Abstract
Objective
Spinal epidural lipomatosis (EL) represents an excessive deposition of unencapsulated adipose tissue in the spinal canal that can result in chronic back pain in patients who are obese with and without diabetes. We aim to calculate the total volumetric epidural fat on lumbar spine MRI in a predominately obese population and correlate total epidural fat to lower back pain (LBP) and body mass index (BMI).
Research design and methods
We developed a program (Fat Finder) to quantify volumetric distribution of epidural fat throughout the lumbar spine. Eleven patients with LBP were imaged using two MRI protocols: parallel axial slices and conventional clinical protocol. The distribution of epidural fat per level was analyzed and normalized to the spinal canal size.
Results
Our sample had an average age of 59.9 years and BMI of 31.57 kg/m2. EL subgroup consisted of seven patients. The L2–L5 total fat volume was 3477.6 mm3 (1431.1–5595.9) in the EL group versus 1783.8 mm3 (815.0–2717.5) in the age-similar non-EL group. A higher percentage of fat volume in the canal was associated with higher LBP scores. The fat percentage was 32.2% among patients with EL versus 15.4% for age-similar non-EL with LBP score of 6.1 and 4.0, respectively.
Conclusions
The Fat Finder is a novel volumetric method to quantify epidural lumbar spinal fat. The epidural fat favors the lower spinal segment with direct proportionality between the fat volume and LBP score, independent of BMI.
Keywords: MRI, algorithms, low back pain, adipose tissue biology
Significance of this study.
What is already known about this subject?
Epidural lipomatosis consists of the deformation of the dural sac caused by adipose fat accumulation in the epidural space of the lumbar spine that may restrict the physical space through which the nerve roots pass leading to symptoms such as back pain.
This can be seen as a comorbidity of diabetes and obesity. Controversy exists on how to diagnose and quantitatively grade epidural lipomatosis.
What are the new findings?
The Fat Finder program provides precise and reliable measurement of the total or individual-level fat volume.
Epidural fat volume correlates proportionally with the degree of back pain. Also, we learned that epidural fat increases towards the lower aspect of the lumbar spine.
How might these results change the focus of research or clinical practice?
The ability to provide volumetric calculation of the epidural fat is game changing. Until now, the inability to grade this condition was due in part to the reference made to single-level linear measurements.
A reliable algorithm can provide a standard mechanism to quantify epidural volume to better understand adipose fat distribution and establish a way to follow patients after treatment induction or behavior modification.
Introduction
Obesity is common and approximately 80%–90% of patients with type 2 diabetes are overweight or obese.1 2 The probability of overweight or obese people presenting with back pain has been reported as high as three times as likely as those who are not obese.3 4 Patients with back pain are absent from work-related duties for longer than a month in more than 30% of cases and 6.7% remain out for longer than 6 months.5 The incidence of low back pain has been linked to higher body mass index (BMI), with an increased risk of 7.7% for obese and 11.6% for severely obese individuals in the USA according to a cohort study of 6796 adults.6 A survey of over 1 million people in the USA showed a strong association between chronic back pain and BMI.7 In this study, when compared with normal weight individuals, people in the overweight BMI range manifested 20% higher rates of persistent back pain. The rate of pain continued to increase as BMI increased with a 68% higher rate for people with class I obesity compared with normal BMI, 136% for people with class II obesity and 254% for people with class III obesity reported back pain.7
Back pain can be seen in association with a variety of etiologies that can result in abnormal compression on the spinal cord and exiting nerve roots. A rare and poorly characterized etiology is spinal epidural lipomatosis (EL), which was originally reported in the literature in 1975 in a renal transplant patient treated with long-term corticosteroids.8 Spinal EL is characterized by excessive unencapsulated adipose tissue accumulation in the epidural space resulting in distortion of the thecal sac, with predominance in the lumbar/sacral spine.9 The most difficult problem facing characterization of EL is that no standard reliable method for measuring epidural adipose tissue has ever been documented. Previous reports were based on single axial or sagittal MRI measurements of the epidural fat thickness and observed discrepancies in the measurements based on the orientation of the image slices.10 11
Accordingly, we propose a reliable way to volumetrically quantify epidural fat using a semiautomated custom image processing ‘Fat Finder’ program. We assessed the efficacy of this approach on the conventional clinical MRI protocol that follows the normal slanted anatomy of the lower lumbar spine. In addition, we evaluated the relationship between total epidural fat, lower back pain and BMI.
Research design and methods
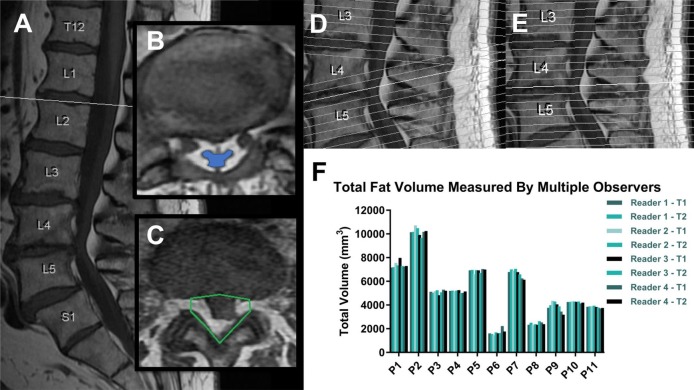
This pilot study included 11 patients who presented with lower back pain from February 2015 to March 2015. The patients were referred for a standard non-contrast lumbar spine MRI with a low back pain. BMI and back pain scores were recorded. Pain was reported using the visual analog scale. We included patients over 18 years of age, without a history of spinal surgical intervention or recent trauma. Two different MRI scanning techniques were performed on the same day to generate T1-weighted axial images with the only difference being a change in the axial slice orientations. Due to the normal lordotic curvature, the clinically conventional technique for lumbar spine MRIs includes a slant in the orientation of the axial slices beginning around L4 that differs from the axial slices above it (figure 1A). These MRIs were classified as the ‘normal imaging group’. The secondary MRIs taken, known as the ‘parallel imaging group’, had no change in the orientation of their axial slices so that there were no overlapping or larger unsampled regions considered in the image set, making it more ideal for a volume calculation. The parallel method thus offers no overlap or excess gap at L4-L5, and provides a more accurate approach to estimate volumetric epidural fat content. figure 1D,E compares side-by-side the locations and changing slant of all the axial slices (drawn as white lines) in these two different imaging protocols with each white line representing the 3D location where the MRI images cross the center of the spine. The left side (figure 1D) shows how the images from the normal protocol change how slanted they are around L4 to follow the change in curvature of the spine, while the right side (figure 1E) shows how all the images from the parallel protocol are evenly spaced and do not change their slant.
Figure 1.
Differences in spinal T1 imaging with epidural fat region of interest selection and validation. (A) Sagittal T1-weighted image slices were used to determine the boundaries of the lumbar spine from mid-L1 to mid-S1. (B, C) Axial image slices were used to locate the thecal sac and the surrounding epidural fat to draw our eight-point region of interest on each slice. In patients with epidural lipomatosis, the condition is diagnosed by observing the epidural fat’s deformation of the thecal sac which is highlighted in blue on image B. This portion of the thecal sac is severely compressed. (D, E) Two different MRI techniques were used: one following the conventional clinical protocol with a slanted correction at mid-L4 (D) and one without (E). (F) Graph showing interobserver and intraobserver variability for total fat volume calculations among four different readers with two trials each.
MR T-weighted images are ideal for this application because the epidural fat can be distinguished from other dural contents with high specificity and allow measurement of the fat content.12 The volume of lumbar epidural fat was measured on T1-weighted MR images in the axial plane between the mid-L1 and the mid-S1 levels. MRIs were analyzed for the presence of EL based on direct deformation of the thecal sac by adipose tissue on the axial T1 films as seen in figure 1B,C. Figure 1B shows a severely compressed thecal sac identified on the image by the blue region encased in epidural fat. The MRI exams were screened for other causes of spinal canal narrowing including disc herniation.
A customized user interface and algorithm, named Fat Finder, was developed to aid in the determination and isolation of the spinal canal boundaries and the volume of epidural fat from each MR axial image slice within the proper spine section. The exported Digital Imaging and Communication in Medicine image files were used to display each T1-weighted axial slice to the user zoomed in on the spinal canal to allow the user to draw an outline of the region of interest (ROI), see figure 1C. After defining a ROI on the first image, the program calculated a cut-off intensity threshold to separate out the epidural fat in the images based on half the highest fat intensity in the canal ROI. The identified epidural fat in the ROI was visually shown to the user throughout the process for verification along with the calculated fat volume. This process of readjusting the canal region and verifying the results was done on each axial slice.
The total fat volume was calculated by multiplying the number of identified fat pixels by the volume of each voxel (length and width of each pixel multiplied by the distance between axial slices). A midpoint estimation method was used with each slice so that the information in the image represented half of the distance between slices, both 2 mm above and below each axial slice three dimensionally. For this reason, the top and bottom slices in each volume measurement were considered bordering slices for the region and only the upper or lower half of their volume inside the desired spinal section was included.
To better assess the compressive effect of the quantified epidural fat volume, the total bony spinal canal volume was estimated as a cylindrical region with an approximate height (h) calculated by multiplying the total number of slices by the distance separating the slices. The volume of a cylinder is calculated by:
The diameter of the cylinder (d) was approximated as an average of the anterior to posterior width of the canal at L4/L5 and L5/S1. The widths were measured linearly on the axial slices at these two junctions. The estimated cylindrical volume of the spinal canal allowed us to approximate what percentage of the canal was occupied by epidural fat. The distribution of this fat by level was determined by identifying the image slices that separate each of the discs between L2 and L5.
To test the reliability of volume measurements with different program users, four observers calculated the epidural fat volume using the Fat Finder program for each patient from our sample study of 11 patients. The measurements were again repeated a week later to assess for intraobserver reliability. This multiple observer validation study used the ‘Normal protocol MRI group’ whose images have a slanted orientation corresponding to the lordotic curvature, and the total volume was calculated from mid-L1 to mid-S1. The fat threshold values remained constant for each reader to limit potential bias.
The user interfaces and underlying algorithms were programmed in MATLAB (Release 2017b, The MathWorks) and deployed using the MATLAB Complier (V.9.0). All statistical analyses were performed in IBM SPSS Statistics (V.24) with level of significance set at 0.05. Paired t-tests and correlation coefficient computation were used to compare the individual and total fat volume measurements from the two MRI approaches. Intraclass correlation coefficient (ICC) was used to determine the interobserver and intraobserver reliability.
Results
Our patient population consisted of 11 patients with an average age of 59.9 years (range 31.6–79.6). There were four males and seven females with an average BMI of 31.57 kg/m2 (range 22.7–36.6). Thecal sac deformation indicating EL was determined in seven patients.
The reliability of the Fat Finder volume quantification program was confirmed by ICCs, which showed no statistically significant differences in the intraobserver reliability (0.993–0.999) and interobserver reliability (0.997–0.998) shown in online supplementary table 1. The volumetric results from the observers’ measurements on each patient show considerable diversity in the total fat volume between patients, with values ranging from 1800 to 10 000 mm3. However, there was no significant difference in volume of epidural fat calculated by the program with each trial thus confirming the consistency of this novel technique (figure 1F). Using the paired t-test, there was also no significant difference in the larger scale volume measurements that included multiple vertebrae (p>0.13, correlation >0.97) between the normal and parallel groups (online supplementary table 2). When the volume was separated into individual vertebrae at each disc level, there was no significant difference between the two groups at L2, L3 and L4 (p>0.21, correlation >0.88), but the p value for total fat volume at L5 was 0.05 (correlation=0.96).
bmjdrc-2018-000599supp001.pdf (282.4KB, pdf)
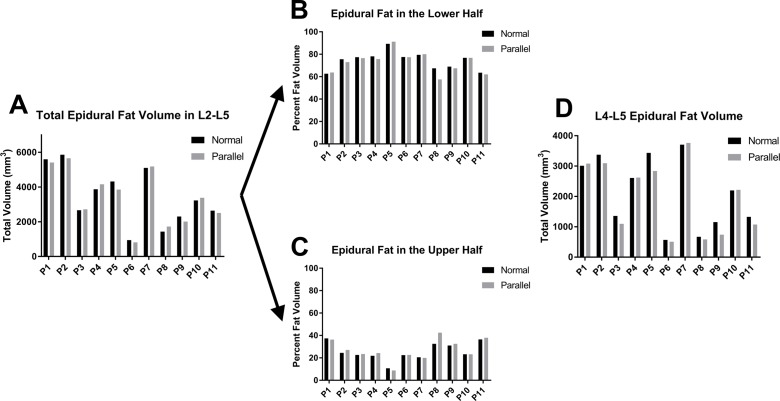
When comparing the volumetric results in figure 2 of each patient’s slanted conventional MRI series (normal group) with the non-slanted ones (parallel group), the differences were seen to be relatively minor with respect to the total scale of the measurements. These differences were typically caused by changing slice orientations that most predominately affect the bottom of the targeted spine section where overlapping axial slices as well as larger unsampled areas were a source of error in measurements. It was also observed that the different orientations of our top and bottom boundary slices in each region account for some variation measured because the normal axial images slant at the lower discs to generate images that are more perpendicular to the direction of the lower spine.
Figure 2.
Comparison of epidural fat quantification among MRI techniques. (A) Graphical comparison of epidural fat between L2 and L5 among the 11 patients using both the conventional MRI (normal) and parallel axial slices showed no significant difference in the fat volume calculations. (B, C) The epidural fat volume was split between L3 and L4 to illustrate the higher portion of fat in the lower section of the spine. (D) The distribution of L4–L5 epidural fat volume, shown here, was specifically compared because that is the location where the two MRI techniques differ the most due to the slant in the conventional protocol.
The differences in MRI scanning technique were amplified by a much larger percentage of the total fat residing in the lower half of the targeted region, averaging 73.6% between L4 and S1 compared with only 26.4% between L1 and L3 (figure 2B,C). In assessing how this change in orientation affects our results, the amount of fat in L4 and L5 was specifically compared between the slanted and non-slanted MRIs to see how much of the differences can be attributed to this region that typically contains a higher percentage of the total fat (figure 2D). Despite minor differences caused by the slice orientations, there was no significant difference in the volume calculated between the two imaging techniques, and therefore, the slanted clinical protocol commonly used is sufficient for making reliable volume measurements.
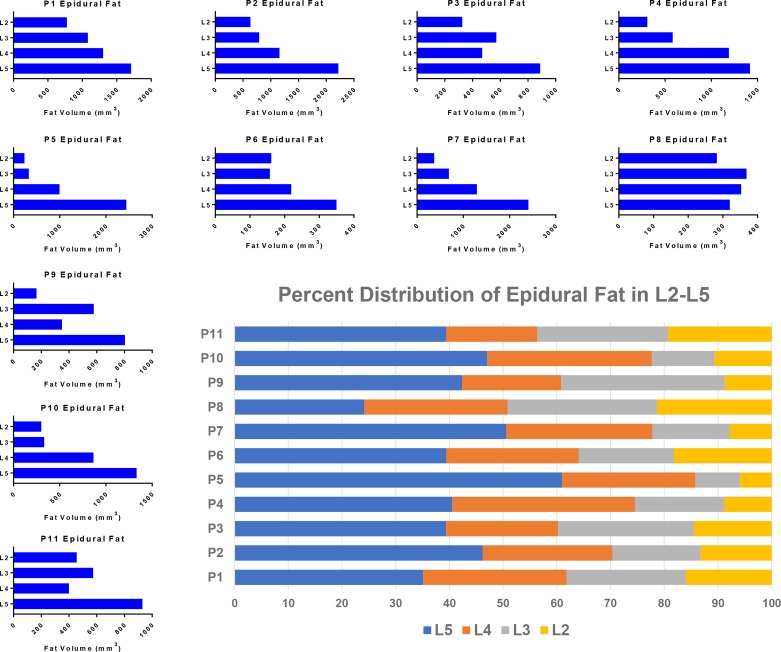
When breaking down the localization of epidural fat, the distribution at each level from L2 to L5 varied between patients and was noticed to be heterogeneous (figure 3). The L5 epidural fat volume consistently measured the highest in all but one patient, and L4 contained the second highest volume in the majority of cases. Gradually increasing fat volume from L2 to L5 was observed in all but one of the patients. In that patient (P8), an unusual near-equal volume distribution was observed. The percentage of volume distribution in figure 3 summarizes the general similarities despite drastically different total fat volumes quantified in the different patients.
Figure 3.
Volumetric localization of epidural fat among the different spinal levels. The smaller blue bar graphs depict the distribution of epidural fat among the different patients broken down by level from L2 to L5. Most of the graphs show an increasing quantity of fat from the upper to lower spine with only a couple having more heterogeneous distributions. The summary chart in the bottom right illustrates these distributions as a percent showing the predominately lower spinal concentration of epidural fat.
The volumetric results are summarized in table 1 by separating the patients into an EL group (EL) and those without (non-EL). A diagnosis of EL was defined as a deformation of the thecal sac at L4/L5 and/or L5/S1. Each group is sorted by their reported pain scores to show the factors associated with a higher reported pain. Two of the patients who did not have EL were noticed to be very different clinically because they are much younger with an average age of 32.3 years old at the time of the study versus 70.8 and 64.7 years old for the rest of the non-EL patients and patients with EL, respectively. Because of these differences, they were assessed separately from the other non-EL patients.
Table 1.
EL and non-EL patient population stratification by pain score and calculated total fat volume. Additionally, the estimated percent of spinal canal epidural fat normalized to the canal diameters at L4–L5 and L5–S1 is shown
| Patient ID | Age (years) | Pain score (1–10) | Total fat volume (mm3) | Spinal canal diameter (mm) | Percent of fat in the canal* | BMI | ||
| Full section | L2–L5 | L4/L5 | L5/S1 | |||||
| Epidural lipomatosis | ||||||||
| P7 Norm | 75.7 | 10 | 6318.5 | 5101.3 | 12.9 | 14.9 | 35.9 | 29.0 |
| P7 Parallel | 75.7 | 10 | 6836.9 | 5183.4 | 12.9 | 14.9 | 38.8 | 29.0 |
| P10 Norm | 71.6 | 7 | 3895.4 | 3228.3 | 15.3 | 13.7 | 22.7 | 34.8 |
| P10 Parallel | 71.6 | 7 | 4407.7 | 3380.6 | 15.3 | 13.7 | 25.7 | 34.8 |
| P8 Norm | 75.0 | 7 | 2119.8 | 1431.1 | 11.4 | 10.9 | 19.4 | 33.6 |
| P8 Parallel | 75.0 | 7 | 2430.7 | 1724.0 | 11.4 | 10.9 | 23.1 | 33.6 |
| P1 Norm | 79.6 | 6 | 6834.5 | 5595.9 | 6.7 | 12.9 | 80.9 | 34.9 |
| P1 Parallel | 79.6 | 6 | 6479.9 | 5408.9 | 6.7 | 12.9 | 79.5 | 34.9 |
| P11 Norm | 46.2 | 5 | 3586.6 | 2640.0 | 13.3 | 11.8 | 22.7 | 36.6 |
| P11 Parallel | 46.2 | 5 | 3390.4 | 2507.0 | 13.3 | 11.8 | 21.4 | 36.6 |
| P5 Norm | 49.5 | 4 | 6497.0 | 4321.7 | 18.0 | 18.7 | 21.9 | 32.3 |
| P5 Parallel | 49.5 | 4 | 6909.5 | 3855.4 | 18.0 | 18.7 | 23.3 | 32.3 |
| P9 Norm | 55.3 | 4 | 3489.2 | 2301.6 | 16.8 | 12.9 | 17.4 | 22.7 |
| P9 Parallel | 55.3 | 4 | 3672.6 | 2007.5 | 16.8 | 12.9 | 18.3 | 22.7 |
| Means | 64.71 | 6.14 | 4776.33 | 3477.62 | 13.49 | 13.69 | 32.21 | 31.99 |
| No epidural lipomatosis | ||||||||
| Older | ||||||||
| P6 Norm | 70.4 | 5 | 1494.6 | 939.8 | 13.7 | 12.6 | 11.5 | 28.9 |
| P6 Parallel | 70.4 | 5 | 1308.4 | 815.0 | 13.7 | 12.6 | 10.5 | 28.9 |
| P3 Norm | 70.6 | 3 | 4763.5 | 2662.6 | 16.4 | 17.6 | 18.1 | 33.8 |
| P3 Parallel | 70.6 | 3 | 5689.0 | 2717.5 | 16.4 | 17.6 | 21.6 | 33.8 |
| Means | 70.48 | 4.00 | 3313.86 | 1783.75 | 15.05 | 15.10 | 15.41 | 31.35 |
| Younger | ||||||||
| P2 Norm | 33.0 | 4 | 9615.5 | 5861.1 | 12.3 | 11.3 | 64.7 | 36.5 |
| P2 Parallel | 33.0 | 4 | 9189.2 | 5654.8 | 12.3 | 11.3 | 61.8 | 36.5 |
| P4 Norm | 31.6 | 2 | 4989.0 | 3872.5 | 9.4 | 9.8 | 52.2 | 24.2 |
| P4 Parallel | 31.6 | 2 | 5234.0 | 4157.5 | 9.4 | 9.8 | 54.8 | 24.2 |
| Means | 32.27 | 3.00 | 7256.91 | 4886.46 | 10.85 | 10.55 | 58.36 | 30.35 |
*Estimated using the mean diameter of the canal measured at L4/L5 and L5/S1 to approximate the total canal size.
BMI, body mass index; EL, epidural lipomatosis.
The mean total fat volume in patients with EL was determined to be 4776.3 mm3 (range: 2119.8–6909.5) when measured from mid-L1 to mid-S1. This decreased to 3477.6 mm3 (range: 1431.1–5595.9) when the spinal section was restricted to L2–L5 vs 1783.75 mm3 (range: 815.0–2717.5) in the age-similar non-EL group. L2–L5 allows the use of only full discs and thus reduces variability that can be introduced from arbitrary selection of mid-L1 and S1 vertebral bodies. When comparing between the two groups, patients with EL experienced significantly higher pain than those without EL, reporting an average score of 6.14 and 3.5, respectively. Higher pain score in the EL group was associated with higher total spinal fat volume as compared with the age-similar non-EL group (table 1). Regardless of similar spinal canal diameters at the levels of L4/L5 and L5/S1, the estimated percentage of epidural fat in the spinal canal at these levels demonstrated double the average percent compared with the non-symptomatic group (32.2% vs 15.4%). It is interesting that the variation in BMI between the EL and non-EL groups and throughout the whole study did not correlate with the epidural fat volume. Three of our patients had diabetes type 2. All three also had EL and high back pain scores—6, 10 and 7 (out of 10 scale). The two patients with the highest percent of fat in the canal in the EL group were both diabetic (80.9% & 35.9%).
Discussion
We report a novel, reliable and reproducible method for measuring epidural adipose tissue in a volumetric multislice manner to aid in the diagnosis of EL. The Fat Finder is a novel volumetric method that assesses total epidural fat volumes throughout the lumbar spine. We found no statistical difference between the four readers when measuring the epidural fat volumes with the Fat Finder program attesting to the reproducibility of this method. The distribution of fat was observed to be primarily concentrated in the lower half of the spine (most pronounced at L5/S1) with a gradual incremental increase from the upper to lower levels in most cases. The Fat Finder algorithm also allows the resulting three-dimensional volume to be broken down anatomically to assess the localized effects of fat compartmentalization. In addition, our findings suggest that a higher estimated percentage of fat volume in the canal (normalized by the anatomic canal diameter at L4/L5 and L5/S1) may be associated with worse pain in patients with EL, and this possible association warrants further research.
Spinal EL involves excessive deposition of unencapsulated adipose tissue in the spinal canal and is recognized as a cause of back pain, claudication, and radiculopathy. This condition has been associated with exogenous steroid administration, Cushing’s syndrome, and obesity.9 13–15 Its pathophysiology is poorly understood with a few isolated case reports linking obesity and steroid excess.8 16–18 A 2005 review of 104 isolated cases of EL revealed four general categorical associations: exogenous and endogenous steroid excess, obesity and idiopathic causes.9 The challenge of characterizing this disease process is that the previous methods used for measuring epidural fat provided a limited assessment of the overall fat volume because they use single dimensional measurements either in the axial or sagittal planes.10 11 Treatments include weight loss, termination of exogenous steroid use, correcting endogenous steroid overproduction and in severe cases surgical decompression.19–21 Weight loss may lead to a reduction in the epidural adipose tissue thus increasing the accommodative ability of the epidural space and preventing compression of neural elements.22
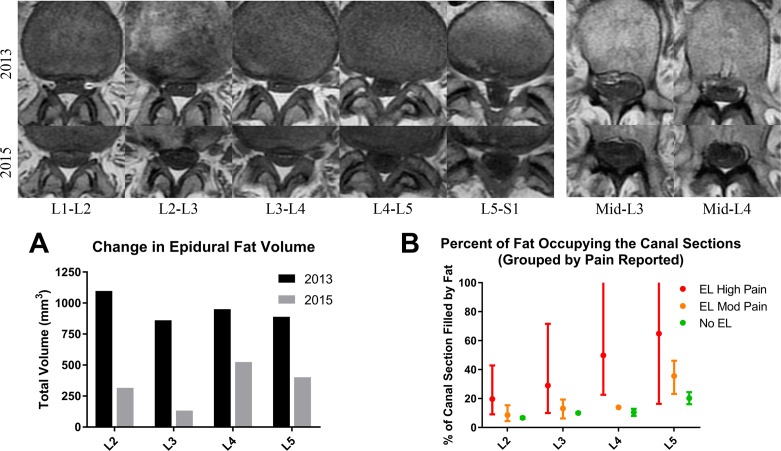
The relevance of this work resides in the ability to volumetrically characterize the epidural fat content in relationship to thecal sac deformation and symptomatology. Better understanding of how the epidural fat is distributed and changes over time along the different lumbar spinal levels may allow us to target and treat patients who are obese and diabetic with EL more effectively. An isolated case with history of obesity and diabetes followed at our institution over a span of almost 2 years showed a significant decrease in the total volume of epidural fat and complete resolution of thecal sac deformation at L1–L2, L2–L3, L3–L4, and L5–S1 levels as his BMI decreased from 31 kg/m2 (moderately obese) to 24.7 kg/m2 (normal weight), which was accompanied by symptomatic resolution. This case suggests that alleviation of symptoms (eg, back pain) can conceivably result from weight loss rather than more invasive interventions. Figure 4 summarizes the spinal canal expansion as BMI decreased including a reduction of fat at all levels with the total volume from L2 to L5 reduced from 4748 to 1715 mm3 (figure 4A).
Figure 4.
Case study showing significant epidural fat contraction after weight loss. The two upper rows show a representative stack of axial T1 images at the different intervertebral disc levels along with mid-L3 and mid-L4 comparisons. The first row of images is from the MRI in 2013 prior to weight loss while the bottom row is from the follow-up MRI in 2015. (A) The change in quantified epidural fat volume from 2013 to 2015 shows a significant volume reduction especially in L2 and L3. (B) Higher estimated percentage of epidural fat in the spinal canal is associated with higher pain scores. The upper scale of the y-axis was restricted to 100% to preserve the detail of the moderate and no epidural lipomatosis (EL) groups, but only one patient had an estimated value extending above 100% due to severely restricted canal size (108% at L4 and 113% at L5). The error bars represent the full range of values in each group with the mean shown as a single point.
The limitations of this pilot study include a small number of subjects and a heterogeneous patient population composition that are mostly older patients (mean age of 59.9) and patients who are obese (mean BMI of 31.57). The EL group comprised older individuals (mean age of 65) with higher back pain, and only patients with EL in our population reported pain scores greater than 6. Surprisingly, the two youngest patients were found to have some of the highest total fat volumes and percentage of fat in the canal although neither had EL. We plan to explore this further in future studies to look at the distribution of epidural fat volume according to age, gender, and BMI in a large sample population. Another limitation is that BMI has been recognized as an incomplete way to characterize obesity.23 However, its prevalence in the literature and value as a well-studied metric still makes it a valid tool for comparison especially when supplemented with other measurements of fat content. To aid in future studies, we will also measure the depth of subcutaneous fat at the L4–L5 and L5–S1 levels to determine whether it provides a more accurate way of characterizing obesity.
We report that while the percent of epidural fat in the spinal canal increases, the associated back pain also increases (figure 4B). Our intention is to apply this method and the Fat Finder program to a large patient population with history of obesity and diabetes to determine which risk factors correlate with excessive deposition of adipose tissue within the epidural space and to assess the association between increasing amounts of epidural adipose tissue and patients’ symptoms.
Acknowledgments
The authors thank Dr Anuj Patel and Dr Catphuong Vu for testing the Fat Finder program and being additional readers for the spinal canal measurements.
Footnotes
Deceased: Dr. Robertson died on 6 June 2017
Presented at: 2018 SSR Annual Meeting (25–28 March 2018).
Contributors: FMG, DDR and MU were integral in the genesis and formulation of the study, and MAW programmed the Fat Finder algorithm with the direction of DDR and Dr Anuj Patel. All authors participated in the development, critical testing, drafting and revision of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional data available for distribution.
References
- 1. Centers for Disease Control and Prevention National Diabetes statistics report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, 2017. [Google Scholar]
- 2. Wing RR. Weight loss in the management of type 2 diabetes : Gerstein HC, Haynes RB, Evidence-based diabetes care. Hamilton, London: BC Decker Inc, 2001: 252. [Google Scholar]
- 3. Koyanagi A, Stickley A, Garin N, et al. The association between obesity and back pain in nine countries: a cross-sectional study. BMC Public Health 2015;15:123 10.1186/s12889-015-1362-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shiri R, Karppinen J, Leino-Arjas P, et al. The association between obesity and low back pain: a meta-analysis. Am J Epidemiol 2010;171:135–54. 10.1093/aje/kwp356 [DOI] [PubMed] [Google Scholar]
- 5. Wynne-Jones G, Cowen J, Jordan JL, et al. Absence from work and return to work in people with back pain: a systematic review and meta-analysis. Occup Environ Med 2014;71:448–56. 10.1136/oemed-2013-101571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smuck M, Kao MC, Brar N, et al. Does physical activity influence the relationship between low back pain and obesity? Spine J 2014;14:209–16. 10.1016/j.spinee.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 7. Stone AA, Broderick JE. Obesity and pain are associated in the United States. Obesity 2012;20:1491–5. 10.1038/oby.2011.397 [DOI] [PubMed] [Google Scholar]
- 8. Lee M, Lekias J, Gubbay SS, et al. Spinal cord compression by extradural fat after renal transplantation. Med J Aust 1975;1:201–3. [DOI] [PubMed] [Google Scholar]
- 9. Fogel GR, Cunningham PY, Esses SI. Spinal epidural lipomatosis: case reports, literature review and meta-analysis. Spine J 2005;5:202–11. 10.1016/j.spinee.2004.05.252 [DOI] [PubMed] [Google Scholar]
- 10. Borré DG, Borré GE, Aude F, et al. Lumbosacral epidural lipomatosis: MRI grading. Eur Radiol 2003;13:1709–21. 10.1007/s00330-002-1716-4 [DOI] [PubMed] [Google Scholar]
- 11. Jaimes R, Rocco AG. Multiple epidural steroid injections and body mass index linked with occurrence of epidural lipomatosis: a case series. BMC Anesthesiol 2014;14:70 10.1186/1471-2253-14-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quint DJ, Boulos RS, Sanders WP, et al. Epidural lipomatosis. Radiology 1988;169:485–90. 10.1148/radiology.169.2.3174998 [DOI] [PubMed] [Google Scholar]
- 13. Chapman PH, Martuza RL, Poletti CE, et al. Symptomatic spinal epidural lipomatosis associated with Cushing's syndrome. Neurosurgery 1981;8:724–7. 10.1227/00006123-198106000-00017 [DOI] [PubMed] [Google Scholar]
- 14. Guegan Y, Fardoun R, Launois B, et al. Spinal cord compression by extradural fat after prolonged corticosteroid therapy. J Neurosurg 1982;2:267–9. 10.3171/jns.1982.56.2.0267 [DOI] [PubMed] [Google Scholar]
- 15. Maillot F, Mulleman D, Mammou S, et al. Is epidural lipomatosis associated with abnormality of body fat distribution? A case report. Eur Spine J 2006;15:105–8. 10.1007/s00586-005-0955-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noël P, Pepersack T, Vanbinst A, et al. Spinal epidural lipomatosis in Cushing's syndrome secondary to an adrenal tumor. Neurology 1992;42:1250 10.1212/WNL.42.6.1250 [DOI] [PubMed] [Google Scholar]
- 17. Buthiau D, Piette JC, Ducerveau MN, et al. Steroid-induced spinal epidural lipomatosis: CT survey. J Comput Assist Tomogr 1988;12:501–3. 10.1097/00004728-198805010-00030 [DOI] [PubMed] [Google Scholar]
- 18. Noh E. An unusual complication of morbid obesity: epidural lipomatosis. Am J Emerg Med 2015;33:742.e3–742.e4. 10.1016/j.ajem.2014.11.043 [DOI] [PubMed] [Google Scholar]
- 19. Pouchot J, Si-Hassen C, Damade R, et al. Cauda equina compression by epidural lipomatosis in obesity. Effectiveness of weight reduction. J Rheumatol 1995;22:1771–5. [PubMed] [Google Scholar]
- 20. Boutsen Y, Donckier J, lipomatosis E. Epidural lipomatosis. Postgrad Med J 2000;76:60–1. 10.1136/pmj.76.891.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pinkhardt EH, Sperfeld AD, Bretschneider V, et al. Is spinal epidural lipomatosis an MRI-based diagnosis with clinical implications? A retrospective analysis. Acta Neurol Scand 2008;117:409–14. 10.1111/j.1600-0404.2007.00964.x [DOI] [PubMed] [Google Scholar]
- 22. Beges C, Rousselin B, Chevrot A, et al. Epidural lipomatosis. Interest of magnetic resonance imaging in a weight-reduction treated case. Spine 1994;19:251–4. [PubMed] [Google Scholar]
- 23. Tomiyama AJ, Hunger JM, Nguyen-Cuu J, et al. Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005-2012. Int J Obes 2016;40:883–6. 10.1038/ijo.2016.17 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2018-000599supp001.pdf (282.4KB, pdf)