Abstract
Objective:
Medium and long-term sequelae of intravitreal bevacizumab (IVB) for type 1 retinopathy of prematurity (ROP) are uncertain. Our aim was to describe the fluorescein angiography (FA) findings in patients who received IVB as a primary treatment for type 1 ROP and compare them to findings in patients with spontaneously regressed ROP.
Design:
Retrospective cohort
Participants:
Patients with a history of retinopathy of prematurity, who underwent fluorescein angiography between December 1st, 2013 and July 31st, 2018. Patients were divided into 2 groups based on whether they had received IVB or had spontaneously regressed.
Methods:
We reviewed the angiograms in the 2 groups for neovascularization (NV) and other abnormal vascular patterns both in the periphery and the posterior pole.
Main Outcome Measures:
FA findings including NV, peripheral and macular vascular abnormalities.
Results:
Forty eyes of 20 infants were included in the IVB group and 16 eyes of 8 infants in the untreated group. Median gestational age was similar in the 2 groups (24.5 & 24.7 weeks respectively; p = 0.44) as was the median birth weight (648.5 & 560.0 g respectively; p=0.26). Median post-menstrual age at the time of FA was 65.1 and 83.9 weeks respectively (p = 0.0002). Review of angiograms demonstrated NV in 30.0% and 37.5% in the IVB and untreated cohorts, respectively (p=0.75). Abnormal vascular patterns in the periphery were similar in both groups (100.0%) while posterior pole finding of vessels encroaching onto the fovea were more prevalent in the IVB cohort (65.0% vs 25.0%; p=0.009).
Conclusion:
FA after bevacizumab for ROP reveals abnormal vascular patterns in all eyes and NV in approximately one-third. Similar abnormal vascular patterns on FA are seen at a similar prevalence after spontaneous regression of ROP. These findings suggest that the abnormal vascular patterns identified by FA in patients with ROP result from the disease process itself rather than as a result of exposure to anti-VEGF medications..
Intravitreal bevacizumab (IVB) for retinopathy of prematurity (ROP) has been widely utilized since the publication of BEAT-ROP in 2011.1 Persistent avascular retina after IVB is common.2, 3 Late recurrence of ROP has been reported after IVB and is thought to be driven by hypoxic avascular peripheral retina.4-6 The long-term risk of late recurrence is unknown. At our institution, persistent avascular retina is imaged with FA and treated with laser when vessels do not extend into zone 3 by 60 weeks post-menstrual age (PMA).
The role of fluorescein angiography (FA) in the management of ROP is still not well established. In recent literature, FA has been utilized to evaluate the fundus of patients who received intravitreal bevacizumab as primary treatment for ROP.7-9 Lepore et al reported peripheral and macular vessel changes seen in eyes receiving intravitreal bevacizumab, but not in eyes undergoing laser treatment.8 Our study aimed to report the fluorescein angiography findings at ≥60 weeks PMA following intravitreal bevacizumab for type 1 ROP. In addition, we compared our findings in patients treated with IVB to a cohort of untreated patients with spontaneously regressed ROP.
Methods
This is a retrospective cohort study of all infants with a history of retinopathy of prematurity who underwent fluorescein angiography between December 1, 2013 and July 31, 2018. The study was approved by the institutional review board of Emory University, and conformed to HIPAA regulations. We searched the fluorescein angiography record database for the diagnosis of ‘retinopathy of prematurity’ (ROP) to identify the participants. At our institute, infants received intravitreal bevacizumab as the first line of management if they had type 1 ROP in zone I or posterior zone II, aggressive posterior retinopathy of prematurity, media opacity, or were poor candidates for general anesthesia. Fluorescein angiography was performed in a subset of these patients, as needed for incomplete vascularization beyond 60 weeks of post-menstrual age or if there was recurrence of stage 3 ROP noted on clinical examination. We also performed FA on infants not meeting criteria for treatment on indirect ophthalmoscopy, but who had large areas of avascular ROP persisting after 60 weeks GA, or in whom other concerning clinical findings were present such as vitreous hemorrhage. We excluded patients receiving laser as primary treatment.
We divided patients into 2 cohorts based on whether they had received IVB (group 1) or had spontaneous regression (group 2). We collected demographic information and compared the BW, GA, lowest zone, highest stage, presence of plus disease and timing of FA. Comparison between the 2 cohorts was performed using 2 sided Wilcoxon ranked sum test and Fisher’s exact test.
We documented abnormal FA findings of the posterior pole and of the retinal periphery including pattern of vascularization at the vascular-avascular junction, presence of shunts, presence of neovascularization (NV), and macular abnormalities. NV was defined as an angiographic lesion that showed early hyperfluorescence with late leakage. The frequency of these findings was compared between groups 1 and 2, using Fisher’s exact test. Statistics were performed on software JMP Pro (JMP®, Version 13.0.0. SAS Institute Inc., Cary, NC, 2016).
Results
Fifty-six eyes of 28 infants were included during the 56-month study period, of which 40 eyes of 20 infants received intravitreal bevacizumab (Group 1), and 16 eyes of 8 infants spontaneously regressed (Group 2). The median gestational ages in groups 1 and 2 were 24.5 weeks (range, 22.7 to 28 weeks), and 24.7 weeks (range, 22.9 to 26.1 weeks), respectively. The groups did not significantly differ in median gestational age, sex, birth weight. In group 1 the median post-menstrual age at the time of intravitreal bevacizumab was 35.2 weeks (range, 32.3 to 41.1 weeks) and the median post-menstrual age at the time of fluorescein angiography (FA) was 65.1 weeks (range, 42.9 to 96.1 weeks). FA was performed significantly later in the untreated cohort, at a median post-menstrual age of 84 weeks (range, 62.9 weeks to 9.8 years; p =0.0002). The severity of ROP based on highest stage and lowest zone was significantly worse in the anti-VEGF cohort in comparison to the untreated cohort as expected. Table 1 depicts the baseline characteristics for the two groups.
Table 1.
Baseline characteristics of intravitreal bevacizumab and untreated patients between 1st December 2013 and 31st July 2018
| Baseline characteristics | Anti-VEGF cohort (20 patients) |
Untreated cohort (8 patients) |
p-value |
|---|---|---|---|
| Median gestational age in weeks (range) | 24.5 (22.7 to28.0) | 24.7 (22.9 to 26.1) | 0.44 |
| Median weight at birth in g (range) | 648.5 (440 to 1025) | 560.0 (482 to 1080) | 0.26 |
| Sex | |||
| Male (%) | 15 (75.0) | 6 (75.0) | - |
| Female (%) | 5 (25.0) | 2 (25.0) | - |
| Race | |||
| Caucasian (%) | 12 (60.0) | 2 (25.0) | - |
| African American (%) | 7 (35.0) | 5 (62.5) | - |
| Hispanic (%) | 1 (5.0) | - | - |
| Asian (%) | - | 1 (12.5) | - |
| Median timing of IVB in weeks (range) | 35.2 (32.3 to 41.1) | - | - |
| Median timing of FA in weeks (range) | 65.1 | 83.9 | 0.0002 |
| Severity of ROP* | |||
| Median highest stage (median) | 2 (2 to 3) | 2 (1 to 3) | <.0001 |
| Median lowest zone (median) | 1 (1 to 2) | 2 (2 to 3) | <.0001 |
| Plus (%) | 32 (88.9) | 0 (0) | - |
| Pre-plus (%) | 4 (11.1) | 0 (0) | - |
among 36 eyes for the anti-VEGF cohort (aggressive posterior disease in 1 patient, missing data on 1 patient) and 14 eyes for the untreated cohort (missing data on 1 patient)
The most common indication for FA was persistent avascular retina at ≥60 weeks of post-menstrual age in both the groups (Table 2). NV was present on FA in 12 (30.0%) and 6 eyes (37.5%) in groups 1 and 2, respectively. Occult NV, applied as a descriptor when the leakage was visible on FA, but the abnormal vessels were not detected on indirect ophthalmoscopy, was present in 5 (12.5%) and 5 (31.3%) of the two groups (Figure 1-2) (Table 3). Laser treatment following FA was performed in all but 1 patient (2 eyes) in group 1. Reasons for laser treatment were NV in 12 of 38 eyes (31.6%), and for persistent avascular retina in zone II for 26 of 38 eyes (68.4%). In Group 2, laser treatment was administered following FA in a total of 6 eyes (37.5%) and the indication was NV in all 6.
Table 2.
Indications of fluorescein angiography among the treated and untreated cohorts between 1st December 2013 and 31st July 2018
| Anti-VEGF cohort | Untreated cohort |
|---|---|
| • Persistent avascular retina at >60 weeks post-menstrual age in 16 (80.0%) • Recurrence of stage 3 ROP or plus disease in 4 (20.0%) |
• Persistent avascular retina at >60 weeks post-menstrual age in 5 (62.5%) • Evaluation of a chorioretinal lesion in 1(12.5 %) • Evaluation of an optic nerve lesion in 1 (12.5%) • Spontaneous vitreous hemorrhage in 1 (12.5%) |
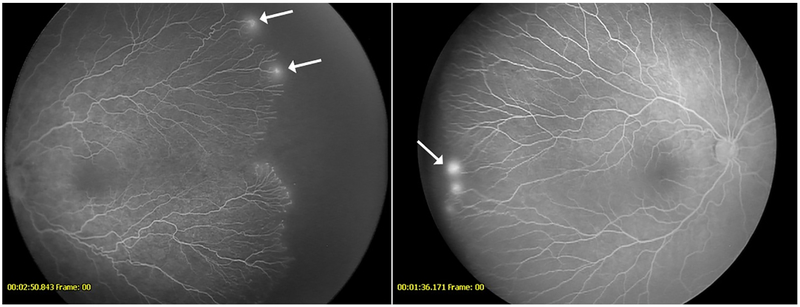
Figure 1-.
Fluorescein angiogram showing leakage (arrows) from vessels at the vascular-avascular junction in a patient from the anti-VEGF cohort (left) and untreated cohort (right)
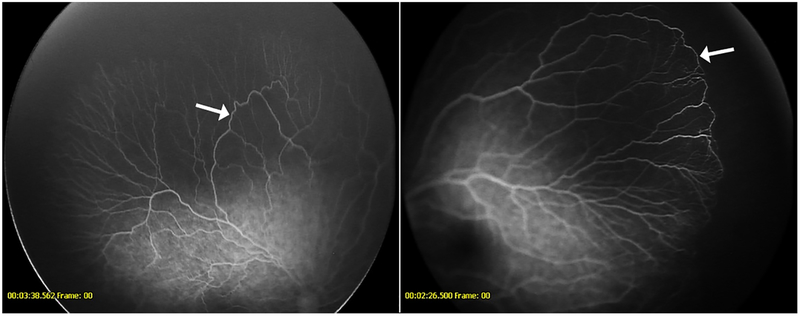
Figure 2-.
Fluorescein angiogram demonstrating recurrent neovascularization (arrows) in a patient with retinopathy of prematurity following intravitreal bevacizumab (left) Fluorescein angiogram showing neovascularization (arrow) in a 9-year-old from the untreated cohort presenting with spontaneous vitreous hemorrhage (right)
Table 3.
Fluorescein angiography findings among the treated and untreated cohorts between 1st December 2013 and 31st July 2018
| No. (%) | |||
|---|---|---|---|
| Findings | Anti-VEGF cohort (40 eyes) |
Untreated cohort (16 eyes) |
p-value |
| Neovascularization | 12 (30.0) | 6 (37.5) | 0.75 |
| Occult neovascularization | 5 (12.5) | 5 (31.3) | 0.12 |
| Peripheral findings | |||
| Anomalous branching pattern | 40 (100.0) | 16 (100.0) | - |
| Blunting | 38 (95.0) | 14 (87.5) | 0.56 |
| Double blunting | 3 (7.5) | 0 (0.0) | 0.54 |
| Abnormal capillary bed | 12 (30.0) | 5 (31.3) | 1 |
| Vascular shunt | 34 (85.0) | 10 (62.5) | 0.08 |
| Shunt crossing horizontal raphe | 11 (27.5) | 1 (6.3) | 0.08 |
| Shunt at junction | 2 (5.0) | 4 (25.0) | 0.05 |
| Posterior pole findings | |||
| Vessel encroaching onto fovea | 26 (65.0) | 4 (25.0) | 0.009 |
| Vessel crossing fovea | 5 (12.5) | 1 (6.3) | 0.66 |
| Tortuosity | 7 (17.5) | 5 (31.3) | 0.29 |
Peripheral findings
These included abnormalities detected at or near the vascular- avascular junction. An anomalous non-dichotomous, finger-like branching pattern near the junction was detected in all eyes across both groups (Figure 3). Blunted vascular terminal was the next most common finding, where the vessels ended abruptly in a stunted or blunted fashion, instead of the normal tapered endings (Figure 3). In a few eyes a ‘double blunted’ pattern of two circumferential rows of blunted vessel terminals was noted (Figure 4). Other findings seen in both groups included an abnormal capillary bed, vascular shunts, including some crossing the horizontal raphe, and shunt vessels running for varying distances exactly at the junction of vascular and avascular retina (Figures 5-6). No significant difference was detected in the prevalence of any peripheral findings between groups 1 and 2, although vascular shunts overall and shunts at junction of avascular retina tended to be more prevalent in group 2 (p=0.08 & p=0.05) and shunts crossing the horizontal raphe tended to be more prevalent in group 1 (p=0.08). Table 3 compares the peripheral findings between the groups.
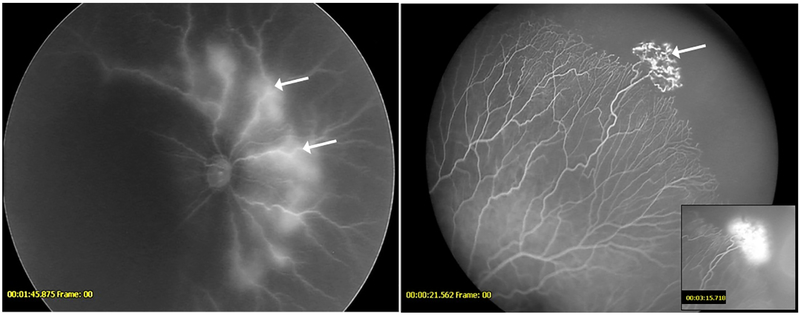
Figure 3-.
Fluorescein angiogram demonstrating anomalous non-dichotomous branching and blunted vessel terminals in a patient from the anti-VEGF cohort (left) and untreated cohort (right)
Figure 4-.
Fluorescein angiogram demonstrating an unusual double- blunted vascular pattern seen only in the anti-VEGF cohort
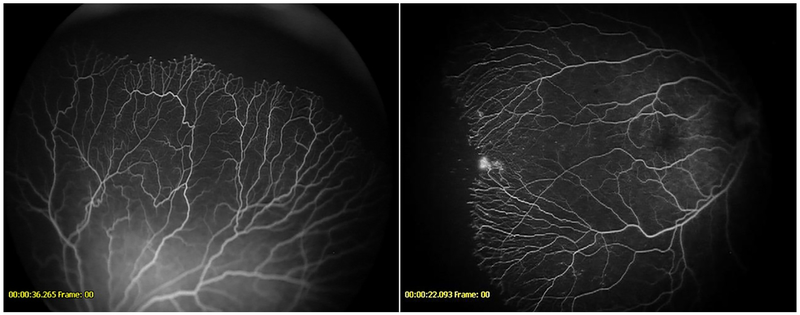
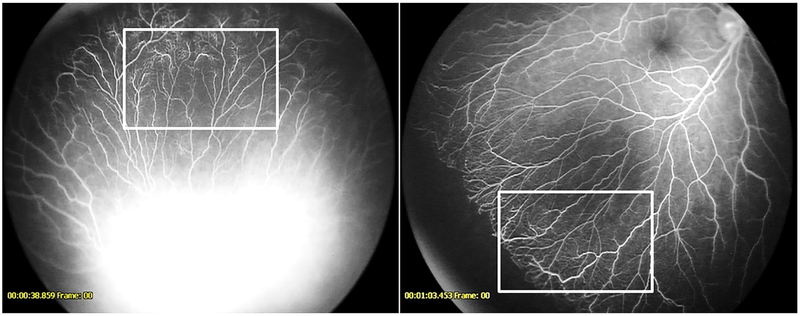
Figure 5-.
Fluorescein angiogram demonstrating abnormal lacy or feathery capillary bed (seen best in the boxed area) in a patient from the anti-VEGF cohort (left) and untreated cohort (right)
Figure 6-.
Fluorescein angiogram demonstrating vessel shunt (arrow) in a patient from the anti-VEGF cohort (left) and shunt vessel along vascular-avascular junction (arrow) in a patient from the untreated cohort (right)
Macular and posterior pole findings
Vessels were seen encroaching onto the fovea in 26 eyes (65.0%) and 4 eyes (25.0%) among groups 1 and 2 respectively, and this difference was statistically significant (p = 0.009). Other findings included vessels crossing the fovea, hyperfluorescent window defect over the macula, and tortuosity of vessels over the posterior pole (Figures 7-8). No significant difference was detected in the prevalence of these features between groups 1 and 2. Table 3 summarizes the posterior pole findings.
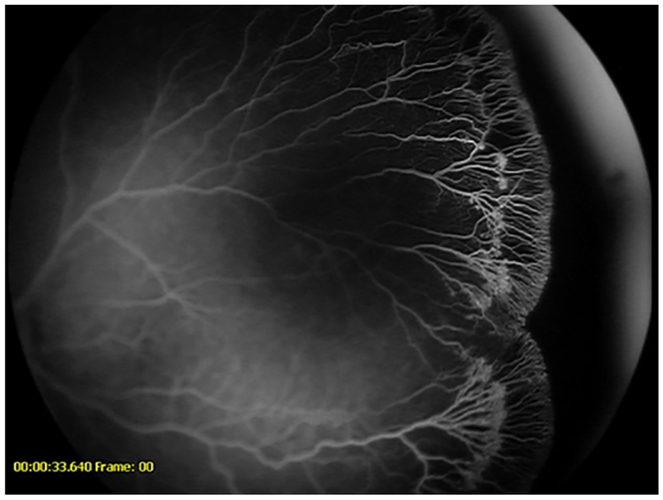
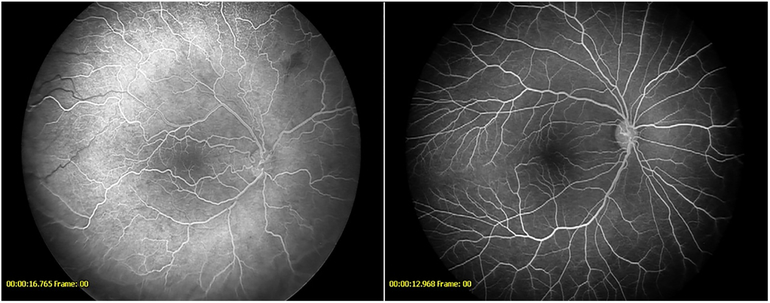
Figure 7-.
Fluorescein angiogram demonstrating vessels encroaching onto the fovea in a patient from the anti-VEGF cohort (left) and the untreated cohort (right)
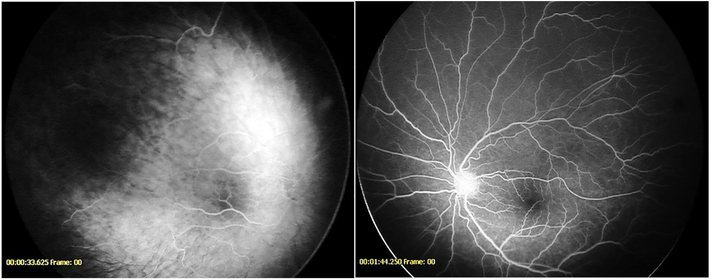
Figure 8-.
Fluorescein angiogram demonstrating vessels crossing the fovea in a patient from the anti-VEGF cohort (left) and from the untreated cohort (right)
Discussion
In this cohort study describing the fluorescein angiography (FA) findings following intravitreal bevacizumab for type 1 retinopathy of prematurity (ROP) we found that just under one-third of the eyes had NV. Of these, about 40% were not detected on indirect ophthalmoscopy, and were thus deemed “occult neovascularization”. All bevacizumab treated eyes demonstrated peripheral vessel abnormalities, while two-thirds had macular vascular changes. Among the untreated cohort who had spontaneous regression of ROP, over one-third of the eyes exhibited NV, of which the majority were occult. Like the bevacizumab treated eyes, all those with spontaneous regression had abnormal peripheral vessels. Abnormal macular vessels were present in one-fourth of untreated eyes. Comparison between groups 1 and 2 showed similar peripheral findings in both groups. Group 1 had more prevalent findings in the macula and this difference was statistically significant. Whether this difference is related to IVB exposure or to differences in disease severity is unknown; however, it has been clearly demonstrated that macular abnormalities are common in infants with gestational age less than 28 weeks, regardless of severity of ROP.10
Previous studies in the literature have reported similar findings. Tahija et al focused on completeness of vascularization after bevacizumab, but also reported fluorescein leakage in just under half of the eyes along with irregular branching and circumferential vessels.9 Henaine-Berra et al argued that even when the abnormal vessel pattern persisted in their patients after the anti-VEGF therapy, they found that there was creation of small vessels and adjustment of vascular density.2 Garcia et al, comparing peripheral FA findings following bevacizumab therapy in aggressive posterior ROP and classic ROP, described leakage in 100% and 57.9% eyes, respectively.3 They also described terminal bulbs, circumferential vessels and abnormal branching. Toy et al reported that only 9% of patients receiving an anti-VEGF agent developed complete vascular maturity when evaluated by FA by 54 weeks.11 They described a characteristic scalloped regression pattern that was not seen in the eyes receiving laser. Lorenz et al described vessel branching abnormalities and circumferential vessel formation in a subset of their 17 infants studied with FA at various time points after treatment with IVB.12 Chen et al described 4 outcomes in the spectrum of regression following IVB characterized on FA including complete vascularization, vascular arrest alone, vascular arrest with tortuosity and reactivation, proposing better prognosis with arrest alone versus arrest with persistent tortuosity.13 Our study reported tortuosity in the IVB group (17.5%), but also in the spontaneous regressed group (31.3%). Lepore et al performed a randomized controlled trial in which one eye received bevacizumab and the other laser, and they compared the FA findings 9 months and 4 years after treatment. 7, 8 They found that a majority of the eyes receiving anti-vascular endothelial growth factor (VEGF) and very few eyes treated with laser had abnormalities of leakage, tangles, shunts and decreased foveal avascular zone. These authors conclude that the use of FA “allowed the description of potentially serious and long-term ocular effects from treatment” of ROP that are more frequent with IVB than with laser.
None of the studies above reported on FA findings following spontaneous regression. There are limited reports in the literature on the medium and long-term FA findings following spontaneous regression. Purcaro et al describe FA findings in children who underwent serial FA every 2 weeks until treatment or discharge.14 They report that arteriovenous shunts and circumferential vessels often persisted even after ROP regression. They also mention hyperfluorescent macular lesions and decreased foveal avascular zone; however, they did not specify which of these findings were seen in the later angiograms and how often they occurred. Similarly, an early study looking at sequelae of ROP by Mintz-Hittner and Kretzer reported on FA done at 2 −16.2 years of age and described abnormal leakage, non-dichotomous branching, and shunts; however they did not comment on the frequency of these findings and their study focused on the temporal retina and the cicatricial outcomes in comparison with the worst stage.15
What is the significance of the abnormal vascular lesions that we and others have identified in anti-VEGF treated ROP eyes, and in ROP eyes undergoing spontaneous regression? Additional studies will be necessary to answer this question. The finding of occult NV in both groups is especially intriguing. One might argue that the lesions we are calling NV may in fact be angiographic leakage resulting from endothelial cell dysfunction and that these lesions may not actually be NV. Given the vasoproliferative nature of ROP however, we believe it is reasonable to assume that lesions with early hyperfluorescence and late leakage are indeed NV. Whether all NV in ROP requires treatment is another question. We do not necessarily treat all NV in other vasoproliferative conditions such as diabetic retinopathy. In addition, the presence of NV does not necessarily imply active vasoproliferation. Inactive or regressed NV may be stable indefinitely in other vasoproliferative disorders and ROP may be similar. Additional study will be necessary to determine which eyes are at risk for reactivation and proliferation. This is particularly true for eyes previously treated with anti-VEGF but it may also be true for select ROP eyes that never met criteria for treatment. Thus, our research brings up several important questions worthy of additional study. Should all babies with more severe ROP undergo FA? When should FA be performed in both treated and untreated infants with arrest of vascular development? Should any occult NV or leakage on FA be treated with laser regardless of history of treatment with anti-VEGF? In general, our approach it to treat, at approximately 60 weeks PMA, persistent avascular retina in ROP eyes previously treated with anti-VEGF. We do not believe there is evidence to support routine treatment of persistent avascular retina in most ROP eyes that undergo spontaneous regression.
The limitations of this study include its retrospective design and the relatively small number of eyes available for analysis. In addition, during the time of the study, not all babies receiving bevacizumab, or having spontaneous regression, underwent FA. As a result, selection bias may have affected the cases that were studied. Since infants deemed to be low risk by indirect ophthalmoscopy were not imaged, the anti-VEGF group was likely enriched for more pronounced FA findings than would have been seen if every treated infant had undergone imaging. The FA images were captured on the Retcam and resolution of Retcam images is lower than some other types of cameras. Some macular findings are best visualized during the transit phase of the FA. Since only one eye can undergo transit phase imaging, it is possible that some findings may have been missed in the other eye. In addition, image reviewers were not masked to the treatment group. Both eyes of the patients were included in the analysis, thus there is potential correlation between the two eyes. As well, our comparison cohort of untreated patients underwent FA for an assortment of indications, with a large range of age at the time of FA. Finally, the untreated cohort by nature had ROP that was less severe at baseline. The strength of the study is the analysis of both untreated and anti-VEGF treated ROP eyes in a way that allows for direct comparison of the abnormal vascular patterns in each group.
In conclusion, this study shows that FA after bevacizumab for ROP reveals abnormal vascular patterns in all eyes and NV (often occult) in approximately one third. Similar abnormal vascular patterns on FA are seen at a comparable prevalence after spontaneous regression of ROP. These findings suggest that the abnormal vascular patterns identified by FA in patients with ROP result from the disease process itself rather than as a result of exposure to anti-VEGF medications. The long-term significance of these vascular patterns, both in anti-VEGF treated and untreated eyes, warrants further investigation.
Precis/Highlights.
Fluorescein angiography in infants with retinopathy of prematurity shows abnormal vascular patterns that are similar in both those treated with intravitreal anti-VEGF and in those who underwent spontaneous regression.
Acknowledgments
Financial Support: National Eye Institute Core Grant P30 EY006360
Abbreviations:
- (IVB)
intravitreal bevacizumab
- (ROP)
retinopathy of prematurity
- (FA)
fluorescein angiography
- (NV)
neovascularization
- (VEGF)
vascular endothelial growth factor
- (PMA)
weeks post-menstrual age
Footnotes
Meeting Presentation: Presented in part at the American Association of Pediatric Ophthalmology & Strabismus, Washington DC, March 2018
Conflict of Interest: No conflicting relationship exists for any author
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011;364(7):603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henaine-Berra A, Garcia-Aguirre G, Quiroz-Mercado H, Martinez-Castellanos MA. Retinal fluorescein angiographic changes following intravitreal anti-VEGF therapy. J aapos 2014;18(2):120–3. [DOI] [PubMed] [Google Scholar]
- 3.Garcia Gonzalez JM, Snyder L, Blair M, et al. PROPHYLACTIC PERIPHERAL LASER AND FLUORESCEIN ANGIOGRAPHY AFTER BEVACIZUMAB FOR RETINOPATHY OF PREMATURITY. Retina 2018;38(4):764–72. [DOI] [PubMed] [Google Scholar]
- 4.Hu J, Blair MP, Shapiro MJ, et al. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol 2012;130(8):1000–6. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal-Sinha S, Guevara JG, Amin SM. Late-onset tractional fibrovascular proliferation post-intravitreal bevacizumab following treatment of retinopathy of prematurity. Can J Ophthalmol 2018;53(3):e99–el03. [DOI] [PubMed] [Google Scholar]
- 6.Yonekawa Y, Thomas BJ, Thanos A, et al. THE CUTTING EDGE OF RETINOPATHY OF PREMATURITY CARE: Expanding the Boundaries of Diagnosis and Treatment. Retina 2017;37(12):2208–25. [DOI] [PubMed] [Google Scholar]
- 7.Lepore D, Quinn GE, Molle F, et al. Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity: report on fluorescein angiographic findings. Ophthalmology 2014;121(11):2212–9. [DOI] [PubMed] [Google Scholar]
- 8.Lepore D, Quinn GE, Molle F, et al. Follow-up to Age 4 Years of Treatment of Type 1 Retinopathy of Prematurity Intravitreal Bevacizumab Injection versus Laser: Fluorescein Angiographic Findings. Ophthalmology 2018;125(2):218–26. [DOI] [PubMed] [Google Scholar]
- 9.Tahija SG, Hersetyati R, Lam GC, et al. Fluorescein angiographic observations of peripheral retinal vessel growth in infants after intravitreal injection of bevacizumab as sole therapy for zone I and posterior zone II retinopathy of prematurity. Br J Ophthalmol 2014;98(4):507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maldonado RS, O'Connell RV, Sarin N, et al. Dynamics of human foveal development after premature birth. Ophthalmology 2011;118(12):2315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toy BC, Schachar IH, Tan GS, Moshfeghi DM. Chronic Vascular Arrest as a Predictor of Bevacizumab Treatment Failure in Retinopathy of Prematurity. Ophthalmology 2016;123(10):2166–75. [DOI] [PubMed] [Google Scholar]
- 12.Lorenz B, Stieger K, Jager M, et al. RETINAL VASCULAR DEVELOPMENT WITH 0.312 MG INTRAVITREAL BEVACIZUMAB TO TREAT SEVERE POSTERIOR RETINOPATHY OF PREMATURITY: A Longitudinal Fluorescein Angiographic Study. Retina 2017;37(1):97–111. [DOI] [PubMed] [Google Scholar]
- 13.Chen TA, Shields RA, Bodnar ZH, et al. A spectrum of regression following intravitreal bevacizumab in retinopathy of prematurity. Am J Ophthalmol 2018. [DOI] [PubMed] [Google Scholar]
- 14.Purcaro V, Baldascino A, Papacci P, et al. Fluorescein angiography and retinal vascular development in premature infants. J Matern Fetal Neonatal Med 2012;25 Suppl 3:53–6. [DOI] [PubMed] [Google Scholar]
- 15.Mintz-Hittner HA, Kretzer FL. Postnatal retinal vascularization in former preterm infants with retinopathy of prematurity. Ophthalmology 1994;101(3):548–58. [DOI] [PubMed] [Google Scholar]