Abstract
Sclerotome is the embryonic progenitor of the axial skeleton. It was previously shown that Tgfbr2 is required in sclerotome for differentiation of fibrous skeletal tissues including the annulus fibrosus of the intervertebral disc. Alternatively, BMP signaling is required to form the vertebral body through chondrogenesis. In addition, TGFβ added to sclerotome cultures induces expression of markers for fibrous tissue differentiation but not cartilage or bone. The mechanism of how TGFβ signaling regulates this lineage decision in sclerotome is not known and could be due to the production of instructive or inhibitory signals or a combination of the two. Here we show that TGFβ antagonizes BMP/ Smad1/5 signaling in primary sclerotome likely through regulation of Noggin, an extracellular BMP antagonist, to prevent chondrogenesis. We then tested whether inhibition of BMP signaling, and inhibition of chondrogenesis, is sufficient to push cells toward the fibrous cell fate. While Noggin inhibited BMP/ Smad1/5 signaling and the formation of chondrogenic nodules in sclerotome cultures; Noggin and inhibition of BMP signaling through Gremlin or DMH2 were insufficient to induce fibrous tissue differentiation. The results suggest inhibition of BMP signaling is not sufficient to stimulate fibrous tissue differentiation and additional signals are likely required. We propose that TGFβ has a dual role in regulating sclerotome fate. First, it inhibits BMP signaling potentially through Noggin to prevent chondrogenesis and, second, it provides an unknown instructive signal to promote fibrous tissue differentiation in sclerotome. The results have implications for the design of stem cell-based therapies for skeletal diseases.
Keywords: TGFβ, fibrogenesis, chondrogenesis, axial skeleton, sclerotome, Noggin, Adamtsl2, Fmod, Scx
Graphical Abstract
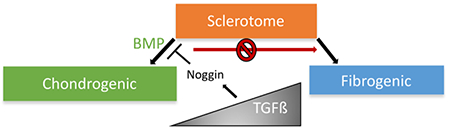
Introduction
Degeneration of fibrous tissues in the spine including the Annulus Fibrosus (AF) of the Intervertebral Disc (IVD) contributes to back pain [1]. The IVD is composed of two tissues, the AF and Nucleus Pulposus (NP), having different origins. The NP is derived from an embryonic structure called the notochord. All of the other connective tissues of the axial skeleton including the AF, tendon, ligament, and the vertebral body (VB) are derived from an embryonic tissue called the sclerotome [2–4]. The VB is made through a hyaline cartilage model by endochondral bone formation [5]. In contrast, AF, ligament, and tendon are primarily fibrous tissues with AF composed of fibrocartilage. Differentiation of sclerotome into various components of the axial skeleton, whether cartilaginous or fibrous, is determined by the location of the cells within the embryo and a complex interaction of growth factors. [6, 7]
Transforming Growth Factor Beta (TGFβ) and Bone Morphogenic Protein (BMP) are important factors that regulate development and cell fate decisions. TGFβ binds to serine/threonine kinase receptors on the cell surface and transfers signals through transcription factors Smad2 and Smad3. BMP primarily binds to a different set of serine/threonine kinase receptors and uses Smad1 and Smad5 proteins to transmit its signal. BMP signaling is critical for chondrogenesis and formation of the VB [8, 9]. Previous data showed that BMP receptors type 1a and 1b are crucial for chondrogenesis in limb [10] and conditional knock down for BMP ligands 2 or 4, and mutation in GDF5, another member of the BMP family, have general defects in the development of hyaline cartilage [11, 12]. TGFβ signaling is known to play an important role in the formation of fibrous skeletal tissues in mice [13–17]. Our laboratory previously showed that mice with a deletion of the TGFβ type 2 receptor (Tgfbr2) in Collagen type 2a (Col2a) expressing cells of the axial skeleton demonstrated a failure in AF formation while development of the VB was minimally affected [13]. Global analysis of gene expression via microarray assay comparing control and 7g/br2-deleted IVD indicated that mutant IVD had a similar molecular profile to the VB. The results suggested that in the absence of Tgfbr2, the cells that were supposed to form AF did not differentiate correctly and took on characteristics of hyaline cartilage [18]. Similarly, absence of Tgfbr2 in limb mesenchyme resulted in failure in the formation of the interzone, another fibrous tissue, and increased cartilage formation in the joint area resulting in joint fusion [16, 19]. In addition, limb mesenchyme cultured from Tgfbr2 knock out mice demonstrated increased cartilage formation suggesting TGFβ prevents chondrogenesis in early undifferentiated mesenchyme [16]. Furthermore, we and others have shown that TGFβ induces markers of fibrous differentiation, including Scx, Adamtsl2 and Fmod, but not markers for cartilage in cultures of mesenchymal cells [18, 20–24]. Based on these data from both in vivo and in vitro models we proposed that TGFβ mediates cell fate decisions in the sclerotome by favoring the formation of fibrous cell types (AF, tendon, ligament) while BMP favors chondrogenesis and formation of the VB [18, 21]. The mechanisms of how TGFβ and BMP act to influence cell fate decisions in the sclerotome are not clear.
Extracellular BMP antagonists have been identified and are key to limiting BMP activity during embryonic development [25]. Many of these BMP antagonists, including Noggin and Gremlin, are highly expressed in the IVD forming region of the sclerotome and interzone region in the limb [19, 26, 27]. Even though these antagonists inhibit BMP signaling, each has distinct functions in skeletal development. For example, mice with deletion of Noggin demonstrate a failure in joint formation including a failure to form IVD Joints are fused in these mice presumably due to excessive BMP signaling and endochondral bone formation in the joint space [28]. Nevertheless, the specific effects of Noggin on differentiation of sclerotome are not well understood.
Understanding how TGFβ and BMP interact in sclerotome will be important to decipher how the axial skeleton forms. Here, we address the mechanism of TGFβ action during cell fate decisions in cultured primary sclerotome. We propose that TGFβ acts in part by antagonizing BMP activity and test the hypothesis that inhibition of BMP signaling and subsequent inhibition of chondrogenesis is sufficient to flip cell fate towards fibrogenesis. We show that TGFβ1 antagonizes BMP/Smad1/5 signaling and that cartilage nodule formation is reduced in TGFβ1 treated cultures. Noggin, a known antagonist of BMP signaling, was induced by TGFβ1. However, Noggin was not sufficient to induce fibrous differentiation, as measured by expression of Adamtsl2, Fmod, and Scx, without TGFβ signaling. Likewise, Gremlin and DMH2, additional inhibitors of BMP signaling, did not induce expression of fibrous markers indicating that inhibiting BMP/Smad1/5 signaling alone is not enough to switch cell fate in the sclerotome. Another instructive signal, likely from TGFβ, is also necessary. We propose TGFβ antagonizes BMP signaling in part through Noggin to prevent chondrogenesis providing a permissive environment for fibrous differentiation.
Methods
The University of Alabama at Birmingham’s Institutional Animal Care and Use Committee approved all animal use. All animal use was carried out in accordance with IACUC guidelines and regulations.
Sclerotome cell culture
Sclerotome cells were isolated from wild type (B1/6 × ICR) mouse embryos at age E11.5. The cells were plated in micromass culture [18, 29]. Specifically, 1.5 × 107 cells were plated in 20ul drops of culture medium, which consisted of 40% DMEM, 60% Ham’s F-12, 10% heat-inactivated FBS, 0.5mM L-glutamine, 1% Penicillin/Streptomycin, 0.25mM Ascorbic Acid and 1mM β-glycerophosphate. After one hour, culture dishes were flooded with medium that contained either TGFβ1 (R&D system), BMP4 (R&D system), GDF5 (Pepro Tech), Noggin (R&D), Gremlin (R&D), DMH2 (Selleckchem), and SB154382 (Tocris science) and cells were cultured at 37°C in a humidified incubator with 5% CO2 for indicated times. Some cultures were fixed with 4% PFA and either stained with Alcian Blue solution (1% Alcian Blue in 0.1M HCL) or removed from the culture dishes and embedded in paraffin for sectioning (sections were 5um thick). Sections were stained with Alcian Blue solution and Fast Red solution (0.1% Nuclear Fast Red in 5% aluminum sulfate). Some cultures were processed for Quantitative Real Time RT-PCR (qPCR) or Western blot.
Western blot
Sclerotome cultures were washed with PBS and lysed with RIPA buffer containing protease and phosphatase inhibitors (Roche). Protein concentrations were determined with the DC protein Assay kit (Bio-Rad). 30-50ug of protein were loaded into 4-20% polyacrylamide gels (Bio-Rad Laboratories) and separated by electrophoresis. The separated protein was transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad) using a Trans-Blot Turbo Transfer System (Bio-Rad). 3% Bovine Serum Albumin (BSA) (Fisher Scientific) was used to block membranes for 1 hour and the membranes were treated with the following antibodies overnight: pSmad1/5 (cell signaling #9516), Smad1 (cell signaling #9743), pSmad2 (cell signaling #3104) Smad2/3 (cell signaling #8685), α-Tubulin (Rockland Immunochemicals), Noggin (R&D #AF719), GAPDH (Rockland Immunochemicals) and CRE (Novagen #69050). After washing with Tris buffer, membranes were treated with secondary antibody (cell signaling) for 1 hr. Western blot images were acquired on a ChemiDoc MP System (Bio-Rad Laboratories). The western blot images were analyzed with ChemiDoc MP system.
Quantitative Real Time RT-PCR (qPCR)
mRNA was isolated using TRIzol Reagent (Thermo Fisher Scientific) and a Direct-zol RNA MiniPrep kit (Zymo Research). The QuantiFast SYBR Green RT-PCR kit (Quiagen) and a Roche LightCycler 480 were used to perform qPCR. Relative mRNA expression levels were determined using the Relative Expression Software Tool (REST) 2009 (QIAGEN). Hypoxanthine Phosphoribosyltransferase (HPRT) was used as the reference/normalization gene. The REST software determined statistical significance using a nonparametric pair-wise fixed reallocation randomization test [30]. The REST program provides fold difference between experimental groups and 68% and 95% confidence intervals. Primers used for this study are shown in Supplementary Table 1 Table S1).
Adenovirus
Adenoviruses encoding for GFP and CRE were amplified in 293A cells. Virus titers were determined by dilution assay and counting GFP-positive cells in adenovirus-infected 293A cell cultures. Sclerotome cells were isolated from Tgfbr2 flox/flox mice at E11.5 [31] and incubated with GFP or CRE adenovirus for 48hrs. Following incubation, cells were processed for qPCR or Western blot.
Small interference RNA transfection
C3H10T1/2 cells were transfected with 30pmole of siRNA Noggin (Santa Cruz; Si-Nog) or scrambled siRNA (Santa Cruz; Si-CTRL) using Lipofectamine RNAi (Thermo Fisher Scientific) following the manufacturer’s protocol. The cells were incubated with Opti MEM medium (Gibco) for 24hrs, then DMEM medium (Gibco) was added to the cells for 24 hours before treatment with vehicle or 5ng/ml of TGFβ1 for 8hrs.
Results
TGFβ1 antagonizes BMP/Smad1/5 signaling and cartilage formation in cultured sclerotome
Cell fate decisions during development often require both instructive signals to directly induce differentiation into a specific cell type and inhibitory signals to prevent unwanted cell fates [32]. We previously showed that loss of Tgfbr2 resulted in cartilage formation in areas where fibrous tissue would normally be located in the joints of the digits and in the AF of the IVD [16, 18]. Furthermore, addition of TGFβ to cultured mesenchyme promoted differentiation of fibrous tissues and reduced the formation of cartilage nodules [18, 20–24]. Based on these observations, it was proposed that TGFβ normally promotes formation of fibrous tissues and inhibits chondrogenesis in early undifferentiated mesenchyme in vivo and in culture. It was not clear if fibrous tissue formation was the result of TGFβ inhibiting cartilage formation, TGFβ promoting fibrous differentiation, or if a combination of both processes was required. BMP is a strong chondrogenic factor [11, 18] so we hypothesized that TGFβ antagonizes BMP signaling in sclerotome. The level of pSmad1/5 as measured by immunoblot was used to evaluate BMP signaling.
Sclerotome cultures contained a basal level of endogenous BMP activity that could be seen as high levels of pSmad1/5 during the first 8 hours of culture and lower but still easily detectable levels after that (Fig. S1). When cells were treated with TGFβ1 for 8 hours the level of pSmad1/5 was reduced compared to vehicle treated control samples when normalized to the amount of total Smad1 (Fig. 1A) indicating an antagonistic effect of TGFβ1 on endogenous BMP signaling. TGFβ1 also reduced pSmad1/5 levels that were induced by exogenously added BMP4 (Fig. 1B), again indicating TGFβ antagonized BMP signaling.
Figure 1. TGFβ1 antagonizes BMP/Smad1/5 signaling.
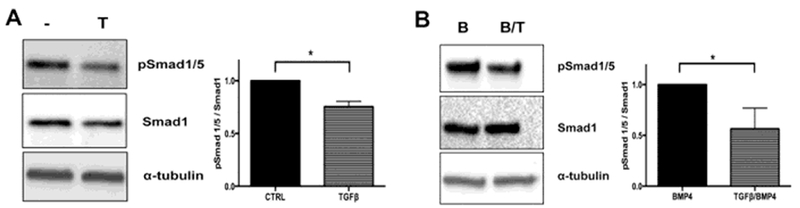
Primary sclerotome was treated with (A) vehicle control (−) or TGFβ1 (T) for 8hrs or (B) BMP4 (B) and BMP4 plus TGFβ1(B/T) for 24hrs. Relative pSmad1/5 levels were determined by Immunoblot assay. Bands on the blots were quantified by densitometry and pSmad1/5 levels were normalized to total Smad1 α-Tubulin was used as a protein loading control. One-way ANOVA was used for statistical analysis between independent samples. (n=3).
Previously, we and others showed that TGFβ inhibited the formation of discrete Alcian Blue stained nodules representing cartilage in mesenchymal cells grown in micromass culture [16, 18, 21–23]. Alcian Blue used under acidic conditions stains sulfated glycosamino glycans on proteoglycans, mostly Acan, in the extracellular matrix so that cartilage stains very deeply with Alcian Blue (Fig. 2B, white arrow). Treament with BMP4, which is a known chondrogenic factor, resulted in an increase in these types of discrete and deeply Alcian Blue stained nodules [18, 21]. To determine if TGFβ inhibited BMP-mediated chondrogenesis, we looked at cartilage nodule formation in primary sclerotome grown for 3 days in micromass culture containing vehicle, TGFβ1, BMP4, or TGFβ and BMP4 together (Fig. 2 A, B). Sclerotome was microdissected from E11.5 wild type embryos and cells were isolated and cultured under high density conditions as previously characterized.22 [18, 29]. Chondrogenic differentiation was first visualized qualitatively. Cartilage nodules were observed under phase contrast microscopy (Fig. 2A) and as discrete areas stained with Alcian Blue (Fig. 2B, white arrow). Alcian Blue used under acidic conditions stains sulfated glycosamino glycans on proteoglycans, mostly Acan, in the extracellular matrix so that cartilage stains very deeply with Alcian Blue (Fig. 2B, white arrow). When sectioned, cartilage nodules demonstrated deep Alcian Blue staining, round cells at high density, and a sharp border with an outer layer of fibroblasts resembling perichondrium, suggestive of hyaline cartilage (Fig. S2). Under basal conditions several cartilage nodules formed in each culture (Fig. 2 Ai and Bi, arrows; quantified in Fig. 2E). Nodule formation under basal conditions was dependent on endogenous BMP signaling since treatment with Noggin, a well-known antagonist of BMP, resulted in fewer nodules (Fig. 2C) [33]. As previously reported [13, 16, 18, 20–23], cultures treated with TGFβ1 demonstrated some diffuse Alcian Blue staining visible throughout the culture (Fig. 2 Bii), but, discrete nodules were not observed suggesting cartilage formation was reduced by TGFβ1. When cultures were treated with BMP4, there was an increase in nodules with the characteristics of hyaline cartilage (high density of round cells, deeply stained, perichondrium) (Fig. 2 Aiii, Biii, arrows and Fig. 2E and Supplementary Fig. S2A). When BMP treated cultures were also treated with TGFβ1, discrete nodules were not seen under phase contrast microscopy (Fig. 2 Aiv). Alcian Blue staining was still visible; however, discrete nodules with perichondrium were not observed suggesting reduced hyaline cartilage in the presence of TGFβ1 (Fig 2 Biv and Fig. 2E and Supplementary Fig. S2B). Growth and differentiation factor 5 (GDF5), a member of the BMP protein family, has also been shown to have chondrogenic activity and, similar to BMP4, signals through Smad1/5 [34, 35]. To determine if TGFβ1 could also inhibit GDF5-mediated chondrogenesis, cultures were maintained with either GDF5 or GDF5 and TGFβ1 together for 3 days. Addition of TGFβ1 resulted in fewer cartilage nodules (Fig. 2D and 2E).
Figure 2. TGFβ1 inhibits formation of cartilage.
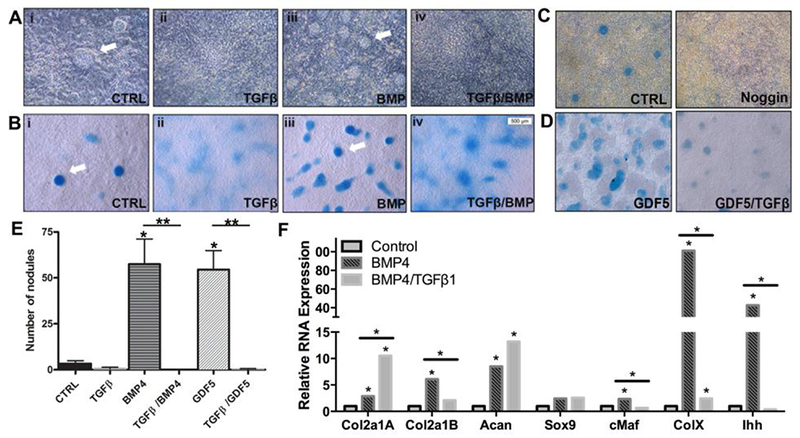
Discrete cartilage nodules are visible in (A) phase contrast images and (B) Alcian Blue staining. Primary sclerotome was treated with (i) vehicle control (CTRL), (ii) TGFβ1, (iii) BMP4, or (iv) BMP4 and TGFβ1 for 3 days. Arrows indicate representative cartilage nodules. Representative images from n=3 biological replicates shown. (C) Alcian Blue staining for Control (CTRL) and Noggin treated cultures after 3 days of treatment. Representative images from n=3 biological replicates shown. (D) Alcian Blue staining for GDF5 treated and GDF5 plus TGFβ1 treated cultures at 3 days of treatment. Representative images from n=3 biological replicates shown. (E) Quantification of nodules in sclerotome cultures after 3 days of indicated treatment. From n=3 biological replicates of each condition. (F) Primary sclerotome was treated with vehicle (CTRL) TGFβ1, BMP4, or TGFβ1 and BMP4 together for 5 days. Relative mRNA levels for the indicated cartilage markers were determined by qPCR. The mRNA levels were normalized to HPRT and analyzed with REST software. All data from REST analysis is shown in Table S2A. (*) indicates significance, p<0.05 (n=3 biological replicates).
It was previously shown that Sox9 and Collagen Type MB were down-regulated by TGFβ in limb micromass cultures after 4 days of treatment [22]. In addition, we previously reported that while BMP up-regulated cartilage markers, Sox9, Sox5, Acan, and Ihh in sclerotome by 8 hours of treatment, TGFβ did not regulate expression of any of these genes at that time while fibrous markers were stimulated [18]. To further determine if TGFβ antagonized BMP signaling and thus chondrogenesis, we used expression of Sox9, Type IIA and Type MB Collagen, Acan, Ihh, c-Maf, and Col10 as measured by real time quantitative RT- PCR (qPCR) as markers for cartilage differentiation (Fig. 2F). mRNA for Collagen Type II is spliced and has two primary transcripts coding for Collagen Type IIA (Col2a1A) and Collagen Type MB (Col2a1B) proteins. Col2a1A contains sequences from exon 2 and is expressed in noncartilagenous and precartilagenous fibroblastic cells. Exon 2 is spliced out of Col2a1B and this is the transcript that is enriched in cartilage [36, 37]. Acan is a proteoglycan that is expressed at high levels in cartilage but is also expressed in AF, tendon and ligament [38]. Sox9, along with Sox5 and Sox6, are major transcriptional regulators of cartilage enriched genes [39]. c-Maf is a transcription factor that is enriched in developing vertebral bodies and is required for normal cartilage development [18, 40]. Col 10a is a marker of cartilage maturation that is regulated by BMP [41]. Indian hedgehog (Ihh) is a downstream target gene for BMP and it is also used as a marker of cartilage [42]. Cultures were untreated or treated with BMP4 or BMP4 with TGFβ1 together for 5 days and relative mRNA levels were determined (Fig. 2F and Table S2A). As expected, when cells were treated with BMP4, Col2a1B, Acan, Ihh, c-Maf, and Col10 expression was increased. Sox9 mRNA levels were not significantly increased by BMP treatment at this time point. Furthermore, addition of TGFβ1 to BMP treated cultures resulted in a significant attenuation of BMP-mediated expression of Col2a1B, c-Maf, Col10, and Ihh mRNA levels. In contrast, BMP-induced levels of Acan mRNA were not affected by treatment with TGFβ1. This fits with the diffuse staining of Alcian Blue throughout the TGFβ1 treated cultures described above (Fig. 2 B). In addition, Col2a1A mRNA, the fibroblast enriched transcript for Type II Collagen, was significantly increased by addition of TGFβ1 to the BMP treated cultures, likely due to TGFβ’ε fibrogenic activity. Together the results suggest that TGFβ antagonizes BMP signaling and thus chondrogenic activity. Expression of markers for early or late osteoblasts or osteocytes, Runx2, Bglap, and DMP1 respectively, were not different in control, BMP4, or BMP4 plus TGFβ1 treated cultures. (Fig. S3 and Table S2B).
Noggin induced by TGFβ1 in cultured sclerotome antagonizes BMP signaling
Previously, it was shown that TGFβ can inhibit BMP signaling through regulation of extracellular antagonists of BMP [43]. Noggin is a well-known extracellular antagonist of BMP that is expressed in early IVD [27]; therefore, we tested the hypothesis that TGFβ regulates Noggin expression. We used western blot analysis to determine if Noggin protein levels were changed after treatment with TGFβ1. We showed that Noggin protein levels were increased after 2 hours of treatment with TGFβ1 (Fig. 3A). Next, we determined if TGFβ1 regulated Noggin mRNA. Cells were treated with TGFβ1 for two hours, RNA was isolated, and qPCR was used to determine the relative levels of Noggin and Scleraxis (Scx), a positive control for TGFβ1 activity. Noggin mRNA was increased 3.5-fold after treatment with TGFβ1 (Fig. 3B and Table S3A).
Figure 3. TGFβ1 regulates Noggin expression.
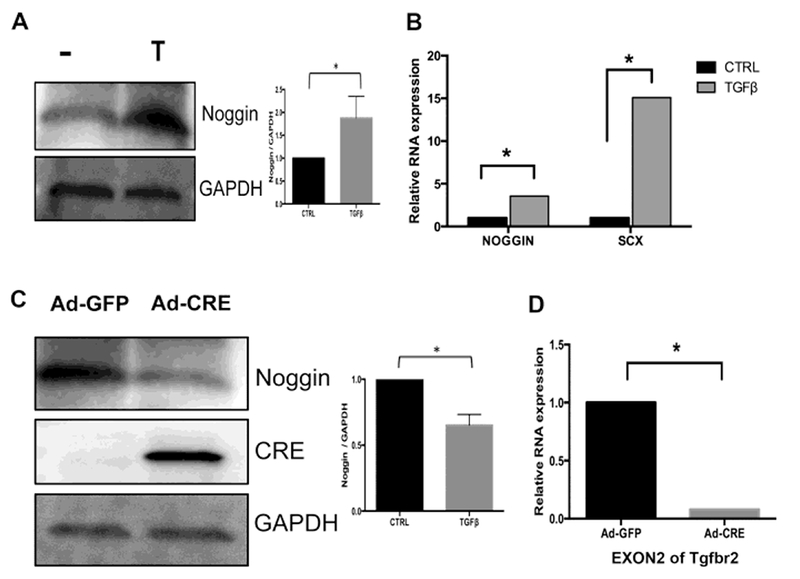
(A) Protein lysates were harvested from primary sclerotome after 2hrs treatment with vehicle control (−) or TGFβ1 (T). Immunoblot was used to detect the relative amount of Noggin protein in the samples. GAPDH was used as a normalization control. One-way ANOVA was used for statistical analysis between independent samples. (n=3). (B) RNA was extracted from cells treated with vehicle (CTRL) or TGFβ1 for 2hrs. Relative Noggin mRNA expression was measured with qPCR and analyzed with REST software. Scleraxis (SCX) RNA was used as a positive control. (*) indicates significant, (n=4). Full qPCR results are shown in Table S3A. (C) Sclerotome from Tgfbr2flox/flox mice was placed in culture and infected with Ad-GFP or Ad-Cre for 48hrs. Relative Noggin protein levels were determined by immunoblot assay. GAPDH was used as a normalization control. Bands were quantified using densitometry. One-way ANOVA was used for statistical analysis between independent samples. p<0.05 (n=3). (D) RNA was isolated from cells treated with either Ad-GFP or Ad-Cre for 48hrs. The relative levels of Tgfbr2 mRNA were measured to confirm knock down with Ad-Cre. (*) indicates significance (n=3). Full qPCR results are shown in Table S3B.
To determine if Tgfbr2 was required for Noggin expression, we isolated and cultured sclerotome from mice containing a floxed allele of Tgfbr2 [31]. The Tgfbr2 gene was deleted by infecting the cells with an Adenovirus that expressed the Cre recombinase (Ad-Cre). Expression of Cre was confirmed by Western blot (Fig. 3C) and the deletion of Tgfbr2 was confirmed by qPCR (Fig. 3D and Table S3B). Noggin protein expression was decreased in Ad-Cre infected cells compared with control, Ad-GFP infected cells, indicating that Tgfbr2 is required for Noggin expression in sclerotome (Fig. 3C). These data indicate that Noggin, a known antagonist of BMP/ Smad1/5 signaling, is regulated by TGFβ.
Since Noggin is an antagonist of BMP, we hypothesize that Noggin is required for TGFβ to inhibit BMP signaling. C3H10T1/2 can be transfected with DNA or siRNA and it was previously shown that C3H10T1/2 cells can differentiate along both chondrogenic and fibrogenic lineages [34, 44]. Furthermore, we showed that C3H10T1/2 cells behave similarly to primary sclerotome in that Noggin as well as the control gene Scx were regulated by TGFβ1 in the C3H10T12 cells (Fig. 4A and Table S4A). We transfected siRNA Noggin (Si-Nog) or a control scrambled RNA (Si-CTRL) into C3H10T1/2 cells and then after 48 hours cells were treated with TGFβ1 or vehicle control for an additional 8 hours. Partial knock down of Noggin was confirmed by western blot (Fig. 4B). As expected, pSmad1/5 was down-regulated in cells that were treated with TGFβ1 in the presence of Si-CTRL (Fig. 4C). In contrast, pSmad1/5 was not down-regulated by TGFβ1 treatment in the presence of Si-Nog (Fig. 4C). Total Smad1 and α-tubulin were used as normalization controls for the western blot assay. This result indicated that Noggin is required for TGFβ-mediated antagonism of BMP signaling.
Figure 4. Noggin is required for TGFβ to inhibit BMP signaling.
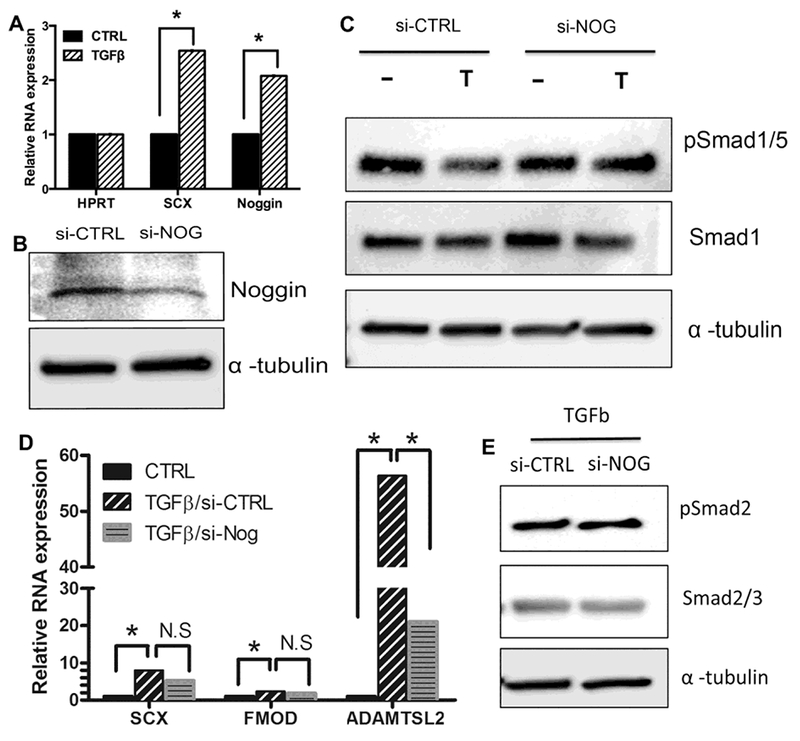
(A) C3T10H1/2 cells were treated with vehicle (CTRL) or TGFβ1 for 8 hours. RNA was collected and relative levels of mRNA for Scx and Noggin were determined by qPCR and analyzed with REST software. n= 4 biological replicates, * = p < 0.05. REST analysis shown in Table S4A. (B) C3T10H1/2 cells were transfected 30 pmole of siRNA control (si-CTRL) or siRNA directed to noggin (si-NOG) then incubated for 48 hours. Immunoblot of Noggin protein levels confirmed knockdown by si-NOG. α-tubulin was used as a loading control. (C) C3T10H1/2 cells were transfected siRNA control (si-CTRL) or siRNA directed to noggin (si-NOG). After 48hrs, cells were treated with vehicle control (−) or TGFβ1 (T) for 8hrs. Protein extracts were collected and pSmad1/5 and Smad1 levels were determined by Immunoblot. α-tubulin was used as a loading control. Representative of n=5 replicates shown (D) C3T10H1/2 were transfected with si-CTRL or si-NOG for 48hrs. RNA was harvested after treatment with TGFβ1 for 8hrs. Relative mRNA levels of SCX, ADAMTSL2, and FMOD was measured with qPCR and normalized with HPRT. Data was analyzed using REST software, * = p< 0.05, (n=5). REST analysis shown in Table S4B. (E) TGFβ signaling was measured by immunoblot of pSmad2 and Smad2/3 in Si-CTRL and Si-NOG treated cells. Representative from n=3 replicates shown.
Previously, our lab and others identified genes that are highly expressed in developing AF including Scx, Fibromodulin (Fmod) and Adamts like-2 (Adamtsl2) [13, 18, 45–47]. Tendon/ligament and AF are closely related fibrous tissues and these markers are also expressed in tendon/ligament. We next asked if Noggin was required for TGFβ mediated expression of these fibrous markers. C3H10T1/2 cells were transfected with Si-CTRL or Si-Nog for 48 hours and then treated with vehicle control or TGFβ1 for an additional 8 hours. In cells expressing Si-Nog, TGFβ-mediated up-regulation of Adamtsl2 was significantly attenuated when compared to the induction observed in control samples containing Si-CTRL (Fig. 4D and Table S4B). TGFβ-mediated up-regulation of Scx and Fmod appeared partially attenuated but did not reach the level of significance, perhap because we could only achieve partial knock down of Noggin in these cells. The results suggest that Noggin is at least partially required for TGFβ-mediated regulation of fibrous differentiation. We then confirmed that the activity of pSmad2/3 was not changed in cells treated with Si-Nog when compared to control cells, indicating that partial knock down of Noggin did not simply inhibit TGFβ signaling (Fig. 4E). The results lead us to hypothesize that inhibition of BMP signaling through Noggin may be at least partially involved in TGFβ-mediated regulation of fibrous differentiation.
Inhibition of BMP/Smad1/5 signaling is not sufficient to promote fibrous differentiation
We next asked if Noggin itself was sufficient to regulate expression of fibrous markers, Scx, Fmod, or Adamtsl2. First, we determined the amount of Noggin necessary to inhibit BMP/Smad1/5 signaling in primary sclerotome. Cells were treated with 0, 0.5, and 1 μg/ml of Noggin for 48 hours and pSmad1/5, Smad1, and α tubulin levels were determined by western blot. pSmad1/5 levels were significantly inhibited by 1 μg/ml of Noggin (Fig. 5A and Supplementary Fig. S4A). Next, RNA was isolated from cells treated with 0, 0.5, or 1 μg/ml Noggin and the relative expression of Scx, Fmod, and Adamtsl2 was examined. Expression levels were also compared to those after treatment with TGFβ1. Treatment with 1 μg/ml Noggin significantly increased Scx, Fmod and Adamtsl2 mRNA levels although induction was much less than that observed with TGFβ1 treatment (Fig. 5B and Table S5A).
Figure 5. Noggin cooperates with TGFβ1 but is not sufficient to regulate AF differentiation.
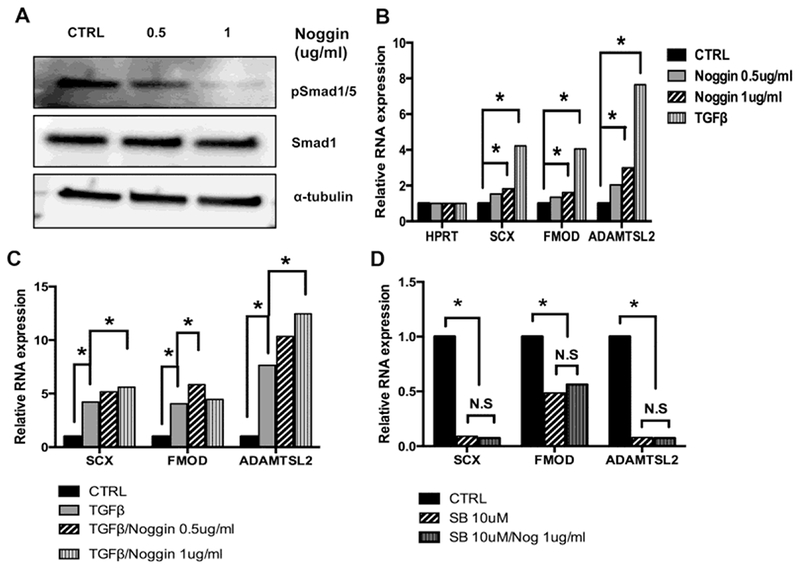
(A) Sclerotome cultures were treated with Noggin for 48hrs. pSmad1/5 and Smad1 levels were detected by Immunoblot and α-tubulin was used as the loading control. Quantification of pSmad1/5 relative to total Smad1 is shown in Figure S4. (B) Sclerotome cultures were treated with vehicle (Ctrl), Noggin or TGFβ1 for 48 hrs. Relative expression of Scx, Fmod, and Adamtsl2 was determined by qPCR and normalized to HPRT. (C) Sclerotome cultures were treated with vehicle (Ctrl), TGFβ1, or Noggin and TGFβ1 together for 48 hrs. Relative expression of Scx, Fmod, and Adamtsl2 was determined by qPCR and normalized to HPRT. (D) Cells were treated for 48 hrs with vehicle (Ctrl), SB154382 (SB), or SB and Noggin together. Relative expression of Scx, Fmod, and Adamtsl2 mRNA was determined by qPCR and normalized to HPRT. All qPCR data was analyzed using REST software. (N.S) denotes non-significant, (*) indicates p, 0.05, (n=3 biological replicates for each condition). Full qPCR results are shown in Tables S5A–C.
We then tested whether TGFβ1 and Noggin cooperated to regulate expression of the fibrous markers. Cells were treated with both Noggin and TGFβ1 for 48 hours and relative mRNA levels of Scx, Fmod, and Adamtsl2 were determined by qPCR (Fig. 5C and Table S5B). Treatment with Noggin and TGFβ1 together enhanced expression of all three markers relative to either treatment with TGFβ1 or Noggin alone. Cooperation between Noggin and TGFβ1 was most evident in regulation of Adamtls2 (Fig. 5C and Table S5B).
Finally, we determined if Noggin could up-regulate Scx, Fmod, and Adamtls2 in the absence of endogenous TGFβ signaling. We used an inhibitor for TGFβ type 1 receptor, SB431542 (SB), to block endogenous TGFβ signaling in primary cells. As expected, treatment with SB was sufficient to down-regulate expression of Scx, Fmod, and Adamtsl2 mRNA indicating its efficacy in the primary cells (Fig. 5D and Table S5C). In contrast to what was observed after adding Noggin to cultures with intact endogenous TGFβ signaling, when Noggin was added to cultures in which TGFβ signaling was blocked, Scx, Fmod, and Adamtsl2 were not up-regulated, (Fig. 5D and Table S5C). The results suggest that Noggin activity itself is not sufficient to regulate gene expression and that additional signals from TGFβ are also required.
To determine if inhibition of BMP signaling by other means was sufficient to promote fibrous cell fate in primary sclerotome, another peptide antagonist of BMP, Gremlin, was tested for its ability to inhibit BMP signaling and regulate fibrous differentiation. It was previously shown that Gremlin can inhibit BMP activity to initiate sclerotome formation from the somite [26]. The concentration of Gremlin to use was determined by measuring the level of pSmad1/5 in response to 0, 0.1, 0.5, and 1 μg/ml of Gremlin after 48 hours. Treatment with 1 μg/ml Gremlin resulted in significant down-regulation of pSmad1/5 (Fig. 6A and Supplementary Fig. S4B). Next, cells were treated with vehicle or 1 μg/ml of Gremlin for 48 hours and the relative levels of Scx, Fmod, and Adamtsl2 mRNA were determined (Fig. 6B and Table S6A). In contrast to Noggin, even in the presence of endogenous TGFβ activity, Gremlin did not regulate Scx, Fmod, or Adamtsl2. Thus, fibrous differentiation was not regulated by Gremlin, suggesting that simply inhibiting BMP/Smad1/5 signaling is not sufficient to make sclerotome chose the fibrous fate. Furthermore, when cells were treated with Gremlin and TGFβ1 together, there was no cooperation (Fig. 6C and Table S6B) suggesting Noggin has some unique activity that cooperates with TGFβ to regulate gene expression distinct from its BMP inhibiting activity.
Figure 6. Inhibition of BMP/Smad1/5 signaling is not sufficient to induce fibrous differentiation.
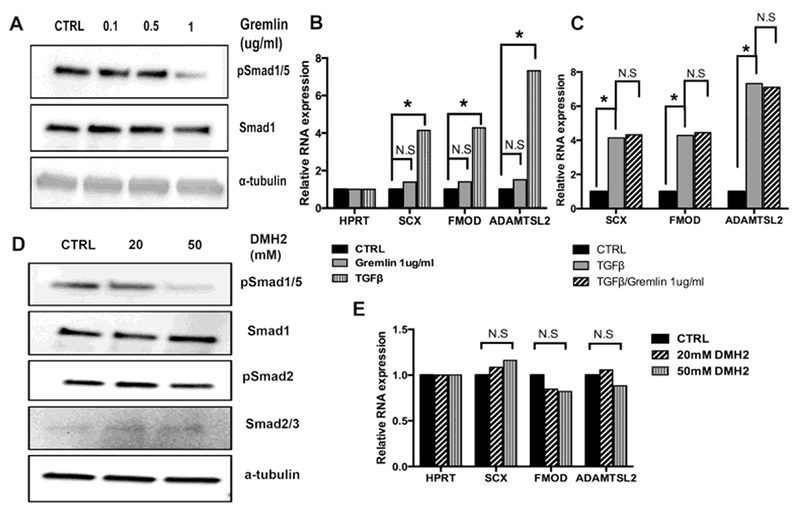
(A) Primary sclerotome was treated with the indicated amount of Gemlin protein for 48 hrs. Relative levels of pSmad1/5 and Smad1 were determined by immnoblot. α-tubulin was used as a loading control. Quantification of immunoblots in Fig. S4. (B) Sclerotome was treated with vehicle (CTRL), Gremlin, or TGF-ß1 for 48 hrs. Relative mRNA levels for Scx, Fmod, and Adamtsl2 were determined by qPCR and quantified using REST software. (N.S) denotes non-significant, (*) indicates P < 0.05, (n=3) (C) Sclerotome was treated with vehicle (CTRL), TGF-β1, or TGF-β1 and Gremlin together for 48 hrs. Relative mRNA levels for Scx, Fmod, and Adamtsl2 were determined by qPCR and quantified using REST software. (N.S) denotes non-significant, (*) indicates significant p < 0.05, (n=3). (D) Primary sclerotome was treated with the indicated amount of DMH2 protein for 48 hrs. Relative levels of pSmad1/5 and Smad1 were determined by immnoblot. α-tubulin was used as a loading control. Immunoblots quantified in Fig. S4. (E) Sclerotome cultures were treated with vehicle control or indicated concentrations of DMH2 for 48hrs. RNA was extracted from cells and used in qPCR to determine the relative levels of Scx, Fmod, and Adamtsl2 mRNA. Expression was normalized to Hprt and quantified using REST software. (N.S) denotes nonsignificant, (*) indicates significant, (n=3) Full qPCR analysis is shown in TableS6A–C.
We tested another BMP inhibitor, DMH2, which at low concentrations inhibits activity of type 1 BMP receptors. DMH2 can also inhibit Tgfbr2 at higher concentrations [48]; therefore, we first determined optimized DMH2 levels to inhibit BMP signaling without affecting signaling through Tgfbr2. Cells were treated with 0, 20 and 50nM DMH2 for 48 hours and pSmad1/5 levels were determined by western blot (Fig. 6D and Fig. S4C). pSmad1/5 levels were significantly reduced with the 50nM concentration. To ensure Tgfbr2 signaling was not disrupted at this concentration of DMH2, pSmad2/3 levels were also determined by immunoblot. pSmad2/3 levels were not altered at 20 or 50nM DMH2 (Fig. 6D and Fig. S4D). Next, RNA was isolated from cells treated with 20 or 50nM DMH2 for 48 hours and relative levels of Scx, Fmod, and Adamtsl2 mRNA were determined by qPCR. Similar to that observed after treatment with Gremlin, gene expression was not altered by treatment with DMH2 (Fig. 6E and Table S6C). The results suggest inhibition of BMP alone is not sufficient to induce fibrous differentiation in sclerotome.
Discussion
Development of tissues and organs is orchestrated by series of cell fate decisions. Cell fate decisions are controlled not only by instructive factors, which direct cell fate to a specific lineage, but also by inhibitory factors, which block the unwanted cell fate. In vitro models of differentiation can be used to study instructive and inhibitory signals as well as how these two types of signals cooperate to determine cell fate. Loh et al. used a human pluripotent stem cell line (H7) to show that inhibitory signals are imperative during the pairwise choices that lead from pluripotency to specific cell types. They were able to logically block differentiation toward unwanted fates, steering the cells through branchpoints to rapidly generate skeletal or cardiac tissues [32]. Likewise, Barske et al. showed that Nuclear Receptor 2f (Nr2f) selectively represses early cartilage lineages in skeletal progenitor cells resulting in the direct formation of dermal bone in the zebrafish. The results suggested that the Nr2f inhibits chondrogenesis so that membranous bone formation is favored [49]. In the axial skeleton, sclerotome is the common progenitor for bone, through endochondral ossification, and fibrous tissues like the AF and tendon. We hypothesized that the inhibitory activity of TGFβ on chondrogenesis in sclerotome could be critical during development of the fibrous parts of the axial skeleton.
We and others have shown that loss of Tgfbr2 in mice results in failure in development and maintenance of fibrous tissues with increase in cartilage and bone formation [6, 13– 15, 19]. The mechanisms of how TGFβ mediates cell fate decisions in the axial skeleton are not clear. In this study we show that 1) TGFβ antagonizes BMP/ Smad1/5 signaling and inhibits chondrogenic differentiation, 2) TGFβ stimulates the expression of Noggin, a well-known BMP antagonist, which is at least partially required for TGFβ to inhibit BMP signaling, and 3) Inhibition of BMP/Smad1/5 signaling is not sufficient for fibrous differentiation suggesting other factors are involved. We propose that in addition to inhibiting BMP signaling in sclerotome, TGFβ likely provides an instructive signal to generate fibrous tissues.
Previously, our laboratory showed, using global transcript profiling of the putative AF that loss of Tgfbr2 in this compartment resulted in a switch in the molecular profile of the cells so that they resembled the hyaline cartilage of the vertebrae. Genes associated with fibrous differentiation were not expressed and genes typical of cartilage were present. In addition, early in development the boundaries between the putative vertebrae and AF were not well defined [13, 50]. The results suggested that Tgfbr2 was required for the formation of the AF in that compartment either through inhibition of the cartilage cell fate or instruction to the AF cell fate or a combination of both. Here, we used primary sclerotome to model cell fate decisions. When grown under micromass conditions, differentiation to hyaline cartilage is the default as indicated by well-defined Alcian Blue stained nodules [18, 29]. Treatment with BMP, a well-known chondrogenic factor, resulted in an increase in the number of discrete cartilage nodules with a perichondrium-like structure surrounding them. In contrast, treatment with TGFβ1 resulted in loss of the well-defined nodules [18, 21–23]. This result is in agreement with other studies showing that TGFβ does not regulate cartilage markers in early undifferentiated mesenchymal cells, instead, markers of fibrous differentiation are up-regulated [18, 20–24]. Here, we showed that TGFβ antagonized BMP activity and attenuated BMP-induced cartilage formation. We propose that TGFβ inhibits chondrogenic differentiation in the developing spine by antagonizing BMP activity, thus providing a space for fibrous tissues to form.
We then sought to determine the mechanism whereby TGFβ inhibited BMP signaling It has been shown in several model systems that BMP and TGFβ /Activin can antagonize each other’s activity. For example, in early patterning of Xenopus embryos Activin and BMPs antagonize each other to establish the dorsal-ventral polarity of the embryo. In this case, it was shown that antagonism occurs intra-cellularly at the level of the common Smad, Smad4 [51]. It was proposed that the Activin signal, which, like TGFβ, is mediated by Smad2/3 and the BMP signal, mediated by Smad1/5/8, compete for Smad4 during signaling. Noggin, an extracellular antagonist of BMP, is expressed in the developing IVD [21]. It was previously shown that Noggin inhibits the onset and progression of ankylosing enthesitis in mice, a condition with some similarities to the phenotype observed in adult mice with disrupted TGFβ signaling [14, 15, 52, 53]. Noggin is a well-known antagonist of BMP signaling, thus, we tested the hypothesis that TGFβ regulated Noggin expression to suppress BMP signaling and thereby cartilage formation. We found that Noggin mRNA and protein levels were regulated by TGFβ1 in sclerotome and that Tgfbr2 was necessary for basal expression of Noggin.
Initially we proposed that inhibition of BMP signaling and thus inhibition of chondrogenesis would be sufficient to shift the balance so that cells would chose fibrous cell fates. While Noggin treatment significantly increased expression of markers for fibrous differentiation, induction was far less than what was seen with TGFβ alone and was dependent on endogenous TGFβ signaling. Additional BMP antagonist, Gremlin and DMH2, did not significantly regulate Scx, Fmod, or Adamtsl2 expression. The results suggested that, while inhibition of chondrocyte cell fate may be required for fibrous differentiation in sclerotome, it is not sufficient, and additional instructive factors are also required.
In addition, in contrast to Noggin, Gremlin and TGFβ1 did not cooperate to regulate gene expression. Similar results to those using Gremlin were observed with another BMP inhibitor, DMH2, which inhibits BMP type 1 receptor activity. The results suggest that Noggin may have some unique functions outside of antagonizing BMP activity that cooperate with TGFβ to regulate gene expression. Previous studies showed that Noggin cooperates with GDF5 to regulate cartilage differentiation whereas Gremlin does not [25, 33]. Another study showed that GDF5 can bind to ROR2 to regulate long bone development [54]. It is possible that Noggin has specific effects on cell fate determination via its interaction with GDF5.
In summary, this study provides evidence that TGFβ has complex functions in regulating cell fate decisions in the axial skeleton. We hypothesize that TGFβ has a dual function. First, it antagonizes BMP/Smad1/5 signaling and chondrogenesis to allow development of fibrous tissues. Second, it promotes fibrous differentiation through a yet unidentified instructive signal. This information will be useful for developing stem-cell based therapies for repair or regeneration of skeletal tissues.
Supplementary Material
Figure S1. Sclerotome in culture has endogenous BMP activity.
(A) Proteins was collected from cells at varying time points as indicated. BMP signaling was measured by immunoblot as the level of pSmad1/5. Smad1 and α-tubulin were used as normalization controls. Representative of n=3 biological replicates is shown. (B) pSmad1/5 density was normalized to total Smad1 using ImageJ. One-way ANOVA was used for statistical analysis between independent samples from 3 separate biological replicates.
Figure S2. Histology of cartilage nodules.
Sections of paraffin embedded sclerotome micromass cultures that were treated with BMP4 (A) or BMP4 plus TGFβ1 (B). Sections were stained with Alcian Blue and Fast Red. Representatives from n=3 separate cultures are shown.
Figure S3. Osteoblast gene expression.
RNA was extracted from cells that has been treated with vehicle (CTRL), TGFβ1, BMP4, or TGFβ1 and BMP4 for 48 hrs. Relative levels of Runx2, Bglap, DMP1, and Ihh were measured by QPCR and analyzed with REST software. Ihh was used as a positive control since it is known to be regulated by BMP and TGFβ. Expression was normalized to HPRT. (N.S) denotes non-significant, (*) indicates significant, p< 0.05 (n=3 biological replicates) Full qPCR analysis is shown in Table S2B.
Figure S4. Quantification of Smad blots.
Cells were treated with Noggin (A), Gremlin (B), or DMH2 (C, D) for 48 hours. Protein was extracted and BMP signaling was measured by immunoblot as the level of pSmad1/5 (A-C) and TGFβ signaling was measures as the level of pSmad2/3. Smad1 or Smad3 were used as normalization controls. Band density of pSmad was normalized with total Smad protein. One-way ANOVA was used for statistical analysis between independent samples (n=3).
Highlights.
TGFβ inhibits BMP signaling in primary sclerotome.
TGFβ inhibits chondrogenesis in primary sclerotome.
TGFβ induces expression of BMP antagonist, Noggin.
Inhibition of BMP signaling is not sufficient to promote fibrous differentiation.
Acknowledgements
This study was supported by a grant from NIH R01 AR053860 to RS. SW was supported by T32 AR069516 (PI Bridges).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interests
The authors have no competing interests to declare.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
References
- [1].Moore RJ, Vernon-Roberts B, Fraser RD, Osti OL, Schembri M, The origin and fate of herniated lumbar intervertebral disc tissue, Spine (Phila Pa 1976) 21 (1996) 2149–2155. [DOI] [PubMed] [Google Scholar]
- [2].Christ B, Huang R, Scaal M, Amniote somite derivatives, Dev Dyn 236 (2007) 2382–2396. [DOI] [PubMed] [Google Scholar]
- [3].Christ B, Huang R, Wilting J, The development of the avian vertebral column, Anat Embryol (Berl) 202 (2000) 179–194. [DOI] [PubMed] [Google Scholar]
- [4].Monsoro-Burq AH, Sclerotome development and morphogenesis: when experimental embryology meets genetics, Int J Dev Biol 49 (2005) 301–308. [DOI] [PubMed] [Google Scholar]
- [5].Long F, Ornitz DM, Development of the endochondral skeleton, Cold Spring Harb Perspect Biol 5 (2013) a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Serra R, Development of the Intervertebral Disc, in: Irving MVR. Shapiro M(Ed.), The Intervertebral Disc, Springer, 2013. [Google Scholar]
- [7].Alkhatib B, Ban GI, Williams S, Serra R, IVD Development: Nucleus pulposus development and sclerotome specification, Curr Mol Biol Rep 4 (2018) 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Serra R, Chang C, TGF-beta signaling in human skeletal and patterning disorders, Birth Defects Res C Embryo Today 69 (2003) 333–351. [DOI] [PubMed] [Google Scholar]
- [9].Wu M, Chen G, Li YP, TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease, Bone Res 4 (2016) 16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM, Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo, Proc Natl Acad Sci U S A 102 (2005) 5062–5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ, Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis, PLoS Genet 2 (2006) e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Thomas JT, Kilpatrick MW, Lin K, Erlacher L, Lembessis P, Costa T, Tsipouras P, Luyten FP, Disruption of human limb morphogenesis by a dominant negative mutation in CDMP1, Nat Genet 17 (1997) 58–64. [DOI] [PubMed] [Google Scholar]
- [13].Baffi MO, Slattery E, Sohn P, Moses HL, Chytil A, Serra R, Conditional deletion of the TGF-beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones, Dev Biol 276 (2004) 124–142. [DOI] [PubMed] [Google Scholar]
- [14].Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL, Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis, J Cell Biol 139 (1997) 541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jin H, Shen J, Wang B, Wang M, Shu B, Chen D, TGF-beta signaling plays an essential role in the growth and maintenance of intervertebral disc tissue, FEBS Lett 585 (2011) 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Seo HS, Serra R, Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints, Dev Biol 310 (2007) 304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dunker N, Schweitzer R, Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation, Development 136 (2009) 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sohn P, Cox M, Chen D, Serra R, Molecular profiling of the developing mouse axial skeleton: a role for Tgfbr2 in the development of the intervertebral disc, BMC Dev Biol 10 (2010) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Spagnoli A, O’Rear L, Chandler RL, Granero-Molto F, Mortlock DP, Gorska AE, Weis JA, Longobardi L, Chytil A, Shimer K, Moses HL, TGF-beta signaling is essential for joint morphogenesis, J Cell Biol 177 (2007) 1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W, Induction of intervertebral disc-like cells from adult mesenchymal stem cells, Stem Cells 23 (2005) 403–411. [DOI] [PubMed] [Google Scholar]
- [21].Cox MK, Appelboom BL, Ban GI, Serra R, Erg cooperates with TGF-beta to control mesenchymal differentiation, Exp Cell Res 328 (2014) 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lorda-Diez CI, Montero JA, Diaz-Mendoza MJ, Garcia-Porrero JA, Hurle JM, betaig-h3 potentiates the profibrogenic effect of TGFbeta signaling on connective tissue progenitor cells through the negative regulation of master chondrogenic genes, Tissue Eng Part A 19 (2013) 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lorda-Diez CI, Montero JA, Martinez-Cue C, Garcia-Porrero JA, Hurle JM, Transforming growth factors beta coordinate cartilage and tendon differentiation in the developing limb mesenchyme, J Biol Chem 284 (2009) 29988–29996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Havis E, Bonnin MA, Olivera-Martinez I, Nazaret N, Ruggiu M, Weibel J, Durand C, Guerquin MJ, Bonod-Bidaud C, Ruggiero F, Schweitzer R, Duprez D, Transcriptomic analysis of mouse limb tendon cells during development, Development 141 (2014) 3683–3696. [DOI] [PubMed] [Google Scholar]
- [25].Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A, Bone morphogenetic proteins: a critical review, Cell Signal 23 (2011) 609–620. [DOI] [PubMed] [Google Scholar]
- [26].Stafford DA, Brunet LJ, Khokha MK, Economides AN, Harland RM, Cooperative activity of noggin and gremlin 1 in axial skeleton development, Development 138 (2011) 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].DiPaola CP, Farmer JC, Manova K, Niswander LA, Molecular signaling in intervertebral disk development, J Orthop Res 23 (2005) 1112–1119. [DOI] [PubMed] [Google Scholar]
- [28].Brunet LJ, McMahon JA, McMahon AP, Harland RM, Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton, Science 280 (1998) 1455–1457. [DOI] [PubMed] [Google Scholar]
- [29].Ahrens PB, Solursh M, Reiter RS, Stage-related capacity for limb chondrogenesis in cell culture, Dev Biol 60 (1977) 69–82. [DOI] [PubMed] [Google Scholar]
- [30].Pfaffl MW, Horgan GW, Dempfle L, Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR, Nucleic Acids Res 30 (2002) e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chytil A, Magnuson MA, Wright CV, Moses HL, Conditional inactivation of the TGF-beta type II receptor using Cre:Lox, Genesis 32 (2002) 73–75. [DOI] [PubMed] [Google Scholar]
- [32].Loh KM, Chen A, Koh PW, Deng TZ, Sinha R, Tsai JM, Barkal AA, Shen KY, Jain R, Morganti RM, Shyh-Chang N, Fernhoff NB, George BM, Wernig G, Salomon REA, Chen Z, Vogel H, Epstein JA, Kundaje A, Talbot WS, Beachy PA, Ang LT, Weissman IL, Mapping the Pairwise Choices Leading from Pluripotency to Human Bone, Heart, and Other Mesoderm Cell Types, Cell 166 (2016) 451–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Seemann P, Brehm A, Konig J, Reissner C, Stricker S, Kuss P, Haupt J, Renninger S, Nickel J, Sebald W, Groppe JC, Ploger F, Pohl J, Schmidt-von Kegler M, Walther M, Gassner I, Rusu C, Janecke AR, Dathe K, Mundlos S, Mutations in GDF5 reveal a key residue mediating BMP inhibition by NOGGIN, PLoS Genet 5 (2009) e1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hatakeyama Y, Tuan RS, Shum L, Distinct functions of BMP4 and GDF5 in the regulation of chondrogenesis, J Cell Biochem 91 (2004) 1204–1217. [DOI] [PubMed] [Google Scholar]
- [35].Storm EE, Kingsley DM, GDF5 coordinates bone and joint formation during digit development, Dev Biol 209 (1999) 11–27. [DOI] [PubMed] [Google Scholar]
- [36].Sandell LJ, Morris N, Robbins JR, Goldring MB, Alternatively spliced type II procollagen mRNAs define distinct populations of cells during vertebral development: differential expression of the amino-propeptide, J Cell Biol 114 (1991) 1307–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ng LJ, Tam PP, Cheah KS, Preferential expression of alternatively spliced mRNAs encoding type II procollagen with a cysteine-rich amino-propeptide indifferentiating cartilage and nonchondrogenic tissues during early mouse development, Dev Biol 159 (1993) 403–417. [DOI] [PubMed] [Google Scholar]
- [38].Yoon JH, Halper J, Tendon proteoglycans: biochemistry and function, J Musculoskelet Neuronal Interact 5 (2005) 22–34. [PubMed] [Google Scholar]
- [39].Liu CF, Samsa WE, Zhou G, Lefebvre V, Transcriptional control of chondrocyte specification and differentiation, Semin Cell Dev Biol 62 (2017) 34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].MacLean HE, Kim JI, Glimcher MJ, Wang J, Kronenberg HM, Glimcher LH, Absence of transcription factor c-maf causes abnormal terminal differentiation of hypertrophic chondrocytes during endochondral bone development, Dev Biol 262 (2003) 51–63. [DOI] [PubMed] [Google Scholar]
- [41].Volk SW, Luvalle P, Leask T, Leboy PS, A BMP responsive transcriptional region in the chicken type × collagen gene, J Bone Miner Res 13 (1998) 1521–1529. [DOI] [PubMed] [Google Scholar]
- [42].Minina E, Wenzel HM, Kreschel C, Karp S, Gaffield W, McMahon AP, Vortkamp A, BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation, Development 128 (2001) 4523–4534. [DOI] [PubMed] [Google Scholar]
- [43].Oshimori N, Fuchs E, Paracrine TGF-beta signaling counterbalances BMP-mediated repression in hair follicle stem cell activation, Cell Stem Cell 10 (2012) 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nakamichi R, Ito Y, Inui M, Onizuka N, Kayama T, Kataoka K, Suzuki H, Mori M, Inagawa M, Ichinose S, Lotz MK, Sakai D, Masuda K, Ozaki T, Asahara H, Mohawk promotes the maintenance and regeneration of the outer annulus fibrosus of intervertebral discs, Nat Commun 7 (2016) 12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Smits P, Lefebvre V, Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs, Development 130 (2003) 1135–1148. [DOI] [PubMed] [Google Scholar]
- [46].Koo BH, Le Goff C, Jungers KA, Vasanji A, O’Flaherty J, Weyman CM, Apte SS, ADAMTS-like 2 (ADAMTSL2) is a secreted glycoprotein that is widely expressed during mouse embryogenesis and is regulated during skeletal myogenesis, Matrix Biol 26 (2007) 431–441. [DOI] [PubMed] [Google Scholar]
- [47].Yoshimoto Y, Takimoto A, Watanabe H, Hiraki Y, Kondoh G, Shukunami C, Scleraxis is required for maturation of tissue domains for proper integration of the musculoskeletal system, Sci Rep 7 (2017) 45010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, Hopkins CR, Lindsley CW, Hong CC, In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors, ACS Chem Biol 5 (2010) 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Barske L, Rataud P, Behizad K, Del Rio L, Cox SG, Crump JG, Essential Role of Nr2f Nuclear Receptors in Patterning the Vertebrate Upper Jaw, Dev Cell 44 (2018) 337–347 e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Baffi MO, Moran MA, Serra R, Tgfbr2 regulates the maintenance of boundaries in the axial skeleton, Dev Biol 296 (2006) 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Candia AF, Watabe T, Hawley SH, Onichtchouk D, Zhang Y, Derynck R, Niehrs C, Cho KW, Cellular interpretation of multiple TGF-beta signals: intracellular antagonism between activin/BVg1 and BMP-2/4 signaling mediated by Smads, Development 124 (1997) 4467–4480. [DOI] [PubMed] [Google Scholar]
- [52].Lories RJ, Derese I, Luyten FP, Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis, J Clin Invest 115 (2005) 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lories RJ, Luyten FP, Bone morphogenetic protein signaling in joint homeostasis and disease, Cytokine Growth Factor Rev 16 (2005) 287–298. [DOI] [PubMed] [Google Scholar]
- [54].Sammar M, Stricker S, Schwabe GC, Sieber C, Hartung A, Hanke M, Oishi I, Pohl J, Minami Y, Sebald W, Mundlos S, Knaus P, Modulation of GDF5/BRI-b signalling through interaction with the tyrosine kinase receptor Ror2, Genes Cells 9 (2004) 1227–1238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Sclerotome in culture has endogenous BMP activity.
(A) Proteins was collected from cells at varying time points as indicated. BMP signaling was measured by immunoblot as the level of pSmad1/5. Smad1 and α-tubulin were used as normalization controls. Representative of n=3 biological replicates is shown. (B) pSmad1/5 density was normalized to total Smad1 using ImageJ. One-way ANOVA was used for statistical analysis between independent samples from 3 separate biological replicates.
Figure S2. Histology of cartilage nodules.
Sections of paraffin embedded sclerotome micromass cultures that were treated with BMP4 (A) or BMP4 plus TGFβ1 (B). Sections were stained with Alcian Blue and Fast Red. Representatives from n=3 separate cultures are shown.
Figure S3. Osteoblast gene expression.
RNA was extracted from cells that has been treated with vehicle (CTRL), TGFβ1, BMP4, or TGFβ1 and BMP4 for 48 hrs. Relative levels of Runx2, Bglap, DMP1, and Ihh were measured by QPCR and analyzed with REST software. Ihh was used as a positive control since it is known to be regulated by BMP and TGFβ. Expression was normalized to HPRT. (N.S) denotes non-significant, (*) indicates significant, p< 0.05 (n=3 biological replicates) Full qPCR analysis is shown in Table S2B.
Figure S4. Quantification of Smad blots.
Cells were treated with Noggin (A), Gremlin (B), or DMH2 (C, D) for 48 hours. Protein was extracted and BMP signaling was measured by immunoblot as the level of pSmad1/5 (A-C) and TGFβ signaling was measures as the level of pSmad2/3. Smad1 or Smad3 were used as normalization controls. Band density of pSmad was normalized with total Smad protein. One-way ANOVA was used for statistical analysis between independent samples (n=3).


