Abstract
Objective:
To test the effects of LEAF (Life Enhancing Activities for Family caregivers), a 6-week positive emotion regulation intervention, on outcomes of positive emotion, depression, anxiety, and physical health as measured by PROMIS.
Methods:
A randomized controlled trial (N = 170) comparing LEAF (N=86) to an emotion reporting/waitlist condition (N = 84) in dementia caregivers. LEAF was individually delivered online by trained facilitators. Participants in the control condition completed daily online emotion reports then crossed over into the intervention condition after 6 weeks. The study was registered with Clinicaltrials.gov (NCT01825681) and funded by R01NR014435.
Results:
Analyses of difference in change from baseline to 6 weeks demonstrated significantly greater decreases in PROMIS depression, (d = −.25; p = .02) and NeuroQOL anxiety (d = −.33; p< .01), and improvements in PROMIS physical health (d = .24; p = .02) in the intervention condition compared to the emotion reporting/waitlist control. The intervention also showed greater improvements in positive emotion (d = .58; p < .01) and positive aspects of caregiving (d = .36; p < .01). Increases in positive emotion significantly mediated the effect of LEAF on depression over time.
Conclusions:
This randomized controlled trial of the online facilitated positive emotion regulation intervention in dementia caregivers demonstrated small to medium effect sizes on caregiver well being and shows promise for remotely delivered programs to improve psychological well-being in caregivers of people with dementia and other chronic illnesses.
Keywords: Caregivers, stress, randomized controlled trial, positive emotion, patient reported outcomes
The prevalence of Alzheimer’s Disease and other dementias is steadily climbing and predicted to affect as many as 16 million Americans by 2050 (Alzheimer’s Association, 2017). If the current trend continues, more than 90% of those with dementia will receive care from a family member or friend (Kasper, Freedman, Spillman, & Wolff, 2015). In a nationwide poll in 2016, 59% of dementia caregivers reported experiencing high levels of emotional and physical stress (Association, 2017) and the chronic stress of dementia caregiving is associated with a range of deleterious physical and mental health consequences (Chattillion et al., 2012; Gouin, Glaser, Malarkey, Beversdorf, & Kiecolt-Glaser, 2012; Kiecolt-Glaser, Dura, Speicher, Trask, & Glaser, 1991; Kiecolt-Glaser, Marucha, Mercado, Malarkey, & Glaser, 1995; Mausbach et al., 2012; Roepke et al., 2012; Schulz & Beach, 1999; Von Känel et al., 2006; von Känel et al., 2012). In addition, caregiving stress and burden negatively impact quality of care and lead to poorer quality of life in the care recipient (Hébert, Dubois, Wolfson, Chambers, & Cohen, 2001; Mittelman, Haley, Clay, & Roth, 2006).
Interventions designed to reduce burden for dementia caregivers have primarily consisted of education or training in caregiving skills, (e.g., Chu, Edwards, Levin, & Thomson, 2000; McCallion, Toseland, & Freeman, 1999; Quayhagen & Quayhagen, 1989), social support (e.g., McCurry, Logsdon, Vitiello, & Teri, 1998; Mittelman, Ferris, Shulman, Steinberg, & Levin, 1996), or stress management, (e.g., Morris, Woods, Davies, Berry, & Morris, 1992; Zarit, Anthony, & Boutselis, 1987) with a primary focus on reducing negative emotion and stress. However, over the past few decades, it has become clear that positive affect or positive emotions, defined as subjective positively valenced feelings that range from happy, calm, and satisfied, to excited and thrilled, are uniquely related to better psychological and physical well-being, independent of the effects of negative emotion (Folkman, 1997; Folkman & Moskowitz, 2000; Fredrickson, 1998; Fredrickson, Cohn, Coffey, Pek, & Finkel, 2008; Tice, Baumeister, Shmueli, & Muraven, 2007; Wichers et al., 2007; Zautra, Johnson, & Davis, 2005). Positive emotion is associated with a host of beneficial outcomes including better relationships, more creativity, better quality of work, higher likelihood of prosocial behavior (Lyubomirsky, King, & Diener, 2005), better physical health (Pressman & Cohen, 2005), and even a lower risk of mortality in healthy as well as chronically ill samples (Chida & Steptoe, 2008; Liu et al., 2016; Moskowitz, 2003; Moskowitz, Epel, & Acree, 2008; Steptoe & Wardle, 2011).
Among caregivers, positive emotion predicts lower frailty over a two-year follow-up (Park-Lee, Fredman, Hochberg, & Faulkner, 2009) and engagement in pleasant activities is associated with higher positive emotion and lower negative emotion (Mausbach, Coon, Patterson, & Grant, 2008; Mausbach, Roepke, Depp, Patterson, & Grant, 2009). Positive caregiver resources such as optimism, resilience, and a sense of coherence are associated with lower levels of burden and higher quality of life (Fianco et al., 2015; Trapp et al., 2015), suggesting that an intervention that specifically targets positive emotion holds promise for improving caregiver well-being, reducing burden, and, ultimately, improving quality of care for the individual living with dementia. Indeed, interventions that specifically target positive emotion are showing efficacy across a range of stressful health conditions such as diabetes (Cohn, Pietrucha, Saslow, Hult, & Moskowitz, 2014; Huffman, DuBois, Millstein, Celano, & Wexler, 2015) heart disease (Huffman et al., 2011; Peterson et al., 2012), hypertension (Boutin-Foster et al., 2016; Ogedegbe et al., 2012), substance use (Carrico et al., 2015; Krentzman et al., 2015), schizophrenia (Caponigro, Moran, Kring, & Moskowitz, 2013), depression (Seligman, Steen, Park, & Peterson, 2005), and HIV (Moskowitz et al., 2017).
Grounded in theory and building on empirical findings of a link between positive emotion and adaptive outcomes for people coping with significant stress, we developed a multi-component intervention that focuses on skills for increasing the frequency of positive emotion to better cope with stress (Cheung et al., 2016; Cohn et al., 2014; Dowling et al., 2014; Moskowitz et al., 2014; Moskowitz et al., 2017; Moskowitz et al., 2012) The intervention is based on revised Stress and Coping Theory (Folkman, 1997) and the Broaden-and-Build Theory of positive emotion (Fredrickson, 1998). These theories describe ways positive emotion supports coping and well-being, such as providing a psychological “time-out” from stressful experiences and motivating and sustaining ongoing efforts to cope. Repeated experiences of positive emotion build social, intellectual, and physical resources that may ultimately benefit psychological well-being and physical health. The rapidly growing body of literature on the social, cognitive, psychological, and health benefits of positive emotion argue strongly for programs targeted at increasing positive emotions and positive experiences as a mechanism for mitigating depression, stress, and burden, and subsequently increasing the likelihood of higher quality care, improved self-care, and improved physical and psychological well-being for dementia caregivers. A feasibility pilot test of a positive emotion regulation intervention in a small sample of caregivers of dementia patients demonstrated increases in positive emotion and decreases in negative emotion, burden, and stress compared to an active control condition (Dowling et al., 2014).
As in other areas of research on health-related stress, the literature on dementia caregiving has suffered from a lack of precision and standardization of measures used to assess important constructs such as stress, depression, health and well-being. Given the variety of measures used across different research teams, it has become difficult to compare these scores in dementia caregiver samples with the general population or other chronically-stressed samples. Moreover, the lack of standardized measures used across research teams has made it difficult to compare the effects of different behavioral or psychological interventions.
The Patient Reported Outcomes Measurement Information System (PROMIS) is a collection of highly flexible, precise, and responsive self-report measures of physical, mental, and social well-being that aims to address the lack of precision, standardization, and comparability of measures that plague the dementia caregiving literature as well as the literature in health and medicine more broadly (Cella et al., 2007). PROMIS is part of the larger person centered HealthMeasures system that includes a number of self-report measures of well-being and functioning (www.healthmeasures.net.) The National Institutes of Health funded a team of leading experts in clinical and patient-reported outcomes research to develop state of the art, psychometrically robust systems to measure patient-centered (or person-centered) outcomes efficiently in patients with a wide range of chronic diseases as well as the general population. Within domains, these person-centered measures have been created through the use of qualitative and quantitative methods that have leveraged both classical test theory and item response theory approaches. Moreover, these measures have been rigorously reviewed and tested for reliability and validity and can be compared across domains and diseases using standardized metrics. There are now more than 800 published studies with PROMIS measures alone, in a number of different chronically ill and general population samples. The majority of the studies are cross sectional, however, with fewer studies reporting longitudinal change or responsiveness to behavioral or psychological interventions. Although there have been a few studies that used PROMIS measures in caregivers (e.g., Daly, Douglas, Lipson, & Foley, 2009; Romero, Flood, Gasiewicz, Rovin, & Conklin, 2015), none have examined change in these outcomes over time or in response to interventions and none have been in dementia caregivers. In the present paper, we report results from an emotion regulation intervention for caregivers of dementia patients and, as part of a set of self-report measures, examine PROMIS and related HealthMeasures constructs (depression, physical health, and anxiety) as outcomes.
In the present study we address a number of concerns in the burgeoning field of positive emotion regulation interventions. Many of the studies thus far suffer from methodological weaknesses including small sample sizes, lack of randomized trials, failure to report intent-to-treat analyses (Bolier et al., 2013), and imprecise measurement of outcomes (Moskowitz et al., 2017). In addition, few studies of positive interventions address questions of whether improvements in the targeted positive psychological construct mediate the effects of the intervention on more distal outcomes. We present results from a randomized controlled trial of a theory based positive emotion regulation intervention, delivered online by trained facilitators, for caregivers of people with dementia. The intervention, Life Enhancing Activities for Family caregivers (LEAF) was delivered remotely, via the internet which allowed us to reach a wide range of caregivers from all over the country, many of whom would not otherwise have been able to participate.
We examine outcomes of depression, anxiety, and physical health as measured by PROMIS (and related HealthMeasures systems), precise and responsive measures that facilitate meaningful comparisons across studies and to other populations, and test whether positive emotion, the proximal target of the intervention, mediated effects on outcomes of depression and burden. We hypothesized that the participants in the LEAF condition would show increases in our primary outcome of interest, positive emotion, compared to the control condition. In addition, we hypothesized that intervention participants would show significantly greater improvements in our secondary outcomes of interest, namely: depression, mental and physical health, anxiety, stress, and caregiving burden relative to controls, and that these improvements would be mediated by increases in positive emotion.
Methods
We conducted a randomized controlled trial of a 6-session positive emotion regulation intervention (LEAF) compared to an emotion-reporting waitlist control. All sessions were delivered live by trained facilitators via the internet on study-supplied tablet computers. Assessments were completed online.
Participants, recruitment, screening, and randomization.
In order to be eligible, participants had to 1) be a primary family caregiver of a person diagnosed with a degenerative dementia condition; 2) live with the care recipient or visit daily; 3) be able to speak and read English; and 4) have reliable WiFi internet at home or access from another location (e.g., a library) convenient for them. Exclusion criteria were active psychosis or significant cognitive impairment as evidenced in the screening phone call. All study procedures were approved by the University of California San Francisco and Northwestern University IRBs, all participants provided verbal and online informed consent, and the study was registered with Clinicaltrials.gov (NCT01825681).
Participants were recruited in person through brochures in clinic waiting rooms and at caregiver events, and online through caregiver support groups, bulletin boards, and other internet postings such as clinical trial matching sites and Facebook. Recruitment started in August, 2014 and was completed in November, 2016. Follow up data collection was completed in June, 2017. Interested participants filled out a brief contact form on the study website. The project coordinator then followed up with a phone call to ask additional screening questions and provide a more detailed description of study procedures. Eligible participants were sent a link to the online consent form and subsequent baseline questionnaire.
Once the baseline questionnaire was completed, participants were randomized 1:1 to either receive the LEAF content immediately (LEAF condition) or to complete daily emotion reports for 6 weeks followed by the LEAF content (emotion reporting-waitlist control.) Randomization was stratified by gender and by three categories of population density: rural (1–999 persons per mi²), suburban (1000–3000 persons per mi²), and urban (>3000 persons per mi²). The study statistician devised the randomization table and participants were automatically randomized upon completing their baseline assessment via a FileMaker database that contained their demographic information and population classification.
Prior to beginning LEAF sessions, the participants were sent a package that contained study materials and an 8” tablet computer. Before scheduling LEAF sessions, the study coordinator had a tech setup meeting by phone with each participant to walk them through the steps of joining a WebEx meeting and to familiarize them with the other uses of the tablet. Each participant was invited to keep their tablet for their own personal use at the end of study participation, when all follow-up data had been collected. Participants who withdrew from the study early were given instructions and mailing supplies to return the tablet and study workbook to the study office.
We minimized attrition by reaching out to the participants often and taking extra time to thoroughly explain study procedures, answer questions, and attend to concerns. The coordinator helped each participant assess whether or not they could successfully integrate the study activities into their schedules and gave each caregiver time to decide to enroll without pressure. If a participant did not complete a study activity, the coordinator made multiple attempts to reach out and connect, making room for the participant to skip that activity but remain in the study.
Intervention sessions
The intervention condition consisted of 6 sessions in which a facilitator taught participants a set of 8 emotion regulation skills intended to increase positive emotion. The intervention has demonstrated feasibility, acceptability, and preliminary efficacy in pilot tests and randomized trials in a number of different samples (Caponigro et al., 2013; Carrico et al., 2015; Cheung et al., 2016; Cohn et al., 2014; Moskowitz et al., 2017), including dementia caregivers (Glenna A Dowling et al., 2014). We briefly review the rationale for inclusion of each of the skills here. Details on development of the intervention are published elsewhere (Dowling et al., 2014; Moskowitz et al., 2014; Moskowitz et al., 2012; Verstaen, Moskowitz, Snowberg, Merrilees, & Dowling, 2018).
In session 1, the facilitators presented the first three skills: noticing positive events, capitalizing on them, and gratitude. Positive life events are associated with increases in positive emotion and intentionally scheduling positive activities is a central part of behavioral activation, an activity commonly used in depression treatment (Cuijpers, Muñoz, Clarke, & Lewinsohn, 2009). Capitalizing, otherwise known as savoring, is an expressive response to a positive event that strengthens the association between positive events and positive emotion (Langston, 1994). Capitalizing includes telling others about it, marking the occurrence in some way, or even thinking about the event again later on (Langston, 1994). The association between intentionally noting things for which one is grateful and increased well-being is supported empirically in a number of different samples (Emmons, 2007; Emmons & McCullough, 2003).
Session 2 focused on the skill of mindfulness. Mindfulness is defined as the ability to intentionally pay attention to and maintain nonjudgmental awareness of thoughts, feelings, and physical sensations in the present moment (Kabat-Zinn, 2003) and mindfulness-based interventions have been demonstrated to improve a number of aspects of psychological and physical well-being, including higher positive emotion (Grossman, Tiefenthaler-Gilmer, Raysz, & Kesper, 2007; Shapiro, Brown, & Biegel, 2007). The LEAF intervention focused specifically on the attention and non-judgment aspects of mindfulness.
In session 3, facilitators presented the skill of positive reappraisal. Positive reappraisal is a reinterpretation of the significance of a potentially stressful event in a more positive, and ultimately less stress-inducing way. For example, seeing the “silver lining” in a stressful event is a common form of positive reappraisal. Positive reappraisal is one of the few ways of coping that is consistently associated with increased positive emotion (Carver & Scheier, 1994; Folkman, 1997; Sears, Stanton, & Danoff-Burg, 2003).
Session 4 contained two skills: personal strengths and attainable goal setting. Focusing on one’s strengths is a form of self-affirmation that can be used as a coping strategy to increase positive emotion and noting one’s strengths is associated with better psychological adjustment to illness (Taylor, Lerner, Sage, & McDowell, 2003; Taylor & Lobel, 1989). Pursuit of attainable goals (vs. more diffuse distant goals) is associated with higher subjective well-being (Emmons, 1986; Emmons, 1992) and an extensive body of research shows that perceptions of goal progress are associated with greater positive emotion. Interventions that encourage attainable goal-setting in students demonstrate greater increases in the ratio of positive to negative emotion over the course of several weeks (Sheldon & Houser-Marko, 2001).
In session 5, the focus was on acts of kindness. Volunteerism and other altruistic behaviors are associated with lower risk of mortality, lower risk of serious illness (Musick & Wilson, 2003; Oman, Thoresen, & McMahon, 1999) and increased positive emotion (Dunn, Aknin, & Norton, 2008; Moen, Dempster-McCain, & Williams, 1993). In this session participants were encouraged to find opportunities to engage in acts of kindness.
In session 6, the final session of the intervention, facilitators worked with participants to plan continued practice of the skills and they provided suggestions for making engagement in the skills an ongoing habit.
Emotion Reporting/Wait list control condition.
In order to control for daily attention to emotional experience, control group participants were asked to complete a daily emotion survey for six weeks (comparable to the time for the LEAF intervention). After 6 weeks, they completed assessment 2, then crossed over into the intervention condition and proceeded as described above.
Assessments.
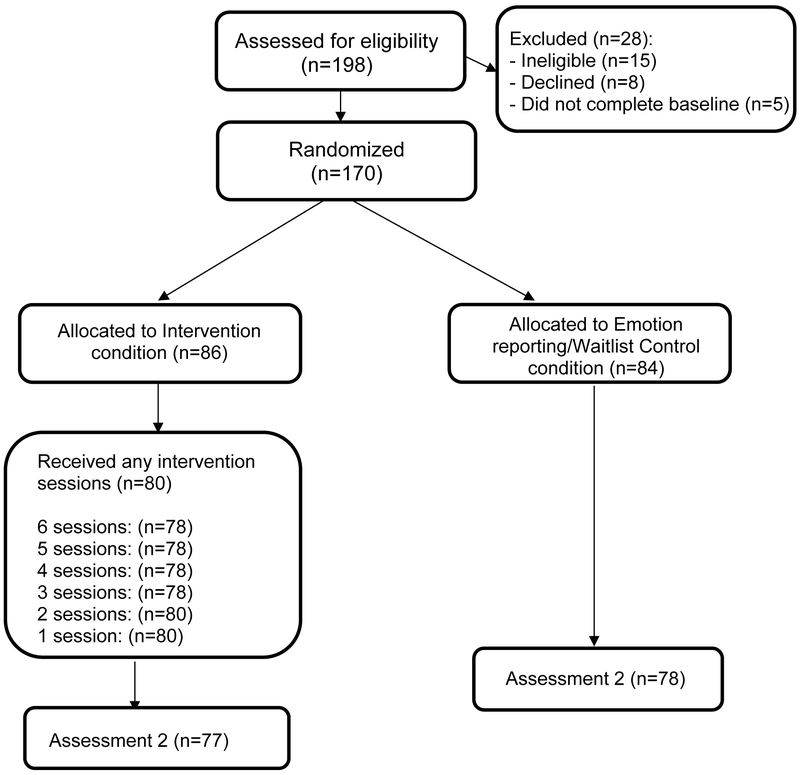
All participants completed assessments at baseline, immediately following the intervention (approximately 6 weeks later), and at three follow up time points: 1 month, 3 months and 6 months post intervention. Emotion reporting/waitlist control participants were assessed at the same intervals: baseline, after an initial wait period of six weeks (equal to the length of the intervention), then at 1-, 3-, and 6-months post intervention. Here we present the data from the first two assessments, prior to the waitlist crossing over to the active LEAF condition. See Figure 1 for CONSORT participant flow diagram.
Figure 1.
CONSORT Participant Flow Diagram.
Measures
Positive and negative emotion.
A modified version of the Differential Emotions Scale (Fredrickson, Tugade, Waugh, & Larkin, 2003) was used to assess positive and negative emotion. The scale includes positive items such as interest, enjoyment, awe, gratitude, hope, and love and negative items such as sadness, anger, disgust, fear, and guilt. In this caregiver sample, the modified DES shows acceptable reliability for both positive emotion (α = .91) and the negative emotion (α = .85.)
Depression.
Emotional Distress – Depression, Patient Reported Outcomes Measurement Information System Item Bank, v. 1.0 (PROMIS; Cella et al., 2010) was used to assess depression. Participants rated 28 items (α = .95) focused on depressive symptoms over the past 7 days.
Anxiety.
The NeuroQOL (Cella et al., 2012) anxiety measure contains 29 items (α = .95) to tap anxiety over the past 7 days. NeuroQOL is one of the HealthMeasures systems, similar to PROMIS.
Global Health.
Participant perceptions of overall physical and mental health were assessed with the Global Health Scale, PROMIS v.1.0/1.1 (Cella et al., 2010) which contains 10 items that are rated on Likert scales reflecting frequency or severity of symptoms and functioning (physical health, α = .68; mental health, α = .77).
Perceived Stress.
The Perceived Stress Scale (PSS;Cohen, 1988) was used to assess stress. The 10 items (α = .88) are designed to identify how unpredictable, uncontrollable and overloaded respondents find their lives.
Dementia Severity.
The Dementia Severity Rating Scale (DSRS; Clark & Ewbank, 1996) was used to characterize the level of functional abilities of the care recipient. The DSRS is comprised of 12 items (α = .90), and scores can range from no impairment (a total score of 0) to extreme impairment for each category assessed (a total score of 54).
Caregiver Burden, Strain, and Positive Aspects of Caregiving.
The Zarit Burden Interview (Zarit, Reever, & Bach-Peterson, 1980) was used to assess caregiver burden. This 22-item inventory (α = .91) assesses caregivers’ subjective feelings of the impact of caregiving on emotional and physical health functioning, social life, and financial status. Caregiver Strain Index (CSI; Robinson, 1983) was used to assess perceived strain. The CSI is a 13-item measure (α = .74) of both objective and subjective elements of caregiver strain. Positive aspects of caregiving was measured with the Positive Aspects of Caregiving scale (Boerner, Schulz, & Horowitz, 2004) an 11-item scale (α = .91) that identifies positive consequences of caregiving such as feeling more useful, feeling appreciated, and strengthening relationships with others.
Analysis
We first examined whether the LEAF and control conditions were comparable at baseline on demographics and outcome variables using t-tests. Next, we conducted intention to treat analyses using multilevel modeling (MLM; Singer & Willett, 2003). MLM offers an approach that accommodates missing data and non-independence in observations. We used MLM to examine whether there were differences in the magnitude of change from the baseline assessment to the post-intervention assessment as a function of intervention condition (LEAF intervention vs. control) as evidenced by a Condition x Time interaction. Specifically, we modeled the fixed effects of time at Level 1 (dummy coded: baseline = 0, post = 1) and intervention condition (dummy coded: control = 0, LEAF intervention = 1) at Level 2. The only random effect that was included in our multilevel models was for the intercept.
Given the LEAF intervention was specifically designed to target positive emotion as the proposed theoretical mechanism, we conducted multilevel moderated mediation analyses (Bauer, Preacher, & Gil, 2006) using a multilevel SEM framework (Preacher, Zyphur, & Zhang, 2010) to examine changes in positive emotion as a mechanism of change. Specifically, we examined whether increases in positive emotion mediated the effects of the intervention for each of the outcome variables that were significantly different between intervention and control conditions. We estimated the specific indirect effects, and conducted Monte Carlo simulations with 20,000 replications to obtain confidence intervals around the indirect effects (MacKinnon, Lockwood, & Williams, 2004; Preacher & Selig, 2012).
Assuming a two-sided alpha of .05 and power of .80, a constant correlation between the repeated assessments of .4, and an overall attrition of 19%, we estimated we would be able to detect a medium effect of d = .5 with a sample size of N = 170.
Results
One hundred and seventy caregivers were randomized to the intervention (N = 86) or the waitlist control (N = 84). Table 1 has the baseline demographics. Participants were aged 34–87 (M = 62.91), 84% were female, and 88% were White. The mean duration of caregiving was 4.2 years with 67% of caregivers being the spouse of the person with dementia. As evidenced in Table 2, correlations of PROMIS and related HealthMeasures outcomes and other indicators of burden, stress, and psychological well-being were highly intercorrelated. Compared to general population norms, baseline scores on depression, anxiety, and perceived stress were significantly higher and global health (both physical and mental health subscales) was significantly lower (Dowling, Verstaen, Snowberg, Merrilees, & Moskowitz, 2017) indicating that caregiving is having a deleterious impact in this sample.
Table 1.
Demographics and Caregiving Characteristics by Condition
| Total Sample | LEAF | Control | |
|---|---|---|---|
| N | 170 | 86 | 84 |
| Age Mean (SD) | 62.91 (9.69) | 63.03 (9.36) | 62.77 (10.09) |
| Patient Dementia Severity at Baseline Mean (SD) | 22.81 (9.51) | 23.12 (9.84) | 22.49 (9.21) |
| Length of caregiver in years Mean (SD) | 4.21 (4.18) | 4.53 (4.31) | 3.89 (3.91) |
| Gender (% Female) | 84.1 | 84.9 | 83.3 |
| Race Ethnicity | |||
| Black/African American (%) | 2.4 | 0 | 4.8 |
| White/European (%) | 88.2 | 91.9 | 84.5 |
| Asian/Asian-American/Pacific Islander (%) | 7.1 | 5.8 | 8.3 |
| American Indian/Eskimo (%) | .6 | 1.2 | 0 |
| Mixed/Other (%) | 1.8 | 1.2 | 2.4 |
| Care Recipient Diagnosis (%) | |||
| Alzheimer’s | 30.0 | 25.6 | 34.5 |
| Frontotemporal Dementia | 11.2 | 12.8 | 9.5 |
| Lewy Body Disease | 8.2 | 12.8 | 3.6 |
| Parkinson’s | 15.3 | 15.1 | 15.5 |
| Other | 35.3 | 33.7 | 36.9 |
| Rurality (%) | |||
| Urban | 50.6 | 48.8 | 52.4 |
| Suburban | 32.4 | 32.6 | 32.1 |
| Rural | 17.1 | 18.6 | 15.5 |
| Relationship to care recipient (%) | |||
| Spouse/sig other | 67.1 | 72.1 | 61.9 |
| Parent (Child?) | 27.1 | 26.7 | 27.4 |
| Other Family member | 4.7 | 1.2 | 8.3 |
| Friend | 1.2 | 0 | 2.4 |
| Education (%) | |||
| < High School | .6 | 1.2 | 0 |
| High School | 5.9 | 8.1 | 3.6 |
| Some College | 14.7 | 18.6 | 10.7 |
| College Graduate | 25.3 | 20.9 | 29.8 |
| Associate Degree | 9.4 | 5.8 | 13.1 |
| Some graduate school | 10.0 | 11,6 | 8.3 |
| Masters | 20.0 | 19.8 | 20.2 |
| Some post Masters work | 3.5 | 5.8 | 1.2 |
| PhD, MD, JD Other | 10.6 | 8.1 | 13.1 |
Note. No group differences were found for any of the demographic variables above.
Table 2.
Baseline Correlations Among Measures
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. PROMIS Depression | - | ||||||||||
| 2. PROMIS Mental Health | −.71** | - | |||||||||
| 3. PROMIS Physical Health | −.57** | .68** | - | ||||||||
| 4. NeuroQOL Anxiety | .71** | −.59** | −.55** | - | |||||||
| 5. Caregiver Burden | .62** | −.59** | −.49** | 60** | - | ||||||
| 6. Caregiver Strain | .48** | −.49** | −.50** | .39** | .64** | - | |||||
| 7. Dementia Severity | .19* | −.27** | −.21** | .05 | .30** | .43** | - | ||||
| 8. DES Positive | −.57** | .56** | .33** | −.43** | −.41** | −.30** | −.16* | - | |||
| 9. DES Negative | .79** | −.61** | −.44** | .65** | .63** | .42** | .16* | −.48** | - | ||
| 10. Positive Aspects of Caregiving | −.38** | .31** | .20** | −.27** | −.48** | −.26** | −.16* | .53** | .37** | - | |
| 11. Perceived Stress | .71** | −.64** | −.53** | .69** | .67** | .50** | .10 | −.49** | .64** | −.43** | - |
Note. p<.05,
p<.01
Caregivers in the intervention and control conditions did not differ on any of the outcome measures at baseline. Retention in the study from baseline to the post-intervention assessment was excellent and did not differ by condition: 89% (77 of 86) of participants in the LEAF condition and 92.8% (78 of 84) of the control completed the second assessment, χ2 = 0.58, p = .44.
In Table 3, we present the estimates and the significance tests for the Condition × Time interactions for all outcome measures of interest. These estimates represent the differences in the magnitude of change between the LEAF group relative to the emotion-reporting/waitlist control group, as modeled by the differences in slopes between the LEAF group relative to the control group. As predicted, analyses of difference in change from baseline to the post intervention assessment demonstrated significantly greater increases in our primary outcome of interest: positive emotion (d = .58; p < .01).
Table 3.
Intervention Outcomes
| Baseline M (SE) |
Post intervention M (SE) |
Overall Effect: Condition × Time Interaction (β11) |
Cohen’s d (CI) | |
|---|---|---|---|---|
| PROMIS depression | ||||
| LEAF | 65.85 (2.02) | 55.18 (2.08) | β11 = −4.70, t(141) = −2.28, p = .02 | −.25 (−.55,.06) |
| Control | 65.62 (2.04) | 59.65 (2.15) | ||
| PROMIS Mental Health | ||||
| LEAF | 37.4 (4.10) | 39.8 (4.10) | β11 = −.08, t(160) = −0.70, p = .49 | .21 (−.09,.52) |
| Control | 39.8 (4.10) | 39.8 (4.10) | ||
| PROMIS Physical Health | ||||
| LEAF | 42.3 (4.20) | 44.9 (4.30) | β11 = 0.59, t(146) = 2.24, p = .03 | .24 (−.07,.54) |
| Control | 44.9 (4.30) | 44.9 (4.30) | ||
| NeuroQOL Anxiety | ||||
| LEAF | 52.15 (1.63) | 44.64 (1.68) | β11 = −5.04, t(145) = −2.63, p = .009 | −.32 (−.63,−.02) |
| Control | 51.38 (1.65) | 48.91 (1.76) | ||
| Zarit Caregiver Burden | ||||
| LEAF | 42.19 (1.58) | 38.18 (1.60) | β11 = −2.38, t(142) = −1.86, p = .066 | −.16 (−.46,.14) |
| Control | 42.18 (1.59) | 41.13 (1.65) | ||
| Caregiver Strain | ||||
| LEAF | 8.40 (0.33) | 8.46 (0.33) | β11 = .05, t(160) = 0.33, p = .74 | .01 (−.30,.30) |
| Control | 8.061 (0.35) | 8.55 (0.33) | ||
| Dementia Scale | ||||
| LEAF | 23.12 (1.07) | 23.42 (1.09) | β11 = −0.27, t(154) = −0.37, p = .71 | −0.03 (−.33, 27) |
| Control | 22.49 (1.09) | 23.06 (1.10) | ||
| DES Positive | ||||
| LEAF | 4.64 (0.16) | 4.86 (0.16) | β11 = .84, t(148) = 4.03, p < .001 | .58 (.27,.88) |
| Control | 4.92 (0.17) | 4.31 (0.17) | ||
| DES Negative | ||||
| LEAF | 3.66 (0.13) | 2.6 (0.13) | β11 = −.27, t(148) = −1.51, p = .13 | −.22 (−.52,.08) |
| Control | 3.70 (0.13) | 2.91 (0.14) | ||
| Positive Aspects of Caregiving | ||||
| LEAF | 35.92 (1.09) | 39.04 (1.12) | β11 = 3.59, t(140) = 4.42, p = .001 | .35 (.05,.66) |
| Control | 26.88 (1.11) | 36.41 (1.16) | ||
| Perceived Stress | ||||
| LEAF | 30.64 (0.71) | 27.48 (0.72) | β11 = −1.32, t(145) = −1.66, p = .10 | −.20 (−.50,.11) |
| Control | 31.08 (0.73) | 29.24 (0.76) |
Notes. Baseline sample size=170, post-intervention sample size=155.
In addition, the intervention also showed greater improvements in secondary outcomes including greater decreases in depression, (d = −.25; p = .02) in the LEAF condition compared to the emotion-reporting/waitlist control. The LEAF group showed a 10.67 point decrease, a full standard deviation, on PROMIS depression. In other words, participants in the intervention group decreased from showing moderate symptoms of depression relative to the population norm, to falling within the normal range of depressive symptoms by the post-intervention assessment. In contrast, participants in the control condition showed a smaller decrease in depression scores (M = 6.97 point decrease), which corresponds to a 0.5 SD decrease, remaining within the mild to moderate range (scores between 55–60).
Participants in the LEAF condition also showed significantly greater decreases in anxiety (d = −.33; p< .01) from baseline to the post intervention assessment, compared to the emotion-reporting/waitlist control. In the current sample, participants in both the intervention and control groups began the study with levels of anxiety that were comparable to the population norm of 50 (MLEAF = 52.15 and MControl = 51.38). Nevertheless, participants in LEAF showed a 7.51 point decrease in their NeuroQOL anxiety T-scores from baseline to post, which corresponds to a 0.75 SD decrease.
Participants in the LEAF condition showed greater improvements in physical health (d = .24; p = .02) from baseline to the post intervention assessment, compared to the emotion-reporting/waitlist control. In addition, the intervention also showed greater improvements in positive aspects of caregiving (d = .36; p < .01). Of note, effects on caregiving burden (d = −.16; p = .07) and perceived stress (d = −.20; p=.10) were in the hypothesized direction but did not reach statistical significance, and LEAF appeared to have negligible effects on caregiver strain, negative emotion, and reports of dementia severity.
Multilevel moderated mediation analyses demonstrated that increased positive emotion significantly mediated the effect of LEAF on depression over time (See Table 4). As reported above, there was a significant direct effect of LEAF on change in depression from baseline to post intervention as well as a significant direct effect of the intervention on change in positive emotion. The change in positive emotion significantly predicted change in depression, b = 2.05, Z = 3.08, p =.002, and when change in positive emotion was entered into the model simultaneously with intervention condition, the total direct effect of the intervention on depression was reduced to non-significance, b = −2.34, Z = −1.08, p = .28. The indirect effect for increased positive emotion mediating the effect of the intervention on depression was significant, 1.71 95% CI [0.63, 2.79], p = .009. As seen in Table 4, the indirect effects for the remaining outcomes were not significant, ps > .24.
Table 4.
Results of the Multilevel Moderated Mediation Analyses Examining Whether Increased Positive Emotion Mediates the Intervention Effects
| Outcome | Indirect Effect (SE) | 95% CI | Z | p |
|---|---|---|---|---|
| PROMIS Depression | 1.71 (0.66) | [0.63, 2.79] | 2.60 | .009 |
| PROMIS Physical Health | 0.01 (0.09) | [−0.13, 0.16] | 0.17 | .86 |
| NeuroQOL Anxiety | 0.75 (0.64) | [−0.30, 1.80] | 1.18 | .24 |
| Zarit Burden Interview | −0.23 (0.42) | [−0.92, 0.46] | −0.54 | .59 |
| Positive Aspects of Caregiving | −0.32 (0.38) | [−0.95, 0.30] | −0.85 | .40 |
Note.
Discussion
The stress of dementia caregiving is associated with a range of physical and psychological health problems and has a deleterious impact on caregiving quality as well as quality of life for the care recipient. Interventions for dementia caregivers have primarily focused on education and skills training with the goal of reducing negative emotions and burden. However, over the past few decades, it has become clear that positive emotions are uniquely related to better psychological and physical well-being, independent of the effects of negative emotion, suggesting that an intervention that specifically targets positive emotion holds promise for improving caregiver well-being and, ultimately, quality of care for the individual living with dementia.
The present randomized controlled trial in dementia caregivers showed that, compared to an emotion reporting control condition, the LEAF positive emotion regulation intervention led to significantly greater increases in the primary outcome of interest, positive emotion, as well as improvements on secondary outcomes including increases in positive aspects of caregiving, decreases in depression and anxiety, and improvements in self-reported physical health. Effects on caregiving burden and perceived stress approached significance. Consistent with the theoretical foundation of the LEAF intervention (Folkman, 1997; Fredrickson, 1998), increases in positive emotion mediated the effects of the intervention on depression. The study was of high quality meeting five of the six Cochrane collaboration quality criteria (Higgins & Green, 2008): 1) randomization concealment, 2) baseline comparability of groups, 3) power analysis and at least 50 participants in the analysis, 4) loss to follow up < 50%, and 5) the use of intent-to-treat analyses. The 6th criteria, blinding of subjects to condition, was not possible once participants started sessions in their assigned condition, although they were blind as to details of the content of the conditions at the time of enrollment and randomization.
Valid and reliable measurement is another important consideration for study quality. The present study included PROMIS and related NeuroQOL self-report measures that follow a systematic and well-documented approach and set of standards for development of new measures. Investigators follow an established protocol that includes guidelines for every step of the process from defining the target concept and conceptual model, composing individual items, constructing and testing the item pool, to determining validity, reliability, interpretability, language translation, and cultural adaptation. The result of this process is a set of valid, highly reliable measures of patient–reported health status for physical, mental, and social well–being. PROMIS measures may be more sensitive to intervention effects than more commonly used caregiving burden/strain measures, and inclusion of highly reliable PROMIS assessments yielded meaningful reports of change. An additional strength of PROMIS is in the scoring metrics that yield easily interpretable T-scores anchored to the distribution of scores in the US general population. This provides meaningful information for our data, because it allows us to have a useful comparison group and to identify the clinical significance of scores.
Overall, the findings of the present study, combined with the growing body of research supporting the efficacy of interventions that focus on positive emotion (e.g., Bolier et al., 2013; Charlson et al., 2007; Charlson et al., 2014; Cheung et al., 2016; Cohn et al., 2014; Moskowitz et al., 2017; Sin & Lyubomirsky, 2009), indicate that such interventions may produce key benefits for individuals coping with health related or other types of life stress. Few randomized trials, however, have examined whether increases in the theorized positive affect target of the intervention actually mediate effects on more distal outcomes such as adherence to recommended health behaviors or depression. For example, Charlson and colleagues tested a positive affect intervention in samples of people with chronic illness and hypothesized that the intervention would have beneficial effects on health behaviors (Boutin-Foster et al., 2016; Mancuso et al., 2012; Ogedegbe et al., 2012; Peterson et al., 2012). Although the intervention was associated with improvements in distal outcomes of achieving exercise recommendations (Peterson et al., 2012) and medication adherence (Ogedegbe et al., 2012), it was not clear whether the intervention influenced positive affect or whether intervention effects were mediated through increased (or maintained) positive affect (Peterson et al., 2013). Moskowitz and colleagues (2017) found that the same positive emotion regulation intervention tested here was associated with improved positive affect, decreased intrusive and avoidant thoughts, and decreased antidepressant use in people newly diagnosed with HIV. However, increased positive emotion did not mediate intervention effects on these outcomes. In contrast, increased positive emotion was a statistically significant mediator of intervention effects on PROMIS depression in the present study.
There are a number of differences between the present sample where positive emotion appears to mediate intervention effects on depression and our previous work where there was no evidence of mediation. First, there are differences in the nature of the stress that may influence the likelihood of the intervention influencing positive emotion, i.e., caregiving is chronic and deteriorating; HIV diagnosis is acute with some chronic elements, and generally improving. There may have been more opportunity for the intervention to influence positive emotion in the caregiving situation that was generally deteriorating compared to HIV diagnosis where the majority of participants show a normative trend toward increased positive emotion as time passes after the diagnosis. A second important difference is in the measures of depression. In the Moskowitz et al (2017) study, in which there was no effect on depression, we used the CES-D. In the present study, we used the PROMIS depression measure and had stronger effects on depression, possible attributable to which may be more precise measurement afforded by the PROMIS measure.
Given that the LEAF intervention was specifically designed to target positive emotion as the proposed theoretical mechanism, we examined changes in positive emotion as a mechanism of change. However, despite finding evidence that positive emotion was a statistically-significant mediator of intervention effects on PROMIS depression, we are limited in the ability to draw causal conclusions about positive emotion as the mechanism of change in this study. Given that we only have data from two assessments collected in the present research (baseline and post), we cannot definitively conclude whether changes in positive emotion temporally preceded changes in depression, or whether changes in depression may have preceded changes in positive emotion (see Winer, Cervone, Bryant, McKinney, Liu, & Nadorff, 2016 for a discussion). Future research that demonstrates change in positive emotion temporally preceding subsequent change in depression would provide a more convincing demonstration of positive emotion as a causal mechanism of change.
To our knowledge, this is the first test of a positive affect regulation intervention in dementia caregivers. The results clearly show that the intervention is acceptable and feasible and holds promise as an efficacious intervention for people in the midst of the stress of caring for a loved one with dementia. Future work should extend to other caregiving groups (e.g. cancer caregivers; (Kent et al., 2016) and explore ways to tailor the intervention content to the individual to potentially increase the strength of the intervention. Furthermore, researchers should consider the possibility of integrating positive emotion skills with other established health behavior interventions to maximize effects on psychological and physical health.
The study had a number of weaknesses. First, the intervention was delivered individually by trained facilitators. Although this individual attention likely contributed to the high levels of retention, such an approach is expensive and may be challenging to implement with fidelity on a wide scale. Future studies should address the question of whether self-guided versions of the intervention have a similar impact on caregiver outcomes and explore ways to incorporate LEAF into clinical care, which would enable more cost efficient and wide-spread implementation. In addition, the present study only had the immediate post-intervention follow-up (6 weeks post baseline) before the control condition crossed over into the intervention so we were unable to examine durability of effects. Instead of a waitlist design, researchers should consider other designs in which it is possible to compare differences of effects between intervention and control arms for a longer period of time.
Finally, a weakness that applies to all interventions with a positive focus, is that proclaiming the importance of positive affect in the stress and coping process may appear to minimize the pain and serious individual and societal consequences associated with major stressful events. We are not advocating a simplistic “don’t worry-be happy” approach, nor do we believe that simply increasing positive emotion will prove to be a cure-all for the very real and complex issues facing dementia caregivers. Such a stance could easily degenerate into blaming the victim for not thinking the positive thoughts that may prevent depression or other negative consequence of enduring stress. However, the present study demonstrates that an intervention that targets positive emotions in caregivers sets the stage for a cascade of adaptive consequences, including reduced depression. Ultimately, given the high levels of stress and depression documented in dementia caregivers, we consider increasing positive emotion to be an inherently worthwhile intervention goal.
Acknowledgments
This work was supported by the National Institute of Nursing Research under Grant R01NR014435 to Glenna A. Dowling and Judith T. Moskowitz.
Footnotes
TRIAL REGISTRATION: ClinicalTrials.gov NCT01825681
References
- Alzheimer’s Association, (2017). Retrieved from http://www.alz.org/
- Bauer DJ, Preacher KJ, & Gil KM (2006). Conceptualizing and testing random indirect effects and moderated mediation in multilevel models: new procedures and recommendations. Psychological methods, 11(2), 142. [DOI] [PubMed] [Google Scholar]
- Boerner K, Schulz R, & Horowitz A (2004). Positive aspects of caregiving and adaptation to bereavement. Psychology and Aging, 19(4), 668. [DOI] [PubMed] [Google Scholar]
- Bolier L, Haverman M, Westerhof GJ, Riper H, Smit F, & Bohlmeijer E (2013). Positive psychology interventions: a meta-analysis of randomized controlled studies. BMC public health, 13(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin-Foster C, Offidani E, Kanna B, Ogedegbe G, Ravenell J, Scott E, . . . Gerber LM (2016). Results from the Trial Using Motivational Interviewing, Positive Affect, and Self-Affirmation in African Americans with Hypertension (TRIUMPH). Ethnicity & disease, 26(1), 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caponigro JM, Moran EK, Kring AM, & Moskowitz JT (2013). Awareness and coping with emotion in schizophrenia: Acceptability, feasibility and case illustrations. Clinical psychology & psychotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Gómez W, Siever MD, Discepola MV, Dilworth SE, & Moskowitz JT (2015). Pilot randomized controlled trial of an integrative intervention with methamphetamine-using men who have sex with men. Archives of Sexual Behavior, 44(7), 1861–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, & Scheier MF (1994). Situational coping and coping dispositions in a stressful transaction. Journal of personality and social psychology, 66, 184–195. [DOI] [PubMed] [Google Scholar]
- Cella D, Lai J-S, Nowinski C, Victorson D, Peterman A, Miller D, . . . Cavazos J. (2012). Neuro-QOL Brief measures of health-related quality of life for clinical research in neurology. Neurology, 78(23), 1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, . . . Choi S. (2010). The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of clinical epidemiology, 63(11), 1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, . . . Rose M. (2007). The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Medical care, 45(5 Suppl 1), S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ME, Boutin-Foster C, Mancuso CA, Peterson JC, Ogedegbe G, Briggs WM, . . . Allegrante JP (2007). Randomized controlled trials of positive affect and self-affirmation to facilitate healthy behaviors in patients with cardiopulmonary diseases: rationale, trial design, and methods. Contemp Clin Trials, 28(6), 748–762. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Wells MT, Peterson JC, Boutin-Foster C, Ogedegbe GO, Mancuso CA, . . . Isen AM (2014). Mediators and moderators of behavior change in patients with chronic cardiopulmonary disease: the impact of positive affect and self-affirmation. Translational behavioral medicine, 4(1), 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattillion EA, Mausbach BT, Roepke SK, von Känel R, Mills PJ, Dimsdale JE, . . . Ancoli-Israel S. (2012). Leisure activities, caregiving demands and catecholamine levels in dementia caregivers. Psychology & Health, 27(10), 1134–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung EO, Cohn MA, Dunn LB, Melisko ME, Morgan S, Penedo FJ, . . . Moskowitz JT (2016). A randomized pilot trial of a positive affect skill intervention (lessons in linking affect and coping) for women with metastatic breast cancer. Psycho-Oncology, n/a-n/a. doi: 10.1002/pon.4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung EO, Cohn MA, Dunn LB, Melisko ME, Morgan S, Penedo FJ, . . . Moskowitz JT (2016). A Randomized Pilot Trial of a Positive Affect Skills Intervention (LILAC) for Women with Metastatic Breast Psycho-Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, & Steptoe A (2008). Positive psychological well-being and mortality: A quantitative review of prospective observational studies. Psychosomatic medicine, 70, 741–756. [DOI] [PubMed] [Google Scholar]
- Chu P, Edwards J, Levin R, & Thomson J (2000). The use of clinical case management for early stage Alzheimer’patients and their families. American Journal of Alzheimer’s Disease and Other Dementias, 15(5), 284–290. [Google Scholar]
- Clark CM, & Ewbank DC (1996). Performance of the dementia severity rating scale: a caregiver questionnaire for rating severity in Alzheimer disease. Alzheimer Disease & Associated Disorders, 10(1), 31–39. [PubMed] [Google Scholar]
- Cohen S (1988). Perceived stress in a probability sample of the United States In Spacapan S & Oskamp S (Eds.), The social psychology of health. The Claremont Symposium on Applied Social Psychology (pp. 31–67). Thousand Oaks, CA: Sage Publications, Inc. [Google Scholar]
- Cohn MA, Pietrucha ME, Saslow LR, Hult JR, & Moskowitz JT (2014). An online positive affect skills intervention reduces depression in adults with type 2 diabetes. The Journal of Positive Psychology, 9(6), 523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, Muñoz RF, Clarke GN, & Lewinsohn PM (2009). Psychoeducational treatment and prevention of depression: the “Coping with Depression” course thirty years later. Clinical psychology review, 29(5), 449–458. [DOI] [PubMed] [Google Scholar]
- Daly BJ, Douglas S, Lipson A, & Foley H (2009). Needs of older caregivers of patients with advanced cancer. Journal of the American Geriatrics Society, 57(s2), s293–s295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling GA, Merrilees J, Mastick J, Chang VY, Hubbard E, & Moskowitz JT (2014). Life enhancing activities for family caregivers of people with frontotemporal dementia. Alzheimer Disease & Associated Disorders, 28(2), 175–181. [DOI] [PubMed] [Google Scholar]
- Dowling GA, Verstaen A, Snowberg K, Merrilees J, & Moskowitz JT (2017). Family Caregivers of People with Dementia have Poorer Psychological and Physical Health Outcomes Compared to Non-caregivers. Neurodegener Dis 17 (suppl 1), 1827. [Google Scholar]
- Dunn EW, Aknin LB, & Norton MI (2008). Spending money on others promotes happiness. Science, 319(5870), 1687–1688. [DOI] [PubMed] [Google Scholar]
- Emmons RA (1986). Personal strivings: An approach to personality and subjective well-being. Journal of Personality & Social Psychology, 51(5), 1058–1068. [Google Scholar]
- Emmons RA (1992). Abstract versus concrete goals: Personal striving level, physical illness, and psychological well-being. Journal of personality and social psychology, 62, 292–300. [DOI] [PubMed] [Google Scholar]
- Emmons RA (2007). Thanks! how the new science of gratitude can make you happier. New York: Houghton Mifflin. [Google Scholar]
- Emmons RA, & McCullough ME (2003). Counting blessings versus burdens: An experimental investigation of gratitude and subjective well-being in daily life. Journal of personality and social psychology, 84, 377–389. [DOI] [PubMed] [Google Scholar]
- Fianco A, Sartori RD, Negri L, Lorini S, Valle G, & Delle Fave A (2015). The relationship between burden and well-being among caregivers of Italian people diagnosed with severe neuromotor and cognitive disorders. Research in developmental disabilities, 39, 43–54. [DOI] [PubMed] [Google Scholar]
- Folkman S (1997). Positive psychological states and coping with severe stress. Social Science and Medicine, 45, 1207–1221. [DOI] [PubMed] [Google Scholar]
- Folkman S, & Moskowitz JT (2000). Positive affect and the other side of coping. American Psychologist, 55(6), 647–654. doi: 10.1037//0003-066x.55.6.647 [DOI] [PubMed] [Google Scholar]
- Fredrickson BL (1998). What good are positive emotions? Review of General Psychology, 2, 300–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Cohn MA, Coffey KA, Pek J, & Finkel SM (2008). Open hearts build lives: Positive emotions, induced through meditation, build consequential personal resources. Journal of personality and social psychology, 95, 1045–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Tugade MM, Waugh CE, & Larkin GR (2003). What good are positive emotions in crises? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. Journal of Personality and Social Psychlogy, 84, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin J-P, Glaser R, Malarkey WB, Beversdorf D, & Kiecolt-Glaser J (2012). Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychology, 31(2), 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Tiefenthaler-Gilmer U, Raysz A, & Kesper U (2007). Mindfulness training as an intervention for fibromyalgia: Evidence of postintervention and 3-year follow-up benefits in well-being. Psychotherapy and Psychosomatics, 76, 226–233. [DOI] [PubMed] [Google Scholar]
- Hébert R, Dubois M-F, Wolfson C, Chambers L, & Cohen C (2001). Factors associated with long-term institutionalization of older people with dementia Data from the Canadian Study of Health and Aging. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 56(11), M693–M699. [DOI] [PubMed] [Google Scholar]
- Huffman JC, DuBois CM, Millstein RA, Celano CM, & Wexler D (2015). Positive Psychological Interventions for Patients with Type 2 Diabetes: Rationale, Theoretical Model, and Intervention Development. Journal of diabetes research, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JC, Mastromauro CA, Boehm JK, Seabrook R, Fricchione GL, Denninger JW, & Lyubomirsky S (2011). Development of a positive psychology intervention for patients with acute cardiovascular disease. Heart International, 6(2), e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J (2003). Mindfulness-Based interventions in context: Past, present, and future. Clinical Psychology: Science and Practice, 10, 144–156. [Google Scholar]
- Kasper JD, Freedman VA, Spillman BC, & Wolff JL (2015). The disproportionate impact of dementia on family and unpaid caregiving to older adults. Health Affairs, 34(10), 1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent EE, Rowland JH, Northouse L, Litzelman K, Chou WYS, Shelburne N, . . . Huss K. (2016). Caring for caregivers and patients: research and clinical priorities for informal cancer caregiving. Cancer, 122(13), 1987–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, & Glaser R (1991). Spousal caregivers of dementia victims: longitudinal changes in immunity and health. Psychosomatic medicine, 53(4), 345–362. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Marucha PT, Mercado A, Malarkey WB, & Glaser R (1995). Slowing of wound healing by psychological stress. The Lancet, 346(8984), 1194–1196. [DOI] [PubMed] [Google Scholar]
- Krentzman AR, Mannella KA, Hassett AL, Barnett NP, Cranford JA, Brower KJ, . . . Meyer PS (2015). Feasibility, acceptability, and impact of a web-based gratitude exercise among individuals in outpatient treatment for alcohol use disorder. The Journal of Positive Psychology, 10(6), 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston CA (1994). Capitalizing on and coping with daily-life events: Expressive responses to positive events. Journal of personality and social psychology, 67, 1112–1125. [Google Scholar]
- Liu B, Floud S, Pirie K, Green J, Peto R, Beral V, & Collaborators MWS (2016). Does happiness itself directly affect mortality? The prospective UK Million Women Study. The Lancet, 387(10021), 874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubomirsky S, King L, & Diener E (2005). The benefits of frequent positive affect: does happiness lead to success? Psychological Bulletin, 131(6), 803–855. doi: 10.1037/0033-2909.131.6.803 [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, & Williams J (2004). Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate behavioral research, 39(1), 99–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso CA, Choi TN, Westermann H, Wenderoth S, Hollenberg JP, Wells MT, . . . Charlson ME (2012). Increasing physical activity in patients with asthma through positive affect and self-affirmation: A randomized trial. Archives of internal medicine, archinternmed. 20111316 v2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach BT, Chattillion E, Roepke SK, Ziegler MG, Milic M, von Känel R, . . . Allison MA (2012). A longitudinal analysis of the relations among stress, depressive symptoms, leisure satisfaction, and endothelial function in caregivers. Health Psychology, 31(4), 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach BT, Coon DW, Patterson TL, & Grant I (2008). Engagement in activities is associated with affective arousal in Alzheimer’s caregivers: A preliminary examination of the temporal relations between activity and affect. Behavior Therapy, 39, 366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach BT, Roepke SK, Depp CA, Patterson TL, & Grant I (2009). Specificity of cognitive and behavioral variables to positive and negative affect. Behaviour Research and Therapy, 47, 608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallion P, Toseland RW, & Freeman K (1999). An evaluation of a family visit education program. Journal of the American Geriatrics Society, 47(2), 203–214. [DOI] [PubMed] [Google Scholar]
- McCurry SM, Logsdon RG, Vitiello MV, & Teri L (1998). Successful behavioral treatment for reported sleep problems in elderly caregivers of dementia patients: a controlled study. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 53(2), P122–P129. [DOI] [PubMed] [Google Scholar]
- Mittelman MS, Ferris SH, Shulman E, Steinberg G, & Levin B (1996). A family intervention to delay nursing home placement of patients with Alzheimer disease: a randomized controlled trial. Jama, 276(21), 1725–1731. [PubMed] [Google Scholar]
- Mittelman MS, Haley WE, Clay OJ, & Roth DL (2006). Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology, 67(9), 1592–1599. [DOI] [PubMed] [Google Scholar]
- Moen P, Dempster-McCain D, & Williams RM (1993). Successful aging. American Journal of Sociology, 97, 1612–1632. [Google Scholar]
- Morris RG, Woods RT, Davies KS, Berry J, & Morris LW (1992). The use of a coping strategy focused support group for carers of dementia sufferers. Counselling Psychology Quarterly, 5(4), 337–348. [Google Scholar]
- Moskowitz JT (2003). Positive Affect Predicts Lower Risk of AIDS Mortality. Psychosomatic medicine, 65(4), 620–626. doi: 10.1097/01.psy.0000073873.74829.23 [DOI] [PubMed] [Google Scholar]
- Moskowitz JT, Carrico AW, Cohn MA, Duncan LG, Bussolari C, Layous K, . . . Folkman S (2014). Randomized controlled trial of a positive affect intervention to reduce stress in people newly diagnosed with HIV; protocol and design for the IRISS study. Open Access Journal of Clinical Trials, 6. [Google Scholar]
- Moskowitz JT, Carrico AW, Duncan LG, Cohn MA, Cheung EO, Batchelder A, . . . Folkman S. (2017). Randomized controlled trial of a positive affect intervention for people newly diagnosed with HIV. Journal of Consulting and Clinical Psychology, Vol 85(5), 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz JT, Epel ES, & Acree M (2008). Positive affect uniquely predicts lower risk of mortality in people with diabetes. Health psychology : official journal of the Division of Health Psychology, American Psychological Association, 27(1 Suppl), S73–82. doi: 10.1037/0278-6133.27.1.S73 [DOI] [PubMed] [Google Scholar]
- Moskowitz JT, Hult JR, Duncan LG, Cohn MA, Maurer SA, Bussolari C, & Acree M (2012). A Positive Affect Intervention for People Experiencing Health-Related Stress: Development and Non-Randomized Pilot Test. Journal of health psychology, 17(5), 677–693. doi: 10.1177/1359105311425275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musick MA, & Wilson J (2003). Volunteering and depression: the role of psychological and social resources in different age groups. Social Science & Medicine, 56, 259–269. [DOI] [PubMed] [Google Scholar]
- Ogedegbe GO, Boutin-Foster C, Wells MT, Allegrante JP, Isen AM, Jobe JB, & Charlson ME (2012). A randomized controlled trial of positive-affect intervention and medication adherence in hypertensive African Americans. Archives of internal medicine, 172(4), 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oman D, Thoresen CE, & McMahon K (1999). Volunteerism and mortality among the community-dwelling elderly. Journal of health psychology, 4, 301–316. [DOI] [PubMed] [Google Scholar]
- Park-Lee E, Fredman L, Hochberg M, & Faulkner K (2009). Positive affect and incidence of frailty in elderly women caregivers and noncaregivers: Results of Caregiver–Study of Osteoporotic Fractures. Journal of the American Geriatrics Society, 57(4), 627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JC, Charlson ME, Hoffman Z, Wells MT, Wong S-C, Hollenberg JP, . . . Allegrante JP (2012). Randomized controlled trial of positive affect induction to promote physical activity after percutaneous coronary intervention. Archives of internal medicine, archinternmed. 20111311 v2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JC, Czajkowski S, Charlson ME, Link AR, Wells MT, Isen AM, . . . Ogedegbe G. (2013). Translating basic behavioral and social science research to clinical application: The EVOLVE mixed methods approach. Journal of consulting and clinical psychology, 81(2), 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Selig JP (2012). Advantages of Monte Carlo confidence intervals for indirect effects. Communication Methods and Measures, 6(2), 77–98. [Google Scholar]
- Preacher KJ, Zyphur MJ, & Zhang Z (2010). A general multilevel SEM framework for assessing multilevel mediation. Psychological methods, 15(3), 209. [DOI] [PubMed] [Google Scholar]
- Pressman SD, & Cohen S (2005). Does positive affect influence health? Psychological Bulletin, 131(6), 925–971. doi: 10.1037/0033-2909.131.6.925 [DOI] [PubMed] [Google Scholar]
- Quayhagen MP, & Quayhagen M (1989). Differential effects of family-based strategies on Alzheimer’s disease. The Gerontologist, 29(2), 150–155. [DOI] [PubMed] [Google Scholar]
- Robinson BC (1983). Validation of a caregiver strain index. Journal of gerontology, 38(3), 344–348. [DOI] [PubMed] [Google Scholar]
- Roepke SK, Allison M, Von Känel R, Mausbach BT, Chattillion EA, Harmell AL, . . . Ziegler MG (2012). Relationship between chronic stress and carotid intima-media thickness (IMT) in elderly Alzheimer’s disease caregivers. Stress, 15(2), 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MM, Flood LS, Gasiewicz NK, Rovin R, & Conklin S (2015). Validation of the National Institutes of Health Patient-Reported Outcomes Measurement Information System Survey as a Quality-of-Life Instrument for Patients with Malignant Brain Tumors and Their Caregivers. Nursing Clinics, 50(4), 679–690. [DOI] [PubMed] [Google Scholar]
- Schulz R, & Beach SR (1999). Caregiving as a risk factor for mortality. JAMA, 282, 2215–2219. [DOI] [PubMed] [Google Scholar]
- Sears SR, Stanton AL, & Danoff-Burg S (2003). The yellow brick road and the emerald city: Benefit finding, positive reappraisal coping and posttraumatic growth in women with early-stage breast cancer. Health Psychology, 22(5), 487–497. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Steen TA, Park N, & Peterson C (2005). Positive psychology progress: empirical validation of interventions. The American psychologist, 60(5), 410–421. doi: 10.1037/0003-066X.60.5.410 [DOI] [PubMed] [Google Scholar]
- Shapiro M, Brown KW, & Biegel GM (2007). Teaching self-care to caregivers: Effects of mindfulness-based stress reduction on the mental health of therapists in training. Training and Education in Professional Psychology, 1, 105–115. [Google Scholar]
- Sheldon KM, & Houser-Marko L (2001). Self-concordance, goal attainment, and the pursuit of happiness: Can there be an upward spiral? Journal of personality and social psychology, 80, 152–165. [PubMed] [Google Scholar]
- Sin NL, & Lyubomirsky S (2009). Enhancing well-being and alleviating depressive symptoms with positive psychology intervention: A practice-friendly meta-analysis. Journal of Clinical Psychology, 65, 467–487. [DOI] [PubMed] [Google Scholar]
- Singer JD, & Willett JB (2003). Applied longitudinal data analysis: Modeling change and event occurrence: Oxford university press. [Google Scholar]
- Steptoe A, & Wardle J (2011). Positive affect measured using ecological momentary assessment and survival in older men and women. Proceedings of the National Academy of Sciences of the United States of America, 108(45), 18244–18248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Lerner JSS, D.K., Sage RM, & McDowell N. K. l. (2003). Are self-enhancing cognitions associated with healthy or unhealthy biological profiles?. Journal of personality and social psychology, 85, 605–615. [DOI] [PubMed] [Google Scholar]
- Taylor SE, & Lobel M (1989). Social comparison activity under threat: Downward evaluation and upward contacts. Psychological Review, 96, 569–575. [DOI] [PubMed] [Google Scholar]
- Tice DM, Baumeister RF, Shmueli D, & Muraven M (2007). Restoring the self: Positive affect helps improve self-regulation following ego depletion. Journal of Experimental Social Psychology, 43, 379–384. [Google Scholar]
- Trapp SK, Perrin PB, Aggarwal R, Peralta SV, Stolfi ME, Morelli E, . . . Arango-Lasprilla JC (2015). Personal strengths and health related quality of life in dementia caregivers from Latin America. Behavioural neurology, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstaen A, Moskowitz JT, Snowberg KE, Merrilees J, & Dowling GA (2018). Life Enhancing Activities for Family Caregivers of people with dementia: protocol for a randomized controlled trial of a positive affect skills intervention. Open Access Journal of Clinical Trials, 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Känel R, Dimsdale JE, Mills PJ, Ancoli-Israel S, Patterson TL, Mausbach BT, & Grant I (2006). Effect of Alzheimer caregiving stress and age on frailty markers interleukin-6, C-reactive protein, and D-dimer. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 61(9), 963–969. [DOI] [PubMed] [Google Scholar]
- von Känel R, Mausbach BT, Dimsdale JE, Mills PJ, Patterson TL, Ancoli-Israel S, . . . Allison M. (2012). Effect of chronic dementia caregiving and major transitions in the caregiving situation on kidney function: A longitudinal study. Psychosomatic medicine, 74(2), 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers MC, Myin-Germeys I, Jacobs N, Peeters F, Kenis G, Derom C, . . . van Os J. (2007). Evidence that moment-to-moment variation in positive emotions buffer genetic risk for depression: a momentary assessment twin study. Acta psychiatrica Scandinavica, 115(6), 451–457. doi: 10.1111/j.1600-0447.2006.00924.x [DOI] [PubMed] [Google Scholar]
- Winer ES, Cervone D, Bryant J, McKinney C, Liu RT, & Nadorff MR (2016). Distinguishing mediational models and analyses in clinical psychology: atemporal associations do not imply causation. Journal of Clinical Psychology, 72(9), 947–955. [DOI] [PubMed] [Google Scholar]
- Zarit SH, Anthony CR, & Boutselis M (1987). Interventions with care givers of dementia patients: comparison of two approaches. Psychology and aging, 2(3), 225. [DOI] [PubMed] [Google Scholar]
- Zarit SH, Reever KE, & Bach-Peterson J (1980). Relatives of the impaired elderly: correlates of feelings of burden. The gerontologist, 20(6), 649–655. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Johnson LM, & Davis MC (2005). Positive affect as a source of resilience for women in chronic pain. Journal of consulting and clinical psychology, 73(2), 212–220. doi: 10.1037/0022-006X.73.2.212 [DOI] [PMC free article] [PubMed] [Google Scholar]