Abstract
Hepatitis E virus recombinant genomes transcribed in vitro from two cDNA clones differing by two nucleotides were infectious for chimpanzees. However, one cDNA clone encoded a virus that was attenuated for chimpanzees and unable to infect rhesus monkeys. The second cDNA clone encoded a virus that infected both chimpanzees and rhesus monkeys and caused acute hepatitis in both. One mutation differentiating the two clones identified a cis-reactive element that appeared to overlap the 3′ end of the capsid gene and part of the 3′ noncoding region. Capping of the RNA transcripts was essential for infectivity.
Hepatitis E virus (HEV) is an unclassified nonenveloped virus that is a major cause of acute hepatitis in many developing countries (1). HEV is usually transmitted via the fecal–oral route, often by drinking contaminated water. The virus was first identified in the feces of an experimentally infected volunteer in the former Soviet Union only 18 years ago (2). The genome from a Burmese strain was the first to be substantially sequenced (3), followed by that of a genetically diverse strain from Mexico (4). Initially only two genotypes, represented by many Asian strains and the single Mexican strain, were recognized. The discovery of virus of a third genotype in swine in the U.S. led to more extensive investigation world-wide and resulted in the discovery of additional strains in developed countries, including the U.S., where the disease is rare, as well as in developing countries, where epidemics and sporadic cases often occur (5). On the basis of sequence analysis of full-length genomes, four genotypes are generally recognized, but analysis of partial sequences suggests these may be subdivided into as many as nine groups (6). Swine are naturally infected with HEV, and a U.S. strain of swine virus was shown to cross species barriers and infect primates (7). Many other animal species have serological evidence of infection, suggesting HEV may be zoonotic (8–10, ‡‡).
HEV has a positive-sense RNA genome of 7.2 kb, which contains three ORFs encoding putative nonstructural proteins (ORF1), a capsid protein (ORF2), and a very small protein of unknown function (ORF3) (3). The ORF1 sequence has a motif that is characteristic of a methyltransferase (11), suggesting that the genomic RNA is capped, because this activity and guanyltransferase activity are responsible for adding m7GTP to the 5′ terminus of mRNA to yield the cap structure m7GpppX. A cap structure has been indirectly identified on HEV genomes by both immunological (12) and molecular techniques (13), and a recombinant HEV protein was shown to have methyltransferase and guanyltransferase activity in vitro (14).
HEV has not been reproducibly grown in cell culture, but the virus in clinical samples has been used to infect primates. Macaques (rhesus and cynomolgus monkeys) have been the most frequently studied animal models; chimpanzees are also susceptible to experimental infection with HEV (15). Animals infected with HEV can develop hepatitis, as demonstrated by elevation of serum liver enzymes and appearance of histopathological changes in the liver. The severity of the hepatitis appears to depend on the initial viral load, and a low dose of virus can infect without causing overt disease (16). Viremia does occur, and virus can be detected also in bile and feces. The development of antibody to ORF2 or ORF3 protein can be used to diagnose an infection (17). The incubation period until seroconversion is ≈3–5 weeks in rhesus macaques inoculated intravenously with 105.5 50% monkey infectious doses (18). Clinical signs normally appear at approximately the time of seroconversion.
Recently it was reported that transfection of cultured Hep G2 cells with noncapped RNA transcripts from a full-length cDNA clone of an Indian strain of HEV resulted in productive replication (19). However, RNA transcripts from the cDNA clone were not infectious for rhesus monkeys when inoculated intrahepatically, as has been done successfully for other positive-sense RNA viruses. When medium harvested from the transfected Hep G2 cells was inoculated into a rhesus monkey, the monkey became infected with HEV. Sequence analysis of the virus recovered from the monkey apparently was not performed to confirm that the virus infecting the rhesus monkey originated from the cDNA clone. The incorporation of a cap structure onto the in vitro synthesized transcripts before transfection was not attempted.
We have constructed two variants of a full-length cDNA clone of the Pakistani (Sar-55) strain of HEV that differ by two nucleotides and have tested in vitro synthesized transcripts from each for their ability to initiate an infection in rhesus monkeys and chimpanzees after intrahepatic inoculation. Capped and uncapped transcripts of one of these clones were compared for infectivity by intrahepatic transfection of chimpanzees.
Methods
Construction of HEV cDNA Clones.
The consensus sequence of the Sar-55 strain was determined by direct analysis of uncloned reverse transcription–PCR (RT-PCR) products amplified from the viral genome; it differed at five positions from that determined by Tsarev et al. (20) (GenBank accession no. M80581) from cloned fragments. Standard molecular techniques were used to assemble a full-length cDNA clone of the Sar-55 strain from cDNA fragments that were produced by RT-PCR. The 5′ terminus of the encoded genome was engineered to conform to the sequence determined by Zhang et al. (13). This was done by inserting a T7 promoter between the XbaI site in the plasmid and the 5′ end of the HEV genome so that transcription by T7 polymerase would result in synthesis of the exact 5′ terminus of HEV. Although this manipulation introduced a second T7 promoter into the plasmid (the one present in the original vector was at the 3′ end of the genome just downstream of the site used for template linearization), the second T7 promoter did not interfere with transcription of the HEV genome. The HEV genome was ligated into the pBlueScript SK(+) vector (Stratagene) between XbaI and EcoRV sites in the polylinker. A BglII restriction site for template linearization was engineered into the clone between the 3′ terminus of the HEV sequence and the EcoRV site in the plasmid polylinker. The first cDNA clone (pSK-HEV-3) differed from the previously reported nucleotide sequence at eight positions and from the newly determined consensus nucleotide sequence at four positions (nucleotides 286 and 4396 in ORF1, nucleotide 7106 in ORF2 nucleotide 7181 in 3′ noncoding). The deduced amino acid sequence was identical to that of the consensus sequence. The T mutation at position 7106 was replaced with the consensus nucleotide G and the C at 7181 was replaced with T to yield clone pSK-HEV-2 (GenBank accession no. AAF444002).
In Vitro Transcription.
Plasmid DNA was amplified in Escherichia coli and purified with a Qiagen (Chatsworth, CA) MaxiPrep kit. Template DNA was linearized with BglII. In the first experiments, the linearized DNA was treated with Exo VII to remove nucleotide overhangs, but this step was subsequently omitted. Capped pSK-HEV-3 RNA (inoculated into rhesus monkeys 407 and 471 and into chimpanzees 1603 and 1609) and pSK-HEV-2 RNA (inoculated into chimpanzee 96A007) were synthesized with mMessage in vitro transcription kits (Ambion, Austin, TX). A typical 100-μl reaction contained 5–7.5 μg linearized template, 10 μl of 10× transcription buffer, 50 μl of 2× capping dNTP mix, 5 μl of 30 mM GTP, and 10 μl of T7 RNA polymerase mix. To compare uncapped and capped RNAs for infectivity, the MEGAscript high-yield transcription kit (Ambion) was used instead of the mMessage kit. A 100-μl capping reaction contained 10 μl each of 75 mM ATP, CTP, and UTP, 10 μl of 15 mM GTP, 15 μl of 40 mM m7G(5′)ppp(5′)G cap analog (Ambion), 10 μl of 10× T7 Reaction Buffer, 5 μg of linearized pSK-HEV-2, and 10 μl of T7 enzyme mix. The components for synthesizing uncapped transcripts were identical, except that the cap analog was replaced with water, and 10 μl of 75 mM GTP rather than 15 mM GTP was used. The reaction tubes in each case were incubated at 37°C for 2 h to permit transcription. Transcription reactions were diluted with 4 volumes of calcium- and magnesium-free PBS and immediately frozen on dry ice, then stored at −80° for up to 3 days.
Intrahepatic Transfection.
Rhesus monkeys (Macaca mulatta) and chimpanzees (Pan troglodytes) were housed at Bioqual (Rockville, MD). The housing, maintenance, and care of the animals met or exceeded all requirements for primate husbandry.
Diluted transcription mixtures were thawed and, guided by ultrasound, were inoculated percutaneously into multiple sites in the liver (21). The rhesus monkeys and chimpanzees inoculated with the pSK-HEV-3 transcripts and chimpanzee 96A007 inoculated with the pSK-HEV-2 transcripts were each injected with 1 ml of diluted transcription mixture. The other animals receiving pSK-HEV-2 transcripts (Rh 622, Rh 624, and chimpanzees 1619, 1620, 1622, and 1627) each received 600 μl of diluted transcripts. Animals were bled weekly, and serum levels of isocitrate dehydrogenase (ICD) and alanine aminotransferase (ALT) were determined by standard methods (Anilytics, Gaithersburg, MD). Baseline levels were calculated as the geometric mean of three weekly samples collected at week zero and before inoculation: serum enzyme levels ≥2 times background were considered elevated and indicative of hepatitis. Antibody to HEV was detected by an in-house ELISA based on recombinant Sar-55 ORF2 protein as previously described, except that the ORF2 protein was more highly purified (22, 23). Percutaneous needle biopsies of the liver were obtained weekly and read under code by a pathologist (S.G.). Histopathology scores of 1+ to 4+ were assigned to denote mild to severe hepatitis, respectively.
Genome Quantification.
RNA was extracted from serum samples with Trizol reagent (Life Technologies, Gaithersburg, MD) and from 10% fecal suspensions in PBS, pH 7.4. HEV genome titer was quantified by Taqman RT-PCR (PE Biosystems). The Taqman assay was standardized with in vitro transcripts of a Sar-55 cDNA clone that were metabolically labeled by incorporation of 3H-UTP. The assay could detect HEV RNA with a sensitivity of 1–10 genomes/ml.
Sequence Analysis.
RT-PCR products amplified from serum or feces were purified on agarose gels and sequenced directly with an automated sequencer to yield the consensus sequence. Secondary structures were predicted with the program mfold.
Results
Transcripts from pSK-HEV-3 Were Infectious for Chimpanzees but Not for Rhesus Monkeys.
The cDNA clone pSK-HEV-3 contained the consensus sequence of the Sar-55 strain except for four introduced mutations that did not change the predicted amino acids. Capped transcripts synthesized in vitro from this cDNA were tested for infectivity by intrahepatic inoculation into the liver of rhesus monkeys 471 and 407. Rhesus monkeys were chosen because they are very susceptible to infection by the Sar-55 strain, and much is known about the natural history of the virus in these animals (15, 18, 22). Although rhesus 407 had elevated serum liver enzymes during 1 week, neither monkey developed antibody to HEV, and the viral genome was not detected in sera by a sensitive manual RT-PCR assay (Table 1), indicating that neither rhesus monkey was demonstrably infected.
Table 1.
Intrahepatic transfection of primates with recombinant HEV genomes: Summary
| Transcripts | Animal* | Peak post/pre-ALT,† week | Peak genome titer,‡ week | Week of seroconversion |
|---|---|---|---|---|
| 7,106 mutant§ | ||||
| (capped) | Rh 471 | 1.5 (1) | ** | Not infected¶ |
| Rh 407 | 2.3 (5) | ** | Not infected¶ | |
| Ch 1609 | 1.4 (14) | 5 (12) | 14 | |
| Ch 1603 | 1.4 (11) | 1.7 (13) | 14 | |
| 7,106 wild type‖ | ||||
| (capped) | Rh 624 | 4.8 (5) | 16,890 (5) | 5 |
| Rh 622 | 2.2 (7) | 126 (7) | 8 | |
| Ch 96A007 | 2.7 (4) | 72 (3) | 5 | |
| Ch 1620 | 1.4 (6) | 18 (5) | 6 | |
| Ch 1619 | 2.0 (8) | 10 (5) | 7 | |
| 7,106 wild type‖ | ||||
| (uncapped) | Ch 1622 | 1.5 (14) | ** | Not infected¶ |
| Ch 1627 | 1.0 (9) | ** | Not infected¶ |
RH, rhesus; Ch, chimpanzee.
Ratio of highest ALT to geometric mean of three pretransfection values.
Per milliliter of serum (Taqman RT-PCR).
pSK-HEV-3 (G mutated to T at position 7,106; T mutated to C at position 7181).
Seroconversion not detected in 20 weeks of followup.
pSK-HEV-2.
Not done.
Because rhesus monkeys are susceptible to infection by many HEV strains, including the Sar-55 strain, and because we have successfully infected both tamarins (hepatitis A virus) and chimpanzees (hepatitis C virus) by the intrahepatic transfection technique, the failure to infect with HEV transcripts suggested that the cDNA clone did not encode an infectious virus (24, 25). However, we had not previously attempted intrahepatic transfection of rhesus monkeys, and it was conceivable that unknown technical problems made it impossible to use the technique in this species. Because the transfection procedure has been validated in chimpanzees, and because they are also susceptible to infection with HEV, the transfection was repeated exactly as before, except two seronegative chimpanzees rather than macaques were inoculated
Although neither animal developed elevated serum liver enzyme levels indicative of hepatitis, each inoculated chimpanzee developed antibody to HEV at week 14 posttransfection, indicating that infection had occurred (Table 1). The first chimpanzee, chimp 1609, had low-level viremia (four to five genomes/ml), which occurred during the 2 weeks preceding seroconversion. Liver histopathology was negative except for biopsies taken the 2 weeks before viremia: they were scored as 1+, suggesting mild hepatitis. Two different regions of the HEV genome, amplified by RT-PCR from serum obtained at week 13, were sequenced. The sequence of the first product (165 nucleotides) confirmed that the silent mutation introduced into the cDNA clone at nucleotide 286 in ORF1 was still present and proved that the infecting virus originated from the cDNA clone. However, the sequence of the second RT-PCR product, 224 nt in length, indicated that the mutations introduced at position 7106 in ORF2 and at 7181 were retained but that the nucleotide at position 7144 (in the 3′ noncoding region) had mutated from a C to an A. The second chimpanzee, chimp 1603, experienced viremia at week 13, but the titer was only 2 genomes/ml. Liver histopathology in this animal was normal for all samples collected weekly during weeks 0–15. The region encompassing nucleotide position 7106 could not be amplified from the serum but was amplified from feces collected from chimp 1603 during week 13. Sequence analysis indicated that the introduced G to T mutation at 7106 and the T to C mutation at 7181 were retained throughout the infection but that the nucleotide at position 7097 had mutated from a G to an A. Although this mutation occurred in the region encoding ORF2 protein, it did not change the predicted amino acid.
Transcripts Containing a Reversion of the 7106 Mutation Encoded a More Virulent Virus.
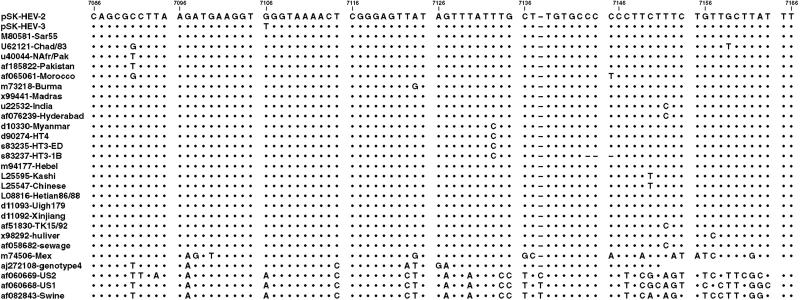
Although the successful infection of chimpanzees demonstrated that the cDNA clone pSK-HEV-3 encoded an infectious virus, the prolonged time to seroconversion suggested that the virus was attenuated for chimpanzees. Comparison of the sequence of the cDNA clone and those of other HEV genomes indicated that the nucleotides introduced into pSK-HEV-3 at positions 286 and 4396 and 7181 were naturally present in some other genotype 1 strains and were, therefore, most likely not detrimental. In contrast, the T introduced at position 7106 was unique to the pSK-HEV-3 cDNA clone and was located in a highly conserved region of the genome. In all other sequences published to date, this position contained either a G or an A (Fig. 1).
Figure 1.
Sequence alignment of 3′ region of HEV genomes. Numbering corresponds to sequence of pSK-HEV-2. A dot denotes identity, and a dash denotes a deletion.
The unique T at position 7106 was corrected to the consensus G to produce a second cDNA clone (pSK-HEV-2) that was identical to the first except for this one difference and that at 7181: capped transcripts from the modified clone were inoculated into the liver of chimpanzee 96A007. The chimpanzee developed hepatitis as indicated by a 2.5-fold rise in the serum ALT level at week 4 (Table 1), but the liver histology remained normal. The animal seroconverted to anti-HEV at week 5. Viremia levels were 15–40 times higher (peak titer 71 genomes/ml at week 3) than previously observed after infection of chimpanzees with the uncorrected clone. The region spanning position 7106 was amplified from feces collected during week 2 from chimp 96A007. In contrast to the genomes recovered from each of the chimps inoculated with the unmodified clone, the 224-nt-long sequence obtained after infection with the corrected genome was identical to that of the cDNA clone used for transcription. The appearance of hepatitis and the normal time to seroconversion suggested that the virus encoded by the corrected cDNA clone, pSK-HEV-2, was more virulent than that from the clone containing the 7106 mutation.
Comparison of Infection in Rhesus Monkeys and Chimpanzees Transfected with the Modified Clone.
To confirm that the virus from the corrected clone, pSK-HEV-2, was robust and to determine whether rhesus monkeys could be infected with it, additional transfections were performed. Identical aliquots of capped transcripts were inoculated intrahepatically into two rhesus monkeys and into two additional chimpanzees. A batch of uncapped transcripts synthesized with the same reagents in a parallel reaction in which the m7G cap analogue was replaced with GTP was similarly inoculated intrahepatically into two other chimpanzees to determine whether the cap structure was required for infectivity. The two RNA preparations appeared to be identical when examined by agarose gel electrophoresis (Fig. 2) except, as expected, slightly more RNA was synthesized in the reaction containing GTP in place of the cap analogue.
Figure 2.
Ethidium bromide agarose gel of 5 μl of capped (Left) or uncapped (Right) RNA transcribed in vitro from pSK-HEV-2.
Both rhesus monkeys and both chimpanzees injected with the capped transcripts became infected, and all four animals seroconverted to anti-HEV (Table 1). Chimpanzees 1620 and 1619 became antibody positive at weeks 6 and 7 posttransfection, respectively. Chimpanzee 1620 experienced a slight elevation of serum ALT around the time of seroconversion when hepatitis would normally occur, but the elevation above baseline was less than the 2-fold elevation indicative of hepatitis. Viremia was detected only at week 5 (18 genomes/ml). Chimpanzee 1619 had an ALT level exactly twice the preinoculation level, suggesting that it had mild hepatitis and that it had viremia during weeks 5 and 6 (10 and 5 genomes/ml, respectively). Similarly, rhesus monkey 624 seroconverted at week 5, and rhesus 622 seroconverted at week 8 posttransfection. Rhesus 624 developed significant hepatitis, with a serum ALT level 4.8 times and an isocitrate dehydrogenase (ICD) level 4.4 times above baseline at week 5, the time of seroconversion. This animal had the longest period of viremia (4 weeks) and the highest titer of virus (16,900 genomes/ml) of the four animals in this experiment. Rhesus 622 had a milder hepatitis, with peak serum ALT and ICD levels just slightly 2-fold above baseline the week before seroconversion. Although viremia was detected in this animal during weeks 5–7, peak titer reached only 126 genomes/ml.
The region surrounding the silent mutation at position 286 was amplified from the serum of all four animals. In each case, the mutation was present, thus confirming that the virus replicating in each animal originated from the cDNA clone. The 224-nt-long region preceding the poly(A) tail was also amplified and sequenced. Once again, in contrast to our detection of mutations in virus recovered from chimps 1603 and 1609, which were transfected with the uncorrected virus clone, mutations were not detected in viruses recovered from any of the four animals, and the consensus sequences of the recovered viruses were identical to that in the pSK-HEV-2 cDNA clone.
Uncapped Transcripts Are Not Infectious.
The two chimpanzees inoculated with uncapped transcripts were followed for 20 weeks. Neither chimpanzee became infected, because they neither developed hepatitis nor seroconverted to anti-HEV.
Discussion
RNA transcribed from the two cDNA clones of HEV described herein, which differed by two nucleotides, was infectious for chimpanzees, but the two clones encoded viruses with quite different phenotypes. Transcripts from the clone pSK-HEV-3, containing a T at position 7106, were not able to infect rhesus monkeys after intrahepatic inoculation but were able to infect chimpanzees. However, the virus was apparently attenuated: hepatitis was not observed in either of the chimpanzees infected with this virus, and the 14-week incubation period preceding seroconversion was prolonged compared with the 3–5 weeks observed previously after infection with wild-type Sar-55 virus contained in clinical samples (22).
In contrast, transcripts from the pSK-HEV-2 cDNA clone, in which the T at position 7106 had been mutated back to the G present in the wild-type strain, produced virus with wild-type phenotype. Whereas the genomes from the unmodified clone were unable to infect rhesus monkeys, the genomes transcribed from the modified cDNA clone infected each of two inoculated rhesus monkeys, and both monkeys developed hepatitis. One rhesus monkey seroconverted at 5 weeks postinoculation and the other at 8 weeks. The time to seroconversion in these two transfected rhesus monkeys was similar to those observed previously when the Sar-55 virus in a clinical sample was titrated in cynomolgus macaques, a species used interchangeably with rhesus macaques for HEV studies (16). The titration study demonstrated that time to seroconversion increased as the dose of virus was decreased, but the severity of hepatitis generally decreased as the dose was decreased. However, severity usually remained in the mild to moderate range even after inoculation with a large dose of virus. Small amounts of wild-type virus could infect animals in some cases without causing disease, consistent with the absence of elevated serum liver enzymes in chimp 1620. In the titration study, the greatest 10-fold dilution that still infected monkeys resulted in seroconversion in the two inoculated monkeys at weeks 8 and 11, respectively, but hepatitis was not observed: inoculation of 100 times more virus caused seroconversion in each of two monkeys at 5 weeks postinoculation and mild hepatitis in one of the monkeys.
Data from the chimpanzee infections further suggested that the virus produced from the corrected clone, pSK-HEV-2, had a wild-type phenotype. Two of the three inoculated chimpanzees (96A007 and 1619) developed hepatitis, and all three chimpanzees seroconverted to anti-HEV between 5 and 7 weeks posttransfection, the time expected on the basis of studies of wild-type Sar-55 virus.
Together, the data indicated that the T mutation at position 7106 was detrimental and attenuated the virus. This attenuation occurred even though the mutation, which was in the coding region of the capsid protein, did not change the encoded amino acid. Therefore, the mutation must be located within a cis-reacting element. The very high degree of nucleotide conservation in the region surrounding the mutation lends support to this conclusion. On the basis of in vitro studies, Agrawal et al. have suggested that the 3′ region of a number of genotype 1 strains (including the Pakistani strain Sar-55) forms a stem–loop structure that is important for binding of the RNA polymerase (26). The introduced G to T mutation in our pSK-HEV-3 cDNA clone is located in a short-stem structure in their model (Fig. 3 A and B).
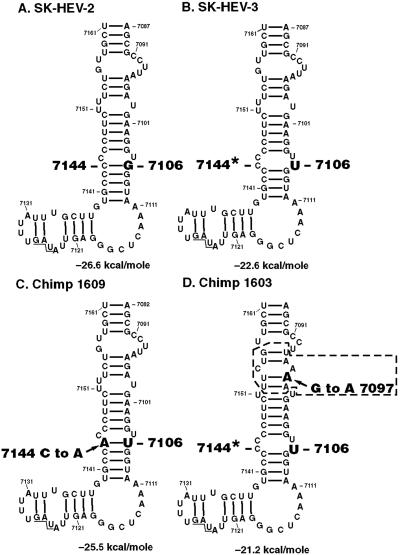
Figure 3.
RNA structure of the 3′ end of Sar-55 genomes as predicted by mfold. Sequences are numbered according to pSK-HEV-2. The sequence between nucleotides 7082–7208 was analyzed, but only nucleotides 7087–7161 are shown: the excluded region had the identical structure in all four cases, but nucleotide 7181, located in a 3-base loop, was a U in SK-HEV-2 compared to a C in the other three cases. (A) SK-HEV-2 (wild-type) sequence; (B) SK-HEV-3 with G to U substitution at nucleotide 7106; (C) sequence recovered from chimp 1609; (D) sequence recovered from chimp 1603. Arrow, new mutation; box, base-pairing affected by new mutation; *, base-paired in wild type; underlined, translation termination codon (7125–7127).
Computer predictions of the 3′ RNA structure are shown for the original genomes and those recovered from the transfected animals (Fig. 3). The new mutation that was selected during replication of the virus in chimp 1609 changed a C residue to an A, with the result that it restored the identical 3′ structure as predicted for wild-type, except that a G/C base pair was replaced with a U/A base pair (Fig. 3C). Selection of this particular “compensatory” mutation strongly suggested that this putative stem was important. The new mutation selected by passage of the virus in the second chimpanzee, chimp 1603, is more difficult to interpret. This G to A mutation would eliminate a G/C base pair predicted by the folding program but not confirmed by chemical probing of in vitro transcripts of the Indian strain (26). However, the new mutation would alter the predicted structure of the Sar-55 strain by removing three bases from a loop just upstream of the mutated site (Fig. 3D). Because this mutation was selected for, it most likely provided a positive replicative advantage. It could be speculated that chimp 1603 had detectable viremia and fecal shedding for only half as long as did chimp 1609, because the new mutation in this case only partially restored the robustness of the virus. That new mutations were not selected in this region in genomes recovered from any of the five animals infected with virus encoded by the corrected clone pSK-HEV-2 suggested the sequence of this clone was optimal for infection of these animals.
The HEV genome within virions has a m7G cap at its 5′ end, and the HEV genome apparently encodes the guanyltransferase and methyltransferase enzymes required to cap the viral RNA (12–14). These data, taken in conjunction with the demonstration that capped Sar-55 transcripts infected chimpanzees, whereas uncapped transcripts did not, suggested that a cap is required for viral viability. The in vivo transfection results provide an explanation for the failure of Panda et al. (19) to infect rhesus monkeys with HEV transcripts from an Indian strain, because they used only uncapped transcripts. However, it is not clear how they were able to infect cultured Hep G2 cells with uncapped transcripts. We have not been able to infect this cell line with any of seven well-characterized strains of HEV we have tested and, thus far, we have not been able to transfect Hep G2 cells with either capped or uncapped transcripts of the pSK-HEV-2 clone (data not shown).
The generation of a cDNA clone of HEV that is able to infect primates provides an opportunity to study the relevant molecular biology of this virus. The ability to use rhesus monkeys rather than chimpanzees is advantageous, because rhesus monkeys are less expensive and easier to manage than are chimps, and some wild rhesus monkeys have antibody to HEV, suggesting that they are a natural host for HEV. Thus, the identification of a cis-reacting element and of an attenuating mutation within it provide an opportunity to study attenuation of this virus in one of its natural hosts.
Acknowledgments
This work was supported in part by National Institute of Allergy and Infectious Diseases Contract No. 1-AO-02733.
Abbreviations
- HEV
hepatitis E virus
- ALT
alanine aminotransferase
- RT-PCR
reverse transcription–PCR
Footnotes
References
- 1.Purcell R H, Emerson S U. In: Fields Virology. Knipe D, Howley P, Griffin D, Lamb R, Martin M, Roizman B, Straus S, editors. Williams & Wilkins, Philadelphia: Lippincott; 2001. pp. 3051–3061. [Google Scholar]
- 2.Balayan M S, Andjaparidze A G, Savinskaya S S, Keliladze E S, Braginsky D M, Savinov A P, Poleschuk V F. Intervirology. 1983;20:23–31. doi: 10.1159/000149370. [DOI] [PubMed] [Google Scholar]
- 3.Tam A W, Smith MM, Guerra M E, Huang C C, Bradley D W, Fry K E, Reyes G R. Virology. 1991;185:120–131. doi: 10.1016/0042-6822(91)90760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C C, Nguyen D, Fernandez J, Yun K Y, Fry K E, Bradley D W, Tam A W, Reyes G. Virology. 1992;191:550–558. doi: 10.1016/0042-6822(92)90230-m. [DOI] [PubMed] [Google Scholar]
- 5.Meng X J, Purcell R H, Halbur P G, Lehman J R, Webb D M, Tsareva T S, Haynes J S, Thacker B J, Emerson S U. Proc Natl Acad Sci USA. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlauder G G, Mushahwar I K. J Med Virol. 2001;65:282–292. doi: 10.1002/jmv.2031. [DOI] [PubMed] [Google Scholar]
- 7.Meng X J, Halbur P G, Shapiro M S, Govindarajan S, Bruna J D, Mushahwar I K, Purcell R H, Emerson S U. J Virol. 1998;72:9714–9721. doi: 10.1128/jvi.72.12.9714-9721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabrane-Lazizi Y, Fine J B, Elm J, Glass G E, Higa H, Diwan A, Gibbs C J, Meng X J, Emerson S U, Purcell R H. Am J Trop Med Hyg. 1999;61:331–335. doi: 10.4269/ajtmh.1999.61.331. [DOI] [PubMed] [Google Scholar]
- 9.Arankalle V A, Goverdhan M K, Banerjee K. J Viral Hepat. 1994;1:125–129. doi: 10.1111/j.1365-2893.1994.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 10.Balayan M S, Usmanov R K, Zamyatina N A, Karas F R. J Med Virol. 1990;32:58–59. doi: 10.1002/jmv.1890320110. [DOI] [PubMed] [Google Scholar]
- 11.Koonin E V, Gorbalenya A E, Purdy M A, Rozanov M N, Reyes G R, Bradley D W. Proc Natl Acad Sci USA. 1992;89:8259–8263. doi: 10.1073/pnas.89.17.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabrane-Lazizi Y, Meng X J, Purcell R H, Emerson S U. J Virol. 1999;73:8848–8850. doi: 10.1128/jvi.73.10.8848-8850.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Purcell R H, Emerson S U. J Med Virol. 2001;65:293–295. doi: 10.1002/jmv.2032. [DOI] [PubMed] [Google Scholar]
- 14.Magden J, Takeda N, Li T, Auvinen P, Ahola T, Miyamura T, Merits A, Kääriäinen L. J Virol. 2001;75:6249–6255. doi: 10.1128/JVI.75.14.6249-6255.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Purcell R H, Emerson S U. ILAR J. 2001;42:161–177. doi: 10.1093/ilar.42.2.161. [DOI] [PubMed] [Google Scholar]
- 16.Tsarev S A, Tsareva T S, Emerson S U, Yarbough P O, Legters L J, Moskal T, Purcell R H. J Med Virol. 1994;43:135–142. doi: 10.1002/jmv.1890430207. [DOI] [PubMed] [Google Scholar]
- 17.Mast E E, Alter M J, Holland P V, Purcell R H. Hepatology. 1998;27:857–861. doi: 10.1002/hep.510270331. [DOI] [PubMed] [Google Scholar]
- 18.Tsarev S A, Tsareva T S, Emerson S U, Rippy M K, Zack P, Shapiro M, Purcell R H. J Infect Dis. 1995;172:31–37. doi: 10.1093/infdis/172.1.31. [DOI] [PubMed] [Google Scholar]
- 19.Panda S K, Ansari I H, Durgapal H, Agrawal S, Jameel S. J Virol. 2000;74:2430–2437. doi: 10.1128/jvi.74.5.2430-2437.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsarev S A, Emerson S U, Reyes G R, Tsareva T S, Legters L J, Malik I A, Iqbal M, Purcell R H. Proc Natl Acad Sci USA. 1992;89:559–563. doi: 10.1073/pnas.89.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanagi M, St. Claire M, Shapiro M, Emerson S U, Purcell R H, Bukh J. Virology. 1998;244:161–172. doi: 10.1006/viro.1998.9092. [DOI] [PubMed] [Google Scholar]
- 22.Tsarev S A, Tsareva T S, Emerson S U, Kapikian A Z, Ticehurst J, London W, Purcell R H. J Infect Dis. 1993;168:369–378. doi: 10.1093/infdis/168.2.369. [DOI] [PubMed] [Google Scholar]
- 23.Robinson R A, Burgess W H, Emerson S U, Leibowitz R S, Sosnovtseva S A, Tsarev S, Purcell R H. Protein Expression Purif. 1998;12:75–84. doi: 10.1006/prep.1997.0817. [DOI] [PubMed] [Google Scholar]
- 24.Yanagi M, Purcell R H, Emerson S U, Bukh J. Proc Natl Acad Sci USA. 1997;94:8738–8743. doi: 10.1073/pnas.94.16.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emerson S U, Lewis M, Govindarajan S, Shapiro M, Moskal T, Purcell R H. J Virol. 1992;66:6649–6654. doi: 10.1128/jvi.66.11.6649-6654.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agrawal S, Gupta D, Panda S K. Virology. 2001;282:87–101. doi: 10.1006/viro.2000.0819. [DOI] [PubMed] [Google Scholar]