Abstract
Background
Patients with recurrent or refractory osteosarcoma have a poor prognosis with less than 30% surviving 2 years. Eribulin is a synthetic analog of halichondrin B, has a novel mechanism of action when compared to other microtubule inhibitors and may have anti-tumor activity in osteosarcoma.
Methods
A prospective study was designed to assess the disease control success at 4 months and objective response rates in patients with recurrent or refractory osteosarcoma treated with eribulin. Eligible patients were between 12 and 50 years of age, had measurable tumor, and met standard organ function requirements. Patients were given eribulin 1.4mg/m2/dose on Day 1 and Day 8 of each 3 week cycle for up to 24 months if there was not progressive disease. Response to therapy was assessed using RECIST 1.1 criteria after cycles 2 and 5 and every 4th cycle thereafter.
Results
Nineteen patients enrolled on the AOST1322 study. The median age of enrollment was 16 years (range 12–25 years). Twelve patients were male and 7 female. Eribulin was well tolerated with neutropenia identified as the most common toxicity. The median progression free survival was 38 days and no patients reached the 4 month time point without progression. No objective responses were seen in any patient..
Conclusion
This study rapidly assessed the clinical activity of a novel agent in this patient population. Eribulin was well tolerated but there were no patients who demonstrated objective response and all patients had progression prior to 4 months.
Keywords: Osteosarcoma, Eribulin
Introduction
Over the past three decades, the 5-year event free survival (EFS) for patients with localized osteosarcoma has been stalled near 65%, with no consistent trend of increase over this time [1]. In addition, the prognosis for the 30–40% of patients who develop disease recurrence, [2] and for those with clinically detectable metastases at the time of initial diagnosis is poor with 2-year survivals of 20–30%.[3–5]. Since the late 1980s, methotrexate, cisplatin and doxorubicin have been the cornerstone of systemic therapy; new active, less toxic therapies are needed for the treatment of osteosarcoma.
Eribulin is a synthetic analog of a natural product, halichondrin B, and has a novel mechanism of action when compared to other microtubule inhibitors. This mechanism includes the polymerization of tubulin and results in accumulation of nonfunctional tubulin aggregates. Unlike other anti-microtubule drugs, such as vincristine, vinblastine and paclitaxel, which suppress the shortening and growth phases of microtubule dynamic instability, eribulin inhibits microtubule growth but does not suppress microtubule shortening[6, 7]. Eribulin also blocks the cell cycle at the G2-M phase and is active in taxane-resistant cell lines with beta-tubulin mutations [8]. In addition, eribulin is metabolized by CYP3A4 but does not significantly inhibit or induce CYP3A4 activity, limiting the likelihood of drug-drug interactions. [7]
Eribulin was evaluated by the Pediatric Preclinical Testing Program (PPTP) and demonstrated activity in a number of pediatric malignancies with significant differences in EFS distribution compared to control in 29 of 35 (83%) of the evaluable solid tumor xenografts and in 8 of 8 (100%) of the evaluable acute lymphoblastic leukemia xenografts[9]. Three of 6 osteosarcoma xenografts had a complete response, 1/6 osteosarcoma xenografts had stable disease, and 2/6 osteosarcoma xenografts had progressive disease[9]. In the osteosarcoma cell line U2OS, eribulin was also shown to affect mitotic spindle centromere dynamics[10]. In adults, eribulin is an FDA-approved agent for the treatment of patients with refractory, metastatic breast cancer. Eribulin has also been assessed in a variety of soft tissue sarcoma histologies. Initially, eribulin was tested in the phase II setting in adults with advanced or progressive soft tissue sarcoma and demonstrated an improvement in 12 week progression free survival and excellent tolerability [11]. Based on this result, a phase III study was completed in order to assess whether the overall survival (OS) in patients with advanced or metastatic soft-tissue sarcoma was improved compared to those who received dacarbazine. The results of this study demonstrated an improved overall survival in the eribulin group (median OS 13.5 months) compared to the dacarbazine group (median OS 11.5 months)[12]. However, a subgroup analysis of patients with liposarcoma indicated a more significant improvement in OS for this particular histology, with a median OS of 15.6 for those treated with eribulin vs 8.4 months for those treated with dacarbazine[13]. These results have contributed to an additional FDA approval for eribulin in the treatment of metastatic or unresectable liposarcoma. However, although eribulin has been tested in a variety of soft tissue sarcoma histologies this is the first study to assess efficacy of eribulin in a primary bone sarcoma.
This report describes the results of COG AOST1322, a phase II study designed to assess the objective response rate of eribulin in pediatric and young adult patients age 12 to 50 with recurrent or refractory osteosarcoma[8].
Patients and Methods
Patient Population
Eligible patients included those ≥12 and < 50 years of age with a diagnosis of relapsed or refractory osteosarcoma with measurable (≥ 10 mm) disease as defined by RECIST 1.1criteria. The lower age limit of 12 was chosen based on a lack of pharmacokinetic data for younger children. Other eligibility criteria included adequate renal, cardiac, and liver function along with adequate bone marrow function as defined by an ANC ≥ 1000, platelet count ≥ 75,000/μL, and hemoglobin ≥ 8.0 g/dL. Patients were also required to have a performance status corresponding to ECOG scores of 0, 1 or 2 and a life expectancy of greater than 8 weeks. Patients were excluded if they had prior use of eribulin or halichondrin B products, prolonged QT syndrome or QT interval ≥501, or peripheral neuropathy ≥ grade 2 using the Common Terminology for Classifying Adverse Events (CTCAE) version 4.0. Patients with recent major surgery within three weeks of enrollment were also excluded.
Based on accrual rates of patients with osteosarcoma from three prior COG studies, ADVL0421, ADVL0524 and ADVL0525[14–16], approximately 18 patients (1.5 patients per month) were expected to enroll annually. This trial was approved by the National Cancer Institute Pediatric Central Institutional Review Board, as well as by local regulatory boards at all participating sites. A document of informed consent was signed by all patients or their parent/legal guardian, and assent was obtained as appropriate according to the local institutional guidelines prior to enrollment.
Drug Administration
Eribulin was supplied by the National Cancer Institute (Bethesda, MD). Patients were given eribulin 1.4mg/m2/dose as an IV infusion over 2–5minutes on Day 1 and Day 8 of each 21 day cycle and treatment could continue for up to 24 months in the absence of disease progression or toxicity requiring discontinuation of treatment. Cycles were repeated provided initial eligibility criteria were met, including an absolute neutrophil count (ANC) ≥ 1,000/μL, platelet count ≥ 75,000/μL, hemoglobin ≥ 8.0 g/dL (may have received RBC transfusions), and normal renal and liver function.
For patients with Grade 4 neutropenia or Grade 4 thrombocytopenia on Day 8 or grade 3 or 4 non-hematologic toxicity, the dose of eribulin was withheld. If the toxicity resolved to meet eligibility or baseline by Day 11, eribulin was reduced to 1.1 mg/m2/dose. If the toxicity did not resolve by Day 11, the dose was omitted and subsequent cycles were given with the 1.1 mg/m2/dose. Dose reductions were not required for grade 3 nausea and vomiting < 3 days duration, grade 3 liver enzyme elevation that returns to grade ≤ 1 or baseline prior to the next dose, grade 3 fever or infection, grade 3 electrolyte disturbances responsive to oral supplementation, or grade 3 ototoxicity in a subject who previously received cisplatin or was enrolled to the trial with grade 3 or greater ototoxicity.
Study Design
Primary Outcome Measures:
Each patient was evaluated for two outcomes: (1) RECIST response as according to RECIST 1.1[17]; and (2) disease control success at four-months (DC4). Any eligible patient who received at least one dose of eribulin was considered evaluable for response assessment, except if the patient received non-protocol therapy after the patient first demonstrated CR or PR, but prior to the time of the confirmatory evaluation. Any evaluable patient who demonstrated a complete or partial response after cycle 2 or before the end of the fifth cycle of therapy was considered a responder; otherwise the patient was considered a non-responder.
Any eligible patient who received at least one dose of eribulin was considered evaluable for DC4. A patient was considered a disease control success if they did not demonstrate disease progression through either five cycles of protocol therapy or four months after study enrollment if eribulin therapy was stopped prior to the fifth cycle for any reason. Otherwise, the patient was considered a disease control failure. The choice of stable disease for four months to characterize a favorable outcome was selected based on the analysis of Lagmay et al.[18]. Response to therapy was planned to be assessed using RECIST 1.1 criteria after cycles 2 and 5 and every 4th cycle thereafter.
Patients who were not evaluable for either response or disease control could be replaced for the application of the statistical rule. Patients who were evaluable for response but not disease control were considered not to have experienced DC4. The definitions above did not provide for a patient to be evaluable for disease control but not RECIST 1.1 response.
The study was planned as a two stage design. Nineteen outcome evaluable patients were to be enrolled. If four or fewer disease control successes and 1 or fewer responses were observed, the study was to be stopped with the conclusion that eribulin was not associated with sufficient activity for further evaluation. Otherwise an additional 10 outcome evaluable patients were to be enrolled. The statistical operating characteristics of this design are presented as part of the Supplemental Materials available at the journal website.
Toxicity Evaluation
Each cycle of protocol therapy that a patient received was evaluated for the presence of dose-limiting toxicity (DLT). Hematological DLT was defined as: (1) ≥ Grade 4 neutropenia for > 7 days; (2) Platelet count < 20,000/μL on 2 separate days, or requiring a platelet transfusion on 2 separate days, within a 7 day period; or (3) Myelosuppression that causes a delay of > 14 days between treatment cycles. Non-hematological DLT was defined as: (1) Day 8 eribulin dose was held due to Grade 3 or Grade 4 non-hematological toxicity attributable to the investigational drug and which did not resolve to meet eligibility or baseline criteria by Day 11; (2) Any ≥ Grade 3 non-hematological toxicity which, according to the treating physician, was considered attributable to the eribulin except Grade 3 nausea and vomiting < 3 days duration, Grade 3 liver enzyme elevation, including ALT/AST/GGT, that returned to Grade ≤ 1 or baseline prior to the time for the next treatment cycle, Grade 3 fever, Grade 3 infection, Grade 3 hypophosphatemia, hypokalemia, hypocalcemia or hypomagnesemia responsive to oral supplementation, or Grade 3 ototoxicity in a subject who previously received cisplatin or was enrolled to the trial with Grade 3 or greater ototoxicity. A Bayesian rule described in the supplementary materials, was used to monitor for an excessive per-cycle DLT rate.
For non-DLT toxicities, reporting of adverse experiences was limited to grade 3 and higher non-hematologic and grade 4 and higher hematologic CTC adverse events. In order to quantify toxicities regardless of grade, all cycles delivered to eligible and evaluable patients enrolled on the study were aggregated and CTCAE toxicities were tabulated. The proportion of all cycles with a particular grade and type of adverse event was calculated.
Results
Patient Characteristics
AOST1322 was activated for enrollment on August 22, 2014 and closed to accrual on December 15, 2014. Data current to June 30, 2015 were used in the preparation of this report. Nineteen patients enrolled on study at a rate of 5.4 per month and none were considered ineligible. All patients were evaluable for toxicity, response evaluation and DC4. The sites of measurable disease and clinical characteristics among the 19 enrolled patients are noted in Table 1. All patients were off protocol therapy at the time of the analysis for this report. The study was closed to further accrual on December 15, 2014 due to a planned interim analysis of the first 19 patients.
Table 1.
Characteristics of Enrolled Patients
| Characteristic | ||
|---|---|---|
| Age in Years at Study Enrollment | Mean | 16.8 Years |
| Median | 16 Years | |
| Range | 12 Years – 25 Years | |
| 21 Years or Older | 2 | |
| Patient Sex | Male | 12 |
| Female | 7 | |
| Race | White | 14 |
| African-American | 3 | |
| American Indian, Aleutian or Eskimo | 1 | |
| Other Race | 1 | |
| Ethnicity | Hispanic | 3 |
| Not Hispanic | 16 | |
| Number of Lesions Measured for RECIST Evaluation | Mean | 1.9 |
| Median | 2 | |
| Range | 1 – 4 | |
| Tumor Burden in millimeters1 | Mean | 78.6 |
| Median | 72 | |
| Range | 10 – 150.6 | |
| Sites of Measurable Disease | ||
| Lung | 11 | |
| Bone | 1 | |
| Other | 3 | |
| Lung + Bone | 2 | |
| Lung + Other | 2 | |
The sum of the longest dimension of all lesions identified by the patient’s physician for assessment of RECIST response.
Antitumor Activity
The median progression free survival was 38 days and no patients reached the 4 month time point without progression; Figure 1. No objective responses were seen in any patient as measured by RECIST 1.1 criteria. Of the 19 patients considered, 5 received one cycle and progressed before the post-cycle 2 assessment as determined by early imaging, 12 received two cycles and 2 received 5 cycles of protocol therapy.
Figure 1.
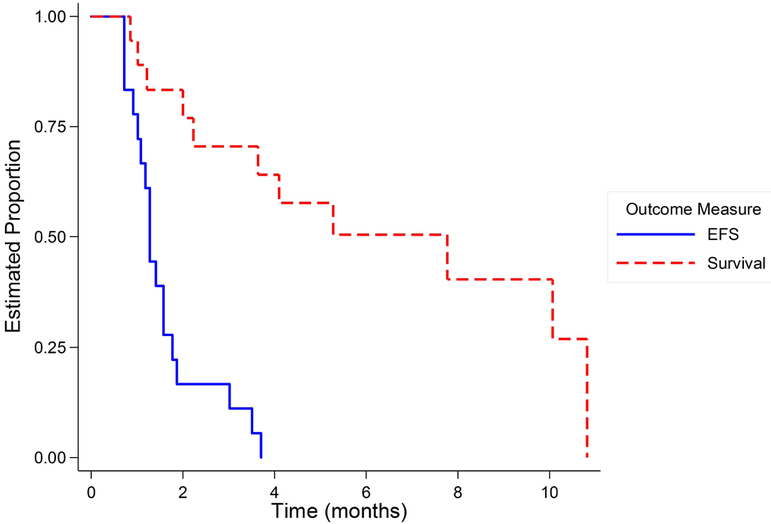
Event Free Survival and Overall Survival for the 19 patients enrolled in AOST1322 demonstrating that no patients were progression free at 4 months.
Toxicity Evaluation
Of 39 cycles administered to the 19 patients, one (1) was associated with dose-limiting toxicity. The patient experienced grade 3 extremity pain possibly related to eribulin. The point estimate of the probability of a DLT is 2.6%. The posterior probability as defined above is less than 0.001. Toxicities observed, both dose-limiting and non-dose limiting, are displayed in Table 2. Neutropenia was the most common toxicity reported, occurring in 18% of the cycles.
Table 2.
Toxicities observed among the 39 cycles of chemotherapy administered to 19 patients enrolled on AOST1322
| Toxicity Grade | |||
|---|---|---|---|
| No toxicity or < grade 3 | Grade 3 or Greater | ||
| Number (Percent) | Number (Percent) | ||
| Organ System | Toxicity Type | ||
| Respiratory/Thoracic/Mediastinal | Atelectasis | 38 (97.4) | 1 (2.6) |
| Dyspnea | 38 (97.4) | 1 (2.6) | |
| Hypoxia | 38 (97.4) | 1 (2.6) | |
| Injury/Poisoning/Procedural | Fracture | 38 (97.4) | 1 (2.6) |
| Metabolism/Nutrition | Hyponatremia | 38 (97.4) | 1 (2.6) |
| Investigations | Neutrophil count decreased | 32 (82.1) | 7 (17.9) |
| White blood cell decreased | 37 (94.9) | 2 (5.1) | |
| Musculoskeletal/Connective | Pain in extremity | 37 (94.9) | 2 (5.1) |
| Cardiac | Pericardial effusion | 38 (97.4) | 1 (2.6) |
Discussion
The rate of disease stability and objective response for eribulin was disappointingly low in this phase II trial for children and young adults with recurrent or refractory osteosarcoma. In fact, we did not observe any complete or partial objective responses and none of the patients remained on study, progression free at 4 months. Eribulin did not meet sufficient activity criteria to warrant further development for the treatment of osteosarcoma.
The decision to conduct this phase II evaluation of eribulin in patients with relapsed or refractory osteosarcoma was made based on multiple lines of preclinical evidence. This included osteosarcoma cell line data indicating cytotoxicity and disruption of the centromere dynamics, leading to mitotic arrest[10]. Additionally, the PPTP data demonstrated that 3 of 6 xenografts had either stable disease or a partial response[9]. This data was similar to the testing of the microtubule inhibitor vincristine which demonstrated high activity with an objective response in 1 of the 2 osteosarcoma xenografts[19]. Additionally, the efficacy demonstrated in patients with soft tissue sarcoma, especially in liposarcoma, supported the importance of the efficacy assessment in the most common primary bone sarcoma.
We note that although microtubule inhibitors have not been commonly used in the treatment of osteosarcoma in recent times, there have been trials in the past that have demonstrated efficacy with combination chemotherapy that included vincristine[20, 21]. However, these studies were not designed to specifically assess single agent efficacy and historical data do not give further clarity since the end point of early single agent studies of vincristine was focused on reduction of tumor size[22]; typically not observed in osteosarcoma. Thus we were disappointed by the ineffectiveness of eribulin.
Although we are unable to identify the reason for the lack of response in our patient population, the PPTP investigators are currently evaluating whether the drug exposures in the preclinical models accurately reflect the actual drug exposure in patients. Additionally, we note that although the PPTP data did not correlate with clinical response in our patient population, there is preliminary data to suggest that eribulin may have clinical activity in patients with Ewing sarcoma[23]. Additionally, the PPTP has demonstrated preclinical activity of the MEK inhibitor selumetinib in the treatment of astrocytoma[24], which has correlated thus far with clinical activity as demonstrated in a phase I clinical trial of low grade astrocytoma[25]. In addition, evaluations are being conducted to consider whether resistant/recurrent preclinical models may demonstrate less tumor activity compared to what was studied in our patient population. Despite the lack of response seen here, the COG bone tumor committee believes that a data driven and highly scrutinized process to help prioritize agents of interest that should be tested in osteosarcoma, as described by Khanna et. al should continue to be used [26]. Certainly, if continued disappointing results are seen, clinical investigators will need to re-evaluate the approach and determine whether a different mechanism to identify agents is needed.
In general, osteosarcoma is a disease that has challenged oncologists for decades with few agents identified with activity. The biology is complicated without a specific target or marker of disease. There also may be a lack of tumor size reduction even with an agent considered to have activity, thus the time to progression may be a better measure[18]. Additionally, studies for new agents are usually in heavily pre-treated patients and may underestimate the response that could be seen in patients with untreated disease[27]. However, collaborative efforts are being made by scientists internationally to increase our understanding of osteosarcoma biology and genomics[28]. We remain hopeful that the likelihood of future success in clinical trials may be improved as we develop a deeper understanding of the biologic mechanisms of osteosarcoma and develop trials that include biomarker selection when a biomarker is known. We also speculate that combination therapy, when appropriate, may also increase the likelihood of success in future trials.
The study design allowed for a rapid assessment of the clinical activity of a novel agent in this patient population and met accrual goals in a faster time period than expected. We have previously proposed a number of potential reasons that may have contributed to this rapid enrollment[29] and expect that if future phase II trials in osteosarcoma have similar enrollment patterns we should be able to test many agents in a short time frame. We also note that the design of this study was successful in answering the primary objective and supports the use of a 4 month progression free survival as an appropriate goal for assessing new therapies in recurrent unresected osteosarcoma.
In conclusion, eribulin was well tolerated but failed to show activity in patients with recurrent osteosarcoma. It remains imperative to work to identify new active therapies for patients with osteosarcoma.
Supplementary Material
Acknowledgements:
This research was supported by the Children’s Oncology Group Statistics & Data Center Grant U10CA098413; Children’s Oncology Group Chair’s Grant U10CA098543 (for the period 2003–2014); NCTN Operations Center Grant U10CA180886 (current grant, as of 3/1/14);NCTN Statistics & Data Center Grant U10CA180899 (current grant, as of 3/1/14). In addition, this research was supported by St. Baldrick’s Foundation. MI also receives funding from the Reid R. Sacco Adolescent and Young Adult Cancer Alliance.
R. Gorlick receives research support to study Lenvatinib by Eisai, Inc.
Grant: U10CA180886/NCTN Operations Center Grant and U10CA180899/NCTN Statistics
Footnotes
Conflicts of Interest: All other authors declare that they have no conflicts of interest.
References
- 1.Smith MA, et al. , Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol, 2010. 28(15): p. 2625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kempf-Bielack B, et al. , Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol, 2005. 23(3): p. 559–68. [DOI] [PubMed] [Google Scholar]
- 3.Bacci G, et al. , Neoadjuvant chemotherapy for osteosarcoma of the extremities with metastases at presentation: recent experience at the Rizzoli Institute in 57 patients treated with cisplatin, doxorubicin, and a high dose of methotrexate and ifosfamide. Ann Oncol, 2003. 14(7): p. 1126–34. [DOI] [PubMed] [Google Scholar]
- 4.Marina NM, et al. , Improved prognosis of children with osteosarcoma metastatic to the lung(s) at the time of diagnosis. Cancer, 1992. 70(11): p. 2722–7. [DOI] [PubMed] [Google Scholar]
- 5.Meyers PA, et al. , Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J Clin Oncol, 1993. 11(3): p. 449–53. [DOI] [PubMed] [Google Scholar]
- 6.Jain S and Vahdat LT, Eribulin mesylate. Clin Cancer Res, 2011. 17(21): p. 6615–22. [DOI] [PubMed] [Google Scholar]
- 7.Preston JN and Trivedi MV, Eribulin: A Novel Cytotoxic Chemotherapy Agent. The Annals of Pharmacotherapy, 2012. 46(6): p. 802–811. [DOI] [PubMed] [Google Scholar]
- 8.Pean E, et al. , The European medicines agency review of eribulin for the treatment of patients with locally advanced or metastatic breast cancer: summary of the scientific assessment of the committee for medicinal products for human use. Clin Cancer Res, 2012. 18(17): p. 4491–7. [DOI] [PubMed] [Google Scholar]
- 9.Kolb EA, et al. , Initial testing (stage 1) of eribulin, a novel tubulin binding agent, by the pediatric preclinical testing program. Pediatr Blood Cancer, 2013. 60(8): p. 1325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okouneva T, et al. , Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase. Mol Cancer Ther, 2008. 7(7): p. 2003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoffski P, et al. , Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histological subtypes. Lancet Oncol, 2011. 12(11): p. 1045–52. [DOI] [PubMed] [Google Scholar]
- 12.Schoffski P, et al. , Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet, 2016. 387(10028): p. 1629–37. [DOI] [PubMed] [Google Scholar]
- 13.Demetri GD, et al. , Activity of Eribulin in Patients With Advanced Liposarcoma Demonstrated in a Subgroup Analysis From a Randomized Phase III Study of Eribulin Versus Dacarbazine. J Clin Oncol, 2017: p. JCO2016716605. [DOI] [PubMed] [Google Scholar]
- 14.Beaty O 3rd, et al. , A phase II trial and pharmacokinetic study of oxaliplatin in children with refractory solid tumors: a Children’s Oncology Group study. Pediatr Blood Cancer, 2010. 55(3): p. 440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs S, et al. , Phase II trial of ixabepilone administered daily for five days in children and young adults with refractory solid tumors: a report from the children’s oncology group. Clin Cancer Res, 2010. 16(2): p. 750–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warwick AB, et al. , Phase 2 trial of pemetrexed in children and adolescents with refractory solid tumors: a Children’s Oncology Group study. Pediatr Blood Cancer, 2013. 60(2): p. 237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, et al. , New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer, 2009. 45(2): p. 228–47. [DOI] [PubMed] [Google Scholar]
- 18.Lagmay JP, et al. , Outcome of Patients With Recurrent Osteosarcoma Enrolled in Seven Phase II Trials Through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: Learning From the Past to Move Forward. J Clin Oncol, 2016. 34(25): p. 3031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houghton PJ, et al. , The pediatric preclinical testing program: description of models and early testing results. Pediatr Blood Cancer, 2007. 49(7): p. 928–40. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe N, et al. , Adjuvant methotrexate and citrovorum-factor treatment of osteogenic sarcoma. N Engl J Med, 1974. 291(19): p. 994–7. [DOI] [PubMed] [Google Scholar]
- 21.Krailo M, et al. , A randomized study comparing high-dose methotrexate with moderate-dose methotrexate as components of adjuvant chemotherapy in childhood nonmetastatic osteosarcoma: a report from the Childrens Cancer Study Group. Med Pediatr Oncol, 1987. 15(2): p. 69–77. [DOI] [PubMed] [Google Scholar]
- 22.James DH Jr. and George P, Vincristine in Children with Malignant Solid Tumors. J Pediatr, 1964. 64: p. 534–41. [DOI] [PubMed] [Google Scholar]
- 23.Schafer ES, et al. , A phase 1 study of eribulin mesylate (E7389), a novel microtubule-targeting chemotherapeutic agent, in children with refractory or recurrent solid tumors: A Children’s Oncology Group Phase 1 Consortium study (ADVL1314). Pediatr Blood Cancer, 2018. 65(8): p. e27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolb EA, et al. , Initial testing (stage 1) of AZD6244 (ARRY-142886) by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer, 2010. 55(4): p. 668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee A, et al. , A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol, 2017. 19(8): p. 1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khanna C, et al. , Toward a drug development path that targets metastatic progression in osteosarcoma. Clin Cancer Res, 2014. 20(16): p. 4200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris MB, et al. , Treatment of osteosarcoma with ifosfamide: comparison of response in pediatric patients with recurrent disease versus patients previously untreated: a Pediatric Oncology Group study. Med Pediatr Oncol, 1995. 24(2): p. 87–92. [DOI] [PubMed] [Google Scholar]
- 28.Isakoff MS, et al. , Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. Journal of Clinical Oncology, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isakoff MS, et al. , Rapid Protocol Enrollment in Osteosarcoma: A Report From the Children’s Oncology Group. Pediatr Blood Cancer, 2016. 63(2): p. 370–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


