SYNOPSIS
Atrial fibrillation (AF) and heart failure (HF) both significantly impact morbidity and mortality, and also account for high symptom burden and impaired health-related quality of life (hrQoL). Several well-designed and broadly-implemented patient-reported outcome (PRO) instruments are available for both AF and HF, and can easily measure hrQoL in each disease process. Disease-specific interventions for AF and HF, separately, have been demonstrated to improve both clinical outcomes and PROs, and emerging data support aggressive treatments for patients with both AF and HF. A better understanding of the diverse phenotypes of AF and HF, as well as the heterogeneous treatment effects of disease-specific interventions, are necessary to further disentangle the complex relationship between symptoms of AF and HF.
Keywords: Atrial fibrillation, heart failure, patient reported outcomes, health-related quality of life
Among patients with heart failure (HF), atrial fibrillation (AF) occurs in more than one third, including up to half of those with severe HF.(1–3) While both diseases are well known to significantly impact morbidity and mortality, they also account for high symptom burden and significantly impair health-related quality of life (hrQoL). While improving quality of life remains a primary goal in the care of patients with AF and HF, measurement and improvement of hrQoL outcomes remains a major challenge. This review will discuss the measurement of hrQoL in AF and HF, including its importance, tools for such measurement and discrimination, and implications for disease management to improve hrQoL in these high-risk patients.
Importance of hrQoL
Separately, AF and HF each reduce hrQoL due to impaired functional status and high symptom burden. For patients with AF without HF, hrQoL has been found to be comparable to that of patients with an acute myocardial infarction.(4) Among patients with HF without AF, hrQoL is very poor for more than 20% of patients with HF, and is associated with increased healthcare utilization, increased costs, and worse clinical prognosis.(5–7) These patients are less likely to report good outlook, more likely to be depressed, and subsequently limit their engagement in care.(8)
However, clinical outcomes among patients with concomitant HF and AF are dramatically worse than either disease alone,(3,9) and there is reason to believe the impact on hrQoL is also compounded.(3) Patients with AF and CHF in the Atrial Fibrillation and Congestive Heart Failure trial demonstrated Short Form 36 Physical Component Summary scores 1.3 standard deviations below the national average. Normal rhythm was associated with improved hrQoL, supporting some independent effects of AF, and the implementation of arrhythmia interventions for these patients (see below).(10,11)
However, the routine and/or systematic measurement of hrQoL in clinical care of patients with AF and HF remains challenging, despite the availability of numerous tools to assess symptoms and hrQoL in these patients.
Measurement of hrQoL in AF and in HF
Patient reported outcomes (PROs) are best defined as “…any report of the status of a patient’s health condition that comes directly from the patient…”(12); they represent the most direct measurement of hrQoL, without influence of interpretation by the clinician or other healthcare personnel. The formal measurement of hrQoL can best be ascertained via structured, validated PROs. Additionally, when performed using well-validated and well-calibrated tools, PROs can provide the well-controlled, quantitative evidence to support interventions to improve hrQoL. For each disease, AF and HF, several PRO tools have been developed to assess symptom status and hrQoL, some of which have been extensively validated and implemented (Table 1).
Table 1.
Summary of Instruments for PRO Measurement in AF and in HF
| PRO Tools | Structure | Sample Clinical Study Implementation |
|---|---|---|
| AF | ||
| AFEQT(14) | 4 Domains: (1) symptoms, (2) daily activities, (3) treatment concerns, (4) treatment satisfaction | ORBIT-AF registry(57), CABANA trial(37) |
| AFSS(4) | 4 Domains: (1) AF burden, (2) global well-being, (3) AF symptom score, (4) healthcare utilization | RACE II trial(58,59), CTAF trial(34) |
| AF-QoL(13) | 3 Domains: (1) Psychological, (2) Physical, (3) Sexual | SARA trial(60) |
| MAFSI(16) | Single inventory of AF symptoms | CABANA trial(37) |
| SCL(61) | Single inventory of AF symptom frequency and severity | AFFIRM trial(17) |
| ASTA(62) | Single inventory of arrhythmia symptoms | SMURF study(63) |
| HF | ||
| KCCQ / KCCQ-12(24,26) | 7 Domains: (1) Physical limitation, (2) symptoms frequency, (3) symptom severity, (4) symptom stability, (5) self-efficacy, (6) limitations to lifestyle, (7) quality of life; KCCQ-12 includes only #1,2,6,7 | STICH(64), PARADIGM-HF(44), TOPCAT(65) trials FDA cleared(25) |
| MLWHF | Cumulative Domains: HF symptoms, physical functioning, sleep, role function, sex, recreation, appetite, psychological/emotional, adverse effects of medication, hospitalization, medical costs | SOLVD(66), A-HeFT(67) |
| CHQ | 3 Domains: (1) Dyspnea, (2) Fatigue, (3) Emotional function | |
PROs: patient reported outcomes; AF: atrial fibrillation; HF: heart failure; AFEQT: AF Effect on Quality of Life; AFSS: University of Toronto AF Severity Scale; AF-QoL: AF Quality of Life scale; MAFSI: Mayo AF-specific Symptom Inventory; SCL: Symptom Checklist; ASTA: Arrhythmia-Specific questionnaire in Tachycardia and Arrhythmia; KCCQ, Kansas City Cardiomyopathy Questionnaire; MLHF, Minnesota Living with Heart Failure; CHQ: chronic heart failure questionnaire.
Patient reported outcome tools developed specifically for HF, and the symptoms they target. Adapted from Mark DB. Assessing quality-of-life outcomes in cardiovascular clinical research. Nat Rev Cardiol 2016;13:286–308 with permission.
AF PROs
Care of patients with AF is multi-dimensional, including the treatment of comorbidities, management of symptoms, and prevention of sequelae, principally stroke. Therefore, AF-specific PROs may target different aspects of the disease, such as: (1) overall health status and impact of the disease (e.g. AFEQT); (2) detailed burden of specific, AF-related symptoms (e.g. the symptom checklist, MAFSI); and (3) the impact of oral anticoagulation on hrQoL (e.g., ACTS). Due to the overlap of AF and HF symptoms, the present review will focus on the first two categories of AF-related PROs.
Several AF-specific PRO instruments have been developed, and they are validated to a varying degree. These PROs have been derived primarily to assess symptom burden in the setting of interventions, such as catheter ablation. Nevertheless, they have been deployed in a variety of clinical and research settings, to assess hrQoL in patients with AF.
Symptom checklists are a major component of AF PROs, and comprise at least a component of the 16-item AF Symptom Checklist (SCL) tool, the Toronto AF Symptom Severity Scale (AFSS), the Mayo AF-specific Symptom Inventory (MAFSI), and the AF Effect on Quality of Life (AFEQT; Table 2).(13–18) Some are more closely tied to AF-specific symptoms than others, and may include additional domains, such as psychological function or sexual activity, in addition to physical function or symptoms. For example, the MAFSI functions as a very specific symptom survey and includes the most common physical symptoms associated with AF, such as palpitations, dizziness, exertional intolerance, sense of low heart rate, and syncope or pre-syncope.(16) Others, such as AF-QoL, are structured more broadly across domains of Psychological, Physical, and Sexual Activity impacts, with many questions that are less specific for AF.(13) As AF clearly affects hrQoL via multiple avenues (e.g., symptoms, anxiety of disease, restriction of activity), different measurement tools for different interventions may be required to demonstrate impact across a variety of domains.
Table 2.
Potential AF-Related Symptoms Included in the Most Common AF-Specific PROs
| ✓ Palpitations | ✓ Anxiety about: | ✓ Weakness |
| ✓ Irregular heart beat | ◦ Impending episodes | ✓ Fatigue at rest |
| ✓ Pause in heart activity | ◦ AF-related sequelae | ✓ Exercise intolerance |
| ✓ Sense of low heart rate | ◦ Procedures | ✓ Dyspnea at rest |
| ✓ Chest pain/pressure | ◦ Medication effects, including | ✓ Dyspnea with exertion |
| ✓ Lightheadedness/dizziness | bleeding | ✓ Treatment satisfaction |
| ✓ Flushing | ◦ Limitations of daily living | ◦ Control of AF |
| ✓ Swelling | ✓ Lack of appetite | ◦ Relief of AF symptoms |
| ✓ Syncope/Pre-Syncope | ✓ Difficulty with concentration | |
| ✓ Sleep disturbance |
AF: atrial fibrillation; PROs: patient reported outcomes.
HF PROs
Similarly, PRO instruments have been developed to capture physical symptom burden, psychological and emotional domains, and overall quality of life among patients with HF. Furthermore, there is robust evidence supporting the use of HF PROs as intermediate endpoints via the FDA’s Expedited Access for Premarket Approval pathway.(19) The most common HF PROs are summarized in Table 1, with the earliest being the Minnesota Living with Heart Failure (MLHF) questionnaire.(20–22) This 21-item instrument is summarized as a single score, without specifically-targeted domains, and assesses the impact of HF on physical, emotional, social, and mental components of quality of life.(23)
The most well-validated, and the only HF PRO that is now qualified as a medical device development tool by the FDA, is the Kansas City Cardiomyopathy Questionnaire (KCCQ).(24,25) The KCCQ includes PRO assessment of HF across 7 domains: Physical Limitation, Symptom Stability, Symptom Frequency, Symptom Burden, Self-Efficacy, Quality of Life, and Social Limitations. A shorter version, the KCCQ-12, has also been developed and validated to target specific domains, but maintain the reliability, responsiveness, and interpretability of the full length-version.(26) Therefore, the KCCQ-12 focuses primarily on domains most related to HF health status (physical limitation, symptom frequency, quality of life, and social limitation), and has been successfully deployed as a routine HF PRO measurement in busy clinical settings.(27) In clinical trials, the KCCQ has been found to be independently predictive of subsequent resource utilization and mortality among HF patients.(5,7,28–30)
Disease-Specific Interventions & Outcomes
Improvement of hrQoL in AF
In studies of AF interventions, PROs often track with clinical and arrhythmia outcomes. For example, in the AFFIRM study testing strategies of rate versus rhythm control, consistent maintenance of sinus rhythm was suboptimal, and clinical outcomes were not significantly different.(31) The PROs and hrQoL among a subset of patients in the two arms were not different.(17). Similarly, the CTAF trial tested several different antiarrhythmic drugs in rhythm control of AF;(32) there was improvement in hrQoL across agents tested, with some of the effect attenuated by recurrence of AF during follow-up.(33,34) In addition, there have been numerous studies comparing catheter ablation of AF to medical therapy (i.e., antiarrhythmic drugs), which have demonstrated favorable results of ablation, with respect to maintaining sinus rhythm and hrQoL.(35,36) Furthermore, many of these studies have included much more aggressive monitoring for arrhythmia, and thus more detailed data on recurrence rates and AF burden. Contemporary data from the CABANA trial have also shown very favorable effects of catheter ablation on hrQoL in a broad population of patients with AF.(37) And in studies with longer follow-up, the effect of ablation on hrQoL appears sustained at 2 years, even among patients with recurrent AF.(16)
It is important to acknowledge that many studies used a variety of PROs, including both disease-specific for AF, as well as more generic hrQoL tools that operate independent of disease state. However, there is robust evidence in support of the specificity of disease-specific PROs, which may better-discriminate improvement targeted at specific disease states and pathophysiology. In a study of 54 patients undergoing ablation, the six-item AF6 questionnaire was found to be more responsive to hrQoL improvement following ablation, compared to the more generic, but longer, standard, SF-36 tool.(38)
Improvement of hrQoL in HF
Across HF studies, several successful interventions for morbidity and mortality have also been shown to improve PROs. In fact, the KCCQ has been found to reliably reflect clinical changes in HF status, and statistically out-performed both the New York Heart Association (NYHA) classification and the 6-minute walk test.(39) Non-pharmacological, non-invasive interventions, such as exercise training, have been shown to improve hrQoL among HF patients.(40) And in the recent PARADIGM-HF study comparing sacubitril/valsartan versus enalapril, the KCCQ was collected in more than 90% of patients.(41) Sacubitril/valsartan was shown not only to improve clinical HF markers and mortality, but there was substantial improvement in nearly all KCCQ physical and social activities. Even more aggressive and invasive HF treatments have also improved hrQoL – patients undergoing implantation of left-ventricular assist devices (LVADs) for end-stage HF again improved mortality, and demonstrated dramatic improvements in hrQoL, despite the morbidity of a major surgical procedure.(42)
Interventions for AF & HF
However, most of these studies did not specifically target patients with concomitant AF and HF – prevalence of AF often hovered around one-third of those enrolled in major HF trials.(43–45) Given the unique risks and challenges of patients with concomitant AF and HF, several studies have specifically targeted these patients, and have reported hrQoL outcomes.
The AF-CHF trial compared strategies of rate-only versus rhythm control among 1,376 patients with AF and HF, and found no differences in the rates of cardiovascular death (the primary endpoint).(46) And while hrQoL also did not differ between the 2 groups overall, maintenance of sinus rhythm was associated with improved NYHA class and hrQoL.(10) In fact, there is mounting evidence that targeted treatment of AF (i.e., restoring and/or maintaining sinus rhythm), dramatically improves PROs among HF patients. Even short-term interventions, such as cardioversion, appear to improve exercise capacity for those who maintain sinus rhythm.(11)
More recently, trials of more definitive interventions for AF, in the setting of HF, have demonstrated favorable long-term results in this high-risk population. Several studies of catheter ablation, specifically in patients with AF and concomitant HF, have shown improvements in clinical outcomes.(47–49) In fact, the first study demonstrating a mortality benefit of ablation in AF, the CASTLE-AF trial, specifically targeted patients with AF and concomitant AF.(50) Subsequent meta-analyses of AF ablation in patients with HF (compared with medical therapy) have shown not only consistent clinical benefits, but improvements in functional status and hrQoL.(51,52)
Discriminating AF vs. HF hrQoL
A major unmet clinical challenge remains the discrimination of effects of AF versus HF in patients with both disease processes, with respect to both adverse clinical events and PROs. Atrial fibrillation may manifest before or after the development of HF, and the temporal relationship may indicate distinct clinical phenotypes and subsequent outcomes. For example, the sequelae of persistent tachycardia, often due to AF, have been well-described to lead to left-ventricular dysfunction and HF;(53,54) rhythm control interventions for AF in these specific patients appear to demonstrate significant benefits, compared with patients manifesting cardiomyopathy due to other causes.(55) Yet, AF may develop later in the HF process, and these patients likely represent different clinical phenotypes. While there remains a role for rhythm control interventions such as ablation, clinical response is often more heterogeneous in this broader group of HF patients.(48)
This variability in disease states limits our ability to identify the relative contributions of AF versus HF to poor hrQoL, across the population of patients with AF & HF. Disentangling the complex relationship between AF & HF is also limited by the significant overlap in symptomatology between the two – each can account for relatively vague symptoms of dyspnea, exertional limitation, and fatigue, to name a few (Figure 1). Therapeutic interventions specifically targeted to either AF or HF (as discussed above) may still provide mechanistic insight through understanding which patients are most likely to respond to specific interventions.
Figure 1.
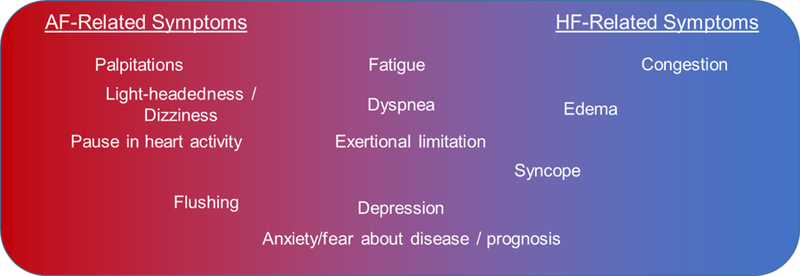
Overlap in symptoms specific for AF versus HF.
Patient Reported Outcomes and Clinician Reported Outcomes
A major concern in the treatment of AF and HF symptoms is the distinction between PROs and clinician reported outcomes (CROs). Several CROs are frequently used in these patients, such as the NYHA classification (for HF) and the European Heart Rhythm Association classification (EHRA; for AF). These scales have been adopted and widely used as a way of classifying disease burden and/or functional status on a simple I-IV scale, as assessed and interpreted by the clinician. They frequently reflect the clinician’s general impression of disease status or burden, and provide little to no detail regarding the patient’s experience and symptoms. While there appears to be general concordance that higher worse status by CROs translates to higher symptom burden by PROs,(15,24) detailed, disease-specific PROs appear to be more sensitive and relevant outcomes for patients, particularly following interventions.(56) In both research and clinical settings, they can provide complementary information on individual patients, and across patient cohorts.
Conclusions
Health-related quality of life is best measured using disease-specific, validated PROs, and patients with AF and HF have significantly impaired hrQoL. Several well-designed and broadly-implemented PRO instruments are available for both AF and HF, and can easily measure hrQoL burden for each disease process. Disease-specific interventions for AF and HF, separately, have demonstrated improvements in both clinical outcomes and PROs, and emerging data support aggressive treatments for patients with both AF and HF. A better understanding of the diverse phenotypes of AF and HF, as well as the heterogeneous treatment effects of disease-specific interventions, are necessary to further disentangle the complex relationship between AF and HF.
KEY POINTS.
AF and HF each represent disease process with significant impacts on health-related quality of life (hrQoL)
hrQoL is best ascertained through the use of structured, well-validated patient reported outcomes (PROs)
Several PRO instruments have been developed and validated for measurement of disease-specific hrQoL domains in AF and in HF.
Some PRO instruments focus on overall AF disease -specific hrQoL, others on symptoms, and others on anticoagulation satisfaction.
Interventions for AF and for HF have demonstrated improvement in hrQoL, as measured by PROs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
Work reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL143156 (to BAS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The following relationships exist related to this presentation: BS reports no other relevant relationships; JPP receives funding for clinical research from Abbott, ARCA biopharma, Boston Scientific, Gilead, Janssen Pharmaceuticals, and NHLBI and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Bayer, Biotronik, GSK, Johnson & Johnson, Medtronic, Motif Bio, Sanofi, and Phillips.
References
- 1.Wang TJ, Larson MG, Levy D et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920–5. [DOI] [PubMed] [Google Scholar]
- 2.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol 2003;91:2D–8D. [DOI] [PubMed] [Google Scholar]
- 3.Khazanie P, Liang L, Qualls LG et al. Outcomes of medicare beneficiaries with heart failure and atrial fibrillation. JACC Heart failure 2014;2:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorian P, Jung W, Newman D et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol 2000;36:1303–9. [DOI] [PubMed] [Google Scholar]
- 5.Chan PS, Soto G, Jones PG et al. Patient health status and costs in heart failure: insights from the eplerenone post-acute myocardial infarction heart failure efficacy and survival study (EPHESUS). Circulation 2009;119:398–407. [DOI] [PubMed] [Google Scholar]
- 6.Hoekstra T, Jaarsma T, van Veldhuisen DJ, Hillege HL, Sanderman R, Lesman-Leegte I. Quality of life and survival in patients with heart failure. Eur J Heart Fail 2013;15:94–102. [DOI] [PubMed] [Google Scholar]
- 7.Heidenreich PA, Spertus JA, Jones PG et al. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol 2006;47:752–6. [DOI] [PubMed] [Google Scholar]
- 8.de Leon CF, Grady KL, Eaton C et al. Quality of life in a diverse population of patients with heart failure: BASELINE FINDINGS FROM THE HEART FAILURE ADHERENCE AND RETENTION TRIAL (HART). Journal of cardiopulmonary rehabilitation and prevention 2009;29:171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsson LG, Swedberg K, Ducharme A et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol 2006;47:1997–2004. [DOI] [PubMed] [Google Scholar]
- 10.Suman-Horduna I, Roy D, Frasure-Smith N et al. Quality of life and functional capacity in patients with atrial fibrillation and congestive heart failure. J Am Coll Cardiol 2013;61:455–60. [DOI] [PubMed] [Google Scholar]
- 11.Wozakowska-Kaplon B, Opolski G. Improvement in exercise performance after successful cardioversion in patients with persistent atrial fibrillation and symptoms of heart failure. Kardiol Pol 2003;59:213–23. [PubMed] [Google Scholar]
- 12.Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 2006;4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arribas F, Ormaetxe JM, Peinado R, Perulero N, Ramirez P, Badia X. Validation of the AF-QoL, a disease-specific quality of life questionnaire for patients with atrial fibrillation. Europace 2010;12:364–70. [DOI] [PubMed] [Google Scholar]
- 14.Spertus J, Dorian P, Bubien R et al. Development and validation of the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) Questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol 2011;4:15–25. [DOI] [PubMed] [Google Scholar]
- 15.Dorian P, Guerra PG, Kerr CR et al. Validation of a new simple scale to measure symptoms in atrial fibrillation: the Canadian Cardiovascular Society Severity in Atrial Fibrillation scale. Circ Arrhythm Electrophysiol 2009;2:218–24. [DOI] [PubMed] [Google Scholar]
- 16.Wokhlu A, Monahan KH, Hodge DO et al. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol 2010;55:2308–16. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins LS, Brodsky M, Schron E et al. Quality of life in atrial fibrillation: the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J 2005;149:112–20. [DOI] [PubMed] [Google Scholar]
- 18.Mark DB. Assessing quality-of-life outcomes in cardiovascular clinical research. Nat Rev Cardiol 2016;13:286–308. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira JP, Duarte K, Graves TL et al. Natriuretic Peptides, 6-Min Walk Test, and Quality-of-Life Questionnaires as Clinically Meaningful Endpoints in HF Trials. J Am Coll Cardiol 2016;68:2690–2707. [DOI] [PubMed] [Google Scholar]
- 20.Rector TS, Francis GS, Cohn JN. Patients’ self-assessment of their congestive heart failure. Part 1: Patient perceived dysfunction and its poor correlation with maximal exercise tests. Heart Fail 1987;3:192–196. [Google Scholar]
- 21.Rector TS, Kubo SH, Cohn JN. Patients’ self-assessment of their congestive heart failure. Part 2: content, reliability and validity of a new measure, The Minnesota Living with Heart Failure Questionnaire. Heart Fail 1987;3:198–209. [Google Scholar]
- 22.Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J 1992;124:1017–25. [DOI] [PubMed] [Google Scholar]
- 23.Rector TS, Tschumperlin LK, Kubo SH et al. Use of the Living With Heart Failure questionnaire to ascertain patients’ perspectives on improvement in quality of life versus risk of drug-induced death. J Card Fail 1995;1:201–6. [DOI] [PubMed] [Google Scholar]
- 24.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245–55. [DOI] [PubMed] [Google Scholar]
- 25.Medical Device Development Tool (MDDT) Qualification Decision Summary For Kansas City Cardiomyopathy Questionnaire (KCCQ). Food and Drug Administration, 2016.
- 26.Spertus JA, Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes 2015;8:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stehlik J, Rodriguez-Correa C, Spertus JA et al. Implementation of Real-Time Assessment of Patient-Reported Outcomes in a Heart Failure Clinic: A Feasibility Study. J Card Fail 2017;23:813–816. [DOI] [PubMed] [Google Scholar]
- 28.Rumsfeld JS, Jones PG, Whooley MA et al. Depression predicts mortality and hospitalization in patients with myocardial infarction complicated by heart failure. Am Heart J 2005;150:961–7. [DOI] [PubMed] [Google Scholar]
- 29.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation 2004;110:546–51. [DOI] [PubMed] [Google Scholar]
- 30.Stehlik J, Estep JD, Selzman CH et al. Patient-Reported Health-Related Quality of Life Is a Predictor of Outcomes in Ambulatory Heart Failure Patients Treated With Left Ventricular Assist Device Compared With Medical Management: Results From the ROADMAP Study (Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management). Circ Heart Fail 2017;10. [DOI] [PubMed] [Google Scholar]
- 31.Wyse DG, Waldo AL, DiMarco JP et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825–33. [DOI] [PubMed] [Google Scholar]
- 32.Lumer GB, Roy D, Talajic M et al. Amiodarone reduces procedures and costs related to atrial fibrillation in a controlled clinical trial. Eur Heart J 2002;23:1050–6. [DOI] [PubMed] [Google Scholar]
- 33.Dorian P, Mangat I. Quality of life variables in the selection of rate versus rhythm control in patients with atrial fibrillation: observations from the Canadian Trial of Atrial Fibrillation. Card Electrophysiol Rev 2003;7:276–9. [DOI] [PubMed] [Google Scholar]
- 34.Dorian P, Paquette M, Newman D et al. Quality of life improves with treatment in the Canadian Trial of Atrial Fibrillation. Am Heart J 2002;143:984–90. [DOI] [PubMed] [Google Scholar]
- 35.Jais P, Cauchemez B, Macle L et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008;118:2498–505. [DOI] [PubMed] [Google Scholar]
- 36.Wazni OM, Marrouche NF, Martin DO et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005;293:2634–40. [DOI] [PubMed] [Google Scholar]
- 37.Packer DL, Mark DB, Robb RA et al. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) Trial: Study Rationale and Design. Am Heart J 2018;199:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjorkenheim A, Brandes A, Magnuson A, Chemnitz A, Edvardsson N, Poci D. Patient-Reported Outcomes in Relation to Continuously Monitored Rhythm Before and During 2 Years After Atrial Fibrillation Ablation Using a Disease-Specific and a Generic Instrument. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spertus J, Peterson E, Conard MW et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005;150:707–15. [DOI] [PubMed] [Google Scholar]
- 40.Ostman C, Jewiss D, Smart NA. The Effect of Exercise Training Intensity on Quality of Life in Heart Failure Patients: A Systematic Review and Meta-Analysis. Cardiology 2017;136:79–89. [DOI] [PubMed] [Google Scholar]
- 41.Chandra A, Lewis EF, Claggett BL et al. Effects of Sacubitril/Valsartan on Physical and Social Activity Limitations in Patients With Heart Failure: A Secondary Analysis of the PARADIGM-HF Trial. JAMA cardiology 2018;3:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers JG, Aaronson KD, Boyle AJ et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol 2010;55:1826–34. [DOI] [PubMed] [Google Scholar]
- 43.Zannad F, McMurray JJ, Krum H et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21. [DOI] [PubMed] [Google Scholar]
- 44.McMurray JJ, Packer M, Desai AS et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 45.Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol 2014;64:710–21. [DOI] [PubMed] [Google Scholar]
- 46.Roy D, Talajic M, Nattel S et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med 2008;358:2667–77. [DOI] [PubMed] [Google Scholar]
- 47.Di Biase L, Mohanty P, Mohanty S et al. Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device: Results From the AATAC Multicenter Randomized Trial. Circulation 2016;133:1637–44. [DOI] [PubMed] [Google Scholar]
- 48.Jones DG, Haldar SK, Hussain W et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol 2013;61:1894–903. [DOI] [PubMed] [Google Scholar]
- 49.Prabhu S, Taylor AJ, Costello BT et al. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J Am Coll Cardiol 2017;70:1949–1961. [DOI] [PubMed] [Google Scholar]
- 50.Marrouche NF, Brachmann J, Andresen D et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N Engl J Med 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 51.Ma Y, Bai F, Qin F et al. Catheter ablation for treatment of patients with atrial fibrillation and heart failure: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord 2018;18:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Briceno DF, Markman TM, Lupercio F et al. Catheter ablation versus conventional treatment of atrial fibrillation in patients with heart failure with reduced ejection fraction: a systematic review and meta-analysis of randomized controlled trials. J Interv Card Electrophysiol 2018;53:19–29. [DOI] [PubMed] [Google Scholar]
- 53.Nerheim P, Birger-Botkin S, Piracha L, Olshansky B. Heart failure and sudden death in patients with tachycardia-induced cardiomyopathy and recurrent tachycardia. Circulation 2004;110:247–52. [DOI] [PubMed] [Google Scholar]
- 54.Packer DL, Bardy GH, Worley SJ et al. Tachycardia-induced cardiomyopathy: a reversible form of left ventricular dysfunction. Am J Cardiol 1986;57:563–70. [DOI] [PubMed] [Google Scholar]
- 55.Calvo N, Bisbal F, Guiu E et al. Impact of atrial fibrillation-induced tachycardiomyopathy in patients undergoing pulmonary vein isolation. Int J Cardiol 2013;168:4093–7. [DOI] [PubMed] [Google Scholar]
- 56.Bjorkenheim A, Brandes A, Magnuson A et al. Assessment of Atrial Fibrillation-Specific Symptoms Before and 2 Years After Atrial Fibrillation Ablation: Do Patients and Physicians Differ in Their Perception of Symptom Relief? JACC Clinical electrophysiology 2017;3:1168–1176. [DOI] [PubMed] [Google Scholar]
- 57.Freeman JV, Simon DN, Go AS et al. Association Between Atrial Fibrillation Symptoms, Quality of Life, and Patient Outcomes: Results From the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circ Cardiovasc Qual Outcomes 2015;8:393–402. [DOI] [PubMed] [Google Scholar]
- 58.Vermond RA, Crijns HJ, Tijssen JG et al. Symptom severity is associated with cardiovascular outcome in patients with permanent atrial fibrillation in the RACE II study. Europace 2014;16:1417–25. [DOI] [PubMed] [Google Scholar]
- 59.Groenveld HF, Crijns HJ, Van den Berg MP et al. The effect of rate control on quality of life in patients with permanent atrial fibrillation: data from the RACE II (Rate Control Efficacy in Permanent Atrial Fibrillation II) study. J Am Coll Cardiol 2011;58:1795–803. [DOI] [PubMed] [Google Scholar]
- 60.Mont L, Bisbal F, Hernandez-Madrid A et al. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). Eur Heart J 2014;35:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bubien RS, Knotts-Dolson SM, Plumb VJ, Kay GN. Effect of radiofrequency catheter ablation on health-related quality of life and activities of daily living in patients with recurrent arrhythmias. Circulation 1996;94:1585–91. [DOI] [PubMed] [Google Scholar]
- 62.Walfridsson U, Arestedt K, Stromberg A. Development and validation of a new Arrhythmia-Specific questionnaire in Tachycardia and Arrhythmia (ASTA) with focus on symptom burden. Health Qual Life Outcomes 2012;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charitakis E, Barmano N, Walfridsson U, Walfridsson H. Factors Predicting Arrhythmia-Related Symptoms and Health-Related Quality of Life in Patients Referred for Radiofrequency Ablation of Atrial Fibrillation: An Observational Study (the SMURF Study). JACC Clinical electrophysiology 2017;3:494–502. [DOI] [PubMed] [Google Scholar]
- 64.Mark DB, Knight JD, Velazquez EJ et al. Quality of life and economic outcomes with surgical ventricular reconstruction in ischemic heart failure: results from the Surgical Treatment for Ischemic Heart Failure trial. Am Heart J 2009;157:837–44, 844.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamo CE, Heitner JF, Pfeffer MA et al. Baseline distribution of participants with depression and impaired quality of life in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial. Circ Heart Fail 2015;8:268–77. [DOI] [PubMed] [Google Scholar]
- 66.Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol 1993;71:1106–7. [DOI] [PubMed] [Google Scholar]
- 67.Taylor AL, Ziesche S, Yancy C et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004;351:2049–57. [DOI] [PubMed] [Google Scholar]


