Abstract
Introduction
Inactivated influenza vaccine is recommended in any stage of pregnancy, but evidence of safety in early pregnancy is limited, including for vaccines containing A/H1N1pdm2009 (pH1N1) antigen. We sought to determine if receipt of vaccine containing pH1N1 was associated with spontaneous abortion (SAB).
Methods
We conducted a case-control study over two influenza seasons (2010–11, 2011–12) in the Vaccine Safety Datalink. Cases had SAB and controls had live births or stillbirths and were matched on site, date of last menstrual period, and age. Of 919 potential cases identified using diagnosis codes, 485 were eligible and confirmed by medical record review. Exposure was defined as vaccination with inactivated influenza vaccine before the SAB date; the primary exposure window was the 1–28 days before the SAB.
Results
The overall adjusted odds ratio (aOR) was 2.0 (95% CI, 1.1–3.6) for vaccine receipt in the 28-day exposure window; there was no association in other exposure windows. In season-specific analyses, the aOR in the 1–28 days was 3.7 (95% CI 1.4–9.4) in 2010–11 and 1.4 (95% CI 0.6–3.3) in 2011–12. The association was modified by influenza vaccination in the prior season (post hoc analysis). Among women who received pH1N1-containing vaccine in the previous influenza season, the aOR in the 1–28 days was 7.7 (95% CI 2.2–27.3); the aOR was 1.3 (95% CI 0.7–2.7) among women not vaccinated in the previous season. This effect modification was observed in each season.
Conclusion
SAB was associated with influenza vaccination in the preceding 28 days. The association was significant only among women vaccinated in the previous influenza season with pH1N1-containing vaccine. This study does not and cannot establish a causal relationship between repeated influenza vaccination and SAB, but further research is warranted.
Keywords: Influenza vaccine, Spontaneous abortion, Influenza, Pregnancy
1. Introduction
Since 2004, the Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) and other organizations have recommended routine influenza vaccination for pregnant women regardless of gestational age [1,2]. Influenza in pregnancy can cause serious, life-threatening illness in both the mother and fetus, as demonstrated during the 2009 pandemic [3,4]. Numerous studies of influenza vaccine during pregnancy have not identified serious safety concerns [5–12], but relatively few investigations have evaluated vaccination in the first trimester, a period when the embryo is highly vulnerable to teratogens and other factors [5,13]. A case-control study conducted by the Vaccine Safety Datalink (VSD) demonstrated that influenza vaccination during early pregnancy in the 2005–06 and 2006–07 influenza seasons was not associated with spontaneous abortion (SAB) [14].
The emergence of a pandemic influenza virus, A/California/7/2009 (H1N1)pdm09 (pH1N1), led to rapid development and widespread use of vaccines containing pH1N1 antigens. Several studies have evaluated the safety of vaccines containing pH1N1 in pregnancy, but few have focused on outcomes in early pregnancy [15–19]. Using a design and protocol similar to the previous study [14], we conducted a case-control study to determine if receipt of influenza vaccine containing pH1N1 was associated with SAB.
2. Methods
2.1. Study population
This study included women who were pregnant during the 2010–11 or 2011–12 influenza seasons and members of one of six integrated healthcare delivery organizations in VSD: Group Health Cooperative, Seattle, WA; Kaiser Permanente (Colorado, Northern California, Southern California, Oregon); and Marshfield Clinic, Marshfield, WI. VSD was established in 1990 as a collaborative project between several healthcare organizations and CDC to monitor vaccine safety.[20].
The study was approved by the Institutional Review Boards of each organization and CDC.
2.2. Cases
Potential cases of SAB (gestational age 5 to <20 weeks) were initially identified at each site through a search of VSD databases for diagnosis codes for spontaneous and unspecified abortion (International Classification of Diseases, 9th Revision, Clinical Modification (ICD9-CM) 634.x and 637.x) (Supplemental Table 1) assigned in ambulatory, urgent care, emergency department, and inpatient settings. Because influenza vaccine is administered seasonally, we required SAB diagnoses be assigned between September 1, 2010 and April 28, 2011 and September 1, 2011 and April 28, 2012 to avoid including cases that had no chance of being vaccinated. Pregnancy was confirmed using information from the medical record: clinic or hospital-based assay, obstetric ultrasound, patient-reported test, or physician diagnosis.
Study eligibility criteria included: (1) SAB confirmed by ultrasound or clinical diagnosis in the absence of ultrasound results; (2) age 18–44 years on date of SAB; (3) date of last menstrual period (LMP) reported in the medical record; and (4) continuous enrollment in the healthcare organization for 12 months prior to LMP. Exclusion criteria included ectopic pregnancy, therapeutic abortion, or SAB occurring <5 weeks of gestation. An ultrasound was not required to minimize selection bias.
Trained abstractors reviewed medical records to collect ultrasound results and other information, including gestational age and biometrics. The estimated date of SAB was based on interpretation of ultrasound results by the investigators who were blinded to vaccination status; ambiguous ultrasound results were adjudicated by an obstetrician (M.A.M.) [21]. For women without ultrasound results, the date was based on the earliest clinical diagnosis. The SAB date was defined as the LMP date plus the gestational age at the time of the SAB. Women were excluded if after careful review of ultrasound and menstrual data, we could not estimate gestational age with reasonable precision (e.g., report of an empty gestational sac not consistent with the LMP date).
2.3. Controls
Controls met the same inclusion criteria as cases (except SAB diagnosis) and had a live delivery or stillbirth (infant born dead ≥20 weeks of gestation) as determined by ICD9-CM codes (Supplemental Table 1). Abstractors reviewed the records of controls to confirm the pregnancy outcome and abstract additional information.
2.4. Matching
Cases and controls were individually matched (1:1 ratio). LMP was a matching variable to ensure nearly identical gestational age relative to calendar time since opportunities for influenza vaccination vary over time. To ensure close matching on LMP, we randomly selected 10 potential controls having LMP dates within seven days of the case LMP; the eligible control with the LMP closest to the case LMP was selected. Cases and controls were also matched by VSD site and maternal age (<30 years, ≥30 years). The reference date for each case-control pair was the estimated SAB date for the case.
2.5. Influenza vaccine exposure
Only women vaccinated with inactivated influenza vaccine (IIV) before the reference date were considered exposed for this study. The composition of the inactivated influenza vaccine (IIV) was identical in each season: A/California/7/2009 (H1N1)pdm09-like, A/Perth/16/2009 (H3N2)-like, and B/Brisbane/60/2008-like [22,23]. Vaccination dates for influenza and other vaccines were abstracted from medical records. We also documented influenza vaccinations administered in the previous season. Women pregnant in 2010–11 could have been vaccinated in 2009–10 with the monovalent (H1N1)pdm09 vaccine, the seasonal vaccine (A/Brisbane/59/2007 (H1N1)-like, A/Brisbane/10/2007 (H3N2)-like, B/Brisbane 60/2008-like), both, or neither [24].
The primary exposure window was defined as 1–28 days before the reference date because the immune response to influenza vaccine peaks in the first four weeks after vaccination [25,26]. We also assessed exposure windows further removed from the reference date (29–56 and >56 days).
2.6. Statistical analysis
Cases and controls were compared using Wilcoxon signed-rank tests for continuous variables, McNemar tests for dichotomous variables, and Bowker’s test of symmetry for categorical variables with >2 levels. All P values were based on two-sided tests. SAS 9.3 (SAS Institute, Cary, NC) was used for the analysis.
We performed conditional logistic regression to estimate the association between SAB and receipt of IIV in each exposure window. The final model included specific covariates selected a priori because they were suspected to be associated with SAB or vaccination: maternal age, smoking during pregnancy, history of type 1 or 2 diabetes, pre-pregnancy body mass index (BMI), and previous health care utilization (defined as the number of days with an outpatient or inpatient encounter in the year before the LMP) [27]. Age, BMI, and health care utilization were included in the model as quadratic splines [28]. In addition, we adjusted for vaccinations given concomitantly with influenza vaccine; the only concomitant vaccine was Tdap. Race variables were excluded from the model because the adjusted and unadjusted odds ratio estimates differed by less than 10% [29]. Other variables omitted because they were not associated with SAB in this study included parity, gravidity, asthma, and hypertension. The referent exposure group in all odds ratio (OR) calculations was women unvaccinated as of the reference date.
Because the OR varied by season of enrollment, we performed a post hoc analysis to determine if receiving pH1N1-containing vaccine in the previous influenza season modified the relationship between SAB and current-season IIV receipt. A dichotomous variable representing receipt of pH1N1-containing vaccine in the previous influenza season was evaluated as an effect modifier. Effect modification was assessed in the model by including a cross-product term for prior season vaccination and current-season IIV receipt in the various risk windows; the main effect terms were also included in this model. We performed this analysis for the 2010–11 and 2011–12 seasons separately and combined. In addition, the association between IIV and SAB was examined within strata defined by prior season receipt of pH1N1-containing vaccines and maternal age ≥35 years by including the appropriate cross-product terms in the model, since advanced maternal age is a well-established risk factor for SAB. We decided a priori not to adjust for history of prior SAB because doing so could result in biased estimates [30]. However, because recurrent miscarriage may have a distinct etiology [31], we performed a separate effect modification analysis in which women with a history of ≥2 SABs were excluded.
Chart-abstracted vaccination data were used in the analyses for the 2010–11 and 2011–12 influenza seasons. For the 2009–10 season, vaccination data extracted from the VSD vaccine database were used. The accuracy of the latter data source was assessed by examining kappa statistics for the 2010–11 and 2011–12 seasons where both chart-abstracted and electronic data were available. In addition, to assess potential protopathic bias[32] (i.e., reverse causality) we extracted and compared all diagnosis codes (ICD-9) assigned to vaccinated cases and controls on the same day as their influenza vaccination.
Using information from the previous study [14], we estimated that 500 matched case-control pairs would provide 80% power to detect an OR of 2.2 in the 28 day exposure window (α = 0.05).
3. Results
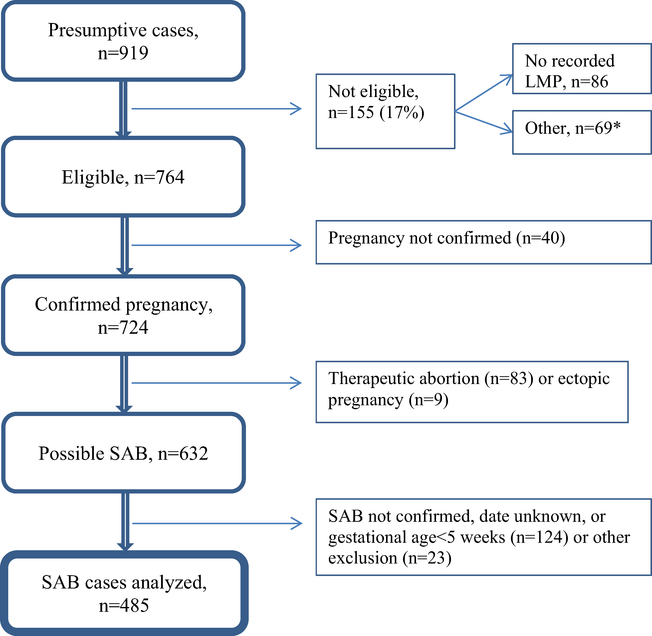
There were 919 presumptive SAB cases identified based on diagnosis codes. Of these, 434 were excluded (Fig. 1); 485 eligible women (53%) were matched to 485 controls. Women enrolled in the 2010–11 and 2011–12 influenza seasons were similar in most respects (Supplemental Table 2), as were cases and controls (Table 1). However, cases were significantly older than controls and more likely to be African-American, to have a history of ≥2 SABs, and to have smoked during pregnancy. The overall proportion of women vaccinated for influenza before the reference date was similar for cases and controls whether they were vaccinated in the previous season or not. Among women not receiving pH1N1-containing influenza vaccine in the prior season, the proportion vaccinated for influenza in the current season was similar for cases and controls within all exposure windows (Table 1). For women that did receive pH1N1-containing influenza vaccine in the prior season, the corresponding proportions were similar in the windows 29–56 and >56 days before the referent date, but there was a fivefold elevation in the 1–28 day exposure window for cases versus controls (17.0% versus 3.1%). As many as seven different manufacturers produced the vaccine administered during the 2010–11 and 2011–12 influenza seasons; the distribution of manufacturer was similar among cases and controls in each season. The mean within-pair difference in LMP between matched cases and controls was −0.55 days; the median was zero. The median gestational age at the time of SAB was 7 weeks (Supplemental Fig. 1). Medical record review included obstetric ultrasounds for most cases (89%).
Fig. 1.
Identification and confirmation of spontaneous abortions. Flow chart describing how spontaneous abortion cases were selected for study. *SAB date outside of the targeted date range for this study (n = 32) or failed to satisfy enrollment criteria (n = 37). SAB: spontaneous abortion, LMP: last menstrual period.
Table 1.
| Cases (n = 485) | Controls (n = 485) | P | |
|---|---|---|---|
| Age in years at reference date | 0.02 | ||
| 18–24 | 87 (18) | 68 (14) | |
| 25–34 | 241 (50) | 289 (60) | |
| 35–44 | 157(32) | 128 (26) | |
| Median (IQR) | 31.8 (27–37) | 31.6 (28–35) | 0.02 |
| BMI | 0.46 | ||
| <18.5 | 13 (3) | 9 (2) | |
| 18.5 to <25 | 203 (42) | 234 (48) | |
| 25 to <30 | 128 (27) | 129 (27) | |
| ≥30 | 134 (28) | 112 (23) | |
| Median (IQR) | 25.7 (22–31) | 24.9 (22–30) | 0.08 |
| Racec | |||
| White | 261 (55) | 268 (57) | 0.77 |
| African American | 42 (9) | 20 (4) | 0.008 |
| Asian Indian | 12 (3) | 13 (3) | 1.00 |
| Chinese | 8 (2) | 18 (4) | 0.06 |
| Native American | 6(1) | 4 (1) | 0.75 |
| Other | 164 (35) | 165(35) | 1.00 |
| Parity (≥1) | 277 (57) | 273 (56) | 0.79 |
| Gravidity, median (IQR) | 1 (0–3) | 1 (0–2) | 0.40 |
| Multiple gestation pregnancy | 5(1) | 7(1) | 0.77 |
| Previous SAB | |||
| ≥1 | 138 (29) | 125 (26) | 0.32 |
| ≥2 | 43 (9) | 26 (5) | 0.03 |
| Smoked during pregnancy | 52 (11) | 34 (7) | 0.05 |
| Same season influenza vaccination before reference date among those vaccinated in the previous seasond | 56 (56.0) | 53 (41.7) | |
| 1–28 days before reference date | 17 (17.0) | 4(3.1) | |
| 29–56 days before reference date | 5 (5.0) | 5 (3.9) | |
| >56 days before reference date | 34 (34.0) | 44 (34.6) | |
| Same season influenza vaccination before reference date among those not vaccinated in the previous seasond | 71 (18.6) | 70 (19.7) | |
| 1–28 days before reference date | 21 (5.5) | 20 (5.6) | |
| 29–56 days before reference date | 12 (3.1) | 11 (3.1) | |
| >56 days before reference date | 38 (10.0) | 39 (11.0) | |
| Concomitant IIV and Tdap vaccination before reference date | 2 (0) | 4(1) | 0.62 |
| Type I/II diabetes | 4(1) | 12 (2) | 0.08 |
| Asthma | 55 (11) | 56 (12) | 0.92 |
| Hypertension | 11 (2) | 16(3) | 0.44 |
| Febrile illness in the 14 days before reference date | 5(1) | 0 | - |
| Number of days with outpatient diagnoses, vaccinations, or hospitalizations in the 365 days before LMP, median (IQR) | 3 (2–6) | 3 (1–6) | 0.04 |
BMI had missing data for 7 cases and 1 control. Race had missing data for 13 cases and 13 controls. Parity had missing data for 2 cases. Smoking had missing data for 10 cases and 6 controls. Diabetes had missing data for 3 cases and 2 controls. Asthma had missing data for 4 cases and 3 controls. Hypertension had missing data for 3 cases and 2 controls. Previous SAB had missing data for 10 cases and 5 controls. Health care utilization had missing data for 1 control. Gravidity had missing data for 6 cases. Febrile illness had missing data for 5 cases. Vaccination data was missing for 3 cases and 2 controls.
Data are N (%) unless otherwise noted. IQR = Interquartile Range. P values were calculated using a Wilcoxon signed rank test, Bowker’s test of symmetry, or exact McNemar test and excluded observations with missing values.
The frequencies for race exceed 485 for both cases and controls because persons could have been included in more than one race category. The large number of persons in the ‘Other’ category is the result of ethnicity (e.g., Hispanic) being mistakenly coded as race in the medical record.
Statistical tests within prior season influenza vaccination status strata are not presented in Table 1 since the corresponding McNemar’s tests would restrict the analyses to matched pairs that are concordant on prior season influenza vaccination status. This would result in the exclusion of a substantial amount of data from the calculations (overall, 35% of the matched pairs were discordant with respect to prior season vaccination status). Percentages were computed using stratum-specific denominators.
The adjusted OR (aOR) for IIV receipt in the 1–28 day exposure window was 2.0 (95% CI, 1.1–3.6); the aORs were 0.9 for both of the other exposure windows (29–56 and >56 days) (Table 2). The aOR in the 28-day window was 3.7 (95% CI, 1.4–9.4) in the 2010–11 season and 1.4 (95% CI, 0.6–3.3) in the 2011–12 season.
Table 2.
Odds of influenza vaccination in SAB cases compared to controls by timing of vaccination during the 2010–11 and 2011–12 influenza seasons and both seasons combined.a
| Influenza season | 2010–11 |
2011–12 |
Both seasons combined |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Discordant pairsb | Adj. ORc | P | Discordant pairsb | Adj. ORc | P | Discordant pairsb | Adj. ORc | P | |
| Time from vaccination to reference date | |||||||||
| 1–28 days | 25 | 3.7 (1.4–9.4) | 0.007 | 23 | 1.4 (0.6–3.3) | 0.47 | 48 | 2.0 (1.1–3.6) | 0.03 |
| 29–56 days | 14 | 1.7 (0.6–4.8) | 0.31 | 6 | 0.1 (0.0–0.9) | 0.04 | 20 | 0.9 (0.4–2.1) | 0.85 |
| >56 days | 51 | 1.0 (0.5–1.8) | 0.99 | 46 | 0.9 (0.4–1.7) | 0.72 | 97 | 0.9 (0.6–1.4) | 0.67 |
The referent exposure group in all odds ratio calculations was comprised of women unvaccinated as of the reference date.
Number of matched pairs where the case was vaccinated in the relevant exposure window (1–28, 29–56, >56 days before the reference date) and the control was unvaccinated as of the reference date or vice versa.
Adj. OR’ represents the odds ratio adjusted for maternal age (spline), BMI (spline), smoking during pregnancy, maternal diabetes, concomitant Tdap vaccination, and health care utilization in prior 12 months (spline). Numbers in parentheses represent 95% confidence intervals.
In a post-hoc analysis, there was significant effect modification (P = 0.02) by prior season vaccination. The aOR for IIV receipt in the 1–28 day window was significantly elevated among women who had also received pH1N1-containing vaccine in the previous season (Table 3). There was no increased risk among women who did not receive influenza vaccine in the previous season regardless of current season IIV vaccination status. The aORs for the other exposure windows demonstrated no statistically significant association between SAB and IIV, regardless of past exposure to pH1N1-containing vaccine. We also assessed the combined effects of pH1N1-containing vaccine in the previous season and age ≥35 years and observed that older women had a larger aOR (22.1, 95%CI 1.7–281.2) than younger women (4.8, 95% CI 1.022.2), but the number of women in these categories was small and the difference was not statistically significant (P = 0.32). In a secondary analysis, we excluded cases and controls with a history of ≥2 SABs. The aOR in the 1–28 day window for women vaccinated with pH1N1-containing vaccine in the previous season remained significantly elevated (aOR = 6.5, 95% CI 1.7–24.3).
Table 3.
Association between SAB and IIV receipt in the current influenza season (2010–11 or 2011–12), by receipt of pH1N1-containing vaccine in the previous season and advanced maternal age.a
| pH1N1-containing vaccine in the previous seasonc | Age (years) | Time from vaccination to reference date in current seasonb |
|||||
|---|---|---|---|---|---|---|---|
| 1–28 days |
29–56 days |
>56 days |
|||||
| No. of cases/controlsd | aOR (95% CI) | No. of cases/controls | aOR (95% CI) | No. of cases/controls | aOR (95% CI) | ||
| Yes | All | 14/4 | 7.7 (2.2–27.3)e | 3/4 | 1.2 (0.2–6.5) | 34/40 | 1.4 (0.7–3.0) |
| No | All | 21/19 | 1.3 (0.7–2.7) | 12/11 | 1.0 (0.4–2.5) | 37/38 | 0.9 (0.5–1.6) |
| Yes | ≥35 | 7/1 | 22.1 (1.7–281.2)f | 1/2 | 0.4 (0.0–5.3) | 16/10 | 1.3 (0.3–4.8) |
| Yes | <35 | 7/3 | 4.8 (1.0–22.2) | 2/2 | 3.4 (0.4–32.6) | 18/30 | 1.5 (0.6–3.7) |
| No | ≥35 | 3/5 | 1.0 (0.2–6.1) | 3/5 | 0.2 (0.0–1.0) | 12/13 | 0.4 (0.2–1.2) |
| No | <35 | 18/14 | 1.4 (0.6–3.0) | 9/6 | 1.9 (0.6–6.3) | 25/25 | 1.1 (0.5–2.1) |
Adjusted for maternal age (spline), BMI (spline), smoking during pregnancy, maternal diabetes, concomitant Tdap vaccination, and health care utilization in prior 12 months (spline).
The referent exposure group in all odds ratio calculations was comprised of women unvaccinated as of the reference date.
Recipients of the monovalent pH1N1 vaccine in 2009–10 may or may not have also received the seasonal vaccine.
Total number of cases and controls in the specific stratum. Number of discordant pairs could not be computed since the stratum-specific estimates were derived from models with cross-product terms whose components were not matching factors.
P = 0.02 for effect modification by pH1N1-containing vaccine in the previous season (7.7 vs. 1.3).
P = 0.32 for effect modification by advanced maternal age among those that received pH1N1-containing vaccine in the previous season (22.1 vs. 4.8).
In each of the two influenza seasons under study, we observed a similar relationship between SAB and IIV. In the 2010–11 season, the aOR for IIV receipt in the 1–28 day exposure window was 32.5 (95% CI 2.9–359.0) for women who were also vaccinated with the monovalent pH1N1 vaccine with or without seasonal vaccine in 2009–10. In the 2011–12 season, the comparable aOR was elevated, but the lower bound of the confidence interval was close to the null (aOR = 6.4, 95% CI 1.0–41.2) for women who were also vaccinated with the seasonal vaccine (which contained pH1N1 antigens) in 2010–11. Effect modification in the 28-day exposure window due to prior vaccination with pH1N1-containing vaccine was present in both the 2010–11 (P = 0.06) and 2011–12 (P = 0.05) seasons, and the magnitude of aOR in the two seasons was not statistically different (aOR 32.5 vs. 6.4, P = 0.30) (Table 4). The associations in the other windows (29–56 and > 56 days), regardless of prior season influenza vaccination status, were generally smaller and not statistically significant. Finally, the aOR for women enrolled in 2010–11 who received only the seasonal vaccine in 2009–10 was smaller (aOR = 3.3, 95% CI 0.5–20.1) and not statistically significant compared to women who had received the monovalent pH1N1 vaccine in 2009–10.
Table 4.
Association between SAB and IIV receipt in the current influenza season, by receipt of pH1N1-containing vaccine in the previous season, stratified by season of enrollment.a
| Time from vaccination to reference date in current seasonb |
||||||
|---|---|---|---|---|---|---|
| 1–28 daysc |
29–56 days |
>56 days |
||||
| No. of cases/controlsd | aOR (95% CI) | No. of cases/controls | aOR (95% CI) | No. of cases/controls | aOR (95% CI) | |
| Vaccination status in 2009–10 of women enrolled in 2010– 11 | ||||||
| pH1N1 ± seasonal vaccine | 6/2 | 32.5, (2.9–359.0) | 2/1 | 4.1, (0.3–63.1) | 16/18 | 3.2, (1.0–10.5) |
| Both vaccines | 5/2 | 31.5, (2.3–424.8) | 1/1 | 2.4, (0.1–58.0) | 11/12 | 3.0, (0.6–14.0) |
| Seasonal only | 3/3 | 3.3, (0.5–20.1) | 4/3 | 1.5, (0.2–11.9) | 10/9 | 2.8, (0.8–10.2) |
| Unvaccinated | 10/5 | 3.4, (0.8–14.2) | 7/4 | 2.5, (0.6–11.2) | 12/13 | 0.6, (0.2–1.6) |
| Vaccination status in 2010–11among women enrolled in 2011–12 | ||||||
| Vaccinated | 8/2 | 6.4, (1.0–41.2) | 1/3 | 0.3, (0.0–4.0) | 18/22 | 0.9, (0.3–2.5) |
| Unvaccinated | 8/11 | 0.7, (0.3–2.2) | 1/4 | 0.04, (0.0–0.8) | 15/16 | 1.0, (0.4–2.7) |
Adjusted for maternal age (spline), BMI (spline), smoking during pregnancy, maternal diabetes, concomitant Tdap vaccination, and health care utilization in prior 12 months (spline).
The referent exposure group in all odds ratio calculations was comprised of women unvaccinated as of the reference date.
P = 0.06 among women enrolled in 2010–11 and P = 0.05 among women enrolled in 2011–12 for effect modification by pH1N1-containing vaccine in the previous season.
Total number of cases and controls in the specific stratum. Number of discordant pairs could not be computed since the stratum-specific estimates were derived from models with cross-product terms whose components were not matching factors.
Vaccinated cases (n = 74, 58%) were more likely than vaccinated controls (n = 64, 52%) to have ≥ 1 diagnosis on the date of their vaccination, but the difference was not statistically significant (P = 0.39). The mean number of diagnoses was similar for cases and controls (1.7 vs. 1.6, P = 0.64). The most common diagnoses were for routine care; the distribution was similar for cases and controls (see the V codes in Table 5). Ten cases were assigned diagnoses consistent with early signs/symptoms of SAB compared to four controls. Of these, three cases and no controls were assigned the diagnosis in the 1–28 days before the SAB diagnosis, the only exposure window in which there was a statistically significant association. The SAB-IIV association among women vaccinated in the previous influenza season changed minimally after exclusion of these three cases and their matched controls (OR = 7.0, 95% CI 1.9–25.2).
Table 5.
Diagnoses assigned to cases and controls on day of influenza vaccination.a
| ICD-9 code | Diagnosis code description | Cases (n = 127) | Controls (n = 123) |
|---|---|---|---|
| Any code | Any diagnosis on day of vaccination | 211 | 182 |
| V04.81 | Need for prophylactic vaccination and inoculation against certain diseases, influenza | 53 (25) | 45 (25) |
| V22.1 | Supervision of other normal pregnancy | 15 (7) | 7 (4) |
| V70.0 | Routine general medical examination | 10 (5) | 4(2) |
| V72.31 | Routine gynecological examination | 8 (4) | 7 (4) |
| V76.2 | Routine pap smear | 6 (3) | 5 (3) |
| 625.x | Pain/other symptoms associated with female genital organs | 3 (1) | 3 (2) |
| 626.x | Disorders of menstruation & other abnormal bleeding from female genital tract | 3 (1) | 0 |
| 640–649 | Complications related to pregnancy (incl. hemorrhage in early pregnancy) | 4 (2) | 1 (0.5) |
| 240–246 | Disorders of thyroid gland | 0 | 1 (0.5) |
| 249 | Secondary diabetes mellitus | 0 | 0 |
| 250 | Diabetes mellitus | 0 | 1 (0.5) |
| 278.00–01 | Obesity, morbid obesity | 3 (1) | 1 (0.5) |
| 303–304 | Alcohol/drug dependence syndrome | 0 | 0 |
| 401–405 | Hypertensive disease | 0 | 0 |
Restricted to women vaccinated before the reference date. Numbers in cells are frequency and (% of diagnoses). The total number of unique diagnosis codes for cases and controls was 87 and 84, respectively.
4. Discussion
In the primary analysis of this observational investigation, we found a modest but statistically significant association between SAB and IIV in the 28 days before the reference date. A post hoc analysis revealed a significant association only among women who had received pH1N1-containing vaccine in the previous influenza season. This effect modification was present in each season, but confidence intervals were wide. The aORs among women who were not previously vaccinated with pH1N1-containing vaccine approximated the null in nearly all risk windows.
Analyses with relatively small numbers of matched pairs may be more susceptible to chance associations. However, we observed a similar relationship between the influenza vaccine and SAB in each of the two years under study. In addition, we had previously conducted an investigation of SAB and IIV during two pre-pandemic influenza seasons (2005–06 and 2006–07); the study design and implementation were nearly identical to the current study [14]. The prior study did not find an association in the 28-day exposure window (OR= 1.2, 95% CI 0.5–2.9) or any other exposure window. The previous and current study populations are similar in most characteristics (Supplemental Table 3), except that women in the current study were potentially infected with or vaccinated against the pH1N1 virus, which is antigenically distinct from H1N1 viruses that circulated before 2009 [33]. Because influenza vaccination of pregnant women increased substantially during and after the pandemic, another difference is that more women in the current study may have received an influenza vaccine in prior years, whereas most vaccinated women in the first study probably were not previously vaccinated [34].
Although SABs have been reported among pregnant women infected with the pH1N1 virus, studies of pH1N1-containing vaccines have not found excess risks [8–12,35]. One large cohort study investigated pregnancy loss occurring after nine weeks of gestation and found that the monovalent vaccine was associated with a significantly reduced risk of SAB [36]. Unmeasured confounding was a concern because a comparable association was found outside the influenza season. Pasternak, et al. conducted a cohort study of >50,000 pregnant women in 2009–10 and found no association between SAB and adjuvanted monovalent pH1N1 vaccine [16]. A systematic review of 19 studies evaluated fetal deaths and congenital malformations among women vaccinated with influenza vaccine. Of the five studies that reported effect estimates for SAB, the hazard or odds ratio associated with vaccination ranged from 0.45 to 1.23, with 95% confidence intervals that included the null in each case [37]. A recent study of 102 spontaneous, pregnancy-specific reports of adverse events following inactivated influenza vaccination submitted to the United States Vaccine Adverse Events Reporting System (VAERS) during 2010–2016 found no unexpected pattern for any fetal outcome, including SAB [38]. Another passive surveillance study in Taiwan in 2009–10 found no association with the monovalent pH1N1 vaccine, although there was substantial under-ascertainment of SAB cases due to incomplete reporting [39]. None of these studies found an association between SAB and influenza vaccination, and accordingly, their investigations did not include an evaluation of effect modification due to vaccination in previous seasons. Finally, one recent, retrospective investigation examined women in the VSD who were exposed to the influenza vaccine during the first trimester and assessed the risk of selected birth defects among live-born offspring [40]. Of the 426,000 women studied using electronic health data, the rate of birth defects among the nearly 53,000 vaccinated in the first trimester was nearly identical to the rate in unvaccinated women or those vaccinated later in pregnancy. Overall, the weight of evidence in support of the safety of the influenza vaccine in pregnant women found in the literature is substantial, particularly for women vaccinated after the first trimester.
One possible explanation for these findings is that a second or boosting dose of pH1N1-containing vaccine may confer risk in early pregnancy, but receipt of an initial or priming dose does not. While a number of studies have shown potential relationships between vaccination and inflammation, and inflammation and pregnancy loss, the biological basis for our observations has not been established. One study followed pregnant and non-pregnant women who were vaccinated in 2011–12 with pH1N1-containing vaccine and found significant increases (P< 0.001) in pro-inflammatory cytokines soon after vaccination, although the increases were mild and transient [41]. Although over 40% of participants had received an influenza vaccination in the previous year, inflammatory responses did not vary by prior vaccination status. Another study found that pregnant women when compared to non-pregnant women had an enhanced chemokine response to pH1N1 influenza virus both before and especially after vaccination with IIV [42]. Others have shown that infection with pH1N1 virus or vaccination with pH1N1-containing vaccine induces an expansion of T helper type 1 (Th1) cells, which are considered to be pro-inflammatory [43,44]. While some degree of inflammation appears necessary for a successful pregnancy, excessive inflammation is associated with SAB and other pregnancy complications [45–47]. Other investigations have reported significant associations between an increased Th1 response and miscarriage [48,49].
The strengths of our investigation include the relatively large number of women with SAB (n = 485). Cases and controls were closely matched on LMP to ensure nearly equal opportunities for vaccination. Medical records were abstracted to collect information on potential confounders and to confirm pregnancies and SABs. Finally, our data come from six geographically diverse healthcare organizations, the combined membership of which represents ~3% of the U.S. population [20].
This study has several important limitations. First, the most striking findings relate to the association between SAB and IIV in women who previously received pH1N1-containing vaccine. This interaction effect was not an a priori hypothesis; the results were generated in a post hoc analysis with small numbers of women in the various subgroups. Although the interaction was observed in each of the two seasons studied, the point estimates were substantially larger (though not statistically different) in the first season for reasons that are unclear. Second, although most cases had an ultrasound, assignment of a precise date of SAB was challenging. With guidance from an obstetrician we integrated different types of information from the medical record (e.g., ultrasound results, clinical and laboratory findings, provider notes) to estimate the timing of the SAB. Estimation of SAB dates was independent of vaccination status so any error should bias the results toward the null (i.e., non-differential misclassification). Third, we studied only women who had clinically confirmed SAB; the proportion of women with clinically unrecognized pregnancy loss is uncertain but may be substantial [50,51]. Our results could be biased if women who sought care for SAB were more likely to be vaccinated in the 28-day exposure window. However, an earlier study, similarly designed and conducted in the same managed care population, showed no association [14]. Fourth, it is possible that women with certain comorbidities or other risk factors for SAB were preferentially vaccinated. While selected comorbidities were not associated with SAB in our study (e.g., asthma, diabetes), information on other risk factors (e.g., autoimmune disease) was not ascertained [27]. In addition, we attempted to determine if cases had greater opportunity for vaccination because they sought care for symptoms foreshadowing an SAB diagnosis (i.e., protopathic bias). More cases than controls (74 vs. 64, P = 0.39) had a diagnosis on the same date as the influenza vaccination, although the difference was not statistically significant and only three had diagnoses consistent with an impending SAB in the exposure window of interest. Nevertheless, protopathic bias cannot be ruled out in our study. Fifth, vaccination status may have been misclassified if women received a vaccine that was both outside of their health care system and not reported to their provider. However, we would expect that subjects would have been queried and their vaccination status recorded given the strong recommendations for vaccination of pregnant women during the study period. It is also possible that women who were pregnant in the previous influenza season would be more likely to have been vaccinated in the previous season. Although we did not collect information on inter-pregnancy intervals, we would expect that the number of women pregnant in consecutive seasons would be small [52]. Finally, while the odds ratio in a case-control study is generally considered an estimate of the risk ratio, this is only true if the outcome is rare or controls were chosen using incidence density sampling [53]. Since neither was the case in this study, the ORs should not be interpreted as risk ratios.
This study found that the overall odds of vaccine exposure in the 28-day exposure window was increased by a factor of two in women with SAB compared to controls. A secondary analysis suggested that the odds among women who were also vaccinated in the previous year with pH1N1-containing vaccine was almost 8-fold (and statistically significant), while the odds among women who did not receive such a vaccine in the prior year was approximately null. It is important to note that this study does not and cannot confirm a causal association, but the validity of the major findings is supported by the effect modification across two influenza seasons and the observation of elevated odds ratios in the 1–28 day exposure window only. More research is needed regarding the immunologic effects of influenza vaccination during pregnancy. A follow-up study funded by CDC is currently underway to evaluate the risk of SAB after repeated influenza vaccination during the 2012–13, 2013–14, and 2014–15 influenza seasons; results are expected by late 2018.
Supplementary Material
Acknowledgments
We are grateful to the following individuals for the help with this research: Deanna Cole, Tara Johnson, Diane Kohnhorst, Madalyn Minervini, Suellyn Murray, Becky Pilsner, Carla Rottscheit, Cathy Schneider, Sandy Strey (data acquisition/management, compensated, Marshfield Clinic Research Institute); David McClure, PhD (scientific advice, compensated, Marshfield Clinic Research Institute); Pat Ross, Cat Magallon, RN, CPC, Margarita Magallon, RHIT, CPC (data acquisition/management, compensated, Kaiser Permanente Northern California); Donna Gleason and Carmel Wax (data acquisition, compensated, Kaiser Permanente Northwest); Jennie Covey, Lawrence Madziwa, and Patti Benson (data acquisition/management, compensated, Group Health Research Institute); Lina Sy, Denison Ryan, Fernando Barreda, Nancy Canul Jauriga, Bianca Cheung, Ana Espinosa Rydman, Theresa Im, Gina Lee, Jose Pio, Karen Schenk, Cheryl Mercado, and Sungching Glenn (data acquisition/management, compensated, Kaiser Permanente Southern California).
Funding
This work was supported by a contract (200-2012-53587) from the Centers for Disease Control and Prevention. CDC scientists participated in the design and conduct of the study, analysis and interpretation of the data, and preparation, review, and approval of the manuscript for publication. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of Centers for Disease Control and Prevention.
Potential conflicts of interests
Dr. Klein reports receiving research support from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, MedImmune, Novartis, and Protein Science. Dr. Naleway reports receiving research support from GlaxoSmithKline, MedImmune, and Pfizer. Ms. Irving reports receiving research support from MedImmune. Dr. Jackson reports receiving research support from Novavax. Dr. Belongia, Mr. Kieke, and Ms. King report receiving research support from MedImmune. All other authors report no potential conflicts of interest.
Abbreviations
- SAB
spontaneous abortion
- VSD
Vaccine Safety Datalink
- pH1N1
influenza virus type A/H1N1pdm2009
- aOR
adjusted odds ratio
- LMP
last menstrual period
- IIV
inactivated influenza vaccine
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2017.06.069.
References
- [1].Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep 2004;53:1–40. [PubMed] [Google Scholar]
- [2].ACOG Committee. Opinion No 468: influenza vaccination during pregnancy. Obstet Gynecol 2010;116:1006–7. 10.1097/AOG.0b013e3181fae845. [DOI] [PubMed] [Google Scholar]
- [3].Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol 2011;205:10–8. 10.1016/j.ajog.2010.12.033. [DOI] [PubMed] [Google Scholar]
- [4].Rasmussen SA, Jamieson DJ, Uyeki TM. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol 2012;207:S3–8. 10.1016/j.ajog.2012.06.068. [DOI] [PubMed] [Google Scholar]
- [5].Bednarczyk RA, Adjaye-Gbewonyo D, Omer SB. Safety of influenza immunization during pregnancy for the fetus and the neonate. Am J Obstet Gynecol 2012;207:S38–46. http://dx.doi.ore/10.1016/j.ajoe.2012.07.002, [DOI] [PubMed] [Google Scholar]
- [6].Omer SB, Bednarczyk R, Madhi SA, Klugman KP. Benefits to mother and child of influenza vaccination during pregnancy. Hum Vaccin Immunother 2012;8:130–7. 10.4161/hv.8.1.18601. [DOI] [PubMed] [Google Scholar]
- [7].Naleway AL, Irving SA, Henninger ML, Li DK, Shifflett P, Ball S, et al. Safety of influenza vaccination during pregnancy: a review of subsequent maternal obstetric events and findings from two recent cohort studies. Vaccine 2014;32:3122–7. 10.1016/j.vaccine.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Moro PL, Broder K, Zheteyeva Y, Revzina N, Tepper N, Kissin D, et al. Adverse events following administration to pregnant women of influenza A (H1N1) 2009 monovalent vaccine reported to the vaccine adverse event reporting system. Am J Obstet Gynecol 2011;205(473):e1–9. 10.1016/j.ajog.2011.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bratton KN, Wardle MT, Orenstein WA, Omer SB. Maternal influenza immunization and birth outcomes of stillbirth and spontaneous abortion: A systematic review and meta-analysis. Clin Infect Dis 2014. 10.1093/cid/ciu915. [DOI] [PubMed] [Google Scholar]
- [10].Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010;303:1517–25. 10.1001/jama.2010.479,303/15/1517 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rubinstein F, Micone P, Bonotti A, Wainer V, Schwarcz A, Augustovski F, et al. Influenza A/H1N1 MF59 adiuvanted vaccine in pregnant women and adverse perinatal outcomes: multicentre study. BMJ 2013;346:f393 10.1136/bmi.f393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chambers CD, Johnson DL, Xu R, Luo YJ, Louik C, Mitchell AA, et al. Safety of the 2010–11, 2011–12, 2012–13, and 2013–14 seasonal influenza vaccines in pregnancy: birth defects, spontaneous abortion, preterm delivery, and small for gestational age infants, a study from the cohort arm of VAMPSS. Vaccine 2016;34:4443–9. http://dx.doi.or2/10.1016/j.vaccine.2016.06.054. [DOI] [PubMed] [Google Scholar]
- [13].Skowronski DM, De Serres G. Is routine influenza immunization warranted in early pregnancy? Vaccine 2009;27:4754–70. 10.1016/j.vaccine.2009.03.079. [DOI] [PubMed] [Google Scholar]
- [14].Irving SA, Kieke BA, Donahue JG, Mascola MA, Baggs J, DeStefano F, et al. Trivalent inactivated influenza vaccine and spontaneous abortion. Obstet Gynecol 2013;121:159–65. 10.1097/AOG.0b013e318279f56f10.1097/AOG.0b013e318279f56f. [DOI] [PubMed] [Google Scholar]
- [15].Håberg SE, Trogstad L, Gunnes N, Wilcox AJ, Giessing HK, Samuelsen SO, et al. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med 2013;368:333–40. http://dx.doi.or2/10.1056/NEJMoa1207210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pasternak B, Svanstrom H, Molgaard-Nielsen D, Krause TG, Emborg HD, Melbye M, et al. Vaccination against pandemic A/H1N1 2009 influenza in pregnancy and risk of fetal death: cohort study in Denmark. BMJ 2012;344: e2794 10.1136/bmJ.e2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tavares F, Nazareth I, Monegal JS, Kolte I, Verstraeten T, Bauchau V. Pregnancy and safety outcomes in women vaccinated with an AS03-adiuvanted split virion H1N1 (2009) pandemic influenza vaccine during pregnancy: a prospective cohort study. Vaccine 2011;29:6358–65. 10.1016/j.vacrine.2011.04.114. [DOI] [PubMed] [Google Scholar]
- [18].Fell DB, Platt RW, Lanes A, Wilson K, Kaufman JS, Basso O, et al. Fetal death and preterm birth associated with maternal influenza vaccination: systematic review. Br J Obstet Gynecol 2015;122:17–26. [DOI] [PubMed] [Google Scholar]
- [19].Oppermann M, Fritzsche J, Weber-Schoendorfer C, Keller-Stanislawski B, Allignol A, Meister R, et al. A(H1N1)v2009: a controlled observational prospective cohort study on vaccine safety in pregnancy. Vaccine 2012;30:4445–52. 10.1016/j.vaccine.2012.04.081. [DOI] [PubMed] [Google Scholar]
- [20].Baggs J, Gee J, Lewis E, Fowler G, Benson P, Lieu T, et al. The vaccine safety datalink: a model for monitoring immunization safety. Pediatrics 2011;127 (Suppl 1):S45–53. 10.1542/peds.2010-1722H. [DOI] [PubMed] [Google Scholar]
- [21].Doubilet PM. Should a first trimester dating scan be routine for all pregnancies? Semin Perinatol 2013;37:307–9. 10.1053/j.semperi.2013.06.006. [DOI] [PubMed] [Google Scholar]
- [22].Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010;59:1–62. [PubMed] [Google Scholar]
- [23].Prevention and control of influenza with vaccines. recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011;60:1128–32. [PubMed] [Google Scholar]
- [24].Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009;58:1–52. [PubMed] [Google Scholar]
- [25].Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol 2004;59:1–15. [DOI] [PubMed] [Google Scholar]
- [26].Bischoff AL, Folsgaard NV, Carson CG, Stokholm J, Pedersen L, Holmberg M, et al. Altered response to A(H1N1)pnd09 vaccination in pregnant women: a single blinded randomized controlled trial. PLoS One 2013;8:e56700 10.1371/journal.pone.0056700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tulandi T, Al-Fozan HM. Spontaneous abortion: risk factors, etiology, clinical manifestations, and diagnostic evaluation. UpToDate. Waltham, MA: Wolters Kluwer; 2013. [Google Scholar]
- [28].Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology 1995;6:356–65. [DOI] [PubMed] [Google Scholar]
- [29].Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993;138:923–36. [DOI] [PubMed] [Google Scholar]
- [30].Weinberg CR. Toward a clearer definition of confounding. Am J Epidemiol 1993;137:1–8. [DOI] [PubMed] [Google Scholar]
- [31].Christiansen OB, Steffensen R, Nielsen HS, Varming K. Multifactorial etiology of recurrent miscarriage and its scientific and clinical implications. Gynecol Obstet Invest 2008;66:257–67. 10.1159/000149575. [DOI] [PubMed] [Google Scholar]
- [32].Horwitz RI, Feinstein AR. The problem of “protopathic bias” in case-control studies. Am J Med 1980;68:255–8. [DOI] [PubMed] [Google Scholar]
- [33].Girard MP, Tam JS, Assossou OM, Kieny MP. The 2009 A (H1N1) influenza virus pandemic: a review. Vaccine 2010;28:4895–902. 10.1016/j.vaccine.2010.05.031. [DOI] [PubMed] [Google Scholar]
- [34].Kennedy ED, Ahluwalia IB, Ding H, Lu PJ, Singleton JA, Bridges CB. Monitoring seasonal influenza vaccination coverage among pregnant women in the United States. Am J Obstet Gynecol 2012;207:S9–S16. 10.1016/j.ajog.2012.06.069. [DOI] [PubMed] [Google Scholar]
- [35].Chambers CD, Johnson D, Xu R, Luo Y, Louik C, Mitchell AA, et al. Risks and safety of pandemic H1N1 influenza vaccine in pregnancy: birth defects, spontaneous abortion, preterm delivery, and small for gestational age infants. Vaccine 2013;31:5026–32. 10.1016/j.vaccine.2013.08.097. [DOI] [PubMed] [Google Scholar]
- [36].Sammon CJ, Snowball J, McGrogan A, de Vries CS. Evaluating the hazard of foetal death following H1N1 influenza vaccination; a population based cohort study in the UK GPRD. PLoS One 2012;7:e51734 10.1371/journal.pone.0051734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McMillan M, Porritt K, Kralik D, Costi L, Marshall H. Influenza vaccination during pregnancy: a systematic review of fetal death, spontaneous abortion, and congenital malformation safety outcomes. Vaccine 2015;33:2108–17. http://dx.doi.or2/10.1016/j.vaccine.2015.02.068. [DOI] [PubMed] [Google Scholar]
- [38].Moro P, Baumblatt J, Lewis P, Cragan J, Tepper N, Cano M. Surveillance of adverse events after seasonal influenza vaccination in pregnant women and their infants in the Vaccine Adverse Event Reporting System, July 2010-May 2016. Drug Saf 2017;40:145–52. 10.1007/s40264-016-0482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Huang WT, Chen WC, Teng HJ, Huang WI, Huang YW, Hsu CW, et al. Adverse events following pandemic A (H1N1) 2009 monovalent vaccines in pregnant women-Taiwan, November 2009-August 2010. PLoS One 2011;6:e23049 10.1371/journal.pone.0023049, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kharbanda EO, Vazquez-Benitez G, Romitti PA, Naleway AL, Cheetham TC, Lipkind HS, et al. First trimester influenza vaccination and risks for maior structural birth defects in offspring. J Pediatr 2017. 10.1016/j.jpeds.2017.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Christian LM, Porter K, Karlsson E, Schultz-Cherry S, Iams JD. Serum proinflammatory cytokine responses to influenza virus vaccine among women during pregnancy versus non-pregnancy. Am J Reprod Immunol 2013;70:45–53. 10.1111/aji.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kay AW, Fukuyama J, Aziz N, Dekker CL, Mackey S, Swan GE, et al. Enhanced natural killer-cell and T-cell responses to influenza A virus during pregnancy. Proc Natl Acad Sci USA 2014;111:14506–11. 10.1073/pnas.1416569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yang J, James E, Gates TJ, DeLong JH, LaFond RE, Malhotra U, et al. CD4+ T cells recognize unique and conserved 2009 H1N1 influenza hemagglutinin epitopes after natural infection and vaccination. Int Immunol 2013;25:447–57. 10.1093/intimm/dxt005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Schmidt T, Dirks J, Enders M, Gartner BC, Uhlmann-Schiffler H, Sester U, et al. CD4+ T-cell immunity after pandemic influenza vaccination cross-reacts with seasonal antigens and functionally differs from active influenza infection. Eur J Immunol 2012;42:1755–66. 10.1002/eji.201242393. [DOI] [PubMed] [Google Scholar]
- [45].Christiansen OB, Nielsen HS, Kolte AM. Inflammation and miscarriage. Semin Fetal Neonatal Med 2006;11:302–8. 10.1016/j.siny.2006.03.001. [DOI] [PubMed] [Google Scholar]
- [46].Szarka A, Rigo J Jr, Lazar L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol 2010;11:59 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol 2010;116:393–401. 10.1097/A0G.0b013e3181e6dbc0. [DOI] [PubMed] [Google Scholar]
- [48].Calleja-Agius J, Muttukrishna S, Pizzey AR, Jauniaux E. Pro- and antiinflammatory cytokines in threatened miscarriages. Am J Obstet Gynecol 2011;205(83):e8–e16. 10.1016/j.ajog.2011.02.051. [DOI] [PubMed] [Google Scholar]
- [49].Lissauer D, Goodyear O, Khanum R, Moss PA, Kilby MD. Profile of maternal CD4 T-cell effector function during normal pregnancy and in women with a history of recurrent miscarriage. Clin Sci (Lond) 2014;126:347–54. 10.1042/CS20130247. [DOI] [PubMed] [Google Scholar]
- [50].Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med 1988;319:189–94. 10.1056/NEIM198807283190401. [DOI] [PubMed] [Google Scholar]
- [51].Jones RK, Kost K. Underreporting of induced and spontaneous abortion in the United States: an analysis of the 2002 national survey of family growth. Stud Fam Plann 2007;38:187–97. [DOI] [PubMed] [Google Scholar]
- [52].Copen CE, Thoma ME, Kirmeyer S. Interpregnancy intervals in the United States: Data from the birth certificate and the National Survey of Family Growth. Natl Vital Stat Rep 2015;64:1–10. [PubMed] [Google Scholar]
- [53].Pearce N What does the odds ratio estimate in a case-control study? Int J Epidemiol 1993;22:1189–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.