Abstract
Epstein–Barr virus (EBV) and Kaposi sarcoma-associated herpesvirus (KSHV) comprise the oncogenic human γ-herpesvirus family and are responsible for 2–3% of all tumours in man. With their prominent growth-transforming abilities and high prevalence in the human population, these pathogens have probably shaped the human immune system throughout evolution for near perfect immune control of the respective chronic infections in the vast majority of healthy pathogen carriers. The exclusive tropism of EBV and KSHV for humans has, however, made it difficult in the past to study their infection, tumourigenesis and immune control in vivo. Mice with reconstituted human immune system components (humanized mice) support replication of both viruses with both persisting latent and productive lytic infection. Moreover, B-cell lymphomas can be induced by EBV alone and KSHV co-infection with gene expression hallmarks of human malignancies that are associated with both viruses. Furthermore, cell-mediated immune control by primarily cytotoxic lymphocytes is induced upon infection and can be probed for its functional characteristics as well as putative requirements for its priming. Insights that have been gained from this model and remaining questions will be discussed in this review.
This article is part of the theme issue ‘Silent cancer agents: multi-disciplinary modelling of human DNA oncoviruses’.
Keywords: Epstein–Barr virus, Kaposi sarcoma-associated herpesvirus, lymphoma, anti-viral immune control, humanized mice
1. Introduction
The two human γ-herpesviruses Epstein–Barr virus (EBV or HHV4) and Kaposi sarcoma-associated herpesvirus (KSHV or HHV8) are among the seven viruses that the World Health Organization has classified as class I carcinogens (besides human papilloma virus (HPV), Merkel cell polyomavirus (MCPyV), hepatitis B and C viruses and human T-cell lymphotropic virus 1 (HTLV-1)) [1–3]. In addition, only one bacterium (Helicobacter pylori) and three parasites (Schistosoma haematobium, Opisthorchis viverrini and Clonorchis sinensis) have been suggested to cause cancers in humans [4]. These infection-associated malignancies are estimated to make up 20% of the tumour load in humans and of the 11 implicated pathogens only five are thought to have direct growth-transforming properties, namely encode oncogenes (EBV, KSHV, HPV, MCPyV and HTLV-1). In vivo models that would allow for tumour induction upon infection exist so far only for EBV, KSHV and HTLV-1 [5–8], and most of those that use the human pathogens and not related viruses in monkeys and rodents, are based on mice with reconstituted human immune system components (humanized mice). In order to reconstitute most human immune system compartments with minimal graft-versus-host disease, hematopoietic progenitor cells (HPCs) are injected into genetically modified mice that lack mouse lymphocytes either with or without a human thymic transplant, and these give rise to most human leucocyte compartments [9].
By conservative estimates, 10% of infection-associated tumours can be attributed to EBV and KSHV with a yearly incidence of 200 000 new virus-associated cancers for EBV alone [10]. EBV is also the only human oncogenic pathogen that can directly transform its main host cell, the human B cell, in vitro and therefore has the strongest directly tumour-inducing abilities of all cancer-associated pathogens [11]. This is particularly surprising, because EBV is also the most widely distributed member of these human oncogenic pathogens and human viruses in general, with more than 90% of the adult population being persistently infected. Moreover, the infection programmes that can be found in EBV-associated malignancies, namely latency I in Burkitt lymphoma and 10% of gastric carcinomas, latency II in the 50% of EBV-associated classical Hodgkin lymphoma and nasopharyngeal carcinoma and latency III in diffuse large B-cell lymphomas and post-transplant lymphoproliferative disorders, are also found in distinct differentiation stages of human B cells in healthy EBV carriers [12,13]. All eight latent EBV proteins and the non-translated RNAs are expressed in infected naive B cells (latency III), whereas only the nuclear antigen 1 (EBNA1) and the latent membrane proteins (LMP1 and 2) plus non-translated RNAs are expressed in germinal centre B cells (latency II). Homeostatically proliferating memory B cells reduce EBV gene expression even further to only EBNA1 and the non-translated RNAs (latency I). Infected quiescent memory B cells express only non-translated EBV RNAs (latency 0) and probably serve as the reservoir for long-term viral persistence [14]. From this site, cognate antigen recognition by the B-cell receptor and ensuing plasma cell differentiation are thought to trigger lytic EBV replication [15]. These premalignant states of oncogenic EBV gene expression programmes are seemingly kept in check by immune control, which will be discussed in further detail below.
KSHV, on the other hand, is much less growth-transforming in vitro and has even difficulties establishing persistent infection in its main host cells, namely B and endothelial cells in vitro [16,17]. In vivo it is, however, associated with the vascular tumour Kaposi sarcoma (KS) and the two B-cell neoplasms primary effusion lymphoma (PEL) and multicentric Castleman's disease (MCD). Only in vitro propagated PEL cell lines keep the KSHV genome [18]. This difficulty in establishing persistent infection is also reflected in its low seroprevalence (less than 10%) in most geographical regions of the world, including Europe and the USA. Seroprevalence is, however, significantly higher in sub-Saharan Africa (greater than 50%) [18]. Viral gene expression varies greatly in established PEL cell lines and KSHV-associated malignancies. While the core latency antigens, latency-associated nuclear antigen (LANA), viral cyclin (vCYC) and viral FLICE inhibitory protein (vFLIP) plus non-translated RNAs are expressed in all KSHV-associated tumours, expression of other KSHV genes is variable and often includes at least a minimal set composed of K1, K15 and the viral G-protein-coupled receptor homologue [18]. However, lytic KSHV protein expression is additionally found in many KSHV-associated malignancies and may be required for the respective tumours [19]. As for EBV, the increased incidence of KSHV-associated malignancies in immunocompromised individuals is taken as an indication that immune control constrains KSHV pathogenesis in persistently infected individuals, but the nature of this protective immune response is less well understood than for EBV.
In addition to their growth-transforming abilities, EBV and KSHV have also developed various strategies to escape immune responses [20]. These include targeting of interferon regulatory factors (IRFs) by KSHV and nearly complete EBV episome methylation to avoid innate immune detection, thereby inhibiting type I interferon production [21,22], downregulation of activating ligands for natural killer (NK) cells by viral miRNAs or KSHV ubiquitin ligases [23–25], inhibiting major histocompatibility complex (MHC) restricted antigen presentation by viral miRNAs, KSHV ubiquitin ligases and lytic EBV proteins that block peptide loading or MHC trafficking [26–28] and regulation of the cytokine and chemokine network by virally encoded cytokines, chemokines or soluble cytokine receptors, like KSHV vIL-6 and EBV vIL-10 [29,30]. Despite these immune evasion mechanisms, KSHV and EBV are efficiently immune controlled in most immunocompetent virus carriers.
Thus, EBV and KSHV are two of the 11 oncogenic pathogens in humans and encode oncogenes. These viruses and their new in vivo models of tumourigenesis and immune control will help us gain better understanding of the complexity of herpesviral infection, the characteristics of B-cell lymphomas that are induced by them and which protective entities of the virus-specific immune responses may control these tumour viruses in healthy EBV and KSHV carriers. These aspects will be discussed in this review.
2. Infection of humanized mice by Epstein–Barr virus and Kaposi sarcoma-associated herpesvirus
Both viruses seem to be transmitted predominantly via saliva in humans [31–33]. The next steps, however, remain somewhat enigmatic. Epithelial cell infection has been postulated, but virus in saliva is more prone to infect B cells, and polarized epithelia have been shown to be primarily susceptible to EBV infection from the basolateral side [34,35]. These findings would argue that EBV infects epithelia during shedding and is transported across mucosal epithelia during primary or frequent reinfections in humans [36,37]. Even less is known about how KSHV overcomes the mucosal epithelial barrier. Since humanized mice are only reconstituted with human immune system components, all epithelia are of mouse origin. Accordingly, epithelial cell infection with EBV and KSHV cannot be addressed in this model. However, if systemic infection of humanized mice would occur upon virus deposition at mucosal surfaces, this could indicate that these viruses are indeed transported across mucosal barriers without directly infecting them.
Human B cells are a reservoir for both viruses [14,38], and this part of their life cycle can be modelled for EBV and KSHV in humanized mice. EBV viral load increases in blood and secondary lymphoid organs up to four weeks after intraperitoneal EBV infection of humanized mice and is exclusively localized to human B cells [8,39–41]. Evidence for all EBV latency programmes and limited lytic EBV replication can be found [40,42–44]. By comparing recombinant EBVs that are either sufficient or deficient in the immediate early gene product BZLF1, which is required for lytic cycle transactivation, it was shown that lytic EBV replication only transiently contributes to viral loads in immunocompetent humanized mice [40] (figure 1). However, lytic EBV replication can be increased by using viruses with similarities to those found in nasopharyngeal carcinoma [45,46]. In some instances, increased lytic reactivation could be traced to polymorphisms in the BZLF1 gene. In addition to the BZLF1-deficient virus other recombinant EBV strains have further revealed surprising aspects of EBV infection. While LMP1 is required to transform B cells during EBV infection in vitro, LMP1 knock-out viruses were found to persist in B cells with CD4+ T-cell help in vivo [47] (figure 2 and table 1). Similarly, LMP2-deficiency does not abolish EBV persistence in vivo, and even a knock-out virus for both latent membrane proteins is capable of establishing chronic infection in humanized mice [55]. Even prior to LMP1 expression, which can be delayed by more than one week during primary B-cell infection in vitro, the EBNA3A and predominantly the EBNA3C proteins are required to rescue EBV-infected B cells from EBNA2-induced proliferation triggered cell death [56,57]. Surprisingly, both EBNA3A and 3C-deficient viruses can persist in humanized mice [58,59]. In immunocompetent humanized mice, infection with EBNA3A and 3C-deficient viruses was restricted to secondary lymphoid tissues and persisted for at least three months. At this time point, primarily non-translated EBV RNA without latent protein expression could be detected in EBV-infected B cells. This infection programme is reminiscent of EBV infection in quiescent memory B cells in humans and the reservoir of long-term EBV persistence [14]. Interestingly, this EBV reservoir was reached without any significant LMP1 expression along the way, while EBNA2 expression and associated proliferation was readily detectable after five weeks of infection. This indicates that EBV persistence can be reached without prior establishment of the complete latency III programme (figure 1), possibly via an extrafollicular route. These studies suggest that EBV can access all of its latent and lytic infection programmes after B-cell infection in humanized mice and that mutant EBVs can be used to enhance or diminish the individual components of this complex infection composition. This should allow us to dissect the individual contributions of EBV antigens to EBV infection in much more detail.
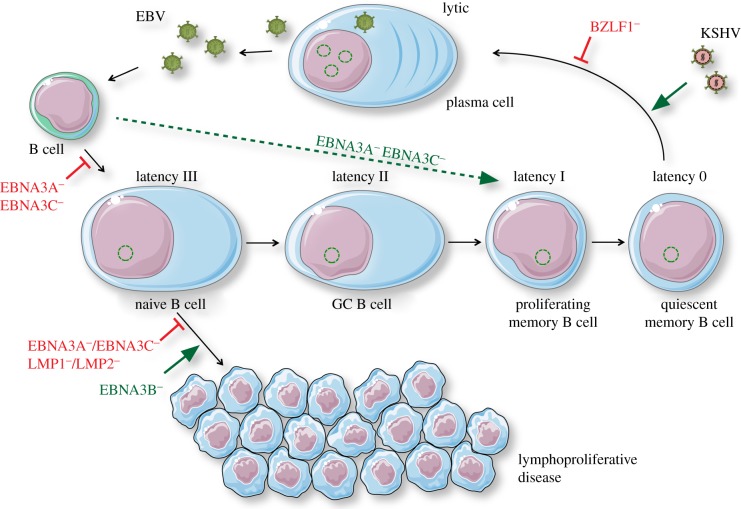
Figure 1.
Requirements for persistence, lytic replication and lymphomagenesis as revealed by mutant EBV infection and KSHV co-infection of humanized mice. EBV infection of B cells establishes different latent EBV infection programmes (0, I, II and III) in B-cell differentiation stages of healthy EBV carriers and reactivates from the memory B-cell pool into lytic replication. Lymphoproliferations develop in humanized mice primarily from latency III infected B cells. EBV nuclear antigen 3A and 3C (EBNA3A and 3C), or latent membrane protein 1 and 2 (LMP1 and 2) deficient viruses are compromised in B-cell lymphoma establishment. EBNA3B-deficient EBV causes lymphomas at increased frequencies. EBNA3A and 3C-deficient viruses block transition into complete latency III but allow direct access to persistence in latency 0. BZLF1-deficient EBV cannot access lytic replication and KSHV co-infection increases lytic replication of wild-type EBV. Inhibitory interactions are indicated by blocked lines, e.g. BZLF1−, and activating interactions by large arrows, e.g. KSHV. This figure was created in part with modified Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 unported license: https://smart.servier.com. (Online version in colour.)
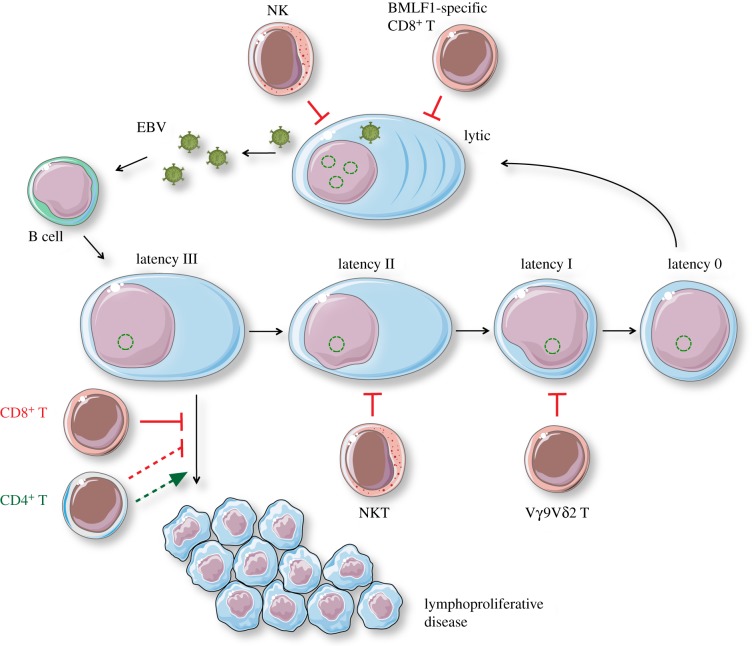
Figure 2.
Components of EBV-specific immune control in humanized mice. Innate and adaptive lymphocyte populations restrict different stages of EBV infection. NK cells and lytic EBV antigen-specific CD8+ T cells like those recognizing BMLF1-derived epitopes restrict virus-producing cells. EBV latency III-driven lymphoproliferations are mainly restricted by cytotoxic CD8+ T cells, while CD4+ T cells may support lymphomagenesis under certain circumstances, like, for example, LMP1-deficient EBV infection. NKT cells might preferentially target EBV latency II and Vγ9 Vδ2T cells recognize mainly B cells with EBV latency I. Immune restrictions are indicated by blocked lines, e.g. NK, and lymphocyte help as large arrows, e.g. CD4+T. Solid lines represent main functions, while broken lines indicate that the respective lymphocytes can have restricting or supporting functions. This figure was created in part with modified Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 unported license: https://smart.servier.com. (Online version in colour.)
Table 1.
Lymphocyte-mediated restriction of EBV infection in humanized mice.
| lymphocyte population | targeted EBV programme | select references |
|---|---|---|
| NK cells | restricting lytic EBV replication | [41,48] |
| NKT cells | stimulated by EBV latency II and restricting EBV lymphomas | [49] |
| Vγ9 Vδ2 T cells | stimulated by EBV latency I and restricting EBV lymphomas | [50,51] |
| CD8+ αβ T cells | restricting lytic and latent EBV infection | [39,52–54] |
| CD4+ αβ T cells | restricting lytic and latent EBV infection, but in some conditions also supporting EBV-associated lymphomagenesis and latency I/II | [39,43,47] |
KSHV seems to only transiently infect humanized mice and persists only in less than 20% of these animals for at least one month [8,60]. However, upon EBV co-infection KSHV is maintained in the majority of animals [8]. Four weeks after infection KSHV is mainly found in B cells, especially in those that also express the non-translated RNAs of EBV. This suggests that KSHV can persist in EBV-infected B cells in humanized mice. These dual-infected B cells can be cultured from double-infected humanized mice in vitro and demonstrate broad expression of KSHV genes, including high levels of the KSHV non-translated RNA pan, lytic KSHV gene products as well as the latent KSHV gene products LANA, vCYC and vFLIP. Therefore, KSHV persistence during EBV co-infection provides us with the opportunity to interrogate the function of a wide variety of KSHV gene products in vivo.
3. Tumours that can be modelled after infection with Epstein–Barr virus and Kaposi sarcoma-associated herpesvirus
Infection of humanized mice with the human tumourviruses EBV and KSHV generates autologous lymphomas and enables us to investigate the requirements for their development and immune control. High-dose EBV infection (105 infectious virus particles) leads to lymphoproliferative lesions in 20–30% of the infected animals [8,40,41]. These tumours are B-cell lymphomas with latency III EBV gene expression which disseminate from the spleen to liver, kidney, lymph nodes and pancreas [8,39]. Although latent EBV antigens are presumably responsible for the transformation of the tumour cells, the competence of the virus to switch into lytic replication seems important for tumour formation [40,44,61,62]. Recombinant BZLF1-deficient EBV establishes fewer tumours, especially in secondary sites like liver and kidney [40,44]. In the tumour microenvironment, mainly early lytic EBV gene expression is detected and this may contribute to inflammation that supports lymphoma establishment. Consistent with this, a virus with increased lytic EBV replication was more tumourigenic than the wild-type [62]. This increased lymphomagenicity owing to lytic EBV replication was also observed during co-infection of EBV with KSHV [8]. While even repeated biweekly infection with KSHV alone did not result in virus-associated pathologies [60], co-infection resulted in tumour formation in the majority of animals. Double-infected cell lines that could be rescued from these infections presented with hallmarks of PELs, a tumour that is always associated with KSHV and in 90% of cases additionally with EBV [17]. A defining characteristic of PEL is plasma cell differentiation [63–66]. Similarly, tumour cell lines derived from KSHV and EBV dual-infected humanized mice exhibited a plasma cell signature, which in turn resembled the gene expression in established human PEL cell lines [8]. Accordingly, the respective tumour cell lines could also be efficiently rescued from the peritoneal cavity. While very broad KSHV gene expression with latent and lytic gene products could be detected in these double-infected B-cell lines, they also displayed increased lytic EBV gene expression [8] (figure 1). This increased lytic replication contributed to lymphomagenesis, because double-infection of KSHV with BZLF1-deficient EBV reduced tumour formation to levels of single infection with wild-type EBV. Furthermore, clinical PEL and dual-infected lymphoproliferative disease samples also displayed increased immediate early (BZLF1) and late (VCA) protein expression, compared to only EBV-associated lymphomas [8]. Moreover, it has been reported that the inhibition of lytic herpesvirus replication was able to prevent KS in patients and has been used to treat individual PEL cases [19,67–70]. These studies suggest that humanized mice develop B-cell lymphomas after EBV single or co-infection with KSHV with similarities to the respective human hematologic malignancies, and that lytic EBV replication contributes to tumour formation.
The tumour microenvironment that is most likely stimulated by lytic reactivation also significantly contributes to the robustness of EBV-associated malignancy development. Along these lines, it was reported that LMP1-deficient EBV can cause lymphomas, but requires CD4+ T-cell help for lymphomagenesis [47]. Since LMP1 mimics constitutive CD40 signalling [71], CD40 L on CD4+ T cells might engage this signalling and provide the necessary tumourigenic microenvironment. Such support from the microenvironment, which seems to be mainly provided by differentiated cord blood-derived T-cell compartments in humanized mice, can even overcome LMP1 and LMP2-deficiency for B-cell lymphoma formation [55]. These cord blood-derived T-cell compartments exert EBV-specific immune control only after blocking inhibitory immune receptors [52], but might mediate microenvironmental help that even allows EBNA3C-deficient EBV to cause B-cell lymphomas [59]. By contrast, in humanized mice with HPC-derived human immune system compartments, EBNA3A and 3C-deficient viruses rarely cause lymphomas, and T cells seem to mediate significant immune control even without inhibitory immune checkpoint blockade [58]. These T cells seem to reconstitute from neonatal EBV-negative HPCs and are primed by EBV infection in vivo [39]. This immune control is also dependent on T-cell homing into the tumour microenvironment, because the loss of T-cell homing after infection with an EBNA3B-deficient EBV leads to increased lymphoma formation [72,73] (figure 1). The resulting tumours resemble diffuse large B-cell lymphomas (DLBCLs), in part owing to their lack of lymphocyte infiltrates. Indeed, EBNA3B mutations have been found in a small subset of DLBCLs [72,74]. The EBV-transformed B cells without EBNA3B that could be expanded from infected humanized mice produced less T-cell chemoattractants, primarily CXCL9 and CXCL10 [72]. Restoring CXCL10 expression rescued immune control of these lymphoma cells. These findings suggest that humanized mice can model B-cell lymphomas with transcriptional similarities to human tumours after EBV and KSHV infection, and, also, that contributions of the tumour microenvironment to lymphomagenesis can be studied.
4. Innate immune control of Epstein–Barr virus in huNSG mice
In contrast with peripheral blood mononuclear cell transfer models in scid or scid common gamma chain deficient mice, which only support transient, mostly T-cell engraftment, often even at the cost of graft-versus-host disease [9], humanized mice allow for the long-term reconstitution of nearly all human immune system compartments, albeit some at lower frequencies than in humans [48,75,76]. With respect to EBV infection, primarily NK cells, innate lymphocyte that recognize tumours and virus-infected cells with a variety of germ-line encoded activating and inhibitory receptors, have been found to expand during both symptomatic primary infection, called infectious mononucleosis (IM), and in humanized mice [41,77–80]. Early differentiated, killer immunoglobulin-like receptor (KIR)-negative NK cells accumulate during IM and remain an enlarged population of the NK cell repertoire for several months after symptomatic primary infection [80,81]. This NK cell population primarily targets lytically EBV replicating B cells [41,80,82] (figure 2 and table 1). NK cell depletion in humanized mice leads to elevated viral loads and tumourigenesis starting at four weeks after EBV infection [41]. KIR-negative NK cells decrease in frequency during the first decade of human life [80,83] and their dwindling numbers might contribute to the enhanced vulnerability towards lytic EBV replication resulting more often in IM when the primary EBV infection is delayed into adolescence. KIR absence can in part be compensated by mismatching KIR recognition of human leucocyte antigens as is the case in semi-allogeneic bone marrow transplantation, and this allows for improved EBV-specific immune control [48]. Thus, NK cells provide important innate immune control of lytic EBV replication, which, when left unchecked, contributes to IM development.
In addition to lytic EBV replication, innate lymphocytes also control latent EBV infection. Vγ9 Vδ2T cells can be expanded in addition to NK cells by Burkitt lymphoma cells from around 50% of healthy donors [84]. This expansion is driven by T-cell receptor (TCR) recognition of mevalonate metabolites restricted by CD277 (BTN3A1) on Burkitt lymphoma cell lines, as well as NKG2D on the innate lymphocytes. Stimulating these Vγ9 Vδ2T cells prior to EBV infection restricts transformed B cells in humanized mice and this lymphoproliferative B cell recognition was also mediated by TCR and NKG2D [50]. Moreover, adoptive transfer of Vγ9 Vδ2T cells also prevented tumourigenesis after EBV infection when given up to five days after virus inoculation and still reduced tumour burden more than three weeks after initial infection [51]. These findings suggest that nearly every second healthy donor can expand Vγ9 Vδ2T cells upon stimulation with EBV-infected B cells and that these innate lymphocytes restrict EBV-associated tumourigenesis, possibly by preferentially targeting latency I (figure 2 and table 1).
In addition to γδ T and NK cells, invariant NKT cells have been described to recognize EBV-positive Hodgkin lymphoma and nasopharyngeal carcinoma cell lines [49]. NKT cells primarily carry invariant Vα24-Jα18/Vβ11 TCRs and recognize glycolipids presented on CD1d molecules [85]. While CD1d is downregulated on transformed B cells with EBV latency III, NKT cells can inhibit B-cell transformation by EBV and target transformed B cells after CD1d expression has been restored pharmacologically [86]. Adoptive transfer of NKT cells restricts tumour outgrowth after EBV-transformed B-cell transfer in a humanized mouse model [49]. Thus, NKT cells might preferentially target B cells with EBV latency II infection (figure 2 and table 1). As such they contribute to a quite comprehensive innate immune control, during which NK cells additionally target lytic EBV replication and γδ T cells EBV latency I.
5. T-cell responses to Epstein–Barr virus infection in huNSG mice
The most dramatic effect of EBV infection in humanized mice is a fulminant 10-fold expansion of CD8+ T cells in blood and secondary lymphoid organs [39]. The extent and the kinetic of this expansion, four to six weeks after primary EBV infection, is quite comparable to IM in college students [78,81]. Cytotoxic CD8+ T cells are considered to be the main protective immune entity against transition from the premalignant latently infected B cells of healthy EBV carriers to EBV-associated lymphomas. This notion stems from increased EBV-associated tumourigenesis after T-cell-targeted immune suppression, as seen, for example, during human immunodeficiency virus (HIV) infection [87], therapeutic immune suppression [88] or in primary immunodeficiencies resulting from genetic mutations in the lymphocyte cytotoxic machinery, TCR signalling molecules and co-stimulatory molecules on cytotoxic T cells [89,90]. Some of the EBV-associated malignancies that emerge under these immune suppressive conditions can be treated by adoptive transfer of in vitro expanded EBV-specific T cells [91]. Furthermore, CD8+ T-cell depletion significantly elevates viral loads and EBV-associated lymphoma formation in humanized mice [39,53,54] (figure 2 and table 1). Fulminant CD8+ T-cell expansion is cut in half when BZLF1-deficient virus is used for infection, suggesting that half of the expanding CD8+ T cells are directed against lytic EBV antigens [40]. Adoptive transfer of CD8+ T-cell clones against one early lytic (BMLF1) and one latent EBV antigen (LMP2) do not dramatically improve this immune control [40]. Only at three weeks after infection of humanized mice, when lytic EBV replication significantly contributes to overall viral loads, adoptive transfer of an early lytic EBV antigen-specific CD8+ T-cell clone (anti-BMLF1) can suppress this lytic EBV replication, while the transfer of an LMP2-specific CD8+ T-cell clone did not influence EBV infection [40] (figure 2). This CD8+ T cell response in humanized mice requires some molecular interactions that have been identified as crucial for protection by primary immunodeficiencies that predispose for EBV pathology. It requires the co-stimulatory receptor 2B4, a member of the signalling lymphocytic activation molecule (SLAM) protein family that is targeted by mutations in the SLAM-associated protein (SAP) leading to X-linked lymphoproliferative disease type 1 (XLP1) [92]. Blocking of 2B4 leads to elevated viral loads and tumourigenesis after EBV infection of humanized mice [53]. This treatment shows no additional effect when CD8+ T cells are depleted, suggesting that 2B4 is primarily required on CD8+ T cells during EBV-specific immune control in humanized mice. Thus, CD8+ T cells are one major protective immune entity against EBV infection in humanized mice.
Depletion of CD4+ T cells mirrors CD8+ T-cell depletion with respect to its effects on viral loads [39]. However, the impact is less pronounced with respect to tumour formation, suggesting that CD8+ T cells can still control EBV-induced lymphomas quite well in humanized mice without much CD4+ T-cell help. Why CD4+ T cells alone do not efficiently control EBV-transformed B cells in vivo is less clear. EBV-specific CD4+ T cells, which are primarily Th1 polarized in healthy EBV carriers [93,94], can efficiently target EBV-transformed B cells [95,96]. This cytotoxic potential applies to both EBV-specific CD4+ T cells of healthy EBV carriers and humanized mice [39,97]. Moreover, the affinity of EBV-specific CD4+ T-cell clones is sufficient to directly recognize naturally processed epitopes on MHC class II molecules and vaccination of humanized mice allows for the priming of EBV-specific CD4+ T cells with exquisite affinity [75]. This high affinity does not require additional external peptide pulsing to reach maximal recognition of EBV-transformed B cells, which is necessary for most CD4+ T-cell clones from healthy EBV carriers [75,98]. However, expansion of these EBV-specific CD4+ T cells after EBV infection or vaccination is moderate [39,75,99] and therefore probably cannot efficiently restrict EBV-driven lymphomagenesis in the absence of CD8+ T cells. Additionally, CD4+ T cells may also play a supportive role in the development of EBV-induced lymphomas as discussed above, and the loss of this help might cancel protective functions out. Thus, humanized mice prime protective CD4+ and CD8+ T-cell responses after EBV infection, but humoral immune responses are poorly developed in this model and only occasionally IgM, but no IgG responses can be observed against EBV antigens [99].
While it is clear that T cells are important for protection in both the primary immune response to EBV as well as in the control of persistent infection, there is variation in the phenotype of memory subsets which are established following infection as well as the localization of these subsets within the body. How this variation contributes to immune control, however, is incompletely understood.
In the acute phase of infection, circulating EBV-specific T cells obtained from the peripheral blood have a uniform effector phenotype (CD45RA−CCR7 −IL-7R−CD27+CD28+), downregulating expression of the CD45RA isotype, as well as the lymphoid homing receptor CCR7 and IL-7R, important in long-term memory T-cell maintenance which is similar to that observed in the primary immune responses to other viruses [100–102]. However, as the primary immune response subsides distinct CD8+ T-cell subsets with phenotypes reminiscent of early (CD45RA−CD27+CD28+) and late (CD45RA+/−CD27−CD28+/−) memory are established. Interestingly, these subsets are enriched for latent and lytic viral epitopes, respectively [103]. A similar distinction has also been observed for CD4+ T cells; while the majority of EBV-specific CD4+ T cells express both CD27 and CD28, those recognizing lytic antigens tend to re-express CD45RA, a hallmark of the effector memory T cells with CD45RA re-expression (TEMRA) subset [94].
Latent and lytic epitope-specific T cells further tend to be preferentially localized to distinct tissue sites following infection of humans. In paired bone marrow and blood samples, CD8+ T cells specific to lytic antigens were enriched in the bone marrow compared to the blood while those specific to latent antigens were equivalent between the two sites [104]. Similarly, the tonsils of long-term EBV carriers (no IM) were enriched for both lytic (2–5 fold) and latent (10–20 fold) epitope-specific CD8+ T cells compared to the blood. Interesting, tonsils from recovered IM patients demonstrated only a minimal enrichment of latent epitope-specific T cells in the tonsils relative to the blood and lytic epitope-specific T cells were present at greater frequencies in the blood compared to the tonsils [102]. Lytic epitope-specific cells further tend not to re-express CCR7, suggesting preferential maintenance in the circulation rather than in the lymphoid tissues [103,105].
Of interest, tonsillar T cells were further found to express the mucosal homing and retention marker CD103, which has since been shown to be a robust marker of the recently described tissue resident memory (TRM) T-cell subset [106]. EBV-specific T cells with a TRM phenotype have been observed in additional studies in both the tonsils and spleen [107,108]. It is additionally worth noting, at least in the circulation, that the virus-specific T-cell repertoire generated in the primary immune responses and maintained in the persistent phase of infection appears to be relatively stable with little variation observed over the course of several years in one study [109]. While it is presently unclear whether differences exist in the abilities of these distinct subsets to protect against long-term viral reactivation or tumourigenesis, a deeper understanding of these factors could have important implications in the design of both prophylactic and therapeutic EBV vaccines.
6. Conclusion and outlook
Humanized mice have allowed us to model infections and tumourigenesis of the human tumour viruses EBV and KSHV in vivo. Both the requirements for persistence of these viruses, their B-cell transformation and restricting immune control are starting to be unravelled. Some of the players in this immune control have been characterized, but their molecular requirements with respect to receptors that mediate this recognition and effector functions that control EBV and KSHV still need to be defined in order to gain insights into immune modulations that could benefit patients with EBV and KSHV-associated diseases. Furthermore, mucosal infections with these two tumour viruses have not been significantly explored but could provide insights into what is required for initial transmission. Finally, antigen specificity of protective immune responses against KSHV remains poorly defined but is a prerequisite for the development of targeted vaccine formulations against this tumour virus. Thus, humanized mice should be explored to develop prophylactic vaccines and therapeutic interventions against EBV- and KSHV-associated pathologies.
For this purpose, we need to develop additional tools and modifications for humanized mice that allow us to genetically manipulate the reconstituted human immune system components or the transferred HPCs before transplantation. These modifications may allow us to restore humoral immunity in these mice, and to generate immortalized sources of hematopoietic stem cells from genetically defined donors, which would vastly facilitate the generation of large cohorts of humanized mice from individual donors. Going forward, EBV and KSHV infections can pose some challenges to newly developed humanized mouse models but at the same time can teach us more about infection, tumourigenesis and immune control of these important human tumour viruses themselves.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed to writing the manuscript and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Research in our laboratory is supported by Cancer Research Switzerland (KFS-4091-02-2017 and KFS-4371-02-2018), KFSPMS and KFSPHHLD of the University of Zurich, the Vontobel Foundation, the Baugarten Foundation, the Sobek Foundation, the Swiss Vaccine Research Institute, the Swiss MS Society and the Swiss National Science Foundation (310030_162560 and CRSII3_160708).
References
- 1.Bouvard V, et al. 2009. A review of human carcinogens—Part B: biological agents. Lancet Oncol. 10, 321–322. ( 10.1016/S1470-2045(09)70096-8) [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM. 2006. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 118, 3030–3044. ( 10.1002/ijc.21731) [DOI] [PubMed] [Google Scholar]
- 3.Morales-Sanchez A, Fuentes-Panana EM. 2014. Human viruses and cancer. Viruses 6, 4047–4079. ( 10.3390/v6104047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandeven N, Nghiem P. 2014. Pathogen-driven cancers and emerging immune therapeutic strategies. Cancer Immunol Res. 2, 9–14. ( 10.1158/2326-6066.CIR-13-0179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peres E, et al. 2018. PDZ domain-binding motif of Tax sustains T-cell proliferation in HTLV-1-infected humanized mice. PLoS Pathog. 14, e1006933 ( 10.1371/journal.ppat.1006933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tezuka K, Xun R, Tei M, Ueno T, Tanaka M, Takenouchi N, Fujisawa J-I. 2014. An animal model of adult T-cell leukemia: humanized mice with HTLV-1-specific immunity. Blood 123, 346–355. ( 10.1182/blood-2013-06-508861) [DOI] [PubMed] [Google Scholar]
- 7.Münz C. 2017. Humanized mouse models for Epstein Barr virus infection. Curr. Opin. Virol. 25, 113–118. ( 10.1016/j.coviro.2017.07.026) [DOI] [PubMed] [Google Scholar]
- 8.McHugh D, et al. 2017. Persistent KSHV infection increases EBV-associated tumor formation in vivo via enhanced EBV lytic gene expression. Cell Host Microbe. 22, 61–73. ( 10.1016/j.chom.2017.06.009) [DOI] [PubMed] [Google Scholar]
- 9.Walsh NC, Kenney LL, Jangalwe S, Aryee KE, Greiner DL, Brehm MA, Shultz LD. 2017. Humanized mouse models of clinical disease. Annu. Rev. Pathol. 12, 187–215. ( 10.1146/annurev-pathol-052016-100332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen JI, Fauci AS, Varmus H, Nabel GJ. 2011. Epstein-Barr virus: an important vaccine target for cancer prevention. Sci. Transl. Med. 3, 107fs7 ( 10.1126/scitranslmed.3002878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young LS, Yap LF, Murray PG. 2016. Epstein-Barr virus: more than 50 years old and still providing surprises. Nat. Rev. Cancer 16, 789–802. ( 10.1038/nrc.2016.92) [DOI] [PubMed] [Google Scholar]
- 12.Babcock JG, Hochberg D, Thorley-Lawson AD. 2000. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 13, 497–506. ( 10.1016/S1074-7613(00)00049-2) [DOI] [PubMed] [Google Scholar]
- 13.Thorley-Lawson DA, Gross A. 2004. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 350, 1328–1337. ( 10.1056/NEJMra032015) [DOI] [PubMed] [Google Scholar]
- 14.Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. 1998. EBV persistence in memory B cells in vivo. Immunity 9, 395–404. ( 10.1016/S1074-7613(00)80622-6) [DOI] [PubMed] [Google Scholar]
- 15.Laichalk LL, Thorley-Lawson DA. 2005. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 79, 1296–1307. ( 10.1128/JVI.79.2.1296-1307.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganem D. 2006. KSHV infection and the pathogenesis of Kaposi's sarcoma. Annu. Rev. Pathol. 1, 273–296. ( 10.1146/annurev.pathol.1.110304.100133) [DOI] [PubMed] [Google Scholar]
- 17.Cesarman E. 2014. Gammaherpesviruses and lymphoproliferative disorders. Annu. Rev. Pathol. 9, 349–372. ( 10.1146/annurev-pathol-012513-104656) [DOI] [PubMed] [Google Scholar]
- 18.Mariggio G, Koch S, Schulz TF. 2017. Kaposi sarcoma herpesvirus pathogenesis. Phil. Trans. R. Soc. B 372, 20160275 ( 10.1098/rstb.2016.0275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin DF, Kuppermann BD, Wolitz RA, Palestine AG, Li H, Robinson CA. 1999. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N. Engl. J. Med. 340, 1063–1070. ( 10.1056/NEJM199904083401402) [DOI] [PubMed] [Google Scholar]
- 20.Jung J, Münz C. 2015. Immune control of oncogenic gamma-herpesviruses. Curr. Opin. Virol. 14, 79–86. ( 10.1016/j.coviro.2015.08.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baresova P, Pitha PM, Lubyova B. 2013. Distinct roles of Kaposi's sarcoma-associated herpesvirus-encoded viral interferon regulatory factors in inflammatory response and cancer. J. Virol. 87, 9398–9410. ( 10.1128/JVI.03315-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woellmer A, Arteaga-Salas JM, Hammerschmidt W. 2012. BZLF1 governs CpG-methylated chromatin of Epstein-Barr virus reversing epigenetic repression. PLoS Pathog. 8, e1002902 ( 10.1371/journal.ppat.1002902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishido S, Choi JK, Lee BS, Wang C, DeMaria M, Johnson RP, Cohen GB, Jung JU. 2000. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi's sarcoma-associated herpesvirus K5 protein. Immunity 13, 365–374. ( 10.1016/S1074-7613(00)00036-4) [DOI] [PubMed] [Google Scholar]
- 24.Thomas M, Boname JM, Field S, Nejentsev S, Salio M, Cerundolo V, Wills M, Lehner PJ. 2008. Down-regulation of NKG2D and NKp80 ligands by Kaposi's sarcoma-associated herpesvirus K5 protects against NK cell cytotoxicity. Proc. Natl Acad. Sci. USA 105, 1656–1661. ( 10.1073/pnas.0707883105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nachmani D, Stern-Ginossar N, Sarid R, Mandelboim O. 2009. Diverse herpesvirus microRNAs target the stress-induced immune ligand MICB to escape recognition by natural killer cells. Cell Host Microbe. 5, 376–385. ( 10.1016/j.chom.2009.03.003) [DOI] [PubMed] [Google Scholar]
- 26.Albanese M, Tagawa T, Buschle A, Hammerschmidt W. 2017. MicroRNAs of Epstein-Barr virus control innate and adaptive antiviral immunity. J. Virol. 91, e01667-16 ( 10.1128/JVI.01667-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishido S, Wang C, Lee BS, Cohen GB, Jung JU. 2000. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 74, 5300–5309. ( 10.1128/JVI.74.11.5300-5309.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ressing ME, van Gent M, Gram AM, Hooykaas MJ, Piersma SJ, Wiertz EJ. 2015. Immune evasion by Epstein-Barr virus. Curr. Top. Microbiol. Immunol. 391, 355–381. [DOI] [PubMed] [Google Scholar]
- 29.Salek-Ardakani S, Arrand JR, Mackett M. 2002. Epstein-Barr virus encoded interleukin-10 inhibits HLA-class I, ICAM-1, and B7 expression on human monocytes: implications for immune evasion by EBV. Virology 304, 342–351. ( 10.1006/viro.2002.1716) [DOI] [PubMed] [Google Scholar]
- 30.Butler LM, Jeffery HC, Wheat RL, Rae PC, Townsend K, Alkharsah KR, Schulz TF, Nash GB, Blackbourn DJ. 2011. Kaposi's sarcoma-associated herpesvirus infection of endothelial cells inhibits neutrophil recruitment through an interleukin-6-dependent mechanism: a new paradigm for viral immune evasion. J. Virol. 85, 7321–7332. ( 10.1128/JVI.00021-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niederman JC, Miller G, Pearson HA, Pagano JS, Dowaliby JM. 1976. Infectious mononucleosis. Epstein-Barr-virus shedding in saliva and the oropharynx. N. Engl. J. Med. 294, 1355–1359. ( 10.1056/NEJM197606172942501) [DOI] [PubMed] [Google Scholar]
- 32.Koelle DM, Huang ML, Chandran B, Vieira J, Piepkorn M, Corey L. 1997. Frequent detection of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in saliva of human immunodeficiency virus-infected men: clinical and immunologic correlates. J. Infect. Dis. 176, 94–102. ( 10.1086/514045) [DOI] [PubMed] [Google Scholar]
- 33.Blackbourn DJ, Lennette ET, Ambroziak J, Mourich DV, Levy JA. 1998. Human herpesvirus 8 detection in nasal secretions and saliva. J. Infect. Dis. 177, 213–216. ( 10.1086/517356) [DOI] [PubMed] [Google Scholar]
- 34.Borza CM, Hutt-Fletcher LM. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8, 594–599. ( 10.1038/nm0602-594) [DOI] [PubMed] [Google Scholar]
- 35.Tugizov SM, Berline JW, Palefsky JM. 2003. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat. Med. 9, 307–314. ( 10.1038/nm830) [DOI] [PubMed] [Google Scholar]
- 36.Sitki-Green D, Covington M, Raab-Traub N. 2003. Compartmentalization and transmission of multiple Epstein-Barr virus strains in asymptomatic carriers. J. Virol. 77, 1840–1847. ( 10.1128/JVI.77.3.1840-1847.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walling DM, Brown AL, Etienne W, Keitel WA, Ling PD. 2003. Multiple Epstein-Barr virus infections in healthy individuals. J. Virol. 77, 6546–6550. ( 10.1128/JVI.77.11.6546-6550.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitby D, et al. 1995. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet 346, 799–802. ( 10.1016/S0140-6736(95)91619-9) [DOI] [PubMed] [Google Scholar]
- 39.Strowig T, et al. 2009. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J. Exp. Med. 206, 1423–1434. ( 10.1084/jem.20081720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antsiferova O, et al. 2014. Adoptive transfer of EBV specific CD8+ T cell clones can transiently control EBV infection in humanized mice. PLoS Pathog. 10, e1004333 ( 10.1371/journal.ppat.1004333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chijioke O, et al. 2013. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Rep. 5, 1489–1498. ( 10.1016/j.celrep.2013.11.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cocco M, et al. 2008. CD34+ cord blood cell-transplanted Rag2−/− gammac−/− mice as a model for Epstein-Barr virus infection. Am. J. Pathol. 173, 1369–1378. ( 10.2353/ajpath.2008.071186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heuts F, Rottenberg ME, Salamon D, Rasul E, Adori M, Klein G, Klein E, Nagy N. 2014. T cells modulate Epstein-Barr virus latency phenotypes during infection of humanized mice. J. Virol. 88, 3235–3245. ( 10.1128/JVI.02885-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma SD, et al. 2011. A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J. Virol. 85, 165–177. ( 10.1128/JVI.01512-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai MH, et al. 2017. The biological properties of different Epstein-Barr virus strains explain their association with various types of cancers. Oncotarget 8, 10 238–10 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai MH, et al. 2013. Spontaneous lytic replication and epitheliotropism define an Epstein-Barr virus strain found in carcinomas. Cell Rep. 5, 458–470. ( 10.1016/j.celrep.2013.09.012) [DOI] [PubMed] [Google Scholar]
- 47.Ma SD, et al. 2015. LMP1-deficient Epstein-Barr virus mutant requires T cells for lymphomagenesis. J. Clin. Invest. 125, 304–315. ( 10.1172/JCI76357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Landtwing V, et al. 2016. Cognate HLA absence in trans diminishes human NK cell education. J. Clin. Invest. 126, 3772–3782. ( 10.1172/JCI86923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuling H, et al. 2009. EBV-induced human CD8+ NKT cells suppress tumorigenesis by EBV-associated malignancies. Cancer Res. 69, 7935–7944. ( 10.1158/0008-5472.CAN-09-0828) [DOI] [PubMed] [Google Scholar]
- 50.Xiang Zet al. 2014. Targeted activation of human Vγ9 Vδ2-T cells controls Epstein-Barr virus-induced B cell lymphoproliferative disease. Cancer Cell. 26, 565–576. ( 10.1016/j.ccr.2014.07.026) [DOI] [PubMed] [Google Scholar]
- 51.Zumwalde NA, et al. 2017. Adoptively transferred Vgamma9Vdelta2T cells show potent antitumor effects in a preclinical B cell lymphomagenesis model. JCI Insight 2, pii. 93179 ( 10.1172/jci.insight.93179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma SD, et al. 2016. PD-1/CTLA-4 blockade inhibits Epstein-Barr virus-induced lymphoma growth in a cord blood humanized-mouse model. PLoS Pathog. 12, e1005642 ( 10.1371/journal.ppat.1005642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chijioke O, Marcenaro E, Moretta A, Capaul R, Münz C. 2015. The SAP-dependent 2B4 receptor mediates CD8+ T cell dependent immune control of Epstein Barr virus infection in mice with reconstituted human immune system components. J. Infect. Dis. 212, 803–807. ( 10.1093/infdis/jiv114) [DOI] [PubMed] [Google Scholar]
- 54.Yajima M, et al. 2009. T cell-mediated control of Epstein-Barr virus infection in humanized mice. J. Infect. Dis. 200, 1611–1615. ( 10.1086/644644) [DOI] [PubMed] [Google Scholar]
- 55.Ma SD, et al. 2017. LMP1 and LMP2A collaborate to promote Epstein-Barr virus (EBV)-induced B cell lymphomas in a cord blood-humanized mouse model but are not essential. J. Virol. 91, e01928-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allday MJ, Bazot Q, White RE. 2015. The EBNA3 family: two oncoproteins and a tumour suppressor that are central to the biology of EBV in B cells. Curr. Top. Microbiol. Immunol. 391, 61–117. [DOI] [PubMed] [Google Scholar]
- 57.Nikitin PA, et al. 2010. An ATM/Chk2-mediated DNA damage-responsive signaling pathway suppresses Epstein-Barr virus transformation of primary human B cells. Cell Host Microbe 8, 510–522. ( 10.1016/j.chom.2010.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murer A, et al. 2018. EBV persistence without its EBNA3A and 3C oncogenes in vivo. PLoS Pathog. 14, e1007039 ( 10.1371/journal.ppat.1007039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romero-Masters JC, et al. 2018. An EBNA3C-deleted Epstein-Barr virus (EBV) mutant causes B-cell lymphomas with delayed onset in a cord blood-humanized mouse model. PLoS Pathog. 14, e1007221 ( 10.1371/journal.ppat.1007221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang LX, et al. 2014. Humanized-BLT mouse model of Kaposi's sarcoma-associated herpesvirus infection. Proc. Natl Acad. Sci. USA 111, 3146–3151. ( 10.1073/pnas.1318175111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong GK, Gulley ML, Feng WH, Delecluse HJ, Holley-Guthrie E, Kenney SC. 2005. Epstein-Barr virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model. J. Virol. 79,13 993–14 003. ( 10.1128/JVI.79.22.13993-14003.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma SD, et al. 2012. An Epstein-Barr virus (EBV) mutant with enhanced BZLF1 expression causes lymphomas with abortive lytic EBV infection in a humanized mouse model. J. Virol. 86, 7976–7987. ( 10.1128/JVI.00770-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klein U, et al. 2003. Gene expression profile analysis of AIDS-related primary effusion lymphoma (PEL) suggests a plasmablastic derivation and identifies PEL-specific transcripts. Blood 101, 4115–4121. ( 10.1182/blood-2002-10-3090) [DOI] [PubMed] [Google Scholar]
- 64.Gaidano G, et al. 1997. Association of Kaposi's sarcoma-associated herpesvirus-positive primary effusion lymphoma with expression of the CD138/syndecan-1 antigen. Blood 90, 4894–4900. [PubMed] [Google Scholar]
- 65.Jenner RG, Maillard K, Cattini N, Weiss RA, Boshoff C, Wooster R, Kellam P. 2003. Kaposi's sarcoma-associated herpesvirus-infected primary effusion lymphoma has a plasma cell gene expression profile. Proc. Natl Acad. Sci. USA 100,10 399–10 404. ( 10.1073/pnas.1630810100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nayar U, et al. 2017. Identification of a nucleoside analog active against adenosine kinase-expressing plasma cell malignancies. J. Clin. Invest. 127, 2066–2080. ( 10.1172/JCI83936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marquet J, Velazquez-Kennedy K, Lopez S, Benito A, Blanchard MJ, Garcia-Vela JA. 2018. Case report of a primary effusion lymphoma successfully treated with oral valganciclovir after failing chemotherapy. Hematol. Oncol. 36, 316–319. ( 10.1002/hon.2445) [DOI] [PubMed] [Google Scholar]
- 68.Robles R, Lugo D, Gee L, Jacobson MA. 1999. Effect of antiviral drugs used to treat cytomegalovirus end-organ disease on subsequent course of previously diagnosed Kaposi's sarcoma in patients with AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20, 34–38. ( 10.1097/00042560-199901010-00005) [DOI] [PubMed] [Google Scholar]
- 69.Xu D, Coleman T, Zhang J, Fagot A, Kotalik C, Zhao L, Trivedi P, Jones C, Zhang L. 2007. Epstein-Barr virus inhibits Kaposi's sarcoma-associated herpesvirus lytic replication in primary effusion lymphomas. J. Virol. 81, 6068–6078. ( 10.1128/JVI.02743-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coen N, Duraffour S, Snoeck R, Andrei G. 2014. KSHV targeted therapy: an update on inhibitors of viral lytic replication. Viruses 6, 4731–4759. ( 10.3390/v6114731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kieser A, Sterz KR. 2015. The latent membrane protein 1 (LMP1). Curr. Top. Microbiol. Immunol. 391, 119–149. [DOI] [PubMed] [Google Scholar]
- 72.White RE, et al. 2012. EBNA3B-deficient EBV promotes B cell lymphomagenesis in humanized mice and is found in human tumors. J. Clin. Invest. 122, 1487–1502. ( 10.1172/JCI58092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen A, Divisconte M, Jiang X, Quink C, Wang F. 2005. Epstein-Barr virus with the latent infection nuclear antigen 3B completely deleted is still competent for B-cell growth transformation in vitro. J. Virol. 79, 4506–4509. ( 10.1128/JVI.79.7.4506-4509.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gottschalk S, et al. 2001. An Epstein-Barr virus deletion mutant associated with fatal lymphoproliferative disease unresponsive to therapy with virus-specific CTLs. Blood 97, 835–843. ( 10.1182/blood.V97.4.835) [DOI] [PubMed] [Google Scholar]
- 75.Meixlsperger S, et al. 2013. CD141+ dendritic cells produce prominent amounts of IFN-alpha after dsRNA recognition and can be targeted via DEC-205 in humanized mice. Blood 121, 5034–5044. ( 10.1182/blood-2012-12-473413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raykova A, et al. 2017. Interleukins 12 and 15 induce cytotoxicity and early NK-cell differentiation in type 3 innate lymphoid cells. Blood Adv. 1, 2679–2691. ( 10.1182/bloodadvances.2017008839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams H, et al. 2005. The immune response to primary EBV infection: a role for natural killer cells. Br. J. Haematol. 129, 266–274. ( 10.1111/j.1365-2141.2005.05452.x) [DOI] [PubMed] [Google Scholar]
- 78.Balfour HHJ, et al. 2013. Behavioral, virologic, and immunologic factors associated with acquisition and severity of primary Epstein-Barr virus infection in university students. J. Infect. Dis. 207, 80–88. ( 10.1093/infdis/jis646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hendricks DW, Balfour HH Jr, Dunmire SK, Schmeling DO, Hogquist KA, Lanier LL. 2014. Cutting edge: NKG2ChiCD57+ NK cells respond specifically to acute infection with cytomegalovirus and not Epstein-Barr virus. J. Immunol. 192, 4492–4496. ( 10.4049/jimmunol.1303211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Azzi T, et al. 2014. Role for early-differentiated natural killer cells in infectious mononucleosis. Blood 124, 2533–2543. ( 10.1182/blood-2014-01-553024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dunmire SK, Grimm JM, Schmeling DO, Balfour HH Jr, Hogquist KA. 2015. The incubation period of primary Epstein-Barr virus infection: viral dynamics and immunologic events. PLoS Pathog. 11, e1005286 ( 10.1371/journal.ppat.1005286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pappworth IY, Wang EC, Rowe M. 2007. The switch from latent to productive infection in Epstein-Barr virus-infected B cells is associated with sensitization to NK cell killing. J. Virol. 81, 474–482. ( 10.1128/JVI.01777-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sundstrom Y, Nilsson C, Lilja G, Karre K, Troye-Blomberg M, Berg L. 2007. The expression of human natural killer cell receptors in early life. Scand. J. Immunol. 66, 335–344. ( 10.1111/j.1365-3083.2007.01980.x) [DOI] [PubMed] [Google Scholar]
- 84.Djaoud Z, et al. 2017. Two alternate strategies for innate immunity to Epstein-Barr virus: one using NK cells and the other NK cells and gammadelta T cells. J. Exp. Med. 214, 1827–1841. ( 10.1084/jem.20161017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chandra S, Kronenberg M. 2015. Activation and function of iNKT and MAIT cells. Adv. Immunol. 127, 145–201. ( 10.1016/bs.ai.2015.03.003) [DOI] [PubMed] [Google Scholar]
- 86.Chung BK, et al. 2013. Innate immune control of EBV-infected B cells by invariant natural killer T cells. Blood 122, 2600–2608. ( 10.1182/blood-2013-01-480665) [DOI] [PubMed] [Google Scholar]
- 87.Totonchy J, Cesarman E. 2016. Does persistent HIV replication explain continued lymphoma incidence in the era of effective antiretroviral therapy? Curr. Opin. Virol. 20, 71–77. ( 10.1016/j.coviro.2016.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gottschalk S, Rooney CM, Heslop HE. 2005. Post-transplant lymphoproliferative disorders. Annu. Rev. Med. 56, 29–44. ( 10.1146/annurev.med.56.082103.104727) [DOI] [PubMed] [Google Scholar]
- 89.Cohen JI. 2015. Primary immunodeficiencies associated with EBV disease. Curr. Top. Microbiol. Immunol. 390, 241–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tangye SG, Palendira U, Edwards ES. 2017. Human immunity against EBV-lessons from the clinic. J. Exp. Med. 214, 269–283. ( 10.1084/jem.20161846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gottschalk S, Rooney CM. 2015. Adoptive T-cell immunotherapy. Curr. Top. Microbiol. Immunol. 391, 427–454. ( 10.1007/978-3-319-22834-1_15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cannons JL, Tangye SG, Schwartzberg PL. 2011. SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 29, 665–705. ( 10.1146/annurev-immunol-030409-101302) [DOI] [PubMed] [Google Scholar]
- 93.Bickham K, et al. 2001. EBNA1-specific CD4+ T cells in healthy carriers of Epstein-Barr virus are primarily Th1 in function. J. Clin. Invest. 107, 121–130. ( 10.1172/JCI10209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Amyes E, et al. 2003. Characterization of the CD4+ T cell response to Epstein-Barr virus during primary and persistent infection. J. Exp. Med. 198, 903–911. ( 10.1084/jem.20022058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paludan C, et al. 2002. EBNA1 specific CD4+ Th1 cells kill Burkitt's lymphoma cells. J. Immunol. 169, 1593–1603. ( 10.4049/jimmunol.169.3.1593) [DOI] [PubMed] [Google Scholar]
- 96.Nikiforow S, Bottomly K, Miller G, Münz C. 2003. Cytolytic CD4+-T-cell clones reactive to EBNA1 inhibit Epstein-Barr virus-induced B-cell proliferation. J. Virol. 77, 12 088–12 104. ( 10.1128/JVI.77.22.12088-12104.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heller KN, Gurer C, Münz C. 2006. Virus-specific CD4+ T cells: ready for direct attack. J. Exp. Med. 203, 805–808. ( 10.1084/jem.20060215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leung CS, et al. 2013. Robust T-cell stimulation by Epstein-Barr virus-transformed B cells after antigen targeting to DEC-205. Blood 121, 1584–1594. ( 10.1182/blood-2012-08-450775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gurer C, et al. 2008. Targeting the nuclear antigen 1 of Epstein Barr virus to the human endocytic receptor DEC-205 stimulates protective T-cell responses. Blood 112, 1231–1239. ( 10.1182/blood-2008-03-148072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Appay V, et al. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8, 379–385. ( 10.1038/nm0402-379) [DOI] [PubMed] [Google Scholar]
- 101.van Leeuwen EM, et al. 2005. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood 106, 2091–2098. ( 10.1182/blood-2005-02-0449) [DOI] [PubMed] [Google Scholar]
- 102.Hislop AD, et al. 2005. Tonsillar homing of Epstein-Barr virus-specific CD8+T cells and the virus–host balance. J. Clin. Invest. 115, 2546–2555. ( 10.1172/JCI24810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hislop AD, Gudgeon NH, Callan MF, Fazou C, Hasegawa H, Salmon M, Rickinson AB. 2001. EBV-specific CD8+ T cell memory: relationships between epitope specificity, cell phenotype, and immediate effector function. J. Immunol. 167, 2019–2029. ( 10.4049/jimmunol.167.4.2019) [DOI] [PubMed] [Google Scholar]
- 104.Palendira U, et al. 2008. Selective accumulation of virus-specific CD8+ T cells with unique homing phenotype within the human bone marrow. Blood 112, 3293–3302. ( 10.1182/blood-2008-02-138040) [DOI] [PubMed] [Google Scholar]
- 105.Hislop AD, Annels NE, Gudgeon NH, Leese AM, Rickinson AB. 2002. Epitope-specific evolution of human CD8+ T cell responses from primary to persistent phases of Epstein-Barr virus infection. J. Exp. Med. 195, 893–905. ( 10.1084/jem.20011692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mueller SN, Mackay LK. 2016. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 16, 79–89. ( 10.1038/nri.2015.3) [DOI] [PubMed] [Google Scholar]
- 107.Woodberry T, et al. 2005. Alpha E beta 7 (CD103) expression identifies a highly active, tonsil-resident effector-memory CTL population. J. Immunol. 175, 4355–4362. ( 10.4049/jimmunol.175.7.4355) [DOI] [PubMed] [Google Scholar]
- 108.Woon HG, et al. 2016. Compartmentalization of total and virus-specific tissue-resident memory CD8+ T cells in human lymphoid organs. PLoS Pathog. 12, e1005799 ( 10.1371/journal.ppat.1005799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klarenbeek PL, et al. 2012. Deep sequencing of antiviral T-cell responses to HCMV and EBV in humans reveals a stable repertoire that is maintained for many years. PLoS Pathog. 8, e1002889 ( 10.1371/journal.ppat.1002889) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.