Abstract
Objective
To estimate the clinical effects and cost-effectiveness of universal prenatal hepatitis C screening, and to calculate potential life expectancy, quality of life, and health care costs associated with universal prenatal hepatitis C screening and linkage to treatment.
Methods
Using a stochastic individual-level microsimulation model, we simulated the lifetimes of 250 million pregnant women matched at baseline with the U.S. childbearing population on age, injection drug use behaviors, and hepatitis C virus (HCV) infection status. Modeled outcomes included hepatitis C diagnosis, treatment and cure, lifetime health care costs, quality-adjusted life years (QALY) and incremental cost-effectiveness ratios (ICERs) comparing universal prenatal hepatitis C screening to current practice. We modeled whether infants exposed to maternal hepatitis C virus (HCV) at birth were identified as such.
Results
Hepatitis C virus–infected pregnant women lived 1.21 years longer and had 16% lower HCV-attributable mortality with universal prenatal hepatitis C screening, which had an ICER of $41,000 per QALY gained compared to current practice. Incremental cost-effectiveness ratios remained below $100,000 per QALY gained in most sensitivity analyses; notable exceptions included ICERs above $100,000 when assuming mean time to cirrhosis of 70 years, a cost greater than $500,000 per false positive diagnosis, or population HCV infection prevalence below 0.16%. Universal prenatal hepatitis C screening increased identification of infants exposed to HCV at birth from 44% to 92%.
Conclusions
In our model, universal prenatal hepatitis C screening improves health outcomes in HCV-infected women, improves identification of HCV exposure in infants born at risk, and is cost-effective.
PRÉCIS
Universal testing for hepatitis C in pregnancy is cost effective and would increase average life expectancy by 1.21 years for infected women.
INTRODUCTION
Hepatitis C virus (HCV) is a virulent, chronic, blood-borne infection which causes progressive liver damage and death.(1) Until recently, the only treatments available for HCV had serious side effects, were contraindicated for many, and cured fewer than 60% of those treated.(2) In 2013, the first direct-acting antiviral (DAA) treatment for HCV was approved. Today, DAAs can cure 95–99% of disease regardless of subtype.(3) However, as treatments improve, HCV incidence is rising among individuals under the age of 30 due to the opioid epidemic.(4, 5)
In reproductive age women, HCV prevalence doubled between 2006 and 2014, but many cases remain unidentified and untreated.(1, 6) For HIV, a reasonable analogy for HCV, guidance began with targeted risk-factor testing, as is currently recommended for HCV. Providers did not adequately identify stigmatized HIV risk behaviors, and targeted testing missed too many cases of HIV. A large proportion of pregnant women with an identified risk factor are not tested for HCV.(7) Eventually, guidance expanded to universal one-time HIV testing, which is both effective and cost-effective.(8, 9) Universal HCV testing in adults is also likely cost-effective.(10, 11)
Prenatal care may provide an ideal venue to diagnose HCV in reproductive age women.(12) Even so, low absolute prevalence, lack of available treatment during pregnancy, and the extremely high cost of HCV treatment raise questions of overall impact and resource allocation. A study conducted before the approval of DAAs found that testing in pregnancy was not likely cost-effective,(13) but with an effective HCV cure, these findings are due for reconsideration.
METHODS
This study did not involve human subjects and therefore did not require IRB approval.
We used a simulation model to investigate the health outcomes and potential cost-effectiveness of universal testing for hepatitis C during prenatal care. We estimated hepatitis C case identification rates in women and their infants, treatment courses initiated, HCV infection cures attained, and the incremental cost-effectiveness ratios (ICERs) of implementing universal hepatitis C testing in prenatal care settings compared to current practice.
The Hepatitis C Cost-Effectiveness (HEP-CE) model is a stochastic microsimulation model that has been previously used to investigate the cost-effectiveness of various strategies for testing for hepatitis C.(10, 14) The model generates a cohort of hypothetical individuals, follows them through the remainder of their life course, and records important outcomes. The generated individuals are representative of the population of interest; in this case, women in their first trimester of pregnancy. We adapted the HEP-CE model to incorporate details of pregnancy including U.S. fertility, live births and miscarriage rates (Table1). Key outcomes from the simulation include life expectancy; periods of reduced quality of life due to HCV infection and liver disease; whether an HCV infection is identified, treated, and cured; number of live births; number of infants born to women with HCV infection; whether such exposures are identified; and total healthcare costs. For each simulation, we generated hundreds of millions of hypothetical people. These individuals’ data were aggregated to make population-level estimates of life expectancy, quality-adjusted life expectancy, total healthcare costs, and care cascade outcomes under the strategies considered.
Table 1.
Simulation model parameters. HCV = hepatitis C virus; SMR = standardized mortality ratio; PWID = person who injects drugs; p-y = person-year; RNA = ribonucleic acid; SVR = sustained virologic response.
| Parameter | Point Estimate | Sensitivity Analysis Range | Source |
|---|---|---|---|
| Age of cohort at simulation start | 15–44 | -- | |
| Risk behavior prevalence | 1.25% | 0–100% | (42) |
| HCV infection attributable to risk behavior | 64.2% | (43) | |
| HCV infection prevalence | |||
| Overall | 0.38% | 0.152%−0.76% | (15) |
| With risk behavior | 19.5% | Calculation | |
| Without risk behavior | 0.138% | Calculation | |
| SMR, risk behavior | 6.1 | (44) | |
| SMR, former risk behavior | 1.8 | (44) | |
| Monthly cessation, PWID | 0.0519 | (45) | |
| Monthly relapse, PWID | 0.0561 | (45) | |
| HCV infection rate (PWID only, per 100 p-y) | 12.3 | 2.7–17.1 | (29) |
| Pregnancy rates (per 1,000 person-years) | (16, 25) | ||
| 15–19 | 20.6 | ||
| 20–24 | 74.7 | ||
| 25–29 | 103.3 | ||
| 30–34 | 104.0 | ||
| 35–39 | 53.3 | ||
| 40–44 | 11.6 | ||
| 45–49 | 0.9 | ||
| 50+ | 0.0 | ||
| Hepatitis C testing | |||
| During pregnancy | 14% | 14–50% | Unpublished CDC |
| No risk behavior, per 100 p-y | 4 | 2–5.8 | (26) |
| With risk behavior, per 100 p-y | 40 | 18–46 | (46) |
| Antibody sensitivity (%) | 99.5 | (39, 40, 47) | |
| Antibody specificity (%) | 89.1 | 80–100 | (39, 40) |
| RNA sensitivity (%) | 96.6 | (39, 40) | |
| RNA specificity (%) | 99.9 | (39, 40) | |
| Probability of linking to care if identified | 0.25 | 0.20–0.30 | (27) |
| Probability of initiating treatment if linked | 0.92 | 0.645–0.92 | (48) |
| Probability of completing treatment if initiated | 0.993 | (22) | |
| Probability of achieving SVR if treated | 0.93–0.99 | (3) | |
| Relative utility with HCV infection | (19) | ||
| Early HCV infection (f0-f3) | 0.94 | 0.94–1.0 | |
| Cirrhosis f4 | 0.75 | ||
| Decompensated cirrhosis | 0.60 | ||
| Relative utility with hepatitis C cure | |||
| Early HCV infection (f0-f3) | 0.97 | 0.94–1.0 | |
| Cirrhosis f4 | 0.94 | ||
| Decompensated cirrhosis | 0.75 | ||
| HCV Treatment Cost ($) | |||
| Glecaprevir and pibrentasvir | 39,600 | 19,800–59,400 | (49) |
| Sofosbuvir and velpatasvir | 68,773 | 47,833–89,712 | (49) |
| RNA Test Cost ($) | 78 | 59–117 | (32) |
For this analysis, we considered two scenarios. The first scenario was current practice: a small percentage of women would be screened during prenatal care. This proportion was drawn from current CDC reports of hepatitis C testing in pregnancy.(15) The second scenario considered the effects of universal testing for HCV infection at the first clinical encounter for each pregnancy. For both scenarios, all women could be tested throughout their lifetimes for HCV infection in venues other than prenatal care. This simulated current practice of hepatitis C testing in the U.S., and featured higher rates of testing among people who inject drugs (PWID).
The simulation begins during pregnancy. We excluded induced abortions from consideration and assumed all pregnancies resulted in a live birth except in the case of a) maternal death or b) miscarriage. After the first pregnancy and until the end of reproductive years at age 49, all individuals simulated may become pregnant again at a rate consistent with U.S. fertility patterns (Appendix 1, available online at http://links.lww.com/xxx). Our fertility data came from the U.S. Vital Statistics 2017 preliminary report on births.(16)
We tracked the number of infants born to women with HCV infection and calculated the proportion of those exposed infants in whom maternal HCV infection was identified such that the infant could be appropriately screened. Because infant and childhood HCV infection are clinically distinct entities from adult infection, and because there are no data to inform linkage rates in the future when infants born with infection become eligible for HCV treatment, we did not simulate the lifetime of HCV-infected infants, nor did we include infant outcomes in calculations of ICERs. We did, however, conduct sensitivity analyses on the cost of testing among infants born to mothers identified with HCV infection while pregnant.
We modeled HCV disease progression through stages of liver fibrosis categorized by METAVIR scores, which range from 0 to 4 based on liver biopsy or calculated from laboratory or imaging data (Appendix 2, available online at http://links.lww.com/xxx).(17) Progression through each stage is stochastic, such that some individuals never progress to late-stage liver disease, whereas others progress quickly. In every disease stage, HCV infection is associated with decreased quality of life and increased healthcare costs, as reflected in previous studies.(18, 19) Only when patients reach the stage of cirrhosis (METAVIR F4), do they become exposed to a monthly risk of HCV-related death. When patients reach the stage of decompensated cirrhosis, their quality of life falls further, healthcare costs increase, and morality increases to a rate that is higher than those with compensated cirrhosis.
We modeled hepatitis C testing at the first prenatal visit with serum HCV antibody testing followed by confirmatory HCV RNA testing. A positive antibody result triggers a reflex HCV RNA testing for confirmatory diagnosis of chronic HCV infection. Reflex testing is encouraged in the U.S. to increase identification of HCV-infected individuals. It reduces patient inconvenience by eliminating the need to return for a second blood draw to confirm the diagnosis and eliminates provider uncertainty about result interpretation. Once identified as HCV-infected, some but not all individuals link to HCV care to be treated. Patients who do not link to care or who are lost to follow-up before starting treatment maintain a probability of spontaneously returning to care in the future, as well as a chance of being screened for HCV infection at other clinical encounters and linking to care through those interactions (Appendix 3, available online at http://links.lww.com/xxx).
No HCV treatment is offered until after six months postpartum; this reflects current practice not to treat during pregnancy or breast-feeding.(20, 21) Throughout this waiting period, there is a probability of loss to follow-up before treatment initiation. We assumed treatment was available to all diagnosed patients without restrictions.
We modeled treatment with a pan-genotypic twelve-week regimen based on sofosbuvir and velpatasvir for those with cirrhosis and an eight-week regimen of glecaprevir and pibrentasvir for those without cirrhosis. The vast majority of patients who initiate treatment achieve sustained virologic response (SVR), and we modeled a rate of treatment default or failure that reflects real-world experience.(3, 22) Untreated HCV infection results in progressive liver disease; liver fibrosis is associated with a reduced quality of life. Upon achieving SVR, fibrosis progression halts and quality of life improves, but liver disease never reverses to full health. After SVR, the cost of providing HCV-related care is reduced by 50%. Among those who had already reached cirrhosis before starting treatment, HCV cure results in a 96% reduction in the rate of HCV-attributable mortality.(23) The residual mortality after cure among people with cirrhosis reflects continued rates of hepatocellular carcinoma and other liver failure. Severe fibrosis and decompensated cirrhosis are associated with increased risk of death, which can be ameliorated but not completely relieved by SVR.
Only people who are currently injecting drugs in the model are at risk for new HCV infection or reinfection after cure. When previously cured patients re-contract HCV, their disease progression resumes at the liver fibrosis stage they had attained during the previous infection. We modeled transitions into and out of injection drug use, allowing for initiation into injection drug use from ages 12 to 25 years. All drug use is associated with increased mortality risk, which we model using standardized mortality ratios (SMR of 6.1 for current injection drug use and 1.8 for historic but not current injection drug use).(24)
We constructed a simulated cohort with demographics and HCV infection prevalence similar to the current population of childbearing women in the U.S. using estimates found in published literature and unpublished CDC communications. The generated cohort had a mean age of 28 years and a standard deviation of 4 years. The overall prevalence of chronic HCV infection in our initial cohort was 0.38% (unpublished CDC data). The large majority of those infections were concentrated among individuals with current or former injection drug use (Table 1). After initial pregnancy, women in the simulation could become pregnant until the age of 49. Because a substantial proportion of pregnancies prove nonviable during the first trimester and spontaneously terminate prior to initiation of routine prenatal care, we modeled only pregnancies that passed beyond the first trimester. We constructed our pregnancy rates by working backwards from the number of live births and adjusting for multiple births and miscarriage and stillbirth rates after the first trimester.(16, 25) The final fertility estimates were highest for women ages 30–34 (approximately 104 pregnancies per 1,000 women) and lowest for women ages 45–49 (approximately 0.9 pregnancies per 1,000 women).
We used unpublished CDC data to estimate that 14% of women would receive a hepatitis C test during pregnancy under current practice; for the intervention scenario, we modeled universal uptake of testing recommendations (100%). We used a published analysis of hepatitis C testing rates in the U.S. to inform rates of testing at other clinical encounters.(26) We chose to model a blood test for HCV antibody followed by a reflex to confirmatory RNA in order to eliminate loss-to-follow up and ensure completion of the diagnostic intervention. In the rare event of a false positive antibody test followed by a false positive confirmatory RNA, we assigned an additional cost including genotype testing, additional confirmatory RNA testing and further care with a physician. This cost was designed to account for the burden on the medical system of remediating an incorrect diagnosis in a healthy individual. We modeled limited patient linkage (25%) to referral for follow-up care.(27) Among women who did not link to care following diagnosis, we assumed that half as many whom originally did link would return to HCV care over the coming 20 years.
We used clinical trial and real-world effectiveness data to determine treatment adherence and SVR rates between 93–99% based on HCV genotype and fibrosis stage at treatment.(28)
We used cohort studies to estimate the rates of reinfection for those with current injection drug use behaviors (12.3 cases per 100 person-years), and for the probability of clearance of HCV during the acute period, which is more likely in women than in men.(1, 29)
We estimated age-stratified healthcare costs related to co-morbidities other than hepatitis C using the Medical Expenditure Panel Survey (MEPS).(30) We estimated treatment costs using the Redbook Online database of pharmaceutical prices. Procedure costs including HCV antibody testing, reflex RNA and physician visits were estimated using the Medicare Reimbursement Fee Schedule.(31, 32)
The simulation continues until each member of the original simulated cohort dies. We also conducted simulations over shorter time horizons of five and ten years to predict the short-term effects of testing for HCV infection. We simulated 250 million individuals to arrive at our final estimates of life expectancy and healthcare costs. Costs and quality-adjusted life expectancy were discounted at a rate of 3% per annum in accordance with best practices defined by the second panel on cost-effectiveness.(33) Discounting is a standard procedure in economic evaluations research that accounts for the theoretical and observed reality that future costs and benefits are less acute and therefore valued less relative to costs in the present.(34, 35) Because discounted costs are not always meaningful to clinical decision makers, we also report undiscounted outcomes to give more information to the healthcare decision-making context. All costs are represented in 2017 USD and are considered from the healthcare payer perspective over a lifetime horizon in order to account for the chronic nature of hepatitis C. We estimated ICERs as the ratio of the difference in discounted, lifetime medical costs between the two strategies and the difference in discounted, quality-adjusted life years (QALY) lived under each strategy. We interpret ICERs assuming a willingness to pay (WTP) threshold of $100,000 per QALY gained. There is no universal WTP threshold, and so we chose $100,000 per QALY to maintain consistency with other analyses and recommendations.(36) For context in benchmarking of cost-effectiveness ratios, comparison to other accepted interventions may be useful. Screening for gestational diabetes, for example, is associated with an ICER of approximately $20,000 per QALY, screening for postpartum depression was found to have an ICER of approximately $14,000 per QALY and fetal echocardiography to detect congenital heart disease was found to have an ICER of $113,000 per QALY.(37, 38)
Once the model was parameterized and tested, we simulated our two scenarios for comparison (i.e. standard of care versus testing at first clinical visit for prenatal care). After analyzing these results, we performed a number of sensitivity analyses and alternative scenarios to illustrate how conclusions could change depending on the parameter values. We were particularly interested in testing how key parameters such as HCV infection prevalence, test performance, the cost of a false positive diagnosis, linkage to care rates, and treatment restrictions would affect the cost-effectiveness conclusions of this analysis. We performed a threshold analysis on HCV prevalence to determine the lowest prevalence below which routine testing is not likely to be cost-effective. Finally, we considered a scenario in which prenatal care providers initiated HCV therapy in office; this was considered in anticipation of trials of HCV therapy in pregnant women.
RESULTS
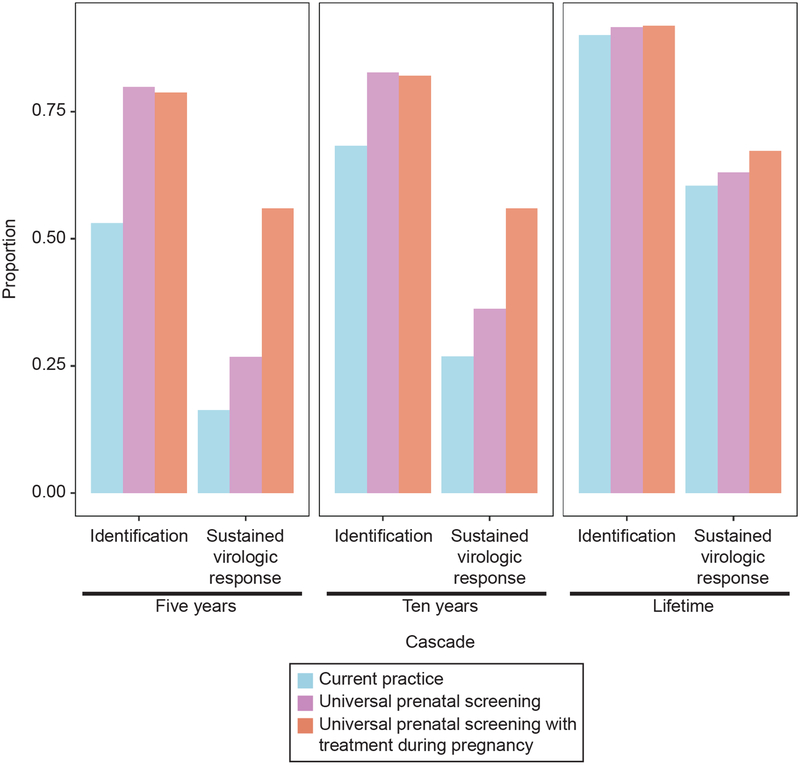
Among women who were HCV-infected at the initiation of the simulation, HCV resulted in 4.70 years of lost life expectancy and 2.88 years of lost discounted quality-adjusted life expectancy under the current practice scenario (Tables 2 and 3). Current practice identified 90% of all HCV infections over the lifetime of the cohort, resulting in 63% initiating treatment, and 60% reaching SVR (Tables 2 and 3). Without change in HCV testing guidance, current practice resulted in only 16% and 27% of infections achieving SVR in the next five and ten years, respectively (Figure 1).
Table 2.
Hepatitis C cascade of care. HCV = hepatitis C virus; SVR = sustained virologic response.
| Strategy | Infections identified (%) | Patients with Infection initiating treatment (%) | Patients with Infections achieving SVR (%) | Reduction in HCV-attributable mortality after cirrhosis* (%) | Infants exposed to HCV at birth identified | Infants exposed to HCV at birth |
|---|---|---|---|---|---|---|
| Current practice | 90.05 | 62.87 | 60.44 | -- | 44% | 0.54% |
| Universal prenatal hepatitis C testing | 91.70 | 65.59 | 63.07 | 16 | 92% | 0.50% |
Table 3.
Life expectancy outcomes
| Strategy | Remaining life expectancy (years) | Life expectancy lost to hepatitis C | Remaining discounted quality-adjusted life expectancy (QALY) | Discounted quality-adjusted life expectancy lost to hepatitis C (QALY) | ||
|---|---|---|---|---|---|---|
| Infected | Uninfected** | Overall | Infected | Uninfected** | Overall | |
| Current practice | 40.05 | 44.75 | 4.70 | 20.68 | 23.56 | 2.88 |
| Universal prenatal hepatitis C testing | 41.26 | 44.75 | 3.49 | 21.18 | 23.56 | 2.38 |
HCV-attributable mortality after cirrhosis represents the proportion of those with HCV infection who reach cirrhosis who ultimately die due to complications of HCV infection, including hepatocellular carcinoma and liver failure.
Remaining life expectancy and remaining QALY for “uninfected” are shown as the average predicted life expectancy for infected individuals had they otherwise not been infected, i.e. counterfactual to what did occur
HCV = hepatitis C virus. QALY = quality-adjusted life year.
Fig. 1.
Proportion of chronic hepatitis C virus infections that are predicted to be identified and achieve sustained virologic response during the simulation for 5 years, 10 years, and lifetime simulation durations in three scenarios for hepatitis C virus screening during prenatal care.
Universal prenatal testing slightly increased the percentage of all HCV infections identified in a lifetime to 92%, with similar small improvements in treatment initiation (66% initiated treatment vs. 63% for current practice) and reaching SVR (63% vs. 60% for current practice). However, universal testing shifted the timing of diagnosis and cure such that 27% (vs. 16% for current practice) and 36% (vs. 27% for current practice) of infections would attain SVR by five and ten years, respectively. Among those who were HCV-infected at the start of the simulation, universal testing resulted in 1.21 additional years of life expectancy and 0.5 additional years of quality-adjusted life expectancy compared to current practice. Universal testing in prenatal settings reduced HCV-attributable mortality by 16% over the lifetime of the cohort.
Universal testing more than doubled the proportion of infants born to HCV-infected women who were identified as exposed to HCV infection (92% with universal testing vs. 44% identified by current practice). Because universal testing identified infected women sooner and shifted the timing of treatment to earlier in life (before subsequent pregnancies), it was also associated with a 6% decrease in the proportion of infants born with HCV exposure.
Lifetime healthcare costs per pregnant woman were, on average, $387,071 and $387,194 for current practice and universal prenatal testing, respectively. Discounted lifetime healthcare costs were $153,168 and $153,246 for current practice and universal prenatal testing strategies, respectively. These cost differences of $123 (undiscounted) and $78 (discounted) represent the total net increase in lifetime healthcare costs per patient. These numbers include the cost of universal HCV prenatal testing, treatment and any other medical care received over the lifetime, and the future cost savings of averted liver failure and further cirrhosis. The small incremental cost difference reflects the fact that for a small portion of the total population – those with HCV infection – the cost of screening and HCV treatment is high, whereas for the large majority who are not HCV-infected, the cost is only the cost of HCV testing. When these two populations are averaged, the resulting incremental cost for the entire population is expectedly small. The cost per SVR attained by universal testing in prenatal care was $154,000.
Compared with current practice, universal prenatal testing resulted in an average gain of 0.002 QALY at an average additional discounted cost of $78, corresponding to an ICER of $41,000 per QALY gained (Table 4).
Table 4.
Cost-effectiveness results
| Strategy | Discounted lifetime healthcare costs ($) | Additional discounted lifetime healthcare costs ($) | Discounted QALY remaining | Gain in discounted QALY | ICER ($/QALY) |
|---|---|---|---|---|---|
| Base Case | |||||
| Current practice | 153,168 | -- | 25.976 | -- | -- |
| Universal prenatal hepatitis C testing | 153,246 | 78 | 25.977 | 0.002 | 41,000 |
| Prenatal hepatitis C treatment available | |||||
| Current practice | 153,168 | -- | 25.976 | -- | -- |
| Universal prenatal hepatitis C testing | 153,280 | 112 | 25.981 | 0.006 | 19,000 |
| Prevalence of HCV infection = 0.19% | |||||
| Current practice | 153,061 | -- | 25.980 | -- | -- |
| Universal prenatal hepatitis C testing | 153,128 | 67 | 25.981 | 0.001 | 83,000 |
| Prevalence of HCV infection = 0.152% | |||||
| Current practice | 153,016 | -- | 25.980 | -- | -- |
| Universal prenatal hepatitis C testing | 153,080 | 65 | 25.981 | 0.0003 | 249,000 |
| HCV antibody test specificity = 80% | |||||
| Current practice | 153,186 | -- | 25.976 | -- | -- |
| Universal prenatal hepatitis C testing | 153,279 | 93 | 25.978 | 0.001 | 69,000 |
| False positive cost = $500,000 | |||||
| Current practice | 153,302 | -- | 25.975 | -- | -- |
| Universal prenatal hepatitis C testing | 153,574 | 272 | 25.977 | 0.002 | $116,000 |
| Years to cirrhosis = 70 | |||||
| Current practice | 153,206 | -- | 25.980 | -- | -- |
| Universal prenatal hepatitis C testing | 153,268 | 62 | 25.981 | 0.0004 | 168,000 |
| Hepatitis C testing for PWID = 50% | |||||
| Current practice | 153,168 | -- | 25.976 | -- | -- |
| Universal prenatal hepatitis C testing | 153,246 | 78 | 25.977 | 0.002 | 50,000 |
| Early hepatitis C Cost = 0 | |||||
| Current practice | 152,527 | -- | 25.986 | -- | -- |
| Universal prenatal hepatitis C testing | 153,613 | 78 | 25.988 | 0.001 | 50,000 |
| Early HCV infection QoL = 1.0 | |||||
| Current practice | 153,168 | -- | 25.986 | -- | -- |
| Universal prenatal hepatitis C testing | 153,246 | 78 | 25.988 | 0.001 | 68,000 |
| Early HCV infection QoL = 1.0 and Cost = 0 | |||||
| Current practice | 152,527 | -- | 25.986 | -- | -- |
| Universal prenatal hepatitis C testing | 152,613 | 86 | 25.987 | 0.001 | 137,000 |
| PWID treatment restriction | |||||
| Current practice | 153,143 | -- | 25.974 | -- | -- |
| Universal prenatal hepatitis C testing | 153,216 | 73 | 25.976 | 0.002 | 34,000 |
| Treatment initiation = 64.5% | |||||
| Current practice | 153,137 | -- | 25.973 | -- | -- |
| Universal prenatal hepatitis C testing | 153,205 | 68 | 25.975 | 0.001 | 49,000 |
| Additional infant testing costs | |||||
| Current practice | 153,168 | -- | 25.976 | -- | -- |
| Universal prenatal hepatitis C testing | 153,246 | 78 | 25.977 | 0.002 | 41,000 |
Calculations based on table may differ from numbers presented due to rounding. HCV = hepatitis C virus; QoL = quality of life; PWID = people who inject drugs.
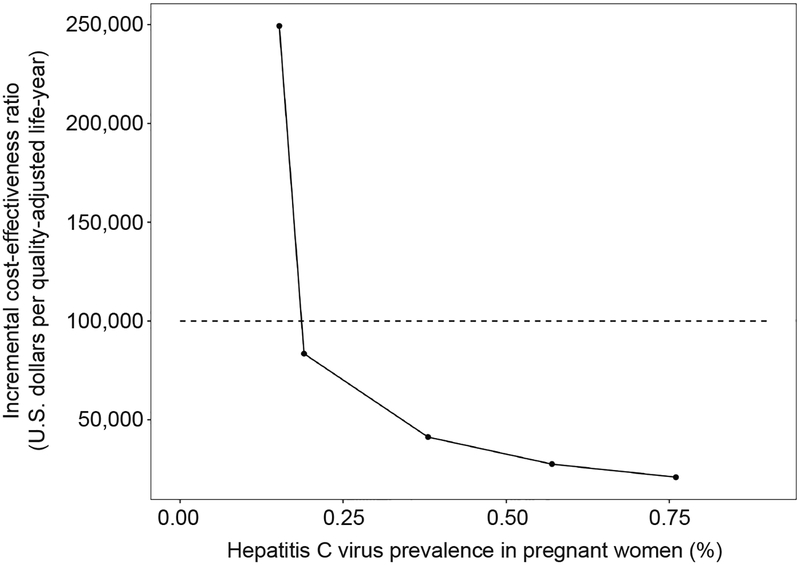
In one-way deterministic sensitivity analyses in which we ranged the population prevalence of HCV, the ICER of universal testing in prenatal settings compared to current practice remained below $100,000 per QALY gained unless the prevalence was less than half the original estimate (Figure 2). If HCV prevalence was 0.19% (half the original estimate) the ICER was $83,000 per QALY gained; if HCV prevalence was 0.152%, the ICER was $249,000 per QALY gained. If HCV prevalence was 0.76%, the ICER was $21,000 per QALY gained.
Fig. 2.
One-way sensitivity analysis of hepatitis C prevalence and its effect on incremental cost-effectiveness ratio of universal hepatitis C testing in prenatal care compared with current practice. Dotted line represents the $100,000 per quality-adjusted life-year willingness-to-pay threshold.
The ICER of universal testing in prenatal care compared to current practice was $69,000 per QALY gained when the specificity of the antibody test was 80% (Table 4). The ICER remained below $100,000 per QALY gained unless the associated cost of false positive diagnosis was greater than $500,000. If we assumed 50% of women with a history of injection drug use were tested during pregnancy in current practice, the ICER associated with universal testing was $50,000 per QALY gained.
If current PWID were not eligible for treatment, universal prenatal HCV screening was cost-effective with an ICER of $34,000 per QALY. If treatment was denied to 35.5% of the linked population, as was found in a recent study, the ICER was $49,000 per QALY. Both of these restricted treatment scenarios resulted in reduced life expectancies compared with the base case, with a population-level average decrease of 0.004 and 0.009 life years for PWID restriction and overall limitation, respectively.
When we added the additional cost of antibody testing for infants identified as being exposed to HCV to the ICER calculation, the ICER rose slightly (from $41,275 to $41,317) but remained $41,000 per QALY when rounded to the nearest thousand (Table 4).
The results of this analysis were largely unchanged when we varied parameters within feasible ranges; ICERs remained under $100,000 per QALY gained when we increased testing outside of prenatal venues, increased testing cost, and reduced quality of life values. A scenario in which hepatitis C treatment was offered to patients during prenatal care increased SVR to 67% and was associated with an ICER of $19,000 per QALY gained compared with current practice. Reducing the rate of fibrosis progression by 50% so that average time to cirrhosis was 70 years decreased the clinical benefit of hepatitis C cure such that the ICER of universal testing in prenatal care increased to $168,000 per QALY gained. In a conservative scenario in which we assumed HCV infection prior to development of cirrhosis has no associated additional cost and no decrease in quality of life, the ICER of universal testing was $137,000 per QALY gained.
DISCUSSION
In our simulation, testing for hepatitis C during pregnancy improved health outcomes for women, increased identification of exposed infants, reduced infant exposure during subsequent pregnancies, and was cost-effective. Women with infection lived 1.2 years longer with universal prenatal HCV testing. Shorter-term simulation results demonstrated the mechanism through which universal prenatal HCV screening provides benefit, despite the relatively small increase in lifetime SVR from 60% to 63%. Eventually, background testing for members of the general population will “catch up” to prenatal testing in terms of cases identified, treated, and cured, but earlier testing saves the patient years of life that would otherwise be lost to poor health or mortality. Although one WHO criterion for ideal screening tests is the availability of treatment, we have shown a benefit even with delayed treatment. Furthermore, our analysis demonstrated that universal hepatitis C testing followed by DAA treatment during pregnancy could improve cure rates from 60% to 67%, at an ICER of $19,000 per QALY gained.
Low absolute prevalence is sometimes cited against universal prenatal hepatitis C testing. However, only when the estimated prevalence of HCV infection dropped below 0.16% did the associated ICER cross the $100,000 per QALY gained benchmark.
Expanding the pool of individuals being tested decreases the positive predictive value of a test, but the two-test diagnostic algorithm for hepatitis C greatly reduces false positive test results. Assuming a population-level prevalence of 0.38%, 89.1% antibody specificity, and 99.9% RNA specificity, only one out of every 10,000 individuals tested would be incorrectly diagnosed with HCV.(39, 40) This rare event did not alter overall cost-effectiveness conclusions; the ICER associated with universal testing remained below $100,000 per QALY gained unless the cost of a false positive was $500,000 or more.
Uncertainty surrounding key parameters is one limitation of this study. We used CDC data as our baseline estimate of HCV prevalence and explored the effect this parameter and others had through sensitivity analyses. Our conclusions were unchanged within available HCV prevalence estimates. A second limitation concerns HCV transmission and drug use. We used available data to model drug use, but with limited data and changes in opioid use in the U.S., this is imperfect. While we included infection and re-infection for those with injection drug use behaviors, we did not consider that community-level treatment may decrease transmission rates. If so, then we likely underestimated positive effects. A third limitation is the uncertainty of cost for early-stage hepatitis C, which vary considerably among studies. We incorporated reasonable estimates and explored these with sensitivity analyses. Finally, our proposed strategy of reflex testing is not available at all venues and may require providers to give explicit orders so that any positive HCV antibody test is followed by a confirmatory RNA test. Without confirmatory testing, linkage rates may decrease.
Although this study demonstrated the likely cost-effectiveness of testing pregnant women, this finding should not be misinterpreted as a recommendation against testing women who are not pregnant. Even when there is substantial hepatitis C testing occurring in other healthcare encounters, prenatal care offers an important opportunity for testing. Secondary benefits to infants through reduced HCV transmission risk should not be considered the main reason to institute this strategy. Although this study focused primarily on cisgender (non-transgender) women, transgender men, nonbinary-identifying individuals and other people who do not present or identify as women also experience pregnancy and should receive prenatal care consistent with any existing guidelines.
Substantial policy changes raise questions surrounding both the intervention itself and its required resources. The Society for Maternal and Fetal Medicine issued a bulletin endorsed by the American Congress of Obstetricians and Gynecologists explicitly mentioning lack of available data on cost-effectiveness as part of their decision not to recommend universal hepatitis C testing in prenatal care.(41) We demonstrated that universal prenatal hepatitis C testing is likely to cost-effective and support this finding with sensitivity analyses. We addressed concerns raised in clinical practice and academic discussion, including false positive results and the prevalence below which universal hepatitis C testing is no longer cost-effective. With clinical trials underway and the possibility of hepatitis C treatment during pregnancy on the horizon, this study can serve as an early hypothesis of what prenatal hepatitis C treatment may look like.(20) We have an opportunity to identify and treat HCV infection in women of reproductive age, to circumvent difficulties of risk-based testing, and to reduce stigma of HCV testing in general. Universal prenatal HCV testing should be considered in plans for the elimination of viral hepatitis C as a public health threat.
Supplementary Material
ACKNOWLEDGEMENTS
This project was funded by the CDC, National Center for HIV, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement (NEEMA, # 5U38PS00-4644) and the Center for Health Economics of Treatment Interventions for Substance Use Disorder, HCV and HIV (NIDA, #P30DA040500). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
Peer Review History
Received September 7, 2018. Received in revised form November 6, 2018. Accepted November 8, 2018. Peer reviews and author correspondence are available at http://links.lww.com/xxx.
Contributor Information
Abriana Tasillo, Boston Medical Center, Section of Infectious Diseases, 801 Massachusetts Avenue Boston, MA 02119, 617-414-7025.
Golnaz Eftekhari Yazdi, Boston Medical Center, Section of Infectious Diseases, 801 Massachusetts Avenue Boston, MA 02119, 617-424-5309.
Shayla Nolen, Boston Medical Center, Section of Infectious Diseases, 801 Massachusetts Avenue Boston, MA 02119, 617-414-7025.
Sarah Schillie, Centers for Disease Control and Prevention, Division of Viral Hepatitis, 1600 Clifton Rd, MS G-37, Atlanta, GA 30333, 404-718-8608.
Claudia Vellozzi, Centers for Disease Control and Prevention, Division of Viral Hepatitis, 1600 Clifton Rd, MS G-37, Atlanta, GA 30333, 404-718-8699.
Rachel Epstein, Boston Medical Center, Section of Infectious Diseases, Section of Pediatric Infectious Diseases, 801 Massachusetts Avenue, Boston, MA 02119, 617-414-5591.
Liisa Randall, Bureau of Infectious Disease and Laboratory Sciences, Massachusetts Department of Public Health, 250 Washington, Boston, MA 02108, (617) 624 5320.
Joshua A. Salomon, Stanford University, Center for Health Policy and Center for Primary Care and Outcomes Research, CHP/PCOR, 117 Encina Commons, Stanford, CA 94305, 650-736-9477.
Benjamin P. Linas, Boston Medical Center, Section of Infectious Diseases, 801 Massachusetts Avenue Boston, MA 02119, 617-414-5238.
References
- 1.Page K, Hahn Judith A, Evans J, Shiboski S, Lum P, Delwart E, et al. Acute Hepatitis C Virus Infection in Young Adult Injection Drug Users: A Prospective Study of Incident Infection, Resolution, and Reinfection. The Journal of Infectious Diseases 2009;200(8):1216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talal AH, LaFleur J, Hoop R, Pandya P, Martin P, Jacobson I, et al. Absolute and relative contraindications to pegylated-interferon or ribavirin in the US general patient population with chronic hepatitis C: results from a US database of over 45 000 HCV-infected, evaluated patients. Alimentary pharmacology & therapeutics 2013. February;37(4):473–81. [DOI] [PubMed] [Google Scholar]
- 3.Kwo PY, Poordad F, Asatryan A, Wang S, Wyles DL, Hassanein T, et al. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1–6 without cirrhosis. J Hepatol 2017. August;67(2):263–71. [DOI] [PubMed] [Google Scholar]
- 4.Zibbell JE, Asher AK, Patel RC, Kupronis B, Iqbal K, Ward JW, et al. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. American Journal of Public Health 2018;108(2):175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. OPTN/SRTR Annual Data Report 2014: Liver. American Journal of Transplantation 2016;16(S2):69–98.26755264 [Google Scholar]
- 6.Ly KN, Jiles RB, Teshale EH, Foster MA, Pesano RL, Holmberg SD. Hepatitis c virus infection among reproductive-aged women and children in the united states, 2006 to 2014. Annals of Internal Medicine 2017;166(11):775–82. [DOI] [PubMed] [Google Scholar]
- 7.Boudova S, Mark K, El-Kamary SS. Risk-Based Hepatitis C Screening in Pregnancy Is Less Reliable Than Universal Screening: A Retrospective Chart Review. Open forum infectious diseases 2018;5(3):ofy043–ofy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006. September 22;55(RR-14):1–17; quiz CE1–4. [PubMed] [Google Scholar]
- 9.Walensky RP, Freedberg KA, Weinstein MC, Paltiel AD. Cost-effectiveness of HIV testing and treatment in the United States. Clin Infect Dis 2007. December 15;45 Suppl 4:S248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barocas JA, Tasillo A, Eftekhari Yazdi G, Wang J, Vellozzi C, Hariri S, et al. Population level outcomes and cost-effectiveness of expanding the recommendation for age-based hepatitis C testing in the United States. Clinical Infectious Diseases 2018:ciy098–ciy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckman MH, Ward JW, Sherman KE. Cost Effectiveness of Universal Screening for HCV Infection in the Era of Direct-Acting, Pangenotypic Treatment Regimens. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 2018. September 8. [DOI] [PubMed] [Google Scholar]
- 12.Jhaveri R, Broder T, Bhattacharya D, Peters MG, Kim AY, Jonas MM. Universal Screening of Pregnant Women for Hepatitis C: The Time Is Now. Clin Infect Dis 2018. September 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plunkett BA, Grobman WA. Routine hepatitis C virus screening in pregnancy: A cost-effectiveness analysis. American Journal of Obstetrics and Gynecology 2005. April//;192(4):1153–61. [DOI] [PubMed] [Google Scholar]
- 14.Assoumou SA, Tasillo A, Leff JA, Schackman BR, Drainoni ML, Horsburgh CR, et al. The cost-effectiveness of one-time hepatitis C screening strategies among adolescents and young adults in primary care settings. Clin Infect Dis 2017. September 09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schillie SF, Canary L, Koneru A, Nelson NP, Tanico W, Kaufman HW, et al. Hepatitis C Virus in Women of Childbearing Age, Pregnant Women, and Children. Am J Prev Med 2018. November;55(5):633–41. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton BE, Martin JA, Oseterman MJK, Driscoll AK, Rossen LM. Births: Provisional Data for 2016: National Center for Health Statistics; 2017. June.
- 17.Smith DJ, Combellick J, Jordan AE, Hagan H. Hepatitis C virus (HCV) disease progression in people who inject drugs (PWID): A systematic review and meta-analysis. International Journal of Drug Policy 2015. 10//;26(10):911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis KL, Mitra D, Medjedovic J, Beam C, Rustgi V. Direct economic burden of chronic hepatitis C virus in a United States managed care population. Journal of Clinical Gastroenterology 2011. February;45(2):e17–24. [DOI] [PubMed] [Google Scholar]
- 19.McLernon DJ, Dillon J, Donnan PT. Health-State Utilities in Liver Disease: A Systematic Review. Medical Decision Making 2008;28:582–92. [DOI] [PubMed] [Google Scholar]
- 20.Spera AM, Eldin TK, Tosone G, Orlando R. Antiviral therapy for hepatitis C: Has anything changed for pregnant/lactating women? World Journal of Hepatology 2016;8(12):557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breastfeeding Among U.S. Children Born 2002–2014, CDC National Immunization Survey: Centers for Disease Control and Prevention; 2017. December 1, 2017. [Google Scholar]
- 22.Feld JJ, Jacobson IM, Hezode C, Asselah T, Ruane PJ, Gruener N, et al. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med 2015. November 16. [DOI] [PubMed] [Google Scholar]
- 23.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. Jama 2012. December 26;308(24):2584–93. [DOI] [PubMed] [Google Scholar]
- 24.Evans E, Li L, Min J, Huang D, Urada D, Liu L, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006–10. Addiction 2015;110(6):996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacDorman M, Gregory E. Fetal and perinatal mortality: United States, 2013. Hyattsville, MD: National Center for Health Statistics; 2015. [PubMed] [Google Scholar]
- 26.Isenhour CJ, Hariri SH, Hales CM, Vellozzi CJ. Hepatitis C Antibody Testing in a Commercially Insured Population, 2005–2014. Am J Prev Med 2017. February 01. [DOI] [PubMed] [Google Scholar]
- 27.Krans EE, Zickmund SL, Rustgi VK, Park SY, Dunn SL, Schwarz EB. Screening and evaluation of hepatitis C virus infection in pregnant women on opioid maintenance therapy: A retrospective cohort study. Substance Abuse 2016 2016/January/02;37(1):88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Younossi ZM, Park H, Gordon SC, Ferguson JR, Ahmed A, Dieterich D, et al. Real-world outcomes of ledipasvir/sofosbuvir in treatment-naive patients with hepatitis C. Am J Manag Care 2016. May;22(6 Spec No.):SP205–11. [PubMed] [Google Scholar]
- 29.Sacks-Davis R, Grebely J, Dore GJ, Osburn W, Cox AL, Rice TM, et al. Hepatitis C Virus Reinfection and Spontaneous Clearance of Reinfection—the InC3 Study. Journal of Infectious Diseases 2015 November 1, 2015;212(9):1407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.AHRQ. Healthcare Cost and Utilization Project National Inpatient Sample: U.S. Health and Human Services; 2015. [Google Scholar]
- 31.United States Department of Health and Human Services Center for Medicare Services. Physician Fee Schedule. [Internet] [cited 2015 22 December]; Available from: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html?redirect=/PhysicianFeeSched
- 32.United States Department of Health and Human Services Center for Medicare Services. Clinical Diagnostic Laboratory Fee Schedule. [Internet] [cited 2015 22 December]; Available from: http://www.cms.gov/ClinicalLabFeesched/ [Google Scholar]
- 33.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. Jama 2016. September 13;316(10):1093–103. [DOI] [PubMed] [Google Scholar]
- 34.Severens JL, Milne RJ. Discounting health outcomes in economic evaluation: the ongoing debate. Value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research 2004. Jul-Aug;7(4):397–401. [DOI] [PubMed] [Google Scholar]
- 35.Attema AE, Brouwer WBF, Claxton K. Discounting in Economic Evaluations. PharmacoEconomics 2018. July 01;36(7):745–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014. August 28;371(9):796–7. [DOI] [PubMed] [Google Scholar]
- 37.Werner EF, Pettker CM, Zuckerwise L, Reel M, Funai EF, Henderson J, et al. Screening for gestational diabetes mellitus: are the criteria proposed by the international association of the Diabetes and Pregnancy Study Groups cost-effective? Diabetes Care 2012. March;35(3):529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson A, Anderson S, Wheeler SB. Screening for and Treating Postpartum Depression and Psychosis: A Cost-Effectiveness Analysis. Matern Child Health J 2017. April;21(4):903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freiman JM, Tran TM, Schumacher SG, White LF, Cohn J, Linas BP, et al. HCV Core Antigen testing for presence of active HCV infection and monitoring for treatment response and cure: A systematic review. 2015.
- 40.Tang W, Chen W, Amini A, Boeras D, Falconer J, Kelly H, et al. PICO 2: Diagnostic accuracy of tests to detect Hepatitis C antibody: A meta-analysis and review of the literature; 2015. [DOI] [PMC free article] [PubMed]
- 41.Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. American Journal of Obstetrics & Gynecology;217(5):B2–B12. [DOI] [PubMed] [Google Scholar]
- 42.Salihu HM, Salemi JL, Aggarwal A, Steele BF, Pepper RC, Mogos MF, et al. Opioid Drug Use and Acute Cardiac Events among Pregnant Women in the United States. The American Journal of Medicine 2017. [DOI] [PubMed] [Google Scholar]
- 43.Division of Viral Hepatitis; National Center for HIV/AIDS VH, STD, and TB Prevention. Surveillance for Viral Hepatitis - United States, 2015; 2017.
- 44.Evans E, Li L, Min J, Huang D, Urada D, Liu L, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006–10. Addiction 2015. June;110(6):996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krebs E, Min JE, Evans E, Li L, Liu L, Huang D, et al. Estimating State Transitions for Opioid Use Disorders. Medical decision making: an international journal of the Society for Medical Decision Making 2017. December 01;37(5):483–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coffin PO, Jin H, Huriaux E, Mirzazadeh A, Raymond HF. Trends in use of health care and HIV prevention services for persons who inject drugs in San Francisco: Results from National HIV Behavioral Surveillance 2005–2012. Drug and Alcohol Dependence 2015. January/1/;146:45–51. [DOI] [PubMed] [Google Scholar]
- 47.Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology 2015;62(5):1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Younossi Z, Henry L. Systematic review: patient-reported outcomes in chronic hepatitis C - the impact of liver disease and new treatment regimens. Alimentary pharmacology & therapeutics 2015. March;41(6):497–520. [DOI] [PubMed] [Google Scholar]
- 49.Micromedex Solutions. Drug Topics Red Book Online. [Internet] 2018. [cited 2018 June 13]; Available from: http://www.micromedexsolutions.com
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.