Abstract
Purpose of Review
Present a conceptual model and review the recent literature on family dynamics, sleep, and hypertension.
Recent Findings
Family dynamics predict hypertension and hypertension risk, in part, due to shared health behaviors. Sleep health behaviors (sleep duration, quality, and efficiency) predict hypertension risk in children and youth, and are emerging as a family-level health behavior. Importantly, both family dynamics and sleep are modifiable. Family members influence one another’s sleep through their physical presence and through psychological and emotional mechanisms. Family members’ sleep patterns may also be coregulated. Negative family dynamics are associated with poor sleep health and predict greater cardiovascular risk. Sleep health behaviors in the family context may also interact with family dynamics to dampen or exacerbate hypertension risk factors in children and youth.
Summary
This review proposes that promoting sleep health in a family context could be one way to reduce long-term hypertension risk.
Keywords: Family relationships, family dynamics, sleep behaviors, sleep health, children, youth, hypertension, hypertension risk
Introduction
Negative family dynamics are strong contributors to the development of hypertension [1]. Hypertension risks are similar among family members, partly due to genetic dispositions inherited by children [2]. However, close family relationships also contribute to individual hypertension risk through daily interactions and the transmission of health behaviors. Cohabitating family members are one of the primary and consistent sources of relationship security or stress, which, in turn, has downstream consequences for health. For example, frequent and negative exchanges within family units are associated with increased risk for chronic illnesses (e.g., cardiovascular disease), while positive family relationships are associated with better physical health outcomes [3]. Children also learn about health behaviors from the family unit [4]. and individuals within a family tend to have concordant health and health behaviors [5]. For example, childhood obesity is strongly linked to parent’s obesity [6]. Therefore, a family- based approach to the study of hypertension can inform important psychosocial targets for intervention [1]. Indeed, family-based interventions can positively influence health behaviors (e.g.,[7]).
One such family-level behavior that influences hypertension risk is sleep health. Sleep health is a multidimensional concept that refers to sleep duration, sleep continuity, timing, sleepiness versus alertness, and quality or satisfaction [8]. Associations between sleep health and cardiovascular outcomes, including hypertension have been reviewed extensively (e.g., see [9] [10]). Briefly, sleep quality, duration, timing, and fragmentation (wake after sleep onset), predict hypertension risk across the age spectrum. For example, short sleep duration (< 6 hours) is associated with increased hypertension via risk factors such as obesity in children and adolescents [11] [12]. Moreover, short sleep duration appears to have a direct association with hypertension. In one of the first longitudinal studies of…, adults with short sleep duration, and without other sleep disorders, were about two times more likely to develop hypertension even after controlling for other prominent risk factors (e.g., obesity, diabetes; [13]). Importantly, sleep behaviors are modifiable, and improving sleep may improve other health behaviors [14]. Thus, examining sleep in a family context allows for a greater understanding of two interrelated, and modifiable, predictors of hypertension: family dynamics and sleep.
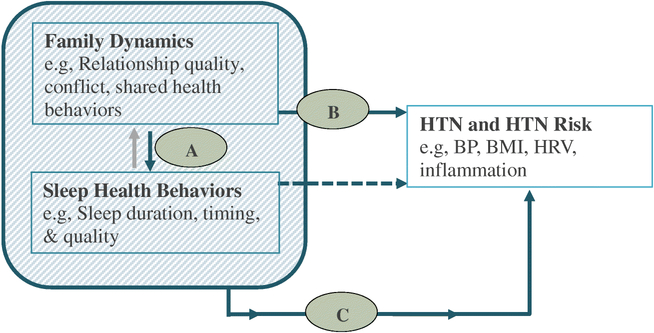
In the following pages, we present and review a conceptual model of sleep, hypertension, and family relationships whereby sleep health and family relationships have a synergistic effect on the development of hypertension (Figure 1). We summarize data published in the last five years. Our summary includes studies that measured constructs of family dynamics (e.g., conflict, cohesion, structure, parenting) and how they relate to sleep behaviors and hypertension outcomes in children and youth of all ages. We focus on children because early life experiences and learned health behaviors lay the foundation for lifelong health outcomes [15-18]. Most of our review focuses on dynamics within the parent-child relationship, which also reflects the majority of the literature on family dynamics and sleep and hypertension. Furthermore, while the correlation between breathing related sleep disorders and hypertension risk is strong, we limited our studies to those focused on sleep behaviors because they are amenable to behavioral intervention and are also independently associated with hypertension [19] and hypertension risk in youth [11].
Figure 1.
A conceptual model of influence of family dynamics and sleep health behaviors on hypertension risk. Bold arrows indicate proposed directions; dashed arrow represents well-documented association between sleep behaviors and hypertension; gray arrow represents bidirectional association to consider in future research. HTN = hypertension; BP = blood pressure; BMI = body mass index; HRV = heart rate variability
The organization of this review corresponds to the conceptual model (Figure 1). We present a framework and review of family dynamics and sleep health (Part A, Figure 1); family dynamics and hypertension risk (Part B, Figure 1); and a review of the few studies measuring family dynamics, sleep health, and hypertension risk (Part C, Figure 1). Finally, we discuss gaps in the literature and future directions for research and necessary steps to develop family-based approaches to improve sleep health, and ultimately, cardiovascular health.
Family relationships and sleep.
We argue that the family environment is an important social construct to consider with respect to sleep and hypertension risk for several reasons. First, there is the opportunity for an individual’s sleep to be influenced by the physical presence of family members. For example, about 25% of children share a bed with their parents [20], and many sleep complaints are due to noise and habits of cohabitating family members [21, 22]. In addition, family members may serve as social zeitgebers (any external or environmental cue that sets biological rhythms) and regulate one another’s sleep timing, and the timing of daily activities that influence circadian rhythms. Second, the presence of family members can influence sleep through psychological or emotional mechanisms. Families are often the first and primary potential source of interpersonal and emotional security, which in turn, is important for sleep [23]. Sleep requires decreased arousal and responsiveness to the external environment, which increases vulnerability. Our ancestors relied on their social network to keep them safe from prey while they slept [23, 24]. Though modern-day and westernized humans are not as susceptible to these threats, relationship insecurity increases arousal [25], which is counterproductive for sleep [26]. This suggests that sleep is vulnerable to social threat. Family relationships, therefore, are especially poised to enhance or dampen the interpersonal security that is necessary for sleep.
Third, emerging evidence suggests that coregulation or synchrony of sleep and circadian processes likely occur within family units. This is because one feature of close relationships is coregulation of psychological and physiological processes [27]. For example, cortisol is co-regulated in mother-child dyads [28] and mothers and infant dyads show parasympathetic synchrony [29]. Coregulation of sleep can be measured by the degree of concordance in sleep and circadian parameters. For example, family members could be concordant in their bed and wake times [30]or in sleep duration [31]. The degree to which families exhibit coregulatory sleep patterns is important for understanding the degree to which family members’ sleep and circadian health are connected.
Thus, sleep appears to be a socially influenced process. Of course, the ways in which family members influence one another’s sleep are interconnected and interdependent. For example, appropriate levels of parental monitoring contributes to increased feelings of security in children [32]. Monitoring also predicts consistent bedtimes and wake times, which is associated with a longer sleep duration [33]. While there are other pathways by which families influence sleep (e.g., genetic predispositions), the purpose of this framework is to highlight primary (and modifiable) targets for intervening to improve sleep and hypertension risk in a family context.
Recent findings on the family dynamics and sleep (Table 1).
Table 1.
Family Dynamics and Sleep
| Author, Year | Sample (n, age, age group, race, population, gender) |
Study Design |
Family variable | Sleep Variable | Covariates | Results |
|---|---|---|---|---|---|---|
| Short, 2013 | US sample: N = 302; Mage = 16.03; ages 13.8-19.9;35.15% male; Australian sample: N = 385; Mage =15.57; ages = 13.3-18.9;59.2% male | Cross sectional | Parent-set bedtime | Adolescent sleep diary: TST | gender, age, school start time, extracurricular load |
Parent-set bedtime ↑ TST |
| Kelly, 2013 [81] | N = 176 US children; 3rd grade at baseline; 32% male; 31% AA, 69% White |
Longitudinal | Marital aggression and emotional insecurity in 3rd and 5th | Child reported: Sleep/wake Problems in 3rd and 5th grade; Actigraphy assessed:SE and TST in 3rd and 5th grade | ethnicity, SES, gender |
Emotional insecurity and marital aggression and TST in 5th grade ns. Emotional insecurity and marital aggression = ↓ SE at 5th grade Emotional insecurity and marital aggression =sleep problems in 5th grade |
| Ray, 2013 [42] | N = 805 Finnish child; ages 10–11 years; 50% male | Cross sectional | Parent practices (e.g. rules and routine); parental warmth/responsiveness | Adolescent reported: TST | gender, gender of parent, grade school, parent’s educational level, employment status | ↑ Parenting practices ↑ TST warmth/responsiveness did not moderate the association between parenting practices and TST |
| Maume, 2013 [40] | N = 974 US adolescents; 6th grade at baseline; follow up at age 15; 50% male; 80% white, 20% nonwhite | Longitudinal | Mother’s partner leaves; mother has new partner; parental-adolescent bond; change in parent-adolescent bond; parental monitoring; change in parental monitoring index; consistent set bedtime | Adolescent reported: TST, sleep disruption | pubertal development, delayed phase preference, race, gender |
Mother’s partner leaves
↓ TST; ↑ sleep disruption Mother has new partner TST ns.; sleep dismption ns. Parental bond TST ns.; sleep dismption ns. Increase in parent-adolescent bond ↑ TST; ↓ sleep disruption ↑ Parental monitoring ↑ TST; sleep disruption ns. ↑ Change parental monitoring ↑ TST; ↓ sleep disruption ↑ Consistent set bedtime on school nights ↑ TST; ↓ sleep disruption |
| Vazsonyi,2015[34] | Georgian population: N =6,992; 40% male; Mage =15.83; ages 15–18; Swiss population: N = 5,575; 49.9% male; Mage = 17.17; ages 15– 18 | Cross sectional | Family structure; parental warmth | Adolescent reported: Sleep quality, TST | age, sex, family structure, parental education |
↑ Parental warmth ↑ Sleep quality ↑ Parental warmth ↑ TST |
| Roblyer, 2015 [38] | N = 91 US adolescents; ages 11 -19; 54.9% male; 12.1% AA, 81.3% White, 6.6% Other | Cross sectional | Parental involvement; conflict; parental control | Adolescent reported: Sleep problems, TST | ethnicity, age, depressive symptoms |
Parental involvement Difficulties initiating sleep ns.; difficulties maintaining sleep ns.; TST ns. ↑Parent-child conflict ↑ Difficulties maintaining sleep; difficulties initiating sleep ns.; TST ns. ↑ Parental control ↑ difficulties initiating sleep; ↑ difficulties maintaining sleep; TST ns. |
| Peltz, 2018 [39] | N = 193 US adolescents; Mage = 15.7; 45.6% male;14% Black, 71% White, 2% multiracial, 2% Latino, 2% Asian | Cross sectional | Family chaos; inconsistent discipline | Adolescent diary:TST, sleep quality | age, gender, SES, daily daytime naps, school start time |
Family chaos ↓ Sleep quality; TST ns. Inconsistent discipline TST ns.; sleep quality ns. |
| Staples, 2015 [41] | N = 87 young US children; 30 months at baseline; 42.53% male; 4% AA, 7% Latino,82% White, 7% mixed/other | Longitudinal | Bedtime routine; inconsistent parenting at all three waves | Actigraph assessed:TST |
At 30 months, routine and inconsistency TST ns. At 36 months,↓ inconsistency, ↑ routine ↑ TST At 42 months, ↑ routine ↑ TST At 42 months, inconsistency TST ns. |
|
| Millikovsky- Ayalon, 2015 [82] | N = 51 father, mother, and young child Israeli triads; (26 children with sleep disturbance and 25 children without sleep disturbance); ages 1-3; 53% male | Cross sectional | Parental stress due to acceptance of the child and restrictions imposed by the parental role; parental bedtime interaction (i.e. measure of parent assistance); parent sensitivity | Parent reported: Child sleep disturbances | matched based on age, gender, birth order, maternal education | ↑ Maternal stress (child acceptance and role restriction) ↑ Sleep disturbance ↑ Paternal stress (child acceptance) ↑ Sleep disturbance Paternal stress (role restriction) Sleep disturbance ns. ↑ Parental bedtime interaction ↑ Sleep disturbance Maternal sensitivity Sleep disturbance ns. ↑ Paternal sensitivity ↓ Sleep disturbance ↑ Parental involvement ↓ Sleep disturbance |
| Fuligni, 2016 [31] | N = 421 adolescents with Mexican heritage; T1age = 15.03; 50%male; follow up =1 year later | Longitudinal | Parental support; conflict; # of people in household | Parent and Adolescent reported: Parent- adolescent sleep concordance at T1 and T2 | parent education | ↑ Household size ↑ Concordance ↑ Parental support ↑ Concordance Conflict Concordance ns. |
| Meijer [35] | N = 650 German adolescents; T1age = 13.36, follow up 1 and 3 years later; 50.46% male | Longitudinal | Quality of parent adolescent relationship, autonomy granting, parental monitoring at all 3 waves | Adolescent reported: bedtime, TIB, sleep quality | sex, age, SES, ethnicity | T1: ↑ Relationship quality Earlier bedtime; ↑ sleep quality; TIB ns. Autonomy Bedtime ns.; TIB ns.; sleep quality ns. ↑ Monitoring Earlier bedtime; ↑ TIB; monitoring ns.; sleep quality ns. T2: ↑ Relationship quality Earlier bedtime;↑ (TIB; ↑ sleep quality ↑ Autonomy ↑ Sleep quality; bedtime ns.; TIB ns. ↑ Monitoring Earlier bedtime; ↑ TIB; ↑ sleep quality T3: ↑ Relationship quality Earlier bedtime; ↑TIB; ↑ sleep quality ↑ Autonomy ↓TIB; ↑ sleep quality; bedtime ns. ↑ Monitoring Earlier bedtime; ↑ TIB; ↑ sleep quality |
| Tetreault,2017 [36] | N = 200 Canadian mother- child dyads; 5 waves from ages 1-4; 49.5% male | Longitudinal | Maternal sensitivity (e.g. cooperation/attunement, positivity, accessibility/availability) | Parent diary: SE, TST | ↑ Cooperation/attunement ↑ SE at age 2, age 3, age 4, TST ns. ↑ Positivity ↑ SE at age 3; TST ns. ↑ Accessibillty/availability ↑ SE at age 3; TST ns. |
|
| Kouros, 2017 [30] | N = 163 US children; Mage = 10.45; 55% male; 25% AA,75% White | Cross sectional | Parent-child concordance | Actigraph assessed:TST, SE, # of wake episodes, WASO, bedtime, morning wake time | age, race, BMI, parental marital status, family income, pubertal status, maternal depressive symptoms |
Mother-child were concordant SE; # of wake episodes; WASO; bedtime; morning wake time Mother-child were not concordant TST Father-child were concordant TST; total WASO; wake time Father-child were not concordant SE; # of wake episode; bedtime |
| Conway, 2018 [37] | N = 820 US children; T1age = age 3, T2 = 6th grade; 10.4% AA, 80.73% White, 5.1% Asian or Native American | Longitudinal | Maternal sensitivity; negative emotionality at age 3 | Parent reported: Sleep problems at 6th grade | child sex, race/ethnicity, family income, maternal age, maternal education, child sleep behaviors at 3rd grade, maternal sensitivity and child negative emotionality at 5th grade, pubertal development status at 6th grade, maternal depressive symptoms at 6th | In adolescents with ↑ negative emotionality at age 3, ↑ maternal sensitivity predicted ↓ sleep problems in 6th In adolescents with ↓ negative emotionality at age 3, ↓ maternal sensitivity predicted ↑ sleep problems in 6th grade |
| Gunn, 2019 [43] | N = 165 US adolescents; Mage= 11.8; ages 10-14; 48% male; 78.6% AA, 15.7% White, 5.7%biracial | Cross sectional | Parental monitoring (parent and adolescent reported); parental knowledge; parental expectations of bedtime | Actigraph assessed:TST, sleep variability | gender, sex | ↑ Parental monitoring (parent reported) ↑ Weekday TST; 7-day TST ns.; weekend TST ns.; sleep variability ns. ↑ Parental monitoring (adolescent reported) ↑ 7-day TST; ↑ Weekday TST; ↑ weekend TST; sleep variability ns. Parental knowledge about bedtime TST ns.; sleep variability ns. Parental expectations of bedtime TST ns; sleep variability ns. |
Note. All sleep parameter results refer to child or adolescent’s sleep parameters; unless otherwise stated, all sleep parameters were either parent-reported or self-reported; ns = not significant; AA = African American; SE = sleep efficiency; TIB = time in bed; BMI = body mass index; TST = total sleep time/sleep duration; WASO = wake after sleep onset.
In cross-sectional and longitudinal studies, family-level variables indicative of cohesion and warmth frequently predict better sleep outcomes in children of all ages [34-37]. For example, in a large, population-based study of Georgian and Swiss adolescents, more parental warmth was associated with better sleep quality and longer sleep duration [34]. Similarly, better parent-child relationship quality predicted better sleep quality over time in young adolescents [35]. In contrast, chaotic households and families with more conflict are associated with difficulties with sleep maintenance [38] and poor sleep quality [39] in children. Family transitions also appear to have negative consequences. Changes in mother’s partner status from age 11 are associated with a shorter sleep duration at age 15 [40].
It is possible that among households with more chaos and transitions, there is less parental structure, which also predicts worse sleep outcomes for children. For example, young adolescents with parent-set bedtimes have longer sleep durations than children without parent-set bedtimes [33] and parent-led bedtime routines are associated with a longer sleep duration in toddlers and preschoolers [41]. Parenting rules and routines about other activities, not just sleep behaviors, appear to be beneficial for sleep. Parenting rules and consistency in routines were associated with longer sleep durations in a large sample of 10-11 year old Finnish children [42]. Moreover, youth-reported parental monitoring was associated with more sleep on school days [43].
Lastly, of the 15 recent studies on family dynamics and sleep, two studies include assessment of concordance among families on sleep behavior. Fuligni and colleagues found that parents and adolescents had concordant sleep durations, and a supportive parent-adolescent relationship was associated with higher concordance [31]. Sleep concordance can vary within family members. Mothers’ sleep fluctuations both predict, and are predicted by their partner’s and their child’s sleep; however father’s sleep was only predicted by mother’s sleep [30]. Importantly, when family members’ sleep patterns were included in models of individual sleep, other health and psychological covariates were no longer significant (e.g., BMI, depression;[30]). Together these findings suggest that the sleep habits and routines of other family members (especially parents), contribute to youth sleep at the individual level, and this may be particularly pronounced in mother-child relationships.
Family dynamics and hypertension risk (Table 2)
Table 2.
Family Dynamics and Hypertension
| Author, Year |
Sample (n, age, age group, race, population, gender) |
Study Design |
Family variable | HT marker | Covariates | Results |
|---|---|---|---|---|---|---|
| Wang,Anderson [57] | N = 1,090 US children; 50.7% male; 12.1% AA 81.2% White,6.7% other | Longitudinal | Maternal depression when child was 1 month, age 2, and age 3 | BMI in grades one, three, and six | birth weight, gender, race, maternal education, SES, breastfed, parent smoker, maternal social support, maternal sensitivity | ↑ Maternal depressive symptoms at 1 month ↑BMI in 6th grade; BMI in 1st and 3rd grade ns. ↑ Maternal depression age 2 ↑ BMI in 3rd and 6th grade ↑ Maternal depression at age 3 ↑ BMI in 3rd grade Maternal depression at more time points ↑ BMI |
| Pretty, 2013 [47] | N = 1,234 Canadians adolescents;Mage =11.8;45% male | Cross sectional | Childhood adversity | Resting BP, HR, BMI, WC | family education, family income, parental history of hypertension, age, sex, physical activity | ≥4 adverse experiences ↑ HR; ↑ BMI; ↑ WC; BP ns. |
| Lumeng,2013 [56] | N = 848 US children; age 4 at baseline, 49.5% male; 82% White; 18% other | Longitudinal | Chronicity of negative events (i.e., negative life events at multiple time points); # of negative life events; impact of negative life events; timing of negative life events; parenting sensitivity | BMI at age 15 | gender, race/ethnicity, maternal education, maternal obesity | ↑ # of negative events ↑ BMI ↑ Chronicity of negative events = ↑ BMI Timing of negative life events BMI ns. Impact of negative life event BMI ns. ↑ Maternal parenting sensitivity ↓ BMI |
| Nikulina,2014 [46] | N = 806 US adults with documented childhood neglect and their matched control, Mage =41; 49% male;59% White, 35% AA, 6% other | Longitudinal | Documented childhood neglect | Resting BP,CRP, pulmonary functioning | gender, smoking, hypertension medication, asthma, BMI or other pulmonary disease diagnoses, neighborhood poverty, childhood family poverty |
Childhood neglect hypertension ns. In White participants, childhood neglect ↑ CRP; ↑ pulmonary functioning |
| Hernandez, 2014 [55] | N = 3,447 US adults; Mage =21.1; 54% male; 72% White; 19% AA, 9% Hispanic | Longitudinal | # of family structure changes throughout childhood (e.g. mother remarrying, mother divorcing) | BMI | Child: race, birth weight, depressive symptoms, self-esteem, # of siblings, duration since last family structure transition, age, year of BMI assessment; Mothers: age, relationship status, education status, change in education status, BMI | Main effects ns. In girls, ↑ family transitions ↑ BMI |
| Su, 2015 [48] | N = 394 US adults; age 5 at baseline; 47% male; 54.1% AA,45.9% White | Longitudinal | Childhood adversity | Resting BP via visits every 1 to 2 years over 23 years from childhood to adulthood | ethnicity, sex, BMI | Main effect = ns; Around age 30, ↑ACE ↑ SBP; ↑ DBP |
| Gupta-Malhotra.2016 [49] | N = 515 African American men; Mage = 47.8 | Retrospective | Lived with both parents from ages 1-12; lived with both parents from ages 13-19 | Resting BP,MAP, PP | age, material status, education, smoker, alcohol, physical activity, BMI, type 2 diabetes, obesity, family history of hypertension, current antihypertensive medication, HDL, LDL, triglycerides, sodium/potassium ratio |
Lived with both parents at any time between 1-12 ↑SBP; DBP ns. Lived with both parents at any time between 13-19 SBP ns.; DBP ns Lived with parents from 1-12 and 13-19 ↓ MAP; ↓PP |
| Boyer. 2015 [50] | N = 1,364 US children; pre-k at baseline; 52% male; 76% White, 24% persons of color | Longitudinal | Parental sensitivity at pre-k and 1st grade | BMI and resting BP at 6th and 9th grade | gender, ethnicity, family income-to-needs ratios | ↑ Both parent’s sensitivity ↓ BMI; ↓ SBP at 9th grade; SPB at 6th grade ns. |
| Gibson,2016 [54] | N = 286 community and clinical Australian population; Mage= 9.43; children; 49% male | Longitudinal | 1 parent vs 2 parents; parental laxness; over-reactivity; verbosity; psychological health/pathology of the family | BMI | Gender, age, SES, mother’s depression, mother’s stress, mother’s negative life, mother’s self-esteem, maternal obesity |
One parent home ↑ BMI Laxness, over-reactivity, verbosity, psychological health/pathology of the family BMI ns. In males, higher verbosity in parent ↑BMI |
| Chan. 2016[7] | N = 259 Canadian adolescents; ages 13-16; 47% male; 49.4% White,15.3% Asian | Cross sectional | Implicit affect; implicit warmth toward family; household crowding | Resting BP, total cholesterol, glycosylated hemoglobin, WC | age, gender, ethnicity | ↑ Early life crowding ↑SBP; DBP ns.; cholesterol ns.; HbA1c ns.; WC ns.; ↑ Implicit negative affect ↑ Total cholesterol; ↑SBP; ↑DBP; HbA1c ns.; WC ns. Implicit warmth SBP ns.; DBP ns.; HbA1c ns.; total cholesterol ns.; HbA1c ns.; WC ns. |
| Dong. 2017[51] | N = 940 Chinese parent-child pairs; ages 7–17, Mage =12.1; 56.17% male | Cross sectional | Resting BP,HbAlc, BMI from child and parent | age, sex, parental age, household structure, income, geographical region, parental education, urban/rural, parental smoking, caloric intake, physical activity | ↑ Parent's HbA1C
↑ child's HbA1c ↑Mother BP ↑ daughter's SBP; ↑daughter's DBP ↑Father CRP ↑child's CRP; ↑ son's obesity |
|
| Yavuz. 2018 [58] | N = 61 normal weight Turkish children and 61 overweight or obese Turkish children; Mage = 5.18 years old; 52.36% male | Cross sectional | Parenting style; food restriction; pressure to eat; food monitoring | Normal weight vs.obese/overweight | mother's education, parent's BMI, child's NA |
↑ authoritarian parenting ↑ obese/overweight Authoritative parenting BMI ns. ↑ Pressure to eat ↑ obese/overweight Food restriction BMI ns. Food monitoring BMI ns. |
| Heredia,2019 [59] | N= 1175 Mexican- American adolescents; ages 11-13 at baseline; 50% male | Longitudinal | Family cohesion; family conflict | BMI | Age, acculturation, household size, parent marital status, parent BMI, parent education, parent acculturation |
In females, ↑ family cohesion Decrease in BMI In females, ↑ family conflict Decrease in BMI |
Note. All results refer to child or adolescent’s cardiovascular outcomes. ns = not significant. AA = African American. BMI = body mass index. SES = social economic status. BP = blood pressure, WC = waist circumference. HR = heart rate. SBP = systolic blood pressure. DBP = diastolic blood pressure. HF = high-frequency bands. LF = low-frequency bands. CRP = C-Reactive protein. MAP = mean arterial pressure. PP = pulse pressure. HDL = high- density lipoprotein. FDF = low- density lipoprotein. HbAlc = hemoglobin Alc.
As illustrated in the conceptual model (Figure 1), the family environment is independently linked to hypertension risk (e.g., BMI, blood pressure). This link has been well documented over the last 10-15 years. A seminal paper by Repetti and colleagues in 2002 highlighted how risky family environments increased risk for poor health, including hypertension [44]. This and other important works advanced the field by emphasizing the family environment and adverse child experiences as prominent factors in the development of cardiovascular disease[45]. Recent findings have added to this work using longitudinal and advanced methodologies. The data, which are summarized in Tables 1-3, overwhelmingly support links between the family dynamics and hypertension risk. However, significant associations between family dynamics and hypertension risk vary across studies.
Table 3.
Family dynamics, Sleep and Hypertension Risk
| Author, year | Sample (n, age, age group, race, population, gender) |
Study Design | Family variable | Sleep variable | HT marker | Covariates | Results |
|---|---|---|---|---|---|---|---|
| Jones,2014[60] | N = English 108 children; age 3; 53% male | Cross sectional | Presents of sleep rule, television viewing rule and/or dietary rule | Parent diary: TST | BMI; WC; triceps skinfold thickness; subscapular skinfold thickness |
Sleep rule ↑ TST, BMI ns; WC ns.; skinfold thickness ns. TV rule ↑ TST; ↓ BMI; ↓ WC; ↓ subscapular skinfold thickness; triceps ns. skinfold thickness ns. Dietary rule ↑ TST; ↓ subscapular skinfold thickness; BMI ns.; WC ns.; triceps ns.; skinfold thickness ns. |
|
| El- Sheikh,2015 [67] | N = US 160 children; age 9.43 at baseline, 1 year follow up 53.1% male; 27% AA, 73% White |
Longitudinal | Marital conflict | Actigraph assessed: TST, night time awakenings, sleep activity,SE | RSA-R | BMI, the presence of an illness and the sleep parameter at W1 | ↑ Marital conflict, ↑ RSA-R, ↓ TST ↑TST ↑ Marital conflict, ↑ RSA-R, and ↓ SE ↑ SE ↑Marital conflict, ↓ RSA-R, and ↓ SE ↓ SE ↑Marital conflict, ↓ RSA-R and ↓ SE ↓ SE ↑Marital conflict, ↑RSA-R, and ↓ sleep activity Sleep activity ↑Marital conflict, ↑ RSA-R; ↓ long wake episodes ↑ Wake episode |
| Kiel,2015 [63] | N = 51 mother-toddler dyads; T1: 18.96 months old at baseline, follow up at ages 2 and 3; 49.02% male; 82.4% White, 2.0% Latino Americans, 3.9% Asian Americans, 11.8% biracial | Longitudinal | Parental overprotection; parental critical control via questionnaires | Parent reported:Sleep problems at ages 2 and 3 | Time 1:Cortisol secretion | sleep problems at age 2, parental overprotection |
Over protection Sleep problems ns. critical control Sleep problems ns. Critical control and cortisol secretion = sleep problems at age 3 |
| Gustafsson,2017[68] | N = 292 pregnant US adolescents; Mage =17.81; 88.28% Latina;3rd trimester fetuses; fetuses = 43% female | Cross sectional | Emotional abuse | Sleep disturbance | Fetal heart rate variability | age, ethnicity, income, gestational age | ↑ Emotional abuse and ↑ sleep disturbances↓ Heart rate variability |
| Zilioli,2017[65] | N = 645 Chinese participants; Mage=10.67 years; ages 8-15;51.9% male | Cross sectional | Loneliness via daily diaries and trait loneliness | Adolescent diary:Subjective sleep quality, SOL, # of awakenings,TIB | CAR | gender, parent death, health status, stressful life events, age, negative affect, depression | ↑ Trait loneliness ↓ Morning cortisol; ↑ TIB; ↓ sle0ep quality ↑ Daily loneliness Flat cortisol slope; ↑ night awakenings; ↓ TIB |
Note. All results refer to child or adolescent’s cardiovascular or sleep outcomes; unless otherwise stated, all sleep parameters were either parent-reported or self-reported; AA = African American; T1 = initial wave of data collection; BMI = body mass index; WC = waist circumference; TST = total sleep time/sleep duration; RSA-R = respiratory sinus arrhythmia- reactivity; SE = sleep efficiency; SOL = sleep onset latency; TIB = time in bed; CAR = cortisol awakening response.
Family dynamics and inflammation and sympathetic activity.
Associations between a family-level variable (neglect) and hypertension were assessed in one prospective study; more childhood neglect was associated with higher inflammation, but not hypertension, 30 years later [46]. Other studies have assessed family level variables and sympathetic and inflammatory predictors of hypertension such as baseline blood pressure (BP) and heart rate (HR). For example, adverse experiences (childhood abuse and household dysfunction), are positively associated with high resting heart rate in a cross-sectional analysis [47], and predict elevated BP in a 23-year longitudinal study [48]. Other family-level predictors in early childhood were also associated with BP at later time points. Lower systolic BP (SBP) in middle-aged men was associated with being raised in a two-parent home [49]. Moreover, parental sensitivity (i.e., observer ratings of emotional support during a challenging task) in early years predicted lower BP when children were 15 years old [50].
A more direct measure of family-level associations with hypertension is concordance of risk factors. Few studies examine degree of concordance in hypertension risk among family members; however, results from one cross-sectional study indicate concordance in BP, hemoglobin A1c (HbA1c), and systemic inflammation [51]. In this study of Chinese children aged 7-17 and their parents, Dong found that BP was positively correlated in mothers and daughters only and CRP was correlated in fathers and sons only. HbA1c was concordant in all dyadic pairs [51]. This study suggests the importance of within family analyses (i.e., mother- child; father-child).
Family dynamics and Body Mass Index (BMI).
BMI is one of the strongest and earliest predictors of hypertension [52, 53]. Similar to findings on family structure and BP, 10-year old children from single-parent homes had higher BMI after two years [54]. Moreover, young adult girls who reported family transitions when they were younger (divorce, mother remarrying, etc.) had higher BMI as adults [55]. Changes in family structure could contribute to more negative events, which are also predictive of BMI. Children with more acute and chronic negative events (e.g., problems with family health and financial stability) from 4–11 years old had higher BMI at age 15 [56]. Maternal depression in early childhood, which is considered a negative family event, strongly predicted BMI in children years later [57]. Negative parenting styles are also linked to BMI. In one cross-sectional study, parents who were more authoritarian (controlling, rule-bound) and who had more rules about eating had children who were more overweight [58]. In contrast, sensitive parenting (involving support and low hostility) in early childhood predicted lower BMI during adolescence [50, 56]. Finally, in a large, longitudinal study on family cohesion and conflict, Heredia and colleagues found that less family cohesion and more conflict predict steeper weight loss declines in Mexican-American girls from age 12 to 17 [59].
Collectively, recent findings yield significant prospective associations between family- level variables and sympathetic activity and BMI, both putative markers for hypertension. In particular, children with two parents home, sensitive parents, and fewer adverse events also had better sympathetic outcomes and lower BMI. However, associations were not always immediately apparent. This highlights the continued importance of longitudinal and prospective study designs, particularly with respect to early markers of hypertension.
Family dynamics, sleep & hypertension risk (Table 3).
To our knowledge, only one study in the last five years measured family dynamics, sleep, and a proximal hypertension risk. Jones and colleagues [60] examined parental rules, short sleep, and body composition in over 100 toddlers and found that parental rules about sleep and television were associated with more total sleep time. BMI was not associated with short sleep in this sample; however, the authors noted that the study may have been underpowered [60]. The remaining studies assessed family relationship characteristics, sleep, and psychophysiological correlates of stress (e.g., cortisol, autonomic imbalance), which have distal, downstream implications for the development of hypertension [61, 62]. For example, toddlers with blunted cortisol and critically controlling mothers had more sleep problems one year later [63]. Blunted cortisol may be a reflection of chronic stress [64]. In a different paradigm of chronic psychosocial stress (children of parents affected by HIV), children who endorsed loneliness also had blunted cortisol and poor sleep quality [65]. These findings suggest that psychosocial stress in childhood is reflected in both sleep and cortisol activity.
Measures of autonomic imbalance also reflect psychophysiological stress [62] and can be assessed through heart rate variability and respiratory sinus arrhythmia (RSA; [66]). El-Sheikh and colleagues [67] demonstrated that good sleep (more total sleep time, better sleep quality) and greater RSA were protective against psychosocial stress (marital conflict) in school-aged children. Another recent study on autonomic imbalance sought to determine whether and how family experiences are transmitted to the next generation. Results yielded an indirect effect in which maternal emotional abuse history was associated with sleep disturbances during pregnancy, which then predicted lower fetal heart rate variability [68].
In sum, examinations of family dynamics, sleep, and proximal hypertension risk are limited. Results from existing studies suggest interactive effects among sleep, cardiovascular markers of psychosocial stress, and family dynamics. In one study, sleep quality moderated a cardiovascular marker of autonomic imbalance [68] and in another, good sleep health appeared to buffer the effects of psychosocial stress in the home [67]. The remaining studies showed that negative or positive family dynamics have similar adverse effects on sleep and psychosocial markers of stress. Thus, it is not yet clear whether and how sleep interacts with family dynamics to influence hypertension risk.
Summary and considerations for future directions.
Summary
The purpose of this review was to highlight recent findings on family dynamics in sleep and hypertension risk. We presented a conceptual framework (Figure 1) for hypertension risk in which family dynamics and sleep behaviors interact to mitigate or amplify hypertension risk. Overall, separate examinations of family dynamics and sleep and family dynamics and hypertension risk suggest similar effects of family dynamics on cardiovascular and sleep health. That is, negative family dynamics, single-parent homes, and multiple transitions within the family tend to predict poor sleep outcomes and are associated with elevations in other hypertension risk factors (e.g., HR, BP, BMI).
It is not yet clear whether and how sleep behaviors interact with family dynamics on hypertension risk; however, based on recent findings, there are a few possibilities. Several studies reported that poor family dynamics were associated with the presence of poor sleep behaviors either concurrently, or within a short time frame (E.g., [34, 38]). On the other hand, the association between family dynamics and hypertension risk was not always immediately apparent (e.g. [49, 50]). In young, healthy children, it is possible that the sleep behaviors are more immediately sensitive to fluctuations in family dynamics than other hypertension risk markers. Limited findings from studies that include measurement of family dynamics, hypertension, and sleep provide some evidence that sleep behaviors moderate the association between family dynamics and distal hypertension risk; however, only two studies [67, 68]tested for interactive associations. Sleep behaviors are now an essential component of cardiovascular health [69], thus, it will be important to continue to measure both sleep and hypertension markers in a family context to understand how these constructs interact.
Considerations for future research
The long-term goal of this research is to improve hypertension. In light of the most recent findings on family dynamics and sleep, there are several considerations for future research that will facilitate this goal. First, advanced methodological and analytic approaches to family-level variables will provide information on gaps in the literature. For example, much of the recent findings are based on aggregated family dynamic characteristics. Measurement of day-to-day family interactions allows for greater specificity on how family relationships contribute to nightly sleep patterns. This design would also allow for rigorous tests of bidirectional and lagged associations between sleep and family dynamics. For example, it is likely that family dynamics are also influenced by sleep (Figure 1) especially in families with infants and toddlers [70]. It is also necessary to consider analytic approaches that take into account the inherent interdependence of family relationships and health behaviors. Family members’ behaviors are likely more similar to one another than to non-family members, and family members’ behaviors are dynamically linked. Thus, behaviors of individuals within family units are nonindependent [71]. Statistical tests with individuals as the unit of analysis, as opposed to dyads or families, do not take into account the shared properties of nested, or interdependent data. Interdependent analytic techniques may result in more accurate estimates of the associations among family dynamics, sleep, and hypertension.
Second, sleep duration and sleep quality were among the most frequently studied components of sleep health. Sleep timing and circadian rhythms in a family context likely warrant further attention. Daily activities such as bedtime, wake time, and meal times (i.e., daily social rhythms) are time cues, zeitgeibers, for the endogenous circadian system [72]. The timing of family-based activities (such as meal time) have the propensity to influence an individual’s daily social rhythmicity, and in turn, their endogenous circadian rhythms. The timing and frequency of engagement with family members can also influence endogenous biological rhythms [72]. Importantly, disruptions to circadian rhythmicity can alter cardiovascular functioning [73]. Moreover, meal timing and eating patterns, which are often family activities, are associated with cardiovascular health [74]. Thus, the timing of family activities (such as mealtime) may have a direct influence on cardiovascular health, and family social rhythms may have an indirect influence on cardiovascular health through circadian rhythms. Family-level assessment of social rhythmicity and circadian rhythms and sleep behaviors, will reveal novel mechanisms for hypertension risk and targets for intervention.
Third, it is also important to consider larger systemic processes that influence family- level sleep and hypertension. We focused our review on within-family dynamics and did not address systemic influences such as socioeconomic status (SES) and race and ethnicity. While several of the studies reviewed here included SES, race, and ethnicity as covariates, few studies reported on significant interactive effects of these social determinants. Nikulina and colleagues [46] found that childhood neglect was associated with CRP in white children only. Chan and colleagues [75] found that more negative feelings about the early family environment was associated with higher resting blood pressure among adolescents with higher SES. Importantly, disparities in sleep health behaviors may contribute to cardiovascular health disparities [76]. To address disparities in hypertension [77] and sleep [76], it is critical to clarify the associations among sleep, family dynamics, and hypertension risk in at-risk populations. Eliminating disparities in hypertension and sleep requires more research with diverse and underrepresented populations, and measurement of factors unique to these groups that could amplify sleep problems and hypertension risk at the family level [76]. For example, shift work and nonstandard work schedules are over represented by ethnic minorities and individuals/families who are economically challenged [78]. Shift work is associated with sleep disruption and hypertension in individuals; however, we know little about how an organizational determinant such as shift work influences sleep and circadian rhythms at the family level. There are multiple interactive systems that have direct and indirect influences on families and their sleep and cardiovascular outcomes [79]. Associations within the family system and between other organizational and community systems will be important to clarify in order to move in the direction of intervention and health promotion.
Finally, to that end, a logical next step in this area of research is to consider family-based approaches to sleep health promotion. We propose that focusing on sleep in a family context may be one way to encourage structure, collaboration, and warmth in the family unit, and that improved connection and sleep, in turn, could improve hypertension risk. One aspect of family dynamics that may be especially critical to target is parenting practices surrounding waking and nighttime behaviors. Parental factors such as monitoring, structure, and sensitivity were associated with sleep outcomes in 11 of the 15 studies on family dynamics and sleep. A focus on modifiable parenting practices has been successful in other health related outcomes. For example, increasing parental warmth, structure, and emotional support in childhood obesity interventions leads to weight reduction in children and parents [80]. Thus, a focus on parenting factors surrounding sleep could ostensibly have other downstream effects on family dynamics, and overall family health.
Conclusion
Components of sleep health such as poor sleep quality and insufficient sleep increase hypertension risk. Family relationship dynamics also contribute to hypertension risk. Understanding sleep health behaviors in a family context provides greater understanding of two potentially modifiable targets for reducing hypertension risk in families. Families can influence one another’s sleep (and potentially circadian rhythms) through shared activities, relationship characteristics, and concordant sleep behaviors. Parenting styles characterized by structure and warmth/sensitivity appear to be associated with better sleep health and hypertension outcomes. However, future research will benefit from clarifying interactions among family dynamics, sleep behaviors, and hypertension risk. Clarification of these processes, in turn, will help to inform family-level approaches to improving sleep, and ultimately, hypertension outcomes.
Acknowledgments
Support for the first author (HEG) was provided by the National Heart Lung and Blood Institute (HL082610).
Footnotes
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
The authors are also grateful to Daniel J. Buysse, MD for valued comments on the conceptual model presented in this manuscript.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.••.Vedanthan R, Bansilal S, Soto AV, Kovacic JC, Latina J, Jaslow R, et al. Family-Based Approaches to Cardiovascular Health Promotion. J Am Coll Cardiol. 2016;67(14): 1725–37.This review highlights how the family contributes to cardiovascular risk and reviews relevant literature on improving cardiovascular health through a family approach.
- 2.Meyler D, Stimpson JP, Peek MK. Health concordance within couples: a systematic review. Soc Sci Med. 2007;64(11):2297–310. [DOI] [PubMed] [Google Scholar]
- 3.•.Chen E, Brody GH, Miller GE. Childhood close family relationships and health. Am Psychol. 2017;72(6):555–66.This is a review of mechanisms by which close family relationships influence health.
- 4.Tinsley BJ. Multiple influences on the acquisition and socialization of children's health attitudes and behavior: an integrative review. Child Dev. 1992;63(5): 1043–69. [DOI] [PubMed] [Google Scholar]
- 5.Rossow I, Rise J. Concordance of parental and adolescent health behaviors. Soc Sci Med. 1994;38(9): 1299–305. [DOI] [PubMed] [Google Scholar]
- 6.Agras WS, Hammer LD, McNicholas F, Kraemer HC. Risk factors for childhood overweight: a prospective study from birth to 9.5 years. J Pediatr. 2004;145(1):20–5. [DOI] [PubMed] [Google Scholar]
- 7.Brown HE, Atkin AJ, Panter J, Wong G, Chinapaw MJ, van Sluijs EM. Family-based interventions to increase physical activity in children: a systematic review, meta-analysis and realist synthesis. Obes Rev. 2016;17(4):345–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.••.Fobian AD, Elliott L, Louie T. A Systematic Review of Sleep, Hypertension, and Cardiovascular Risk in Children and Adolescents. Curr Hypertens Rep. 2018;20(5):42.Recent systematic revew linking children and youth sleep to hypertension and cardiovascular risk and highlighted the need for more systematic research in area.
- 10.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring). 2008;16(2):265–74. [DOI] [PubMed] [Google Scholar]
- 11.Nixon GM, Thompson JM, Han DY, Becroft DM, Clark PM, Robinson E, et al. Short sleep duration in middle childhood: risk factors and consequences. Sleep. 2008;31(1):71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews KA, Pantesco EJM. Sleep characteristics and cardiovascular risk in children and adolescents: an enumerative review. Sleep Medicine. 2016;18:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47(5):833–9. [DOI] [PubMed] [Google Scholar]
- 14.Yoong SL, Chai LK, Williams CM, Wiggers J, Finch M, Wolfenden L. Systematic review and meta-analysis of interventions targeting sleep and their impact on child body mass index, diet, and physical activity. Obesity (Silver Spring). 2016;24(5): 1140–7. [DOI] [PubMed] [Google Scholar]
- 15.Maggi S, Irwin LJ, Siddiqi A, Hertzman C. The social determinants of early child development: an overview. J Paediatr Child Health. 2010;46(11):627–35. [DOI] [PubMed] [Google Scholar]
- 16.Lau RR, Quadrel MJ, Hartman KA. Development and change of young adults' preventive health beliefs and behavior: influence from parents and peers. J Health Soc Behav. 1990;31(3):240–59. [PubMed] [Google Scholar]
- 17.Patrick H, Nicklas TA. A review of family and social determinants of children's eating patterns and diet quality. J Am Coll Nutr. 2005;24(2):83–92. [DOI] [PubMed] [Google Scholar]
- 18.Edwardson CL, Gorely T. Parental influences on different types and intensities of physical activity in youth: A systematic review. Psychol Sport Exerc. 2010; 11(6):522–35. [Google Scholar]
- 19.Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29(8): 1009–14. [DOI] [PubMed] [Google Scholar]
- 20.Mileva-Seitz VR, Bakermans-Kranenburg MJ, Battaini C, Luijk MP. Parent-child bed-sharing: The good, the bad, and the burden of evidence. Sleep Med Rev. 2017;32:4–27. [DOI] [PubMed] [Google Scholar]
- 21.Maume DJ, Sebastian RA, Bardo AR. Gender, Work-Family Responsibilities, and Sleep. Gender Soc. 2010;24(6):746–68. [Google Scholar]
- 22.Buxton OM, Chang AM, Spilsbury JC, Bos T, Emsellem H, Knutson KL. Sleep in the modern family: protective family routines for child and adolescent sleep. Sleep Health. 2015; 1 (1): 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. J Adolesc Health. 2002;31(6 Suppl): 175–84. [DOI] [PubMed] [Google Scholar]
- 24.Worthman CM, Melby MK. Toward a comparative developmental ecology of human sleep. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. 2002:69–117. [Google Scholar]
- 25.Gander M, Buchheim A. Attachment classification, psychophysiology and frontal EEG asymmetry across the lifespan: a review. Front Hum Neurosci. 2015;9:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: a focus on insomnia. Sleep Med Rev. 2010;14(4):227–38. [DOI] [PubMed] [Google Scholar]
- 27.Sbarra DA, Hazan C. Coregulation, dysregulation, self-regulation: an integrative analysis and empirical agenda for understanding adult attachment, separation, loss, and recovery.Pers Soc Psychol Rev. 2008; 12(2): 141–67. [DOI] [PubMed] [Google Scholar]
- 28.Papp LM, Pendry P, Adam EK. Mother-adolescent physiological synchrony in naturalistic settings: within-family cortisol associations and moderators. J Fam Psychol. 2009;23(6):882–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldman R, Eidelman AI. Maternal postpartum behavior and the emergence of infant-mother and infant-father synchrony in preterm and full-term infants: the role of neonatal vagal tone. Dev Psychobiol. 2007;49(3):290–302. [DOI] [PubMed] [Google Scholar]
- 30.•.Kouros CD, El-Sheikh M. Within-Family Relations in Objective Sleep Duration, Quality, and Schedule. Child Dev. 2017;88(6): 1983–2000.This study explored interdependence in sleep among multiple family members and found bidrectional assocations among mothers, fathers, and children.
- 31.•.Fuligni AJ, Tsai KM, Krull JL, Gonzales NA. Daily concordance between parent and adolescent sleep habits. J Adolesc Health. 2015;56(2):244–50.This study demonstrated that adolescent sleep is connected to their parent's sleep even after accounting for common factors that influence sleep patterns.
- 32.Vaughn BE, Waters TE, Steele RD, Roisman GI, Bost KK, Truitt W, et al. Multiple domains of parental secure base support during childhood and adolescence contribute to adolescents' representations of attachment as a secure base script. Attach Hum Dev. 2016;18(4):317–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Short MA, Gradisar M, Lack LC, Wright HR, Dewald JF, Wolfson AR, et al. A Cross-Cultural Comparison of Sleep Duration Between US and Australian Adolescents: The Effect of School Start Time, Parent-Set Bedtimes, and Extracurricular Load. Health Educ Behav. 2013;40(3):323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vazsonyi AT, Harris C, Terveer AM, Pagava K, Phagava H, Michaud PA. Parallel Mediation Effects by Sleep on the Parental Warmth-Problem Behavior Links: Evidence from National Probability Samples of Georgian and Swiss Adolescents. J Youth Adolescence. 2015;44(2):331–45. [DOI] [PubMed] [Google Scholar]
- 35.Meijer AM, Reitz E, Dekovic M. Parenting matters: a longitudinal study into parenting and adolescent sleep. J Sleep Res. 2016;25(5):556–64. [DOI] [PubMed] [Google Scholar]
- 36.Tetreault E, Bouvette-Turcot AA, Bernier A, Bailey H. Associations between early maternal sensitivity and children's sleep throughout early childhood. Infant Child Dev. 2017;26(4). [Google Scholar]
- 37.Conway A, Modrek A, Gorroochum P. Maternal Sensitivity Predicts Fewer Sleep Problems at Early Adolescence for Toddlers with Negative Emotionality: A Case of Differential Susceptibility. Child Psychiat Hum D. 2018;49(1):86–99. [DOI] [PubMed] [Google Scholar]
- 38.Roblyer MIZ, Grzywacz JG. Demographic and Parenting Correlates of Adolescent Sleep Functioning. J Child Fam Stud. 2015;24(11):3331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peltz JS, Rogge RD, O'Connor TG. Adolescent sleep quality mediates family chaos and adolescent mental health: A daily diary-based study. J Fam Psychol. 2018. [DOI] [PubMed] [Google Scholar]
- 40.Maume DJ. Social ties and adolescent sleep disruption. J Health Soc Behav. 2013;54(4):498–515. [DOI] [PubMed] [Google Scholar]
- 41.Staples AD, Bates JE, Petersen IT. Bedtime Routines in Early Childhood: Prevalence, Consistency, and Associations with Nighttime Sleep. Monogr Soc Res Child.2015;80(1): 141–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ray C, Kalland M, Lehto R, Roos E. Does Parental Warmth and Responsiveness Moderate the Associations Between Parenting Practices and Children's Health-related Behaviors? J Nutr Educ Behav. 2013;45(6):602–10. [DOI] [PubMed] [Google Scholar]
- 43.Gunn HE, O'Rourke F, Dahl RE, Goldstein TR, Rofey DL, Forbes EE, et al. Young adolescent sleep is associated with parental monitoring. Sleep Health. 2019;5(1):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128(2):330–66. [PubMed] [Google Scholar]
- 45.Su S, Jimenez MP, Roberts CT, Loucks EB. The role of adverse childhood experiences in cardiovascular disease risk: a review with emphasis on plausible mechanisms. Curr Cardiol Rep. 2015;17(10):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nikulina V, Widom CS. Do race, neglect, and childhood poverty predict physical health in adulthood? A multilevel prospective analysis. Child Abuse Neglect. 2014;38(3):414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pretty C, O'Leary DD, Cairney J, Wade TJ. Adverse childhood experiences and the cardiovascular health of children: a cross-sectional study. Bmc Pediatr. 2013; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.••.Su SY, Wang XL, Pollock JS, Treiber FA, Xu XJ, Snieder H, et al. Adverse Childhood Experiences and Blood Pressure Trajectories From Childhood to Young Adulthood The Georgia Stress and Heart Study. Circulation. 2015;131(19): 1674–U116.Authors of this manuscript found compelling evidence that adverse childhood experience have a lasting effect on blood pressure.
- 49.Gupta-Malhotra M, Hashmi SS, Barratt MS, Milewicz DM, Shete S. Childhood-Onset Essential Hypertension and the Family Structure. J Clin Hypertens. 2016; 18(5):431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyer BP, Nelson JA. Longitudinal Associations of Childhood Parenting and Adolescent Health: The Mediating Influence of Social Competence. Child Development. 2015;86(3):828–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong F, Howard AG, Herring AH, Adair LS, Thompson AL, Popkin BM, et al. Concordance of haemoglobin A1c, blood pressure and C-reactive protein between children and their parents in Chinese households. Pediatr Obes. 2017;12(5):422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelly RK, Magnussen CG, Sabin MA, Cheung M, Juonala M. Development of hypertension in overweight adolescents: a review. Adolesc Health Med Ther. 2015;6:171–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Field AE, Cook NR, Gillman MW. Weight status in childhood as a predictor of becoming overweight or hypertensive in early adulthood. Obes Res. 2005; 13(1): 163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gibson LY, Allen KL, Byrne SM, Clark K, Blair E, Davis E, et al. Childhood Overweight and Obesity: Maternal and Family Factors. J Child Fam Stud. 2016;25(11):3236–46. [Google Scholar]
- 55.Hernandez DC, Pressler E, Dorius C, Mitchell KS. Does Family Instability Make Girls Fat? Gender Differences Between Instability and Weight. J Marriage Fam. 2014;76(1): 175–90. [Google Scholar]
- 56.Lumeng JC, Wendorf K, Pesch MH, Appugliese DP, Kaciroti N, Corwyn RF, et al. Overweight Adolescents and Life Events in Childhood. Pediatrics. 2013;132(6):E1506–E12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L, Anderson JL, Dalton WT, Wu TJ, Liu XC, Zheng SM, et al. Maternal Depressive Symptoms and the Risk of Overweight in Their Children. Matern Child Hlth J. 2013;17(5):940–8. [DOI] [PubMed] [Google Scholar]
- 58.Yavuz HM, Selcuk B. Predictors of obesity and overweight in preschoolers: The role of parenting styles and feeding practices. Appetite. 2018;120:491–9. [DOI] [PubMed] [Google Scholar]
- 59.Heredia NI, Wilkinson AV, Forman MR, Christie IC, Wang J, Daniel CR, et al. Longitudinal associations of family functioning with body mass index in Mexican-origin adolescents living in the U.S. Preventive Medicine. 2019;118:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones CHD, Pollard TM, Summerbell CD, Ball H. Could Parental Rules Play a Role in the Association between Short Sleep and Obesity in Young Children? J Biosoc Sci. 2014;46(3):405–18. [DOI] [PubMed] [Google Scholar]
- 61.Pickering TG. Stress, inflammation, and hypertension. J Clin Hypertens (Greenwich). 2007;9(7):567–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010; 141(2): 122–31. [DOI] [PubMed] [Google Scholar]
- 63.Kiel EJ, Hummel AC, LuebbeMiami AM. Cortisol secretion and change in sleep problems in early childhood: Moderation by maternal overcontrol. Biological Psychology. 2015;107:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80(3):265–78. [DOI] [PubMed] [Google Scholar]
- 65.Zilioli S, Slatcher RB, Chi PL, Li XM, Zhao JF, Zhao GX. The impact of daily and trait loneliness on diurnal cortisol and sleep among children affected by parental HIV/AIDS. Psychoneuroendocrinology. 2017;75:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30(2):183–96. [DOI] [PubMed] [Google Scholar]
- 67.El-Sheikh M, Hinnant JB, Erath SA. Marital Conflict, Vagal Regulation, and Children's Sleep: A Longitudinal Investigation. Monogr Soc Res Child. 2015;80(1):89–106. [DOI] [PubMed] [Google Scholar]
- 68.Gustafsson H, Doyle C, Gilchrist M, Werner E, Monk C. Maternal abuse history and reduced fetal heart rate variability: Abuse-related sleep disturbance is a mediator. Development and Psychopathology. 2017;29(3): 1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.St-Onge MP, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, et al. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation. 2016;134(18):e367–e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meltzer LJ, Montgomery-Downs HE. Sleep in the family. Pediatr Clin North Am. 2011;58(3):765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kenny DA, Kashy DA, Cook WL. Dyadic data analysis. New York: Guilford Press; 2006. xix, 458 p. p. [Google Scholar]
- 72.Grandin LD, Alloy LB, Abramson LY. The social zeitgeber theory, circadian rhythms, and mood disorders: review and evaluation. Clin Psychol Rev. 2006;26(6):679–94. [DOI] [PubMed] [Google Scholar]
- 73.Evans JA, Davidson AJ. Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci. 2013;119:283–323. [DOI] [PubMed] [Google Scholar]
- 74.St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, et al. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation. 2017;135(9):e96–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chan M, Miller GE, Chen E. Early Life Socioeconomic Status and Metabolic Outcomes in Adolescents: The Role of Implicit Affect About One's Family. Health Psychology. 2016;35(4):387–96. [DOI] [PubMed] [Google Scholar]
- 76.••.Jackson CL, Redline S, Emmons KM. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health. 2015;36:417–40.This literature review presenting a compelling model on how sleep disparities contribute to disparities in cardiovascular diseases.
- 77.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10): 1233–41. [DOI] [PubMed] [Google Scholar]
- 78.Enchautegui ME. Nonstandard Work Schedules and the Well-Being of Low-Income Families. Urban Institute; 2013. [Google Scholar]
- 79.Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010; 14(3): 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Golan M Parents as agents of change in childhood obesity - from research to practice. Int J Pediatr Obes. 2006;l(2):66–76. [DOI] [PubMed] [Google Scholar]
- 81.Kelly RJ, El-Sheikh M. Longitudinal Relations Between Marital Aggression and Children's Sleep: The Role of Emotional Insecurity. Journal of Family Psychology. 2013;27(2):282–92. [DOI] [PubMed] [Google Scholar]
- 82.Millikovsky-Ayalon M, Atzaba-Poria N, Meiri G. The Role of the Father in Child Sleep Disturbance: Child, Parent, and Parent-Child Relationship. Infant Ment Health J. 2015;36(1):114–27. [DOI] [PubMed] [Google Scholar]