Abstract
Objective:
This study aimed to evaluate the association between subclinical hypothyroidism and thyroid autoantibodies with clinical pregnancy rate after intrauterine insemination (IUI) in euthyroid women.
Methods:
In this prospective cohort study, we recruited 497 women who underwent IUI treatment. We assessed thyroid function tests, thyroid antibodies and clinical pregnancy rates of the patients.
Results:
The patients were divided into two groups according to TSH values: normal group, n=387, and subclinical hypothyroidism group 2, n=110. The clinical pregnancy rate was 15.2% in the Control Group and 17.3% in the study group (p=0.656). In the Study Group, 35% of the patients had anti-TPO positivity (p=0.531) and 42.1% of the patients had anti-TG positivity (p=0.285). There was no statistically significant difference in clinical pregnancy rates between the groups in terms of antithyroid antibody positivity (p=0.54; p=0.559, respectively).
Conclusion:
Anti-TPO antibodies and subclinical hypothyroidism had no impact on clinical pregnancy rates in the women submitted to IUI.
Keywords: autoantibody, clinical pregnancy, intrauterine insemination, subclinical hypothyroidism, thyroglobulin, thyroid peroxidase
INTRODUCTION
Thyroid disorders are one of the most common endocrinological diseases affecting women of reproductive age (Negro & Mestman, 2011; Krassas et al., 2010; Sarac & Koc, 2018). They are associated with adverse reproductive outcomes such as spontaneous abortion and infertility (Krassas et al., 2010; Kalem et al., 2016; Liu et al., 2005). Hypothyroidism is responsible for harmful effects on fetal health, the guidelines suggest that TSH (thyroid stimulating hormone) levels should be <2.5 mIU/L in pregnant or first trimester pregnant women (Burman, 2009; Garber et al., 2012). The 2012 guidelines of the American Thyroid Association and the American Society of Clinical Endocrinologists recommend limiting serum TSH to 2.5 mIU/L in euthyroid patients planning to become pregnant (Garber et al., 2012). However, in 2017 the American Society of Thyroid Guidelines recommended the upper reference limit of TSH to 4.0 mIU/L (Alexander et al., 2017). There is no clear consensus on the efficacy of an upper value for TSH and the effects on fertility outcomes (Miko et al., 2017).
Chronic autoimmune thyroiditis (named Hashimoto thyroiditis) (HT), is the most common endocrinopathy in premenopausal women in developed countries (Friedrich et al., 2008). All over the world, iodine deficiency is still the most common cause of thyroid dysfunction (Hayashi et al., 1986). Various studies have shown that HT is associated with various gynecological problems, recurrent miscarriage, unexplained infertility, and in vitro fertilization failure (Dendrinos et al., 2000; Poppe et al., 2007; van den Boogaard et al., 2011; Aydın et al., 2008).
There is a negative association between maternal thyroid dysfunction and low birth weight, preterm birth, preeclampsia and decreased intelligence (De Groot et al., 2012). Subclinical hypothyroidism without thyroid autoantibodies seems to be related to the problems mentioned above (Negro et al., 2010; Benhadi et al., 2009). Therefore, hypothyroidism treatment during pregnancy is essential. Recommendations for the treatment of subclinical hypothyroidism before and during pregnancy also differ (Vila et al., 2014).
Intrauterine insemination (IUI) is widely used to treat infertility, and it is considered a non-invasive and less expensive treatment when compared to assisted reproduction techniques (ART) such as in vitro fertilization (IVF) (Dilbaz et al., 2011). The clinical pregnancy rates differ between indications in 8-20% per cycle (Merviel et al., 2014; Dilbaz et al., 2011). Fertility treatment outcome in the presence of thyroid problems is challenging (Practice Committee of the American Society for Reproductive, 2015; Tan et al., 2014; Tuncay et al., 2018).
In this study, we aimed to evaluate a possible association between subclinical hypothyroidism and thyroid autoantibodies with clinical pregnancy rates after intrauterine insemination in euthyroid women.
MATERIALS AND METHODS
Sample and Data
We recruited 497 women who applied to the reproductive endocrinology and infertility clinics of the Zekai Tahir Burak Women's Health Education and Research Hospital from October 2015 to June 2017 in this prospective cohort study. The study was approved by the Local Ethics Committee of the institution (05.27.2015 #20), and the universal principles of the Declaration of Helsinki were applied (World Medical Association, 2013). We excluded those with tubal factor infertility, male infertility, endometriosis, and systemic disorders such as overt diabetes mellitus, cardiac pathologies and known thyroid diseases (medications such as levothyroxine or anti-thyroid drugs). Semen samples were obtained by masturbation after 2-3 days of sexual abstinence. Sperm preparation was undertaken using the swim-up technique and stored at room temperature until the time of insemination. IUI was performed using a soft IUI catheter. Semen parameters were analyzed according to the WHO 2010 criteria (Cooper et al., 2010). All patients received clomiphene citrate treatment (50-100 mg/day) (Klomen@, Kocak Farma, Istanbul, Turkey) orally or 37.5-150 IU of pure FSH or human menopausal gonadotropin (hMG) (Gonal-F@, Merck Sereno/ Menogon@, Ferring Pharmaceuticals Istanbul, Turkey), respectively starting on 3-5 cycle days of menstruation and lasting until 7-9 cycle days of menstruation for ovulation induction. The drug dosage was individualized according to patient response and/or the data from the previous cycles. We performed serial transvaginal ultrasonography (TVUS) examinations. We administered a dose of 10,000 IU of urinary HCG or 250 mg of recombinant HCG (Pregnyl@, Organon, Istanbul, Turkey, respectively) when at least one follicle of ≥18 mm was seen upon transvaginal ultrasonography. To prevent multiple pregnancies, we included only cycles with mono and bi-follicular growth (>18 mm) in the analysis.
We evaluated the demographic features, infertility types, infertility duration, endometrial thickness on HCG day, basal hormonal parameters (FSH, E2), thyroid function tests [free tri-iodothyronine (fT3) and free thyroxine (fT4) and TSH], thyroid antibodies [antithyroid peroxidase (anti-TPO) and antithyroglobulin (anti-TG) antibodies] and clinical pregnancy rates of the patients. We ran a qualitative serum β-HCG test 14 days after insemination if menstruation had not started. Clinical pregnancy was defined as the presence of a gestational sac with accompanying fetal heartbeat by ultrasound at least 4 weeks after IUI.
Laboratory Analysis
Blood samples were taken from the participants' antecubital veins. All serum parameters included in this analysis were obtained on the 3rd to the 5th day of the menstrual cycle with IUI. In our department, the normal range for TSH is 0.34-5.6µIU/ml; for fT3 it is 2.5-3.9pg/ml; for fT4 it is 0.61-1.12ng/dl; 0-9IU/ml for anti-TPO; and 0-4IU/ml for anti-TG. These normal ranges were calculated by the laboratory and all examined serum parameters were determined in the ISO-certified central laboratory of the Dr. Zekai Tahir Burak Women's Health Care University of Health Sciences, Education and Research Hospital, Ankara, TURKEY, using commercially available assays using the Elecsys electrochemiluminescence immunoassays on a Cobas 6000 immunoanalyzer (Roche Diagnostics, Mannheim, Germany). The inter and intra assay CVs were <2% and <6.5% for TSH, ≤2% and <5% for fT4, ≤2% and <5% for fT3, <5% and ≤7% for anti-TPO, <2% and ≤5% for anti-TG. The TSH measuring range was 0.005-100 µIU/mL; for fT3 it was 0.3-10 nmol/L; for fT4 it was 0.101-7.77 ng/dL; for anti-TPO it was 5-600 IU/ml; for anti-TG it was 10-4000 IU/ml.
Statistical Analysis
We used the statistical Package for the Social Sciences, version 23.0 (SPSS Inc., Chicago, IL) for the statistical analysis. The sample size calculation for the entire study population, a two-sample comparison with a 5% level of significance (alpha) and a power of 0.80 with an allocation ratio of 3:1, gave a study population of 315 vs. 104 women in each group. Sample size calculations were performed using the G*Power v3.1.5 general power analysis program (Faul et al., 2007). For quantitative data, we used mean values and standard deviations, whereas for quantitative data we used numbers and percentages. We used the Kolmogorov-Smirnov and Shapiro-Wilk tests to assess the normal distribution of the univariate variables. In order to analyze the variables that did not have normal distribution we used non-parametric methods. Non-parametric variables between groups were compared through the Mann-Whitney U test. For categorical variables we used the Fisher's exact or the Pearson Chi-Square test, where appropriate. We ran an ROC curve analysis to determine a cut-off value for pregnancy prediction. p values less than 0.05 indicated statistical significance. The p-value presented in our statistical analysis are for two-tailed tests.
RESULTS
The area under the ROC curve revealed that no cut-off value of TSH can predict pregnancy in intrauterine insemination cycles (the area under the ROC curve was 0.495 (%95 CI: 0.424-0.566) (p-value=0.887). Therefore, the patients were divided into two groups as TSH values between 0.35-2.49mIU/L (group 1, n=387) and 2.52-4.88 mIU/L (group 2, n=110). The groups were statistically comparable in terms of the variables mentioned.
Demographic features of the subjects are shown in Table 1. Of these, 387 women (77.2%) had TSH values between 0.35-2.49 mIU/L (control group), and 110 women (22.8%) had TSH values between 2.52-4.88 mIU/L (study group). There were no statistically significant differences between the groups in terms of age, BMI, infertility duration, infertility type (primer/seconder), ovulation induction protocol and clinical pregnancy rates (p>0.05). The clinical pregnancy rate was 15.2% in the Control Group and 17.3% in the study group (p=0.656). There was no statistically significant difference between the groups in terms of FSH, E2 and endometrial thickness on the ovulation trigger day (Table 2).
Table 1.
Comparison of the subjects' demographic features
| Control Group (n=387) | Study Group (n=110) | p-value | |
|---|---|---|---|
| Age (years) | 27 (18-44) | 26 (19-34) | 0.395** |
| BMI (kg/m2) | 24.44 (16.44-40.39) | 24.69 (18.73-38.71) | 0.313** |
| Infertility duration | 3 (1-14) | 3 (1-14) | 0.176** |
| Infertility | 1.00* | ||
| -Primary | 298 (77%) | 85 (77.3%) | |
| -Secondary | 89 (23%) | 25 (22.7%) | |
| Ovulation Induction | 0.813*** | ||
| clomiphene citrate | 248 (64.1%) | 67 (60.9%) | |
| rFSH | 128 (33.1%) | 40 (36.4%) | |
| hMG | 11 (2.8%) | 3 (2.7%) | |
| Clinical pregnancy rate, n (%) | 59 (15.2%) | 19 (17.3%) | 0.656* |
Fisher’s exact
Mann Whitney U
Table 2.
Laboratory parameters
| Control group (n=387) | Study group (n=110) | p-value | |
|---|---|---|---|
| FSH | 6.51 (4-15) | 6.7 (3.2-55.7) | 0.879** |
| E2 | 42 (5-163) | 39.77 (5-84) | 0.567** |
| Endometrial Thickness | 9 (4-15) | 8.5 (5-17) | 0.528** |
| TSH | 1.36 (0.35-2.49) | 3.15 (2.52-4.88) | <0.0001** |
| fT3 | 3.09 (2.12-4.65) | 3.16 (2.3-4.8) | 0.54** |
| fT4 | 0.91 (0.56-3.24) | 0.92 (0.68-1.54) | 0.559** |
| Anti-TPO (%) | 29.2% | 35% | 0.531* |
| Anti-TG (%) | 29% | 42.1% | 0.285* |
Fisher’s exact
Mann Whitney U
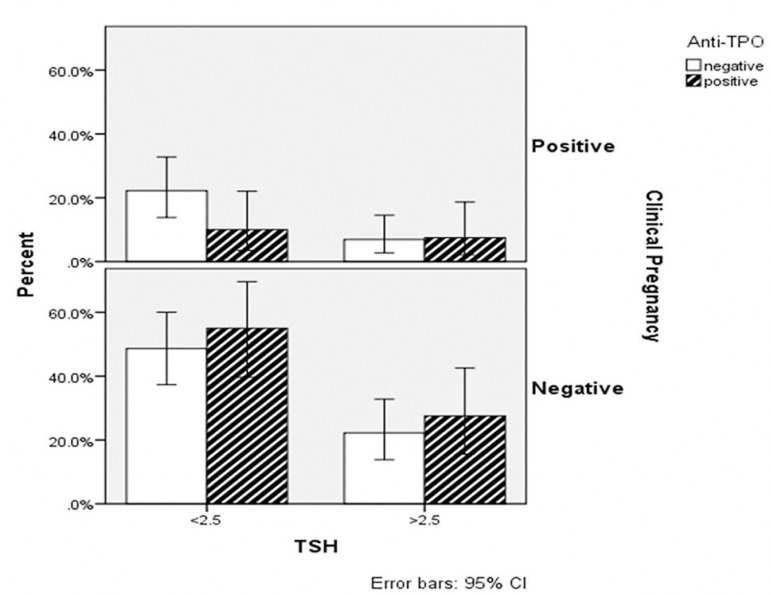
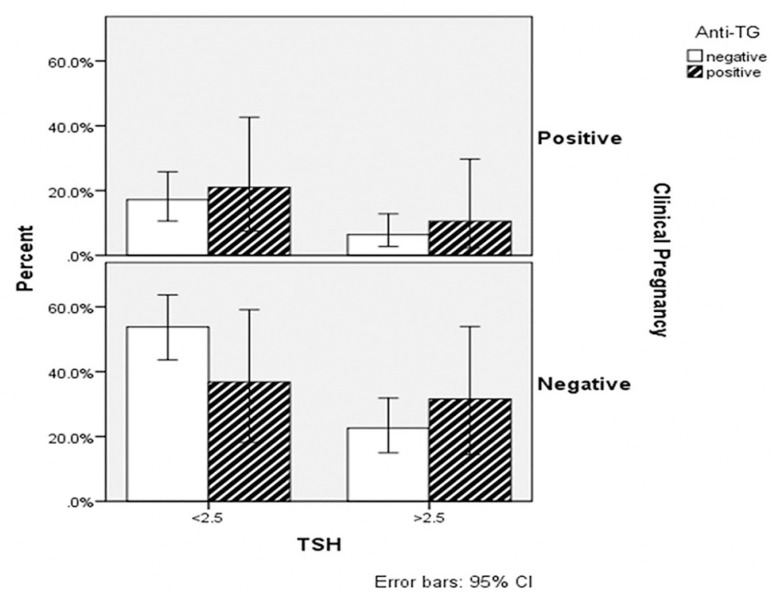
Anti-TPO positivity was present in 35% vs. 29.2% of patients in the Study and Control groups, respectively (p=0.531); while anti-TG positivity was present in 42.1% vs. 29% in the Study and Control groups, respectively (p=0.285) (Table 2). There was no statistically significant difference for clinical pregnancy rates between the groups in terms of antithyroid antibody positivity (Figures 1 and 2). No statistically significant difference between the groups was seen in terms of fT3 and fT4 results (p=0.54; p=0.559, respectively) (Table 2).
Figure 1.
The clinical pregnancy rate of patients concerning TSH and anti-TPO positivity
Figure 2.
The clinical pregnancy rate of patients about TSH and anti-TG positivity
DISCUSSION
The fertility treatment outcome in the presence of thyroid autoimmunity (TAI) and subclinical hypothyroidism is contradictory (Unuane et al., 2017; Medenica et al., 2015; Karmon et al., 2015; Jatzko et al., 2014; Tuncay et al., 2018). In this study, we investigated the fertility outcome in euthyroid women treated with IUI concerning the TSH threshold and antithyroid antibodies. We found no significant differences in fertility outcomes among euthyroid women between the groups. The clinical pregnancy rate was similar between the two groups. 59 patients (15.2%) out of the 397 patients in the low-TSH group (Control Group) became pregnant, whereas the clinical pregnancy rate was 19/110 (17.3%) in the subclinical hypothyroidism group (Study Group).
In the euthyroid patient group with women of normal upper TSH values we have found similar IUI outcomes compared to women with baseline TSH <2.5 mIU/L. Unexpectedly, some of the previous studies also showed results similar to those from our study; the women with a TSH score of >2.5 mIU/L before IUI had a higher birth rate after a clinical pregnancy and lower spontaneous abortion risk (Tuncay et al., 2018; Jatzko et al., 2014). Reh et al. (2010) observed that there was no significant difference in clinical pregnancy or birth rates between TSH levels of 0.4-2.4 mIU/L and women above 2.5 mIU/L in the infertile population. They did not report any difference in miscarriage rates in the low and high TSH groups (Reh et al., 2010). In another study carried out by Karmon et al. (2015), there was no significant difference in clinical pregnancy rates among women with TSH levels of 0.4-2.4 mIU/L and levels > 2.5 mIU/L. In addition, they found that preconceptional TSH levels were inversely associated with spontaneous abortion and positively associated with live birth after clinical pregnancy (Karmon et al., 2015).
The American Thyroid Association supported the 2012 guidelines on hypothyroidism management in pregnancy (Garber et al., 2012). The document strengthens the idea of keeping TSH levels at <2.5 mIU/L in women with hypothyroidism during the first trimester of pregnancy. Guidelines should also recommend treatment if TSH levels for euthyroid women are 2.5 mIU/L or higher in the first trimester or in those planning a pregnancy. This supports the view that physiologically HCG cross-reacts with the TSH receptor and causes a decrease in TSH levels (Gilbert et al., 2008).
In addition, many studies have redefined the TSH reference intervals in pregnancy and argued that there should be lower values in the first trimester (Springer et al., 2009; Garber et al., 2012; Ödöl et al., 2009). However, there is no evidence that pre-pregnancy outcome in early euthyroid women with high normal TSH levels has altered early cycle and pregnancy outcomes. Furthermore, since general screening is not recommended, it is difficult to make a decision to intervene in the high normal TSH values found incidentally in a non-pregnant asymptomatic patient (Committee on Patient Safety and Quality Improvement; Committee on Professional Liability, 2007).
Recent studies in pregnant women in Asia (China, Korea, and India) have shown that there is only a minimal reduction in the upper reference level (Li et al., 2014; Moon et al., 2015). According to these results, in the recent guidelines of the American Thyroid Association, the lower reference range of TSH decreased by about 0.4 mIU/L, the upper reference range decreased by about 0.5 mIU/L. This corresponds to a TSH upper limit of 4.0 mIU/L for patients in the first trimester (Alexander et al., 2017). In our study, no cut-off limit for TSH can be found to predict pregnancy. A recent guide from the Practice Committee of the American Society for Reproductive Medicine (2015) states that there is insufficient data to indicate that TSH levels between 2.5 and 4 mIU/L are associated with abortion and pregnancy side effects.
In a study by Negro et al. (2010), in 4,123 thyroid antibody-negative women, it was reported that the loss of pregnancy below 11 weeks was higher in people with TSH levels of 2.5-5 mIU/L. There may be a few reasons for this. The authors did not work on the infertile population, but included women who were in their first trimester with spontaneous pregnancies. In their study, all TSH levels were measured in the preconceptional period. This difference can be explained in part as follows; women with TSH levels ≥2.5 mIU/L in the first trimester may have higher levels before pregnancy if TSH drops in early gestation, as suggested in the literature (Gilbert et al., 2008). In addition, over-stimulation appears to influence TSH levels (Gracia et al., 2012). Future studies should clarify the potential benefits of treatment of women with high normal TSH levels who are already pregnant and asymptomatic, or who plan to become pregnant (naturally or otherwise).
The strengths of this study include the large sample size and its unique population of women undergoing IUI, which allowed the uniform assessment of preconceptional levels of TSH. A related point is that all the patients in our center routinely undergo TSH measurement before receiving IUI treatments.
Despite these advantages, an associated limitation is that the live birth, spontaneous abortion and other obstetric or fetal end points of our subjects were not available. Further evaluation of this relation is necessary to rule out the possibility of chance and unmeasured confounding.
TSH and TAI were independently associated with pregnancy outcomes after spontaneous conception or ART (Thangaratinam et al., 2011). One review showed antithyroid antibodies were not associated with increased reproductive loss in patients submitted to ART treatments (Leiva et al., 2017). In two meta-analyses carried out with ART (Busnelli et al., 2016; Toulis et al., 2010), TAI has a potentially harmful effect on pregnancy. In a meta-analysis involving twelve studies, Busnelli et al. (2016) showed a negative TAI effect of in terms of an increased risk of miscarriage and a decreased chance of live birth. In another meta-analysis involving four studies, Toulis et al. (2010) showed a 2-fold increase in risk of miscarriage for TAI-positive patients, but no significant effect on clinical pregnancy and live birth rates. In another study, pregnancy outcomes of 114 TAI-positive and 495 TAI-negative infertile women were compared and there was no significant difference in implantation, fertilization rate, pregnancy rates and live birth rates (Łukaszuk et al., 2015). Tan et al. (2014) concluded that pregnancy outcome was comparable between women with and without TAI after-ICSI, but TAI status did not affect ICSI outcomes alone.
Several hypotheses have been proposed to explain the possible causal relationship between TAI and negative obstetric outcome. First, TAI can lead to a general immune imbalance, implantation failure targeting the reproductive tract. Thus, thyroid antibodies are considered to be among the causes of fertility problems and recurrent pregnancy loss. Second, thyroid antibodies may cause thyroid function decline as an undesirable pregnancy outcome. A positive TAI status increases the risk of developing (sub) clinical hypothyroidism (Medici et al., 2014). In Unuane's study, the baseline characteristics of both patient groups were similar. There was a significant higher mean TSH in the anti-TPO positive group upon the fertility treatment onset (Unuane et al., 2017).
In conclusion, with this large prospective cohort study we could not find any significant difference in clinical pregnancy rates in women with and without anti-TPO antibodies and subclinical hypothyroidism who underwent IUI. We could not confirm that a TSH level above 2.5 mIU/l has a negative effect on pregnancy rates. More prospective studies are needed to confirm our results, which will shed new light on the impact of thyroid function on IUI success. Future studies will also be useful to clarify which TSH threshold for thyroid hormone replacement should be used for infertile women.
REFERENCES
- Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, Peeters RP, Sullivan S. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27:315–389. doi: 10.1089/thy.2016.0457. [DOI] [PubMed] [Google Scholar]
- Aydın A, Dönmez M, Aydın Y, Karatekin G, Özdemir G, Oruç Ö. Thyroid Storm Complicating Pregnancy, A Case Report and Management. Gynecol Obstet Reprod Med. 2008;14:121–124. [Google Scholar]
- Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol. 2009;160:985–991. doi: 10.1530/EJE-08-0953. [DOI] [PubMed] [Google Scholar]
- Burman KD. Controversies surrounding pregnancy, maternal thyroid status, and fetal outcome. Thyroid. 2009;19:323–326. doi: 10.1089/thy.2009.1570. [DOI] [PubMed] [Google Scholar]
- Busnelli A, Paffoni A, Fedele L, Somigliana E. The impact of thyroid autoimmunity on IVF/ICSI outcome: a systematic review and meta-analysis. Hum Reprod Update. 2016;22:793–794. doi: 10.1093/humupd/dmw034. [DOI] [PubMed] [Google Scholar]
- Committee on Patient Safety and Quality Improvement. Committee on Professional Liability ACOG Committee Opinion No. 381: Subclinical hypothyroidism in pregnancy. Obstet Gynecol. 2007;110:959–960. doi: 10.1097/01.AOG.0000263932.05511.d4. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2543–2565. doi: 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- Dendrinos S, Papasteriades C, Tarassi K, Christodoulakos G, Prasinos G, Creatsas G. Thyroid autoimmunity in patients with recurrent spontaneous miscarriages. Gynecol Endocrinol. 2000;14:270–274. doi: 10.3109/09513590009167693. [DOI] [PubMed] [Google Scholar]
- Dilbaz B, Özkaya E, Çınar M, Çakır E, Dilbaz S. Predictors of Total Gonadotropin Dose Required for Follicular Growth in Controlled Ovarian Stimulation with Intrauterin Insemination Cycles in Patients with Unexplained Infertility or Male Subfertility. Gynecol Obstet Reprod Med. 2011;17:28–34. [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Friedrich N, Schwarz S, Thonack J, John U, Wallaschofski H, Völzke H. Association between parity and autoimmune thyroiditis in a general female population. Autoimmunity. 2008;41:174–180. doi: 10.1080/08916930701777629. [DOI] [PubMed] [Google Scholar]
- Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA, American Association of Clinical Endocrinologists. American Thyroid Association Taskforce on Hypothyroidism in Adults Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988–1028. doi: 10.4158/EP12280.GL. [DOI] [PubMed] [Google Scholar]
- Gilbert RM, Hadlow NC, Walsh JP, Fletcher SJ, Brown SJ, Stuckey BG, Lim EM. Assessment of thyroid function during pregnancy: first-trimester (weeks 9-13) reference intervals derived from Western Australian women. Med J Aust. 2008;189:250–253. doi: 10.5694/j.1326-5377.2008.tb02015.x. [DOI] [PubMed] [Google Scholar]
- Gracia CR, Morse CB, Chan G, Schilling S, Prewitt M, Sammel MD, Mandel SJ. Thyroid function during controlled ovarian hyperstimulation as part of in vitro fertilization. Fertil Steril. 2012;97:585–591. doi: 10.1016/j.fertnstert.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi N, Tamaki N, Konishi J, Yonekura Y, Senda M, Kasagi K, Yamamoto K, Iida Y, Misaki T, Endo K, Torizuka K, Mori T. Sonography of Hashimoto's thyroiditis. J Clin Ultrasound. 1986;14:123–126. doi: 10.1002/jcu.1870140208. [DOI] [PubMed] [Google Scholar]
- Jatzko B, Vytiska-Bistorfer E, Pawlik A, Promberger R, Mayerhofer K, Ott J. The impact of thyroid function on intrauterine insemination outcome--a retrospective analysis. Reprod Biol Endocrinol. 2014;12:28–28. doi: 10.1186/1477-7827-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalem MN, Kalem Z, Gürgan T. Problems and Complications During the Treatment of Infertility in Women with Polycystic Ovary Syndrome. Gynecol Obstet Reprod Med. 2016;22:113–124. doi: 10.21613/GORM.2016.54. [DOI] [Google Scholar]
- Karmon AE, Batsis M, Chavarro JE, Souter I. Preconceptional thyroid-stimulating hormone levels and outcomes of intrauterine insemination among euthyroid infertile women. Fertil Steril. 2015;103:258–63e1. doi: 10.1016/j.fertnstert.2014.09.035. [DOI] [PubMed] [Google Scholar]
- Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31:702–755. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- Leiva P, Schwarze JE, Vasquez P, Ortega C, Villa S, Crosby J, Balmaceda J, Pommer R. There is no association between the presence of anti-thyroid antibodies and increased reproductive loss in pregnant women after ART: a systematic review and meta-analysis. JBRA Assist Reprod. 2017;21:361–365. doi: 10.5935/1518-0557.20170057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Shan Z, Mao J, Wang W, Xie X, Zhou W, Li C, Xu B, Bi L, Meng T, Du J, Zhang S, Gao Z, Zhang X, Yang L, Fan C, Teng W. Assessment of thyroid function during first-trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women? J Clin Endocrinol Metab. 2014;99:73–79. doi: 10.1210/jc.2013-1674. [DOI] [PubMed] [Google Scholar]
- Liu J, Larsen U, Wyshak G. Prevalence of primary infertility in China: in-depth analysis of infertility differentials in three minority province/autonomous regions. J Biosoc Sci. 2005;37:55–74. doi: 10.1017/S0021932003006461. [DOI] [PubMed] [Google Scholar]
- Łukaszuk K, Kunicki M, Kulwikowska P, Liss J, Pastuszek E, Jaszczołt M, Męczekalski B, Skowroński K. The impact of the presence of antithyroid antibodies on pregnancy outcome following intracytoplasmatic sperm injection-ICSI and embryo transfer in women with normal thyreotropine levels. J Endocrinol Invest. 2015;38:1335–1343. doi: 10.1007/s40618-015-0377-5. [DOI] [PubMed] [Google Scholar]
- Medenica S, Nedeljkovic O, Radojevic N, Stojkovic M, Trbojevic B, Pajovic B. Thyroid dysfunction and thyroid autoimmunity in euthyroid women in achieving fertility. Eur Rev Med Pharmacol Sci. 2015;19:977–987. [PubMed] [Google Scholar]
- Medici M, Korevaar TI, Schalekamp-Timmermans S, Gaillard R, de Rijke YB, Visser WE, Visser W, de Muinck Keizer-Schrama SM, Hofman A, Hooijkaas H, Bongers-Schokking JJ, Tiemeier H, Jaddoe VW, Visser TJ, Peeters RP, Steegers EA. Maternal early-pregnancy thyroid function is associated with subsequent hypertensive disorders of pregnancy: the generation R study. J Clin Endocrinol Metab. 2014;99:E2591–E2598. doi: 10.1210/jc.2014-1505. [DOI] [PubMed] [Google Scholar]
- Merviel P, Cabry R, Lourdel E, Barbier F, Scheffler F, Mansouri N, Devaux A, Benkhalifa M, Copin H. Intrauterine insemination. Rev Prat. 2014;64:87–91. [PubMed] [Google Scholar]
- Miko E, Meggyes M, Doba K, Farkas N, Bogar B, Barakonyi A, Szereday L, Szekeres-Bartho J, Mezosi E. Characteristics of peripheral blood NK and NKT-like cells in euthyroid and subclinical hypothyroid women with thyroid autoimmunity experiencing reproductive failure. J Reprod Immunol. 2017;124:62–70. doi: 10.1016/j.jri.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Moon HW, Chung HJ, Park CM, Hur M, Yun YM. Establishment of trimester-specific reference intervals for thyroid hormones in Korean pregnant women. Ann Lab Med. 2015;35:198–204. doi: 10.3343/alm.2015.35.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab. 2010;95:E44–E48. doi: 10.1210/jc.2010-0340. [DOI] [PubMed] [Google Scholar]
- Negro R, Mestman JH. Thyroid disease in pregnancy. Best Pract Res Clin Endocrinol Metab. 2011;25:927–943. doi: 10.1016/j.beem.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Ödöl E, Tosun M, Torumtay B, Alper T, Malatyalıoğlu E, Çetinkaya M, Kökçü A. Antenatal Screening for the Frequency of Subclinic Hypothroidism. Gynecol Obstet Reprod Med. 2009;15:130–132. [Google Scholar]
- Poppe K, Velkeniers B, Glinoer D. Thyroid disease and female reproduction. Clin Endocrinol (Oxf) 2007;66:309–321. doi: 10.1111/j.1365-2265.2007.02752.x. [DOI] [PubMed] [Google Scholar]
- Practice Committee of The American Society For Reproductive Medicine Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104:545–553. doi: 10.1016/j.fertnstert.2015.05.028. [DOI] [PubMed] [Google Scholar]
- Reh A, Grifo J, Danoff A. What is a normal thyroid-stimulating hormone (TSH) level? Effects of stricter TSH thresholds on pregnancy outcomes after in vitro fertilization. Fertil Steril. 2010;94:2920–2922. doi: 10.1016/j.fertnstert.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Sarac M, Koc I. Prevalence and Risk Factors of Infertility in Turkey: Evidence from Demographic and Health Surveys, 1993-2013. J Biosoc Sci. 2018;50:472–490. doi: 10.1017/S0021932017000244. [DOI] [PubMed] [Google Scholar]
- Springer D, Zima T, Limanova Z. Reference intervals in evaluation of maternal thyroid function during the first trimester of pregnancy. Eur J Endocrinol. 2009;160:791–797. doi: 10.1530/EJE-08-0890. [DOI] [PubMed] [Google Scholar]
- Tan S, Dieterle S, Pechlavanis S, Janssen OE, Fuhrer D. Thyroid autoantibodies per se do not impair intracytoplasmic sperm injection outcome in euthyroid healthy women. Eur J Endocrinol. 2014;170:495–500. doi: 10.1530/EJE-13-0790. [DOI] [PubMed] [Google Scholar]
- Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ. 2011;342:d2616–d2616. doi: 10.1136/bmj.d2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulis KA, Goulis DG, Venetis CA, Kolibianakis EM, Negro R, Tarlatzis BC, Papadimas I. Risk of spontaneous miscarriage in euthyroid women with thyroid autoimmunity undergoing IVF: a meta-analysis. Eur J Endocrinol. 2010;162:643–652. doi: 10.1136/bmj.d2616. [DOI] [PubMed] [Google Scholar]
- Tuncay G, Karaer A, İnci Coşkun E, Baloğlu D, Tecellioğlu AN. The impact of thyroid-stimulating hormone levels in euthyroid women on intrauterine insemination outcome. BMC Womens Health. 2018;18:51–51. doi: 10.1186/s12905-018-0541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unuane D, Velkeniers B, Bravenboer B, Drakopoulos P, Tournaye H, Parra J, De Brucker M. Impact of thyroid autoimmunity in euthyroid women on live birth rate after IUI. Hum Reprod. 2017;32:915–922. doi: 10.1093/humrep/dex033. [DOI] [PubMed] [Google Scholar]
- van den Boogaard E, Vissenberg R, Land JA, van Wely M, van der Post JA, Goddijn M, Bisschop PH. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update. 2011;17:605–619. doi: 10.1093/humupd/dmr024. [DOI] [PubMed] [Google Scholar]
- Vila L, Velasco I, González S, Morales F, Sánchez E, Torrejón S, Soldevila B, Stagnaro-Green A, Puig-Domingo M. Controversies in endocrinology: On the need for universal thyroid screening in pregnant women. Eur J Endocrinol. 2014;170:R17–R30. doi: 10.1530/EJE-13-0561. [DOI] [PubMed] [Google Scholar]
- World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]