Abstract
Cottontail rabbit papillomavirus (CRPV) was the first DNA virus shown to be tumorigenic. The virus has since been renamed and is officially known as Sylvilagus floridanus papillomavirus 1 (SfPV1). Since its inception as a surrogate preclinical model for high-risk human papillomavirus (HPV) infections, the SfPV1/rabbit model has been widely used to study viral–host interactions and has played a pivotal role in the successful development of three prophylactic virus-like particle vaccines. In this review, we will focus on the use of the model to gain a better understanding of viral pathogenesis, gene function and host immune responses to viral infections. We will discuss the application of the model in HPV-associated vaccine testing, in therapeutic vaccine development (using our novel HLA-A2.1 transgenic rabbits) and in the development and validation of novel anti-viral and anti-tumour compounds. Our goal is to demonstrate the role the SfPV1/rabbit model has played, and continues to play, in helping to unravel the intricacies of papillomavirus infections and to develop tools to thwart the disease.
This article is part of the theme issue ‘Silent cancer agents: multi-disciplinary modelling of human DNA oncoviruses’.
Keywords: DNA vaccine, immunization, anti-viral treatment, rabbit, papillomavirus, infection
1. The cottontail rabbit papillomavirus/rabbit model system: a historical perspective
Cottontail rabbits (Sylvilagus floridanus) of the midwest United States often manifest large warty structures on cutaneous skin sites [1] (figure 1). In 1933, Shope & Hurst [2] reported the isolation of an infectious agent from these structures. This agent retained its infectivity upon filtration and a virus responsible for the pathology was soon identified [3]. The virus was first called Shope papillomavirus (SPV) but later became known as the cottontail rabbit papillomavirus (CRPV) [4], and is now known as Sylvilagus floridanus papillomavirus 1 (SfPV1). Early studies focused on the progression of lesions to carcinomas and demonstrated that the virus could also infect domestic rabbits (Oryctolagus cuniculus) [5]. The latter discovery opened the door for a small animal model to investigate papillomavirus pathology. Among important findings in the early years were the following: (i) wild rabbit lesions were far more viral DNA and virus particle-rich than domestic rabbit lesions [6] (figure 1); (ii) naked viral DNA is infectious [7], including plasmid DNA cloned between the early and late regions of the viral genome [8]; (iii) tumours in domestic rabbits were more prone to cancers and a subset of these metastasized to the lung and other organs [7–10]; (iv) viral DNA copy number in benign lesions was correspondingly higher in wild rabbit lesions (figure 1) [9,10]. DNA in cancers, however, was hard to detect in both species, although viral transcripts could be found [11,12]; (v) sera from both SfPV1-infected wild and domestic rabbits could neutralize the virus in vitro and in vivo [6,13,14]; (vi) the genomic structure was determined. Strongest homologies were found with HPV1 [15,16]; (vii) viral transcripts have been mapped and the cap sites have been determined [17]. The virus encodes 10 genes, two of which (LE6, the corresponding protein is translated from the first open reading frame starting at 154 bp, with 272aa in length and E7) code for two essential transforming proteins [18,19], two of which are capsid proteins (L1 and L2) [14,20], two of which are involved in the control of replication and gene regulation (E1 and E2) [21–26] and two others, E9^E2C (renamed E8^E2) which has no effect on growth of the lesions but acts as a transcriptional repressor in mammalian cells [26] (https://pave.niaid.nih.gov/) and E4 which plays a role in DNA amplification and L1 expression [27] (figure 2); (viii) unlike LE6 and E7 that are essential for SfPV1 infection, two additional oncogenes SE6, the corresponding protein is translated from the first open reading frame starting at 445 bp, with 175aa in length and E8 (renamed E10) (https://pave.niaid.nih.gov/) both have an effect on tumour growth but not on viral production [31–33]; (ix) early genes E1 and E2 are also absolutely required for infection [24,28,34] (figure 2); (x) E6 and E7 are expressed in malignant lesions and have been posited to be targets for therapeutic interventions [17,31]; (xi) both episomal and integrated DNAs were found in SfPV1-induced carcinomas, and DNA was highly methylated in malignancies in domestic rabbits [32]; (xii) both progressive and regressive variants of the virus exist [33,35,36]; and (xiii) cells infected with SfPV1 were found to co-localize with hair follicle stem cells [37].
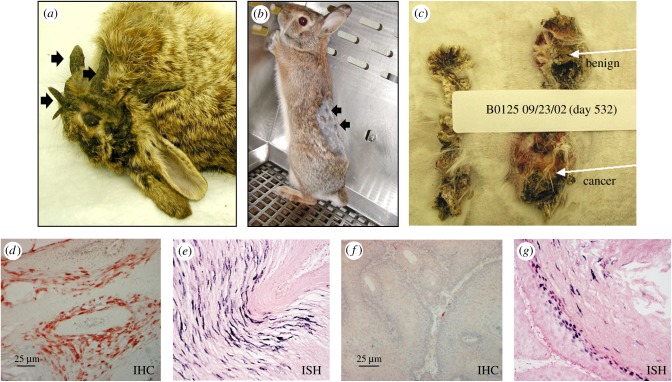
Figure 1.
Discovery of Sylvalagus floridanus papillomavirus 1 (SfPV1) and the development of the SfPV1/domestic rabbit model. Wild cottontail rabbits with long horny growths around the ears and chin and on the torso are common in the midwest (a). The growths are actually warts induced by papillomavirus infections. The virus isolated from these warts is infectious and induces tumour growth in the wild rabbit (b, arrows) and domestic New Zealand white (NZW) rabbits (c). Most tumours on domestic rabbits progress to cancer within a year or more of infection (c). Viral capsid protein and viral DNA are detected in infected tissues of cottontail rabbit lesions (d,e) and NZW domestic rabbit (f,g) by immunohistochemistry (IHC) and by in situ hybridization (ISH), respectively.
Figure 2.
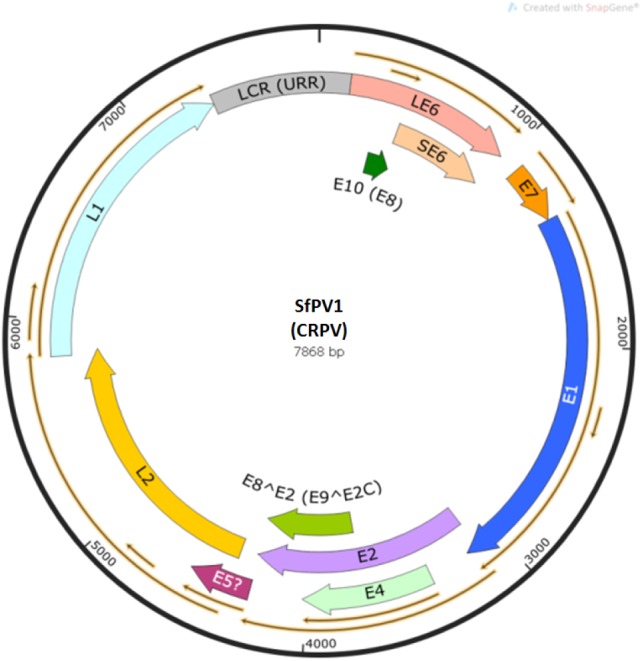
The SfPV1 (CRPV) genome. Genes found to be essential to produce papillomas include LE6, E7, E1 and E2 [19]. Two late genes L1 and L2 are required for the viral life cycle [14]. Other genes including E4, SE6, E8 (renamed E10) [16,27] and E9 ^E2C (renamed E8 ^E2) [22] (https://pave.niaid.nih.gov/) are not essential for SfPV1 infection and tumour growth in vivo [28]. E5 is not considered a functional gene, although it showed transformation activity in vitro [29,30].
2. Investigation of virus–host interactions using the Sylvilagus floridanus papillomavirus 1/rabbit model
(a). Sylvilagus floridanus papillomavirus 1 pathogenesis
Much has been learned about viral pathogenesis using the SfPV1 model. Studies have been greatly facilitated by the fact that viral DNA is capable of producing infections [8,38,39]. Viral infections are initiated in the basal cells and thus must gain access to these cells via abrasions in the skin. Wounding has thus been recognized as essential for papillomavirus infections. Initially, our laboratory used a combination of turpentine and acetone to induce hyperplasticity in the skin and then worked the DNA (in the form of plasmid) or virus into the skin with a needle [8]. We subsequently investigated simple wounding without chemicals and developed a protocol that is far more benign for both technician and animal [40]. Using this protocol, we have achieved infectivity efficiencies orders of magnitude higher and results that are highly reproducible from experiment to experiment [41]. Other laboratories have employed a gene-gun to induce infections from DNA [35,42,43]. In these laboratories, the viral DNA—excised, re-ligated or in plasmid form—was coated onto the ‘bullets’ for delivery into the skin [27]. In our hands, this technique was less efficient and less reproducible; however, it has the advantage of delivering DNA that contains no extraneous intervening sequences [31].
Many studies have been conducted using plasmid DNA and much important information has been gleaned. A key feature of the system is that mutations can be easily inserted into the viral genome and functionally tested in vivo. Using this strategy, the E4 gene was found to be essential for the completion of the productive cycle, although not essential for infection [27]. The E5 gene start codon was mutated and found to be dispensable for infection [15,16]. The E8 gene was reported to be essential by one research group [44] and dispensable by another [16]; differences in methodology probably played a significant role in these outcomes [41]. SiRNA-expressing cassettes were engineered into the viral genome to monitor infectious outcome in situ [45].
Both progressive and regressive variants of SfPV1 are extant. Genetic mutations were used to determine that a 15 bp region at the C terminus of the E6 gene was responsible for this difference [36]. This region contains a PDZ binding domain that is important for the functioning of E6 [46]. Interestingly, a single mutation in wild-type (progressive) E6 that corresponds to one of the changes in the regressive E6 results in different outcomes in inbred and outbred laboratory rabbits [47]. Thus, genetic makeup of both host and virus play a crucial role in the outcome of infection.
Multiple SfPV1 mutants have been generated in our laboratory including many with mutations in the upstream regulation region [28]. All mutants have been tested in vivo to yield interesting insights into the function and plasticity of the SfPV1 genome and its interaction with the host. A novel ‘tandem repeat’ strategy has been established to enhance tumour growth in cases where mutations have created genomes that resulted in diminished tumour growth. The mutant genomes that were cloned with an additional fragment of SfPV1 demonstrated improved tumour growth when compared with those without this tandem repeat [28,48].
Papillomaviruses have developed multiple strategies to escape host immune surveillance. One is the use of rare codons [49–51]. To explore whether synonymous codon modifications in the SfPV1 genome would impact disease outcome in rabbits, we engineered a panel of mutants with synonymous codon changes in two oncogenes (E6 and E7) to make them more mammalian-like [52]. This strategy has been used to increase L1 expression of different human papillomavirus (HPV) types in vitro [53,54]. We detected dramatic phenotypic changes as a result of the codon optimizations [52]. Intriguingly, codon-modified E6 synergized with codon-modified E7 and induced early cancer development in domestic rabbits [52]. Some of these constructs, by contrast, showed higher regression rates than those of the wild-type [55]. These findings suggest that synonymous codon modifications can alter the immunogenicity of the virus and lead to either rapid progression or eventual regression depending upon the changes made. Our unpublished observations (N. M. Cladel 2008) include a cancer, which spontaneously regressed. Codon modifications of papillomavirus genes hold promise for future studies of host immune response, cancer development and viral gene functions. Synonymous mutations were once considered to be of little or no functional importance until recent studies demonstrated the significant function or phenotypical changes in difference model systems [56–59]. The findings reported here for SfPV1 contribute to the growing body of evidence that, in fact, synonymous changes can result in profound changes in gene expression.
(b). Host immune response to Sylvilagus floridanus papillomavirus 1 infections
Host immunity, including innate and adaptive immune responses, contributes to disease control as well as progression when circumvented [35,60–65]. A particular major histocompatibility complex class II (MHCII) genotype has been linked to the regressive phenotype for SfPV1 infections [66]. Further studies demonstrated that the rabbit MHCII (DRA–DQA) haplotype plays a role in tumour regression [35]. An inbred rabbit line (EIII/JC) mounts a vigorous response to SfPV1 infections and animals experience a regression rate of about 10%. By contrast, the outbred rabbits have a very low regression rate (about 1%) [47]. The human leukocyte antigens (HLA)-A2.1 transgenic rabbits also demonstrated higher immunogenicity when compared with outbred domestic rabbits [67]. Host T and B-cell-mediated immune responses are important factors in SfPV1-associated infections and diseases [60,64,68]. Tumour regression was correlated with infiltration of CD4 and CD8T cells that target early proteins E2 and E6 [60,69]. Taking advantage of these findings, prophylactic and therapeutic vaccines composed of DNA, peptides or protein were designed to target early genes as amplified below [31,70–72]. When host immune responses were suppressed with cyclosporine A, the regressive strain became persistent, supporting the observations that CD4 and CD8T cells play a critical role in controlling disease progression [47,69].
3. Prophylactic and therapeutic vaccine development using the Sylvilagus floridanus papillomavirus 1/rabbit model
(a). Prophylactic vaccines using the early and late genes as targets
A study in domestic rabbits using tumour suspensions to immunize animals demonstrated that tumour cells contained an antigen or antigens that could stimulate immune responses in the host [6]. Furthermore, sera harvested from these immunized animals neutralized the virions both in vitro and in vivo [73,74]. These studies demonstrated that the host could generate anti- SfPV1 antibodies and eventually led to the finding that immunization with L1 virus-like particles (VLPs) of rabbits could yield long-lasting protection from infection [75–77]. This finding, in turn, led to the development of the current L1 VLP vaccines, which are highly effective and which are projected to markedly reduce the incidence of HPV infection and subsequent cervical cancer in recipients [78]. In addition to stimulating strong humoral immune response, L1 protein was also found to elicit broad cellular immune responses in humans. The cellular immune responses resulting from the L1 vaccination have been further demonstrated in the rabbit model [79,80]. The L2 protein is far less immunogenic than the L1 protein, but has the advantage of producing antibodies that are cross-protective across a number of viral species [81,82]. Recent developments in pseudovirus and quasi-virus production have paved the way for the testing of prophylactic vaccines against multiple HPV types in the SfPV1/rabbit model [83,84]. We produced infectious hybrid viruses by encapsidating the SfPV1 genome into capsids of several oncogenic HPVs (HPV16, 18, 31, 45 and 58). All of these hybrid viruses were infectious in rabbits as determined by the generation of tumours [84,85].
Protective immunity by early gene products has been tested in the rabbit model as well as in other animal models for more than a decade. Owing to the poor immunogenicity of these proteins, low or undetectable antibody levels were found in most immunized animals [86,87]. By contrast, cell-mediated immune responses were found to play a critical role in the host protection against viral infections [63,86–89]. Interestingly, the method of delivering the antigen also plays an important role in the outcome [90–92]. Antigens can be delivered as protein products, peptides or as DNA and the outcomes can be different depending upon the mode of delivery. For example, DNA delivered by gene-gun promoted strong cell-mediated immune response while intramuscular injection of DNA offered no protection, even though some cell-mediated immune responses were generated in the immunized animals [87].
(b). Therapeutic vaccines using the early and late genes as targets
Although prophylactic vaccines provide excellent protection against new infections, they offer no protection against established infections. Therefore, a vaccine that could resolve active infections would be highly desirable. The SfPV1 rabbit model has been used to investigate effective targets for therapeutic purposes [17,72,93,94]. Because early genes promote cell-mediated immune responses in the host and hold promise to clear the infection, early proteins (E1, E2, E6 and E7) have been tested for their therapeutic potential in different formats. These include DNA vaccines delivered by gene-gun or by viral vector [31,95] (figure 3). E1 and E6 proteins have proved to be more effective for eliminating established tumours than E7 and E2, while the combination of the four early genes yielded the best results [70,89,96]. Recent promising studies used long peptides of E6 and E7 proteins as immunogens [72,94]. This immunization strategy showed a strong therapeutic effect in the SfPV1/rabbit model as well as in human clinical trials [94,97–99].
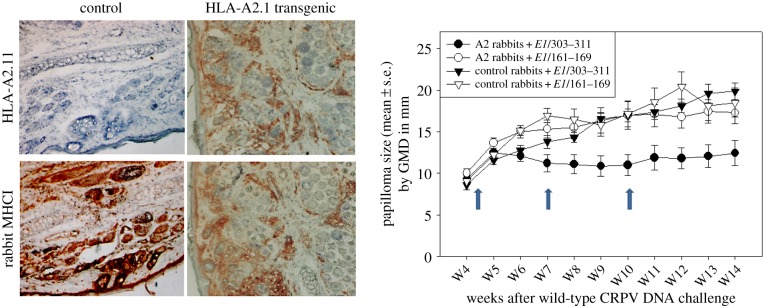
Figure 3.
Therapeutic vaccination with HLA-A2.1 restricted epitope DNA vaccines targeting early gene E1. Control (HLA-A2.1-negative) and HLA-A2.1 transgenic rabbits (HLA-A2.1-positive) rabbits are both positive for rabbit MHCI as shown by immunohistochemistry on the left. Animals infected with SfPV1 were divided into four groups and vaccinated with two different E1 epitope DNA vaccines (E1/303–311 and E1/161–169) at week 4 post-infection [95]. Two booster immunizations were conducted at three-week intervals (arrows). Significantly smaller tumours were found in the transgenic rabbit group immunized with epitope E1/303–311 when compared with the other three groups (by geometric mean diameter (GMD) in mm, p < 0.05, unpaired Student t-test) indicating the immune response was effective and specific.
4. The Sylvilagus floridanus papillomavirus 1/rabbit model for the testing of anti-viral compounds
The SfPV1/rabbit model is valued for its reproducibility and has been widely used for many years to test anti-tumour compounds [100,101]. Topical cidofovir treatment has been shown to be effective in resolving the tumours, but the recurrence rate is high [101] (figure 4). The effect can be improved by combining cidofovir treatment with DNA vaccination by gene-gun [102]. Our laboratory has tested many other compounds over the last several decades. Unfortunately, the efficacy was not optimal in most studies [55,103]. New strategies involving changes in methods of delivery, formulations and combination treatments hold promise in the coming years [101–105]. Our ultimate goal is to find ways to eradicate papillomavirus-associated diseases and cancers; it is our expectation that the SfPV1/rabbit model will continue to prove very useful in this quest.
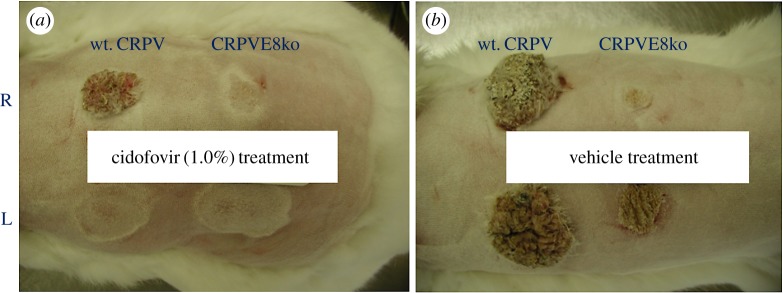
Figure 4.
Therapeutic treatment with cidofovir and vehicle (cremophor) at weeks 2–4 post-viral infections [101]. Two groups of animals were infected with wild-type (wt.) SfPV1 (induces large tumours) and SfPV1E8ko (induce small and persistent tumours) at two back sites, respectively. Two tumours induced by either wt. SfPV1or SfPV1E8ko on the left (L) side of the rabbits were treated with either 1.0% cidofovir formulated in cremophor (a) or cremophor (b) from week 2 to week 4 post-infection. The right (R) two sites were not treated and served as controls for the treated sites on the same animal. The animals were monitored for tumour growth weekly and pictures were recorded. Cidofovir-treated sites were free of tumours induced by both wt. SfPV1 and SfPV1E8ko at week 7 post-infection, while no reduction in tumours was found on the vehicle-treated sites (p < 0.05, unpaired Student t-test).
5. Limitations and future directions
The SfPV1/rabbit papillomavirus model induces infection with long-term persistence and malignant progression of lesions both consistently and reproducibly. Eighty years of studies with this unique model have proved it to be one of the best surrogate models for studying high-risk HPV infection [55,100]. Over the past several decades, we and others have generated a large panel of mutants to aid in the study of the pathogenesis of papillomavirus [55]. These mutant constructs will allow us to dissect the function of different genes and their role in the pathogenesis of papillomavirus-associated skin infections. However, SfPV1 only infects cutaneous sites and therefore is not the ideal model to mimic anogenital infections and diseases. For future directions, we look forward to more comparative studies with human samples. These additional studies will help to uncover mechanisms that could ultimately lead to a novel treatment for HPV-associated diseases and cancers.
Acknowledgements
We acknowledge Karla Balogh, Jingwei Li, Sarah Brendle, Lynn Budgeon, Ricai Han, Tim Culp and Callie Bounds who have contributed to our studies.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
Research reported in this publication was supported by the National Cancer Institute under award number R01 CA47622 (N.C.), NIH contract HHSN272201000020I (N.C.) and the Jake Gittlen Memorial Golf Tournament.
References
- 1.Duch CE, Williams RA, Timm RM, Perez-Tris J, Benitez L. 2015. A century of Shope papillomavirus in museum rabbit specimens. PLoS ONE 10, e0132172 ( 10.1371/journal.pone.0132172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shope RE, Hurst EW. 1933. Infectious papillomatosis of rabbits; with a note on the histopathology. J. Exp. Med. 58, 607–624. ( 10.1084/jem.58.5.607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shope RE. 1935. Serial transmission of virus of infectious papillomatosis in domestic rabbits. Proc. Soc. Exp. Biol. Med. 32, 830–832. ( 10.3181/00379727-32-7875) [DOI] [Google Scholar]
- 4.Syverton JT, Berry GP. 1935. Carcinoma in cottontail rabbit following spontaneous virus papilloma (Shope). Proc. Soc. Exp. Biol. Med. 33, 399–400. ( 10.3181/00379727-33-8386P) [DOI] [Google Scholar]
- 5.Kidd JG, Rous P. 1938. The carcinogenic effect of a papilloma virus on the tarred skin of rabbits. II. Major factors determining the phenomenon: the manifold effects of tarring. J. Exp. Med. 68, 529–562. ( 10.1084/jem.68.4.529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidd JG. 1938. Immunological reactions with a virus causing papillomas in rabbits. I. Demonstration of a complement fixation reaction: relation of virus-neutralizing and complement binding antibodies. J. Exp. Med. 68, 703–724. ( 10.1084/jem.68.5.703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito Y, Evans CA. 1961. Induction of tumors in domestic rabbits with nucleic acid preparations from partially purified Shope papilloma virus and from extracts of the papillomas of domestic and cottontail rabbits. J. Exp. Med. 114, 485–500. ( 10.1084/jem.114.4.485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreider JW, Cladel NM, Patrick SD, Welsh PA, DiAngelo SL, Bower JM, Christensen ND. 1995. High efficiency induction of papillomas in vivo using recombinant cottontail rabbit papillomavirus DNA. J. Virol. Methods 55, 233–244. ( 10.1016/0166-0934(95)00062-Y) [DOI] [PubMed] [Google Scholar]
- 9.Stevens JG, Wettstein FO. 1979. Multiple copies of malignant non-virus-producing neoplasms. J. Virol. 30, 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wettstein FO, Stevens JG.. 1981. Distribution and state of viral nucleic acid in tumors induced by Shope papilloma virus. In Viruses in naturally occurring cancers (eds Essex M, Todaro G, zur Hausen H), pp. 301–307. New York, NY: Cold Spring Harbor. [Google Scholar]
- 11.Wettstein FO, Stevens JG. 1981. Transcription of the viral genome in papillomas and carcinomas induced by the Shope virus. Virology 109, 448–451. ( 10.1016/0042-6822(81)90517-1) [DOI] [PubMed] [Google Scholar]
- 12.Wettstein FO. 1990. State of viral DNA and gene expression in benign vs. malignant tumors. In Papillomaviruses and human cancer (ed. Pfister H.), pp. 155–180. Boca Raton, FL: CRC Press. [Google Scholar]
- 13.Christensen ND, Kreider JW. 1991. Neutralization of CRPV infectivity by monoclonal antibodies that identify conformational epitopes on intact virions. Virus Res. 21, 169–179. ( 10.1016/0168-1702(91)90031-P) [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Budgeon LR, Cladel NM, Culp TD, Balogh KK, Christensen ND. 2007. Detection of L1, infectious virions and anti-L1 antibody in domestic rabbits infected with cottontail rabbit papillomavirus. J. Gen. Virol. 88(Pt 12), 3286–3293. ( 10.1099/vir.0.82879-0) [DOI] [PubMed] [Google Scholar]
- 15.Brandsma JL, Yang Z-H, DiMaio D, Barthold SW, Johnson E, Xiao W. 1992. The putative E5 open reading frame of cottontail rabbit papillomavirus is dispensable for papilloma formation in domestic rabbits. J. Virol. 66, 6204–6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu J, Han R, Cladel NM, Pickel MD, Christensen ND. 2002. Intracutaneous DNA vaccination with the E8 gene of cottontail rabbit papillomavirus induces protective immunity against virus challenge in rabbits. J. Virol. 76, 6453–6459. ( 10.1128/JVI.76.13.6453-6459.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandsma JL, Shlyankevich M, Buonocore L, Roberts A, Becker SM, Rose JK. 2007. Therapeutic efficacy of vesicular stomatitis virus-based E6 vaccination in rabbits. Vaccine 25, 751–762. ( 10.1016/j.vaccine.2006.08.012) [DOI] [PubMed] [Google Scholar]
- 18.Meyers C, Harry J, Lin Y-L, Wettstein FO. 1992. Identification of three transforming proteins encoded by cottontail rabbit papillomavirus. J. Virol. 66, 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harry JB, Wettstein FO. 1996. Transforming properties of the cottontail rabbit papillomavirus oncoproteins LE6 and SE6 and of the E8 protein. J. Virol. 70, 3355–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyers C, Wettstein FO. 1991. The late region differentially regulates the in vitro transformation by cottontail rabbit papillomavirus DNA in different cell types. Virology 181, 637–646. ( 10.1016/0042-6822(91)90897-K) [DOI] [PubMed] [Google Scholar]
- 21.Giri I, Yaniv M. 1988. Study of the E2 gene product of the cottontail rabbit papillomavirus reveals a common mechanism of transactivation among papillomaviruses. J. Virol. 62, 1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeckel S, Loetzsch E, Huber E, Stubenrauch F, Iftner T. 2003. Identification of the E9/E2C cDNA and functional characterization of the gene product reveal a new repressor of transcription and replication in cottontail rabbit papillomavirus. J. Virol. 77, 8736–8744. ( 10.1128/JVI.77.16.8736-8744.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeckel S, Huber E, Stubenrauch F, Iftner T. 2002. A transactivator function of cottontail rabbit papillomavirus e2 is essential for tumor induction in rabbits. J. Virol. 76, 11 209–11 215. ( 10.1128/JVI.76.22.11209-11215.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X, Xiao W, Brandsma J. 1994. Papilloma formation by cottontail rabbit papillomavirus requires E1 and E2 regulatory genes in addition to E6 and E7 transforming genes. J. Virol. 68, 6097–6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danos O, Mulligan RC, Yaniv M. 1986. Production of spliced DNA copies of the cottontail rabbit papillomavirus genome in a retroviral vector. Ciba Found. Symp. 120, 68–82. [DOI] [PubMed] [Google Scholar]
- 26.Fujii T, Brandsma JL, Peng X, Srimatkandada S, Li L, Canaan A, Deisseroth AB. 2001. High and low levels of cottontail rabbit papillomavirus E2 protein generate opposite effects on gene expression. J. Biol. Chem. 276, 867–874. ( 10.1074/jbc.M007120200) [DOI] [PubMed] [Google Scholar]
- 27.Peh WL, Brandsma JL, Christensen ND, Cladel NM, Wu X, Doorbar J. 2004. The viral E4 protein is required for the completion of the cottontail rabbit papillomavirus productive cycle in vivo. J. Virol. 78, 2142–2151. ( 10.1128/JVI.78.4.2142-2151.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu J, Cladel NM, Balogh K, Budgeon L, Christensen ND. 2007. Impact of genetic changes to the CRPV genome and their application to the study of pathogenesis in vivo. Virology 358, 384–390. ( 10.1016/j.virol.2006.08.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han R, Cladel NM, Reed CA, Christensen ND. 1998. Characterization of transformation function of cottontail rabbit papillomavirus E5 and E8 genes. Virology 251, 253–263. ( 10.1006/viro.1998.9416) [DOI] [PubMed] [Google Scholar]
- 30.Hu J, Cladel NM, Christensen ND. 2007. Increased immunity to cottontail rabbit papillomavirus infection in EIII/JC inbred rabbits after vaccination with a mutant E6 that correlates with spontaneous regression. Viral Immunol. 20, 320–325. ( 10.1089/vim.2006.0104) [DOI] [PubMed] [Google Scholar]
- 31.Han R, Cladel NM, Reed CA, Peng X, Christensen ND. 1999. Protection of rabbits from viral challenge by gene gun-based intracutaneous vaccination with a combination of cottontail rabbit papillomavirus E1, E2, E6, and E7 genes. J. Virol. 73, 7039–7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wettstein FO, Barbosa MS, Nasseri M. 1987. Identification of the major cottontail rabbit papillomavirus late RNA cap site and mapping and quantitation of an E2 and minor E6 coding mRNA in papillomas and carcinomas. Virology 159, 321–328. ( 10.1016/0042-6822(87)90470-3) [DOI] [PubMed] [Google Scholar]
- 33.Salmon J, Ramoz N, Cassonnet P, Orth G, Breitburd F. 1997. A cottontail rabbit papillomavirus strain (CRPVb) with strikingly divergent E6 and E7 oncoproteins: an insight in the evolution of papillomaviruses. Virology 235, 228–234. ( 10.1006/viro.1997.8680) [DOI] [PubMed] [Google Scholar]
- 34.Defeo-Jones DI, et al. 1993. Papillomavirus E7 protein binding to the retinoblastoma protein is not required for viral induction of warts. J. Virol. 67, 716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salmon J, Nonnenmacher M, Caze S, Flamant P, Croissant O, Orth G, Breitburd F. 2000. Variation in the nucleotide sequence of cottontail rabbit papillomavirus a and b subtypes affects wart regression and malignant transformation and level of viral replication in domestic rabbits. J. Virol. 74, 10 766–10 777. ( 10.1128/JVI.74.22.10766-10777.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu J, Cladel NM, Pickel MD, Christensen ND. 2002. Amino acid residues in the carboxy-terminal region of cottontail rabbit papillomavirus e6 influence spontaneous regression of cutaneous papillomas. J. Virol. 76, 11 801–11 808. ( 10.1128/JVI.76.23.11801-11808.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitt A, Rochat A, Zeltner R, Borenstein L, Barrandon Y, Wettstein FO, Iftner T. 1996. The primary target cells of the high-risk cottontail rabbit papillomavirus colocalize with hair folicle stem cells. J. Virol. 70, 1912–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ito Y. 1963. Studies on subviral tumorigenesis: carcinoma derived from nucleic acid-induced papillomas of rabbit skin. Acta Unio Int. Contra Cancrum 19, 280–283. [PubMed] [Google Scholar]
- 39.Xiao W, Brandsma JL. 1996. High efficiency, long-term clinical expression of cottontail rabbit papillomavirus (CRPV) DNA in rabbit skin following particle-mediated DNA transfer. Nucleic Acids Res. 24, 2620–2622. ( 10.1093/nar/24.13.2620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cladel NM, Hu J, Balogh K, Mejia A, Christensen ND. 2008. Wounding prior to challenge substantially improves infectivity of cottontail rabbit papillomavirus and allows for standardization of infection. J. Virol. Methods 148, 34–39. ( 10.1016/j.jviromet.2007.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cladel NM, Hu J, Balogh KK, Christensen ND. 2010. Differences in methodology, but not differences in viral strain, account for variable experimental outcomes in laboratories utilizing the cottontail rabbit papillomavirus model. J. Virol. Methods 165, 36–41. ( 10.1016/j.jviromet.2009.12.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reuter JD, Gomez D, Brandsma JL, Rose JK, Roberts A. 2001. Optimization of cottontail rabbit papilloma virus challenge technique. J. Virol. Methods 98, 127–134. ( 10.1016/S0166-0934(01)00370-6) [DOI] [PubMed] [Google Scholar]
- 43.Stubenrauch F, Hummel M, Iftner T, Laimins LA. 2000. The E8E2C protein, a negative regulator of viral transcription and replication, is required for extrachromosomal maintenance of human papillomavirus type 31 in keratinocytes. J. Virol. 74, 1178–1186. ( 10.1128/JVI.74.3.1178-1186.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nonnenmacher M, Salmon J, Jacob Y, Orth G, Breitburd F. 2006. Cottontail rabbit papillomavirus E8 protein is essential for wart formation and provides new insights into viral pathogenesis. J. Virol. 80, 4890–4900. ( 10.1128/JVI.80.10.4890-4900.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huber E, Vlasny D, Jeckel S, Stubenrauch F, Iftner T. 2004. Gene profiling of cottontail rabbit papillomavirus-induced carcinomas identifies upregulated genes directly involved in stroma invasion as shown by small interfering RNA-mediated gene silencing. J. Virol. 78, 7478–7489. ( 10.1128/JVI.78.14.7478-7489.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muench P, Probst S, Schuetz J, Leiprecht N, Busch M, Wesselborg S, Stubenrauch F, Iftner T. 2010. Cutaneous papillomavirus E6 proteins must interact with p300 and block p53-mediated apoptosis for cellular immortalization and tumorigenesis. Cancer Res. 70, 6913–6924. ( 10.1158/0008-5472.CAN-10-1307) [DOI] [PubMed] [Google Scholar]
- 47.Hu J, Peng X, Cladel NM, Pickel MD, Christensen ND. 2005. Large cutaneous rabbit papillomas that persist during cyclosporin A treatment can regress spontaneously after cessation of immunosuppression. J. Gen. Virol. 86(Pt 1), 55–63. ( 10.1099/vir.0.80448-0) [DOI] [PubMed] [Google Scholar]
- 48.Bounds CE, Hu J, Cladel NM, Balogh K, Christensen ND. 2011. Vaccine generated immunity targets an HPV16 E7 HLA-A2.1-restricted CD8(+) T cell epitope relocated to an early gene or a late gene of the cottontail rabbit papillomavirus (CRPV) genome in HLA-A2.1 transgenic rabbits. Vaccine 29, 1194–1200. ( 10.1016/j.vaccine.2010.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao KN, Gu W, Fang NX, Saunders NA, Frazer IH. 2005. Gene codon composition determines differentiation-dependent expression of a viral capsid gene in keratinocytes in vitro and in vivo. Mol. Cell. Biol. 25, 8643–8655. ( 10.1128/MCB.25.19.8643-8655.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao KN, Liu WJ, Frazer IH. 2003. Codon usage bias and A+T content variation in human papillomavirus genomes. Virus Res. 98, 95–104. ( 10.1016/j.virusres.2003.08.019) [DOI] [PubMed] [Google Scholar]
- 51.Zhou J, Liu WJ, Peng SW, Sun XY, Frazer I. 1999. Papillomavirus capsid protein expression level depends on the match between codon usage and tRNA availability. J. Virol. 73, 4972–4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cladel NM, Hu J, Balogh KK, Christensen ND. 2008. CRPV genomes with synonymous codon optimizations in the CRPV E7 gene show phenotypic differences in growth and altered immunity upon E7 vaccination. PLoS ONE 3, e2947 ( 10.1371/journal.pone.0002947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baud D, Ponci F, Bobst M, De Grandi P, Nardelli-Haefliger D. 2004. Improved efficiency of a salmonella-based vaccine against human papillomavirus type 16 virus-like particles achieved by using a codon-optimized version of L1. J. Virol. 78, 12 901–12 909. ( 10.1128/JVI.78.23.12901-12909.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mossadegh N, Gissmann L, Muller M, Zentgraf H, Alonso A, Tomakidi P. 2004. Codon optimization of the human papillomavirus 11 (HPV 11) L1 gene leads to increased gene expression and formation of virus-like particles in mammalian epithelial cells. Virology 326, 57–66. ( 10.1016/j.virol.2004.04.050) [DOI] [PubMed] [Google Scholar]
- 55.Christensen ND, Budgeon LR, Cladel NM, Hu J. 2017. Recent advances in preclinical model systems for papillomaviruses. Virus Res. 231, 108–118. ( 10.1016/j.virusres.2016.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. 2007. A ‘silent’ polymorphism in the MDR1 gene changes substrate specificity. Science 315, 525–528. ( 10.1126/science.1135308) [DOI] [PubMed] [Google Scholar]
- 57.Carlini DB. 2004. Experimental reduction of codon bias in the Drosophila alcohol dehydrogenase gene results in decreased ethanol tolerance of adult flies. J. Evol. Biol. 17, 779–785. ( 10.1111/j.1420-9101.2004.00725.x) [DOI] [PubMed] [Google Scholar]
- 58.Chamary JV, Parmley JL, Hurst LD. 2006. Hearing silence: non-neutral evolution at synonymous sites in mammals. Nat. Rev. Genet. 7, 98–108. ( 10.1038/nrg1770) [DOI] [PubMed] [Google Scholar]
- 59.Burns CC, Shaw J, Campagnoli R, Jorba J, Vincent A, Quay J, Kew O. 2006. Modulation of poliovirus replicative fitness in HeLa cells by deoptimization of synonymous codon usage in the capsid region. J. Virol. 80, 3259–3272. ( 10.1128/JVI.80.7.3259-3272.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okabayashi M, Angell MG, Budgeon LR, Kreider JW. 1993. Shope papilloma cell and leukocyte proliferation in regressing and progressing lesions. Am. J. Pathol. 142, 489–496. [PMC free article] [PubMed] [Google Scholar]
- 61.Höpfl RM, Christensen ND, Angell MG, Kreider JW. 1993. Skin test to assess immunity against cottontail rabbit papillomavirus antigens in rabbits with progressing papillomas or after papilloma regression. J. Invest. Dermatol. 101, 227–231. ( 10.1111/1523-1747.ep12364825) [DOI] [PubMed] [Google Scholar]
- 62.Hagari Y, Budgeon LR, Pickel MD, Kreider JW. 1995. Association of tumor necrosis factor-α gene expression and apoptotic cell death with regression of shope papillomas. J. Invest. Dermatol. 104, 526–529. ( 10.1111/1523-1747.ep12606028) [DOI] [PubMed] [Google Scholar]
- 63.Selvakumar R, Borenstein LA, Lin YL, Ahmed R, Wettstein FO. 1995. Immunization with nonstructural proteins E1 and E2 of cottontail rabbit papillomavirus stimulates regression of virus-induced papillomas. J. Virol. 69, 602–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin YL, Borenstein LA, Selvakumar R, Ahmed R, Wettstein FO. 1993. Progression from papilloma to carcinoma is accompanied by changes in antibody response to papillomavirus proteins. J. Virol. 67, 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Breitburd F, Ramoz N, Salmon J, Orth G. 1997. HLA control in the progression of human papillomavirus infections. Sem. Cancer Biol. 7, 359 ( 10.1006/scbi.1996.0045) [DOI] [PubMed] [Google Scholar]
- 66.Han R, Breitburd F, Marche PN, Orth G. 1992. Linkage of regression and malignant conversion of rabbit viral papillomas to MHC class II genes. Nature 356, 66–68. ( 10.1038/356066a0) [DOI] [PubMed] [Google Scholar]
- 67.Hu J, Peng X, Budgeon LR, Cladel NM, Balogh KK, Christensen ND. 2007. Establishment of a cottontail rabbit papillomavirus/HLA-A2.1 transgenic rabbit model. J. Virol. 81, 7171–7177. ( 10.1128/JVI.00200-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hopfl R, Christensen ND, Angell MG, Kreider JW. 1995. Leukocyte proliferation in vitro against cottontail rabbit papillomavirus in rabbits with persisting papillomas/cancer or after regression. Arch. Dermatol. Res. 287, 652–658. ( 10.1007/BF00371738) [DOI] [PubMed] [Google Scholar]
- 69.Selvakumar R, Schmitt A, Iftner T, Ahmed R, Wettstein FO. 1997. Regression of papillomas induced by cottontail rabbit papillomavirus is associated with infiltration of CD8+ cells and persistence of viral DNA after regression. J. Virol. 71, 5540–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brandsma JL, Shylankevich M, Su Y, Roberts A, Rose JK, Zelterman D, Buonocore L. 2007. Vesicular stomatitis virus-based therapeutic vaccination targeted to the E1, E2, E6, and E7 proteins of cottontail rabbit papillomavirus. J. Virol. 81, 5749–5758. ( 10.1128/JVI.02835-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Welters MJ, et al. 2008. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin. Cancer Res. 14, 178–187. ( 10.1158/1078-0432.CCR-07-1880) [DOI] [PubMed] [Google Scholar]
- 72.Hu J, Budgeon LR, Balogh KK, Peng X, Cladel NM, Christensen ND. 2014. Long-peptide therapeutic vaccination against CRPV-induced papillomas in HLA-A2.1 transgenic rabbits. Trials Vaccinol. 3, 134–142. ( 10.1016/j.trivac.2014.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shope RE. 1937. Immunization of rabbits to infectious papillomatosis. J. Exp. Med. 65, 219–231. ( 10.1084/jem.65.2.219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beard JW, Wyckoff RW. 1937. Isolation of homogeneous heavy protein from virus-induced rabbit papillomas. Science 85, 201–202. ( 10.1126/science.85.2199.201) [DOI] [PubMed] [Google Scholar]
- 75.Lin Y-L, Borenstein LA, Ahmed R, Wettstein FO. 1993. Cottontail rabbit papillomavirus L1 protein-based vaccines: protection is achieved only with a full-length, nondenatured product. J. Virol. 67, 4154–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller JT, Lowy DR. 1995. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 69, 3959–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Christensen ND, Reed CA, Cladel NM, Han R, Kreider JW. 1996. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J. Virol. 70, 960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lowy DR, Schiller JT. 2006. Prophylactic human papillomavirus vaccines. J. Clin. Invest. 116, 1167–1173. ( 10.1172/JCI28607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sundaram P, Tigelaar RE, Brandsma JL. 1997. Intracutaneous vaccination of rabbits with the cottontail rabbit papillomavirus (CRPV) L1 gene protects against virus challenge. Vaccine 15, 664–671. ( 10.1016/S0264-410X(96)00237-X) [DOI] [PubMed] [Google Scholar]
- 80.Hu J, Cladel NM, Budgeon LR, Reed CA, Pickel MD, Christensen ND. 2006. Protective cell-mediated immunity by DNA vaccination against papillomavirus L1 capsid protein in the cottontail rabbit papillomavirus model. Viral Immunol. 19, 492–507. ( 10.1089/vim.2006.19.492) [DOI] [PubMed] [Google Scholar]
- 81.Christensen ND, Kreider JW, Kan NC, DiAngelo SL. 1991. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology 181, 572–579. ( 10.1016/0042-6822(91)90890-N) [DOI] [PubMed] [Google Scholar]
- 82.Embers ME, Budgeon LR, Pickel M, Christensen ND. 2002. Protective immunity to rabbit oral and cutaneous papillomaviruses by immunization with short peptides of l2, the minor capsid protein. J. Virol. 76, 9798–9805. ( 10.1128/JVI.76.19.9798-9805.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kalnin K, et al. 2014. Low doses of flagellin-L2 multimer vaccines protect against challenge with diverse papillomavirus genotypes. Vaccine 32, 3540–3547. ( 10.1016/j.vaccine.2014.04.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Culp TD, Cladel NM, Balogh KK, Budgeon LR, Mejia AF, Christensen ND. 2006. Papillomavirus particles assembled in 293TT cells are infectious in vivo. J. Virol. 80, 11 381–11 384. ( 10.1128/JVI.01328-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gambhira R, et al. 2007. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J. Virol. 81, 13 927–13 931. ( 10.1128/JVI.00936-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sundaram P, Tigelaar RE, Xiao W, Brandsma JL. 1998. Intracutaneous vaccination of rabbits with the E6 gene of cottontail rabbit papillomavirus provides partial protection against virus challenge. Vaccine 16, 613–623. ( 10.1016/S0264-410X(97)84510-0) [DOI] [PubMed] [Google Scholar]
- 87.Han R, Reed CA, Cladel NM, Christensen ND. 1999. Intramuscular injection of plasmid DNA encoding cottontail rabbit papillomavirus E1, E2, E6 and E7 induces T cell-mediated but not humoral immune responses in rabbits. Vaccine 17, 1558–1566. ( 10.1016/S0264-410X(98)00356-9) [DOI] [PubMed] [Google Scholar]
- 88.Leachman SA, et al. 2000. Granulocyte-macrophage colony-stimulating factor priming plus papillomavirus E6 DNA vaccination: effects on papilloma formation and regression in the cottontail rabbit papillomavirus—rabbit model. J. Virol. 74, 8700–8708. ( 10.1128/JVI.74.18.8700-8708.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Han R, Reed CA, Cladel NM, Christensen ND. 2000. Immunization of rabbits with cottontail rabbit papillomavirus E1 and E2 genes: protective immunity induced by gene gun-mediated intracutaneous delivery but not by intramuscular injection. Vaccine 18, 2937–2944. ( 10.1016/S0264-410X(00)00110-9) [DOI] [PubMed] [Google Scholar]
- 90.Yang A, Farmer E, Wu TC, Hung CF. 2016. Perspectives for therapeutic HPV vaccine development. J. Biomed. Sci. 23, 75 ( 10.1186/s12929-016-0293-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu Y, Yuen PW, Lam JK. 2014. Intranasal DNA vaccine for protection against respiratory infectious diseases: the delivery perspectives. Pharmaceutics 6, 378–415. ( 10.3390/pharmaceutics6030378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pertmer TM, Eisenbraun MD, McCabe D, Prayaga SK, Fuller DH, Haynes JR. 1995. Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte responses following epidermal delivery of nanogram quantities of DNA. Vaccine 13, 1427–1430. ( 10.1016/0264-410X(95)00069-D) [DOI] [PubMed] [Google Scholar]
- 93.Brandsma JL, Shlyankevich M, Su Y, Zelterman D, Rose JK, Buonocore L. 2009. Reversal of papilloma growth in rabbits therapeutically vaccinated against E6 with naked DNA and/or vesicular stomatitis virus vectors. Vaccine 28, 8345–8351. ( 10.1016/j.vaccine.2009.04.082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vambutas A, DeVoti J, Nouri M, Drijfhout JW, Lipford GB, Bonagura VR, Van der Burg SH, Melief CJ. 2005. Therapeutic vaccination with papillomavirus E6 and E7 long peptides results in the control of both established virus-induced lesions and latently infected sites in a pre-clinical cottontail rabbit papillomavirus model. Vaccine 23, 5271–5280. ( 10.1016/j.vaccine.2005.04.049) [DOI] [PubMed] [Google Scholar]
- 95.Hu J ST, Peng X, Cladel NM, Balogh K, Christensen ND. 2009. Strong and specific protective and therapeutic immunity induced by single HLA-A2.1 restricted epitope DNA vaccine in rabbits. Procedia Vaccinol. 1, 4–14. [Google Scholar]
- 96.Brandsma JL, Shlyankevich M, Zelterman D, Su Y. 2007. Therapeutic vaccination of rabbits with a ubiquitin-fused papillomavirus E1, E2, E6 and E7 DNA vaccine. Vaccine 25, 6158–6163. ( 10.1016/j.vaccine.2007.06.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Melief CJ, Van der Burg SH. 2008. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat. Rev. Cancer 8, 351–360. ( 10.1038/nrc2373) [DOI] [PubMed] [Google Scholar]
- 98.Kenter GG, et al. 2008. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin. Cancer Res. 14, 169–177. ( 10.1158/1078-0432.CCR-07-1881) [DOI] [PubMed] [Google Scholar]
- 99.Melief CJ. 2011. Synthetic vaccine for the treatment of lesions caused by high risk human papilloma virus. Cancer J. 17, 300–301. ( 10.1097/PPO.0b013e318235e0fe) [DOI] [PubMed] [Google Scholar]
- 100.Doorbar J. 2016. Model systems of human papillomavirus-associated disease. J. Pathol. 238, 166–179. ( 10.1002/path.4656) [DOI] [PubMed] [Google Scholar]
- 101.Christensen ND, Cladel NM, Hu J, Balogh KK. 2014. Formulation of cidofovir improves the anti-papillomaviral activity of topical treatments in the CRPV/rabbit model. Antiviral Res. 108C, 148–155. ( 10.1016/j.antiviral.2014.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Christensen ND, Han R, Cladel NM, Pickel MD. 2001. Combination treatment with intralesional cidofovir and viral-DNA vaccination cures large cottontail rabbit papillomavirus-induced papillomas and reduces recurrences. Antimicrob. Agents Chemother. 45, 1201–1209. ( 10.1128/AAC.45.4.1201-1209.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Christensen ND. 2005. Cottontail rabbit papillomavirus (CRPV) model system to test antiviral and immunotherapeutic strategies. Antivir. Chem. Chemother. 16, 355–362. ( 10.1177/095632020501600602) [DOI] [PubMed] [Google Scholar]
- 104.Bins AD, van den Berg JH, Oosterhuis K, Haanen JB. 2013. Recent advances towards the clinical application of DNA vaccines. Neth. J. Med. 71, 109–117. [PubMed] [Google Scholar]
- 105.Albers AE, Kaufmann AM. 2009. Therapeutic human papillomavirus vaccination. Public Health Genomics 12, 331–342. ( 10.1159/000214923) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.