55 | F | B | Multiple | Solid | Not assessed |
| 21 | Thachil et al. [32] | 2007 | 26 | F | R | Single | Hypoechoic area representing an area of fibrocystic change | Not assessed |
| 22 | Delporte et al. [33] | 2010 | 65 | F | R | Single | Irregular hypoechogenic mass | Increased |
| 23 | Fu et al. [34] | 2014 | 59 | F | L | Single | Solid hypoechoic mass with a clear boundary | Increased |
| 24 | Fu et al. [34] | 2014 | 37 | F | L | Single | Solid hypoechoic mass | Not assessed |
| 25 | Goncalves et al. [35] | 2014 | 35 | F | L | Single | Solid heterogeneous nodule | Not assessed |
| 26 | Gunduz et al. [36] | 2014 | 33 | F | R | Single | Irregular mass with heterogeneous internal echo | Not assessed |
| 27 | Stewart et al. [3] | 2015 | 46 | F | R | Single | Indistinct, hypoechoic mass with posterior acoustic shadowing | Increased |
| 28 | Gomaa et al. [16] | 2018 | 29 | F | L | Single | Well-defined mass which has a consistency of parenchymal ducts and fat | Not assessed |
When describing sonographic findings, terms not strictly following the Bi-RADS lexicon were often used in previous reports, and these are shown in Table 1 as described. Although the sonographic findings of myeloid sarcoma are usually not specific, some emphasized on suspicious findings characterized by an irregular shape, spiculated or angular or indistinct margins, and posterior shadowing [4,20,21,24,25,33]. In the present case, the myeloid sarcoma involving the breast exhibited a characteristic heterogeneous echotexture on sonography, which somewhat resembled the typical internal echotexture of breast hamartoma [37]. Breast hamartomas predominantly appear as circumscribed, oval masses with mixed iso- and hyperechogenicity, sonographically [37]. The heterogeneous internal echotexture of hamartoma reflects its intrinsic histologic characteristics, comprising a mixture of different tissues, and depending on the relative proportion of adipose, glandular, fibrous tissues, is variably mixed with hypoechoic and band-like or nodular hyperechoic areas [37]. Hamartoma is not hypervascular on color-Doppler imaging [37], inconsistent with our case. Although data on the same is limited, breast hamartomas are usually less elastic (Italian elastosonographic score of 3 or 4) than the tissues that surround them, and their appearance largely depends on the amount of fibrous tissue [37]. Interestingly, through our own re-evaluation of the sonographic images obtained from previous case reports, we observed, not infrequently, similar hamartoma-like patterns of the internal echotexture in breast myeloid sarcoma [2,12,13,16,22,26].
Some reports clearly specified a hamartoma-like appearance when describing the sonographic features [2,16]. Furthermore, across a vast number of reports, the internal echotextures of breast myeloid sarcoma were found to be at least heterogeneous [2–4,12–16,20–24,26,27,30,35,36].
The elastography findings of intramammary hematologic malignancy have rarely been reported on [38–42]. Aslan et al. conducted a study in which they assessed the elasticity of intramammary hematologic malignancies in 9 patients; the study revealed that the elasticities of intramammary hematologic malignancy are variablesoft in 2 patients, intermediate in 5, and high in 2 [39]. Of the 9 hematologic malignancy cases, only 1 was myeloid sarcoma, which demonstrated intermediate elasticity (shear-wave velocity: 4.68 to 5.74 m/s, virtual touch imaging: diffuse dark, same size with B-mode ultrasonography) [39]. The published cutoff shear-wave velocity value for malignancies ranges from 4.1 to 5.2 m/s [39]. Another report also described the elastography findings of myeloid sarcoma of the breast, in which the Tsukuba elasticity score, strain index, and elasticity ratio were 2, 2.3, and 0.71, respectively; these values indicated a benign nature [38]. To the best of our knowledge, the current case report is the second of its kind to report on the strain elastography features of myeloid sarcoma involving the breast. In the present case, the myeloid sarcoma exhibited apparent hard elasticity based on both the qualitative assessment of general color-coded maps and quantitative assessment of the strain ratio. All other sporadic case reports on the elasticity of intramammary hematologic malignancies exclusively included lymphoma cases in which the elasticities were also variably reported as soft [40,42] or hard [38,41].
Breast leukemias are known to have 3 mammographic patterns: breast masses, architectural distortions, and no abnormalities [18]. Most breast masses are hyperdense, have a round shape and microlobulated margins, and occasionally accompany internal microcalcifications [18]. Architectural distortions were observed less frequently on mammography and no abnormalities were demonstrated on mammography in some breast leukemia cases [18].
On magnetic resonance imaging, breast leukemias also exhibited nonspecific findings, most of which were masses (often markedly enhancing); non-mass enhancements were rarely observed [26].
Once leukemia relapses in the breast, the extramedullary disease is highly resistant to treatment and the prognosis is deteriorated [13]. Although optimal consensus treatment strategies have not determined, surgical resection (lumpectomy or mastectomy) with systemic chemotherapy is generally recommended [4,13]. Treatments with concurrent radiation therapy are controversial [13]. However, early diagnosis and the prompt initiation of systemic chemotherapy may result in a long disease-free survival duration and may even prove curative in some cases [13]. In cases of myeloid sarcoma of the breast after stem-cell transplantation, disease-free survival durations of 3, 4, and 12 years were observed when systemic therapy was performed [13].
Conclusions
In this report, we described a rare case of myeloid sarcoma as a breast relapse of AML after stem-cell transplantation. In this case, the presence of a prior history of AML was helpful in the diagnosis of breast relapse associated with AML. However, breast manifestation is rarely the initial diagnosis in AML. Familiarity with the associated radiologic features may aid in the provision of a differential diagnosis when faced with this unusual breast lesion. Although the conventional sonographic features somewhat resembled the characteristic internal echotexture of breast hamartoma, the color-Doppler sonography and strain elastography findings further raised suspicions of malignancy.
This case report highlights certain sonographic features of myeloid sarcoma that mimic benign hamartoma, and stresses on the added value of color-Doppler sonography and sonoelastography in further assessment. When faced with an unusual breast lesion, it is important to perform appropriate clinicopathologic correlation and consider broad differential diagnoses.
References:
- 1.Valbuena JR, Admirand JH, Gualco G, Medeiros LJ. Myeloid sarcoma involving the breast. Arch Pathol Lab Med. 2005;129(1):32–38. doi: 10.5858/2005-129-32-MSITB. [DOI] [PubMed] [Google Scholar]
- 2.Lim HS, Park MH, Heo SH, et al. Myeloid sarcoma of the breast mimicking hamartoma on sonography. J Ultrasound Med. 2008;27(12):1777–80. doi: 10.7863/jum.2008.27.12.1777. [DOI] [PubMed] [Google Scholar]
- 3.Stewart RL, Dell CM, Samayoa L. Myeloid sarcoma of the breast misdiagnosed as poorly differentiated mammary carcinoma with lobular features. Breast J. 2015;21(2):192–93. doi: 10.1111/tbj.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhai J, Kong X, Yang X, et al. An uncommon granulocytic sarcoma of the breast: A case report and literature review. Onco Targets Ther. 2018;11:3685–90. doi: 10.2147/OTT.S149149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas A, Degenhardt F, Farrokh A, et al. Significant differentiation of focal breast lesions: Calculation of strain ratio in breast sonoelastography. Acad Radiol. 2010;17:558–63. doi: 10.1016/j.acra.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Fischer T, Peisker U, Fiedor S, et al. Significant differentiation of focal breast lesions: Raw data-based calculation of strain ratio. Ultraschall Med. 2012;33:372–79. doi: 10.1055/s-0031-1273222. [DOI] [PubMed] [Google Scholar]
- 7.Zhao QL, Ruan LT, Zhang H, et al. Diagnosis of solid breast lesions by elastography 5-point score and strain ratio method. Eur J Radiol. 2012;81:3245–49. doi: 10.1016/j.ejrad.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Cho N, Moon WK, Kim HY, et al. Sonoelastographic strain index for differentiation of benign and malignant nonpalpable breast masses. J Ultrasound Med. 2010;29:1–7. doi: 10.7863/jum.2010.29.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Zhi H, Xiao XY, Yang HY, et al. Ultrasonic elastography in breast cancer diagnosis: Strain ratio vs. 5-point scale. Acad Radiol. 2010;17:1227–33. doi: 10.1016/j.acra.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Joo M, Lee HK, Kang YK, Kim JH. Granulocytic sarcoma of the breast preceding acute myelogenous leukemia: A case report. J Korean Med Sci. 2000;15:457–59. doi: 10.3346/jkms.2000.15.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dialani V, Mani K, Johnson NB. Chronic lymphocytic leukemia involving the breast parenchyma, mimicker of invasive breast cancer: Differentiation on breast MRI. Case Rep Med. 2013;2013:603614. doi: 10.1155/2013/603614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozsoy A, Akdal Dolek B, Barca N, et al. Ultrasound findings in a case of myeloid sarcoma of the breast. J Belg Soc Radiol. 2016;100:15. doi: 10.5334/jbr-btr.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toumeh A, Phinney R, Kobalka P, Mohamed I. Bilateral myeloid sarcoma of the breast and cerebrospinal fluid as a relapse of acute myeloid leukemia after stem-cell transplantation: A case report. J Clin Oncol. 2012;30:e199–201. doi: 10.1200/JCO.2011.40.2255. [DOI] [PubMed] [Google Scholar]
- 14.Kim SJ, Hong WS, Jun SH, et al. Granulocytic sarcoma in breast after bone marrow transplantation. J Breast Cancer. 2013;16:112–16. doi: 10.4048/jbc.2013.16.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng KCA, Li YL, Lam T. Acute myeloid leukaemia presenting with bilateral breast masses. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2018-225735. pii: bcr-2018-225735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomaa W, Ghanim A, Emam E, et al. Primary myeloid sarcoma of the breast: A case report and review of literature. J Microsc Ultrastruct. 2018;6:212–14. doi: 10.4103/JMAU.JMAU_15_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham I. A clinical review of breast involvement in acute leukemia. Leuk Lymphoma. 2006;47:2517–26. doi: 10.1080/10428190600967022. [DOI] [PubMed] [Google Scholar]
- 18.Surov A, Wienke A, Abbas J. Breast leukemia: An update. Acta Radiol. 2012;53:261–66. doi: 10.1258/ar.2011.110470. [DOI] [PubMed] [Google Scholar]
- 19.Wienbeck S, Meyer HJ, Uhlig J, et al. Radiological imaging characteristics of intramammary hematological malignancies: Results from a German multi-center study. Sci Rep. 2017;7:7435. doi: 10.1038/s41598-017-07409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiorns MP, Murfitt J. Granulocytic sarcoma (chloroma) of the breast: Sonographic findings. Am J Roentgenol. 1997;169:1639–40. doi: 10.2214/ajr.169.6.9393183. [DOI] [PubMed] [Google Scholar]
- 21.Ahrar K, McLeary MS, Young LW, et al. Granulocytic sarcoma (chloroma) of the breast in an adolescent patient: ultrasonographic findings. J Ultrasound Med. 1998;17:383–84. doi: 10.7863/jum.1998.17.6.383. [DOI] [PubMed] [Google Scholar]
- 22.Son HJ, Oh KK. Multicentric granulocytic sarcoma of the breast: Mammographic and sonographic findings. Am J Roentgenol. 1998;171:274–75. doi: 10.2214/ajr.171.1.9648817. [DOI] [PubMed] [Google Scholar]
- 23.Fitoz S, Atasoy C, Yavuz K, et al. Granulocytic sarcoma. Cranial and breast involvement. Clin Imaging. 2002;26:166–69. doi: 10.1016/s0899-7071(01)00388-6. [DOI] [PubMed] [Google Scholar]
- 24.Guermazi A, Feger C, Rousselot P, et al. Granulocytic sarcoma (chloroma): Imaging findings in adults and children. Am J Roentgenol. 2002;178:319–25. doi: 10.2214/ajr.178.2.1780319. [DOI] [PubMed] [Google Scholar]
- 25.Kinoshita T, Yokokawa M, Yashiro N, et al. Multicentric granulocytic sarcoma of the breast: mammographic, sonographic, and MR findings of granulocytic sarcoma of the breasts. Clin Imaging. 2006;30:271–74. doi: 10.1016/j.clinimag.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Kim SJ. Magnetic resonance imaging features of breast leukemia. Magn Reson Med Sci. 2013;12:309–13. doi: 10.2463/mrms.2012-0091. [DOI] [PubMed] [Google Scholar]
- 27.Edison MN, O’Dell MC, Letter HP, et al. Juvenile myelomonocytic leukemia presenting as bilateral breast masses. Pediatr Radiol. 2017;47:104–7. doi: 10.1007/s00247-016-3710-z. [DOI] [PubMed] [Google Scholar]
- 28.Khoury NJ, Hanna Al-Kass FM, Jaafar HN, et al. Bilateral breast involvement in acute myelogenous leukemia. Eur Radiol. 2000;10:1031. doi: 10.1007/s003300051059. [DOI] [PubMed] [Google Scholar]
- 29.Ngu IW, Sinclair EC, Greenaway S, Greenberg ML. Unusual presentation of granulocytic sarcoma in the breast: A case report and review of the literature. Diagn Cytopathol. 2001;24:53–57. doi: 10.1002/1097-0339(200101)24:1<53::aid-dc1009>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 30.Dutta Roy S, Stafford JS, Scally J, Selvachandran SN. Granulocytic sarcoma of the breast antedating acute myelogenous leukemia. Breast. 2004;13:242–46. doi: 10.1016/j.breast.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Shea B, Reddy V, Abbitt P, et al. Granulocytic sarcoma (chloroma) of the breast: A diagnostic dilemma and review of the literature. Breast J. 2004;10:48–53. doi: 10.1111/j.1524-4741.2004.09612.x. [DOI] [PubMed] [Google Scholar]
- 32.Thachil J, Richards RM, Copeland G. Granulocytic sarcoma – a rare presentation of a breast lump. Ann R Coll Surg Engl. 2007;89:W7–9. doi: 10.1308/147870807X227827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delporte F, Voorhoopf LJ, Lodewyck T, De Paepe P. Primary granulocytic sarcoma of the breast: A case report and review of the literature. Eur J Gynaecol Oncol. 2011;32:435–38. [PubMed] [Google Scholar]
- 34.Fu J, Luo J. Granulocytic sarcoma of the breast in acute myeloid leukemia: Two case reports. Oncol Lett. 2014;7:145–47. doi: 10.3892/ol.2013.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goncalves J, Louro LV, Ribeiro I, et al. Radiotherapy for granulocytic sarcoma of the breast – Case report and review of the literature. Rep Pract Oncol Radiother. 2014;19:343–46. doi: 10.1016/j.rpor.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunduz E, Akay MO, Karagulle M, Ak IS. Isolated granulocytic sarcoma of the breast after allogeneic stem cell transplantation: A rare involvement also detected by 18FDG-PET/CT. Turk J Haematol. 2014;31:88–91. doi: 10.4274/Tjh.2012.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Presazzi A, Di Giulio G, Calliada F. Breast hamartoma: Ultrasound, elastosonographic, and mammographic features. Mini pictorial essay. J Ultrasound. 2015;18:373–77. doi: 10.1007/s40477-015-0175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdulkadir E, Gulhan E, Ulus S. Differentiation of secondary involvement of the breast by lymphoreticular malignancy from fibroadenoma using ultrasound elastography: A report of two cases. Indian J Radiol Imaging. 2017;27:237–40. doi: 10.4103/ijri.IJRI_238_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aslan H, Pourbagher A. Breast involvement by hematologic malignancies: Ultrasound and elastography findings with clinical outcomes. J Clin Imaging Sci. 2017;7:42. doi: 10.4103/jcis.JCIS_65_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barr RG, Zhang Z. Shear-wave elastography of the breast: Value of a quality measure and comparison with strain elastography. Radiology. 2015;275:45–53. doi: 10.1148/radiol.14132404. [DOI] [PubMed] [Google Scholar]
- 41.Gkali CA, Chalazonitis AN, Feida E, et al. Primary non-Hodgkin lymphoma of the breast: ultrasonography, elastography, digital mammography, contrast-enhanced digital mammography, and pathology findings. Ultrasound Q. 2015;31:279–82. doi: 10.1097/RUQ.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 42.Sousaris N, Barr RG. Sonoelastography of breast lymphoma. Ultrasound Q. 2016;32:208–11. doi: 10.1097/RUQ.0000000000000213. [DOI] [PubMed] [Google Scholar]