Abstract
Background:
Concentrations of circulating apolipoproteins are strongly linked to risk for coronary artery disease (CAD). Relative importance of the additional knowledge of apolipoprotein concentrations within specific lipoprotein species for CAD risk prediction is limited.
Objectives:
The authors sought to evaluate the performance of an HDL apolipoproteomic score, based on targeted mass spectrometry of HDL-associated apolipoproteins, for the detection of angiographic CAD and outcomes.
Methods:
HDL-associated apoA-1, apoC-1, apoC-2, apoC-3, and apoC-4 were measured in 943 participants without prevalent myocardial infarction (MI) referred for coronary angiography in the Catheter Sampled Blood Archive in Cardiovascular Diseases (CASABLANCA) study. A composite HDL apolipoproteomic score (pCAD) was associated with likelihood of obstructive CAD (≥70% lesion in ≥1 vessel) and with incident cardiovascular outcomes over 4-year follow-up.
Results:
There were 587 (62.2%) patients with coronary stenosis. The pCAD score was associated with the presence of obstructive CAD (OR: 1.39; 95% confidence interval [CI]: 1.14–1.69; P<0.001), independently of conventional cardiovascular risk factors including circulating plasma apoA-1 and apoB. The C-index for pCAD was 0.63 (95% CI: 0.59–0.67) for the presence of obstructive CAD. While pCAD was not associated with cardiovascular mortality among all individuals (HR: 1.24, 95% CI: 0.93–1.66, P=0.15), there was evidence of association for individuals with obstructive CAD (HR: 1.48; 95% CI: 1.07–2.05, P=0.019).
Conclusions:
An HDL apolipoproteomic score is associated with the presence of CAD, independently of circulating apoA-1 and apoB concentrations and other conventional cardiovascular risk factors. Among individuals with CAD, this score may be independently associated cardiovascular death. (CASABLANCA; NCT00842868)
Clinical Trial:
CASABLANCA, https://clinicaltrials.gov/ct2/show/NCT00842868, NCT00842868
Keywords: coronary artery disease, lipids, HDL, prevention, proteomics
Condensed Abstract:
Plasma lipids are strongly predictive for coronary artery disease (CAD) but are comprised of various molecules, including apolipoproteins which determine their functions. Whether knowledge of apolipoprotein concentrations within plasma lipids has incremental clinical utility is unknown. In this study, we used a novel approach to profile the apolipoproteins within high-density lipoprotein (HDL), an important reservoir of apolipoproteins. An HDL apolipoproteomic score (pCAD) was independently associated with angiographic CAD and, among those with CAD, was associated with future risk of cardiovascular death. These findings suggest that pCAD may have utility for CAD diagnosis as well as prognosis when CAD is present.
Introduction
Genetic or pharmacologic modulation of high-density lipoprotein (HDL) cholesterol (HDL-C) concentrations do not appreciably alter CAD risk (1–3). Nevertheless, recent evidence suggests that genetic variation influencing plasma triglyceride-rich lipoproteins (TRLs) influences risk of CAD (4,5). A better understanding of plasma lipid biology might therefore better inform risk.
Plasma lipoproteins are complex and comprise of various molecules, including apolipoproteins, cholesterol esters, and triglycerides. Apolipoprotein B (apoB) is the primary determinant of atherogenic lipoproteins, including low-density lipoprotein (LDL), and apolipoprotein A-1 (apoA-1) is the primary determinant of HDL. The ratio of circulating apoB/apoA-1 outperforms conventional plasma lipid measures for the prediction of CAD events (6). Recently, multiple-reaction monitoring mass spectrometry was used to evaluate 13 circulating apolipoproteins with cardiovascular disease events among 688 individuals.(7) These data demonstrated independent associations for circulating apoC-2, apoC-3, and apoE, which are primarily linked to very low-density lipoprotein (VLDL), a TRL.
The presence of apolipoproteins within plasma lipid particles themselves is not static, contributing to the heterogeneity of plasma lipids themselves, with potential clinical consequences.(8) HDL is an important circulating reservoir of apolipoproteins and cholesterol esters, shuttling these molecules with atherogenic lipoproteins as well as to the liver for excretion.(9) To investigate the clinical potential of apoA-1-associated proteins in relationship to CAD, we developed, characterized, and validated a rapid enrichment technique complemented by a mass spectrometry-based discovery pipeline according to best practices.(10,11) A composite HDL apolipoproteomic score (pCAD), based on five apolipoproteins within HDL (i.e., apoA-1, apoC-1, apoC-2, apoC-3, and apoC-4), was separately derived to distinguish individuals with angiographic CAD from controls (12).
These technological advances permitted us to 1) efficiently and reliably characterize the HDL apolipoproteome in nearly 1,000 patients, and 2) evaluate these features with both the presence of CAD defined by coronary angiography and with incident cardiovascular death. We hypothesized that the information contained within the pCAD score would be associated with the presence of CAD and incident cardiovascular events, even when accounting for traditional cardiovascular risk factors, including circulating apoB and apoA-1.
Methods
CASABLANCA study
The design of the Catheter Sampled Blood Archive in Cardiovascular Diseases (CASABLANCA) (ClinicalTrials.gov NCT00842868) study has previously been described.(13) The study was approved by the Partners HealthCare/Massachusetts General Hospital Institutional Review Board, and all study participants provided written informed consent. Briefly, 1,251 patients aged ≥ 18 years referred for coronary or peripheral angiography with or without intervention between 2008 and 2011 at the Massachusetts General Hospital (Boston, MA) were prospectively enrolled. Angiography referral indications included acute myocardial infarction (MI), stable or unstable angina pectoris, congestive heart failure, abnormal noninvasive cardiovascular testing, or pre-operative non-coronary cardiac surgery. Detailed clinical and historical variables and reason for referral for angiography were recorded at the time of the procedure. Patients referred for coronary angiography due to acute MI or those who did not undergo coronary angiography were excluded from the present study, leaving 943 participants.
HDL apoliproteomic assessment
After study enrollment, fifteen milliliters of blood were taken immediately before and immediately after the angiographic procedure through a centrally placed vascular access sheath. The blood was immediately centrifuged for 15 minutes; serum and plasma were aliquoted on ice and frozen at −80°C until biomarker measurement. These samples were used to assess for plasma lipids and circulating apoB and apoA-1 (Roche Diagnostics, Indianapolis, IN). A validated method for measurement of apoA-1, apoC-1, apo-C2, apoC-3, and apoC-4 and calculation of pCAD score was recently described.(10,12) All analytical measurements were performed on blinded samples at Cleveland Heart Lab (Cleveland, Ohio).
Enrichment for Apo A-1-associated lipoproteins.
Recombinant 15NHis6ApoA-1 was added to human serum, incubated, diluted then purified using PhyTips (Phynexus, San Jose, CA), packed with Ni-NTA HisBind Superflow stationary phase. The diluted samples were bound to the Phytip columns followed by washing. Resin-bound His6-ApoA-1 and associated proteins were then eluted with 300 mM imidazole, 50 mM Tris-HCl, pH 9.0, 25% methanol. Analyses indicate that exogenous ApoA-1 efficiently incorporates into HDL and does not distort nor change the distribution of previously defined HDL subclasses.(10) Tecan Freedom Evo automated liquid handler (Tecan, Mannedorf, Switzerland) was used for end-to-end automation.
Targeted proteomic analysis.
Enriched apoA-1-associated lipoproteins were heat-denatured prior to addition of Endoproteinase LysC (Wako Chemicals USA, Richmond, VA). Samples were digested by incubation in an Eppendorf PCR thermocycler for 4 h at 37 °C, and a defined mixture of 13C6, 15N2-lysine-labeled internal standard peptides was added to the protein-digest. Peptides were detected from 10 uL of mixture using an Agilent 6490 triple quadrupole mass spectrometer operating in dynamic MRM mode, allowing for the targeted detection of peptide targets from apolipoprotein A-1, C-1, C-2, C-3, and C-4. Two transitions were monitored per peptide, and up to two peptides per protein. Peptide signal intensities were obtained via integrating the chromatographic peak for the quantifier transition using MassHunter Quantitative Analysis (Agilent, Santa Clara, CA). The relative abundances of HDL-associated proteins from apoA-1-associated lipoproteins and UC-prepared HDL were highly correlated, with Pearson r values ranging from 0.81 (apoA-2) to 0.99 (apoC-2 and apoC-3) (10).
pCAD score derivation and calculation.
Of 21 measurable HDL-associated from this platform, 5 proteins (i.e., those included in pCAD) were correlated with ex vivo cholesterol efflux capacity in 70 separate samples and prioritized by elastic net regression as recently descried.(12) Using multivariable logistic regression in a separate dataset 157 CAD cases and 74 matched asymptomatic controls, weights for each of the 5 apolipoproteins were derived.(12) From the individual measurements of HDL-associated apolipoprotein A-1, C-1, C-2, C-3, and C-4 in this study, pCAD was calculated as a weighted sum. The pCAD score is presented such that higher values reflect increased CAD risk, and is thus inversely correlated with HDL-C.
We also evaluated the influence of heparin on pCAD interpretation. We selected 17 random serum samples to cover a pCAD range of approximately −3 to +2. We separately supplemented each serum sample with heparin 0.1 U/mL and 0.5 U/mL. We also separately aliquoted into a heparin plasma tube (15 U/mL). We measured HDL apolipoproteins and calculated pCAD with these three conditions.
Follow-up and Outcomes
Results of coronary angiography (based on visual estimation at the time of the procedure by the primary proceduralist) were recorded; the left main, left anterior descending, left circumflex, and right coronary artery were each considered major coronary arteries, and the highest percent stenosis within each major coronary artery or their branches was recorded. For our primary outcome, we characterized the presence of coronary stenosis as ≥70% luminal obstruction, a widely held standard for angiographic “significance” as before, in at least one major coronary arter.(14) In secondary analyses, we also evaluated ≥30% stenosis in ≥1, ≥50% stenosis in ≥1, and ≥70% stenoses in ≥2 coronary arteries.
From the date of enrollment until completion of follow-up (mean 4 years, maximum 8 years), electronic medical records were reviewed. We reviewed electronic medical records and telephone called patients and/or their treating physicians to determine clinical endpoints as previously detailed (13). Vital status was assessed using the Social Security Death Index and/or death announcement postings. Deaths were adjudicated for the presence or absence of a cardiovascular cause. Our primary outcome for incident events analysis was the composite of myocardial infarction or cardiovascular death. In secondary analyses, we considered these two sub-categories separately. Assessment of outcomes was performed independently of HDL apolipoproteomic scores.
Statistical analysis
Baseline demographics and cardiovascular risk factors are presented as mean (standard deviation, SD), median (interquartile range, IQR), or count (percentage). We evaluated the association of these variables with the continuous pCAD score using Pearson chi-square test for dichotomous risk factors, Cochran–Mantel–Haenszel ANOVA for categorical variables with >2 groups, and general ANOVA for continuous variables. For continuous biomarkers we use Kruskal-Wallis test to evaluate the association. For all analyses, apoA-1 and apoB were log-transformed. All continuous variables (including log-transformed biomarkers), including pCAD, were then standardized to mean=0 and SD=1.
We also evaluated pairwise correlation of plasma lipids and related circulating biomarkers, including apoA-1, apoB, and high sensitivity C-reactive protein (hsCRP), with the composite pCAD score and its composite HDL apolipoproteins (apoA-1, apoC-1, apoC-2, apoC-3, and apoC-4); statistical significance, accounting for multiple pairwise tests, was assigned at , or 5.5×10−4. Positively correlated clusters were identified using Ward’s minimum variance method of hierarchical cluster analysis.
Multiple linear regression models were constructed to determine how conventional cardiovascular risk factors associated with pCAD. Covariates included age, sex, statin use, diabetes mellitus, current smoker, systolic blood pressure, history of hypertension, apoB, and apoA-1. A second model contained the same except non-HDL-C and HDL-C were used instead of apoB and apoA-1, respectively.
Next, we performed a logistic regression associating the presence of angiographic coronary stenosis. Both univariate analysis, and multivariable analysis adjusting for age, sex, statin use, diabetes mellitus, current smoker, systolic blood pressure, history of hypertension, apoB, and apoA-1 were performed.
Incident event analyses for pCAD were examined using Cox regression. The primary outcome for these analyses was cardiovascular death. All individuals were included in these analyses. In addition to adjustment of conventional cardiovascular risk factors (i.e., age, sex, statin use, diabetes mellitus, current smoker, systolic blood pressure, history of hypertension, apoB, and apoA-1), we also adjusted for prevalent CAD (based on coronary angiography findings as above, prior percutaneous coronary intervention, or prior coronary artery bypass graft surgery). We also assessed whether the effect of pCAD on events was different in the presence or absence of CAD by testing the interaction of pCAD with CAD status. We further generated a receiver operating characteristic (ROC) curve and assessed change in area under the curve (AUC) from conventional cardiovascular risk factors. For all Cox models, the proportional hazards assumption was tested and was not refuted.
Statistical tests were performed in R (version 3.4.2, R Foundation for Statistical Computing, Vienna, Austria) and SAS (version 9.4, SAS Institute, Cary, North Carolina, UA). For these analyses, an alpha level of 0.05 was considered statistically significant.
Results
Baseline characteristics
Baseline characteristics of all 943 study subjects are presented in Online Table 1. The mean age (standard deviation, SD) was 66.6 (11.3) years and 678 (71.9%) were male. As this represents patients clinically referred for angiography, there is a high prevalence of cardiometabolic risk factors and atherosclerotic cardiovascular disease Approximately half of participants carried a clinical diagnosis of CAD at presentation of coronary angiography. Overall, 553 (58.6%) had clinical atherosclerosis in at least one of coronary, peripheral, or cerebrovascular beds. The majority (71.1%) of participants were prescribed statin medications.
Correlation of HDL proteins with plasma lipids
The pairwise correlations between HDL-associated apolipoproteins, pCAD, plasma lipids, plasma apoB, plasma apoA-1, and hsCRP are shown in Figure 1. Hierarchical clustering separately identified a cluster comprised of plasma apoA-1, HDL apoA-1, and HDL-C, as expected, since apoA-1 is the primary determinant of HDL. HDL-associated and circulating plasma apoA-1 were strongly but not perfectly correlated (r = 0.86, P < 0.001). A separate cluster was of the four HDL apoC proteins. Another cluster consisted of total cholesterol, LDL-C, non-HDL-C, and apoB. Triglycerides, while correlated with HDL-C (r = −0.40) and non-HDL-C (r = 0.5), represented a separate cluster in our analysis.
Figure 1. Intercorrelation of HDL proteins and plasma lipoprotein biomarkers.
Each cell represents pairwise Pearson correlation for magnitude of correlation through color and r correlation coefficient. White cells have P>0.05/91 pairwise tests=5.5×10−4, and r correlation coefficients are still presented. Variables are arranged through hierarchical clustering. Clusters of positively correlated variables are represented with black squares.
The pCAD score was also correlated with HDL-C (r = −0.66, P < 0.001), triglycerides (r = 0.39, P < 0.001), and plasma apoA-1 (r = −0.61, P = < 0.001). While pCAD was not correlated with LDL-C or non-HDL-C, there was more modest correlation with plasma apoB (r = 0.11, P = < 0.001). The pCAD score was normally distributed across study participants (Online Figure 1). Among HDL apolipoproteins, pCAD was correlated with HDL apoA-1 (r = −0.71, P < 0.001), HDL apoC-1 (r = −0.52, P < 0.001), and HDL apoC-4 (r = 0.18, P < 0.001) (Online Figure 2).
Since the HDL apolipoproteins were measured within HDL, as expected, they all showed strong correlation with HDL-C. Among these HDL apolipoproteins, HDL apoA-1 and HDL apoC3 concentration were most strongly correlated with triglycerides (r = −0.32, P < 0.001; r = 0.25, P < 0.001, respectively).
Across 17 serum samples representing a range of pCAD values (approximately −3 to +2), pCAD values were not significantly different when compared with heparin 0.1 U/mL (Beta 0.94, 95% CI: 0.76–1.13) or heparin 0.5 U/mL (Beta 0.95, 95% CI: 0.80–1.10) addition (one-way ANOVA P = 0.36) (Online Figure 3). Proportional bias was modestly greater with much higher doses of heparin (i.e. 15 U/mL) in a heparin plasma tube but the difference was not significant (Beta 0.87, 95% CI: 0.48–1.26; paired t-test P = 0.33).
Association of HDL pCAD with cardiovascular risk factors
The distributions of baseline cardiovascular risk factors and pertinent medical history by HDL pCAD quartiles are described in Table 1. Individuals with elevated pCAD values appeared to more likely be male, with hypertension, to have prevalent histories of CAD, myocardial infarction and diabetes mellitus, and to have elevated hsCRP concentrations. They also were more likely to have lower total cholesterol concentrations, largely driven by concomitant lower HDL-C and apoA-1 concentrations. While LDL-C concentrations were similar across pCAD quartiles, apoB concentrations were greater with increasing pCAD scores.
Table 1.
Distribution of cardiovascular risk factors by pCAD quartile.
| Characteristic | Q1 (N = 235) | Q2 (N = 236) | Q3 (N = 236) | Q4 (N = 236) | P |
|---|---|---|---|---|---|
| Age, years | 67.4 (11.2) | 67.0 (10.8) | 66.3 (12.1) | 65.3 (11.0) | 0.12 |
| Female sex | 94 (40.0%) | 63 (26.7%) | 49 (20.8%) | 59 (25.0%) | <0.001 |
| Ethnicity | 0.03 | ||||
| Caucasian | 227 (96.6%) | 224 (94.9%) | 220 (93.2%) | 213 (90.3%) | |
| African | 3 (1.3%) | 6 (2.5%) | 5 (2.1%) | 8 (3.4%) | |
| Asian/Pacific | 2 (0.9%) | 0 (0%) | 4 (1.7%) | 5 (2.1%) | |
| Hispanic | 3 (1.3%) | 3 (1.3%) | 5 (2.1%) | 8 (3.4%) | |
| Native American | 0 (0%) | 0 (0%) | 1 (0.4%) | 1 (0.4%) | |
| Other/Unknown | 0 (0%) | 3 (1.3%) | 1 (0.4%) | 1 (0.4%) | |
| Current smoker | 27 (11.6%) | 28 (12.0%) | 36 (15.5%) | 27 (11.5%) | 0.51 |
| Hypertension | 153 (65.1%) | 166 (71.2%) | 181 (76.7%) | 190 (80.5%) | <0.001 |
| Coronary artery disease | 95 (40.4%) | 106 (44.9%) | 142 (60.2%) | 138 (58.5%) | <0.001 |
| Prior myocardial infarction | 33 (14.0%) | 48 (20.3%) | 64 (27.1%) | 81 (34.3%) | <0.001 |
| Prior CVA/TIA | 20 (8.5%) | 29 (12.3%) | 21 (8.9%) | 25 (10.6%) | 0.51 |
| Diabetes mellitus | 31 (13.2%) | 54 (22.9%) | 64 (27.1%) | 88 (37.3%) | <0.001 |
| Systolic blood pressure, mmHg | 139.3 (23.5) | 137.3 (23.0) | 136.2 (21.7) | 134.7 (21.4) | 0.17 |
| Diastolic blood pressure, mmHg | 72.6 (11.9) | 73.4 (11.6) | 73.0 (11.7) | 72.5 (10.7) | 0.81 |
| Plasma lipids, mg/dL | |||||
| Total cholesterol | 162.8 (33.0) | 153.8 (33.7) | 144.5 (34.9) | 149.4 (46.0) | <0.001 |
| LDL cholesterol | 85.4 (27.6) | 84.6 (29.2) | 80.8 (30.3) | 84.6 (38.6) | 0.06 |
| HDL cholesterol | 61.0 (16.4) | 46.7 (10.1) | 38.9 (8.9) | 35.0 (11.4) | <0.001 |
| Triglycerides | 82.1 (34.2) | 112.5 (57.1) | 123.8 (61.2) | 149.5 (64.1) | <0.001 |
| Apolipoprotein A1, mg/dL | 151.8 (26.2) | 132.4 (19.1) | 117.7 (19.0) | 109.7 (23.8) | <0.001 |
| Apolipoprotein B, mg/dL | 81.3 (19.7) | 85.4 (22.9) | 84.1 (23.7) | 90.1 (28.7) | 0.007 |
| hsCRP, mg/L | 3.9 (7.6) | 5.9 (16.6) | 5.8 (10.7) | 12.7 (27.8) | <0.001 |
| Statin prescription | 149 (63.7%) | 170 (73.0%) | 170 (72.3%) | 178 (75.4%) | 0.03 |
Continuous variables are expressed as mean (standard deviation) and categorical variables as count (proportion). CVA/TIA = cerebrovascular accident/transient ischemic attack; HDL = high-density lipoprotein; LDL = low-density lipoprotein; Q = quartile.
In a multivariable model including age, sex, statin prescription, diabetes mellitus, smoking status, systolic blood pressure, history of hypertension, HDL-C, and non-HDL-C, variables demonstrating at least nominal association (P < 0.05), in decreasing order of significance, included HDL-C, diabetes mellitus, systolic blood pressure, statin prescription, and non-HDL-C (Online Table 2). In a model replacing HDL-C for plasma apoA-1 and non-HDL-C for plasma apoB the effect from statin prescription increased, and the association of apoB was with pCAD was stronger (0.21 pCAD score/apoB SD, P < 0.001) than that of non-HDL-C (0.060 pCAD score/non-HDL-C SD, P = 0.025) (Online Table 3). The increased pCAD score associated with statin prescription likely reflects reverse confounding since introducing prevalent CAD into the model, rendered the association of statin prescription non-significant (P > 0.05). These terms, including plasma apoA-1 and apoB, represent 45.3% of the variability of pCAD; 24.6% of pCAD’s variability is explained by plasma apoA-1.
Association of pCAD with angiographic CAD
Of the 943 study participants, 587 (62.2%) had CAD, defined as ≥70% lesion in ≥1 vessel. In an unadjusted model, HDL pCAD was associated with CAD (OR 1.64; 95% CI: 1.42–1.90; P < 0.001). In a multivariable model including age, sex, statin prescription, diabetes mellitus, smoking status, systolic blood pressure, hypertension, apoA-1, and apoB, the association persisted (OR 1.39; 95% CI: 1.14–1.69; P = 0.001). The association of pCAD with CAD was not different across plasma apoA-1 concentrations (Pinteraction = 0.47) (Figure 2). Among the 800 (84.8%) individuals not receiving systemic intravenous heparin, the association of pCAD with CAD odds was similar in a multivariable model (OR 1.38; 95% CI: 1.10–1.73; P = 0.005). While the presence of diabetes influences the mean, the association of pCAD with CAD was not different in the presence or absence of prevalent diabetes mellitus (Pinteraction = 0.40). Across varying degrees of angiographic CAD severity, the associations persisted with similar effects (Online Tables 4 and 5). For example, the HDL pCAD association with ≥30% lesion in ≥1 vessel (OR 1.45; 95% CI: 1.15–1.82; P = 0.002) was similar to the association with ≥70% lesion in ≥2 vessels (OR 1.40; 95% CI: 1.14–1.73; P = 0.002).
Figure 2. pCAD distributions by plasma apoA-1 quantile and presence of angiographic obstructive CAD.
Boxplots of pCAD scores separated by plasma apoA-1 quantile and presence of obstructive CAD by angiography are depicted. For each box, the middle line represents the 50th quantile (Q2), and lower and upper box edges represent the 25th (Q1) and 75th (Q3) quantiles, respectively. The lower whisker extends to the smallest observation ≥ Q1 − 1.5 * IQR (where IQR = Q3 − Q1). The upper whisker extends to the largest observation ≤ Q3 + 1.5 * IQR. Depicted points represent values extending beyond the whiskers.
We also evaluated the association of each of the individual unweighted HDL-associated apolipoproteins comprising pCAD with angiographic CAD in a multivariable model accounting for conventional cardiovascular risk factors (Online Table 6). In this model, only HDL apoC-1 showed evidence of association with CAD (OR 0.64; 95% CI: 0.47–0.87; P = 0.004).
For clinical CAD risk factors, plasma apoB, plasma apoA-1, and pCAD, individual C-statistics were computed for presence of CAD and compared (Online Table 7). The pCAD score performed similar to or better than all factors assessed in the present study with respect to presence of CAD (Figure 3). The C-statistic for pCAD was 0.63 (95% CI: 0.59–0.67). In a model that includes all risk factors and plasma apolipoproteins, the C-statistic was 0.717 (95% CI: 0.683–0.751). The addition of pCAD to this composite did not significantly improve the C-statistic (0.724, 95% CI: 0.690–0.758, P = 0.22); however, model fit improved with the introduction of pCAD into the multivariable model (Likelihood ratio test P = 0.001) (Online Table 8).
Figure 3. Comparisons of C-statistics for obstructive CAD across clinical variables, plasma apolipoproteins, and pCAD.
Individual C-statistics and 95% confidence intervals were computed for CAD in the present study across CAD clinical risk factors, apoB, apoA-1, and an HDL apolipoproteomic score (pCAD). Horizontal lines represent the upper and lower bounds of the 95% confidence interval for pCAD. The y-axis is truncated to allow for comparisons across the individual variables assessed. Variables are ordered along the x-axis by C-statistic estimation.
Association of pCAD with cardiovascular mortality
A total of 101 cardiovascular deaths (10.7%) were sustained during the follow-up period. Among those with obstructive CAD at angiography, 76 (12.9%) died from cardiovascular causes. Among those without obstructive CAD at angiography, 24 (6.8%) died from cardiovascular causes.
In an unadjusted model, pCAD was associated with cardiovascular death (HR 1.33; 95% CI: 1.09–1.63; P = 0.005). The association was not present among those without obstructive CAD (HR 1.00; 95% CI 0.66–1.52; P = 1.0) but became evident among those with obstructive CAD (HR 1.36; 95% CI 1.07–1.73; P = 0.01). In a multivariable model, adjusting for clinical risk factors, apoA-1, apoB, and presence of coronary artery stenosis, there was no significant association of pCAD with cardiovascular death among all individuals (HR 1.24; 95% CI 0.93–1.66; P = 0.15). However, in the multivariable model, there was evidence supporting association with cardiovascular death among those with obstructive CAD (HR 1.48; 95% CI: 1.07–2.05; P = 0.019) but not without obstructive CAD (HR 0.75; 95% CI 0.38–1.49; P = 0.42) (Online Table 9). Exploratory analyses among those with obstructive CAD indicate that pCAD differences between those who did and did not have cardiovascular death may be maximal among those with higher plasma apoA-1 (and, thus, HDL-C) concentrations, particularly among diabetics (Online Figure 4).
In a multivariable analysis comprised of the five individual unweighted HDL apolipoproteins, HDL apoC-3 concentrations showed association with incident cardiovascular mortality among those without obstructive CAD (HR 2.38; 95% CI: 1.48–3.82; P < 0.001) and with obstructive CAD (HR 1.60; 95CI: 1.16–2.22; P = 0.004) (Online Table 10). HDL apoC-2 showed association with incident cardiovascular mortality among those without obstructive CAD (HR 0.11; 95% CI: 0.03–0.52; P = 0.005) but not with obstructive CAD.
Discussion
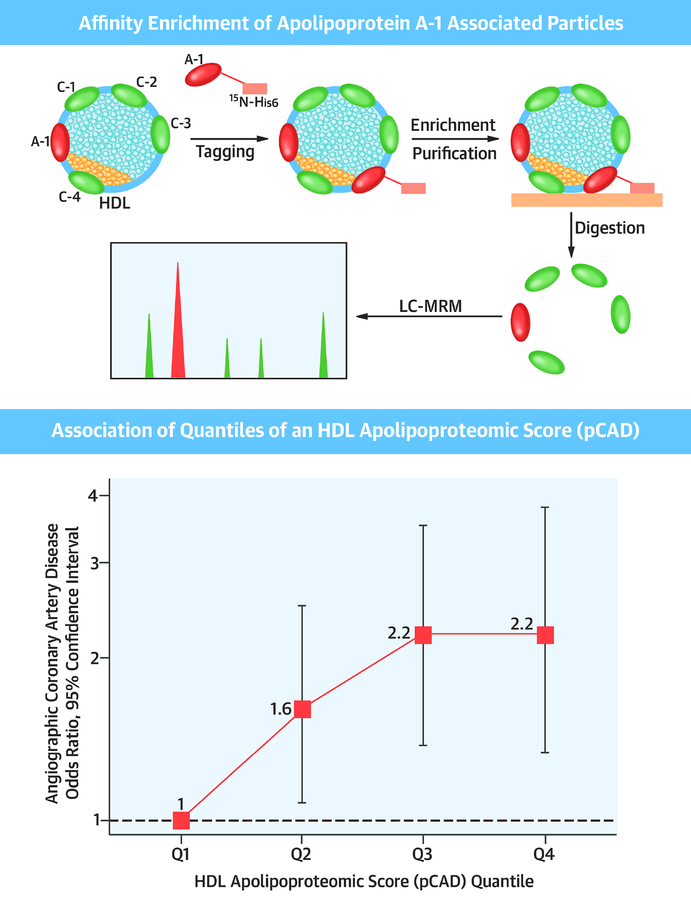
Among 943 patients without myocardial infarction referred for coronary angiography, the HDL apolipoproteome was assessed through a novel, high-throughput assay (Central Illustration). Independent of HDL-C or plasma apoA-1 concentrations, the concentrations of apolipoproteins in HDL particles (quantified as a composite pCAD score) associated with the presence of obstructive CAD during angiography by 1.39-fold per score standard deviation. Furthermore, this score may identify individuals who are heightened risk for cardiovascular death when obstructive CAD is present.
Central Illustration: HDL Apolipoproteomic Score and Coronary Artery Disease.
Top.Schematic of affinity enrichment of apolipoprotein A-1 associated lipoproteins and subsequent apolipoproteomic analysis. Bottom. Association of quantiles of an HDL apolipoproteomic score (pCAD) with angiographic coronary artery disease, adjusted for age, sex, history of myocardial infarction, statin use, hypertension, current smoking, diabetes mellitus, plasma apolipoprotein A-1, and plasma apolipoprotein B.
These results may have important implications for CAD risk prediction. HDL particles are not homogeneous entities between individuals; several prior efforts have associated other metrics of HDL heterogeneity with cardiovascular disease. HDL subclasses are currently measured as HDL2-C and HDL3-C by vertical-spin density gradient UC or nuclear magnetic resonance. Among patients with acute myocardial infarction or electively referred for coronary angiography, lower HDL3-C may predict risk of death better than HDL-C.(15) Prior proteomic analyses of HDL2 and HDL3 further correlated with putative functional properties of the HDL particle in small studies (16–18). Separately, heightened HDL reverse cholesterol transport, as measured by ex vivo cholesterol efflux, was associated with reduced odds of obstructive CAD by coronary angiography and reduced risk of a first atherosclerotic cardiovascular disease event.(19,20) HDL proteomic assessments in murine models showed that HDL inflammatory proteins better differentially determine hepatic cholesterol trafficking under different dietary fat conditions than efflux capacity (21,22). Such data indicate opportunities to extend assessment beyond simple HDL-C measurement to inform risk.
We found that an HDL apolipoproteomic score associates with the presence of angiographically-significant CAD. To advance the potential for clinical application, we used lipid-free apoA-1 as an affinity tag as opposed to prior approaches which may influence HDL composition or are challenging to scale (10). Surprisingly, risk discrimination for CAD with pCAD was similar to or superior than conventional risk factors. The HDL apoliproteome is a complex exchangeable reservoir of proteins which may influence intrinsic function and reflect alterations in lipoproteins or cells that HDL interacts with. While more recent murine HDL proteomic analyses focus on inflammatory HDL proteins (21,22), our results highlight that intrinsic HDL apolipoproteins, may separately influence atherosclerosis. To what degree this represents a putative atheroprotective intrinsic HDL function, such as cholesterol efflux, or biology across diverse lipoproteins is currently unclear and cannot be inferred from these results.
With respect to individual HDL apolipoproteins, in multivariable models, HDL apoC-1 showed association with reduced odds of angiographic CAD while HDL apoC-3 showed association with incident cardiovascular mortality. ApoC-1 is the endogenous inhibitor of cholesterol ester transfer protein (CETP), limiting the exchange of neutral lipids between plasma lipoproteins; the extent of inhibition is associated with apoC-1 concentration within HDL.(23) In an atherogenic murine model, apoC-1-enriched HDL promotes atherosclerosis in response to inflammatory stimuli.(24) However, in a study of 130 CAD cases and 130 CAD-free controls, HDL apoC-1 concentration was lower among CAD cases.(25) ApoC-3 is the endogenous inhibitor of lipoprotein lipase, and genetic deficiency is associated with a reduced risk of CAD.(4) Prior work suggests that HDL enrichment of apoC-3 promotes endothelial apoptosis and diminished cholesterol efflux (26,27).
Despite improving model calibration for association with CAD, incorporation of pCAD with a composite of conventional risk factors did not clearly improve its discrimination. For purposes of prognosis and resultant management modification, calibration is a key feature. Notably, pCAD is similarly altered even in the presence of non-obstructive CAD, and its utility is not impacted by diabetes mellitus status. Therefore, an elevated pCAD score in the primary prevention setting may prompt more aggressive management. Whether novel strategies to “rectify” the HDL apolipoproteome may be particularly advantageous is currently unclear.
The HDL apolipoproteome may additionally be a marker of residual cardiovascular risk when angiographically-significant CAD is present. While this secondary finding requires confirmation, these data suggest that when conventional cardiovascular risk factors are adequately addressed, the presence of HDL apolipoproteome abnormalities identifies patients at heightened risk for recurrent events. Additional exploratory analyses indicate that the risk difference is particularly marked when HDL-C concentrations are not low. The metabolic disturbances often associated with low HDL-C were previously associated with HDL subclasses.(28,29) Concordantly, when HDL-C is not low, elevated pCAD (versus not elevated) may represent a more meaningful risk difference.
An important limitation of this study is the inability to make causal inference. Much of the efforts evaluating atheroprotective properties of HDL stem from earlier observations strongly inversely correlating HDL-C concentrations with CAD risk. While several studies show that the relationship between HDL-C concentrations and CAD risk is not causal, the components of HDL may reflect complex lipoprotein biology relevant to atherosclerosis. While the actionability of an elevated pCAD score requires an adequately-powered randomized controlled trial, the appropriate management strategy may include therapies that are currently not yet in clinical practice. Further limitations include the cross-sectional nature of CAD association and generalizability since the study population was referred for coronary angiography. A prospective analysis in a large epidemiological study for the development of a first clinical CAD event is necessary. Additionally, while the current sample size is the largest of any HDL apolipoproteomic study, the non-significant concordant effect direction of pCAD with cardiovascular mortality among all individuals suggests that statistical power may be a limitation. Lastly, the platform used to measure HDL proteins can currently detect 21 HDL proteins.(12) It is not known whether the incorporation of additional HDL proteins into the pCAD will meaningfully improve performance of the score, and should be balanced by cost and scalability considerations.
In conclusion, within a contemporary cohort, we evaluated the relationship of the HDL apolipoproteome with angiographic coronary atherosclerosis and cardiovascular death. Independent of conventional plasma lipids and circulating plasma apolipoproteins, an HDL apolipoproteomic score independently associated with the presence of obstructive CAD and may predict risk of cardiovascular death among those with CAD. Our study highlights that atherosclerosis is influenced by a complex interplay of apolipoproteins across lipoprotein species; quantification of these components can inform risk and prognosis.
Supplementary Material
Clinical Perspectives.
Competency in Medical Knowledge:
Plasma lipids are complex macromolecules comprised of apolipoproteins, cholesterol, and triglycerides. Apolipoprotein concentrations are associated with the risk of developing CAD and with prognosis.
Translational Outlook:
Additional research is required to disclose the mechanisms linking HDL apolipoprotein concentration with CAD and define the optimum management of patients with elevated HDL apolipoprotein levels.
Acknowledgements:
The authors would like to acknowledge and thank the participants of the CASABLANCA study.
Funding: This investigator-initiated study was sponsored by Cleveland Heart Labs. The sponsor measured HDL proteins and calculated the pCAD score for each participant in a blinded fashion. The sponsor was not involved in the study design or data interpretation; the sponsor participated in drafting methods related to HDL proteomic assessment. P.N. is supported by an award from the National Heart, Lung, and Blood Institute (K08HL140203).
Disclosures: Supported by a research grant from Cleveland Heart Labs. P.N. is the co-inventor of helical synthetic peptides that stimulate cellular cholesterol efflux (US7691965B2, WO2005058938A3), reports research grants from Amgen and Boston Scientific, and consulting income from Apple. T.S.C., Z.J., C.B., M.S.P. are employees of Cleveland Heart Labs. J.L.J. has received grant support from Roche Diagnostics, Abbott Diagnostics, Singulex, Prevencio, and Cleveland Heart Labs, consulting income from Roche Diagnostics and Critical Diagnostics, and participates in clinical endpoint committees/data safety monitoring boards for Siemens Diagnostics. The remaining authors do not have relevant disclosures.
Abbreviations
- CAD
coronary artery disease
- CVA/TIA
cerebrovascular accident/transient ischemic attack
- HDL
high-density lipoprotein
- HDL-C
high-density lipoprotein cholesterol
- hsCRP
high sensitivity C-reactive protein
- LC-MRM
liquid chromatography-multiple reaction monitoring mass spectrometry
- LDL
low-density lipoprotein
- LDL-C
low-density lipoprotein cholesterol;
- MI
myocardial infarction
- ROC
receiver operating characteristic
- TRL
triglyceride-rich lipoproteins
- VLDL
very low-density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Voight BF, Peloso GM, Orho-Melander M et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 2012;380:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz GG, Olsson AG, Abt M et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J med. 2012;367:2089–99. [DOI] [PubMed] [Google Scholar]
- 3.Boden WE, Probstfield JL, Anderson T et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J med. 2011;365:2255–67. [DOI] [PubMed] [Google Scholar]
- 4.Crosby J, Peloso GM, Auer PL et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J med. 2014;371:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Do R, Willer CJ, Schmidt EM et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet 2013;45:1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yusuf S, Hawken S, Ounpuu S et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–52. [DOI] [PubMed] [Google Scholar]
- 7.Pechlaner R, Tsimikas S, Yin X et al. Very-Low-Density Lipoprotein-Associated Apolipoproteins Predict Cardiovascular Events and Are Lowered by Inhibition of APOC-III. J Am Coll Cardiol 2017;69:789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stegemann C, Pechlaner R, Willeit P et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 2014;129:1821–31. [DOI] [PubMed] [Google Scholar]
- 9.Shah AS, Tan L, Long JL, Davidson WS. Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J lipid research 2013;54:2575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collier TS, Jin Z, Topbas C, Bystrom C. Rapid Affinity Enrichment of Human Apolipoprotein A-I Associated Lipoproteins for Proteome Analysis. J Proteome Res 2018;17:1183–1193. [DOI] [PubMed] [Google Scholar]
- 11.In: Micheel CM, Nass SJ, Omenn GS, editors. Evolution of Translational Omics: Lessons Learned and the Path Forward. Washington (DC), 2012. [PubMed] [Google Scholar]
- 12.Jin Z, Collier TS, Dai DLY et al. Development and Validation of Apolipoprotein AI-Associated Lipoprotein Proteome Panel for the Prediction of Cholesterol Efflux Capacity and Coronary Artery Disease. Clinical chemistry 2018. [DOI] [PubMed] [Google Scholar]
- 13.Gaggin HK, Bhardwaj A, Belcher AM et al. Design, methods, baseline characteristics and interim results of the Catheter Sampled Blood Archive in Cardiovascular Diseases (CASABLANCA) study. IJC Metabolic & Endocrine 2014;5:11–18. [Google Scholar]
- 14.Ibrahim NE, Januzzi JL Jr., Magaret CA et al. A Clinical and Biomarker Scoring System to Predict the Presence of Obstructive Coronary Artery Disease. J Am Coll Cardiol 2017;69:1147–1156. [DOI] [PubMed] [Google Scholar]
- 15.Martin SS, Khokhar AA, May HT et al. HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: the Lipoprotein Investigators Collaborative. European heart journal 2015;36:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson WS, Silva RA, Chantepie S, Lagor WR, Chapman MJ, Kontush A. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arteriosclerosis, thrombosis, vascular biology 2009;29:870–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaisar T, Mayer P, Nilsson E, Zhao XQ, Knopp R, Prazen BJ. HDL in humans with cardiovascular disease exhibits a proteomic signature. Clin Chim Acta 2010;411:972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaisar T, Pennathur S, Green PS et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J clinical investigation 2007;117:746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khera AV, Cuchel M, de la Llera-Moya M et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl j med 2011;364:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohatgi A, Khera A, Berry JD et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl j med 2014;371:2383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Reilly M, Dillon E, Guo W et al. High-Density Lipoprotein Proteomic Composition, and not Efflux Capacity, Reflects Differential Modulation of Reverse Cholesterol Transport by Saturated and Monounsaturated Fat Diets. Circulation 2016;133:1838–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaisar T, Tang C, Babenko I et al. Inflammatory remodeling of the HDL proteome impairs cholesterol efflux capacity. J lipid research 2015;56:1519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dumont L, Gautier T, de Barros JP et al. Molecular mechanism of the blockade of plasma cholesteryl ester transfer protein by its physiological inhibitor apolipoprotein CI. J biological chemistry 2005;280:38108–16. [DOI] [PubMed] [Google Scholar]
- 24.Westerterp M, Berbee JF, Pires NM et al. Apolipoprotein C-I is crucially involved in lipopolysaccharide-induced atherosclerosis development in apolipoprotein E-knockout mice. Circulation 2007;116:2173–81. [DOI] [PubMed] [Google Scholar]
- 25.Yan LR, Wang DX, Liu H et al. A pro-atherogenic HDL profile in coronary heart disease patients: an iTRAQ labelling-based proteomic approach. PloS one 2014;9:e98368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo M, Liu A, Wang S et al. ApoCIII enrichment in HDL impairs HDL-mediated cholesterol efflux capacity. Sci Rep 2017;7:2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riwanto M, Rohrer L, Roschitzki B et al. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation 2013;127:891–904. [DOI] [PubMed] [Google Scholar]
- 28.Festa A, Williams K, Hanley AJ et al. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation 2005;111:3465–72. [DOI] [PubMed] [Google Scholar]
- 29.Garvey WT, Kwon S, Zheng D et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 2003;52:453–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.