Abstract
Objectives:
To determine if children with laryngeal penetration on videofluoroscopic swallow study (VFSS) who received feeding interventions (thickened liquids, change in liquid flow rate and/or method of liquid delivery) had improved symptoms and decreased hospitalizations compared to those without intervention.
Methods:
We performed a retrospective cohort study of children under 2 years with laryngeal penetration on VFSS at our institution in 2015 to determine initial and follow-up VFSS findings, symptom improvement at follow-up, and hospitalization risk before and after VFSS. Proportions were compared with Fisher’s exact test and hospitalizations with paired t-tests.
Results:
We evaluated 137 subjects with age 8.93±0.59 months who had laryngeal penetration without aspiration on VFSS. 55% had change in management, with 40% receiving thickening and 15% a change in flow rate. There was significant improvement in symptoms for children that had feeding intervention and this improvement was greatest with thickening (OR 41.8, 95% CI 12.34-141.69, p<0.001). On repeat VFSS, 26% had evidence of aspiration that was not captured on initial VFSS. Subjects had decreased total and pulmonary hospitalizations with feeding intervention and decreased pulmonary nights with thickening (p<0.05).
Conclusions:
Laryngeal penetration appears to be clinically significant in children with oropharyngeal dysphagia and interventions to decrease its occurrence are associated with improved outcomes including decreased symptoms of concern and hospitalization nights. Thickening or other feeding intervention should be considered for all symptomatic children with laryngeal penetration on swallow study.
Keywords: oropharyngeal dysphagia, swallowing disorders, thickened feeds
Introduction
There is debate about the clinical significance of laryngeal penetration on videofluoroscopic swallow study (VFSS) and as a result there is great variability in the clinical approach to these patients (1, 2). With the increasing survival of premature infants and other children with chronic medical conditions, feeding and swallowing disorders are increasing in prevalence (3–6). Patients with these conditions are frequently managed by both specialist and generalist medical providers and have instrumental or clinical swallow evaluations performed in a variety of settings; therefore, an understanding of swallow study abnormalities is essential for their appropriate management (3, 7, 8). However, there is limited evidence to guide treatment for children with oropharyngeal dysphagia and isolated laryngeal penetration; many providers feel that penetration is a normal finding that does not merit intervention whereas other providers consider these patients at risk for future aspiration and manage their swallowing issues as they would in a patient who frankly aspirated during VFSS.
The gold standard test for evaluation of swallow function in children is the VFSS, a radiologic exam in which the patient is fed different consistencies of barium via various methods of delivery to formally test for oropharyngeal dysphagia (8, 9). Laryngeal penetration occurs when material enters the laryngeal inlet but does not extend below the level of the vocal cords and can vary in terms of frequency and depth. Penetration can occur for a variety of reasons including anatomic abnormalities, laryngopharyngeal sensory deficits, and developmental/neurologic conditions, but is thought to primarily occur due to disrupted swallow function in which some amount of material from the oral cavity travels inappropriately to the laryngeal inlet instead of esophagus (10–13).
Many clinicians focus their attention on aspiration and are often reassured when only laryngeal penetration is seen on VFSS. Similarly, most of the scientific literature has also narrowly focused on aspiration, leading to a relative lack of understanding of the clinical significance of penetration. In studies that have considered penetration to be an abnormal finding, children with penetration are typically analyzed in combination with aspiration, making it difficult to discern the actual contribution of patients with isolated laryngeal penetration (11–16).
Several small studies in adults and children have suggested that penetration is associated with negative clinical outcomes, including increased risk of pneumonia and occurrence of aspiration (2, 17–19). Other studies, however, have suggested that penetration is a normal variant, but much of this work is in the older adult population and it is difficult to know how to apply these findings to pediatrics (20–22).
While there continues to be active debate about the clinical significance of penetration seen on VFSS, few groups have studied whether interventions specifically targeted at decreasing penetration can improve clinical outcomes and no prior studies have examined the benefits of feeding interventions in these children. The primary aim of this study was to determine if children with laryngeal penetration who received feeding interventions (thickened liquids, change in liquid flow rate and/or method of liquid delivery) after their swallow study had improved symptoms and decreased hospitalizations compared to those without intervention. Secondary aims were to determine if presenting characteristic could predict finding laryngeal penetration on VFSS and if penetration was persistent over time. We hypothesized that children receiving feeding interventions would have improved symptoms and fewer hospitalizations compared to those who did not receive interventions.
Methods
We reviewed the records of all children under 2 years who had isolated laryngeal penetration and no aspiration on VFSS performed at Boston Children’s Hospital in 2015. Subjects were identified by Informatics for Integrating Biology and the Bedside software(23). The primary aims were to determine if there was symptom improvement and change in hospitalization in patients who were and were not treated with feeding interventions (i.e. change in liquid flow rate or thickening of formula) for their laryngeal penetration. Additional aims were to determine if any presenting characteristics could predict finding penetration on VFSS and if penetration was persistent over time. Records were reviewed to determine subject characteristics, presenting symptoms, initial and follow-up VFSS findings, whether there was symptom improvement at follow-up, and hospitalization risk before and after VFSS. All VFSS were performed by speech-language pathologists (SLP) with pediatric radiologists in standard fashion (8, 9, 24). Proportions were compared with Fisher’s exact test and hospitalizations were compared with paired t-tests. Data were analyzed using SPSS Statistics (version 23). The present study was approved by the Institutional Review Board at Boston Children’s Hospital (IRB-P00023746, approved 10/19/16). For detailed methods, see Supplemental Methods section.
Results
We evaluated 137 subjects with mean age 8.93±0.59 months who had isolated laryngeal penetration without evidence of aspiration on their first VFSS. Baseline characteristics, subspecialty of provider that ordered the VFSS, subject comorbidities, and symptoms present at time of referral are in Table 1. These are shown for the cohort overall and also compared between those with and those without a feeding intervention after they were found to have penetration. Subjects that received intervention were 2.5 months younger than those that did not receive an intervention (p=0.029). Subjects with oxygen requirement were less likely to receive intervention (p=0.04). Of note, none of the other baseline characteristics, including ordering providers, comorbidities or presenting symptoms differed significantly between the groups.
Table 1: Baseline Characteristics, VFSS Ordering Provider and Presenting Symptoms.
Demographic characteristics, provider that ordered VFSS, comorbidities, and symptoms present at time of referral to VFSS are shown with a comparison between subjects that received feeding intervention for their laryngeal penetration and those that did not receive a feeding intervention. Data are expressed as % (n) and mean ± standard error.
| All Subjects (n=137) | Feeding Intervention (n=75) | No Feeding Intervention (n=62) | P-value | |
|---|---|---|---|---|
| Baseline Characteristics | ||||
| Male | 61% (84) | 57% (43) | 66% (41) | 0.378 |
| Age at VFSS (months) | 8.93 + 0.59 | 7.73 ± 0.69 | 10.37 ± 0.97 | 0.029 |
| Symptom Duration Prior to VFSS (months) | 6.28 ±0.93 | 6.82 ± 1.28 | 5.71 ± 1.39 | 0.565 |
| VFSS Ordering Provider | ||||
| Gastroenterology | 34% (47) | 33% (25) | 36% (22) | 0.857 |
| General Pediatrics | 26% (35) | 28% (21) | 23% (14) | 0.556 |
| Pulmonology | 18% (25) | 17% (13) | 19% (12) | 0.826 |
| Otolaryngology | 10% (14) | 11% (8) | 10% (6) | 1.000 |
| Other Subspecialty | 8% (11) | 9% (7) | 7% (4) | 0.754 |
| Neurology | 4% (5) | 1% (1) | 7% (4) | 0.176 |
| Comorbidities | ||||
| Prematurity | 35% (48) | 37% (28) | 32% (20) | 0.592 |
| Neurologic | 22% (30) | 16% (12) | 30% (18) | 0.065 |
| Gastrointestinal | 18% (24) | 20% (15) | 15% (9) | 0.500 |
| Pulmonary | 15% (21) | 13% (10) | 18% (11) | 0.487 |
| Cardiac | 10% (13) | 11% (8) | 8% (5) | 0.772 |
| Metabolic | 10% (13) | 8% (6) | 11% (7) | 0.567 |
| Presenting Symptom | ||||
| Choking/Gagging | 41% (56) | 40% (30) | 42% (26) | 0.862 |
| Reflux | 34% (47) | 35% (26) | 34% (21) | 1.000 |
| Vomiting | 30% (41) | 33% (25) | 26% (16) | 0.356 |
| Poor Feeding | 23% (31) | 24% (18) | 21% (13) | 0.688 |
| Slow Feeding | 9% (12) | 8% (6) | 10% (6) | 0.769 |
| Coughing | 66% (91) | 69% (52) | 63% (39) | 0.470 |
| Noisy Breathing | 29% (39) | 32% (24) | 24% (15) | 0.346 |
| Congestion | 22% (30) | 24% (18) | 19% (12) | 0.541 |
| Spells | 19% (26) | 21% (16) | 16% (10) | 0.515 |
| Respiratory Distress | 14% (19) | 12% (9) | 16% (10) | 0.621 |
| Recurrent Pneumonia | 12% (17) | 12% (9) | 13% (8) | 1.000 |
| Oxygen Requirement | 3% (4) | 0% (0) | 7% (4) | 0.040 |
| Symptoms During Meals | 78% (105) | 77% (57) | 78% (48) | 0.839 |
| Symptoms After Meals | 34% (47) | 41% (30) | 27% (17) | 0.147 |
| Symptoms Both During and After Meals | 24% (33) | 30% (22) | 18% (11) | 0.113 |
| No Relation to Meals | 12% (16) | 12% (9) | 11% (7) | 1.000 |
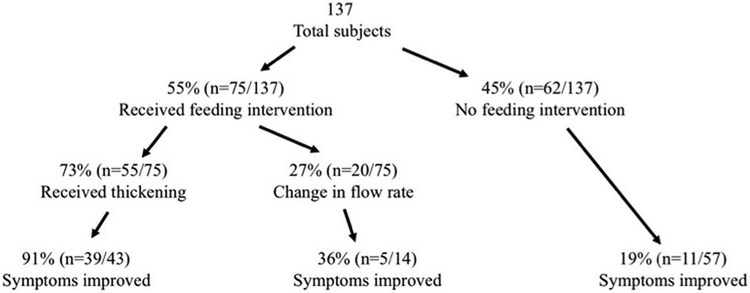
There was significant variability in the management of these patients, as shown in Figure 1. Overall, 60% (n=82) were maintained on thin liquids, 9% (n=12) were treated with half-nectar, 29% (n=39) were treated with nectar, and 3% (n=4) were treated with honey consistency. Approaches varied depending on which specialty cared for the patient and ordered the VFSS, with thickening rates of 37% for general pediatrics, 38% for gastroenterology, 48% for pulmonology, 43% for otolaryngology, and 0% for neurology.
Figure 1: Study Population.
The flow diagram shows the percentage and numbers of subjects in each group, how many subjects had each type of feeding intervention for their laryngeal penetration, and how many subjects, of those that were seen in follow-up and following their prescribed feeding intervention, that had symptom improvement in the follow-up period.
The depth and consistency of laryngeal penetration was varied in the cohort. 62% (n=80) of subjects had deep penetration and 32% (41) had shallow penetration. 31% (n=40) had consistent penetration, 54% (n=70) had inconsistent penetration, and 15% (n=20) had only 1 episode of penetration, as shown in Figure, Supplemental Digital Content 1. Subjects with deep/consistent penetration were more likely to receive intervention compared to shallow/inconsistent or only one episode (p<0.0001). 36% (n=47) described the penetration that was observed as “clinically insignificant” while the other characteristics of these subjects’ penetration was quite varied, with 60% shallow, 40% deep, 57% inconsistent, 13% consistent, and 30% just one episode.
Change in management largely depended on depth/frequency of penetration observed, with subjects having deep/consistent penetration most likely to receive thickening compared to those with shallow/inconsistent penetration, but even subjects with inconsistent penetration had symptom improvement after receiving thickening (n=41 vs n=3, p<0.0001 for deep and n=13 vs n=6, p=0.008 for inconsistent), as shown in Figure, Supplemental Digital Content 2.
Of the 137 subjects, 84% (n=115) were seen in clinic for follow-up during the study period at a mean of 78.3±12.5 days following first VFSS. Of these, 88% (n=101) were reportedly following their recommended intervention (thickening, change in flow rate, some combination of these two approaches, or no intervention). There was significant improvement in presenting symptoms for children that had feeding intervention and improvement was greatest for those that received thickening, as shown in Table 2. In a comparison of symptom improvement between subjects that received thickening vs change in liquid flow rate alone, those that received thickening had significantly higher rates of improvement (OR 17.55, 95% CI 3.91-78.76, p<0.001).
Table 2: Symptom Improvement at Follow-up Compared by Feeding Intervention.
At follow-up visits, subjects that received feeding intervention for their laryngeal penetration had significantly higher rates of symptom improvement compared to those that did not receive intervention and this difference was most striking for subjects that received thickening. Data are expressed as percent (n) or odds ratio (95% confidence interval).
| Symptom Improved | Symptom Not Improved | Odds Ratio (95% CI) | p-value | |
|---|---|---|---|---|
| Any Feeding Intervention vs No Feeding Intervention | ||||
| Any Feeding Intervention | 77% (44) | 23% (13) | 17.89 (6.47–49.49) | p<0.001 |
| No Feeding Intervention | 16% (7) | 84% (37) | ||
| Thickening Formula vs No Feeding Intervention | ||||
| Thickening Formula | 91% (40) | 9% (4) | 41.8 (12.34–141.69) | p<0.001 |
| No Feeding Intervention | 19% (11) | 81% (46) | ||
| Thickening Formula vs Change in Flow Rate | ||||
| Thickening Formula | 91% (39) | 9% (4) | 17.55 (3.91–78.76) | p<0.001 |
| Change in Flow Rate | 36% (5) | 64% (9) | ||
Subjects receiving any feeding intervention (change in flow rate or thickening) had decreased total and pulmonary hospitalizations and patients receiving thickening had decreased pulmonary hospitalization nights as shown in Table 3. In patients who did not receive an intervention at the time of VFSS, there was no significant decrease in hospitalizations after VFSS compared to the time period before VFSS, suggesting that symptom improvement does not improve naturally over time. Notably, 30% (n=6) of the subjects that were reported to have a single episode of laryngeal penetration on their VFSS and 26% (n=12) of the subjects that were described as having penetration that was thought to be “clinically insignificant” had at least one hospital admission during the follow-up period.
Table 3: Hospitalization Risk Compared by Interventions for Laryngeal Penetration.
Hospitalization numbers were found to be decreased after feeding interventions and formula thickening but not for subjects that did not receive intervention for their laryngeal penetration. Data are expressed as mean ± standard error.
| Prior to Thickening | After Thickening | p-value | |
|---|---|---|---|
| Total Admissions | 0.60 ± 0.12 | 0.44 ± 0.10 | 0.261 |
| Total Nights | 2.27 ± 0.69 | 1.00 ± 0.29 | 0.077 |
| Pulmonary Admissions | 0.33 ± 0.09 | 0.15 ± 0.07 | 0.058 |
| Pulmonary Nights | 1.20 ± 0.43 | 0.31 ± 0.17 | 0.048 |
| GI Admissions | 0.16 ± 0.07 | 0.09 ± 0.05 | 0.322 |
| GI Nights | 1.00 ± 0.57 | 0.41 ± 0.23 | 0.307 |
| Prior to Thickening | After Thickening | p-value | |
| Total Admissions | 0.60 ± 0.12 | 0.44 ± 0.10 | 0.261 |
| Total Nights | 2.27 ± 0.69 | 1.00 ± 0.29 | 0.077 |
| Pulmonary Admissions | 0.33 ± 0.09 | 0.15 ± 0.07 | 0.058 |
| Pulmonary Nights | 1.20 ± 0.43 | 0.31 ± 0.17 | 0.048 |
| GI Admissions | 0.16 ± 0.07 | 0.09 ± 0.05 | 0.322 |
| GI Nights | 1.00 ± 0.57 | 0.41 ± 0.23 | 0.307 |
| Prior to VFSS | After VFSS | p-value | |
| Total Admissions | 0.53 ± 0.16 | 0.45 ± 0.13 | 0.639 |
| Total Nights | 2.03 ± 0.72 | 1.36 ± 0.51 | 0.431 |
| Pulmonary Admissions | 0.19 ± 0.06 | 0.16 ± 0.07 | 0.718 |
| Pulmonary Nights | 0.52 ± 0.20 | 0.58 ± 0.39 | 0.867 |
| GI Admissions | 0.00 ± 0.00 | 0.03 ± 0.02 | 0.159 |
| GI Nights | 0.00 ± 0.00 | 0.07 ± 0.05 | 0.208 |
In the cohort overall, there was a mean of 1.44±0.07 swallow studies performed per subject during the study period. Of the 137 subjects, 31% (n=42) went on to have a second VFSS and on that study, 31% (n=13) had normal swallow function, 41% (n=17) had persistent penetration, and notably 26% (n=11) now had evidence of aspiration that was not captured on their initial VFSS. Of these, 91% (n=10) did not have neurologic impairments or other comorbidities that might have been expected to cause a decline in swallow function.
We additionally evaluated each presenting symptom for evidence of association with laryngeal penetration. Coughing was the only symptom that was significantly associated with laryngeal penetration (OR 1.792, 95% CI 1.166-2.753, p=0.008). Odds ratios for all other symptoms ranged from 0.497 to 2.008 (p>0.10).
Discussion
Laryngeal penetration is a common finding in the field of pediatric swallow disorders with a relevance that remains fiercely debated, perhaps due to the paucity of studies addressing its true clinical significance (1, 2, 20, 25). In the current study, we showed that interventions for penetration are associated with improvements in presenting symptoms and hospitalization risk in young children, both of which are clinically significant markers that suggest a potentially large effect of such a small intervention. While all feeding interventions were associated with improved outcomes, thickening of formula seemed to have the single greatest impact on symptom improvement. This therapeutic approach can make these children feel better, relieve parental concern, and actually improve clinical outcomes with an inexpensive intervention that would likely decrease costs by preventing expensive hospitalizations. In contrast, we found that outcomes are not changed for patients who do not get interventions for their penetration. Thus without intervention there is no improvement in hospitalizations and limited symptom improvement at follow-up.
One finding in this study was the diverse range of providers ordering these studies, which may result in great variability in management approaches to penetration. Swallowing abnormalities such laryngeal penetration are increasing in frequency in the pediatric population as more premature infants and other children with chronic medical conditions survive (4, 13, 14). Therefore, this issue will likely become only more important in the future and, as our results suggest, more medical providers will be involved in management of these patients.
There is a wide range of approaches to laryngeal penetration and some SLPs and/or radiologists will comment in their reports that non-clinically significant penetration is present which may result in providers not offering intervention to these patients, even when such patients are persistently symptomatic in our clinical experience, and we show in this study that this assumption of normalcy may affect clinical outcomes. In many institutions, interventions are not commonly pursued after finding penetration on swallow study for several possible reasons(20–22). Some providers consider penetration a normal variant and therefore do not believe it needs treatment. Others feel that it is not indicative of aspiration risk. Still others might worry that the risk of thickening agents or other feeding interventions might outweigh any benefit of intervention. Based on the results of the present study, we feel that any finding of laryngeal penetration in a symptomatic child should be considered clinically significant and a change in management should be considered.
There are several important practical considerations from our study. First, the current approach to laryngeal penetration is variable. In our cohort, only 55% of subjects had change in their management, with most receiving thickening but others receiving a change in liquid flow rate. Feeding interventions are typically made at the discretion and recommendation of the SLP that performs the study or by the ordering physician. One limitation to our study may be that only the most symptomatic patients received thickening and thickening was not needed in the non-intervention patients. Our data suggests this is not the case with the patients who did not receive interventions showing no improvement in symptoms over time; 91% of subjects had symptom improvement with thickening, compared to only 19% improvement at follow-up in the group that received no intervention. Also notably, even some of the patients who were thought to have only transient penetration on their swallow study and who therefore did not receive treatment ultimately went on to have both pulmonary/gastrointestinal hospitalizations, emphasizing the importance of treating any degree of laryngeal penetration.
Another important finding from the current study is that laryngeal penetration is not transient and may actually be indicative of aspiration risk. To our knowledge, no prior studies have evaluated whether the penetration can predict future aspiration; on repeat VFSS, 26% of subjects with prior laryngeal penetration were found to have frank aspiration that was not captured on initial VFSS and, importantly, the great majority of these patients were not neurologically impaired and did not have any other comorbidity, such as cardiac disease, that would have been expected to cause a decline in swallow function. This is consistent with the results of Friedman et al, who showed that deep laryngeal penetration might also predict the occurrence of aspiration in children within individual swallow studies (2). These results combined highlight the transient nature of VFSS that captures only a very limited moment in time.
An additional practical consideration is our analysis of which presenting symptoms are suggestive of laryngeal penetration. While multiple studies have shown that oropharyngeal dysphagia with aspiration is typically silent in this age group, including recent large studies from our own institution, it is less clear if children with penetration have clear symptoms that can be tied to their type of oropharyngeal dysphagia (16, 26–28). Silva-Munhoz found an association between choking and finding isolated laryngeal penetration in a group of 15 children(29). Weir also examined symptoms predictive of isolated laryngeal penetration and found no consistent correlation (26). In the present study with a larger cohort, we found an association between cough and the presence of laryngeal penetration.
Lastly, and perhaps of greatest clinical significance, we showed that children who receive interventions for their laryngeal penetration have decreased hospitalizations. In particular, thickening and other feeding interventions were seen to affect pulmonary hospitalizations, suggesting additionally that laryngeal penetration puts children at increased risk for respiratory exacerbations. These results complement and add to prior studies in this field. Few studies have evaluated the impact of feeding interventions on improvement in symptoms and outcomes in any type of oropharyngeal dysphagia(25). Jackson showed symptom improvements from feeding interventions in a population of Down syndrome patients with aspiration (30). Coon questioned the benefits of thickening for oropharyngeal dysphagia patients but found decreased acute respiratory hospitalizations for children with silent aspiration that received thickening (31). Khoshoo showed that thickening decreased the occurrence of aspiration in a small group of infants with swallowing dysfunction associated with bronchiolitis (32, 33). Krummrich used a parental reporting tool and found that parents of young children with dysphagia reported a significant decrease in symptoms and increase in oral intake when their children were started on thickened liquids(34). None of these studies, however, evaluated outcomes in isolated laryngeal penetration.
There are a small number of other studies showing that there is clinical significance to penetration, though ours is the first to show that treatment is associated with improved outcomes. Gurberg reported on 235 children with oropharyngeal dysphagia with a mean age of 5.6 years, of whom only 59 had laryngeal penetration, that children with laryngeal penetration have increased rates of pneumonia over the 3 years after the diagnosis of laryngeal penetration (17). Serel Arslan also showed a positive correlation between penetration and recurrent pneumonia in a population of 274 children with a median age of 2.75 years with oropharyngeal dysphagia, of whom 89 had isolated laryngeal penetration (18). This result is similar to adult studies showing a 4 times increased risk of pneumonia in patients with penetration (35). We also previously showed that oropharyngeal dysphagia is associated with the occurrence of brief resolved unexplained events (BRUE, formerly known as apparent life threatening events) and in that study 43% of subjects admitted after BRUE had isolated laryngeal penetration on VFSS, suggesting this is a clinically relevant entity(36).
There are a number of limitations to the present study, including the retrospective nature of this study. At our institution, the SLP performing the VFSS in conjunction with the ordering physician makes decisions about what interventions to pursue during the course of the VFSS. The retrospective nature of this study limited our ability to consider any unmeasured factors that might have determined which patients received feeding interventions, thickening or no intervention for their penetration (i.e. parent or provider preference in addition to results of prior clinical feeding evaluations, other medical/feeding history, or the medical team’s understanding of the underlying pathophysiology of swallowing dysfunction) but importantly, apart from age and oxygen requirement, none of the other baseline characteristics, including ordering providers, comorbidities or presenting symptoms differed significantly between the groups. It is also possible that thickening itself might have had other beneficial effects such as decreasing gastroesophageal reflux. In measuring symptom improvement, we relied on caregiver report, which is likely subjective but we believe still clinically relevant; for this reason, we also used hospitalization as an objective measure of comparison. Additionally, while we have the SLP and radiologists’ categorization of severity of penetration, validated scoring such as the Penetration-Aspiration Score, was not used at the time of VFSS; however, lack of standardization in pediatric fluoroscopy is a national problem and our institution is not alone(37, 38). Future studies will be needed to delineate outcomes for intervening based on specific degrees of penetration, nutritional symptoms such as failure to thrive, and use of standardized protocols in the approach to isolated laryngeal penetration.
Conclusion
Laryngeal penetration appears to be clinically significant in symptomatic children and feeding interventions are associated with improved outcomes. Thickening or other feeding intervention should be considered for all symptomatic children with laryngeal penetration on VFSS. Close collaboration and follow-up with SLPs, ordering providers and patients is needed to insure that different feeding interventions are considered and to monitor efficacy and any side effects from interventions.
Supplementary Material
The proportion of subjects that received feeding intervention for each penetration type. Subjects with deep/consistent penetration were more likely to receive intervention compared to shallow/inconsistent or only one episode (p<0.0001).
The proportion of subjects with symptom improvement after feeding intervention for each penetration type. Significant p-values are shown.
What is Known
There is debate about the clinical significance of laryngeal penetration on videofluoroscopic swallow study and there is great variability in the clinical approach to these patients.
No prior studies have examined the benefits of feeding interventions in these patients.
What This Study Adds
Laryngeal penetration is not transient in children under 2 years of age and may be indicative of aspiration risk.
Symptomatic patients with laryngeal penetration who receive feeding interventions, and especially those who receive thickening, have improved symptoms and decreased hospitalizations compared to those with no change in management.
Acknowledgements
This work was supported by The Translational Research Program at Boston Children’s Hospital, NIH R01 DK097112-01, and NIH T32 DK007477-33. The authors have no conflicts of interest to disclose. This work was previously presented in part at Digestive Diseases Week in Washington, DC, June 2018.
Conflicts of Interest and Source of Funding: This work was supported by The Translational Research Program at Boston Children’s Hospital, NIH R01 DK097112-01, and NIH T32 DK007477-33. The authors have no conflicts of interest to disclose.
References
- 1.Delzell PB, Kraus RA, Gaisie G, et al. Laryngeal penetration: a predictor of aspiration in infants? Pediatr Radiol 1999;29:762–5. [DOI] [PubMed] [Google Scholar]
- 2.Friedman B, Frazier JB. Deep laryngeal penetration as a predictor of aspiration. Dysphagia 2000;15:153–8. [DOI] [PubMed] [Google Scholar]
- 3.Borowitz KC, Borowitz SM. Feeding Problems in Infants and Children: Assessment and Etiology. Pediatr Clin North Am 2018;65:59–72. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya N The prevalence of pediatric voice and swallowing problems in the United States. Laryngoscope 2015;125:746–50. [DOI] [PubMed] [Google Scholar]
- 5.Benfer KA, Weir KA, Bell KL, et al. Oropharyngeal Dysphagia and Cerebral Palsy. Pediatrics 2017;140. [DOI] [PubMed] [Google Scholar]
- 6.McGrattan KE, McGhee H, DeToma A, et al. Dysphagia in infants with single ventricle anatomy following stage 1 palliation: Physiologic correlates and response to treatment. Congenit Heart Dis 2017;12:382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suterwala MS, Reynolds J, Carroll S, et al. Using fiberoptic endoscopic evaluation of swallowing to detect laryngeal penetration and aspiration in infants in the neonatal intensive care unit. J Perinatol 2017;37:404–8. [DOI] [PubMed] [Google Scholar]
- 8.Arvedson JC, Lefton-Greif MA. Instrumental Assessment of Pediatric Dysphagia. Semin Speech Lang 2017;38:135–46. [DOI] [PubMed] [Google Scholar]
- 9.Hiorns MP, Ryan MM. Current practice in paediatric videofluoroscopy. Pediatr Radiol 2006;36:911–9. [DOI] [PubMed] [Google Scholar]
- 10.Aviv JE, Spitzer J, Cohen M, et al. Laryngeal adductor reflex and pharyngeal squeeze as predictors of laryngeal penetration and aspiration. Laryngoscope 2002;112:338–41. [DOI] [PubMed] [Google Scholar]
- 11.Durvasula VS, O’Neill AC, Richter GT. Oropharyngeal Dysphagia in children: mechanism, source, and management. Otolaryngol Clin North Am 2014;47:691–720. [DOI] [PubMed] [Google Scholar]
- 12.Lefton-Greif MA, Carroll JL, Loughlin GM. Long-term follow-up of oropharyngeal dysphagia in children without apparent risk factors. Pediatr Pulmonol 2006;41:1040–8. [DOI] [PubMed] [Google Scholar]
- 13.Svystun O, Johannsen W, Persad R, et al. Dysphagia in healthy children: Characteristics and management of a consecutive cohort at a tertiary centre. Int J Pediatr Otorhinolaryngol 2017;99:54–9. [DOI] [PubMed] [Google Scholar]
- 14.Newman LA, Keckley C, Petersen MC, et al. Swallowing function and medical diagnoses in infants suspected of Dysphagia. Pediatrics 2001;108:E106. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Chang YS, Yoo HS, et al. Swallowing dysfunction in very low birth weight infants with oral feeding desaturation. World J Pediatr 2011;7:337–43. [DOI] [PubMed] [Google Scholar]
- 16.Weir KA, McMahon S, Taylor S, et al. Oropharyngeal aspiration and silent aspiration in children. Chest 2011;140:589–97. [DOI] [PubMed] [Google Scholar]
- 17.Gurberg J, Birnbaum R, Daniel SJ. Laryngeal penetration on videofluoroscopic swallowing study is associated with increased pneumonia in children. Int J Pediatr Otorhinolaryngol 2015;79:1827–30. [DOI] [PubMed] [Google Scholar]
- 18.Serel Arslan S, Demir N, Karaduman AA. Both pharyngeal and esophageal phases of swallowing are associated with recurrent pneumonia in pediatric patients. Clin Respir J 2018;12:767–71. [DOI] [PubMed] [Google Scholar]
- 19.Strychowsky JE, Dodrill P, Moritz E, et al. Swallowing dysfunction among patients with laryngeal cleft: More than just aspiration? Int J Pediatr Otorhinolaryngol 2016;82:38–42. [DOI] [PubMed] [Google Scholar]
- 20.Allen JE, White CJ, Leonard RJ, et al. Prevalence of penetration and aspiration on videofluoroscopy in normal individuals without dysphagia. Otolaryngol Head Neck Surg 2010;142:208–13. [DOI] [PubMed] [Google Scholar]
- 21.Molfenter SM, Hsu CY, Lu Y, et al. Alterations to Swallowing Physiology as the Result of Effortful Swallowing in Healthy Seniors. Dysphagia 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daggett A, Logemann J, Rademaker A, et al. Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia 2006;21:270–4. [DOI] [PubMed] [Google Scholar]
- 23.Murphy SN, Weber G, Mendis M, et al. Serving the enterprise and beyond with informatics for integrating biology and the bedside (i2b2). J Am Med Inform Assoc 2010;17:124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jadcherla SR, Stoner E, Gupta A, et al. Evaluation and management of neonatal dysphagia: impact of pharyngoesophageal motility studies and multidisciplinary feeding strategy. J Pediatr Gastroenterol Nutr 2009;48:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gosa MM, Carden HT, Jacks CC, et al. Evidence to support treatment options for children with swallowing and feeding disorders: A systematic review. J Pediatr Rehabil Med 2017;10:107–36. [DOI] [PubMed] [Google Scholar]
- 26.Weir K, McMahon S, Barry L, et al. Clinical signs and symptoms of oropharyngeal aspiration and dysphagia in children. Eur Respir J 2009;33:604–11. [DOI] [PubMed] [Google Scholar]
- 27.Velayutham P, Irace AL, Kawai K, et al. Silent aspiration: Who is at risk? Laryngoscope 2018;128:1952–7. [DOI] [PubMed] [Google Scholar]
- 28.Duncan DR, Mitchell PD, Larson K, et al. Presenting Signs and Symptoms do not Predict Aspiration Risk in Children. J Pediatr 2018;201:141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva-Munhoz Lde F, Buhler KE, Limongi SC. Comparison between clinical and videofluoroscopic evaluation of swallowing in children with suspected dysphagia. Codas 2015;27:186–92. [DOI] [PubMed] [Google Scholar]
- 30.Jackson A, Maybee J, Moran MK, et al. Clinical Characteristics of Dysphagia in Children with Down Syndrome. Dysphagia 2016;31:663–71. [DOI] [PubMed] [Google Scholar]
- 31.Coon ER, Srivastava R, Stoddard GJ, et al. Infant Videofluoroscopic Swallow Study Testing, Swallowing Interventions, and Future Acute Respiratory Illness. Hosp Pediatr 2016;6:707–13. [DOI] [PubMed] [Google Scholar]
- 32.Khoshoo V, Ross G, Kelly B, et al. Benefits of thickened feeds in previously healthy infants with respiratory syncytial viral bronchiolitis. Pediatr Pulmonol 2001;31:301–2. [DOI] [PubMed] [Google Scholar]
- 33.Khoshoo V, Edell D. Previously healthy infants may have increased risk of aspiration during respiratory syncytial viral bronchiolitis. Pediatrics 1999;104:1389–90. [DOI] [PubMed] [Google Scholar]
- 34.Krummrich P, Kline B, Krival K, et al. Parent perception of the impact of using thickened fluids in children with dysphagia. Pediatr Pulmonol 2017;52:1486–94. [DOI] [PubMed] [Google Scholar]
- 35.Pikus L, Levine MS, Yang YX, et al. Videofluoroscopic studies of swallowing dysfunction and the relative risk of pneumonia. AJR Am J Roentgenol 2003;180:1613–6. [DOI] [PubMed] [Google Scholar]
- 36.Duncan DR, Amirault J, Mitchell PD, et al. Oropharyngeal Dysphagia Is Strongly Correlated With Apparent Life-Threatening Events. J Pediatr Gastroenterol Nutr 2017;65:168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hind JA, Gensler G, Brandt DK, et al. Comparison of trained clinician ratings with expert ratings of aspiration on videofluoroscopic images from a randomized clinical trial. Dysphagia 2009;24:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration-aspiration scale. Dysphagia 1996;11:93–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The proportion of subjects that received feeding intervention for each penetration type. Subjects with deep/consistent penetration were more likely to receive intervention compared to shallow/inconsistent or only one episode (p<0.0001).
The proportion of subjects with symptom improvement after feeding intervention for each penetration type. Significant p-values are shown.