Abstract
Patient: Male, 28
Final Diagnosis: Bartonella endocarditis
Symptoms: Abdominal pain • cough • weight loss
Medication: —
Clinical Procedure: —
Specialty: Infectious Diseases
Objective:
Rare co-existance of disease or pathology
Background:
Culture-negative Bartonella quintana endocarditis is challenging to diagnose and is associated with high mortality rates. Diagnostic confirmation of Bartonella quintana infection requires specialized assays, as identifying Bartonella henselae endocarditis by serology can be difficult due to the high rate of serological cross-reactivity. This is a case report of culture-negative Bartonella quintana endocarditis that was diagnosed with epidemio-logic data, histology, and nucleic acid amplification testing.
Case Report:
A 28-year-old man with a history of homelessness was admitted to hospital with worsening productive cough, weight loss, and abdominal pain. A transthoracic echocardiogram (TTE) showed pulmonary valve vegetation and several aortic valve vegetations. His hospital course was complicated by cardiogenic shock and septic shock requiring transfer to a tertiary care medical intensive care unit. Although blood cultures remained negative for bacterial infection, serology testing was positive for Bartonella henselae and Bartonella quintana IgM and IgG. Nucleic acid amplification testing for 16S ribosomal RNA (rRNA) using valve tissue was diagnostic for Bartonella quintana.
Conclusions:
This case of culture-negative Bartonella quintana endocarditis demonstrates the use of diagnostic nucleic acid amplification methods to confirm the diagnosis.
MeSH Keywords: Aortic Valve Insufficiency; Bartonella Infections; Bartonella Quintana; Endocarditis, Bacterial; RNA, Ribosomal, 16S
Background
Bartonella bacilli are small intracellular Gram-negative organisms that are mainly transmitted by lice and fleas and can infect immunocompetent and immunocompromised individuals [1–3]. These bacilli are endemic to specific regions in Asia, Europe, and Africa but may be found in any part of the world. Bartonella quintana and Bartonella henselae are the two most common species of Bartonella, both of which can cause culture-negative endocarditis [1–3]. The reported incidence of culture-negative endocarditis due to Bartonella has been reported to be increasing [1].
Bartonella henselae and Bartonella quintana are often difficult to distinguish by serologic testing due to serological cross-reactivity [2]. However, there are distinct differences in epidemiologic risk factors and disease complications between the two species that may assist in determining the most likely infectious organism. Bartonella quintana infection is the cause of trench fever, which may lead to endocarditis and chronic bacteremia in homeless individuals, and infection with Bartonella henselae is more common in younger patients who have had recent contact with cats [1,3]. Given the prolonged incubation time and fastidious growth on culture media, endocarditis due to Bartonella species is usually culture-negative and may be undiagnosed, leading to delays in treatment that can result in increased mortality [4].
In this report, a case is presented of a patient with culture-negative Bartonella quintana endocarditis diagnosed using a combined diagnostic approach that included clinical evaluation, imaging, epidemiology, microbiology, serology, histopathology, and 16S ribosomal RNA (rRNA) nucleic acid sequence-based amplification testing.
Case Report
A 28-year-old homeless Nepalese man with no known comorbid diseases, who had immigrated to the United States six years previously presented to the emergency department with six months of productive cough, weight loss, and intermittent abdominal pain. On physical examination, he was found to have a grade 3/6 systolic murmur heard at the right sternal border. Laboratory results initially showed elevated hepatic transaminases. Computed tomography (CT) of the chest showed bilateral pleural effusions, and CT of the abdomen and pelvis were normal. General surgery and infectious disease consultations were obtained. Initially, antibiotics were not given due to a low index of suspicion for infection, and surgical intervention was not recommended given the patient’s overall hemodynamic stability.
Transthoracic echocardiogram (TTE) showed a normal ejection fraction (EF) of 50–55%, a mobile vegetation attached to the pulmonary valve, pulmonary insufficiency, and elevated right heart pressures. The TTE also showed several bulky lesions attached to the aortic valve with associated moderate to severe aortic insufficiency. During the first week of hospitalization, the patient decompensated and developed cardiogenic and septic shock and acute hypoxic respiratory failure, which were all thought to be secondary to complicated subacute bacterial endocarditis. He was treated with intravenous vancomycin and ceftriaxone and was transferred to a tertiary care facility for evaluation for cardiac surgery. He was found to have multi-organ failure with an increased serum lactate level and worsening oliguria requiring initiation of continuous renal replacement therapy (CRRT). Given his worsening clinical course and hemodynamic instability, the patient was considered to be a high-risk surgical candidate, and the decision was made to medically stabilize the patient before surgical intervention.
Because the patient had a history of homelessness and was a recent immigrant from Nepal, following admission to the medical intensive care unit, serology and molecular testing were performed as an infection screen. Table 1 summarizes the serology results from the infection screen. QuantiFERONTB Gold In-Tube® testing showed evidence of latent tuberculosis infection. However, the chest x-ray findings were not consistent with active pulmonary tuberculosis, and three sputum specimens were negative for acid-fast bacilli on smear and culture. Serology was positive for Bartonella henselae IgM (1: 64) and IgG (1: 1024) and Bartonella quintana IgM (1: 64) and IgG (1: 256). Following these results, intravenous doxycycline and gentamicin treatment began on hospital day 13. He was found to have only two out of 33 blood cultures that were positive throughout his four-month period of hospitalization. Candida albicans was grown at about two months into his hospital course, and because the source of infection was most likely to have been secondary to an indwelling catheter used for total parenteral nutrition (TPN), he was subsequently treated with intravenous micafungin.
Table 1.
Summary of the results from the infection screen in a 28-year-old homeless man with culture-negative endocarditis.
| B. henselae IgG | 1: 1024 |
| B. henselae IgM | 1: 64 |
| RMSF IgG | Positive |
| RMSF IgM | Negative |
| Coxiella burnetti Phase 1 and 2 IgM and IgG | Negative |
| Brucella Ab | Negative |
| Mycoplasma pneumonia PCR | Negative |
| Urine Legionella Ag | Negative |
| Quantiferon TB gold | Positive |
| Respiratory syncytial virus A | Positive |
| Epstein Barr virus PCR | Positive |
| HSV1 PCR | Positive |
| HAV Ab | Positive |
| HBsAb | Positive |
| HBsAg | Negative |
| HBcAb | Negative |
| HCV Ab | Negative |
| Cytomegalovirus PCR | 62,356 |
| Influenza PCR | Negative |
Ab – antibody; B. henselae – Bartonella henselae; Brucella Ab – Brucella antibody; B. quintana – Bartonella quintana; HAV Ab – hepatitis A virus antibody; HbcAb – hepatitis B core antibody; HBsAb – hepatitis B surface antibody; HBsAg – hepatitis B surface antigen; HCV Ab – hepatitis C virus antibody; HSV1 – herpes simplex virus 1; IFA – indirect immunofluorescence antibody assay; PCR – polymerase chain reaction; RMSF – Rocky Mountain spotted fever; TB – tuberculosis.
After 43 days of medical optimization, the patient underwent mechanical aortic valve replacement and mitral valve repair.
During surgery, tissue was obtained from the aortic valve and mitral valve, and 16S ribosomal RNA (rRNA) polymerase chain reaction (PCR) testing of aortic valve tissue was positive for Bartonella quintana. Histopathology of the aortic valve and pulmonary valve showed vegetations and destruction of the valve tissue, with fibrinoid necrosis and inflammation (Figures 1, 2). Histochemical staining was negative for intracellular organisms using Warthin-Starry silver stain. His postoperative course was complicated by peri-aortic thrombosis and surgical wound dehiscence requiring multiple thoracotomies. The patient died after four months of hospitalization after suffering from postoperative hemothorax and gastrointestinal bleeding complicated by hemorrhagic shock.
Figure 1.
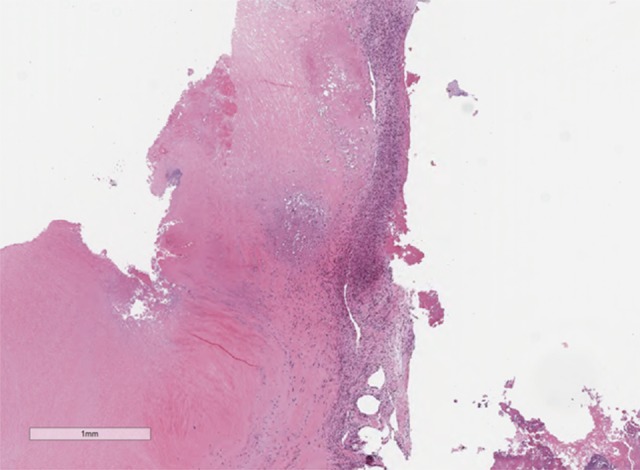
Photomicrograph of the histology of the pulmonary valve. Low power image of the histology of the pulmonary valve shows an inflammatory infiltrate and fibrinoid necrosis. No viable tissue is present. Hematoxylin and eosin (H&E). Magnification, ×2.
Figure 2.
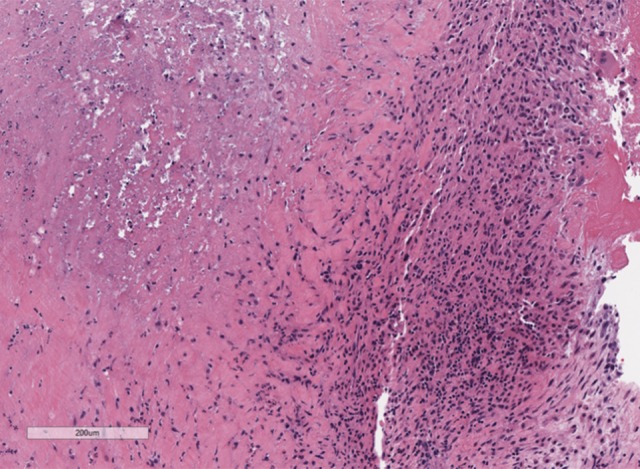
Photomicrograph of the histology of the pulmonary valve. Higher power image of the histology of the pulmonary valve shows an inflammatory infiltrate and fibrinoid necrosis. Hematoxylin and eosin (H&E). Magnification, ×8.
Discussion
This case report illustrates the importance of a multidisciplinary diagnostic and management approach that used a combination of epidemiology, serology, and histopathology in the diagnosis of Bartonella quintana culture-negative endocarditis. A species of Bartonella was the likely pathogen given the presence of multiple serum samples that were positive for Bartonella IgG and IgM. Candida albicans was unlikely the source of endocarditis as cultures were positive two months into the patient’s hospitalization and occurred after the initiation of total parenteral nutrition (TPN). The patient had a history of homelessness, increasing his risk for endocarditis due to Bartonella quintana [1,5]. Histopathology of the valvular tissue showed extensive fibrinoid necrosis, and inflammation, consistent with Bartonella endocarditis [6]. Most importantly, valvular tissue was positive for Bartonella quintana by 16S ribosomal RNA (rRNA) polymerase chain reaction (PCR), a nucleic acid amplification test that has become increasingly used in the diagnosis of culture-negative endocarditis [7]. Therefore, Bartonella quintana was confirmed as the cause of culture-negative endocarditis in this patient.
Culture-negative endocarditis is increasingly reported in the literature, and it has been estimated that up to 55% of cases of infective endocarditis are culture-negative [8]. In 1994, new echocardiographic criteria were established by the Duke Endocarditis Service for the diagnosis of infective endocarditis, which is referred to as the Duke criteria [9]. When infective endocarditis is difficult to diagnose, the modified Duke criteria may also be negative, and the diagnosis requires support with additional methods. The findings from a study by Lamas and Eykyn reported that only 21% of patients with blood culture-negative endocarditis were initially diagnosed using the Duke criteria [10]. The evaluation for organisms commonly associated with culture-negative endocarditis, including Bartonella, Coxiella, and Chlamydia, is crucial for definitive diagnosis. In France, Bartonella is the causative organism in between 20–30% of all documented cases of blood culture-negative endocarditis [11,12].
Commonly associated risk factors for Bartonella endocarditis include exposure to poor sanitary conditions, a history of cat scratch disease, or the presence of a cardiac murmur [4]. Patients at highest risk for Bartonella infection have limited access to medical resources, resulting in adverse outcomes due to delays in diagnosis. Treatment generally consists of at least two weeks of antibiotic therapy, specifically including combination therapy with an aminoglycoside, as well as surgery, depending on the degree of valvular involvement and hemodynamic stability of the patient [13]. This patient’s history of homelessness predisposed him to Bartonella quintana infection due to his exposure to lice [3]. Bartonella quintana is most commonly found in patients living in areas of high population density with poor sanitary conditions. In a study by Dalton et al., 30% of 71 homeless individuals who were tested in France had seropositivity to Bartonella quintana [5]. In a study reported by Fournier et al., 38 of 48 cases of Bartonella endocarditis were positive for Bartonella quintana, and only ten cases were positive for Bartonella henselae. Despite testing positive for both Bartonella henselae and Bartonella quintana by serology, this patient’s epidemiologic risk factors suggested a diagnosis of Bartonella quintana endocarditis.
This case demonstrates the importance of utilizing multiple diagnostic approaches to distinguish between species of Bartonella in cases of infection. Previous studies have supported that the examination of valve tissue may be more sensitive than blood culture, due to the higher bacterial content in the tissue [14,15]. Although histochemical staining using the Warthin–Starry silver stain was negative, this is a non-specific histochemical stain that did not exclude infection with Bartonella [2]. Lepidi et al. investigated the characteristic Bartonella infection involving the cardiac valves using histopathology and described the characteristic findings of extensive fibrinoid necrosis, and inflammation [6]. These findings are similar to those found in the histology in this case. Bartonella quintana has also been shown to be more frequently associated with infective endocarditis in individuals with previously normal native valves when compared with Bartonella henselae, which may indicate that patients with Bartonella quintana endocarditis may have been bacteremic for long periods before developing endocarditis [16].
According to the 2015 European Society for Cardiology (ESC) guidelines for the management of infective endocarditis, cases of culture-negative endocarditis should raise suspicion for infection due to fastidious bacteria, and serology testing should be performed for Coxiella burnetii, Bartonella henselae, Bartonella quintana, Legionella pneumophila, Brucella, Mycoplasma, and Aspergillus [17]. This patient had positive serology results for both Bartonella henselae and Bartonella quintana, and a high rate of cross-reactivity with serology testing has previously been reported for these organisms [2,16]. McGill et al. found extensive cross-reactivity between Bartonella henselae and Rickettsia rickettsii or Rocky mountain spotted fever (RMSF), and also with Chlamydia, Mycoplasma pneumoniae, and Escherichia coli [2]. Therefore, serology studies are unlikely to distinguish between different species of Bartonella.
Nucleic acid amplification testing can help to distinguish between bacterial species when serology testing is inconclusive [17]. Bacteria have three genes that code for rRNA functionality, including 5S, 16S, and 23S [14]. The 16S rRNA gene is highly conserved but has variable regions that allow for discrimination between species and is now increasingly used for the molecular diagnosis of infectious diseases, including culture-negative endocarditis [14]. A proposal has been made to incorporate 16S rRNA positivity as an additional factor within the modified Duke criteria [1]. 16S rRNA polymerase chain reaction (PCR) is also useful in detecting the presence of non-viable bacterial DNA in culture-negative endocarditis in cases in which antimicrobial therapy has already been initiated [7]. Akram et al. compared the utility of 16S rRNA PCR with microbiologic testing, and PCR testing of the cardiac valves was positive in 31.2% of cases compared with 21.9% by culture [7]. Therefore, 16S rRNA PCR should be considered to be a valuable diagnostic tool for the identification of infectious organisms in culture-negative endocarditis.
Conclusions
Culture-negative Bartonella endocarditis remains difficult to diagnose due to the low diagnostic yield of available serology, microbiology, and histopathology methods. This case has demonstrated the challenging clinical issues associated with the serology cross-reactivity between Bartonella and other species that may also cause culture-negative endocarditis. Also, delays in diagnosis in patients with culture-negative endocarditis results in a relatively high mortality rate. This case has demonstrated the importance of using a multidisciplinary approach to diagnose culture-negative endocarditis and has reviewed the use of serology assays and 16S ribosomal RNA (rRNA) polymerase chain reaction (PCR) for diagnosis compared to blood cultures and histology of tissue samples. In cases of culture-negative endocarditis, a diagnostic approach involving assessment of clinical symptoms and epidemiologic risk factors in conjunction with microbiology, serology, histopathology, and molecular testing are crucial to arrive at a definitive diagnosis that allows timely and optimal patient management.
Footnotes
Conflict of interest
None.
References:
- 1.Edouard S, Nabet C, Lepidi H, et al. Bartonella, a common cause of endocarditis: A report on 106 cases and review. J Clin Microbiol. 2015;53:824–29. doi: 10.1128/JCM.02827-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGill SL, Regnery RL, Karem KL. Characterization of human immunoglobulin (Ig) isotype and IgG subclass response to Bartonella henselae infection. Infect Immun. 1998;66:5915–20. doi: 10.1128/iai.66.12.5915-5920.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fournier PE, Lelievre H, Eykyn SJ, et al. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: A study of 48 patients. Medicine (Baltimore) 2001;80:245–51. doi: 10.1097/00005792-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Raoult D, Fournier PE, Drancourt M, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125:646–52. doi: 10.7326/0003-4819-125-8-199610150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Dalton M, Robinson L, Cooper J. Use of Bartonella antigens for serologic diagnosis of cat-scratch disease at a national referral center. Arch Intern Med. 1995;155:1670–76. [PubMed] [Google Scholar]
- 6.Lepidi H, Fournier PE, Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol. 2000;114:880–89. doi: 10.1309/R0KQ-823A-BTC7-MUUJ. [DOI] [PubMed] [Google Scholar]
- 7.Akram A, Maley M, Gosbell I. Utility of 16S rRNA PCR performed on clinical specimens in patient management. Int J Infect Dis. 2017;57:144–49. doi: 10.1016/j.ijid.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Schabereiter-Gurtner C, Nehr M, Apfalter P, et al. Evaluation of a protocol for molecular broad-range diagnosis of culture-negative bacterial infections in clinical routine diagnosis. J Appl Microbiol. 2008;104:1228–37. doi: 10.1111/j.1365-2672.2007.03648.x. [DOI] [PubMed] [Google Scholar]
- 9.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: Utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200–9. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 10.Lamas CC, Eykyn SJ. Blood culture-negative endocarditis: Analysis of 63 cases presenting over 25 years. Heart. 2003;89(3):258–62. doi: 10.1136/heart.89.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fournier PE, Thuny F, Richert H, et al. Comprehensive diagnostic strategy for blood culture-negative endocarditis: A prospective study of 819 new cases. Clin Infect Dis. 2010;51:131–40. doi: 10.1086/653675. [DOI] [PubMed] [Google Scholar]
- 12.Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: Etiologic diagnosis of 348 cases. Medicine (Baltimore) 2005;84(3):162–73. doi: 10.1097/01.md.0000165658.82869.17. [DOI] [PubMed] [Google Scholar]
- 13.Raoult D, Fournier P-E, Vandenesch F. Outcome and treatment of Bartonella endocarditis. Arch Intern Med. 2003;163:226–30. doi: 10.1001/archinte.163.2.226. [DOI] [PubMed] [Google Scholar]
- 14.Millar BC, Moore JE. Current trends in the molecular diagnosis of infective endocarditis. Eur J Clin Microbiol Infect Dis. 2004;23:353–65. doi: 10.1007/s10096-004-1132-6. [DOI] [PubMed] [Google Scholar]
- 15.Chin YT, Hasan R, Qamruddin A. 16S rRNA PCR for the diagnosis of culture-negative Bartonella quintana endocarditis: The importance of sample type. Indian J Med Microbiol. 2015;33:185–86. doi: 10.4103/0255-0857.148429. [DOI] [PubMed] [Google Scholar]
- 16.Fournier PE, Mainardi JL, Raoult D. Value of microimmunofluorescence for diagnosis and follow-up of Bartonella endocarditis. Clin Diagn Lab Immunol. 2002;9:795–801. doi: 10.1128/CDLI.9.4.795-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC) Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]


