Abstract
Objective
This study investigates how the excess risk of lower extremity amputations (amputations) in people with type 1 diabetes mellitus (DM) differs from the general population by diabetes duration, glycemic control, and renal complications.
Research design and methods
We analyzed data from people with type 1 DM from the Swedish National Diabetes Register without prior amputation from January 1998 to December 2013. Each person (n=36 872) was randomly matched with five controls by sex, age, and county (n=184 360) from the population without diabetes. All were followed until first amputation, death or end of follow-up.
Results
The overall adjusted HR for all amputation was 40.1 (95% CI 32.8 to 49.1) for type 1 DM versus controls. HR increased with longer diabetes duration. The incidence of amputation/1000 patient-years was 3.18 (95% CI 2.99 to 3.38) for type 1 DM and 0.07 (95% CI 0.05 to 0.08) for controls. The incidence decreased from 1998–2001 (3.09, 95% CI 2.56 to 3.62) to 2011–2013 (2.64, 95% CI 2.31 to 2.98). The HR for major amputations was lower than for minor amputations and decreased over the time period (p=0.0045). Worsening in glycemic control among patients with diabetes led to increased risk for amputation with an HR of 1.80 (95% CI 1.72 to 1.88) per 10 mmol/mol (1%) increase in hemoglobin A1c.
Conclusions
Although the absolute risk of amputation is relatively low, the overall excess risk was 40 times that of controls. Excess risk was substantially lower for those with good glycemic control and without renal complications, but excess risk still existed and is greatest for minor amputations.
Keywords: type 1 diabetes, lower extremity amputations, minor amputations, renal failure, HbA1c
Significance of this study.
What is already known about this subject?
Earlier studies have shown decreasing incidence of lower extremity amputations (amputations) and that they are related to hemoglobin A1c (HbA1c) and to diabetes complications.
What are the new findings?
This nationwide study of virtually all people with type 1 diabetes mellitus (DM) in Sweden and their matched controls found that there was a high excess risk of amputation for people with type 1 DM compared with the general population (40-fold), and only the risk for major amputations (above knee) has decreased substantially during the 1998–2013 study period.
There was a much lower excess risk for persons with good glycemic control and no renal complications, but the risk was still over six times greater than for the general population.
How might these results change the focus of research or clinical practice?
Special focus has to be given to persons with very poor glycemic control and renal complications, but it is important to screen all people with type 1 DM as even those who reach the target HbA1c level and without renal complications have an excess risk of lower extremity amputation compared with the general population.
The uniquely strong relationship found between HbA1c and amputations indicates that amputations would substantially decrease if the long-term glycemic control can be at least moderately improved in the population of people with type 1 DM and if the proportion of patients with very poor glycemic control could be reduced.
Introduction
People with diabetes have higher risk for lower extremity amputations (amputations), and 40%–50% of all non-traumatic amputations have reportedly been due to diabetes.1 2 People with type 1 diabetes mellitus (type 1 DM) and amputation have reduced survival rates.2 Amputation also leads to high medical and social costs.3 Several studies have evaluated the risk of amputation in people with type 2 diabetes, but limited population-based studies have been performed in type 1 DM.4–7 In people with diabetes poor glycemic control has been associated with amputation8 as well as renal complications.9 Diabetes renal failure and foot ulcers are often coexisting complications. People with diabetes renal failure have a higher incidence of foot ulcers and increased risk of amputation,10 a risk strongly associated with declining estimated glomerular filtration rates.11 Renal complications, resulting from history of poor glycemic control, might be a marker for advanced complications such as foot ulcers and amputations, although other crucial pathophysiologic mechanisms may play a role.12
With improved glucose-lowering therapies for people with type 1 DM and stricter guidelines for controlling risk factors such as lipids and blood pressure in recent decades, it is important to determine the current prognosis for amputations in people with type 1 DM. However, it is unknown whether people with type 1 DM who achieve the recommended glycemic control and avoid renal complications, which are thought to be associated with lower risk of complications,13 have prognoses similar to the general population.
In this study, we evaluated the overall excess risk for amputation by glycemic control, renal complications, and duration of diabetes in people with type 1 DM versus controls.
Participants and methods
Participants
Data were analyzed from the Swedish National Diabetes Register (NDR), which contains data on people with both types 1 and 2 diabetes since 1996. NDR is a nationwide register that receives data from both primary care and diabetes outpatient clinics. Virtually all people in Sweden age >18 with type 1 DM are included, and information on hemoglobin A1c (HbA1c), diabetes risk factors, complications, and diabetes medications is collected. Each person provides informed consent. To ensure higher validity of data on type 1 DM diagnosis, type 1 DM in NDR was defined as treatment with insulin and diagnosis at age ≤30.14 NDR has been described in detail in previous publications.15 16
All people with type 1 DM with ≥1 record in NDR from January 1, 1998 until December 31, 2012 were included. For each person, five controls were randomly selected from the general population matched by age, sex, and county. Both people with diabetes and the controls were followed from study start or first record until death, first amputation, or study end.
To identify coexisting conditions, each person’s unique identification number was linked to the Swedish Inpatient and Cause of Death Register.17 Education, country, and birth data were retrieved from the Longitudinal Integration Database for Health Insurance and Labour Market Studies. Country of birth categories were Sweden or other. Education was classified as low (compulsory only), intermediate, or high (university level or similar).18 19
To identify amputations, data from the NDR were matched with data from the Swedish National Patient Register (NPR), which includes both inpatient and outpatient care data. Persons with inconsistent vital status data among patients with diabetes (n=4, and their controls n=20) and controls (n=237) were excluded from the cohort, as were both people with type 1 DM (n=291, and their controls n=1448) and controls (n=38) with documented amputations before study start. In total, 36 577 persons were analyzed within the type 1 DM group and 182 617 within the control group (see figure 1 in online supplementary material).
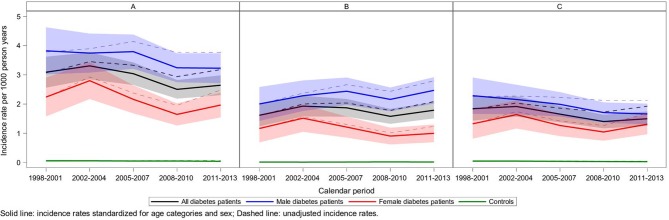
Figure 1.
Crude age-adjusted and sex-adjusted incidence rates of amputations over time. (A) Overall, (B) minor amputations and (C) major amputations. The exact estimates with 95% CI are reported in online supplementary table 3.
bmjdrc-2018-000602supp001.htm (1.3MB, htm)
Outcomes
Primary outcomes
The primary outcomes were HRs of lower extremity amputation for persons with type 1 DM compared with the general population in relation to HbA1c and renal complications.
Secondary outcomes
The secondary outcomes were the risk of amputations during different time periods and risk of amputations in relation to 10 mmol/mol (1%) increase in HbA1c.
Procedures
To identify amputations in the NPR, we used operation codes NGQ09, NFQ19, and NFQ99, and the International Classification of Diseases 10th Revision (ICD10) codes Z89.6 and Z89.7 for amputations above the knee (major amputations); operation codes NGQ19, NGQ99, NHQ09, and NHQ11, and ICD10 code Z89.5 for amputations below the knee but above the ankle (major amputations); and operation codes NHQ12, NHQ13, NHQ14, NHQ16, NHQ17, and NHQ99, and ICD10 code Z89.4 for amputations below the ankle (minor amputations). ICD codes for comorbidities are listed in the online supplementary material.
HbA1c, blood pressure, lipid levels, body mass index (BMI), diabetes duration, smoking, microalbuminuria, and creatinine levels were retrieved from NDR. HbA1c was measured in accordance with the International Federation of Clinical Chemistry standard and reported in percentages according to the National Glycohemoglobin Standardization Program.20 Updated mean was used.21 HbA1c was categorized in five predefined groups of 10 mmol/mol (approximately 1 percentage unit): ≤52 mmol/mol (≤6.9%), 53–62 mmol/mol (7.0%–7.8%), 63–72 mmol/mol (7.9%–8.7%), 73–82 mmol/mol (8.8%–9.6%), and ≥83 mmol/mol (≥9.7%).
Microalbuminuria was defined as two positive tests from three samples taken within 1 year, with albumin:creatinine ratio of 3–30 mg/mmol (approximately 30–300 mg/g) or urinary albumin clearance of 20–200 µg/min (20–300 mg/L), and macroalbuminuria as an albumin:creatinine ratio >30 mg/mmol (>300 mg/g) or urinary albumin clearance >200 µg/min (>300 mg/L). Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation.22 Chronic kidney disease (CKD) stage 1 was defined as eGFR ≥90 mL/min, CKD stage 2 as eGFR=60–89 mL/min, CKD stage 3 as eGFR=30–59 mL/min, CKD stage 4 as eGFR=15–29 mL/min, and CKD stage 5 as the need for renal dialysis, renal transplantation, or as an eGFR <15 mL/min.22
Statistical analysis
Continuous variables are presented as mean±SD or median (minimum, maximum). Categorical variables are described as numbers with percentages. For test between two groups, Fisher’s exact test was used for dichotomous variables, Mantel-Haenszel χ2 test for ordered categorical variables and Mann-Whitney U test for continuous variables. Event rates of amputations per 1000 patient-years with 95% Poisson CIs were calculated overall, by sex, by age category (18–49, 50–64 and 65+ years) and by calendar period (1998–2001, 2002–2004, 2005–2007, 2008–2010, and 2011–2013). Both unstandardized and event rates standardized by age and sex of the first period (for the diabetes cohort) are presented. Survival analyses estimating the risk of amputation were also performed for these five time periods within the diabetes group and versus controls using Cox regression (online supplementary 4), adjusted for time-updated age and gender (model 1), additionally including time-updated diabetes duration (model 2) and additionally adjusted for education category, birth in Sweden, and comorbidities (acute myocardial infarction, atrial fibrillation, coronary heart disease, heart failure, valve disease, stroke, cancer, and previous foot ulcer; model 3). Diabetes duration was added as a continuous covariate in the model. For all controls, the diabetes duration was set to 0. For patients with type 1 DM, the estimates for the group, HbA1c, albuminuria, and eGFR categories were retrieved from the models for diabetes duration at 30, 40, 43 (median time to amputation, called overall analysis), 50, and 60 years.
Cox regression (model 3) was used to estimate the risk of amputation in people with type 1 DM versus controls in relation to time-updated mean HbA1c categories, time-updated albuminuria categories, time-updated eGFR categories, and time-updated mean HbA1c categories, together with measures of renal impairment (albuminuria and normoalbuminuria; eGFR <60 and ≥60 mL/min). The above analyses were presented for diabetes duration 30, 40, 50, and 60 years.
The effect per 10 mmol/mol (1%) higher updated mean HbA1c on events was evaluated among people with diabetes, adjusted for all variables included in model 3 as specified above, and time-updated mean systolic blood pressure, time-updated mean BMI, time-updated smoking status, time-updated use of blood pressure-lowering medications, time-updated mean high-density lipoprotein (HDL) cholesterol, time-updated mean low-density lipoprotein (LDL) cholesterol, time-updated use of lipid-lowering medications, and time-updated insulin method (injection or use of insulin pump) in one model and adding time-updated albuminuria categories in a second model.
A post-hoc analysis was performed using Cox regression (models 1–3) to evaluate the risk of amputation over time divided into three time periods for more amputation events per time period.
The proportional hazards assumption was fulfilled and was investigated by reviewing the log(-log(survival)) vs log(time) curves for categories of main effect variables.
All tests were two-tailed and conducted at the 0.05 significance level. All analyses were performed using SAS V.9.4.
Results
Baseline characteristics
We evaluated 36 577 people with type 1 DM and 182 617 matched controls. The mean age and proportion of women was the same in both groups, 35 years and 45%, respectively (table 1 and online supplementary table 1). Ninety-three percent of people in the diabetes group were born in Sweden compared with 86.7% of controls. For people with type 1 DM, the mean HbA1c was 66.1 mmol/mol (8.2%), the mean diabetes duration was 20 years, the mean BMI was 25.9 kg/m2, 12.3% were smokers, and 3.7% (n=1349) had previous foot ulcers (table 1 and online supplementary table 1). The mean age at the time of amputation was 59 years, the median diabetes duration was 43 years, and the mean diabetes duration was 44 years. The median follow-up time was 9.8 years for type 1 DM and 10.4 years for controls.
Table 1.
Baseline characteristics of people with type 1 diabetes and controls with no prior amputation by categories of HbA1c at first inclusion in the National Diabetes Register from 1998 to 2013
| Controls n=182 617 |
All type 1 diabetes n=36 577 |
P value | |
| Women* | 82 738 (45.3%) | 16 567 (45.3%) | 0.97 |
| Age (years)* | 35.2 (14.6) n=182 617 |
35.2 (14.6) n=36 577 |
0.79 |
| Age category | |||
| 18–34 | 102 427 (56.1%) | 20 495 (56.0%) | |
| 35–49 | 45 377 (24.8%) | 9083 (24.8%) | |
| 50–64 | 27 588 (15.1%) | 5533 (15.1%) | |
| 65+ | 7225 (4.0%) | 1466 (4.0%) | 0.72 |
| Born in Sweden | 158 353 (86.7%) | 34 028 (93.0%) | <0.0001 |
| Education category | |||
| Low | 38 640 (21.5%) | 8091 (22.4%) | |
| Mid | 92 477 (51.6%) | 19 378 (53.7%) | |
| High | 48 234 (26.9%) | 8629 (23.9%) | <0.0001 |
| Variables in the National Diabetes Register only | |||
| HbA1c (mmol/mol, IFCC) | 66.1 (14.7) n=36 340 |
||
| HbA1c (%, NGSP) | 8.20 (1.35) n=36 340 |
||
| Diabetes duration (years) | 19.9 (14.7) n=36 577 |
||
| Insulin method | |||
| Injection | 27 972 (80.3%) | ||
| Pump | 6841 (19.7%) | ||
| BMI (kg/m2) | 25.9 (4.6) n=35 343 |
||
| LDL (mmol/L) | 2.63 (0.82) n=33 009 |
||
| Systolic BP (mm Hg) | 126.1 (15.7) n=36 121 |
||
| Diastolic BP (mm Hg) | 72.6 (9.3) n=36 121 |
||
| Smoking | 4435 (12.3%) | ||
| Blood pressure-lowering medication | 14 171 (39.3%) | ||
| Lipid-lowering medication | 12 978 (35.9%) | ||
| eGFR | 93.5 (31.6) n=34 328 |
||
| Albuminuria | |||
| Normoalbuminuria | 26 797 (80.0%) | ||
| Microalbuminuria | 3796 (11.3%) | ||
| Macroalbuminuria | 217 (6.3%) | ||
| CKD stage 5 | 794 (2.4%) | ||
| Registrations in the inpatient register prior to baseline | |||
| Acute myocardial infarction (I21) | 835 (0.5%) | 808 (2.2%) | <0.0001 |
| Atrial fibrillation (I48) | 811 (0.4%) | 215 (0.6%) | 0.0004 |
| Coronary heart disease (I20–I25) | 1667 (0.9%) | 1605 (4.4%) | <0.0001 |
| Heart failure (I50) | 438 (0.2%) | 491 (1.3%) | <0.0001 |
| Valve disease (I05–I09, I34–I36) | 359 (0.2%) | 134 (0.4%) | <0.0001 |
| Stroke (I61–I64) | 726 (0.4%) | 548 (1.5%) | <0.0001 |
| Cancer (C00–C97) | 2093 (1.1%) | 515 (1.4%) | <0.0001 |
| Foot ulcer (circulatory complications) (E10.5, E11.5, E12.5, E13.5, E14.5) | 17 (0.0%) | 1349 (3.7%) | <0.0001 |
For categorical variables, n (%) is presented. For continuous variables, mean (SD) is presented.
*Variables used in the matching process.
BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; IFCC, International Federation of Clinical Chemistry; LDL, low-density lipoprotein; NGSP, National Glycohemoglobin Standardization Program.
Incidence rate of amputation
The total amputation/1000 patient-years for all types of amputations was 3.18 (95% CI 2.99 to 3.38) for people with type 1 DM and 0.07 (95% CI 0.05 to 0.08) for controls (table 2). Incidence per 1000 patient-years increased with age. People aged 18–49 years had an incidence rate of 1.54 (95% CI 1.40 to 1.70) compared with 18.65 (95% CI 16.02 to 21.60) in people ≥65 years. In contrast, the rate ratio (incidence of amputation compared between people with type 1 DM and controls) for amputations was higher for younger than older people, and ranged from 77.5 for ages 18–49 to 25.2 for people ≥65 years. Both major and minor amputations were substantially more common in people with type 1 DM. The incidence increased with age, while the rate ratio showed the opposite patterns, with higher estimates in younger individuals. Men had a higher overall incidence rate of all amputations regardless of age for both people with type 1 DM and controls. This was even evident for major and minor amputations (table 2).
Table 2.
Incidence of amputations per 1000 patient-years by sex and age categories at baseline with 95% CIs estimated by exact Poisson confidence limits
| Event | Subgroup | Statistics | All | Men | Women | |||
| Type 1 diabetes | Controls | Type 1 diabetes | Controls | Type 1 diabetes | Controls | |||
| Any amputation | All | n (%, N) | 1081 (3.0, 36 577) | 117 (0.1, 182 617) | 711 (3.6, 20 010) | 80 (0.1, 99 879) | 370 (2.2, 16 567) | 37 (0.0, 82 738) |
| Event rate (95% CI) | 3.18 (2.99 to 3.38) | 0.07 (0.05 to 0.08) | 3.87 (3.59 to 4.17) | 0.08 (0.07 to 0.10) | 2.37 (2.13 to 2.62) | 0.05 (0.03 to 0.06) | ||
| 18–49 years | n (%, N) | 428 (1.4, 29 578) | 31 (0.0, 147 804) | 276 (1.7, 16 305) | 23 (0.0, 81 463) | 152 (1.1, 13 273) | 8 (0.0, 66 341) | |
| Event rate (95% CI) | 1.54 (1.40 to 1.70) | 0.02 (0.01 to 0.03) | 1.82 (1.62 to 2.05) | 0.03 (0.02 to 0.04) | 1.21 (1.02 to 1.42) | 0.01 (0.01 to 0.02) | ||
| 50–64 years | n (%, N) | 474 (8.6, 5533) | 41 (0.1, 27 588) | 325 (11.0, 2968) | 26 (0.2, 14 792) | 149 (5.8, 2565) | 15 (0.1, 12 796) | |
| Event rate (95% CI) | 8.91 (8.13 to 9.75) | 0.14 (0.10 to 0.18) | 11.70 (10.46 to 13.04) | 0.16 (0.11 to 0.24) | 5.87 (4.96 to 6.89) | 0.11 (0.06 to 0.17) | ||
| 65+ years | n (%, N) | 179 (12.2, 1466) | 45 (0.6, 7225) | 110 (14.9, 737) | 31 (0.9, 3624) | 69 (9.5, 729) | 14 (0.4, 3601) | |
| Event rate (95% CI) | 18.65 (16.02 to 21.60) | 0.74 (0.54 to 0.99) | 24.05 (19.77 to 28.99) | 1.06 (0.72 to 1.51) | 13.74 (10.69 to 17.39) | 0.44 (0.24 to 0.75) | ||
| Minor amputations | All | n (%, N) | 656 (1.8, 36 577) | 33 (0.0, 182 617) | 462 (2.3, 20 010) | 27 (0.0, 99 879) | 194 (1.2, 16 567) | 6 (0.0, 82 738) |
| Event rate (95% CI) | 1.92 (1.78 to 2.08) | 0.02 (0.01 to 0.03) | 2.50 (2.28 to 2.74) | 0.03 (0.02 to 0.04) | 1.24 (1.07 to 1.42) | 0.01 (0.00 to 0.02) | ||
| 18–49 years | n (%, N) | 308 (1.0, 29 578) | 9 (0.0, 147 804) | 208 (1.3, 16 305) | 9 (0.0, 81 463) | 100 (0.8, 13 273) | 0 (0.0, 66 341) | |
| Event rate (95% CI) | 1.11 (0.99 to 1.24) | 0.01 (0.00 to 0.01) | 1.37 (1.19 to 1.57) | 0.01 (0.01 to 0.02) | 0.79 (0.65 to 0.97) | 0.00 (0.00 to 0.01) | ||
| 50–64 years | n (%, N) | 276 (5.0, 5533) | 12 (0.0, 27 588) | 208 (7.0, 2968) | 8 (0.1, 14 792) | 68 (2.7, 2565) | 4 (0.0, 12 796) | |
| Event rate (95% CI) | 5.12 (4.54 to 5.76) | 0.04 (0.02 to 0.07) | 7.37 (6.40 to 8.44) | 0.05 (0.02 to 0.10) | 2.65 (2.06 to 3.36) | 0.03 (0.01 to 0.07) | ||
| 65+ years | n (%, N) | 72 (4.9, 1466) | 12 (0.2, 7225) | 46 (6.2, 737) | 10 (0.3, 3624) | 26 (3.6, 729) | 2 (0.1, 3601) | |
| Event rate (95% CI) | 7.29 (5.71 to 9.19) | 0.20 (0.10 to 0.35) | 9.63 (7.05 to 12.85) | 0.34 (0.16 to 0.63) | 5.10 (3.33 to 7.48) | 0.06 (0.01 to 0.23) | ||
| Major amputations | All | n (%, N) | 638 (1.7, 36 577) | 87 (0.0, 182 617) | 405 (2.0, 20 010) | 56 (0.1, 99 879) | 233 (1.4, 16 567) | 31 (0.0, 82 738) |
| Event rate (95% CI) | 1.87 (1.72 to 2.02) | 0.05 (0.04 to 0.06) | 2.19 (1.98 to 2.41) | 0.06 (0.04 to 0.08) | 1.49 (1.30 to 1.69) | 0.04 (0.03 to 0.05) | ||
| 18–49 years | n (%, N) | 209 (0.7, 29 578) | 22 (0.0, 147 804) | 124 (0.8, 16 305) | 14 (0.0, 81 463) | 85 (0.6, 13 273) | 8 (0.0, 66 341) | |
| Event rate (95% CI) | 0.75 (0.65 to 0.86) | 0.02 (0.01 to 0.02) | 0.82 (0.68 to 0.97) | 0.02 (0.01 to 0.03) | 0.67 (0.54 to 0.83) | 0.01 (0.01 to 0.02) | ||
| 50–64 years | n (%, N) | 295 (5.3, 5533) | 30 (0.1, 27 588) | 198 (6.7, 2968) | 19 (0.1, 14 792) | 97 (3.8, 2565) | 11 (0.1, 12 796) | |
| Event rate (95% CI) | 5.45 (4.85 to 6.11) | 0.10 (0.07 to 0.14) | 6.96 (6.02 to 8.00) | 0.12 (0.07 to 0.19) | 3.78 (3.07 to 4.62) | 0.08 (0.04 to 0.14) | ||
| 65+ years | n (%, N) | 134 (9.1, 1466) | 35 (0.5, 7225) | 83 (11.3, 737) | 23 (0.6, 3624) | 51 (7.0, 729) | 12 (0.3, 3601) | |
| Event rate (95% CI) | 13.74 (11.51 to 16.28) | 0.58 (0.40 to 0.80) | 17.75 (14.14 to 22.01) | 0.79 (0.50 to 1.18) | 10.05 (7.48 to 13.21) | 0.38 (0.20 to 0.67) | ||
Event rates are expressed per 1000 patient-years.
In Cox regression analysis, the overall HR of amputations in people with type 1 DM compared with controls was 53.6 (95% CI 44.3 to 64.8) when adjusted for time-updated age and sex. For minor amputations, the HR was 112.3 (95% CI 79.2 to 159.4) and 42.6 (95% CI 34.0 to 53.2) for major amputations. When adjusted according to model 3, the overall HR for a patient with diabetes was 40.1 (95% CI 32.8 to 49.1) for all amputations. For diabetes duration of 40 years, it was 86.3 (95% CI 60.2 to 123.8) for minor amputations and 27.3 (95% CI 21.5 to 34.5) for major amputations. The overall excess risk of amputations increased with longer diabetes duration (table 3A,B; online supplementary tables 5,8 and 9).
Table 3A.
Adjusted HR for minor amputations and 95% CI for type 1 diabetes vs controls, time-updated mean HbA1c categories, albuminuria categories, and eGFR categories examined by Cox regression
| Amputation below ankle | HR (95% CI), p value | |||
| Diabetes duration 30years | Diabetes duration 40years | Diabetes duration 50years | Diabetes duration 60years | |
| Overall | ||||
| Controls (reference) | 1.00 | 1.00 | 1.00 | 1.00 |
| Group all type 1 diabetes with no prior amputation | 75.7 (52.6 to 109.0)* | 86.3 (60.2 to 123.8)* | 98.4 (67.3 to 143.9)* | 112.3 (73.7 to 171.0)* |
| Time-updated mean HbA1c categories | ||||
| ≤52 mmol/mol (≤6.9%) | 27.3 (16.7 to 44.7)* | 32.0 (19.7 to 52.0)* | 37.4 (22.8 to 61.5)* | 43.7 (25.9 to 74.0)* |
| 53–62 mmol/mol (7.0%–7.8%) | 45.0 (30.2 to 67.2)* | 52.7 (35.6 to 77.9)* | 61.6 (41.1 to 92.4)* | 72.0 (46.5 to 111.7)* |
| 63–72 mmol/mol (7.9%–8.7%) | 65.7 (44.6 to 96.7)* | 76.8 (52.5 to 112.2)* | 89.8 (60.5 to 133.2)* | 105.0 (68.3 to 161.5)* |
| 73–82 mmol/mol (8.8%–9.6%) | 123.4 (83.4 to 182.6)* | 144.4 (98.3 to 212.1)* | 168.9 (113.1 to 252.0)* | 197.5 (127.6 to 305.6)* |
| ≥83 mmol/mol (≥9.7%) | 299.7 (201.5 to 445.6)* | 350.5 (237.2 to 517.8)* | 409.9 (273.0 to 615.4)* | 479.4 (308.0 to 746.3)* |
| Time-updated albuminuria categories | ||||
| Normoalbuminuria | 48.6 (33.1 to 71.4)* | 50.4 (34.5 to 73.7)* | 52.4 (35.1 to 78.2)* | 54.3 (34.8 to 85.0)* |
| Microalbuminuria | 130.6 (87.5 to 195.1)* | 135.5 (91.7 to 200.2)* | 140.6 (93.6 to 211.2)* | 145.9 (93.3 to 228.1)* |
| Macroalbuminuria | 209.2 (139.4 to 314.1)* | 217.0 (146.2 to 322.0)* | 225.2 (149.5 to 339.4)* | 233.7 (149.2 to 366.2)* |
| CKD stage 5 | 623.7 (408.4 to 952.6)* | 646.8 (428.6 to 975.9)* | 671.5 (439.1 to 1026.8)* | 696.7 (439.2 to 1105.2)* |
| Time-updated eGFR categories | ||||
| CKD stage 1 (eGFR ≥90) | 45.9 (30.6 to 69.0)* | 47.2 (31.5 to 70.7)* | 48.5 (31.6 to 74.5)* | 49.8 (31.0 to 80.2)* |
| CKD stage 2 (eGFR 60–89) | 75.3 (51.2 to 110.8)* | 77.3 (52.9 to 113.2)* | 79.5 (53.1 to 118.9)* | 81.7 (52.1 to 128.0)* |
| CKD stage 3 (eGFR 30–59) | 160.5 (106.4 to 242.1)* | 164.9 (110.6 to 245.9)* | 169.5 (111.9 to 256.7)* | 174.2 (110.6 to 274.5)* |
| CKD stage 4 (eGFR 15–29) | 190.8 (111.7 to 325.9)* | 196.0 (115.7 to 332.2)* | 201.5 (117.4 to 345.7)* | 207.2 (117.0 to 366.8)* |
| CKD stage 5 (eGFR <15, dialysis or transplantation) | 623.1 (408.2 to 950.9)* | 640.1 (424.0 to 966.4)* | 658.0 (429.3 to 1008.5)* | 676.2 (424.5 to 1077.1)* |
All models are adjusted for time-updated age, sex, born in Sweden, maximum educational level, baseline comorbidities and time-updated diabetes duration centered at 30, 40, 50 and 60 years.
*Significant at p<0.0001.
Table 3B.
Adjusted HR for major amputations and 95% CIs for type 1 diabetes vs controls, time-updated mean HbA1c categories, albuminuria categories, and eGFR categories examined by Cox regression
| Amputation above ankle | HR (95% CI), p value | |||
| Diabetes duration 30years | Diabetes duration 40years | Diabetes duration 50years | Diabetes duration 60years | |
| Overall | ||||
| Controls (reference) | 1.00 | 1.00 | 1.00 | 1.00 |
| Group all type 1 diabetes with no prior amputation | 21.4 (16.6 to 27.5)* | 27.3 (21.5 to 34.5)* | 34.8 (27.1 to 44.7)* | 44.4 (33.2 to 59.4)* |
| Time-updated mean HbA1c categories | ||||
| ≤52 mmol/mol (≤6.9%) | 8.1 (5.3 to 12.2)* | 10.8 (7.3 to 16.0)* | 14.4 (9.7 to 21.5)* | 19.3 (12.6 to 29.4)* |
| 53–62 mmol/mol (7.0%–7.8%) | 13.2 (9.8 to 17.8)* | 17.6 (13.3 to 23.3)* | 23.5 (17.7 to 31.4)* | 31.5 (22.8 to 43.3)* |
| 63–72 mmol/mol (7.9%–8.7%) | 18.6 (14.0 to 24.8)* | 24.9 (19.1 to 32.5)* | 33.3 (25.3 to 43.8)* | 44.5 (32.6 to 60.7)* |
| 73–82 mmol/mol (8.8%–9.6%) | 30.6 (22.8 to 41.1)* | 40.9 (30.9 to 54.0)* | 54.6 (40.9 to 73.0)* | 73.0 (52.7 to 101.2)* |
| ≥83 mmol/mol (≥9.7%) | 78.4 (58.1 to 105.9)* | 104.8 (78.8 to 139.4)* | 140.0 (104.0 to 188.5)* | 187.1 (134.1 to 261.1)* |
| Time-updated albuminuria categories | ||||
| Normoalbuminuria | 13.6 (10.2 to 18.1)* | 16.5 (12.6 to 21.6)* | 20.1 (15.2 to 26.6)* | 24.4 (17.6 to 33.7)* |
| Microalbuminuria | 26.2 (19.0 to 36.2)* | 31.8 (23.6 to 42.9)* | 38.7 (28.5 to 52.4)* | 47.0 (33.5 to 65.9)* |
| Macroalbuminuria | 59.3 (43.4 to 80.9)* | 72.0 (54.1 to 95.9)* | 87.6 (65.3 to 117.4)* | 106.4 (76.5 to 148.0)* |
| CKD stage 5 | 194.1 (139.8 to 269.5)* | 235.9 (173.9 to 320.0)* | 286.7 (210.1 to 391.4)* | 348.5 (246.7 to 492.3)* |
| Time-updated eGFR categories | ||||
| CKD stage 1 (eGFR ≥90) | 15.4 (11.2 to 21.2)* | 19.0 (14.0 to 25.7)* | 23.3 (16.9 to 32.1)* | 28.6 (19.9 to 41.1)* |
| CKD stage 2 (eGFR 60–89) | 16.7 (12.4 to 22.6)* | 20.6 (15.6 to 27.1)* | 25.2 (19.0 to 33.6)* | 31.0 (22.4 to 43.0)* |
| CKD stage 3 (eGFR 30–59) | 26.7 (19.1 to 37.3)* | 32.8 (24.1 to 44.5)* | 40.2 (29.6 to 54.7)* | 49.5 (35.3 to 69.3)* |
| CKD stage 4 (eGFR 15–29) | 55.0 (35.5 to 85.4)* | 67.6 (44.5 to 102.8)* | 83.1 (54.5 to 126.6)* | 102.1 (65.3 to 159.6)* |
| CKD stage 5 (eGFR <15, dialysis or transplantation) | 185.3 (132.9 to 258.5)* | 227.7 (167.5 to 309.6)* | 279.6 (204.6 to 382.2)* | 343.7 (242.9 to 486.3)* |
All models are adjusted for time-updated age, sex, born in Sweden, maximum educational level, baseline comorbidities and time-updated diabetes duration centered at 30, 40, 50 and 60 years.
*Significant at p<0.0001.
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c.
Change in risk of amputation over time
The incidence of amputations was evaluated for five predefined time periods 1998–2001, 2002–2004, 2005–2007, 2008–2010, and 2011–2013. The mean age in men was 39.9 years in the first time period compared with 41.3 years in the last period, and 40.3 and 42.3 years, respectively, in women (online supplementary table 2). Figure 1 shows the crude incidence rates and incidence rates adjusted for age and sex for all amputations in people with type 1 DM and controls, as well as for major and minor amputations for the five time periods. Age-adjusted and sex-adjusted incidence rates of all amputations were numerically lower for people with type 1 DM during the last compared with the first time period, but there were no significant differences, 3.09 (95% CI 2.56 to 3.62) vs 2.64 (95% CI 2.31 to 2.98). The lower rate of amputations during the last time period was mainly due to fewer major amputations in men over time (figure 1 and online supplementary table 3).
In Cox regression adjusting for risk factors in addition to age and sex, there were no significant differences in amputations over time among people with type 1 DM or when compared with controls over time, although the fully adjusted model (model 3) showed such a tendency within the diabetes group for the last versus the first time period (HR 0.75, 95% CI 0.56 to 1.01, p=0.060; online supplementary table 4). There was no significant change in minor amputations, but major amputations had decreased significantly for the last versus the first time period (HR 0.57, 95% CI 0.39 to 0.84, p=0.0045; online supplementary table 4). In a post-hoc analysis of amputations over time for the time periods 1998–2003, 2004–2008, and 2009–2013, there was a significant reduction in amputations within the diabetes group in the fully adjusted model (model 3), with an HR of 0.75 (95% CI 0.61 to 0.94), p=0.011, whereas the other two models showed no significant reduction (online supplementary table 4). There was no significant change for minor amputations, but major amputations showed a significant change between all periods (online supplementary table 4).
Excess risk of amputations in relation to glycemic control and renal complications
As there was an interaction with diabetes duration, HRs were stratified by diabetes duration of 30, 40, 50, and 60 years. The excess risk of all, major, and minor amputations in people with type 1 DM versus controls estimated by HR increased with higher HbA1c and longer diabetes duration (table 3A,B and online supplementary table 5). At 30 years diabetes duration and HbA1c ≤52 mmol/mol (6.9%), the HR for any type of amputation was 11.8 (95% CI 8.5 to 16.3) and increased to 116.9 (95% CI 91.8 to 148.9), with HbA1c levels ≥83 mmol/mol (9.7%). At 60 years diabetes duration, the corresponding HRs were 23.2 (95% CI 16.4 to 32.6) and 229.9 (95% CI 174.6 to 302.6). The excess risk of amputations for people with type 1 DM versus controls estimated by HR also increased with severity of renal complications, both when renal complications were defined by grade of albuminuria (normoalbuminuria microalbuminuria and macroalbuminuria) as well as for CKD stages defined by level of eGFR. This was evident in all models (online supplementary table 5).
The excess risk for minor amputations for people with type 1 DM compared with controls was higher than for major amputations and increased from 27.3 (95% CI 16.7 to 44.7) with HbA1c ≤52 mmol/mol (6.9%) and 30 years diabetes duration to 479.4 (95% CI 308.0 to 746.3) with HbA1c ≥83 mmol/mol (9.7%) and 60 years diabetes duration, while the HR increased from 8.1 (95% CI 5.3 to 12.2) to 187.1 (95% CI 134.1 to 261.1) for major amputations. This excess risk even increased with the severity of renal complications and was evident for all models (table 3A,B and online supplementary tables 8 and 9).
A significantly increased risk of amputation was found among people with type 1 DM compared with controls when good glycemic control (HbA1c ≤52 mmol/mol, 6.9%) existed simultaneously with normoalbuminuria. The HR was 6.2 (95% CI 3.7 to 10.4) at 30 years diabetes duration and 10.8 (95% CI 6.3 to 18.3) at 60 years diabetes duration (online supplementary table 6). At the same level of HbA1c (≤52 mmol/mol, 6.9%) but with albuminuria, the HR increased approximately fivefold, to 32.6 (95% CI 21.5 to 49.4) at 30 years diabetes duration and 56.5 (95% CI 37.0 to 86.2) at 60 years diabetes duration. The HR for both those with normal albuminuria and albuminuria increased with worsening glycemic control. For people with normoalbuminuria and HbA1c ≥83 mmol/mol (9.7%), the HR was 106.7 (95% CI 76.5 to 148.8) at 30 years diabetes duration and 184.8 (95% CI 127.6 to 267.5) at 60 years diabetes duration, while it was 207.3 (95% CI 158.5 to 271.2) and 359.0 (95% CI 265.8 to 485.0), respectively, for people with albuminuria. Similar results were found when the risk of amputation for people with type 1 DM versus controls was evaluated in relation to HbA1c with eGFR above and below 60 (online supplementary table 7). The HRs were higher for model 2 (adjusted for age, sex, and diabetes duration) than for model 3, where the analysis was adjusted for age, sex, diabetes duration, birth in Sweden, educational level, and comorbidities (online supplementary tables 6 and 7).
The HR for minor amputations increased from 11.3 (95% CI 5.2 to 24.6) with good glycemic control and normoalbuminuria to 87.9 (95% CI 48.9 to 157.9) when albuminuria was present at 30 years diabetes duration. At 60 years diabetes duration the corresponding figures were 15.4 (95% CI 6.8 to 34.6) and 119.9 (95% CI 65.4 to 219.7). For major amputations the corresponding figures were 5.6 (95% CI 3.1 to 10.2) and 17.1 (95% CI 9.7 to 30.1) for 30 years diabetes duration and 12.4 (95% CI 6.7 to 22.8) and 37.5 (95% CI 21.4 to 65.8) for 60 years diabetes duration (table 4A,B).
Table 4A.
Adjusted HRs of minor amputations for people with type 1 diabetes vs controls and 95% CIs for time-updated mean HbA1c categories and coexisting normoalbuminuria and eGFR >60 mL/min
| Amputation below ankle | HR (95% CI), p value | |||
| Diabetes duration 30years | Diabetes duration 40years | Diabetes duration 50years | Diabetes duration 60years | |
| Time-updated mean HbA1c categories and albuminuria | ||||
| Controls (reference) | 1.00 | 1.00 | 1.00 | 1.00 |
| ≤52 mmol/mol (≤6.9%)—normoalbuminuria | 11.3 (5.2 to 24.6)* | 12.5 (5.8 to 27.2)* | 13.9 (6.3 to 30.5)* | 15.4 (6.8 to 34.6)* |
| 53–62 mmol/mol (7.0%–7.8%)—normoalbuminuria | 34.9 (22.2 to 54.9)* | 38.7 (24.8 to 60.4)* | 43.0 (27.1 to 68.1)* | 47.6 (29.0 to 78.3)* |
| 63–72 mmol/mol (7.9%–8.7%)—normoalbuminuria | 49.6 (32.2 to 76.5)* | 55.0 (35.9 to 84.2)* | 61.0 (39.2 to 95.1)* | 67.7 (41.7 to 109.7)* |
| 73–82 mmol/mol (8.8%–9.6%)—normoalbuminuria | 61.8 (37.2 to 102.6)* | 68.5 (41.5 to 113.1)* | 76.0 (45.3 to 127.5)* | 84.3 (48.6 to 146.3)* |
| ≥83 mmol/mol (≥9.7%)—normoalbuminuria | 221.8 (134.0 to 367.0)* | 245.8 (149.0 to 405.3)* | 272.8 (162.5 to 457.8)* | 302.4 (173.8 to 526.3)* |
| ≤52 mmol/mol (≤6.9%)—not normoalbuminuria | 87.9 (48.9 to 157.9)* | 97.4 (54.8 to 173.0)* | 108.1 (60.4 to 193.4)* | 119.9 (65.4 to 219.7)* |
| 53–62 mmol/mol (7.0%–7.8%)—not normoalbuminuria | 91.7 (58.0 to 144.9)* | 101.6 (65.2 to 158.2)* | 112.7 (71.7 to 177.2)* | 125.0 (77.1 to 202.7)* |
| 63–72 mmol/mol (7.9%–8.7%)—not normoalbuminuria | 139.2 (91.5 to 211.5)* | 154.2 (102.7 to 231.5)* | 171.1 (112.5 to 260.4)* | 189.8 (120.2 to 299.5)* |
| 73–82 mmol/mol (8.8%–9.6%)—not normoalbuminuria | 252.0 (166.4 to 381.5)* | 279.2 (186.4 to 418.2)* | 309.9 (203.8 to 471.4)* | 343.6 (217.4 to 543.1)* |
| ≥83 mmol/mol (≥9.7%)—not normoalbuminuria | 547.9 (359.9 to 834.1)* | 607.1 (402.6 to 915.6)* | 673.8 (439.6 to 1032.8)* | 747.1 (468.9 to 1190.4)* |
| Time-updated mean HbA1c categories and eGFR | ||||
| ≤52 mmol/mol (≤6.9%)—eGFR ≥60 | 14.7 (7.4 to 29.4)* | 16.0 (8.0 to 31.8)* | 17.3 (8.6 to 34.9)* | 18.8 (9.1 to 38.9)* |
| 53–62 mmol/mol (7.0%–7.8%)—eGFR ≥60 | 34.7 (22.2 to 54.1)* | 37.6 (24.3 to 58.3)* | 40.9 (26.0 to 64.3)* | 44.4 (27.2 to 72.5)* |
| 63–72 mmol/mol (7.9%–8.7%)—eGFR ≥60 | 55.7 (36.7 to 84.4)* | 60.5 (40.2 to 90.9)* | 65.6 (42.8 to 100.5)* | 71.2 (44.6 to 113.8)* |
| 73–82 mmol/mol (8.8%–9.6%)—eGFR ≥60 | 98.1 (63.6 to 151.3)* | 106.5 (69.5 to 163.2)* | 115.6 (74.0 to 180.5)* | 125.5 (77.2 to 204.1)* |
| ≥83 mmol/mol (≥9.7%)—eGFR ≥60 | 297.7 (191.8 to 462.2)* | 323.2 (209.0 to 499.8)* | 350.8 (222.3 to 553.6)* | 380.9 (231.5 to 626.6)* |
| ≤52 mmol/mol (≤6.9%)—eGFR <60 | 91.1 (48.4 to 171.6)* | 98.9 (53.1 to 184.2)* | 107.4 (57.3 to 201.1)* | 116.6 (60.8 to 223.4)* |
| 53–62 mmol/mol (7.0%–7.8%)—eGFR <60 | 117.3 (73.0 to 188.5)* | 127.3 (80.4 to 201.6)* | 138.2 (86.5 to 220.8)* | 150.0 (91.1 to 247.1)* |
| 63–72 mmol/mol (7.9%–8.7%)—eGFR <60 | 178.2 (114.6 to 276.9)* | 193.4 (126.0 to 296.8)* | 209.9 (135.2 to 326.0)* | 227.9 (141.7 to 366.5)* |
| 73–82 mmol/mol (8.8%–9.6%)—eGFR <60 | 299.9 (191.0 to 471.0)* | 325.6 (209.9 to 505.0)* | 353.4 (225.2 to 554.7)* | 383.7 (236.2 to 623.3)* |
| ≥83 mmol/mol (≥9.7%)—eGFR <60 | 693.3 (442.0 to 1087.5)* | 752.6 (485.0 to 1167.8)* | 816.9 (519.4 to 1284.8)* | 886.9 (544.1 to 1445.5)* |
Adjusted for time-updated age, sex, born in Sweden, maximum educational level, baseline comorbidities and time-updated diabetes duration centered at 30, 40, 50 and 60 years.
*Significant at p<0.0001.
Table 4B.
Adjusted HRs of major amputations for people with type 1 diabetes vs controls and 95% CIs for time-updated mean HbA1c categories and coexisting normoalbuminuria and eGFR >60 mL/min
| Amputation above ankle | HR (95% CI), p value | |||
| Diabetes duration 30years | Diabetes duration 40years | Diabetes duration 50years | Diabetes duration 60years | |
| Time-updated mean HbA1c categories and albuminuria | ||||
| Controls (reference) | 1.00 | 1.00 | 1.00 | 1.00 |
| ≤52 mmol/mol (≤6.9%)—normoalbuminuria | 5.6 (3.1 to 10.2)* | 7.3 (4.1 to 13.2)* | 9.5 (5.3 to 17.2)* | 12.4 (6.7 to 22.8)* |
| 53–62 mmol/mol (7.0%–7.8%)—normoalbuminuria | 8.9 (6.0 to 13.1)* | 11.6 (8.0 to 16.7)* | 15.0 (10.3 to 21.9)* | 19.5 (13.0 to 29.3)* |
| 63–72 mmol/mol (7.9%–8.7%)—normoalbuminuria | 11.1 (7.7 to 16.2)* | 14.5 (10.1 to 20.7)* | 18.8 (13.0 to 27.1)* | 24.4 (16.3 to 36.5)* |
| 73–82 mmol/mol (8.8%–9.6%)—normoalbuminuria | 17.6 (11.3 to 27.4)* | 22.9 (14.9 to 35.2)* | 29.7 (19.1 to 46.1)* | 38.6 (24.1 to 61.7)* |
| ≥83 mmol/mol (≥9.7%)—normoalbuminuria | 71.7 (46.6 to 110.3)* | 93.2 (61.1 to 142.1)* | 121.0 (78.3 to 186.9)* | 157.2 (98.5 to 251.1)* |
| ≤52 mmol/mol (≤6.9%)—not normoalbuminuria | 17.1 (9.7 to 30.1)* | 22.2 (12.9 to 38.4)* | 28.9 (16.7 to 49.8)* | 37.5 (21.4 to 65.8)* |
| 53–62 mmol/mol (7.0%–7.8%)—not normoalbuminuria | 25.9 (18.0 to 37.4)* | 33.7 (23.9 to 47.4)* | 43.7 (31.1 to 61.5)* | 56.8 (39.3 to 82.2)* |
| 63–72 mmol/mol (7.9%–8.7%)—not normoalbuminuria | 41.2 (29.9 to 56.8)* | 53.5 (39.7 to 72.0)* | 69.5 (51.3 to 94.1)* | 90.3 (64.3 to 126.7)* |
| 73–82 mmol/mol (8.8%–9.6%)—not normoalbuminuria | 55.0 (39.4 to 76.9)* | 71.5 (52.1 to 98.1)* | 92.8 (67.0 to 128.6)* | 120.7 (84.0 to 173.3)* |
| ≥83 mmol/mol (≥9.7%)—not normoalbuminuria | 139.2 (99.4 to 195.0)* | 180.9 (131.4 to 248.9)* | 234.8 (168.7 to 326.7)* | 305.2 (211.4 to 440.7)* |
| Time-updated mean HbA1c categories and eGFR | ||||
| ≤52 mmol/mol (≤6.9%)—eGFR ≥60 | 6.8 (3.9 to 11.7)* | 8.6 (5.0 to 14.8)* | 11.0 (6.4 to 19.0)* | 14.1 (8.0 to 24.8)* |
| 53–62 mmol/mol (7.0%–7.8%)—eGFR ≥60 | 10.3 (7.1 to14.8)* | 13.1 (9.3 to 18.5)* | 16.8 (11.8 to 23.9)* | 21.4 (14.5 to 31.5)* |
| 63–72 mmol/mol (7.9%–8.7%)—eGFR ≥60 | 16.0 (11.5 to 22.2)* | 20.4 (15.0 to 27.9)* | 26.1 (18.9 to 36.0)* | 33.3 (23.2 to 47.8)* |
| 73–82 mmol/mol (8.8%–9.6%)—eGFR ≥60 | 19.4 (13.1 to 28.7)* | 24.8 (17.0 to 36.2)* | 31.7 (21.5 to 46.7)* | 40.4 (26.5 to 61.8)* |
| ≥83 mmol/mol (≥9.7%)—eGFR ≥60 | 67.3 (45.3 to 99.8)* | 85.9 (58.4 to 126.3)* | 109.6 (73.4 to 163.8)* | 140.0 (90.2 to 217.2)* |
| ≤52 mmol/mol (≤6.9%)—eGFR <60 | 17.2 (9.4 to 31.2)* | 21.9 (12.3 to 39.1)* | 28.0 (15.7 to 49.8)* | 35.7 (19.7 to 64.6)* |
| 53–62 mmol/mol (7.0%–7.8%)—eGFR <60 | 24.6 (16.5 to 36.7)* | 31.4 (21.6 to 45.7)* | 40.1 (27.6 to 58.3)* | 51.2 (34.5 to 76.2)* |
| 63–72 mmol/mol (7.9%–8.7%)—eGFR <60 | 39.4 (27.6 to 56.4)* | 50.4 (36.1 to 70.2)* | 64.3 (45.9 to 90.0)* | 82.1 (56.8 to 118.5)* |
| 73–82 mmol/mol (8.8%–9.6%)—eGFR <60 | 69.0 (47.7 to 99.9)* | 88.1 (62.3 to 124.8)* | 112.5 (79.1 to 160.0)* | 143.6 (97.9 to 210.7)* |
| ≥83 mmol/mol (≥9.7%)—eGFR <60 | 166.2 (114.5 to 241.4)* | 212.3 (149.3 to 301.9)* | 271.0 (189.4 to 387.6)* | 346.0 (234.3 to 510.8)* |
Adjusted for time-updated age, sex, born in Sweden, maximum educational level, baseline comorbidities and time-updated diabetes duration centered at 30, 40, 50 and 60 years.
*Significant at p<0.0001.
eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c.
Risk of amputation among people with type 1 DM in relation to 10 mmol/mol (1%) increase in HbA1c
Cox regression was used to analyze the effect of 10 mmol/mol (1%) change in HbA1c on the risk of amputation within the diabetes cohort. When adjusted for sex, age, diabetes duration, birthplace, educational level, baseline comorbidities, time-updated systolic blood pressure, BMI, smoking, blood pressure-lowering and lipid-lowering medications, HDL cholesterol, LDL cholesterol, and method of insulin use, the HR was 1.80 (95% CI 1.72 to 1.88) for every 10 mmol/mol (1%) increase for all amputations. After adjusting further for time-updated urine albuminuria, the HR was 1.72 (95% CI 1.64 to 1.81) for every 10 mmol/mol (1%) increase in HbA1c. For major amputations the HR was 1.68 (95% CI 1.57 to 1.79) without adjusting for urine albuminuria and 1.61 (95% CI 1.50 to 1.72) when adjusted for albuminuria. For minor amputations the corresponding results were 1.80 (95% CI 1.70 to 1.90) and 1.73 (95% CI 1.63 to 1.84), respectively.
Discussion
This nationwide study including 36 577 people with type 1 DM and 182 617 matched controls from the general population in Sweden shows a 40-fold excess risk of lower extremity amputations in people with type 1 DM. The excess risk of amputations approached that of the general population with better glycemic control and fewer renal complications, but an excess risk remained for people with type 1 DM at normoalbuminuria and who reached the target levels of HbA1c. Excess risk was greater for minor than major amputations and only decreased over time for major amputations. The incidence of amputations was also higher for men than women and in older compared with younger individuals.
To our knowledge this is the first study of principally all people with type 1 DM in a single country and comparing the risk of amputations with the general population in relation to risk factors. Earlier studies have also identified an excess risk of amputations in people with versus without type 1 DM,4 7 and others performing analyses within the diabetes group found that impaired glycemic control and renal complications were associated with increased risk of amputations.8 9 The current incidence of amputations (3.18/1000 patient-years) is within the same range of that from similar cohorts evaluating coronary events (5.7/1000 patient-years) and heart failure (4.0/1000 patient-years).16 23 Hence, it is notable that although the relative risk of amputations in people with type 1 DM compared with the general population is around 40 times compared with 4 times for coronary events and heart failure, the risk of experiencing an amputation for people with type 1 DM is somewhat lower. These results also show that amputation is a diabetes-specific complication to a greater extent than cardiovascular disease, which is more common in the general population.
People with normoalbuminuria and mean HbA1c levels below targets had an excess risk for all amputations that was approximately 8 times at 40 years diabetes duration and increased with longer diabetes duration, this was 7 times greater for major amputations and 13 times greater for minor amputations. These findings should be interpreted with caution with regard to whether targeting HbA1c and avoiding renal complications is sufficient to reducing the risk of amputations to that of the general population. The current data include information on HbA1c levels for approximately 10 years, and it is possible that people with diabetes had worse glycemic control before participating in NDR. Although we used normoalbuminuria as a marker for earlier glycemic control, this is only a rough measure of historic glucose levels. However, in clinical practice it is important for caregivers to be aware that people with on-target HbA1c and no renal complications still have an excess risk of amputation, although it is considerably lower than for other people with diabetes.
Although there were some indications of lower risk of amputations in people with type 1 DM over time as shown by numerically lower rates and a significant reduction in a post-hoc analysis, this was due to a significant change in major amputations and not in minor amputations. Therefore, improved prevention of amputations in people with type 1 DM is urgently needed. Glycemic control seems to be of special concern for preventing amputations. Besides the high excess risk of amputations in people with type 1 DM compared with the general population, indicating a diabetes-specific condition, the 80% increase in risk of all and minor amputations by 10 mmol/mol (1%) higher HbA1c estimated within the diabetes group was considerably higher than for coronary events (30% for men and 41% for women) and heart failure (30%) reported in similar cohorts.15 23 An earlier meta-analysis showed a 15% increase in risk of myocardial infarction for a similar increase in HbA1c,24 and few analyses of microvascular complications have shown such a high gradient of risk as found here for amputation.21 Since hyperglycemia is a prerequisite for renal complications, the dramatic increase in risk of amputation with severity of renal complications indicates the same phenomenon. Therefore, from a longer term perspective, improving glycemic control in people with type 1 DM may lead to a substantial decrease in amputations. Not only hyperglycemia, but other risk factors for diabetes complications such as hypertension and smoking cessation are also important strategies to control for in people with type 1 DM to decrease the incidence of amputations both from a medical and health economics perspective.13
Although the relative risk in type 1 DM is higher for younger people due to few events in the general population, the risk of amputations increases substantially with age, and the mean age and diabetes duration were 59 and 44 years, respectively, at the time of first amputation in the current study. Why renal complications are such a strong risk factor for amputations may be partly due to being a marker of high historic glycemic control and an overall high risk of complications. However, it is also possible that other mechanisms associated with renal complications such as hypertension, reduced albumin levels, inflammatory processes, and factors of the dialysis process may play an essential role for lower extremity amputations.8 9 25–27 To prevent amputations, screening for neuropathy and ulcers and performing preventive foot care are of importance, which often require specialist foot teams for people with diabetes and severe ulcers.13
A strength of this study is the large cohort, both for people with type 1 DM and the availability of five matched controls. Further adjustment for several variables could be performed, including comorbidities when comparing the risk of amputations in people with type 1 DM with that of the general population. Moreover, well-known risk factors for diabetes complications existed for virtually all people with type 1 DM and during a long time period, which are essential when estimating the excess risk of amputations compared with controls and the risk gradient between HbA1c and amputations within the diabetes group. Limitations include that some potential risk factors for amputations such as BMI, blood pressure levels, lipid-lowering medication, and smoking were not available among controls. Moreover, since this is an observational study, residual confounding cannot be excluded.
In conclusion, the absolute risk of experiencing an amputation for people with type 1 DM is relatively low, especially at younger ages, whereas the relative risk compared with that of the general population remains high. To reduce the excess risk of amputations, improving glycemic control in people with type 1 DM is crucial. Longer diabetes duration, renal complications, and poor glycemic control are strong risk factors for amputations. Although people with diabetes, good glycemic control and no renal complications have considerably lower risks for amputations, excess risk of amputations remains, and thus this group also requires continued prevention.
Acknowledgments
We thank all of the clinicians who care for individuals with type 1 diabetes in Sweden, the personnel at the National Diabetes Register, and Joseph W Murphy for editorial assistance.
Footnotes
Presented at: This study has been presented at ADA 2017 in San Diego.
Contributors: Design and conception: AFÓ, A-MS, AP, SG, AR and ML. Acquisition, analysis and interpretation of data: all authors. Drafting of the manuscript: AFÓ and ML. Critical revision of the manuscript for important intellectual content: all authors. Final approval of the version to be published: all authors. AFÓ, A-MS, AP, SG, and ML had access to raw data. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Funding: This study was supported by an unrestricted grant from the Novo Nordisk Foundation, the Swedish Government (under the Avtal om Läkarutbildning och Medicinsk Forskning [Agreement for Medical Education and Research]), the Swedish Society of Medicine, the Swedish Heart and Lung Foundation, the Swedish Research Council, and the Swedish Diabetes Foundation.
Competing interests: ML has received grants from AstraZeneca, Dexcom, Novo Nordisk and Pfizer; and consulting fees from AstraZeneca, Eli Lilly, Dexcom, MSD, Novo Nordisk, and Rubin Medical. TN has received unrestricted grants from AstraZeneca and consulting fees from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Merck and Sanofi-Aventis. AFÓ, A-MS, SG, AR and AP declared no competing interests.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the Regional Ethical Review Board of the University of Gothenburg, Sweden.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data may be obtained from the registries named in the method section and are not publicly available
References
- 1.Berne C, Agardh C-D. 2009 diabetes. 4 ed Stockholm: Liber. [Google Scholar]
- 2.Mohammedi K, Potier L, Belhatem N, et al. Lower-extremity amputation as a marker for renal and cardiovascular events and mortality in patients with long standing type 1 diabetes. Cardiovasc Diabetol 2016;15 10.1186/s12933-015-0322-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerdtham UG, Clarke P, Hayes A, et al. Estimating the cost of diabetes mellitus-related events from inpatient admissions in Sweden using administrative hospitalization data. Pharmacoeconomics 2009;27:81–90. 10.2165/00019053-200927010-00008 [DOI] [PubMed] [Google Scholar]
- 4.Jonasson JM, Ye W, Sparén P, et al. Risks of nontraumatic lower-extremity amputations in patients with type 1 diabetes: a population-based cohort study in Sweden. Diabetes Care 2008;31:1536–40. 10.2337/dc08-0344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jørgensen ME, Almdal TP, Faerch K. Reduced incidence of lower-extremity amputations in a Danish diabetes population from 2000 to 2011. Diabet Med 2014;31:443–7. 10.1111/dme.12320 [DOI] [PubMed] [Google Scholar]
- 6.Kurowski JR, Nedkoff L, Schoen DE, et al. Temporal trends in initial and recurrent lower extremity amputations in people with and without diabetes in Western Australia from 2000 to 2010. Diabetes Res Clin Pract 2015;108:280–7. 10.1016/j.diabres.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 7.Lopez-de-Andres A, Jiménez-García R, Aragón-Sánchez J, et al. National trends in incidence and outcomes in lower extremity amputations in people with and without diabetes in Spain, 2001-2012. Diabetes Res Clin Pract 2015;108:499–507. 10.1016/j.diabres.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 8.Sahakyan K, Klein BE, Lee KE, et al. The 25-year cumulative incidence of lower extremity amputations in people with type 1 diabetes. Diabetes Care 2011;34:649–51. 10.2337/dc10-1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margolis DJ, Hofstad O, Feldman HI. Association between renal failure and foot ulcer or lower-extremity amputation in patients with diabetes. Diabetes Care 2008;31:1331–6. 10.2337/dc07-2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavery LA, Hunt NA, Ndip A, et al. Impact of chronic kidney disease on survival after amputation in individuals with diabetes. Diabetes Care 2010;33:2365–9. 10.2337/dc10-1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Y, Qian H, Xu L, et al. Association between estimated glomerular filtration rate and outcomes in patients with diabetic foot ulcers: a 3-year follow-up study. Eur J Endocrinol 2017;177:41–50. 10.1530/EJE-17-0070 [DOI] [PubMed] [Google Scholar]
- 12.Game FL, Selby NM, McIntyre CW. Chronic kidney disease and the foot in diabetes--is inflammation the missing link? Nephron Clin Pract 2013;123:36–40. 10.1159/000351813 [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association Standards of medical care for patients with diabetes mellitus. Diabetes Care 2018;41(Suppl 1). [Google Scholar]
- 14.Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Glycemic control and cardiovascular disease in 7,454 patients with type 1 diabetes: an observational study from the Swedish national diabetes Register (NDR). Diabetes Care 2010;33:1640–6. 10.2337/dc10-0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lind M, Bounias I, Olsson M, et al. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet 2011;378:140–6. 10.1016/S0140-6736(11)60471-6 [DOI] [PubMed] [Google Scholar]
- 16.Rosengren A, Vestberg D, Svensson A-M, et al. Long-term excess risk of heart failure in people with type 1 diabetes: a prospective case-control study. Lancet Diabetes Endocrinol 2015;3:876–85. 10.1016/S2213-8587(15)00292-2 [DOI] [PubMed] [Google Scholar]
- 17.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emilsson L, Smith JG, West J, et al. Increased risk of atrial fibrillation in patients with coeliac disease: a nationwide cohort study. Eur Heart J 2011;32:2430–7. 10.1093/eurheartj/ehr167 [DOI] [PubMed] [Google Scholar]
- 19.Olén O, Bihagen E, Rasmussen F, et al. Socioeconomic position and education in patients with coeliac disease. Dig Liver Dis 2012;44:471–6. 10.1016/j.dld.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 20.Jeppsson JO, Kobold U, Barr J, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med 2002;40:78–89. 10.1515/CCLM.2002.016 [DOI] [PubMed] [Google Scholar]
- 21.Lind M, Odén A, Fahlén M, et al. A systematic review of HbA1c variables used in the study of diabetic complications. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2008;2:282–93. 10.1016/j.dsx.2008.04.006 [DOI] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal Disease Study Group. Ann Intern Med 1999;130:461–70. [DOI] [PubMed] [Google Scholar]
- 23.Matuleviciene-Anängen V, Rosengren A, Svensson A-M, et al. Glycaemic control and excess risk of major coronary events in persons with type 1 diabetes. Heart 2017;103:1687–95. 10.1136/heartjnl-2016-311050 [DOI] [PubMed] [Google Scholar]
- 24.Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: Glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004;141:421–31. 10.7326/0003-4819-141-6-200409210-00007 [DOI] [PubMed] [Google Scholar]
- 25.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, et al. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989;32:219–26. [DOI] [PubMed] [Google Scholar]
- 26.Kaminski MR, Raspovic A, McMahon LP, et al. Factors associated with foot ulceration and amputation in adults on dialysis: a cross-sectional observational study. BMC Nephrol 2017;18 10.1186/s12882-017-0711-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinchliffe RJ, Kirk B, Bhattacharjee D, et al. The effect of haemodialysis on transcutaneous oxygen tension in patients with diabetes-a pilot study. Nephrol Dial Transplant 2006;21:1981–3. 10.1093/ndt/gfl241 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2018-000602supp001.htm (1.3MB, htm)