Abstract
Objective
Assessment of total energy expenditure (TEE) is essential for appropriate recommendations regarding dietary intake and physical activity in patients with and without diabetes mellitus (DM). However, few reports have focused on TEE in patients with DM, particularly in Asian countries. Therefore, we evaluated TEE in Japanese patients with DM using the doubly labeled water (DLW) method and physical activity level (PAL).
Research design and methods
In this cross-sectional observational study, we evaluated 52 patients with type 2 DM and 15 patients without DM. Free-living TEE was measured over 12–16 days by the DLW method, and PAL was calculated as TEE divided by the basal metabolic rate (BMR) as assessed by indirect calorimetry. The equivalence margin was defined as 5 kcal/kg/day.
Results
The numbers of patients with DM treated with insulin, oral antidiabetic drugs, and diet were 18 (34.6%), 20 (38.5%), and 14 (26.9%), respectively. The mean±SD level of glycated hemoglobin was 6.9%±0.8% and 5.5%±0.3% in the DM and non-DM group, respectively (p<0.001). The mean body mass index was 23.3±3.0 and 22.7±2.1 kg/m2 in the DM and non-DM group, respectively. The mean TEE per kilogram body weight adjusted for sex and age was 36.5 kcal/kg/day and 37.5 kcal/kg/day in the DM and non-DM group, respectively, with no significant difference (mean difference, −1.0 kcal/kg/day; 95% CI -4.2 to 2.3 kcal/kg/day). The BMR tended to be higher in the DM than in the non-DM group (mean difference, 33 kcal/day; 95% CI, −15 to 80 kcal/day). The mean PAL adjusted for sex and age was 1.71 and 1.81 in the DM and non-DM group, respectively, without a significant difference (mean difference, −0.10; 95% CI −0.21 to 0.01).
Conclusion
TEE was comparable between Japanese patients with and without DM.
Trial registration number
Keywords: energy expenditure, physical activity, type 2 diabetes
Significance of this study.
What is already known about this subject?
The basal metabolic rate may be higher in patients with than without diabetes mellitus.
The impact of diabetes mellitus on total energy expenditure remains unclear.
What are the new findings?
Total energy expenditure seems to be comparable between patients with and without diabetes mellitus.
The basal metabolic rate tends to be higher in patients with than without diabetes mellitus.
The physical activity level may be slightly lower in patients with than without diabetes mellitus.
How might these results change the focus of research or clinical practice?
Total energy expenditure is comparable between patients with and without diabetes mellitus; thus, it is likely to provide similar calories per kilogram in both populations.
Introduction
Diet therapy and physical activity are fundamental for patients with diabetes mellitus (DM).1 Total energy expenditure (TEE) is variable because of many factors. The ideal daily caloric intake is an essential information for appropriate recommendations regarding dietary intake and physical activity both in patients with DM and in healthy people.
TEE comprises the basal metabolic rate (BMR), diet-induced thermogenesis, and activity energy expenditure. The BMR is affected by many factors, including age, sex, height, body weight, fat-free mass (FFM), and hormonal factors such as the thyroid hormone and catecholamine levels. The estimated BMR is calculated with a formula that includes age, sex, height, and body weight.2 3 Diet-induced thermogenesis is the energy expenditure required for digestion, absorption, and conversion of food or nutrients, and consists of about 10% of TEE in the general population. Activity energy expenditure is the most variable component of TEE in each individual and consists of non-exercise energy expenditure and exercise energy expenditure.
TEE can be measured or estimated by various methods. Dietary surveys are often used in the clinical setting based on the assumption that TEE is equal to the total energy intake while the body weight is stable; however, the accuracy of the dietary survey has been reconsidered, especially in obese patients.4 Various wearable devices with triaxial accelerometers can measure physical activity and calculate TEE using the estimated BMR according to age and sex.5 Although these methods may be useful and cost-effective for personal monitoring, the metabolic chamber method and the doubly labeled water (DLW) method are the gold standard measurement techniques for TEE. Direct comparison of TEE between subjects with and without DM using either the metabolic chamber method or DLW method has also been reported. A Danish study using the metabolic chamber method showed higher TEE in subjects with DM than without DM after matching for age, sex, body weight, and activity level.6 7 The metabolic chamber method allows for precise measurement of diet-induced thermogenesis with multiple blood sampling; however, subjects’ physical activity in such studies may differ from their habitual level. The DLW method is very expensive but can measure free-living TEE in many populations according to various attributes such as sex, age, occupation, residential area, and pathologic condition.8 However, few reports to date have shown the impact of DM on TEE, particularly in Asian countries.4 9–11 Therefore, we evaluated TEE in Japanese patients with and without DM using the DLW method. We also calculated the physical activity level (PAL) in the free-living condition.
Methods
Study participants
Patients with and without DM at Shiga University of Medical Science (SUMS) Hospital, Shiga, Japan were invited to participate in this study. Before we checked patients’ eligibility, we screened them by analyzing the distribution of age, body mass index (BMI), sex, glycated hemoglobin (HbA1c) level, and treatment regimen in patients with DM (DM group), and the distribution of age, BMI, and sex in patients without DM (non-DM group), among all patients who visited the outpatient clinic of our endocrinology department from August 2016 to May 2017. The inclusion criteria for the DM group were as follows: outpatients with type 2 DM, age 60–79 years, and BMI of 18 to <35 kg/m2. The inclusion criteria for the non-DM group were as follows: outpatients who were visiting the SUMS Hospital for dyslipidemia, hypertension, and obesity; age 60–79 years; and BMI of 18 to <35 kg/m2. The non-DM group comprised patients without diabetic medications and without past or current DM, or with an HbA1c level of <6.0% within 1 year. The exclusion criteria are presented in online supplementary information 1.
bmjdrc-2019-000648supp001.docx (23.8KB, docx)
The DM group comprised three subgroups of patients: those with DM treated by insulin (DM-Insulin), DM treated by oral antidiabetic drugs (OADs) (DM-OAD), and DM treated by diet (DM-Diet). Each group had a 1:1 sex ratio and an HbA1c distribution similar to that of each treatment population at the SUMS Hospital. The non-DM group also had a 1:1 sex ratio and a BMI distribution similar to that of the DM group. The nature and potential risks of the study were explained to all participants, and written informed consent was obtained. The study is registered at UMIN Clinical Trials Registry (http://www.umin.ac.jp/ctr/index.htm).
Study schedule
This study involved two scheduled visits separated by 12–16 days. At visit 1, patients’ body weight and other baseline information were obtained. The BMR was measured by indirect calorimetry, and the body composition was measured by bioelectrical impedance analysis. Fasting blood and urine samples were collected to evaluate patients’ metabolic conditions. The DLW method was started at visit 1 for measurement of TEE. Physical activity was measured using a triaxial accelerometer between visits 1 and 2. Dietary intake was recorded on 3 of the 12–16 days. At visit 2, blood and urine samples were taken for TEE measurement.
Weight and body composition
The body weight of each participant, without shoes and with light clothing weighing a maximum of 0.1 kg, was recorded using an electronic scale (BF-220; Tanita, Tokyo, Japan). The percentage of body fat was determined by a bioelectrical impedance analyzer (SFB7; ImpediMed, Queensland, Australia).
Laboratory analyses
Blood samples were taken after an overnight fast at both the beginning (visit 1) and end of the 2-week observation period (visit 2). The plasma and urinary glucose levels were measured using the hexokinase glucose 6-phosphate dehydrogenase ultraviolet method. The serum insulin level was measured by a chemiluminescent enzyme immunoassay. The serum triglyceride and total cholesterol levels were determined enzymatically and by the cholesterol dehydrogenase ultraviolet method, respectively. The serum high-density lipoprotein cholesterol level was determined by a direct method. The low-density lipoprotein cholesterol level was calculated using the Friedewald equation (total cholesterol − [high-density lipoprotein cholesterol + triglyceride/5]). The HbA1c level was measured by the latex agglutination method.
Measurement of TEE by the DLW method
TEE was measured by the DLW method (modified two-point approach). An oral dose of 0.1 g 2H2O (2H2O 99.9 atom %; Taiyo Nippon Sanso, Tokyo, Japan) and 2.0 g H218O (H218O 10.0 atom %; Taiyo Nippon Sanso) per kilogram of estimated total body water was given on visit 1. The DLW was sterilized by filtering it through a 0.22 µm filtering system and sealed in a sterile 125 mL bottle (Nalgene, Rochester, New York, USA). Baseline blood (BLB) and urine (BLU) samples were collected before a dose of DLW. An oral dose of DLW was given at around 09:00 (0 hour). After the dose of DLW, the bottle was rinsed twice with 25 mL of tap water that was also consumed. A BLB sample was collected before the dose of DLW. The patients voided urine at 2 hours, and 3-hour and 4-hour postdose blood samples (PD3B and PD4B, respectively) and 3-hour and 4-hour postdose urine samples (PD3U and PD4U, respectively) were collected. The morning after visit 1, a urine sample was collected at home (D1U). On the mornings of days 12–16 (visit 2), samples at the end of the period were collected once for blood (ED1B) and twice for urine (ED1U and ED2U) at a 1-hour interval. The plasma was separated by centrifugation for 15 min at 4°C, then transferred to an airtight screw-capped container and immediately frozen at −30°C. Isotope analyses of the blood and urine samples were performed in duplicate using an isotope-ratio mass spectrometer (Hydra 20-20 Stable Isotope Mass Spectrometer; Sercon, Crewe, UK). The 2H:1H ratio was analyzed by hydrogen gas equilibration using a platinum catalyst. The 18O:16O ratio was analyzed after carbon dioxide equilibration. Isotope analyses were carried out at ESTech Kyoto (Kyoto, Japan). The average SD for the analyses was 1.2‰ for 2H and 0.10‰ for 18O. The urine samples (BLU, PD4U, and ED2U) and the blood samples (BLB, PD4B, and ED1B) were used to calculate TEE, and the average value was used for each patient (a modified two-point approach based on a handbook for the DLW method). For the first five patients, a blood sample at ED1B was not obtained. Therefore, TEE was calculated from the urine samples (BLU, PD4U, and ED1U) and those from day 1 (D1U) and ED2U. The 18O and 2H dilution spaces were determined by the plateau method when using PD4 samples, while the intercept method using D1U and ED1U samples was adopted for the first five participants. Total body water was calculated as the mean of the dilution space estimated by 2H and 18O (No and Nd) calculated from the mean value of the isotope pool size of 2H divided by 1.041 and that of 18O divided by 1.007.12 Nd/No in the present study was 1.025±0.006 (range, 1.015–1.037). The carbon dioxide production rate (rCO2) was calculated according to the following equation: rCO2=0.4554 × total body water × (1.007 × 18O elimination rate − 1.041 × 2H elimination rate).
TEE was calculated according to the following equation:
The food quotient was calculated using a brief self-administered diet history questionnaire.13 If the two calculated TEE values obtained by urine and blood differed by >8%, all samples for the participant were reanalyzed (n=9). Additionally, when the error of the duplicate analyses of each isotopic abundance analysis was large (n=5) or the results were suspicious for other reasons (eg, larger at ED2 than at ED1) (n=7), all of these samples were reanalyzed even if the agreement of the two TEE values was within 8%. For two participants, the difference remained large after the reanalysis; thus, TEE was calculated in the same way as for the first five participants. The average difference in TEE obtained by urine and blood samples was 16±114 kcal/day (0.9%±5.1%). We used two different samples (urine + blood and urine only) to calculate TEE in the present study, and the degree of agreement was examined in patients from whom both samples were obtained. The average difference was 14±41 kcal/day (0.7%±1.9%).
Measurement of BMR
The BMR was measured by indirect calorimetry (Quark RMR; COSMED, Rome, Italy). Before measurement of BMR, the patients were instructed to ingest only water for 12 hours. The test was performed between 08:30 and 10:00. The Quark RMR measures the volume of oxygen consumed and the volume of carbon dioxide expired and calculates the BMR using the modified Weir equation.14 Before measurement, the procedure was explained to the patients, who had comfortably rested on a bed for 30 min. After the machine was calibrated, a canopy was placed to cover the patient’s face and upper body. A steady state was achieved for more than 5 min by the Quark BMR after 10–15 min of breathing while the patient lay awake in the supine position.
Evaluation of PAL
The PAL was calculated using the following equation: PAL=(TEE estimated by DLW method) / (BMR measured by indirect calorimetry).8 In addition, physical activity and sedentary behavior were measured with a triaxial accelerometer (Active Style Pro, HJA-750C; Omron Healthcare, Kyoto, Japan). The patients wore the accelerometer on their waist for 2 weeks except under special circumstances, such as dressing, bathing, and swimming. The device is described in detail elsewhere.15 16 Metabolic equivalents (METs) were calculated by two different equations for ambulatory and non-ambulatory activities.
Step counts were also measured because they have been widely used in many studies and investigations to objectively evaluate physical activity. Moreover, total physical activity of light, moderate, and vigorous intensity was obtained as a sum of the ambulatory time and non-ambulatory time.
Statistical analysis
We calculated the sample size based on the results of a study performed in Scotland,9 which reported the TEE in patients with DM as measured with the DLW method. For application of this calculation to Japanese patients, we selected the data of patients with a BMI of <30 kg/m2, and the mean TEE of these patients was 39.5 kcal/kg/day with an SD of 5.95. We defined 5 kcal/kg/day as the equivalence margin. Twenty-three patients per group were required to detect a difference between the DM and non-DM groups (power=0.8, α=0.05, 1:1 ratio). Because we wanted to include three different therapeutic subgroups, we set the final sample size as 60 for the DM group and 20 for the non-DM group.
Data are expressed as mean±SD or median (IQR) for continuous variables and n (%) for categorical variables. Normal distribution was tested using the Anderson-Darling test. TEE, BMR, and PAL were compared using analysis of covariance between the DM and non-DM groups and are expressed as mean (95% CI). Subgroup analysis was performed in each of the three DM treatment groups: DM-Insulin, DM-OAD, and DM-Diet. Multivariable models adjusted for sex, age, FFM, fat mass (FM), and average METs were used to control confounders. A two-tailed p value of <0.05 was considered statistically significant for superiority. For the equivalence, 5 kcal/kg/day was set as the equivalence margin according to the mean difference between the DM and non-DM groups with 95% CI.17 Statistical analyses were performed with SAS V.9.4.
Results
Study participants
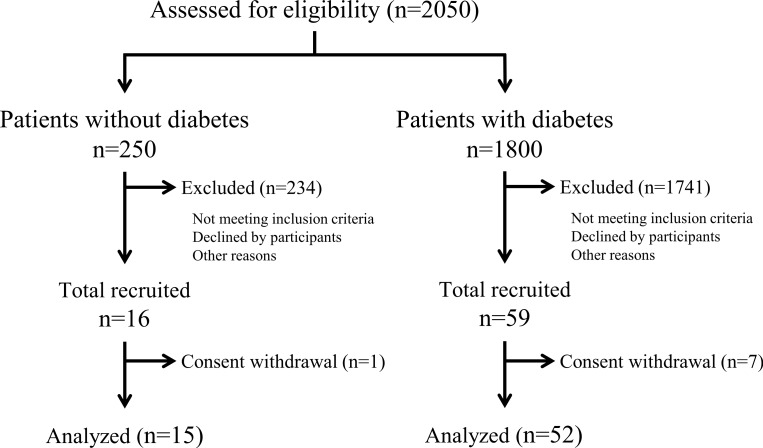
The flow diagram of the study participants is shown in figure 1. Before enrollment, we screened 2050 outpatients to evaluate the prevalence of DM and its treatment, as well as the distribution of age, sex, BMI, and HbA1c. Of these 2050 patients, 16 and 59 patients were enrolled in the non-DM and DM groups according to the inclusion criteria explained in the Methods section. After exclusion of 1 patient from the non-DM group and 7 patients from the DM group who withdrew their consent, 15 and 52 patients were included for analysis in the non-DM and DM groups, respectively.
Figure 1.
Study flow diagram.
The characteristics of the study participants are shown in table 1. Although all patients were 60–79 years old, the mean age was significantly higher in the DM than in the non-DM group. BMI, FM, and FFM were not different between the two groups. The fasting plasma glucose, HbA1c, and glycoalbumin levels were higher in the DM than in the non-DM group. The total cholesterol and low-density lipoprotein cholesterol levels were lower in the DM than in the non-DM group. Among all patients with DM, the percentages of patients in the DM-Insulin, DM-OAD, and DM-Diet subgroups were 34.6%, 38.5%, and 26.9%, respectively.
Table 1.
Characteristics of study participants
| Non-DM (n=15) | DM (n=52) | P value | |
| Male sex | 6 (40.0) | 28 (53.8) | 0.392 |
| Age, years | 67.1±4.7 | 70.2±5.1 | 0.039 |
| Height, cm | 158.9±9.8 | 160.2±8.5 | 0.614 |
| Body weight, kg | 57.5±8.1 | 59.7±8.8 | 0.368 |
| Body mass index, kg/m2 | 22.7±2.1 | 23.3±3.0 | 0.490 |
| Waist circumference, cm | 86.7±7.2 | 84.6±8.4 | 0.379 |
| Fat-free mass, kg | 42.2±7.9 | 44.8±7.2 | 0.236 |
| Fat mass, kg | 15.2±4.3 | 15.0±4.9 | 0.836 |
| HbA1c, % | 5.5±0.3 | 6.9±0.8 | <0.001 |
| Glycoalbumin, % | 13.9±0.9 | 18.6±3.6 | <0.001 |
| 1,5-AG, µg/mL | 19.2±6.0 | 12.3±7.1 | 0.001 |
| Fasting plasma glucose, mg/dL | 94.6±5.9 | 127.2±31.0 | <0.001 |
| Total cholesterol, mg/dL | 215.1±27.7 | 191.3±30.2 | 0.008 |
| HDL cholesterol, mg/dL | 66.9±19.4 | 64.2±15.4 | 0.566 |
| LDL cholesterol, mg/dL | 122.1±19.8 | 106.0±24.4 | 0.023 |
| Triglycerides, mg/dL | 119.0 (59.0–198.0) | 87.5 (71.0–133.5) | 0.685 |
| Blood urea nitrogen, mg/dL | 15.4±4.4 | 16.2±3.4 | 0.461 |
| Creatinine, mg/dL | 0.75±0.11 | 0.77±0.18 | 0.661 |
| Average METs | 1.42±0.14 | 1.39±0.15 | 0.490 |
| Anti-DM medication | |||
| Insulin | – | 18 (34.6) | – |
| Oral antidiabetic drugs | – | 20 (38.5) | – |
| Diet and exercise | – | 14 (26.9) | – |
Data are presented as n (%), mean±SD, or median (IQR).
1,5-AG, 1,5-anhydroglucitol;DM, diabetes mellitus;HDL, high-density lipoprotein;HbA1c, glycated hemoglobin;LDL, low-density lipoprotein;METs, metabolic equivalents.
Energy expenditure
Unadjusted TEE/kg/day was 37.8 kcal/kg/day (95% CI 34.9 to 40.7) in the non-DM group and 36.4 kcal/kg/day (95% CI 34.8 to 37.9) in the DM group as assessed by the DLW method (table 2). After adjustment for sex, age, FFM, and FM, TEE/kg/day was 37.5 kcal/kg/day (95% CI 35.2 to 39.9) in the non-DM group and 36.5 kcal/kg/day (95% CI 35.2 to 37.7) in the DM group, showing that the DM group had a slightly lower mean value (mean difference, −1.1; 95% CI −3.8 to 1.6; p=0.425). After further adjustment with the average METs to overcome individual PALs, the difference in TEE/kg/day between the two groups showed no significant difference (mean difference, −1.1; 95% CI −3.9 to 1.6; p=0.415). The equivalence margin was set at 5 kcal/kg/day to indicate clinical significance. Therefore, we considered that energy expenditure between the two groups was comparable.
Table 2.
Total energy expenditure, basal metabolic rate, and physical activity level in the non-DM and DM groups
| Non-DM (n=15) Mean (95% CI) |
DM (n=52) Mean (95% CI) |
Mean difference (95% CI) |
P value | |
| Total energy expenditure (kcal/kg/day) | ||||
| Unadjusted | 37.8 (34.9 to 40.7) | 36.4 (34.8 to 37.9) | −1.4 (−4.7 to 1.8) | 0.386 |
| Model 1 (adjusted for sex and age) | 37.5 (34.6 to 40.3) | 36.5 (35.0 to 38.0) | −1.0 (−4.2 to 2.3) | 0.546 |
| Model 2 (model 1+ fat-free mass) | 37.2 (34.3 to 40.0) | 36.6 (35.1 to 38.1) | −0.6 (−3.8 to 2.7) | 0.717 |
| Model 3 (model 2 + fat mass) | 37.5 (35.2 to 39.9) | 36.5 (35.2 to 37.7) | −1.1 (−3.8 to 1.6) | 0.425 |
| Model 4 (model 3 + METs) | 37.6 (35.2 to 40.0) | 36.5 (35.2 to 37.7) | −1.1 (−3.9 to 1.6) | 0.415 |
| Total energy expenditure (kcal/kg IBW/day) | ||||
| Unadjusted | 38.9 (36.2 to 41.6) | 38.1 (36.7 to 39.5) | −0.8 (−3.8 to 2.3) | 0.606 |
| Model 1 (adjusted for sex and age) | 39.0 (36.3 to 41.7) | 38.1 (36.6 to 39.5) | −0.9 (−4.1 to 2.2) | 0.56 |
| Model 2 (model 1+ fat-free mass) | 39.4 (36.7 to 42.0) | 38.0 (36.6 to 39.4) | −1.4 (−4.5 to 1.7) | 0.717 |
| Model 3 (model 2 + fat mass) | 39.4 (36.6 to 42.1) | 38.0 (36.6 to 39.4) | −1.4 (−4.5 to 1.7) | 0.371 |
| Model 4 (model 3 + METs) | 39.3 (36.6 to 42.1) | 38.0 (36.5 to 39.4) | −1.4 (−4.5 to 1.8) | 0.396 |
| Total energy expenditure (kcal/day) | ||||
| Unadjusted | 2168 (1971 to 2366) | 2159 (2053 to 2264) | −10 (−234 to 214) | 0.930 |
| Model 1 (adjusted for sex and age) | 2181 (2030 to 2333) | 2155 (2075 to 2234) | −27 (−200 to 146) | 0.758 |
| Model 2 (model 1+ fat-free mass) | 2220 (2087 to 2353) | 2144 (2074 to 2213) | −76 (−228 to 76) | 0.323 |
| Model 3 (model 2 + fat mass) | 2221 (2087 to 2355) | 2143 (2073 to 2214) | −77 (−231 to 77) | 0.319 |
| Model 4 (model 3 + METs) | 2222 (2086 to 2358) | 2143 (2072 to 2214) | −79 (−235 to 78) | 0.319 |
| Basal metabolic rate (kcal/day) | ||||
| Unadjusted | 1194 (1111 to 1276) | 1260 (1215 to 1304) | 66 (−28 to 160) | 0.165 |
| Model 1 (adjusted for sex and age) | 1202 (1143 to 1260) | 1257 (1226 to 1288) | 55 (−12 to 122) | 0.104 |
| Model 2 (model 1+ fat-free mass) | 1222 (1179 to 1265) | 1251 (1229 to 1274) | 29 (−20 to 78) | 0.245 |
| Model 3 (model 2 + fat mass) | 1220 (1178 to 1261) | 1252 (1230 to 1274) | 33 (−15 to 80) | 0.180 |
| Physical activity level | ||||
| Unadjusted | 1.81 (1.72 to 1.90) | 1.71 (1.66 to 1.76) | −0.10 (−0.21 to 0.00) | 0.059 |
| Model 1 (adjusted for sex and age) | 1.81 (1.72 to 1.90) | 1.71 (1.66 to 1.76) | −0.10 (−0.21 to 0.01) | 0.069 |
| Model 2 (model 1+ METs) | 1.81 (1.72 to 1.91) | 1.71 (1.66 to 1.76) | −0.10 (−0.21 to 0.01) | 0.068 |
DM, diabetes mellitus;METs, metabolic equivalents;IBW, ideal body weight.
Unadjusted TEE was 2168 kcal/day (95% CI 1971 to 2366) in the non-DM group and 2159 kcal/day (95% CI 2053 to 2264) in the DM group as assessed by the DLW method (table 2, online supplementary figure 1). After adjustment for sex, age, FFM, and FM, TEE was 2221 kcal/day (95% CI 2087 to 2355) in the non-DM group and 2143 kcal/day (95% CI 2073 to 2214) in the DM group, showing that the DM group had a slightly lower mean value (mean difference, −77; 95% CI −231 to 77; p=0.319). After further adjustment with the average METs, the difference in TEE between the two groups remained statistically non-significant (mean difference, −79; 95% CI −235 to 78; p=0.319).
bmjdrc-2019-000648supp002.pdf (180.5KB, pdf)
BMR and PAL
The unadjusted BMR tended to be higher in the DM than in the non-DM group (mean difference, 66; 95% CI −28 to 160; p=0.165). After adjustment for sex, age, and FFM, the DM group had a slightly higher mean value (mean difference, 29; 95% CI −20 to 78; p=0.245). Conversely, PAL adjusted for sex and age tended to be lower in the DM than in the non-DM group (mean difference, −0.10; 95% CI −0.21 to 0.01; p=0.069).
Subgroup analysis by DM treatment
A similar analysis was performed in each DM treatment subgroup (table 3). TEE/kg/day adjusted by age, sex, FFM, FM, and METs was not significantly different among the DM-Insulin, DM-OAD, and DM-Diet subgroups. In contrast, the BMR tended to be higher in the DM-Insulin than DM-OAD and DM-Diet subgroups, although there was no significant difference (table 3).
Table 3.
Total energy expenditure, basal metabolic rate, and physical activity level in the non-DM group and three DM subgroups
| Non-DM (n=15) | DM-Insulin (n=18) | DM-OAD (n=20) | DM-Diet (n=14) | P value | |
| Total energy expenditure (kcal/day) | |||||
| Unadjusted | 2168 (1969 to 2368) | 2113 (1931 to 2295) | 2215 (2042 to 2387) | 2137 (1931 to 2343) | 0.868 |
| Model 1 (adjusted for sex and age) | 2185 (2032 to 2338) | 2108 (1971 to 2245) | 2200 (2070 to 2329) | 2147 (1988 to 2305) | 0.794 |
| Model 2 (model 1+ fat-free mass) | 2220 (2086 to 2355) | 2111 (1992 to 2231) | 2164 (2050 to 2279) | 2155 (2017 to 2294) | 0.701 |
| Model 3 (model 2 + fat mass) | 2224 (2088 to 2360) | 2099 (1971 to 2227) | 2175 (2054 to 2297) | 2152 (2012 to 2292) | 0.636 |
| Model 4 (model 3 + METs) | 2223 (2086 to 2361) | 2098 (1968 to 2228) | 2177 (2045 to 2309) | 2151 (2005 to 2297) | 0.642 |
| Basal metabolic rate (kcal/day) | |||||
| Unadjusted | 1194 (1111 to 1277) | 1256 (1180 to 1332) | 1289 (1218 to 1361) | 1221 (1135 to 1307) | 0.336 |
| Model 1 (adjusted for sex and age) | 1205 (1147 to 1264) | 1252 (1199 to 1304) | 1285 (1235 to 1334) | 1222 (1161 to 1282) | 0.165 |
| Model 2 (model 1+ fat-free mass) | 1224 (1181 to 1267) | 1253 (1215 to 1292) | 1266 (1229 to 1302) | 1226 (1182 to 1271) | 0.365 |
| Model 3 (model 2 + fat mass) | 1220 (1178 to 1262) | 1268 (1228 to 1307) | 1253 (1216 to 1291) | 1230 (1187 to 1273) | 0.312 |
| Physical activity level | |||||
| Unadjusted | 1.81 (1.72 to 1.91) | 1.68 (1.60 to 1.77) | 1.72 (1.64 to 1.80) | 1.74 (1.65 to 1.84) | 0.211 |
| Model 1 (adjusted for sex and age) | 1.81 (1.71 to 1.90) | 1.68 (1.60 to 1.77) | 1.71 (1.63 to 1.79) | 1.75 (1.65 to 1.85) | 0.217 |
| Model 2 (model 1+ METs) | 1.81 (1.72 to 1.91) | 1.68 (1.60 to 1.77) | 1.71 (1.62 to 1.79) | 1.76 (1.66 to 1.86) | 0.199 |
Data are presented as mean (95% CI).
DM, diabetes mellitus;OAD, oral antidiabetic drug;METs, metabolic equivalents.
Discussion
The current study revealed three important findings. First, TEE was comparable between patients with and without DM. Second, the mean TEE was slightly lower and the mean BMR was slightly higher in patients with than without DM, although the difference was not statistically significant. Third, the PAL tended to be lower in patients with than without DM. These data suggest that recommendations regarding dietary intake in patients with DM can be determined using an equation similar to that used in patients without DM.
TEE was comparable between patients with and without DM (table 2). This is inconsistent with a previous study of Pima Indians,10 which showed no significant difference in TEE as measured by the metabolic chamber method between 49 patients with DM and 102 participants without DM; however, the patients with DM had significantly higher TEE after adjustment for age, sex, FFM, and FM. A Danish study11 also showed no significant difference in unadjusted TEE as measured by the metabolic chamber method between 31 patients with DM and 61 patients without DM, but TEE was significantly higher in patients with DM after adjustment for sex, age, FFM, FM, and physical activity (mean difference, 164 kcal/day; SD, 31 kcal/day; p<0.01). In a US study using the DLW method, TEE was not significantly different between 9 obese subjects without DM and 12 obese subjects with DM (p=0.496).4 Direct comparison by the DLW method was recently reported in 10 Japanese patients without DM and 12 with DM, showing no significant difference between the two groups.18Online supplementary table 1 summarizes the TEE values measured either by the metabolic chamber method or DLW method in patients with DM. The reason for the inconsistency is uncertain, but we speculate that it occurred partly because of the difference between the metabolic chamber and DLW methods. The metabolic chamber method provides TEE in a confined space; instead, the DLW method provides more information regarding physical activity under free-living conditions. Each study involves different ethnicities, BMIs, and models for adjustment. Because TEE may vary by individual physical activity and our results may not be sufficient to make conclusions between two groups, we tested TEE using a METs-adjusted model in which METs were directly estimated by an accelerometer. As a result, METs-adjusted TEE was also comparable between patients with and without DM (table 2, model 4).
The BMR was slightly higher in patients with than without DM. This is consistent with previous studies that showed a higher BMR in subjects with DM.11 19 20 In one of these studies, the BMR was significantly higher among participants with an abnormal HbA1c level.20 In contrast, the BMR adjusted for age, sex, HbA1c level, and fasting glucose level tended to be higher in patients with than without DM in the present study (mean difference, 80 kcal/day; 95% CI −5 to 166; p=0.065) (online supplementary table 2). Interestingly, a recent study showed that lower BMRs were found in subjects with a family history of DM who developed DM later in life.21 In the above-mentioned study of Pima Indians, an increased BMR was observed in subjects with DM and in those with impaired glucose intolerance,22 suggesting that a threshold of an increase in the BMR exists between individuals with normal and impaired glucose tolerance and that hyperglycemia is not a sole cause of an increased BMR. Another study showed that insulin treatment was negatively correlated with the BMR in Japanese patients with type 2 DM. In the same study, endogenous insulin secretion determined by the glucagon test was also an independent factor for the BMR.23 Our study produced a similar finding in that the DM-Insulin and DM-OAD subgroups had a slightly higher BMR than the DM-Diet subgroup and non-DM group, although the difference was not statistically significant (table 3).
The PAL tended to be lower in patients with than without DM (1.71 vs 1.81, respectively) (table 2). Previous reports using the DLW method showed similar PALs in healthy older Caucasians (male, 1.65; female, 1.51) and African–Americans (male, 1.62; female, 1.41).24 A recent study of 99 older patients also showed a similar PAL level (1.68).25 In the present study, the mean difference was 0.1, which equates to a maximum of about 120–150 kcal/day in a normal adult. In addition, physical activity varies among study participants; thus, caution is needed when generalizing this difference to other populations because of selection bias. However, this difference may be ignorable.
This study has three main strengths. First, a comprehensive analysis was employed to measure energy expenditure between patients with and without DM, including the DLW method, indirect calorimetry, bioelectrical impedance analysis, and triaxial accelerometry. Second, equivalence was determined by the threshold of 5 kcal/kg/day with the 95% CI of the mean difference. Third, our patients with DM consisted of DM-Insulin, DM-OAD and DM-Diet subgroups (table 3). Among these subgroups, TEE was comparable, but the BMR tended to be higher in the DM-Insulin subgroup than in the DM-OAD and DM-Diet subgroups, although there was no significant difference.
This study also has some limitations. First, the number of participants was relatively small due to the cost of the DLW method. Further study is necessary to determine the statistical difference between the two groups with enough statistical power. Although we carefully selected participants to mimic the distribution of our entire outpatient population, selection bias is a major concern in this study. We selected patients aged 60–79 years and avoided those with an extremely high or low BMI. The HbA1c distribution was carefully matched to each treatment cohort, and the BMI distribution was matched between the DM and non-DM groups. Second, we observed a significant difference in age between the DM and non-DM groups (70.2 vs 67.1 years, respectively). Thus, we statistically adjusted patients’ age throughout the study when we compared the two groups. Third, the body composition was determined by the bioelectrical impedance method instead of a dual-energy X-ray absorptiometry scan. The adjusted TEE by FFM measured with the bioelectrical impedance method may be inaccurate. Fourth, the patients in the non-DM group were outpatients who did not have DM but who regularly visited the same hospital for dyslipidemia, hypertension, and other conditions. These patients might have had a different lifestyle than healthy volunteers. This point is both a limitation and strength of this study. As another potential bias, the study participants were recruited at a single hospital. Therefore, any generalizations must be made with caution.
In conclusion, TEE was comparable between Japanese patients with and without DM, although the BMR and PAL were slightly different between the two groups. Thus, it may be reasonable to apply the same dietary recommendations of the general population to patients with DM. Further study is necessary to confirm these findings for application to clinical practice.
Acknowledgments
The authors thank the participants and the CLEVER-DM Study investigators for their involvement in this study. CLEVER-DM: Clinical Evaluation of Energy Requirements in Patients with Diabetes Melitus.
Footnotes
Presented at: Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, Florida, June 22–26, 2018.
Collaborators: A complete list of the members of the CLEVER-DM Study is provided in online supplementary information 2. The authors also thank Keiko Kosaka, Eriko Naiki, Chikako Ikeuchi, Takako Fujii, Noriko Yamamoto, Kaori Nagano, Mayumi Yotsushika, Mai Nakaizumi, Akiko Sakata, and Hiroko Kogure for their expert technical assistance. Finally, the authors thank Angela Morben, DVM, ELS, from Edanz Group (www.edanzediting.com/ac), for editing the English text of a draft of this manuscript.
Contributors: KM and KK contributed to the planning and conduct of the study, and drafted and revised the manuscript. ST contributed to the planning of the study and revised the manuscript. AO, KN, MK, KF, and IM contributed to the conduct of the study. YN, SN, YY, SU, MS, FK, HM, and NE contributed to the planning of the study and revised the manuscript. NE and SS contributed to the planning of the study. All authors read and approved the final manuscript.
Funding: This study was funded by AMED under Grant Numbers JP17ek0210045 and JP18ek0210112. This study was also supported in part by the Japan Foundation for Applied Enzymology (to KK).
Competing interests: ST received consigned research funds from Omron Healthcare. The remaining authors declare no competing interests.
Patient consent for publication: Not required.
Ethics approval: The study was performed in accordance with the principles contained within the Declaration of Helsinki. The protocol was approved by the ethics committee of Keio University (Protocol No 2015–03), National Institutes of Biomedical Innovation, Health and Nutrition (Protocol No 29), and Shiga University of Medical Science (Protocol No 28–062).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available upon reasonable request.
References
- 1.Association AD 4. lifestyle management. Diabetes Care 2018;41(Suppl 1):S38–S50. [DOI] [PubMed] [Google Scholar]
- 2.Roza AM, Shizgal HM. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am J Clin Nutr 1984;40:168–82. 10.1093/ajcn/40.1.168 [DOI] [PubMed] [Google Scholar]
- 3.Ganpule AA, Tanaka S, Ishikawa-Takata K, et al. . Interindividual variability in sleeping metabolic rate in Japanese subjects. Eur J Clin Nutr 2007;61:1256–61. 10.1038/sj.ejcn.1602645 [DOI] [PubMed] [Google Scholar]
- 4.Sallé A, Ryan M, Ritz P. Underreporting of food intake in obese diabetic and nondiabetic patients. Diabetes Care 2006;29:2726–7. 10.2337/dc06-1582 [DOI] [PubMed] [Google Scholar]
- 5.Dowd KP, Szeklicki R, Minetto MA, et al. . A systematic literature review of reviews on techniques for physical activity measurement in adults: a DEDIPAC study. Int J Behav Nutr Phys Act 2018;15 10.1186/s12966-017-0636-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravussin E, Harper IT, Rising R, et al. . Energy expenditure by doubly labeled water: validation in lean and obese subjects. Am J Physiol 1991;261:E402–E409. 10.1152/ajpendo.1991.261.3.E402 [DOI] [PubMed] [Google Scholar]
- 7.Gibney ER, Murgatroyd P, Wright A, et al. . Measurement of total energy expenditure in grossly obese women: comparison of the bicarbonate-urea method with whole-body calorimetry and free-living doubly labelled water. Int J Obes Relat Metab Disord 2003;27:641–7. 10.1038/sj.ijo.0802302 [DOI] [PubMed] [Google Scholar]
- 8.Pannemans DL, Westerterp KR. Energy expenditure, physical activity and basal metabolic rate of elderly subjects. Br J Nutr 1995;73:571–81. 10.1079/BJN19950059 [DOI] [PubMed] [Google Scholar]
- 9.Chong PK, Jung RT, Rennie MJ, et al. . Energy expenditure in lean and obese diabetic patients using the doubly labelled water method. Diabet Med 1993;10:729–35. 10.1111/j.1464-5491.1993.tb00156.x [DOI] [PubMed] [Google Scholar]
- 10.Fontvieille AM, Lillioja S, Ferraro RT, et al. . Twenty-four-hour energy expenditure in Pima Indians with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1992;35:753–9. [DOI] [PubMed] [Google Scholar]
- 11.Bitz C, Toubro S, Larsen TM, et al. . Increased 24-h energy expenditure in type 2 diabetes. Diabetes Care 2004;27:2416–21. 10.2337/diacare.27.10.2416 [DOI] [PubMed] [Google Scholar]
- 12.Racette SB, Schoeller DA, Luke AH, et al. . Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol 1994;267:E585–E590. 10.1152/ajpendo.1994.267.4.E585 [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi S, Murakami K, Sasaki S, et al. . Comparison of relative validity of Food Group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 D dietary records in Japanese adults. Public Health Nutr 2011;14:1200–11. 10.1017/S1368980011000504 [DOI] [PubMed] [Google Scholar]
- 14.Weir Jbdeb. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9. 10.1113/jphysiol.1949.sp004363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oshima Y, Kawaguchi K, Tanaka S, et al. . Classifying household and locomotive activities using a triaxial accelerometer. Gait Posture 2010;31:370–4. 10.1016/j.gaitpost.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 16.Ohkawara K, Oshima Y, Hikihara Y, et al. . Real-time estimation of daily physical activity intensity by a triaxial accelerometer and a gravity-removal classification algorithm. Br J Nutr 2011;105:1681–91. 10.1017/S0007114510005441 [DOI] [PubMed] [Google Scholar]
- 17.Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med 2011;26:192–6. 10.1007/s11606-010-1513-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshimura E, Ohkawara K, Ishikawa-Takata K, et al. . Assessment of energy expenditure using doubly labeled water, physical activity by accelerometer and reported dietary intake in Japanese men with type 2 diabetes: a preliminary study. J Diabetes Investig 2018;37 10.1111/jdi.12921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyake R, Ohkawara K, Ishikawa-Takata K, et al. . Obese Japanese adults with type 2 diabetes have higher basal metabolic rates than non-diabetic adults. J Nutr Sci Vitaminol 2011;57:348–54. 10.3177/jnsv.57.348 [DOI] [PubMed] [Google Scholar]
- 20.Alawad AO, Merghani TH, Ballal MA. Resting metabolic rate in obese diabetic and obese non-diabetic subjects and its relation to glycaemic control. BMC Res Notes 2013;6 10.1186/1756-0500-6-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyenwe EA, Ogwo CC, Owei I, et al. . Parental history of type 2 diabetes is associated with lower resting energy expenditure in normoglycemic subjects. BMJ Open Diabetes Res Care 2018;6:e000511 10.1136/bmjdrc-2018-000511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weyer C, Bogardus C, Pratley RE. Metabolic factors contributing to increased resting metabolic rate and decreased insulin-induced thermogenesis during the development of type 2 diabetes. Diabetes 1999;48:1607–14. 10.2337/diabetes.48.8.1607 [DOI] [PubMed] [Google Scholar]
- 23.Ikeda K, Fujimoto S, Goto M, et al. . Impact of endogenous and exogenous insulin on basal energy expenditure in patients with type 2 diabetes under standard treatment. Am J Clin Nutr 2011;94:1513–8. 10.3945/ajcn.111.017889 [DOI] [PubMed] [Google Scholar]
- 24.Carpenter WH, Fonong T, Toth MJ, et al. . Total daily energy expenditure in free-living older African-Americans and Caucasians. Am J Physiol 1998;274:E96–E101. 10.1152/ajpendo.1998.274.1.E96 [DOI] [PubMed] [Google Scholar]
- 25.Starling RD, Toth MJ, Carpenter WH, et al. . Energy requirements and physical activity in free-living older women and men: a doubly labeled water study. J Appl Physiol 1998;85:1063–9. 10.1152/jappl.1998.85.3.1063 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2019-000648supp001.docx (23.8KB, docx)
bmjdrc-2019-000648supp002.pdf (180.5KB, pdf)