Abstract
Antibodies to the E6 and E7 oncoproteins of high-risk human papillomavirus (HPV) types are strongly associated with HPV-driven cancer, while antibodies against the capsid protein L1 are considered cumulative exposure markers. To test the hypothesis that L1 antibody levels are stable over time, whereas E6 and E7 levels undergo decay after cervical cancer (CxCa) treatment, we performed multiplex serology for HPV16 and 18 antigens E6, E7 and L1 in a post-treatment study of 184 patients with invasive CxCa that were characterized with a median follow-up time of 725 days, and 2–12 sera per patient. Antibody titers significantly decreased within the first six months for HPV16 E6 and E7 but not L1, and stabilized for the following 12 months on a high level, with few patients showing seroreversion. Of 67 patients seropositive for HPV16 E6 at diagnosis, 28 (41.8%) showed a decrease in antibody titers of at least 50% within the first 18 months. Similarly, of 50 HPV16 E7 seropositives, 33 (66.0%) showed decreasing antibody levels, whereas antibody decay was less frequent for HPV16 L1 (12 of 47, 25.5%). Using a power-law mathematical model to characterize antibody decay kinetics, the mean (±s.e.) durations to a 50% reduction in antibody titers within individual patients were estimated to be 56.9 (±26.1) and 56.3 (±19.0) days for HPV16 E6 and E7, respectively. In summary, HPV16 E6 and E7 antibodies undergo a slow but significant decrease in antibody titers within the first 6–18 months following CxCa treatment. However, larger studies are needed to confirm the utility of serology for prediction of disease progression and time to relapse based on antibody decay kinetics.
This article is part of the theme issue ‘Silent cancer agents: multi-disciplinary modelling of human DNA oncoviruses'.
Keywords: human papillomavirus, serology, cervical cancer, antibody, kinetics, decay, mathematical modelling
1. Introduction
Specific human papillomavirus (HPV) types are classified as Class I human carcinogens and are associated with carcinoma of the cervix, vulva, vagina, penis, anus, and the head and neck, particularly the oropharynx [1]. In all of these cancers, HPV16 is the predominant type, causing about half of all cervical cancers (CxCa) and more than 80% of all other HPV-attributable cancer cases [2]. Persistent HPV infection is considered a requirement for the development of CxCa (i.e. all cases are by definition attributed to HPV), and approximately 15 high-risk HPV types involved in the development of CxCa have been identified [3]. Following HPV16, HPV18 is the second most frequent type in CxCa with an attributable fraction of approximately 15%.
After natural infection of mucosal epithelia by HPV, several early (E1, E2, E4, E6, E7) and late (L1) viral proteins are expressed. Antibodies against the major capsid protein L1 can be detected upon infection (although not every infected individual seroconverts) and persist for many years; this is thought to be based on bone-marrow B memory cells that are independent of antigenic challenge [4]. Thus, these antibodies are considered markers of past or present infection, i.e. cumulative exposure markers [5]. By contrast, antibodies to the oncoproteins E6 and E7 are usually not elicited upon mere infection; they result from oncoprotein overexpression in HPV-driven invasive carcinomas (and potentially their precursor lesions), and are thus strongly associated with invasive cancer. This immune response is presumably based on long-lived plasma cells that depend on antigenic challenge [4]. Seroconversion for E6 and E7 antibodies is a late event in CxCa, i.e. the antibodies can be detected at the time of cancer diagnosis [6,7] but not years before [8], or in women with high-grade CxCa precursor lesions, i.e. cervical intraepithelial neoplasia grade 2 or higher (CIN2+) [9,10]. Thus, we hypothesized that upon removal of tumours, and thus the antigenic source for challenge of plasma cells, E6 and E7 antibodies should undergo a significant decay, while L1 antibodies should be largely unaffected by treatment.
However, the available data to support or refute this hypothesis are scarce, both in CxCa and in oropharyngeal cancer (OPC), an HPV-driven cancer that has recently been subject to extensive research [11]. This is partially based on the finding that in contrast to CxCa, antibodies to HPV16 E6 and E7 are prospective markers for OPC that can be detected 10+ years prior to diagnosis [12,13]. In OPC, antibody presence or their levels at the time of diagnosis [14–19] as well as antibody kinetics during patient follow-up after primary tumour treatment [15,16,20] have been investigated as markers for prediction of treatment success (i.e. complete tumour removal) and the likelihood of disease recurrence. However, these studies were mostly small with few recurrence events, and most studies that have investigated post-treatment antibody kinetics suffered from short follow-up and/or too few serial blood samples taken after diagnosis. Taken together, these studies were largely inconclusive [21]. Moreover, it is difficult to interpret these data given the lack of knowledge about the natural history of oral HPV infection [22].
Decay dynamics of oncoprotein antibody titers have been found to be indicative of cancer relapse for other oncoviruses, such as Merkel cell polyomavirus (MCV) causing Merkel cell carcinoma (MCC) [23]. In comparison to the data-driven classification of patients performed so far, mathematical models that use mechanistic frameworks to describe antibody dynamics provide a more detailed quantification and prediction of the kinetics of the response for individual patients. Although being used frequently to predict the longevity of vaccine-induced antibodies, such as against HPV [24] or other viruses and pathogens [25,26], these models have neither been used to characterize antibody kinetics after tumour removal nor tested for the possibility to predict tumour relapse.
Most studies that have investigated HPV antibodies following CxCa diagnosis were published in the 1990s and included only single HPV proteins or peptide antigens derived thereof with none of these studies investigating the exact timing of the antibody dynamics [10,27–35]. Thus, we conducted a systematic investigation of HPV seroprevalence at diagnosis, and quantification of post-treatment HPV antibody kinetics, using multiplex serology to detect antibodies against HPV16 and 18 E1, E2, E4, E6, E7 and L1, in a clinically well characterized, large cohort of incident CxCa patients with long-term follow-up and multiple sampling time points. Using mathematical models to quantify antibody decay characteristics for HPV16 E6 and E7, we then also tested the ability to predict the possibility of tumour relapse based on the obtained kinetics.
2. Methods
(a). Study population
The study investigated HPV antibody kinetics in 184 cervical cancer (CxCa) patients treated by surgery at Jena University Hospital between 1995 and 2015. The clinical and pathological parameters of the patients are shown in table 1. Serum samples were taken at the time of primary surgery and at individual post-operative intervals to determine routine clinical parameters. Serum samples were pseudonymized and stored at −30°C in the biobank of the Department of Gynaecology of Jena University Hospital. Informed consent was provided by all patients. Approval for this study was given by the Ethics Committee of the Friedrich-Schiller University Jena (reference nos. 0077-7/98).
Table 1.
Baseline clinical characteristics of the study population (N = 184). SD, standard deviation; SCC, squamous cell carcinoma.
| n (%) | |
|---|---|
| age at diagnosis (years) | |
| mean (s.d.) | 48 (13) |
| median (range) | 46 (17–78) |
| serum samples per woman (n) | |
| mean (s.d.) | 4 (2.5) |
| median (range) | 3 (2–12) |
| follow-up time (days) | |
| mean (s.d.) | 907 (864) |
| median (range) | 725 (18–6637) |
| tumour HPV DNAa | |
| HPV16 | 69 (57.5) |
| HPV18 | 27 (22.5) |
| other HPV types | 24 (20.0) |
| tumour stageb | |
| T1 | 87 (47.3) |
| T2 | 60 (32.6) |
| T3 | 15 (8.2) |
| T4 | 12 (6.5) |
| nodal stageb | |
| N0 | 108 (58.7) |
| N1 | 52 (28.3) |
| resection marginb | |
| R0 | 98 (53.3) |
| R1 | 21 (11.4) |
| R2 | 2 (1.1) |
| histologyb | |
| SCC | 138 (75.0) |
| adenocarcinoma | 31 (16.8) |
| follow-up statusb | |
| no relapse/residual disease | 110 (59.8) |
| relapse/residual disease | 63 (34.2) |
a120 HPV genotyping results for 114 women available (due to multiple HPV DNA positivity in four tumours).
bDoes not add up to 100% due to missing data.
(b). Human papillomavirus genotyping
HPV genotyping of tumour tissue specimens was performed at baseline using the GP5+/6+ EIA approach as described [36], detecting 14 high-risk and six low-risk HPV types of the α genus.
(c). Human papillomavirus serology
Serum samples of the study participants were analysed by multiplex HPV serology as previously described [37,38]. Briefly, HPV antigens were expressed as glutathione S-transferase (GST) fusion proteins in Escherichia coli and in situ affinity purified on glutathione-derivatized fluorescent polystyrene beads (Luminex Corp., Austin, TX, US). Bead sets carrying different antigens were mixed and incubated with pre-diluted human serum (1 : 100 in blocking buffer). Primary antibodies bound to the antigens were detected with biotinylated secondary antibody and streptavidin-R-phycoerythrin as fluorescent reporter dye. Antibody quantity was determined as the median fluorescence intensity (MFI) of at least 100 beads per set. Antibodies against the E1, E2, E4, E6, E7 and L1 proteins of the two most frequent HPV types in CxCa (HPV 16 and 18) as well as the E6, E7 and L1 proteins of the less frequent high-risk HPV types 31, 33, 35, 45, 52 and 58 were detected. Where possible, cut-off values for seropositivity for each antigen were defined based on HPV DNA tumour status using receiver operating characteristic (ROC) analysis maximizing sensitivity and specificity for HPV16 E1 (313 MFI), E2 (235 MFI), E4 (451 MFI), E6 (355 MFI), E7 (249 MFI) and HPV18 E6 (107 MFI) and E7 (98 MFI). Where HPV seroprevalence and/or HPV tumour DNA prevalence were too low, or in case of L1 antibodies where seropositivity is not strongly linked to tumour HPV DNA status [39], cut-offs were determined using visual inspection of percentile plots [40–42] (HPV16 L1: 250 MFI and HPV18 E1: 200 MFI, E2: 250 MFI, E4: 150 MFI, L1: 250 MFI).
(d). Statistical analysis
(i). Categorization of follow-up time
Based on the observation that titers to HPV16 E6 and E7 in HPV16 DNA-positive cases decrease significantly in the first six months and continue to decay within the following 12 months, follow-up time was categorized into four groups: 0 (baseline), one to six months (using the serum sample that was collected closest to six months of follow-up), seven to 18 months (using the serum sample that was collected closest to 18 months of follow-up) and greater than 18 months (last available serum sample during at least 18 months of follow-up).
(ii). Definition of antibody decrease
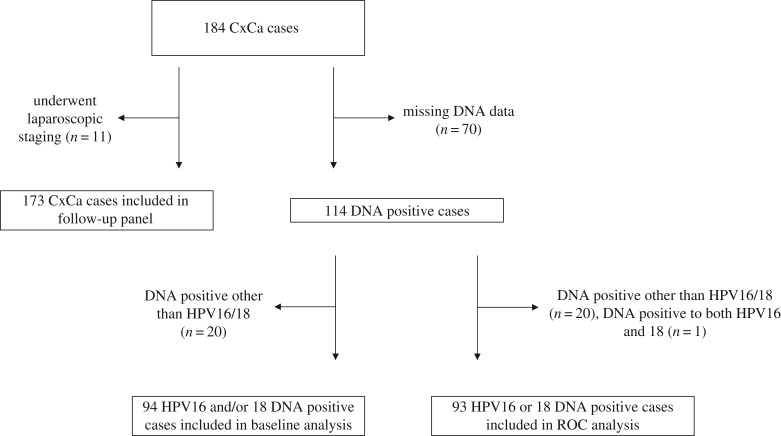
Based on the observed antibody kinetics, a decreasing antibody trend was defined for serial samples that were seropositive at baseline and showed a reduction in antibody reactivity of at least 50% of the baseline MFI within the first 18 months. Every other trend was considered to be either stable or increasing. Of the 184 patients, only 11 patients underwent laparoscopic staging without surgery which were excluded in this analysis (figure 1). For analysis of serological responses to HPV16 with regard to follow-up status (i.e. relapse versus no relapse), patients without short-term follow-up samples, i.e. those with the first follow-up sample collected after more than 18 months, were analysed separately.
Figure 1.
CONSORT flow diagram. Of the 184 patients, only 11 patients underwent laparoscopic staging without surgery. CxCa, cervical carcinoma; ROC, receiver operating characteristics.
Statistical significance of differences between continuous variables (i.e. MFI values) was assessed by Wilcoxon rank sum tests, and by Fisher's exact test for categorical variables (i.e. seroprevalence). All tests were performed two-sided, statistical analyses were performed using SAS 9.3, and p-values < 0.05 were considered statistically significant. The p-values were corrected for multiple significance testing with the Bonferroni method where applicable. Linear mixed-effect model analysis was performed in R v. 3.5.0 (https://www.r-project.org) using the lme4 package.
(e). Mathematical analysis of antibody decay kinetics
(i). Mathematical models for antibody kinetics
Three mathematical models of different complexity were analysed with regard to their ability to describe and quantify the decreasing antibody kinetics observed in the data: (i) an exponential model, (ii) a power-law model and (iii) a two-phase decay model.
The exponential model assumes that the antibody level, A(t), measured in MFI, decays over time with a constant rate λ. Thus,
| 2.1 |
where A0 defines the initial MFI at baseline, i.e. t = 0.
The second model extends the exponential model by assuming that the antibody decay rate is not constant but rather follows a Gamma distribution. This model has been previously used in HPV vaccination studies to account for heterogeneity in the decay rates of B-cells [39] and is defined as the power-law model with A(t) described by
| 2.2 |
where θ defines an arbitrary small constant and the rate of decay [24].
Finally, the two-phase decay model extends the exponential model by additionally accounting for the turnover of antibody-producing plasma cells. Plasma cells, P, are assumed to produce antibodies at a constant rate α and decay at a rate δ. The turnover of the concentration of antibodies and plasma cells after tumour resection is then described by
| 2.3 |
Solving equation (2.3), and normalizing the concentration of plasma cells at baseline to 1, P0 = 1, the antibody decay curve shows a characteristic two-phase decay which is given by
| 2.4 |
(ii). Parameter fitting
For each patient, the MFI data for HPV16 E6 and E7 antibodies were normalized for the corresponding MFI measured in the baseline serum sample, leading to A0 = 1. This was done to address the relative antibody decay kinetics from baseline for each patient classified with decreasing antibody kinetics, which is more informative for the progression of the patient from a clinical perspective. Relationship of absolute MFI values to disease progression has been investigated as well, which provided no added information. The models were implemented in R v. 3.5.0 (https://www.r-project.org) and fitted to the combined population data using the functions ‘lm' (exponential model) and ‘nls' (power-law model and two-phase decay model, algorithm ‘port'). For parameter estimation, only values with A ≤ 1 were considered to ensure antibody decay, neglecting one measurement of patient 120 in HPV16 E7 which was considered an outlier.
To additionally account for individual patient kinetics, nonlinear mixed-effects models of the power-law and two-phase decay model were fitted to the data using Monolix (2018 R1, www.lixoft.com). The parameter estimates obtained for the previous population-based analyses were used for parameter initialization.
(iii). Calculation of time to 50% reduction in antibody titers
The estimated parameters of the nonlinear mixed-effects model analysis by Monolix were used to predict the individual antibody dynamics for HPV16 E6 and E7 of the power-law and two-phase decay model. For the power-law model, the time to achieve 50% reduction in the antibody titer for each patient was then determined using the exact solution given by
| 2.5 |
For the two-phase decay model, t1/2 was calculated by minimizing the equation
| 2.6 |
with A(t) defined as in equation (2.4). Minimization was performed by the mle2-function (R package: bbmle, using the optim function) and using the parameters λ, α and δ determined for each patient (see electronic supplementary material, tables S6 and S7).
3. Results
(a). Study population
Clinical characteristics of the 184 women participating in the follow-up study are shown in table 1. Median age at diagnosis was 46 (range 17–78). The median number of serum samples per woman was 3 (range 2–12), and the median follow-up time was 725 days (range 18–6637 days). Tumour HPV DNA status at baseline was available for 114 women (62% of the study population), 110 with single infections and four with multiple infections (figure 1). Two women harboured tumours positive for two HPV types, and another two women harboured tumours positive for three HPV types, yielding 120 genotyping results in total. The majority of tumours were DNA-positive for HPV16 (n = 69, 57.5%), followed by HPV18 (n = 27, 22.5%); other HPV types were detected in 24 tumours (20.0%). Approximately half of the patients had T1-sized tumours (47.3%). The majority showed no lymph node involvement (58.7%) and microscopically confirmed tumour-free resection margins (53.3%). Histologically confirmed squamous cell carcinoma was reported in 75.0% of cases, and residual disease or relapse was found in approximately one-third of patients (34.2%) during follow-up. Overall, 166 (90.2%) of all 184 women were seropositive at least at one time point during this study against at least one of the 40 tested HPV antigens. As seroprevalences for several of these antigens were very low, we focused on comparisons with sufficient statistical power to obtain meaningful estimates, specifically E6, E7 and L1 of HPV16 and, where possible, HPV18.
(b). Comparison of human papillomavirus serology with tumour DNA status at baseline
Seropositivity against E6, E7 and L1 of HPV16 according to HPV16 and 18 tumour DNA status is shown in table 2. One patient was excluded due to double HPV16 and HPV18 DNA positivity in the tumour sample. Among the 68 cases DNA positive for only HPV16, the highest seroprevalence was observed for HPV16 E6 (n = 42, 61.8%), followed by HPV16 E7 (n = 32, 47.1%) and HPV16 L1 (n = 27, 39.7%). Both HPV16 E6 and E7 antibodies were almost absent in cases with HPV18 DNA-positive tumours (each n = 1 out of 26, 3.8%) while HPV16 L1 antibodies were detectable in 3 (11.5%) of these women. Similarly, specific antibody patterns were detected for HPV18 E6, E7 and L1 antibodies (electronic supplementary material, table S1).
Table 2.
HPV16 serology and tumour HPV DNA status at baseline.
|
DNA statusa |
||
|---|---|---|
| seropositive for | HPV16 pos. (n = 68) | HPV18 pos. (n = 26) |
| HPV16 E6 | 42 (61.8) | 1 (3.8) |
| HPV16 E7 | 32 (47.1) | 1 (3.8) |
| HPV16 L1 | 27 (39.7) | 3 (11.5) |
aOne patient excluded due to double HPV16 and 18 positivity.
(c). Comparison of human papillomavirus serology with nodal status at baseline
HPV16 E6 seropositivity was elevated among HPV16 DNA-positive patients with lymph node involvement (N1, n = 24, 75% seropositive) compared to patients without positive nodal stage (N0, n = 38, 50% seropositive). This difference was however not statistically significant (p = 0.066).
(d). Antibody decay of baseline DNA-positive and seropositive cases
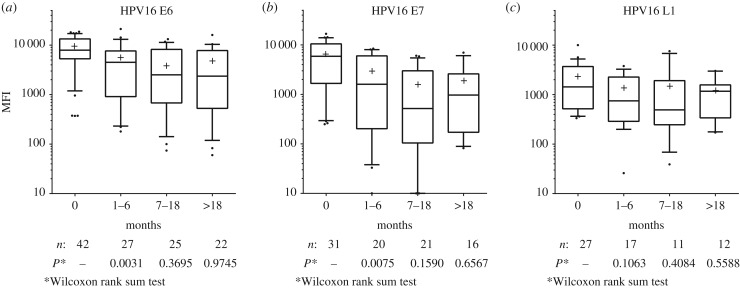
To investigate type-specific HPV antibody decay, we examined follow-up samples of patients positive for HPV antibodies at baseline and tumour HPV DNA of the same type (figure 2). Among the patients with HPV16 DNA-positive tumours (n = 68), 42 and 31 were positive for HPV16 E6 and E7 antibodies at baseline, respectively. Within the first six months of follow-up, a 43% decrease in E6 antibody reactivity (median MFI) was observed (from 7911 to 4493 MFI, p = 0.009). Similarly, E7 antibody reactivity decreased by 73% (from 5886 to 1602 MFI, p = 0.024). Fitting a linear mixed-effect model to the patient data for the first six months after baseline corroborated a significant decrease in the antibody titers of HPV16 E6 and E7 over this time period (E6: slope −23.54 ± 5.28 d−1; E7: slope −16.38 ± 3.97 d−1; population estimate ± s.e.). Within the next 12 months (until 18 months of follow-up), antibody reactivities against the HPV16 oncoproteins continued to decrease (E6: 2514 MFI, E7: 517 MFI); however, the difference was not statistically significant compared to six months of follow-up (p = 0.370 and p = 0.160, respectively). After 18 months of follow-up, antibody reactivities to HPV16 E6 and E7 stabilized at high levels (E6: 2359 MFI, E7: 971 MFI), without further changes in antibody reactivities (p = 0.975 and p = 0.657). Antibody reactivities to HPV16 L1 were initially lower than for the oncoproteins (1492 MFI at baseline), and showed a similar pattern over time, although changes in antibody reactivity were less pronounced (until six months of follow-up: 747 MFI, until 18 months: 492 MFI, beyond 18 months: 1168 MFI; p = 0.106, p = 0.480, p = 0.559, respectively; linear mixed-effect model for the first six months L1: slope −6.26 ± 2.29 d−1). Antibody reactivities to HPV16 E1, E2 and E4 showed similar, statistically insignificant patterns (electronic supplementary material, figure S1). Due to the low numbers of concordantly HPV18 DNA and HPV18 seropositive patients, no consistent patterns could be detected (electronic supplementary material, figure S2).
Figure 2.
Antibody decay of baseline HPV16 DNA-positive and seropositive cases. The three panels show antibody reactivity to HPV16 proteins E6 (a), E7 (b) and L1 (c) during follow-up. Serial serum samples were categorized as follows: baseline (month 0); the sample collected closest to six months follow-up (months 1–6); the sample collected closest to 18 months follow-up (months 7–18); the last follow-up sample available (greater than 18 months). Whiskers represent 90th and 10th percentiles, respectively; medians are indicated by horizontal lines; means are displayed by crosses. Patient numbers with sera available at the given time points vary due to heterogeneous follow-up sampling. The p-values indicate statistical significance compared to previous category.
(e). Human papillomavirus antibody kinetics during follow-up
Of 67 and 50 patients initially seropositive for HPV16 E6 and E7, 13 (19.4%) and 13 (26.0%) showed seroreversion, respectively (electronic supplementary material, table S3). Based on the observed antibody kinetics among all patients concordantly positive for HPV16 E6 or E7 antibodies and tumour HPV16 DNA (figure 2), serial samples of individual patients were considered to show a decreasing antibody trend if they were seropositive at baseline and had a reduction in antibody reactivity (MFI) of at least 50% of the baseline MFI within the first 18 months. Every other trend was considered to be either stable or increasing.
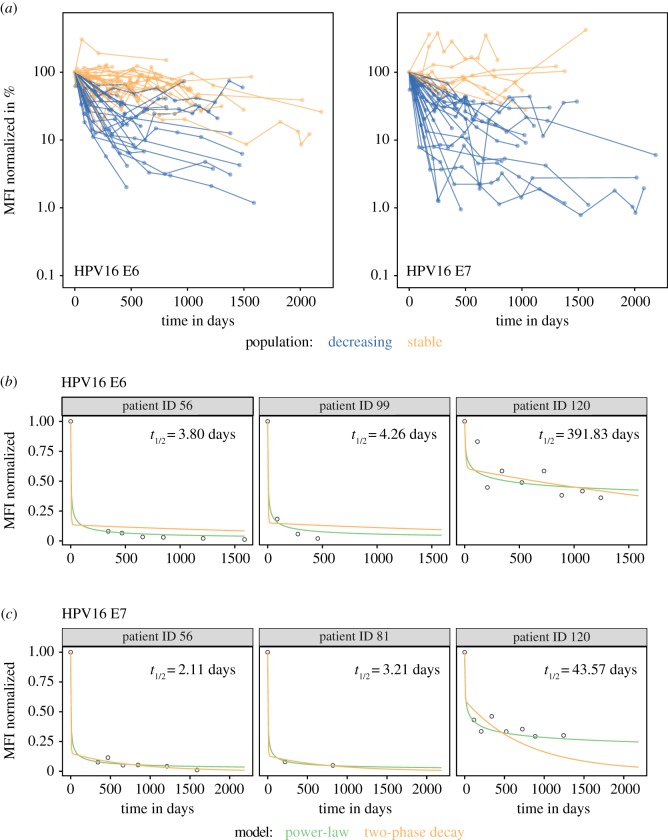
Individual patients' antibody kinetics for HPV16 E6 and E7 are shown in figure 3. Of the 67 patients seropositive for HPV16 E6 at baseline, 28 (41.8%) showed a decrease in antibody reactivity over the first 18 months (table 3), whereas more than half of the patients (n = 39, 58.2%) showed stable or increasing antibodies with time. The maximum number of patients showing decreasing antibody reactivity after treatment were those seropositive for HPV16 E7 (33 out of 50, 66.7%), while only 12 out of 47 (25.5%) patients seropositive for HPV16 L1 had a decay in antibody titers with time (L1 versus E6: p = 0.11, L1 versus E7: p < 0.0001). The 45, 31 and 49 patients seropositive for the HPV16 early proteins E1, E2 and E4 showed similar distributions with 14 (31.1%), 10 (32.3%) and 16 (32.7%) patients characterized with decreasing trends, and 31 (68.9%), 21 (67.7%) and 33 (67.3%) patients with stable or increasing antibodies. Only four of 22 (18.2%) patients seropositive for HPV18 E6 showed decreasing titers during follow-up (electronic supplementary material, table S2). Of 22 patients seropositive for HPV18 E7, nine (41.0%) showed decreasing trends, whereas 6 of 38 (15.8%) patients positive for L1 antibodies showed decreasing antibody reactivity. Among women seropositive for HPV18 E1, E2 and E4, 9 of 50 (18%), 7 of 27 (25.9%) and 1 of 14 (7.1%) showed decreasing antibody reactivity.
Figure 3.
Changes in HPV16 E6 and E7 MFI levels for baseline seropositive cases over time and selected individual fits for nonlinear mixed-effect models. (a) A decreasing trend is defined by a reduction in antibody reactivity (MFI) of at least 50% of the baseline MFI within the first 18 months (coloured blue). Every other trend is considered to be either stable or increasing (coloured orange). Four patients for HPV16 E7 were excluded from the plot (but not the analysis) since they showed normalized MFI values of either greater than 500% or less than 0.1%. (b,c) Representative individual fits from nonlinear mixed-effect models of the power-law (green) and two-phase decay (orange) model for HPV16 E6 (b) and HPV16 E7 (c). Individual patients were selected to be representative for having a long follow-up (patient 56 in E6 and E7), a short follow-up (E6: patient 99, E7: patient 81) and a high plateau during follow-up (patient 120 in both E6 and E7). Times to 50% reduction of individual antibody titers are based on the predictions from the power-law model. Individual parameter estimates are shown in electronic supplementary material, tables S6 and S7.
Table 3.
HPV16 serology during follow-up (n = 173).
| antigen | seropositive n (%) | decreasing n (%)a | stable or increasing n (%)a |
|---|---|---|---|
| HPV16 E1 | 45 (26.0) | 14 (31.1) | 31 (68.9) |
| HPV16 E2 | 31 (17.9) | 10 (32.3) | 21 (67.7) |
| HPV16 E4 | 49 (28.3) | 16 (32.7) | 33 (67.3) |
| HPV16 E6 | 67 (38.7) | 28 (41.8) | 39 (58.2) |
| HPV16 E7 | 50 (28.9) | 33 (66.0) | 17 (34.0) |
| HPV16 L1 | 47 (27.2) | 12 (25.5) | 35 (74.5) |
aA decreasing trend shows reduction in antibody reactivity of at least 50% of the baseline MFI within the first 18 months. Every other trend is considered to be either stable or increasing. Of the 184 patients, 11 patients who underwent laparoscopic staging without surgery were excluded in this analysis.
(f). Correlation of HPV16 E6 and E7 antibodies
Among the 28 patients characterized as having decreasing HPV16 E6 antibodies, 15 were also seropositive for E7 antibodies: 14 showed decreasing E7 antibodies, and one had stable E7 antibodies. By contrast, among the 33 patients with decreasing HPV16 E7 antibodies, 25 were HPV16 E6 seropositive, and about half of these had decreasing and half had stable HPV16 E6 antibodies (n = 14 and n = 11, respectively).
This suggests that decaying kinetics in HPV16 E6 antibodies are associated with antibody decay for E7, but not vice versa.
(g). Association of antibody kinetics with relapse
Clinical data with regard to relapse or residual disease during follow-up was available for 173 women (table 1); however, a substantial fraction of the patients with relapse (22 of 63, 35%) showed relapse only after the last serological follow-up sample had been collected (n = 9) or already entered the study with a relapse diagnosis (n = 13). Changes in HPV16 E6, E7 and L1 antibody levels for baseline seropositive cases over time, stratified by relapse status, are shown in electronic supplementary material, figure S3. For none of the antigens, was a clear antibody kinetic pattern associated with relapse. Similarly, in patients with relapse or residual disease during serological follow-up, no clear patterns with regard to stable or decaying antibodies could be determined (electronic supplementary material, table S4). Including all patients with a relapse diagnosis, the proportions of patients showing decreasing HPV16 E6 and E7 antibodies were slightly lower among those patients with relapse versus those without, but this was not statistically significant (E6: 34.5% versus 45.0%, p = 0.53; E7: 57.1% versus 66.7%, p = 0.11).
(h). Mathematical modelling of antibody decay kinetics
To better quantify the kinetics of E6 and E7 antibodies after biopsy, three mathematical models of different complexity were fit to the data of those patients that were classified as having decreasing antibody titers (i.e. for E6: 28 of 67 patients, and for E7: 33 of 50, with 14 patients appearing in both selections). To select appropriate model structures, individual patient kinetics were neglected and models were fitted to the combined population data (see Methods for a detailed description of the different models considered). As has been observed previously [24,43,44], a simple model assuming a constant rate of antibody decay does not provide a good description of the observed antibody kinetics (electronic supplementary material, figure S4). By contrast, models assuming Gamma-distributed decay rates (power-law model) or a two-phase decay kinetics accounting for antibody and plasma cell decay (two-phase decay model) fit the data substantially better than the exponential model as determined by the Akaike information criterion (AIC), with lowest values indicating the best fit (exponential: E6 351.8, E7 643.2; power-law: E6–92.1, E7–115.9; two-phase decay: E6–87.9, E7–118.3; see electronic supplementary material, figure S4). Based on the obtained AIC values, both the power-law and two-phase decay model are considered equally likely in describing the observed kinetics. Therefore, both models were used to re-analyse the data based on a nonlinear mixed-effect model approach, which accounts for overall population dynamics and individual patient kinetics. Although both models are comparable in their ability to explain the observed dynamics for individual patients (figure 3; electronic supplementary material, figures S5 and S6) the power-law model was preferred as it provided more stable and robust estimates for the individual parameters (compare table 4 and electronic supplementary material, table S5). Concentrating on the results from the power-law model, the analysis indicated similar estimates for the decay rates, , for HPV16 E6 and E7 (table 4). Based on the obtained estimates, the corresponding durations to a 50% reduction in antibody levels within the individual patients are predicted to be 56.9 ± 26.1 days (mean ± s.e.) and 56.3 ± 19.0 days for E6 and E7, respectively (see electronic supplementary material, figures S5 and S6 & tables S6 and S7 for individual patient predictions and parameter estimates). For the 14 patients that were classified with decaying antibody kinetics for both HPV16 E6 and E7, estimated times to 50% reduction were found to be correlated with comparable estimates for both antibodies (Spearman's ρ = 0.78, p-value = 0.0015; electronic supplementary material, figure S7).
Table 4.
Parameter estimates of the nonlinear mixed-effect models for the power-law model for the antigens HPV16 E6 and E7. For each antigen, the parameter estimates and relative standard errors (r.s.e.) are given, as well as the AIC value of the model. In addition, the corresponding mean estimates and standard errors of the time to 50% reduction of the individual antibody levels based on the predicted dynamics, A(t), for each patient are presented. For the individual patient estimates see electronic supplementary material, tables S6 and S7.
|
parameter |
HPV16 E6 |
HPV16 E7 |
||||
|---|---|---|---|---|---|---|
| (unit) | estimate | r.s.e. (in %) | estimate | r.s.e. (in %) | ||
| power-law | decay rate | (d−1) | 0.242 | 9.04 | 0.268 | 11.0 |
| scaling constant | θ (d) | 1.083 | 7.77 | 1.379 | 15.6 | |
| AIC | −144.58 | −322.42 | ||||
| mean | s.e. | mean | s.e. | |||
| time to 50% reduction of individual antibody levels A(t) | t1/2 (d) | 56.94 | 26.12 | 56.29 | 18.97 | |
4. Discussion
In this study, we conducted a comprehensive analysis of the post-treatment dynamics of antibodies to the E6 and E7 oncoproteins and the L1 capsid proteins of HPV 16 and 18, in a clinically well-characterized large cohort of incident CxCa patients. Analysing these data in detail and using a mathematical model to quantify antibody decay kinetics, we found that HPV16 E6 and E7 antibodies undergo a slow but significant decrease in antibody titers within the first six months following CxCa treatment. These titers then stabilize on a high level until 18 months of follow-up, while L1 antibodies generally undergo significantly less decay. This supports our initial hypothesis that oncoprotein antibodies wane following surgical tumour removal, while L1 antibodies are less affected. Patients who suffered from residual disease or relapse during or after the follow-up showed lower HPV16 E6 antibody decay proportions than those who did not recur, but this was not statistically significant, and not consistent across the tested antigens. This may be explained by less interaction of the recurring lesion with lymphatic tissue due to radical lymphadenectomy in the course of surgery. A higher number of seropositive cases in nodal stage-positive patients compared to nodal stage-negative patients supports this assumption.
The decay of antibodies to oncoproteins probably comes as a result of a reduced challenge of plasma cells after antigen removal. However, HPV oncoprotein antibodies undergo a much less complete decay than those to, e.g. the MCV [23]. MCV causes a rare but very aggressive skin cancer, i.e. MCC. After surgical MCC treatment, antibodies to the large and small T-antigens of MCV (the polyomavirus analogue to the HPV E6 and E7 oncoproteins) undergo a quick decay, including complete seroreversion in most patients after approx. 8 months [23]. In the case of tumour recurrence, T-antigen antibodies re-appear prior to clinical diagnosis of the relapse, and can be thus used for prediction of treatment success. The fast kinetics in MCV T-antigen antibodies is probably possible because no long-lived plasma, or memory B cells are generated. However, the striking difference in antibody dynamics between these two close relatives (HPV and MCV) is not fully understood [23].
Prior studies could show a decrease in antibody reactivity after treatment of CxCa. For example, Baay et al. [45] described a decay in titers against native HPV16 E6 and E7 detected by RIPA in patients with CxCa following radical hysterectomy. The authors observed a decrease in HPV16 E7 titers after 100 days, ranging between 10 and 70% of the baseline titer. In patients with recurrence, a less pronounced decrease was found. Other authors described a decrease in titers after treatment compared to baseline and pre-treatment antibody levels, respectively [10,27–35]. However, no study evaluated the exact timing in which the change in titers took place. The majority of the more recent publications in clinical use of HPV serology is focused on OPC and not CxCa. A notable difference between CxCa and OPC is the difference in detectable antibody lead times: in CxCa, seroconversion for E6 and E7 antibodies is a late event, i.e. the antibodies can only be detected at the time of cancer diagnosis [6,7], while in OPC patients, E6 and E7 antibodies can be detected 10 and more years prior to diagnosis [12,13]. This difference has been attributed to the fact that the main sites of HPV-driven OPC, i.e. the palatine and lingual tonsils, belong to the Waldeyer's ring of head and neck lymphoid tissue epithelium which may trigger early seroconversion [46], while immunosurveillance of the cervix uteri is much less pronounced. Another notable difference is that the natural history of genital HPV infection and the subsequent development of CxCa have been studied in detail, and CxCa precursor lesions are well known, while the natural history of oral HPV infection is poorly understood, and OPC precursor lesions have not been described yet. In addition, CxCa is primarily surgically treated, while OPC patients may undergo primary radiotherapy, or chemoradiotherapy. It remains to be seen whether the antibody dynamics we describe also hold true for OPC patients following treatment.
The main strengths of our study are the long follow-up of the patient cohort with an average of approximately 2 years, and follow-up for more than 5 years for some of the patients included in the analysis of antibody dynamics. Repeated blood sampling throughout the follow-up allowed a comprehensive mathematical modelling approach to quantify patient-specific antibody kinetics. In addition, the use of state of the art multiplex serology allowed us to measure antibodies to multiple HPV types and full-length proteins simultaneously. We were, however, not able to use the previously established standard cut-offs for HPV seropositivity [12] as the signal intensity observed in this serological analysis was somewhat reduced, an effect that is most likely based on prolonged serum storage times and repeated analysis of the serum samples (i.e. several freeze–thaw cycles). However, we based our cut-offs on a ROC analysis, maximizing sensitivity and specificity of the measurement against tumour HPV DNA status as the gold-standard. In addition, the specificity of antibodies against HPV16 and 18, especially those against the oncoproteins, for tumours driven by these types was very high, supporting the general validity of our approach.
Serum samples included in this study were collected on an individual basis to determine routine clinical parameters. Thus, the collection intervals were heterogeneous. For the analysis of HPV antibody dynamics, we categorized individual follow-up into a baseline category, and three follow-up categories, always using the sample collected closest to the end of the respective follow-up period (six months, 18 months and the last available follow-up sample taken after 18 months or more). One alternative to this approach would have been to take the average of the available samples within these time periods to use the full range of available data. However, we decided against this approach as, obviously, HPV antibodies are dynamic within these first 6, and the following 12 months, i.e. assuming a step-wise function for antibody decay would be inappropriate. Thus, taking the last available sample in every time period category described the observed kinetics better in the categorical analysis. However, all available data have been used in the mathematical models to quantify antibody decay kinetics.
Our estimates predict durations for a 50% reduction in antibody titers within individual patients of 56.9 ± 26.1 days (mean ± s.e.) and 56.3 ± 19.0 days for E6 and E7, respectively. As calculation of the mean is affected by outliers characterized by insufficient clearance or early saturation of antibody levels (e.g. patient 120 for E6; electronic supplementary material, figure S4), we also determined the median durations which are given by 13.8 (E6) and 14.8 (E7) days (see also electronic supplementary material, tables S6 and S7). Although our estimates describe the net loss rate of antibodies including de novo antibody production and loss, the estimates for the time to 50% reduction in antibody titers are comparable to estimates of the general half-life of IgG (t1/2 approx. 21–24 days) [47] and slightly lower than the estimates obtained for maternal IgG antibodies (t1/2 approx. 35–40 days) [48]. Comparable to previous results, we observed that a power-law model assuming Gamma-distributed decay rates of plasma cells is able to describe the observed data better than a model assuming simple exponential decay. The power-law model also provides more robust parameter estimates than the two-phase decay model as it contains less parameters to estimate. However, as both models predict similar dynamics, estimates for the time to 50% reduction in antibody titers are comparable between both models. In cases where denser sampling of antibody titers within the early decay kinetics can be achieved, parameter identification for the two-phase decay model could be improved allowing corroboration of the determined decay characteristics. A denser sampling of antibody levels for each patient would also help to apply the identified mathematical models to each patient independently. The current approach relies on a nonlinear mixed-effect model which simultaneously accounts for population and individual patient dynamics and thus allows predicting individual kinetics of patients with insufficient numbers of measurements (see electronic supplementary material, figure S5, e.g. patient 2, 26 or 45) due to population-based parameter constraints. However, this approach requires the pre-categorization of patients in stable or decreasing populations in order to provide reliable results. Here, we used an observed MFI decrease by 50% or more within the first 18 months of follow-up to categorize a patient as showing decreasing antibody kinetics, which is stricter than previous definitions [23]. Although this definition might be arbitrary, it provided an appropriate separation of the observed dynamics (figure 3). By contrast, absolute MFI values were not informative for the prediction of antibody dynamics and we found no significant difference in the baseline values between patients classified as stable or decreasing (E6: p = 0.69, E7: p = 0.12). High-density sampling comprising at least 4–5 follow-up measurements for each patient would allow a direct estimation of individual decay rates and the time to 50% reduction in antibody titers, which would enable us to categorize each patient with either decreasing or stable dynamics based on their estimates. Within the limited number of patients that were classified as decreasing and experienced a tumour relapse during or after the study, we found evidence for a correlation between the estimated time to 50% reduction in antibody titers and the time to relapse, i.e. faster decays determining later relapse (E6: n = 8, Spearman's ρ = −0.76, p = 0.036; E7: n = 10, Spearman's ρ = −0.32, p = 0.37; electronic supplementary material, figure S8). However, the current patient cohort does not provide sufficient evidence for the use of absolute MFI values or antibody decay kinetics as predictors for the occurrence of time to a potential relapse. Larger studies including patients with sufficient numbers of follow-up measurements to determine antibody decay characteristics might help to address this point more conclusively.
Another potential weakness of our study is that HPV DNA status of tumours was not available for every patient. However, this is not routinely performed as every CxCa case is considered HPV-driven, and HPV genotyping is not mandatory as it has no consequence for tumour treatment. The proportions of tumours driven by HPV16 and HPV18 were somewhat elevated in our study (i.e. about 61% for HPV16 and 24% for HPV18) but are well in line with expectations (i.e. about 50% for HPV16 and 15% for HPV18).
Within this study we have generated an analytical framework to analyse antibody kinetics in HPV-related cancers. Dense sampling of antibody dynamics within the very beginning of follow-up is required to inform mathematical models used to quantify antibody kinetics, which is potentially useful to characterize individual progression of a patient. Larger clinical studies are needed to corroborate the trends observed in the study including the association of lower decay proportions in relapsing patients, of elevated HPV16 E6 seroprevalence with increased nodal status, and of HPV16 E6 antibody decay with decaying kinetics in HPV16 E7 (but not vice versa). Such studies would help to determine the general utility of HPV-associated antibody kinetics for patient care and clinical decision-making.
Supplementary Material
Data accessibility
The raw data for this publication has been made publicly available at http://doi.org/10.5281/zenodo.2555612.
Authors' contributions
T.P. carried out the serological laboratory work, performed descriptive data analysis and drafted the manuscript; C.H. performed mathematical modelling and contributed to the manuscript; M.P. gave input to study design and the manuscript; K.C. helped with molecular laboratory work; J.S. contributed to the mathematical modelling; I.B.R. collected field data; M.D. supervised field data collection and HPV genotyping; F.G. supervised mathematical modelling and contributed to the manuscript; T.W. conceived and coordinated the study, supervised the descriptive data analysis and drafted the manuscript. All authors gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
This work was supported by DKFZ intramural funding to T.W. F.G. was supported by BIOMS (Center for Modelling and Simulation in the Biosciences) and the Chica and Heinz Schaller Foundation.
References
- 1.Bouvard V, et al. 2009. Cogliano V; WHO international agency for research on cancer monograph working group. A review of human carcinogens–part B: biological agents. Lancet Oncol. 10, 321–322. ( 10.1016/S1470-2045(09)70096-8) [DOI] [PubMed] [Google Scholar]
- 2.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. 2016. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Global Health 4, e609–e616. ( 10.1016/S2214-109X(16)30143-7) [DOI] [PubMed] [Google Scholar]
- 3.Roden RBS, Stern PL. 2018. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat. Rev. Cancer 18, 240–254. ( 10.1038/nrc.2018.13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanzavecchia A, Sallusto F. 2009. Human B cell memory. Curr. Opin Immunol. 21, 298–304. ( 10.1016/j.coi.2009.05.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dillner J. 1999. The serological response to papillomaviruses. Semin. Cancer Biol. 9, 423–430. ( 10.1006/scbi.1999.0146) [DOI] [PubMed] [Google Scholar]
- 6.Meschede W, Zumbach K, Braspenning J, Scheffner M, Benitez-Bribiesca L, Luande J, Gissmann L, Pawlita M. 1998. Antibodies against early proteins of human papillomaviruses as diagnostic markers for invasive cervical cancer. J. Clin. Microbiol. 36, 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreimer AR, et al. 2015. Human papillomavirus antibodies and future risk of anogenital cancer: a nested case-control study in the European prospective investigation into cancer and nutrition study. J. Clin. Oncol. 33, 877–884. ( 10.1200/JCO.2014.57.8435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehtinen M, et al. 2003. Evaluation of antibody response to human papillomavirus early proteins in women in whom cervical cancer developed 1 to 20 years later. Am. J. Obstet. Gynecol. 188, 49–55. ( 10.1067/mob.2003.98) [DOI] [PubMed] [Google Scholar]
- 9.Clifford GM, et al. 2016. Immunodeficiency and the risk of cervical intraepithelial neoplasia 2/3 and cervical cancer: a nested case-control study in the Swiss HIV cohort study. Int. J. Cancer 138, 1732–1740. ( 10.1002/ijc.29913) [DOI] [PubMed] [Google Scholar]
- 10.Chee YH, Namkoong SE, Kim DH, Kim SJ, Park JS. 1995. Immunologic diagnosis and monitoring of cervical cancers using in vitro translated HPV proteins. Gynecol. Oncol. 57, 226–231. ( 10.1006/gyno.1995.1130) [DOI] [PubMed] [Google Scholar]
- 11.Gooi Z, Chan JY, Fakhry C. 2016. The epidemiology of the human papillomavirus related to oropharyngeal head and neck cancer. Laryngoscope 126, 894–900. ( 10.1002/lary.25767) [DOI] [PubMed] [Google Scholar]
- 12.Kreimer AR, et al. 2013. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J. Clin. Oncol. 31, 2708–2715. ( 10.1200/JCO.2012.47.2738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreimer AR, et al. 2017. Kinetics of the human papillomavirus type 16 E6 antibody response prior to oropharyngeal cancer. J. Natl Cancer Inst. 109, djx005 ( 10.1093/jnci/djx005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlstrom KR, Anderson KS, Cheng JN, Chowell D, Li G, Posner M, Sturgis EM. 2015. HPV serum antibodies as predictors of survival and disease progression in patients with HPV-positive squamous cell carcinoma of the oropharynx. Clin. Cancer Res. 21, 2861–2869. ( 10.1158/1078-0432.CCR-14-3323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fakhry C, et al. 2016. Serum antibodies to HPV16 early proteins warrant investigation as potential biomarkers for risk stratification and recurrence of HPV-associated oropharyngeal cancer. Cancer Prevent. Res. 9, 135–141. ( 10.1158/1940-6207.CAPR-15-0299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang Kuhs KA, et al. 2017. Human papillomavirus 16 E6 antibodies are sensitive for human papillomavirus-driven oropharyngeal cancer and are associated with recurrence. Cancer 123, 4382–4390. ( 10.1002/cncr.30966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, et al. 2017. Human papillomavirus (HPV) 16 antibodies at diagnosis of HPV-related oropharyngeal cancer and antibody trajectories after treatment. Oral Oncol. 67, 77–82. ( 10.1016/j.oraloncology.2017.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spector ME, et al. 2017. E6 and E7 antibody levels are potential biomarkers of recurrence in patients with advanced-stage human papillomavirus-positive oropharyngeal squamous cell carcinoma. Clin. Cancer Res. 23, 2723–2729. ( 10.1158/1078-0432.CCR-16-1617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang CG, et al. 2017. Molecular and serologic markers of HPV 16 infection are associated with local recurrence in patients with oral cavity squamous cell carcinoma. Oncotarget 8, 34 820–34 835. ( 10.18632/oncotarget.16747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koslabova E, Hamsikova E, Salakova M, Klozar J, Foltynova E, Salkova E, Rotnaglova E, Ludvikova V, Tachezy R. 2013. Markers of HPV infection and survival in patients with head and neck tumors. Int. J. Cancer 133, 1832–1839. ( 10.1002/ijc.28194) [DOI] [PubMed] [Google Scholar]
- 21.Mirghani H, Lang Kuhs KA, Waterboer T. 2018. Biomarkers for early identification of recurrences in HPV-driven oropharyngeal cancer. Oral Oncol. 82, 108–114. ( 10.1016/j.oraloncology.2018.05.015) [DOI] [PubMed] [Google Scholar]
- 22.Tam S, Fu S, Xu L, Krause KJ, Lairson DR, Miao H, Sturgis EM, Dahlstrom KR. 2018. The epidemiology of oral human papillomavirus infection in healthy populations: a systematic review and meta-analysis. Oral Oncol. 82, 91–99. ( 10.1016/j.oraloncology.2018.04.005) [DOI] [PubMed] [Google Scholar]
- 23.Paulson KG, et al. 2017. Viral oncoprotein antibodies as a marker for recurrence of Merkel cell carcinoma: a prospective validation study. Cancer 123, 1464–1474. ( 10.1002/cncr.30475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser C, Tomassini JE, Xi L, Golm G, Watson M, Giuliano AR, Barr E, Ault KA. 2007. Modeling the long-term antibody response of a human papillomavirus (HPV) virus-like particle (VLP) type 16 prophylactic vaccine. Vaccine 25, 4324–4333. ( 10.1016/j.vaccine.2007.02.069) [DOI] [PubMed] [Google Scholar]
- 25.Antia A, Ahmed H, Handel A, Carlson NE, Amanna IJ, Antia R, Slifka M. 2018. Heterogeneity and longevity of antibody memory to viruses and vaccines. PLoS Biol. 16, e2006601 ( 10.1371/journal.pbio.2006601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andraud M, Lejeune O, Musoro JZ, Ogunjimi B, Beutels P, Hens N. 2012. Living on three time scales: the dynamics of plasma cell and antibody populations illustrated for hepatitis A virus. PLoS Comput. Biol. 8, e1002418 ( 10.1371/journal.pcbi.1002418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehtinen M, Leminen A, Kuoppala T, Tiikkainen M, Lehtinen T, Lehtovirta P, Punnonen R, Vesterinen E, Paavonen J. 1992. Pre- and posttreatment serum antibody responses to HPV 16 E2 and HSV 2 ICP8 proteins in women with cervical carcinoma. J. Med. Virol. 37, 180–186. ( 10.1002/jmv.1890370306) [DOI] [PubMed] [Google Scholar]
- 28.Dillner J. 1993. Disappearance of antibodies to HPV 16 E7 after treatment for cervical cancer. Lancet 341, 1594 ( 10.1016/0140-6736(93)90730-5) [DOI] [PubMed] [Google Scholar]
- 29.Baay MF, Duk JM, Burger MP, de Bruijn HW, Stolz E, Herbrink P. 1995. Follow-up of antibody responses to human papillomavirus type 16 E7 in patients treated for cervical carcinoma. J. Med. Virol. 45, 342–347. ( 10.1002/jmv.1890450319) [DOI] [PubMed] [Google Scholar]
- 30.Lenner P, Dillner J, Wiklund F, Hallmans G, Stendahl U. 1995. Serum antibody responses against human papillomavirus in relation to tumor characteristics, response to treatment, and survival in carcinoma of the uterine cervix. Cancer Immunol. Immunother. 40, 201–205. ( 10.1007/BF01517352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher SG, et al. 1996. The association of human papillomavirus type 16 E6 and E7 antibodies with stage of cervical cancer. Gynecol. Oncol. 61, 73–78. ( 10.1006/gyno.1996.0099) [DOI] [PubMed] [Google Scholar]
- 32.Di Lonardo A, Marcante ML, Poggiali F, Venuti A. 1998. HPV 16 E7 antibody levels in cervical cancer patients: before and after treatment. J. Med. Virol. 54, 192–195. () [DOI] [PubMed] [Google Scholar]
- 33.Park JS, Park DC, Kim CJ, Ahn HK, Um SJ, Park SN, Kim SJ, Namkoong SE. 1998. HPV-16-related proteins as the serologic markers in cervical neoplasia. Gynecol. Oncol. 69, 47–55. ( 10.1006/gyno.1998.4963) [DOI] [PubMed] [Google Scholar]
- 34.Hamsíková E, Ludvíková V, Tachezy R, Kovarík J, Brousková L, Vonka V. 2000. Longitudinal follow-up of antibody response to selected antigens of human papillomaviruses and herpesviruses in patients with invasive cervical carcinoma. Int. J. Cancer 86, 351–355. () [DOI] [PubMed] [Google Scholar]
- 35.Zumbach K, Kisseljov F, Sacharova O, Shaichaev G, Semjonova L, Pavlova L, Pawlita M. 2000. Antibodies against oncoproteins E6 and E7 of human papillomavirus types 16 and 18 in cervical-carcinoma patients from Russia. Int. J. Cancer 85, 313–318. () [DOI] [PubMed] [Google Scholar]
- 36.Jacobs MV, Snijders PJ, van den Brule AJ, Helmerhorst TJ, Meijer CJ, Walboomers JM. 1997. A general primer GP5+/GP6+-mediated PCR-enzyme immunoassay method for rapid detection of 14 high-risk and 6 low-risk human papillomavirus genotypes in cervical scrapings. J. Clin. Microbiol. 35, 791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waterboer T, Sehr P, Michael KM, Franceschi S, Nieland JD, Joos TO, Templin MF, Pawlita M. 2005. Multiplex human papillomavirus serology based on in situ-purified glutathione S-transferase fusion proteins. Clin. Chem. 51, 1845–1853. ( 10.1373/clinchem.2005.052381) [DOI] [PubMed] [Google Scholar]
- 38.Waterboer T, Sehr P, Pawlita M. 2006. Suppression of non-specific binding in serological Luminex assays. J. Immunol. Methods 309, 200–204. ( 10.1016/j.jim.2005.11.008) [DOI] [PubMed] [Google Scholar]
- 39.Combes JD, et al. 2014. Antibodies against high-risk human papillomavirus proteins as markers for invasive cervical cancer. Int. J. Cancer 135, 2453–2461. ( 10.1002/ijc.28888) [DOI] [PubMed] [Google Scholar]
- 40.Michael KM, et al. 2008. Seroprevalence of 34 human papillomavirus types in the German general population. PLoS Pathog. 4, e1000091 ( 10.1371/journal.ppat.1000091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carter JJ, et al. 2009. Association of Merkel cell polyomavirus-specific antibodies with Merkel cell carcinoma. J. Natl Cancer Inst. 101, 1510–1522. ( 10.1093/jnci/djp332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Migchelsen SJ, et al. 2017. Defining seropositivity thresholds for use in trachoma elimination studies. PLoS Negl. Trop. Dis. 11, e0005230 ( 10.1371/journal.pntd.0005230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Damme P, Leroux-Roels G, Law B, Diaz-Mitoma F, Desombere I, Collard F, Tornieporth N, Van Herck K. 2001. Long-term persistence of antibodies induced by vaccination and safety follow-up, with the first combined vaccine against hepatitis A and B in children and adults. J. Med. Virol. 65, 6–13. ( 10.1002/jmv.1094) [DOI] [PubMed] [Google Scholar]
- 44.Van Herck K, Van Damme P. 2001. Inactivated hepatitis A vaccine-induced antibodies: follow-up and estimates of long-term persistence. J. Med. Virol. 63, 1–7. () [DOI] [PubMed] [Google Scholar]
- 45.Baay MF, Duk JM, Burger MP, de Bruijn HW, Stolz E, Herbrink P. 1999. Humoral immune response against proteins E6 and E7 in cervical carcinoma patients positive for human papilloma virus type 16 during treatment and follow-up. Eur. J. Clin. Microbiol. Infect. Dis. 18, 126–132. ( 10.1007/s100960050240) [DOI] [PubMed] [Google Scholar]
- 46.Kreimer AR, Shiels MS, Fakhry C, Johansson M, Pawlita M, Brennan P, Hildesheim A, Waterboer T. 2018. Screening for human papillomavirus-driven oropharyngeal cancer: considerations for feasibility and strategies for research. Cancer 124, 1859–1866. ( 10.1002/cncr.31256) [DOI] [PubMed] [Google Scholar]
- 47.William EP. (ed.). 2013. Fundamental immunology, 7th edn Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- 48.Sato H, Albrecht P, Reynolds DW, Stagno S, Ennis FA. 1979. Transfer of measles, mumps, and rubella antibodies from mother to infant. Its effect on measles, mumps, and rubella immunization. Am. J. Dis. Child. 133, 1240–1243. ( 10.1001/archpedi.1979.02130120032005) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data for this publication has been made publicly available at http://doi.org/10.5281/zenodo.2555612.