Abstract
Experimental and epidemiological evidence reveal the profound influence that industrialized modern society has imposed to human social habits and physiology during the past 50 years. This drastic change in lifestyle is thought to be one of the main causes of modern diseases including obesity, type 2 diabetes, mental illness such as depression, sleep disorders, and certain types of cancer. These disorders have been associated to disruption of the circadian clock, an intrinsic time-keeper molecular system present in virtually all cells and tissues. The circadian clock is a key element in homeostatic regulation by controlling a large array of genes implicated in cellular metabolism. Importantly, intimate links between epigenetic regulation and the circadian clock exist and are likely to prominently contribute to the plasticity of the response to the environment. In this review, we summarize some experimental and epidemiological evidence showing how environmental factors such as stress, drugs of abuse and changes in circadian habits, interact through different brain areas to modulate the endogenous clock. Furthermore we point out the pivotal role of the deacetylase SIRT1 as a molecular effector of the environment in shaping the circadian epigenetic landscape.
Modern life-style and disruption of the clock
The highly accelerated industrialization and urbanization during the past 50 years has imposed drastic changes in social lifestyle. This has been particularly significant in urban zones, where the sleeping period has been reduced of ~ 2 hours in the last 50 years (Misra and Khurana, 2008, Lucassen et al., 2012). These new environmental conditions have been associated to chronic stress, which in turn generate certain neuropsychiatric disorders, such as anxiety, depression, cognitive dysfunction and sleep disorders, all that are linked to disruption of circadian rhythms (Chrousos, 2009). The recent development of telecommunications and internet has further expanded these alterations in individual habits and promoted sleep disorders. These modern life-style rhythms are known as ‘social zeitgebers’, and include work and social demands on interpersonal relationships. The new social zeitgebers have forced the population to adapt to specific types of food consumption, as well the feeding and sleep schedule, according to their current needs (Mistlberger and Skene, 2004, Soria and Urretavizcaya, 2009). The consequent alteration of circadian rhythms correlates with the increase in predisposition to develop a variety of chronic diseases, mostly cardiovascular, metabolic and neuropsychiatric. These illnesses are particularly evident in night and shift workers. The environment-morbidity relationship is not well understood; however, studies in humans and animal experimental models are providing evidence towards the identification of molecular and cellular mechanisms involved in these pathologies. In this review we summarize the key molecular mechanisms of the circadian clock and the link with epigenetic control. Specifically, we expand on the critical role of SIRT1 in the modulation of the circadian clock and metabolic homeostasis, as well as its central role in cognitive functions. Finally, we highlight the influence that feeding may have on the central clock at the behavioral and molecular levels.
The central clock
All functions of the central nervous system have evolved in a circadian environment, generating responses essential to adapt and anticipate environmental fluctuations. Circadian clocks are organized hierarchically, with the central clock localized in the suprachiasmatic nucleus (SCN) of the hypothalamus, and peripheral clocks localized in other brain regions and peripheral tissues (Cermakian and Sassone-Corsi, 2002)
The SCN consists of a bilateral nucleus with about 20,000 neurons. It is located in the anterior hypothalamus above the optic chiasm and receives the light input from the retina through the retino-hypothalamic tract (RHT) (Moga and Moore, 1997). Light functions as a prominent zeitgeber, adjusting the endogenous rhythms to maintain the 24 hour-period oscillation. The role of the SCN as master clock was first illustrated in rodents where bilateral destruction of the SCN abolished circadian rhythms (Stephan and Zucker, 1972), whereas transplanting fetal SCN restored the circadian rhythms at behavioral and hormonal levels (Drucker-Colin et al., 1984). This was further sustained from studies in hamsters, in which the altered behavior from tau mutant animals was recovered by transplantation of SCN form wild-type animals (Amir et al., 2004). In addition to the SCN, other brain regions show circadian rhythms including the substantia nigra (SN), the nucleus accumbens (NA) and the ventromedial hypothalamus (VMH) (Dibner et al., 2010). Surgical ablation of the SCN abolishes the rhythmicity of the extra-hypothalamic nuclei (Inouye and Kawamura, 1979). Thus, the SCN appears to orchestrate circadian rhythmicity in other brain nuclei, possibly through both synaptic connections and diffusible molecules. Finally, physiological communication between the SCN and peripheral clocks by autonomic innervations and endocrine signaling has been postulated (Buijs and Kalsbeek, 2001).
Interactions of the SCN with other brain areas
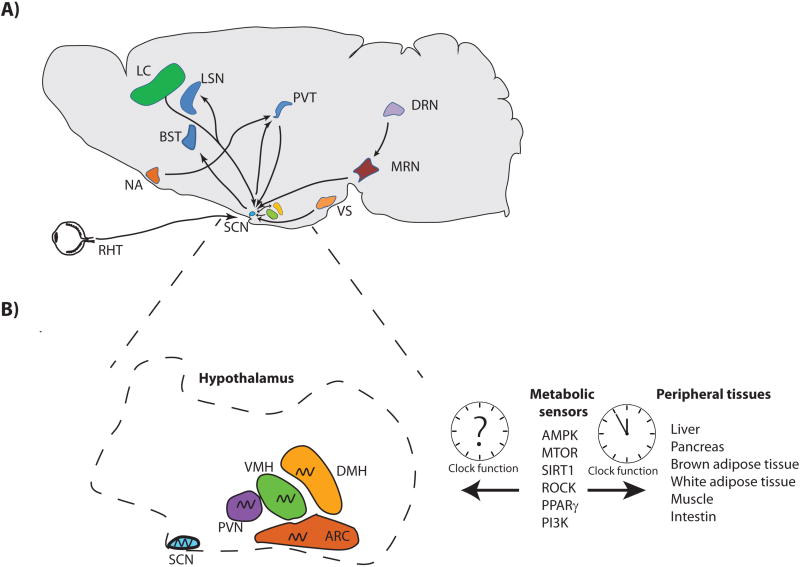
Although light is the dominant zeitgeber and the SCN is the light entrainable oscillator (LEA), some evidence suggests that the central clock could also be influenced by non-photic inputs including behavior, nutritional intake, restricted exercise and social contact (Hastings et al., 1998, Challet, 2010). Thus, the SCN may be considered as an element of a neuronal network in which signals are received from, and sent to other brain regions, including nuclei from the limbic cortex, the basal forebrain, the hypothalamus, the brainstem and the midline thalamus (Moga and Moore, 1997). The afferent projections of the SCN come from six areas: the retina, the limbic system, the hypothalamus, the raphe nuclei, the paraventricular thalamus and the extraretinal visual system. Furthermore, these connections arrive in to two main subdivision of the SCN: i) the core, which contains neurons producing vasoactive intestinal peptide (VIP) and gastrin releasing peptide (GRP). It receives dense visual afferents projections from the retinogeniculo and pretectohypothalamic tracts and non-photic inputs from the raphe and midline thalamus (Leak et al., 1999). ii) The shell that surrounds the core and receives inputs from non-visual sources, including limbic areas (infralimbic cortex, ventral subiculum and lateral septal nucleus), basal forebrain, hypothalamus, brainstem and thalamus and contains neurons producing arginine vasopressin (AVP) as neurotransmitter and calretinin (Leak and Moore, 2001). The core is densely projected to the shell with sparsely reciprocal innervation (Leak et al., 1999). In the case of the efferent projections from the SCN, the core and the shell also differs in the innervated targets, for instance the core connects with the hypothalamus within nuclei including the perisuprachiasmatic nucleus (PSCN), the lateral subparaventricular area (LSPVZ), the ventral tuberal area (VTA), the basal forebrain within the lateral septal nucleus (LSN), and the thalamus in nuclei such as the parataenial nucleus (PT) and the nucleus reuniens, (RE). The shell mainly controls the hypothalamic nucleus including the preoptic area (POA), the dorsomedial hypothalamus (DMH), the paraventricular hypothalamus (PVH), medial subparaventricular area (MSPVZ) and the thalamic regions which include the paraventricular thalamic nucleus (PVT), the zona incerta (ZI), the parataenial nucleus (PT), the nucleus reuniens, (RE) and the basal forebrain in the nucleus of the stria terminalis (BST) (Moga and Moore, 1997, Leak et al., 1999, Leak and Moore, 2001). Hence the core might compute dense light and serotoninergic inputs, meanwhile the shell further modulate the central clock by weak inputs from a wide array of brain areas from the limbic system and the hypothalamus. Interestingly experimental evidence have supported this anatomical organization, for example, acute increase in SCN serotonin released from the dorsal raphe nucleus (DRN) is induced by sleep deprivation, which is mediated by the suppression of DRN GABAergic tone, inducing phase resetting of the central clock (Glass et al., 2003). Hence, it has been postulated that serotonin might modulate the excitability of the SCN to afferent inputs at three levels, i) modulation of afferent visual or nonvisual pathways regulating the phase of circadian response ii) modulation of the excitability of the SCN to afferent photic or non-photic inputs, iii) modulation of the responsiveness of efferent targets to circadian signals (Lowry, 2002) (FIG 1 A).
Figure 1. The SCN interacts with several brain areas.
A) Representative schema depicting the efferent and afferent signaling to the suprachiasmatic nucleus (SCN) from different brain regions. These include limbic structures such as infralimbic cortex (LC), lateral septal nucleus (LSN), basal forebrain of the stria terminalis (BST), ventral subiculum (VS), paraventricular thalamic nuclei (PVT), accumbens nucleus (NA), dorsal raphe nucleus (DRN), median raphe nucleus (MRN), and hypothalamic nuclei such as ventromedial hypothalamus (VMH), dorsomedial hypothalamus (DMH), paraventricular hypothalamus (PVN), and the retino-hypothalamic tract (RHT). B) Schema representing the possible link between the hypothalamic clocks and the metabolic sensors influencing the clock in peripheral tissues (See also Table 1).
Regarding the connections between the SCN and other hypothalamic nuclei controlling food intake and body weight, the ventral part of the arcuate nucleus (ARC) sends peripheral information to the SCN such as feeding related signals (Yi et al., 2006). Hence, ARC lesions induce arrhythmic food intake when animals are exposed to constant darkness (Li et al., 2012). Furthermore, lesions in the SCN in rats decrease the activation of the ARC and a-MSH neuronal activity (Guzmán-Ruiz et al., 2013) and result in uninterrupted feeding (Kalra et al., 1999) and altered diurnal pattern of plasmatic leptin levels (Kalsbeek et al., 2001). These evidence suggest that the hypothalamic nuclei involved in feeding behavior acts in synchrony with the SCN to generate feeding rhythms entrained to photic cues. Furthermore, it has been observed in animals and humans that the preference for certain kinds of food change within the hour of the day. For example in rats the preference of carbohydrates and proteins increases at the onset of the activity period at night, and by the end of their activity phase, the preference for fats is increased (Cagampang and Bruce, 2012). This is also observed in humans, where carbohydrates are preferred during the breakfast, and high fat diet are preferred during the evening (Westerterp-Plantenga et al., 1996). Interestingly, the SCN shares connections with limbic brain areas involved in motivation and reward responses such as the bed nucleus of the stria terminalis (BNST) and the nucleus accumbens (Watts et al., 1987, Amir et al., 2004). This stress out the role of other brain regions of the reward system that have been implicated in both, drugs and food addiction as discussed below. The hypothalamus also controls the energy balance by modulating the energy expenditure as heat production. For example, the ventromedial hypothalamus (VMH) which receives neuronal afferents from the SCN and paraventricular hypothalamus (PVH) has been implicated in the control of the thermogenesis by the brown adipose tissue (Amir, 1990). Interestingly, the stimulation of the SCN by glutamate, activates BAT thermogenesis and this effect is mediated by the VMH (Amir et al., 1989). Finally, the dorsomedial hypothalamus which also receives indirect and direct inputs from the SCN has been implicated in several circadian rhythms such as wakefulness, feeding, locomotor activity, and serum corticosteroid and glucose levels (Nagai et al., 1988, Chou et al., 2003, Cailotto et al., 2005).
The core cellular clock
In virtually every cell of the body, the molecular clock “ticks” through a complex molecular mechanism consisting on a network of interlocked transcriptional-translational feedback loops. Central to the circadian machinery are the core clock proteins Circadian Locomotor Output Cycles Kaput (CLOCK) and Brain and Muscle ARNT-Like 1 (BMAL1). These transcription factors dimerize through their PAS domains and subsequently bind E-box promoter elements, thereby activating the transcription of clock controlled genes (CCGs). The genes Period 1–3 (Per1, Per2 and Per3) and Cryptochrome 1–2 (Cry1 and Cry2) are core clock genes and their products heterodimerize to repress CLOCK-BMAL1, inhibiting their own expression and forming a negative autoregulatory feed-back loop (Sahar and Sassone-Corsi, 2009). A number of CCGs encode for transcription factors, including D-box binding protein (DBP), Thyrotroph Embryonic Factor (TEF), Retinoic Acid-Related Orphan Receptor a (RORα) and Reverse Erithroblastosis Virus α and β (REV-ERBα/β). DBP and TEF bind D-boxes, while RORα and REV-ERBα/β bind the Reb-Erb/ROR promoter elements, insuring additional circadian waves in expression of downstream genes. It is estimated that the circadian machinery controls the cyclic expression of about 10–20% of genes in the cell (Aguilar-Arnal and Sassone-Corsi, 2013). Importantly, epigenetic control plays a central role in the harmonic organization of circadian transcription.
Epigenetics: how the environment shapes the circadian response
Epigenetic control involves a variety of mechanisms, including chromatin remodeling through post-translational modifications of the N-terminal tails of histones. These include methylation, acetylation, ubiquitination, and phosphorylation (Borrelli et al., 2008, Aguilar-Arnal and Sassone-Corsi, 2013). The transcriptional control of a significant fraction of the genome by the clock invokes genome-wide mechanisms of chromatin remodeling. Early findings supported this concept by showing that chromatin remodeling in the SCN is triggered in response to light input (Crosio et al., 2000). Further observation revealed that activation of CCGs by CLOCK-BMAL1 is coupled to circadian changes in histone acetylation at their promoters (Etchegaray et al., 2003). Subsequent studies confirmed and expanded these observations showing that in addition to histone acetylation, histone methylation is also important for clock function (Etchegaray et al., 2003, Curtis et al., 2004, Naruse et al., 2004, Etchegaray et al., 2006). The discovery that the core protein CLOCK has itself intrinsic histone acetyl-transferase (HAT) activity, targeting histone H3 K9 and K14 at CCG promoters, paved the way to unravel the function and structure of the circadian chromatin complex (Doi et al., 2006a). First, CLOCK acetylates its molecular partner BMAL1 at the single aminoacid K537, an event essential for circadian rhythmicity (Hirayama et al., 2007). Moreover, it has been demonstrated that the histone methyltransferase MLL1 directs the cyclic tri-methylation of the H3K4 on CCGs promoters, directing the recruitment of the dimer CLOCK:BMAL1 to genomic targets and promoting transcriptional activation (Katada and Sassone-Corsi, 2010). Other proteins are able to interact with the clock machinery and promote circadian epigenetic changes. The methyl transferase EZH2 interacts with CLOCK and BMAL1, promoting H3K27 di- and tri-methylation and enhances the transcriptional repression mediated by CRY (Etchegaray et al., 2006). The histone demethylases JARID1a and JMJD5 have also been implicated (DiTacchio et al., 2011), whereas other studies further indicated intercorrelations and dynamics within different epigenetic circadian events (Koike et al., 2012, Vollmers et al., 2012). Thus, the core circadian clock appears to be coupled to a variety of epigenetic mechanisms, including the modulation of the nuclear organization (Aguilar-Arnal et al., 2013). These molecular mechanisms might be coupled to changes in the environment through signaling pathways. In this respect, the NAD+ dependent SIRT1 histone deacetylase (HDAC) plays a pivotal role, linking the circadian clock to the intracellular energetic environment.
SIRT1: a deacetylase at the interface between metabolism and circadian control
The enzyme ‘silent mating type information 2 homolog 1’, (SIRT1), is a NAD+-dependent deacetylase, (Bellet et al., 2011). SIRT1 has a wide variety of targets, including histone and non-histones proteins. Because of this, SIRT1 influences several cellular and physiological processes, including DNA repair, cell cycle arrest, cell survival, gluconeogenesis, lipid metabolism, insulin sensitivity, and has been related with both, healthy aging and control of lifespan. Moreover, SIRT1 exerts control on metabolism by deacetylating key metabolism-regulatory factors such as FOXO1, PGC-1a, p53, E2F1, PPARγ, STAT3 and SCREBP-1c (Brooks and Gu, 2009, Peek et al., 2012).
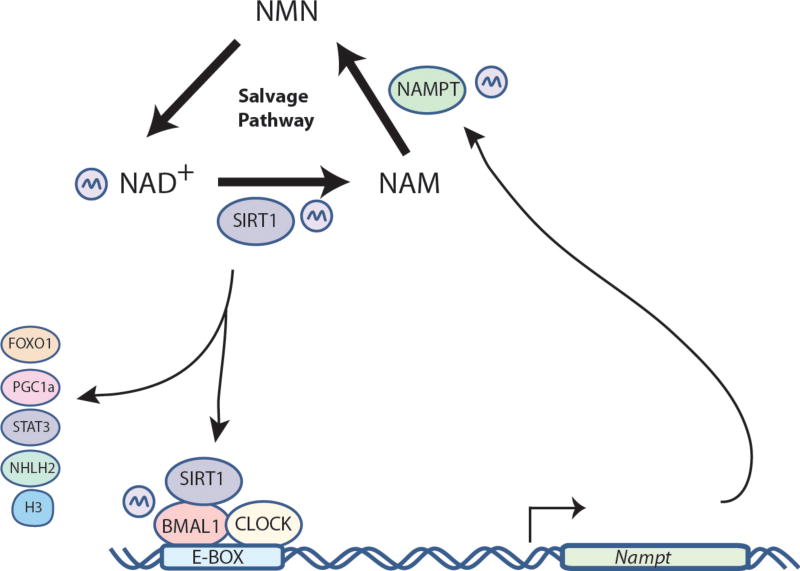
The HDAC activity of SIRT1 oscillates in a circadian manner, rhythmically deacetylating the histone H3K9/K14 at the promoters of CCGs, and the non-histone proteins BMAL1 and PER2 (Asher et al., 2008, Nakahata et al., 2008). Additionally, genetic ablation of Sirt1 or pharmacological inhibition of SIRT1 provokes disturbances in circadian cycles, both in cultured cells and in vivo (Nakahata et al., 2009). It has been suggested that the activity of SIRT1 counterbalances the rhythmic HAT function of CLOCK, although other HATs are likely to be implicated (Masri and Sassone-Corsi, 2010). Importantly, the cyclic activity of SIRT1 is modulated by the circadian levels of its cofactor NAD+ (Nakahata et al., 2008). NAD+ can be synthesized de novo from tryptophan or by the salvage pathway (Revollo et al., 2004). NAD+ can be used for energy transferring with the subsequent production of ATP in the mitochondria, or it is used in regulatory functions as a cofactor for NAD+-consuming enzymes. Remarkably, the circadian clock acts on the synthesis of NAD+, controlling the circadian expression of the nicotinamide phosphoribosyltransferase (NAMPT) gene, a CCG that encodes a key enzyme in the salvage pathway (Nakahata et al., 2009, Ramsey et al., 2009). Thus, the circadian feedback transcriptional loop is tightly linked to an enzymatic feedback loop (FIG. 2). This regulatory pathway appears to be functional in peripheral clocks as well as in the central clock. Indeed, SIRT1 modulates the central clock in a process that appears to become less efficient in aged animals, as observed in jet-lag experiments and gene expression studies. This modulation comprises a direct activation of BMAL1 by SIRT1 through PGC-1α and NAMPT (Chang and Guarente, 2013). Furthermore, it has been demonstrated that SIRT1 has tissue-specific functions on different metabolic tissues such as liver, skeletal and cardiac muscle, pancreas and adipose tissue (Rodgers et al., 2008). For example, SIRT1 is necessary for the adaptations to fasting, and in conditions of caloric restriction SIRT1 triggers lipid mobilization from adipose tissue, a switch from glucose to lipid oxidation in skeletal muscle and liver and an increase in hepatic glucose production (Ramadori et al., 2011). These observations suggest the importance of SIRT1 in the control of both central and peripheral clocks, where it might modulate in a circadian manner a plethora of physiological outputs.
Figure 2. The NAD+ salvage pathway is controlled by the circadian clock.
The biosynthesis of NAD+ follows a circadian pattern, which is caused by the circadian expression of NAMPT, a rate-limiting enzyme in the NAD+ biosynthetic salvage pathway. The Nampt gene contains E-boxes in its promoter, leading to direct transcriptional control by the dimer CLOCK:BMAL1. The fluctuating levels of NAD+ modulate the activity of SIRT1 which in turn regulates the transcriptional activity of CLOCK:BMAL on their targets genes.
Control of energy-balance by SIRT1
The central nervous system (CNS) directs both behavioral and metabolic responses in peripheral tissues to adapt quickly to the changing environment. Specifically, the hypothalamus computes the metabolic information from the body and responds accordingly to meet the body’s energy balance requirement, through the melanocortin system (Cone, 2005, Morton et al., 2006, Dietrich and Horvath, 2013).
Since food consumption in mammals follows a circadian rhythm, it is expected that the neuroendocrine mechanisms controlling feeding behavior will also coherently display daily oscillations. There is growing evidence that the circadian clock directly participates in the hypothalamic control of food intake and energy homeostasis. In the arcuate nucleus (ARC), the expression of the orexigenic Npy/Agrp and anorexigenic Pomc/Cart genes are rhythmic (Xu et al., 1999, Lu et al., 2002, Stutz et al., 2007), an event which is correlated with daily rhythms in food intake. While it is unclear whether the molecular clock controls the expression of these neuropeptides, studies in mouse show that animals with a disrupted clock display hyperphagia, accompanied with altered rhythms in the expression of Cart and ghrelin genes. Both genes contain E-box elements in their promoters, and are thereby bona fide CLOCK-BMAL1 targets (Turek et al., 2005). Control of food intake by the hypothalamus involves the action of metabolic sensors such as the AMP activated kinase (AMPK) and SIRT1 (Minokoshi et al., 2004, Cota et al., 2006, Çakir et al., 2009), which control the circadian clock in peripheral tissues (Nakahata et al., 2008, Lamia et al., 2009, Giebultowicz and Kapahi, 2010). Recently, using two-photon laser microscopy on organotypic slices of SCN, it has been observed that the redox state in the SCN oscillates daily in wild type mice but not in Bmal1−/− mice(Wang et al., 2012). In the hypothalamus the redox state has been implicated in the control of food intake (Benani et al., 2007). The redox state has been also correlated with the daily energetic status of the cell in the hypothalamus and peripheral tissues, such as the liver and adipose tissue. Particularly, the NAD+/NADH ratio changes with feeding in the hypothalamus and peripheral tissues such as liver, inducing cyclic activation in SIRT1 enzymatic potential (Çakir et al., 2009, Dibner et al., 2010). SIRT1 also regulates hypothalamic metabolic functions by modulating the central melanocortin signaling (Çakir et al., 2009) and thereby it is implicated in the generation of food anticipatory activity (FAA), as discussed below (Sutton et al., 2010). In POMC neurons of the ARC of the hypothalamus the lack of SIRT1 leads to hypersensitivity to diet-induce obesity. This is mediated by a perturbation in the PI3K pathway in these neurons, which reduces the sympathetic activity necessary for the metabolic control of the perigonadal white adipose tissue (WAT) (Ramadori et al., 2010). Furthermore, absence of SIRT1 in the SF1 neurons of the ventromedial hypothalamus (VMH), a nucleus highly sensitive to glucose levels, impairs glucose metabolism inducing dietary type 2 diabetes (Ramadori et al., 2010, Ramadori et al., 2011). Finally, SIRT1 also modulates the feeding behavior acting on the melanocortin system (Sasaki et al., 2010, Sasaki and Kitamura, 2010). SIRT1, has been also implicated in neurogenesis, synaptic formation, and exerts protective action against Alzheimer, amyotrophic lateral sclerosis and axonal degeneration (Ramadori et al., 2008, Michan, 2013).
SIRT1 has been also linked to the rewarding processes related to drugs of abuse. Accumulating evidence indicates that mechanisms of drug addiction involve epigenetic changes within brain reward regions (Feng and Nestler, 2013). During repeated cocaine administration, Sirt1 expression increases and is associated to an increment in electrical excitability of the nucleus accumbens, potentiating the rewarding effects of cocaine (Renthal et al., 2009). Moreover, SIRT1 modulates not only the homeostatic process but also non-homeostatic process. Hence, it was shown that SIRT1 regulates anxiety and exploratory behavior by activating Mao-A transcription, a gene that encodes the enzyme monoamine oxidase A (MAO-A), which in turn degrades serotonin and noradrenaline. This effect is obtained through direct deacetylation by SIRT1 of the transcription factor NHLH2 that controls Mao-A expression (Libert et al., 2011). Interestingly it has been observed that the expression of Mao-A follows a circadian rhythm which is controlled by the clock machinery (Hampp et al., 2008). However, whether SIRT1 participates in the circadian control of Mao-A expression remains unexplored.
Addiction and the Circadian Clock
Experimental and epidemiological studies have linked the circadian clock with addiction to drug, alcohol and food. Addict patients show disruption in sleep and circadian rhythmicity, whereas experiments of drug self-administration in rodents show circadian patterns of drug ingestion (Terman and Terman, 1970, Kosobud et al., 2007). The use of mouse models of clock disruption has revealed an increase in cocaine reward and excitability of dopamine neurons in brain reward regions. These effects are associated to an increase of dopamine release and turnover and an increment in dopamine receptors sensibility (Spencer et al., 2012). Similarly, clockΔ19 mutant mice – which express a truncated, inactive form of the CLOCK protein - exhibit an increase in ethanol intake, an effect which is mediated by the ventral tegmental area (VTA) dopamine system. Moreover, chronic alcohol treatment leads to changes in Clock gene expression in the VTA and in the SCN, which could be linked to the pervasive disruption in rhythm and sleep in alcoholics (Chen et al., 2004, Ozburn et al., 2013).
Disruption of circadian behavior is also intimately associated to social stress (Tornatzky and Miczek, 1993, Holmes et al., 1995). For example activation of the stress system stimulates arousal and suppresses sleep (Chrousos, 2007). Importantly, it has been suggested that psychological distress can be reduced by eating high-palatable food (Maniam and Morris, 2012), a notion that is supported by some experimental evidence (Dallman et al., 2005). The reward system has been implicated in the development of eating disorders, including bulimia, anorexia, and binge and night eating disorder (Tanofsky-Kraff and Yanovski, 2004, Adam and Epel, 2007, Zheng et al., 2009). Hence, it is plausible that feeding disorders might have a circadian component, since the feeding regimen and the scheduled feeding are capable to control the endogenous clock. Furthermore, alteration of circadian rhythms caused by mutation of clock genes also leads to changes of normal feeding schedule (Turek et al., 2005, Kohsaka et al., 2007, Challet and Mendoza, 2010, Mendoza et al., 2010, Volkow et al., 2011).
It has been postulated that, as in the addiction to drugs of abuse, the consumption of high palatable food results in an increased reinforced value of food. Importantly, reinforcement learning elicited by either drugs of abuse as well as food, promotes the nuclear accumulation of the ‘dopamine-regulated and cyclic-AMP-regulated phosphoprotein’ (DARPP-32), mediated by a signaling cascade triggered by the dopamine D1 receptor. Consequently DARPP-32 inhibits protein phosphatase 1 (PP1), resulting in events of chromatin reorganization characterized by an increment of H3 phosphorylation (Stipanovich et al., 2008). Interestingly, DARPP-32 also participates in central clock entrainment by photic inputs (Yan et al., 2006). Importantly, signaling, molecular and behavioral connections between dopamine regulatory pathways and the circadian clock have been revealed (Doi et al., 2006b, Yujnovsky et al., 2006, Zocchi and Sassone-Corsi, 2010). These and additional experimental evidence suggest that the reward system might entrain the endogenous clock. For example, some reports have shown that animals fed scheduled high-palatable food under ad-libitum standard chow are capable to entrain the SCN clock in constant darkness (Challet and Mendoza, 2010). Indeed, the SCN receives inputs from three areas in the limbic system, namely the infralimbic cortex, the lateral septal nucleus and the ventral subiculum (Moga and Moore, 1997). Furthermore, the SCN might receive dopaminergic signals indirectly through the paraventricular thalamic nuclei that projects directly to the SCN or via orexinergic neurons (Bubser et al., 2005)
Food as a zeitgeber
Since the circadian machinery serves to anticipate changes in the environment and consequently to adapt to daily variations in food availability, the clock is not only synchronized by the day/night cycle, but also by the feeding schedule. While the light-entrainable oscillator (LEO) is well characterized (the SCN), the exact localization within the brain of the food-entrainable oscillators (FEOs) remains a subject of debate. The behavioral manifestation of the FEO corresponds to changes or adaptation towards food consumption that have been extensively studied in rodents. When food is administered at specific times of the day, the animal displays an increase in locomotor activity few hours before the feeding time. This phenomenon, known as food anticipatory activity (FAA), is accompanied by several physiological changes such as the rise of corticosterone and insulin secretion, body temperature, gastrointestinal motility and activity of digestive enzymes. FAA influences the oscillation of clock genes in peripheral tissues but not in the SCN (Stephan, 2002, Froy, 2007). Thus, FAA appears to function as a zeitgeber since it is characterized by a limited time-frame of entrainment, the persistence of oscillation, and transient resetting after a change in meal time (Screaton et al., 2004, Buhr et al., 2010). Remarkably, neither SCN-ablation nor constant darkness inhibits the FAA and its associated rhythms in peripheral tissues, suggesting the existence of the FEO outside of the SCN. Attempts to localize the FEO in the central nervous system have been carried out by targeting specific nuclei in the hypothalamus including the VMH, PVN, ARC, lateral hypothalamus (LH) and DMH.
The DMH is a possible candidate. Indeed, a compact part of the DMH shows oscillation in Per2 expression under scheduled feeding, which persists 2 days after entrainment by feeding restriction. Furthermore, DMH-lesioned mice fail to anticipate a time meal and premeal rise in body temperature (Gooley et al., 2006), and restoration of Bmal1 in the DMH is able to rescue the FAA (Fuller et al., 2008). Yet, contradictory data contrast the notion that the DMH is necessary for FAA generation (Landry et al., 2006, Moriya et al., 2009). Another hypothalamic nucleus considered to be part of the circuits controlling the FAA is the VMH. This is the first nucleus activated during feeding restriction, and lesions in the VMH abolish the ability to anticipate a food-restricted meal (Ribeiro et al., 2007). Moreover, since feeding is a highly reinforcing behavior, the NAc has been postulated to be part of the FEO. Hence, a lesion in the core part of the NAc reduces the FAA. In agreement with these results, highly palatable food is also able to induce FAA even under conditions of ad libitum food access (Challet and Mendoza, 2010). One attractive hypothesis is that the FEO is not localized in one nucleus. Rather, it is distributed in various hypothalamic nuclei and extra hypothalamic areas, such as the NA, the amygdala, the bed nucleus of the stria terminalis, and the preoptic area nucleus of the solitary tract (Mistlberger, 2011). This would allow a more pleiotropic control of the neuronal pathways involved in FAA.
At molecular level, the core clock has been implicated in the development of FAA. Under restricted feeding clock gene expression is shifted in non-SCN brain regions. Also, mutations in clock genes are able to affect the development of the FAA. For example, brain-specific Bmal1-null mice display a deficit in the development of FAA, accompanied by a reduced food consumption (Mieda and Sakurai, 2011). As mentioned above, the generation of FAA needs an entrainment in a limited window of time. Hence, mice lacking Cry1 (which shows a shorter circadian period than Cry2−/− mice) are entrained only to a shorter period of feeding cycles compared with the Cry2−/− animals. This implies that the intrinsic rhythmicity controlled by the core clock machinery could be a component for the entrainment of FAA (Takasu et al., 2012). However, contrasting results in mice lacking Cry1/Cry2, Bmal1, Per1/Per2, or in the ClockΔ19 mutant mice, show maintenance of the FAA rhythm. (Storch and Weitz, 2009) Thus, it has been suggested that FAA may be independent of the clock system and that it has rather emerged because of the rhythms of metabolic gene expression (Mistlberger, 2011). Interestingly, food entrainment, when accompanied with caloric restriction, causes a shift in the circadian gene expression in the SCN (Mendoza et al., 2005). All together, these observations suggest that the nutritional input acts as a powerful zeitgeber which modulates the core clock system within cells residing in the SCN as well as the peripheral clocks. Moreover, under certain conditions, the food input and the reward inputs might converge to override the dominating effect of light on the central clock.
Finally, most of the molecular mechanism governing the hypothalamic clocks by non-photic cues remains to be analyzed. The hypothalamic responses to the nutritional and metabolic conditions of the body through hormones such as leptin, ghrelin, insulin, or metabolites including glucose, aminoacids, lipids, NAD+ and AMP are triggered by metabolic sensors and signaling pathways including the AMPK, SIRT1, PI3K, PPARγ, etc., (Table 1) which modulate the neuronal responses to body needs. Importantly, these factors are also known to control the circadian clock in peripheral tissues such as liver, muscle, white and brown adipose tissue. The coordinated circadian regulation between the metabolic pathways and transcriptional networks achieved by the nutritional sensors in peripheral tissues (Desvergne et al., 2006, Asher and Schibler, 2011, Eckel-Mahan et al., 2012, Eckel-Mahan et al., 2013), also might be modulating the circadian clock in the different hypothalamic nuclei that compute the metabolic information. Therefore, the understanding of how the circadian clock within these hypothalamic nuclei is modulated by non-photic inputs, to respond to the metabolic necessities is of pivotal importance for the development of treatments against metabolic diseases such as type-2 diabetes, obesity and feeding disorders (FIG. 1B).
TABLE 1.
Metabolic sensors that participate in both, hypothalamic function and the circadian clock.
| PROTEIN | Hypothalamic function | Clock function | Ref. |
|---|---|---|---|
| ROCK (Rho-associated protein kinase) | Mediates leptin action in the ARC | Modulates the circadian rhythmicity of the sensitivity of myofilaments to Ca2+ | (Huang et al., 2012, Su et al., 2012, Saito et al., 2013) |
| AMPK (AMP activated protein kinase) | Its activity is modulated by metabolic signals in the hypothalamus and it is essential for the control of food intake. | AMPK phosphorylates and destabilizes CRY1, thereby regulating the molecular clock | (Minokoshi et al., 2004, Lamia et al., 2009) |
| MTOR (mammalian target of rapamycin) | Activated by L-leucine, mTOR inhibits food intake | In the SCN mTOR shows rhythmic activity, and participates in the entrainment of the central clock. | (Cota et al., 2006, Cao et al., 2013) |
| SIRT1 (silent mating type information regulation 2 homolog 1) | Protects against diet-induce obesity acting in signaling pathways in POMC and SF1 hypothalamic neurons | Regulates the circadian clock thought BMAL1 and PER2 deacetylation | (Asher et al., 2008, Nakahata et al., 2008, Ramadori et al., 2010, Ramadori et al., 2011) |
| PPARγ (Peroxisome proliferator-activated receptor gamma) | Leads to positive energy balance through hyperphagia | Its expression is circadian in peripheral tissues. Participates in the reprogramming of the circadian clock by nutritional stress. | (Yang et al., 2006, Ryan et al., 2011, Eckel-Mahan et al., 2013) |
| PI3K (Phosphatidylinositide 3-kinase) | Needed for the anorexigenic effect of leptin and insulin in the VMH. | Participates in the circadian regulation of L-type voltage-gated calcium channel in the retina. | (Ko et al., 2009, Xu et al., 2010, Klockener et al., 2011) |
Conclusive remarks
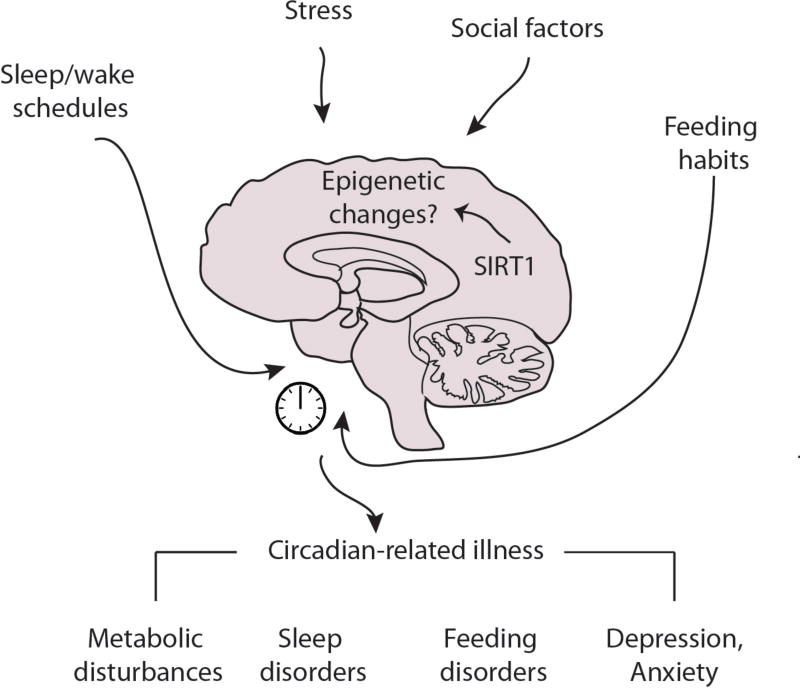
Environment has changed during the past 50 years at a pace that has had a tremendous impact on the physiology and metabolism of all life forms. When considering the varied cycles of activity and resting, the feeding schedule, the transformed diet, the social stressors, alcohol or drug abuse, it is essential to consider how all these may have a zeitgeber effect. This is a very exciting time as we are starting to gain insight within the molecular and epigenetic mechanisms that control these processes. Hence, new insights will allow the design of more effective strategies and pharmacological approaches targeting key proteins and pathways to resynchronize the endogenous clock, and treat the wide spectrum of pathologies such as obesity, type 2 diabetes, cardiovascular disease, mental illness such as depression, drug, alcohol or food addiction and feeding and sleep disorders (FIG. 3).
Figure 3. Social environment acting as zeitgeber.
The central nervous system receives time cues through different pathways. The central clock residing in the suprachiasmatic nucleus (SCN) directly receives light inputs through the retino-hypothalamic tract (RHT). The food entrainable oscillators (FEOs) localized in different areas in the brain receive metabolic and non-metabolic signals. Further signals might modulate the endogenous clock through cortico-limbic structures which are sensible to socio-enviromental factors. These stressors in turn might alter the endogenous clock generating a plethora of circadian-related diseases.
Highlights.
Environmental factors modulate the central clock
The SCN communicates with other brain regions
The circadian clock is modulated by different stimuli such as nutritional inputs
Circadian rhythms affect overall health
Acknowledgments
We thank all the members of the Sassone-Corsi and Borrelli laboratories for discussions and insights. Work in the Center for Epigenetics and Metabolism is supported by the National Institute of Health, the Merieux Fondation and INSERM (Institut National de la Sante et Recherche Medicale, France). R.O-S. is supported by a fellowship from the Government of Mexico and by the Della Martin Foundation.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiology & Behavior. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Aguilar-Arnal L, Hakim O, Patel VR, Baldi P, Hager GL, Sassone-Corsi P. Cycles in spatial and temporal chromosomal organization driven by the circadian clock. Nat Struct Mol Biol. 2013 doi: 10.1038/nsmb.2667. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Arnal L, Sassone-Corsi P. The circadian epigenome: how metabolism talks to chromatin remodeling. Current Opinion in Cell Biology. 2013;25:170–176. doi: 10.1016/j.ceb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir S. Intra-ventromedial hypothalamic injection of glutamate stimulates brown adipose tissue thermogenesis in the rat. Brain Res. 1990;511:341–344. doi: 10.1016/0006-8993(90)90181-a. [DOI] [PubMed] [Google Scholar]
- Amir S, Lamont EW, Robinson B, Stewart J. A Circadian Rhythm in the Expression of PERIOD2 Protein Reveals a Novel SCN-Controlled Oscillator in the Oval Nucleus of the Bed Nucleus of the Stria Terminalis. The Journal of Neuroscience. 2004;24:781–790. doi: 10.1523/JNEUROSCI.4488-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir S, Shizgal P, Rompre PP. Glutamate injection into the suprachiasmatic nucleus stimulates brown fat thermogenesis in the rat. Brain Research. 1989;498:140–144. doi: 10.1016/0006-8993(89)90409-5. [DOI] [PubMed] [Google Scholar]
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- Asher G, Schibler U. Crosstalk between Components of Circadian and Metabolic Cycles in Mammals. Cell Metab. 2011;13:125–137. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Bellet MM, Orozco-Solis R, Sahar S, Eckel-Mahan K, Sassone-Corsi P. The Time of Metabolism: NAD+, SIRT1, and the Circadian Clock. Cold Spring Harbor Symposia on Quantitative Biology. 2011;76:31–38. doi: 10.1101/sqb.2011.76.010520. [DOI] [PubMed] [Google Scholar]
- Benani A, Troy S, Carmona MC, Fioramonti X, Lorsignol A, Leloup C, Casteilla L, Pénicaud L. Role for Mitochondrial Reactive Oxygen Species in Brain Lipid Sensing: Redox Regulation of Food Intake. Diabetes. 2007;56:152–160. doi: 10.2337/db06-0440. [DOI] [PubMed] [Google Scholar]
- Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CL, Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer. 2009;9:123–128. doi: 10.1038/nrc2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubser M, Fadel JR, Jackson LL, Meador-Woodruff JH, Jing D, Deutch AY. Dopaminergic regulation of orexin neurons. European Journal of Neuroscience. 2005;21:2993–3001. doi: 10.1111/j.1460-9568.2005.04121.x. [DOI] [PubMed] [Google Scholar]
- Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2:521–526. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- Cagampang FR, Bruce KD. The role of the circadian clock system in nutrition and metabolism. The British journal of nutrition. 2012;108:381–392. doi: 10.1017/S0007114512002139. [DOI] [PubMed] [Google Scholar]
- Cailotto C, La Fleur SE, Van Heijningen C, Wortel J, Kalsbeek A, Feenstra M, Pevet P, Buijs RM. The suprachiasmatic nucleus controls the daily variation of plasma glucose via the autonomic output to the liver: are the clock genes involved? Eur J Neurosci. 2005;22:2531–2540. doi: 10.1111/j.1460-9568.2005.04439.x. [DOI] [PubMed] [Google Scholar]
- Çakir I, Perello M, Lansari O, Messier NJ, Vaslet CA, Nillni EA. Hypothalamic Sirt1 Regulates Food Intake in a Rodent Model System. PLoS One. 2009;4:e8322. doi: 10.1371/journal.pone.0008322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Robinson B, Xu H, Gkogkas C, Khoutorsky A, Alain T, Yanagiya A, Nevarko T, Liu Andrew C, Amir S, Sonenberg N. Translational Control of Entrainment and Synchrony of the Suprachiasmatic Circadian Clock by mTOR/4E-BP1 Signaling. Neuron. 2013;79:712–724. doi: 10.1016/j.neuron.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermakian N, Sassone-Corsi P. Environmental stimulus perception and control of circadian clocks. Curr Opin Neurobiol. 2002;12:359–365. doi: 10.1016/s0959-4388(02)00347-1. [DOI] [PubMed] [Google Scholar]
- Challet E. Interactions between light, mealtime and calorie restriction to control daily timing in mammals. J Comp Physiol B. 2010;180:631–644. doi: 10.1007/s00360-010-0451-4. [DOI] [PubMed] [Google Scholar]
- Challet E, Mendoza J. Metabolic and reward feeding synchronises the rhythmic brain. Cell and Tissue Research. 2010;341:1–11. doi: 10.1007/s00441-010-1001-9. [DOI] [PubMed] [Google Scholar]
- Chang H-C, Guarente L. SIRT1 Mediates Central Circadian Control in the SCN by a Mechanism that Decays with Aging. Cell. 2013;153:1448–1460. doi: 10.1016/j.cell.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Advis JP, Sarkar DK. Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J Neurochem. 2004;88:1547–1554. doi: 10.1046/j.1471-4159.2003.02300.x. [DOI] [PubMed] [Google Scholar]
- Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Organization and Integration of the Endocrine System. Sleep medicine clinics. 2007;2:125–145. doi: 10.1016/j.jsmc.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Crosio C, Cermakian N, Allis CD, Sassone-Corsi P. Light induces chromatin modification in cells of the mammalian circadian clock. Nat Neurosci. 2000;3:1241–1247. doi: 10.1038/81767. [DOI] [PubMed] [Google Scholar]
- Curtis AM, Seo SB, Westgate EJ, Rudic RD, Smyth EM, Chakravarti D, FitzGerald GA, McNamara P. Histone acetyltransferase-dependent chromatin remodeling and the vascular clock. J Biol Chem. 2004;279:7091–7097. doi: 10.1074/jbc.M311973200. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006;86:465–514. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Horvath TL. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends in Neurosciences. 2013;36:65–73. doi: 10.1016/j.tins.2012.12.005. [DOI] [PubMed] [Google Scholar]
- DiTacchio L, Le HD, Vollmers C, Hatori M, Witcher M, Secombe J, Panda S. Histone lysine demethylase JARID1a activates CLOCK-BMAL1 and influences the circadian clock. Science. 2011;333:1881–1885. doi: 10.1126/science.1206022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Hirayama J, Sassone-Corsi P. Circadian Regulator CLOCK Is a Histone Acetyltransferase. Cell. 2006a;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Doi M, Yujnovsky I, Hirayama J, Malerba M, Tirotta E, Sassone-Corsi P, Borrelli E. Impaired light masking in dopamine D2 receptor-null mice. Nat Neurosci. 2006b;9:732–734. doi: 10.1038/nn1711. [DOI] [PubMed] [Google Scholar]
- Drucker-Colin R, Aguilar-Roblero R, Garcia-Hernandez F, Fernandez-Cancino F, Bermudez Rattoni F. Fetal suprachiasmatic nucleus transplants: diurnal rhythm recovery of lesioned rats. Brain Res. 1984;311:353–357. doi: 10.1016/0006-8993(84)90099-4. [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan Kristin L, Patel Vishal R, de Mateo S, Orozco-Solis R, Ceglia Nicholas J, Sahar S, Dilag-Penilla Sherry A, Dyar Kenneth A, Baldi P, Sassone-Corsi P. Reprogramming of the Circadian Clock by Nutritional Challenge. Cell. 2013;155:1464–1478. doi: 10.1016/j.cell.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel-Mahan KL, Patel VR, Mohney RP, Vignola KS, Baldi P, Sassone-Corsi P. Coordination of the transcriptome and metabolome by the circadian clock. Proceedings of the National Academy of Sciences. 2012;109:5541–5546. doi: 10.1073/pnas.1118726109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchegaray JP, Lee C, Wade PA, Reppert SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature. 2003;421:177–182. doi: 10.1038/nature01314. [DOI] [PubMed] [Google Scholar]
- Etchegaray JP, Yang X, DeBruyne JP, Peters AH, Weaver DR, Jenuwein T, Reppert SM. The polycomb group protein EZH2 is required for mammalian circadian clock function. J Biol Chem. 2006;281:21209–21215. doi: 10.1074/jbc.M603722200. [DOI] [PubMed] [Google Scholar]
- Feng J, Nestler EJ. Epigenetic mechanisms of drug addiction. Curr Opin Neurobiol. 2013;23:521–528. doi: 10.1016/j.conb.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froy O. The relationship between nutrition and circadian rhythms in mammals. Front Neuroendocrinol. 2007;28:61–71. doi: 10.1016/j.yfrne.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Lu J, Saper CB. Differential Rescue of Light- and Food-Entrainable Circadian Rhythms. Science. 2008;320:1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebultowicz J, Kapahi P. Circadian clocks and metabolism: the nutrient-sensing AKT and TOR pathways make the link. Curr Biol. 2010;20:R608–609. doi: 10.1016/j.cub.2010.05.052. [DOI] [PubMed] [Google Scholar]
- Glass JD, Grossman GH, Farnbauch L, DiNardo L. Midbrain raphe modulation of nonphotic circadian clock resetting and 5-HT release in the mammalian suprachiasmatic nucleus. J Neurosci. 2003;23:7451–7460. doi: 10.1523/JNEUROSCI.23-20-07451.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- Guzmán-Ruiz M, Saderi N, Cázarez-Márquez F, Guerrero-Vargas NN, Basualdo MC, Acosta-Galván G, Buijs RM. The Suprachiasmatic Nucleus Changes the Daily Activity of the Arcuate Nucleus α-MSH Neurons in Male Rats. Endocrinology. 2013 doi: 10.1210/en.2013-1604. [DOI] [PubMed] [Google Scholar]
- Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, Brunk I, Spanagel R, Ahnert-Hilger G, Meijer JH, Albrecht U. Regulation of Monoamine Oxidase A by Circadian-Clock Components Implies Clock Influence on Mood. Current Biology. 2008;18:678–683. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Duffield GE, Smith EJ, Maywood ES, Ebling FJ. Entrainment of the circadian system of mammals by nonphotic cues. Chronobiol Int. 1998;15:425–445. doi: 10.3109/07420529808998700. [DOI] [PubMed] [Google Scholar]
- Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450:1086–1090. doi: 10.1038/nature06394. [DOI] [PubMed] [Google Scholar]
- Holmes MC, French KL, Seckl JR. Modulation of serotonin and corticosteroid receptor gene expression in the rat hippocampus with circadian rhythm and stress. Molecular Brain Research. 1995;28:186–192. doi: 10.1016/0169-328x(94)00207-u. [DOI] [PubMed] [Google Scholar]
- Huang H, Kong D, Byun KH, Ye C, Koda S, Lee DH, Oh B-C, Lee SW, Lee B, Zabolotny JM, Kim MS, Bjorbaek C, Lowell BB, Kim Y-B. Rho-kinase regulates energy balance by targeting hypothalamic leptin receptor signaling. Nat Neurosci. 2012;15:1391–1398. doi: 10.1038/nn.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic "island" containing the suprachiasmatic nucleus. Proceedings of the National Academy of Sciences. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocrine reviews. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Fliers E, Romijn JA, La Fleur SE, Wortel J, Bakker O, Endert E, Buijs RM. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology. 2001;142:2677–2685. doi: 10.1210/endo.142.6.8197. [DOI] [PubMed] [Google Scholar]
- Katada S, Sassone-Corsi P. The histone methyltransferase MLL1 permits the oscillation of circadian gene expression. Nat Struct Mol Biol. 2010;17:1414–1421. doi: 10.1038/nsmb.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockener T, Hess S, Belgardt BF, Paeger L, Verhagen LAW, Husch A, Sohn J-W, Hampel B, Dhillon H, Zigman JM, Lowell BB, Williams KW, Elmquist JK, Horvath TL, Kloppenburg P, Bruning JC. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat Neurosci. 2011;14:911–918. doi: 10.1038/nn.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Jian K, Shi L, Ko GY-P. Phosphatidylinositol 3 kinase-Akt signaling serves as a circadian output in the retina. Journal of Neurochemistry. 2009;108:1607–1620. doi: 10.1111/j.1471-4159.2009.05931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Koike N, Yoo S-H, Huang H-C, Kumar V, Lee C, Kim T-K, Takahashi JS. Transcriptional Architecture and Chromatin Landscape of the Core Circadian Clock in Mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosobud AEK, Gillman AG, Leffel JK, Pecoraro NC, Rebec GV, Timberlake W. Drugs of Abuse Can Entrain Circadian Rhythms. The Scientific World JOURNAL. 2007;7:203–212. doi: 10.1100/tsw.2007.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry GJ, Simon MM, Webb IC, Mistlberger RE. Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1527–1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- Leak RK, Card JP, Moore RY. Suprachiasmatic pacemaker organization analyzed by viral transynaptic transport. Brain Research. 1999;819:23–32. doi: 10.1016/s0006-8993(98)01317-1. [DOI] [PubMed] [Google Scholar]
- Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. The Journal of Comparative Neurology. 2001;433:312–334. doi: 10.1002/cne.1142. [DOI] [PubMed] [Google Scholar]
- Li A-J, Wiater MF, Oostrom MT, Smith BR, Wang Q, Dinh TT, Roberts BL, Jansen HT, Ritter S. Leptin-sensitive neurons in the arcuate nuclei contribute to endogenous feeding rhythms. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2012;302:R1313–R1326. doi: 10.1152/ajpregu.00086.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Pointer K, Bell Eric L, Das A, Cohen Dena E, Asara John M, Kapur K, Bergmann S, Preisig M, Otowa T, Kendler Kenneth S, Chen X, Hettema John M, van den Oord Edwin J, Rubio JP, Guarente L. SIRT1 Activates MAO-A in the Brain to Mediate Anxiety and Exploratory Drive. Cell. 2011;147:1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA. Functional Subsets of Serotonergic Neurones: Implications for Control of the Hypothalamic-Pituitary-Adrenal Axis. Journal of Neuroendocrinology. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Lu XY, Shieh KR, Kabbaj M, Barsh GS, Akil H, Watson SJ. Diurnal rhythm of agouti-related protein and its relation to corticosterone and food intake. Endocrinology. 2002;143:3905–3915. doi: 10.1210/en.2002-220150. [DOI] [PubMed] [Google Scholar]
- Lucassen EA, Rother KI, Cizza G. Interacting epidemics? Sleep curtailment, insulin resistance, and obesity. Annals of the New York Academy of Sciences no-no. 2012 doi: 10.1111/j.1749-6632.2012.06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniam J, Morris MJ. The link between stress and feeding behaviour. Neuropharmacology. 2012;63:97–110. doi: 10.1016/j.neuropharm.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Masri S, Sassone-Corsi P. Plasticity and specificity of the circadian epigenome. Nat Neurosci. 2010;13:1324–1329. doi: 10.1038/nn.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza J, Clesse D, Pévet P, Challet E. Food-reward signalling in the suprachiasmatic clock. Journal of Neurochemistry. 2010;9999 doi: 10.1111/j.1471-4159.2010.06570.x. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Graff C, Dardente H, Pevet P, Challet E. Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle. J Neurosci. 2005;25:1514–1522. doi: 10.1523/JNEUROSCI.4397-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michan S. Acetylome Regulation By Sirtuins In The Brain:From Normal Physiology To Aging And Pathology. Current pharmaceutical design. 2013 doi: 10.2174/1381612811319380014. [DOI] [PubMed] [Google Scholar]
- Mieda M, Sakurai T. Bmal1 in the Nervous System Is Essential for Normal Adaptation of Circadian Locomotor Activity and Food Intake to Periodic Feeding. The Journal of Neuroscience. 2011;31:15391–15396. doi: 10.1523/JNEUROSCI.2801-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim Y-B, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Misra A, Khurana L. Obesity and the Metabolic Syndrome in Developing Countries. J Clin Endocrinol Metab. 2008;93:s9–30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Neurobiology of food anticipatory circadian rhythms. Physiology & Behavior. 2011;104:535–545. doi: 10.1016/j.physbeh.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Skene DJ. Social influences on mammalian circadian rhythms: animal and human studies. Biological Reviews. 2004;79:533–556. doi: 10.1017/s1464793103006353. [DOI] [PubMed] [Google Scholar]
- Moga MM, Moore RY. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J Comp Neurol. 1997;389:508–534. doi: 10.1002/(sici)1096-9861(19971222)389:3<508::aid-cne11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Moriya T, Aida R, Kudo T, Akiyama M, Doi M, Hayasaka N, Nakahata N, Mistlberger R, Okamura H, Shibata S. The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice. European Journal of Neuroscience. 2009;29:1447–1460. doi: 10.1111/j.1460-9568.2009.06697.x. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Nagai K, Fujii T, Inoue S, Takamura Y, Nakagawa H. Electrical stimulation of the suprachiasmatic nucleus of the hypothalamus causes hyperglycemia. Hormone and metabolic research. 1988;20:37–39. doi: 10.1055/s-2007-1010743. [DOI] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian Control of the NAD+ Salvage Pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse Y, Oh-hashi K, Iijima N, Naruse M, Yoshioka H, Tanaka M. Circadian and Light-Induced Transcription of Clock Gene Per1 Depends on Histone Acetylation and Deacetylation. Molecular and Cellular Biology. 2004;24:6278–6287. doi: 10.1128/MCB.24.14.6278-6287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Falcon E, Mukherjee S, Gillman A, Arey R, Spencer S, McClung CA. The Role of Clock in Ethanol-Related Behaviors. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek CB, Ramsey KM, Marcheva B, Bass J. Nutrient sensing and the circadian clock. Trends Endocrinol Metab. 2012;23:312–318. doi: 10.1016/j.tem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Fujikawa T, Anderson J, Berglund Eric D, Frazao R, Michán S, Vianna Claudia R, Sinclair David A, Elias Carol F, Coppari R. SIRT1 Deacetylase in SF1 Neurons Protects against Metabolic Imbalance. Cell Metabolism. 2011;14:301–312. doi: 10.1016/j.cmet.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, Rahmouni K, Coppari R. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, Elmquist JK, Coppari R. Brain SIRT1: Anatomical Distribution and Regulation by Energy Availability. J Neurosci. 2008;28:9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong H-K, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S-i, Bass J. Circadian Clock Feedback Cycle Through NAMPT-Mediated NAD+ Biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Kumar A, Xiao G, Wilkinson M, Covington Iii HE, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, Russo SJ, Vialou V, Chakravarty S, Kodadek TJ, Stack A, Kabbaj M, Nestler EJ. Genome-wide Analysis of Chromatin Regulation by Cocaine Reveals a Role for Sirtuins. Neuron. 2009;62:335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- Ribeiro AC, Sawa E, Carren-LeSauter I, LeSauter J, Silver R, Pfaff DW. Two forces for arousal: Pitting hunger versus circadian influences and identifying neurons responsible for changes in behavioral arousal. Proc Natl Acad Sci U S A. 2007;104:20078–20083. doi: 10.1073/pnas.0710096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1[alpha] and SIRT1 pathways. FEBS Letters. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KK, Li B, Grayson BE, Matter EK, Woods SC, Seeley RJ. A role for central nervous system PPAR-[gamma] in the regulation of energy balance. Nat Med. 2011;17:623–626. doi: 10.1038/nm.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar S, Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer. 2009;9:886–896. doi: 10.1038/nrc2747. [DOI] [PubMed] [Google Scholar]
- Saito T, Hirano M, Ide T, Ichiki T, Koibuchi N, Sunagawa K, Hirano K. Pivotal role of Rho-associated kinase 2 in generating the intrinsic circadian rhythm of vascular contractility. Circulation. 2013;127:104–114. doi: 10.1161/CIRCULATIONAHA.112.135608. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kim H-J, Kobayashi M, Kitamura Y-I, Yokota-Hashimoto H, Shiuchi T, Minokoshi Y, Kitamura T. Induction of Hypothalamic Sirt1 Leads to Cessation of Feeding via Agouti-Related Peptide. Endocrinology. 2010;151:2556–2566. doi: 10.1210/en.2009-1319. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kitamura T. Roles of FoxO1 and Sirt1 in the central regulation of food intake. Endocrine Journal. 2010;57:939–946. doi: 10.1507/endocrj.k10e-320. [DOI] [PubMed] [Google Scholar]
- Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, Guzman E, Niessen S, Yates JR, 3rd, Takemori H, Okamoto M, Montminy M. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Soria V, Urretavizcaya M. Circadian rhythms and depression. Actas espanolas de psiquiatria. 2009;37:222–232. [PubMed] [Google Scholar]
- Spencer S, Torres-Altoro MI, Falcon E, Arey R, Marvin M, Goldberg M, Bibb JA, McClung CA. A mutation in CLOCK leads to altered dopamine receptor function. Journal of Neurochemistry. 2012;123:124–134. doi: 10.1111/j.1471-4159.2012.07857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK. The “Other” Circadian System: Food as a Zeitgeber. Journal of Biological Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipanovich A, Valjent E, Matamales M, Nishi A, Ahn J-H, Maroteaux M, Bertran-Gonzalez J, Brami-Cherrier K, Enslen H, Corbille A-G, Filhol O, Nairn AC, Greengard P, Herve D, Girault J-A. A phosphatase cascade by which rewarding stimuli control nucleosomal response. Nature. 2008;453:879–884. doi: 10.1038/nature06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch K-F, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proceedings of the National Academy of Sciences. 2009;106:6808–6813. doi: 10.1073/pnas.0902063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz AM, Staszkiewicz J, Ptitsyn A, Argyropoulos G. Circadian expression of genes regulating food intake. Obesity (Silver Spring) 2007;15:607–615. doi: 10.1038/oby.2007.564. [DOI] [PubMed] [Google Scholar]
- Su W, Xie Z, Guo Z, Duncan MJ, Lutshumba J, Gong MC. Altered clock gene expression and vascular smooth muscle diurnal contractile variations in type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol. 2012;302:H621–633. doi: 10.1152/ajpheart.00825.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton GM, Begriche K, Kumar KG, Gimble JM, Perez-Tilve D, Nogueiras R, McMillan RP, Hulver MW, Tschop MH, Butler AA. Central nervous system melanocortin-3 receptors are required for synchronizing metabolism during entrainment to restricted feeding during the light cycle. FASEB J. 2010;24:862–872. doi: 10.1096/fj.09-142000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasu NN, Kurosawa G, Tokuda IT, Mochizuki A, Todo T, Nakamura W. Circadian Regulation of Food-Anticipatory Activity in Molecular Clock–Deficient Mice. PLoS One. 2012;7:e48892. doi: 10.1371/journal.pone.0048892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Yanovski SZ. Eating disorder or disordered eating? Non-normative eating patterns in obese individuals. Obes Res. 2004;12:1361–1366. doi: 10.1038/oby.2004.171. [DOI] [PubMed] [Google Scholar]
- Terman M, Terman JS. Circadian rhythm of brain self-stimulation behavior. Science. 1970;168:1242–1244. doi: 10.1126/science.168.3936.1242. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiology & Behavior. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science (New York, NY. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends in Cognitive Sciences. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmers C, Schmitz Robert J, Nathanson J, Yeo G, Ecker Joseph R, Panda S. Circadian Oscillations of Protein-Coding and Regulatory RNAs in a Highly Dynamic Mammalian Liver Epigenome. Cell Metabolism. 2012;16:833–845. doi: 10.1016/j.cmet.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TA, Yu YV, Govindaiah G, Ye X, Artinian L, Coleman TP, Sweedler JV, Cox CL, Gillette MU. Circadian Rhythm of Redox State Regulates Excitability in Suprachiasmatic Nucleus Neurons. Science. 2012;337:839–842. doi: 10.1126/science.1222826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. The Journal of Comparative Neurology. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga MS, MJ IJ, Wijckmans-Duijsens NE. The role of macronutrient selection in determining patterns of food intake in obese and non-obese women. Eur J Clin Nutr. 1996;50:580–591. [PubMed] [Google Scholar]
- Xu B, Kalra PS, Farmerie WG, Kalra SP. Daily changes in hypothalamic gene expression of neuropeptide Y, galanin, proopiomelanocortin, and adipocyte leptin gene expression and secretion: effects of food restriction. Endocrinology. 1999;140:2868–2875. doi: 10.1210/endo.140.6.6789. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hill JW, Fukuda M, Gautron L, Sohn JW, Kim KW, Lee CE, Choi MJ, Lauzon DA, Dhillon H, Lowell BB, Zigman JM, Zhao JJ, Elmquist JK. PI3K signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell Metab. 2010;12:88–95. doi: 10.1016/j.cmet.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Bobula JM, Svenningsson P, Greengard P, Silver R. DARPP-32 Involvement in the Photic Pathway of the Circadian System. The Journal of Neuroscience. 2006;26:9434–9438. doi: 10.1523/JNEUROSCI.2538-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- Yi CX, van der Vliet J, Dai J, Yin G, Ru L, Buijs RM. Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology. 2006;147:283–294. doi: 10.1210/en.2005-1051. [DOI] [PubMed] [Google Scholar]
- Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK:BMAL1. Proceedings of the National Academy of Sciences. 2006;103:6386–6391. doi: 10.1073/pnas.0510691103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 2009;33(Suppl 2):S8–13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocchi L, Sassone-Corsi P. Joining the dots: from chromatin remodeling to neuronal plasticity. Current Opinion in Neurobiology. 2010;20:432–440. doi: 10.1016/j.conb.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]