Abstract
Disease emergence occurs within the context of ecological communities, and disease driven declines in host populations can lead to complex direct and indirect ecological effects. Varying effects of a single disease among multiple susceptible hosts could benefit relatively resistant species. Beginning in 2013, an outbreak of sea star wasting disease (SSWD) led to population declines of many sea star species along the west coast of North America. Through field surveys and laboratory experiments, we investigated how and why the relative abundances of two co-occurring sea star species, Evasterias troschelii and Pisaster ochraceus, shifted during the ongoing wasting epidemic in Burrard Inlet, British Columbia, Canada. We hypothesized that Evasterias is competitively inferior to Pisaster but more resistant to SSWD. Thus, we predicted that SSWD-induced declines of Pisaster could mitigate the negative effects of SSWD on Evasterias, as the latter would experience competitive release. We document shifts in sea star abundance from 2008–2017: Pisaster abundance and mean size declined during the outbreak, while Evasterias abundance increased from relatively rare to numerically dominant within the intertidal. When exposed to symptomatic sea stars, Pisaster and Evasterias both showed signs of SSWD, but transmission and susceptibility was lower in Evasterias. Despite diet overlap documented in our field surveys, Evasterias was not outcompeted by Pisaster in laboratory trails conducted with the relatively small Pisaster available after the outbreak. Interference competition with larger Pisaster, or prey exploitation by Pisaster during the summer when Evasterias is primarily subtidal, may explain the rarity of Evasterias prior to Pisaster declines. Our results suggest that indirect effects mediated by competition can mask some of the direct effects of disease outbreaks, and the combination of direct and indirect effects will determine the restructuring of a community after disturbance.
Keywords: disease outbreak, asteroid idiopathic wasting syndrome, competition, Evasterias troschelii, Pisaster ochraceus, population size
1. Introduction
Emerging diseases can cause long-term declines in host populations [1–8]. In turn, host population declines could alter the structure and dynamics of the communities in which they are embedded, with the direction and magnitude of effects on other species dependent on the host's position within the community [9,10]. For example, disease-induced population declines can lead to competitive release of uninfected competitors [4,11], release of resource/prey species from top-down control [1,12], and depleted resource availability for consumers [10]. Further, if multiple hosts share a single disease, outbreaks can lead to complex dynamics and intensified population declines even in the absence of direct interactions between the hosts (e.g. through apparent competition, [13,14]). While all disease-induced population declines have the potential to affect communities, diseases of keystone species in particular could have disproportionate effects on their communities because of the dominant effects of the host on community structure [15,16].
Disease-induced mass mortality events have been linked to introduced and novel diseases [4] and temperature effects on extant diseases that dampen host immune defence [17] or alter parasite transmission and/or production, (e.g. [7,8,18]). Beginning in 2013, sea star (Class Asteroidea) populations along the Californian and British Columbian coasts underwent a sudden mass mortality event. By the summer of 2014 up to 20 species of sea stars along the entire northeastern Pacific coast from Baja Mexico to the Aleutian Islands in Alaska were observed with visible signs of disease [19]. Disease-induced mortalities of sea stars have been documented previously along this Pacific coast [20,21], but the current, ongoing mortality event is unprecedented owing to the number of species affected and the magnitude and large geographical extent of the declines [5,6,19]. Field correlations and laboratory studies suggest that the outbreak could have been intensified by rising ocean temperature [5,19,22,23].
The aetiological agent remains elusive for the set of visible signs of disease known collectively as sea star wasting disease (SSWD; also known as asteroid idiopathic wasting syndrome and sea star wasting syndrome). Signs of SSWD in Pisaster ochraceus (hereafter, Pisaster) can be associated to a dysbiosis of a healthy microbiome, potentially involving a range of different and opportunistic pathogens [23]. Virus-sized particles have been associated with SSWD signs in several species and studies [19,24]. In Pycnopodia helianthoides, wasting asteroid-associated densovirus (WAaDs) is a putative aetiological agent for signs of wasting [19,25], but while WAaDs is present in Pisaster and Evasterias troschelii (hereafter, Evasterias), its presence is not necessary for visible disease progression in Pisaster [23]. Despite the unanswered questions on aetiology, the progression of the disease has been categorized into four stages of visible signs; starting with the appearance of minor lesions, followed by lesions spreading to the sea star's arms, leading to severe tissue deterioration and loss of limbs, and finally tissue damage across the entire body and death [19].
Populations of Pisaster, which is among the 20 species known to be affected, declined substantially owing to SSWD [5,6,19,26]. Pisaster is of particular interest because it is one of the species hardest hit by SSWD alongside the sunflower star P. helianthoides [5,19,27], and because it plays an important ecological role in the rocky coast ecosystem. Pisaster, the inspiration for the keystone species concept [15], has major impacts on the community assemblage of the rocky intertidal ecosystem through top-down control [28–30]. When Pisaster is removed from the rocky intertidal, the primary prey of Pisaster, the mussels Mytilus californianus and Mytilus trossulus, outcompete other primary substrate species such as barnacles and algae [29]. This has large scale effects as this food web based on benthic primary and secondary production may be disrupted [15]. The SSWD-induced decline of Pisaster may result in similar ecosystem cascading effects and changes in ecosystem function.
Efforts have been taken to uncover the ecological consequences of SSWD-induced reductions in the previously coastwide-abundant sea star Pisaster [31–34], with an emphasis on release from top-down control. However, the ways SSWD-induced declines in Pisaster may cause shifts within the sea star community via changing competitive dynamics remains unclear. Pathogens have been shown to have strong effects on competition when competing species have differential susceptibility to a pathogen [13,35,36]. A potential consequence of declines of dominant sea stars like Pisaster, then, is the competitive release [37,38] of more disease-resistant species [4]. One species that could potentially benefit from the decline of Pisaster is the mottled sea star, Evasterias, whose range overlaps with Pisaster, as it is found from Alaska to California, although it is rarely found south of Washington's Puget Sound [39,40]. Along most of its range, Evasterias is considered scarce with the exception of large populations in the sheltered fjords of Alaska and in the inland waters (Salish Sea) of British Columbia and Washington State [39,40]. Both Evasterias and Pisaster inhabit the intertidal and subtidal areas within Burrard Inlet, British Columbia; an area with prominent levels of SSWD [6]. The two species have overlapping habitat use [39] and diet [41], suggesting the potential for competition. This competition is likely to be asymmetrical, as Pisaster is widely considered to be a dominant sea star in the intertidal and has been shown to act aggressively towards other sea stars [42,43]. Therefore, although Evasterias is one of the 20 species to be observed with signs of SSWD [19], Evasterias may be able to expand into the niche previously filled and dominated by Pisaster depending on the competitive relationship between the two species and the degree to which each is vulnerable to persistent population declines in the face of SSWD.
To better understand the potential ecological consequences of SSWD, we investigated how and why the relative abundances of Evasterias and Pisaster have shifted in association with the SSWD outbreak. We hypothesized that declines in the Burrard Inlet Pisaster population from SSWD will mitigate the direct effects of SSWD on Evasterias by releasing Evasterias from competition and allowing it to numerically increase in the intertidal zone. Specifically, we hypothesized that the Evasterias population is less susceptible to SSWD than the Pisaster population, is competitively inferior to Pisaster, and has increased following the decline of Pisaster in the field. To test these hypotheses, we combined long-term monitoring in the field with laboratory disease challenge trials and competition experiments. By using the Burrard Inlet populations of Evasterias and Pisaster as a model system, we assess the degree to which indirect and direct effects of SSWD may change dominance patterns within the sea star predator guild.
2. Methods
(a). Field surveys
First observed in coastal British Columbia in 2013 [44], SSWD symptomatic stars were still present in local populations through to at least 2019 (A.-L.M. Gehman 2019, personal communication), suggesting an ongoing outbreak. We assessed changes in the relative abundances of Evasterias and Pisaster in the intertidal zone before and during the wasting outbreak using a decade-long dataset collected from 2008 to 2017 in Burrard Inlet, British Columbia (electronic supplementary material, table S1 and figure S1). Sites were surveyed on the lowest tide series of the month (electronic supplementary material, table S1), where low tide was below +1 m in tidal height relative to Canadian chart datum (lowest astronomical tide), enabling maximum intertidal access. Sea star abundance was measured by counting the number of sea stars present within a fixed survey area defined by local topography at each site (electronic supplementary material, table S1). We counted all visible sea stars, including ones sheltering in cracks, but no boulders were overturned. At one site Pisaster size was measured intermittently (see the electronic supplementary material, S1.1). Further methods for the field surveys and analysis can be found in the electronic supplementary material, S1.1.
(b). Wasting disease study
To test susceptibility and SSWD progression through populations of Pisaster and Evasterias, tanks of healthy sea stars were exposed to a symptomatic sea star in the laboratory. We used two adult Pisaster with signs of SSWD (minor white lesions on their arms), collected on 7 August 2016 from Campbell Point, Mayne Island, British Columbia, as the source of infection for our experiments, and thus our focal disease agents. To minimize any cross-water contamination, we isolated the focal disease sea stars in a 20 l tank sitting in a seawater table. To infect both Evasterias and Pisaster, we exposed a single individual of each species to the focal disease sea stars. Once infected, they were placed into separate 20 l tanks. These ‘disease agent sea stars’ were used to initiate a controlled transmission of disease to susceptible conspecifics (see below). Mean arm lengths (±s.e.) for the experimentally exposed Pisaster (n = 5) and Evasterias (n = 5) were 54.40 ± 4.97 and 46.20 ± 3.84 mm, respectively.
Susceptible Pisaster and Evasterias were collected on 1 August 2016, at Brockton Point. Mean arm lengths (±s.e.) for Pisaster and Evasterias were 45.56 ± 2.05 and 44.84 ± 1.18 mm, respectively. We acclimated sea stars in an aerated, recirculating seawater table at 27 psu and 13°C for approximately one month. For the experiment, susceptible sea stars of each species were haphazardly selected and placed into 20 l tanks. Each tank contained five sea stars, and there were five replicate tanks for each species (i.e. 50 sea stars overall). Immediately after the initial placement of sea stars into their tanks, we introduced a single disease agent sea star of the same species into each tank. The exposure time to the susceptible sea stars lasted as long as the disease agent star remained alive. The experimental initiation date varied from tank to tank to correspond to the timing of the onset of visible signs of disease (lesions or arm loss) in the sick individual being introduced. All replicates were initiated within 14 days of one another and were observed for 28 days following the introduction of the disease agent sea star. Further methods and analysis can be found in the electronic supplementary material, S1.2.
(c). Dietary overlap
We assessed sea star dietary composition at four sites over five sampling dates: Sharon Cove (1 December 2009 and 10 April 2016), Point Atkinson (10 April 2016), Ferguson Point (11 April 2016), and Dunbar Street (15 November 2016). We followed the methods of [45], where sea stars were turned over at low tide and any prey (in our case, exclusively barnacles and mussels) that had been removed from the rock and were being held against the mouth were identified. Prey identity was recorded, but not prey abundance within a single meal. In a few cases, two different species were observed in a single meal. These cases were too rare to treat as a specific dietary category. Instead, the proportion of all meals that contained barnacles included these multi-prey meals, as did the proportion of all meals that contained mussels. The proportions of Evasterias and Pisaster feeding was compared with a χ2 contingency table analysis, as was the number of meals for each species that contained barnacles or mussels. We also ran laboratory experiments evaluating competition between Evasterias and Pisaster over the mussel M. trossulus (see the electronic supplementary material, S1.3).
3. Results
(a). Inter-annual patterns in abundance and body size
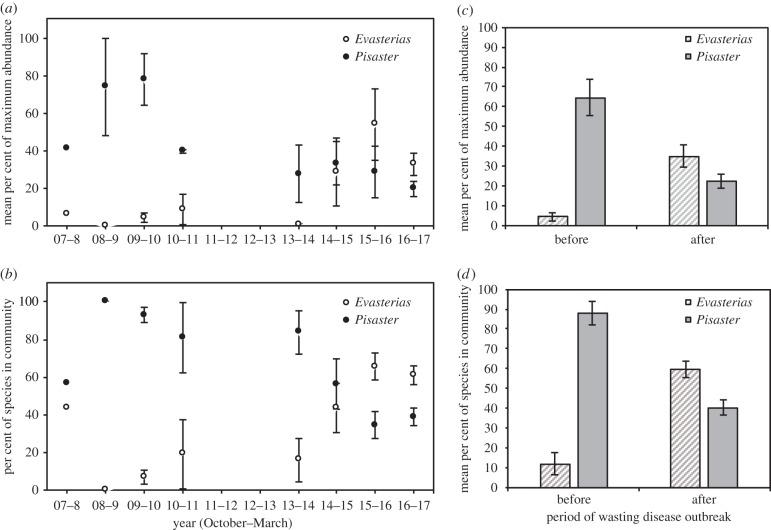
Evasterias abundance, both absolute and relative to Pisaster, remained low and relatively constant for the years of 2008 to 2013 (figure 1a,b; electronic supplementary material, figure S2). Pisaster abundance peaked in 2009–2010 after which point it began a decline that continued into the SSWD outbreak period. During this period of relatively low Pisaster abundance, particularly post-2013, Evasterias numbers increased substantially (figure 1a,b; electronic supplementary material, figure S2), and it became the numerically dominant sea star in the system in the post-SSWD outbreak era (figure 1c,d). This reversal of dominance is reflected by a significant species × time interaction (table 1).
Figure 1.
Long-term Evasterias and Pisaster population data. (a) Mean percentage of maximum abundance for each species and (b) mean percentage of each species within the sea star community, during winter surveys from 2008 to 2017. We binned winter seasons by October–March. Sample size varies across years where n = 1 survey for 2007–2008, n = 2 surveys for the years for 2008–2009 and 2010–2011, n = 4 for 2009–2010 and 2013–2014, n = 5 surveys for 2014–2015 and 2015–2016, and n = 30 surveys for 2016–2017. (c) Mean percentage of maximum abundance for each species and (d) mean percentage of each species within the sea star community, before and after estimated time of outbreak of wasting disease, n = 9 surveys for years before wasting and n = 40 surveys for years after wasting. Note that the single site surveyed in 2007–2008 was naturally high in Evasterias relative to other sites. Error bars represent ±1 s.e.
Table 1.
Repeated measures ANOVA of Evasterias and Pisaster per cent maximum abundance over time.
| degrees of freedom | sum of squares | mean squares | F-value | p-value | |
|---|---|---|---|---|---|
| species | 1 | 6.62 | 6.62 | 5.36 | 0.023 |
| time | 1 | 0.42 | 0.42 | 0.34 | 0.561 |
| species : time | 1 | 43.60 | 43.60 | 35.31 | <0.0001 |
In addition to the general decline in Pisaster density through the study period, there was also a decline in mean Pisaster body size. The radius (arm tip to centre of the oral disc) of Pisaster at Sharon Cove before the outbreak was relatively large: 11.6 ± 0.5 cm (mean ± s.e.) in 2005 and 10.5 ± 0.7 cm in 2008. Three years post-outbreak (2016), mean Pisaster radius at the same site had declined to 7.7 ± 0.9 cm. Size differences among years were significant (ANOVA: F = 9.82, p = 0.0002), with 2005 and 2008 being statistically similar to one another but different from 2016 (Tukey HSD, α = 0.05).
(b). Wasting disease study
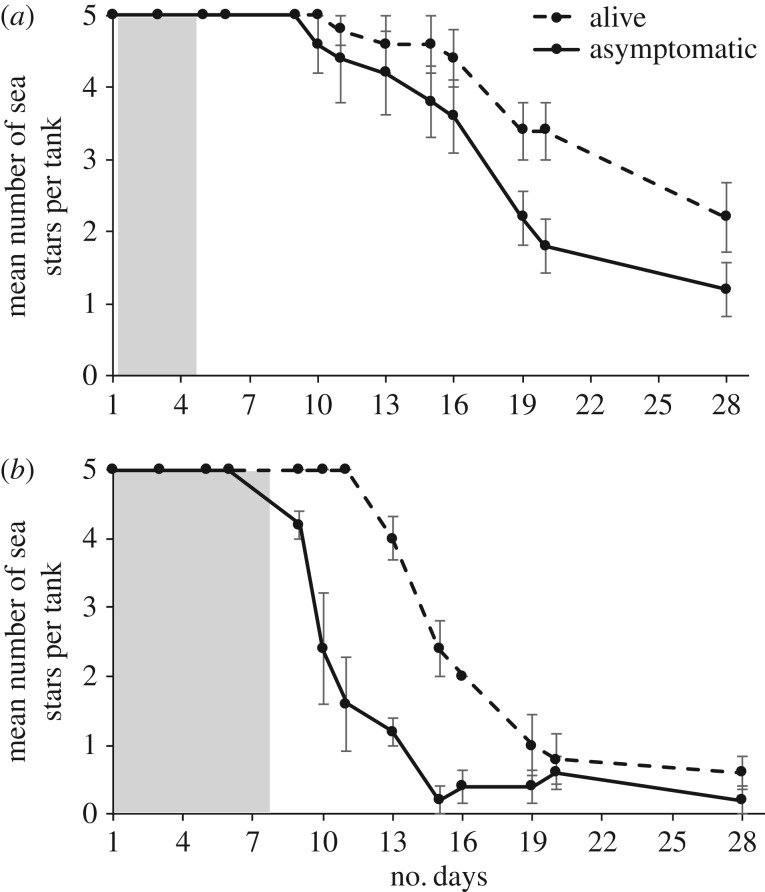
Outbreaks of wasting disease in the initially asymptomatic sea stars occurred in all 10 experimental tanks. After 28 days of exposure to disease agent sea stars, the mean number of susceptible Evasterias that had not shown signs of wasting was 1.2 ± 0.37 s.e. individuals out of five, and the mean number for Pisaster was 0.2 ± 0.44 s.e. individuals out of five (figure 2). Mean number of susceptible Evasterias found alive at the end of 28 days was 2.2 ± 0.49 s.e. individuals, while the mean number of Pisaster found alive was 0.6 ± 0.25 s.e. individuals (figure 2).
Figure 2.
Breakdown of disease progression in (a) Evasterias and (b) Pisaster over 28 days, in terms of number of asymptomatic and the number of surviving individuals remaining in a tank. Each dot represents a mean number across the five tanks, error bars represent ±1 s.e. The mean time each species was exposed to the disease agent sea star is represented by the shaded column.
Wasting disease was present in the recirculating seawater system at an order of magnitude lower prevalence than the infection levels in the experimental tanks. The control sea stars had two separate wasting events and a total of six Pisaster displayed small white lesions and low turgor pressure. Control tank infection prevalence was approximately 7% infection (6 out of 90 control sea stars) and total experimental infection prevalence was 72%. Wasting sea stars were removed from the control areas as soon as signs of disease were observed.
Disease progression timing varied between disease agent Pisaster and disease agent Evasterias; all disease agent Pisaster and two Evasterias displayed signs of disease in synchrony, but three disease agent Evasterias took longer to display signs of disease. The last disease agent Evasterias to develop signs of disease was 14 days after the last Pisaster to develop signs of disease. Survival time after placement in the experimental tanks (i.e. once they became symptomatic), also varied by species for the 10 disease agent sea stars. Disease agent Evasterias mean survival time (i.e. time during which they exhibited signs of disease) during exposure trials was 4.20 (±0.74 s.e.) days, while the disease agent Pisaster mean survival time during exposure trials was 7.40 (±1.72 s.e.) days (figure 2).
Disease progression differed between species within the experimental treatments. There was a significant effect of time (table 2), and a significant species by time interaction (table 2), with lower infection in Evasterias than Pisaster (figure 2). For mortality, there was a significant effect of time, and a significant species by time interaction (table 2), with higher mortality in Pisaster (figure 2).
Table 2.
Statistical results for logistic regression of number of individual Evasterias and Pisaster disease progression (number symptomatic) and mortality (number dead) over time. (Species, time and the interaction of species by time were included as fixed effects, and experimental tank nested in time was included as a random effect. Italics indicate p-values of less than 0.05.)
| disease progression |
mortality |
|||||
|---|---|---|---|---|---|---|
| predictors | odds ratios | CI | p | odds ratios | CI | p |
| (intercept) | 0.00 | 0.00–0.01 | <0.001 | 0.00 | 0.00–0.01 | <0.001 |
| species | 1.95 | 0.29–12.94 | 0.489 | 0.36 | 0.04–3.57 | 0.386 |
| time | 1.35 | 1.24–1.48 | <0.001 | 1.30 | 1.20–1.41 | <0.001 |
| species * time | 1.18 | 1.04–1.35 | 0.013 | 1.21 | 1.06–1.38 | 0.005 |
| random effects | ||||||
| σ2 | 3.29 | 3.29 | ||||
| τ00 | 0.41 Tank | 0.04 Tank | ||||
| τ11 | 0.00 Tank*Time | 0.00 Tank*Time | ||||
| observations | 130 | 130 | ||||
| marginal R2/conditional R2 | 0.723/0.758 | 0.680/0.702 | ||||
Time to 50% infected was 17.6 ± 2.04 (mean ± s.e.) days for Evasterias, and 10.4 ± 0.46 days for Pisaster. Daily transmission rate, or the daily rate at which susceptible sea stars became infected, for Evasterias was 0.27 of the total five sea stars per tank, and for Pisaster was 0.56 sea stars per tank. Time to reach 50% mortality was 24.0 ± 1.98 (mean ± s.e.) days for Evasterias and 14.6 ± 0.24 days for Pisaster. Daily disease-induced mortality, or the rate at which newly infected individuals died per day, was 0.16 out of five sea stars per tank per day for Evasterias, and 0.47 sea stars per tank per day for Pisaster.
(c). Dietary overlap and potential competitive effects
Similar proportions of Evasterias (19 out of 66 feeding; 28.8%) and Pisaster (38 out of 134 feeding; 28.4%) were feeding when surveyed in the field at low tide (contingency table χ2 = 0.004, p = 0.95). Barnacles (primarily Balanus glandula and occasionally Balanus crenatus) and mussels (M. trossulus) were the only prey items observed in our samples (table 3). Of the sea stars that were feeding, the relative proportion of meals that contained barnacles versus mussels was similar between the two sea star species (table 3; contingency table χ2 = 0.59, p = 0.44). In the laboratory experiments Evasterias grew faster than Pisaster, however there was no significant effect of interspecific competition on growth for the size classes of sea stars available for these experiments (electronic supplementary material, S1.4, S2.2 and figure S3).
Table 3.
Per cent of meals that contained barnacles (Balanus spp.) or mussels (Mytilus trossulus), pooled across sampling dates and sites. (Because some meals contained both barnacles and mussels, the totals for each species add to more than 100%.)
| predator | prey | per cent of meals (n) |
|---|---|---|
| Evasterias | barnacles | 47.4 (9) |
| mussels | 57.9 (11) | |
| Pisaster | barnacles | 39.5 (15) |
| mussels | 73.7 (28) |
4. Discussion
With a single set of disease signs (if not a single pathogen), this SSWD disease outbreak has illustrated how differences in the cost of infection across species can lead to altered population response. Here, we show that susceptibility and disease induced mortality differ among species, and that the outbreak is associated with important shifts in sea star assemblages that may be mediated indirectly by other ecological forces.
(a). Long-term abundance trends
In response to the outbreak of SSWD, intertidal Pisaster populations have declined coastwide, including in Burrard Inlet, British Columbia [6]. Unlike some areas, particularly further south where the consequences of SSWD have been more severe [6], the Burrard Inlet Pisaster population did not crash synchronously across all sites in 2014. There is uncertainty around the initial date of intertidal wasting outbreaks in the Burrard Inlet, as sampling once per year will miss important dynamics. That said, dynamics varied from one site to the next. One population (Ferguson Point) declined to its lowest point in December 2013 while others (Point Atkinson and Sharon Cove) did not reach their abundance minima until January 2016. Wasting outbreaks in the nearby San Juan Islands have also exhibited highly patchy dynamics in space and time [26]. Compared to California, where Pisaster populations collapsed across many sites within a narrow time window, spatial and temporal variation in SSWD-related mortality, coupled with continued incidence of symptomatic individuals (C.D.G. Harley 2017, personal observation), has resulted in a steadier decline, when averaged across sites, in Burrard Inlet between 2013 and 2017.
The Pisaster population in Burrard inlet exhibited temporal patterns that appear to be unrelated to disease as well. Pisaster abundance peaked at all sites in 2009–2010, and had already declined considerably from this peak by the time SSWD emerged in 2013. It is unclear whether the population trend from 2009 to 2013 represents a regression to the mean following a recruitment or immigration pulse, or a general decline related to some other factor. We observed very little wasting in Burrard Inlet prior to 2013, making wasting an unlikely culprit. Rather, there is some evidence to suggest that Pisaster densities in 2009–2010 were in fact uncharacteristically high. Specifically, there was an especially heavy settlement of mussels in 2008 that coated the shores of Burrard Inlet (C.D.G. Harley 2008, personal observation), and low freshwater input from the Fraser River in 2009 may have resulted in conditions especially conducive to Pisaster locomotor performance and feeding, which are sensitive to low salinity [46]. This increase in Pisaster's preferred prey coupled with optimal conditions for foraging may have sustained higher predator densities in 2009 [47]. Interestingly, a similar mussel recruitment pulse in 2013 was not followed by an increase in Pisaster, perhaps owing to disease-associated sea star declines from late 2013 onwards.
SSWD-induced declines in Pisaster abundance have been accompanied by a decline in mean body size, either owing to larger sea stars being more susceptible to disease or to pulses of recruitment following the loss of large fractions of the adult population [32,34]. We observed a similar decline in mean body size in the post-outbreak population in Burrard Inlet. Because Pisaster consumption rates increase with body size [48], the reduction in size coupled with a reduction in overall numbers has a very strong potential to reduce the ecological impact of Pisaster as a predator and as a competitor.
Contrary to the abundance trend observed in Pisaster, the abundance of Evasterias has increased markedly since the onset of the SSWD outbreak. After a transient decline from 2011 to 2013, Evasterias numbers increased to levels approximately five times higher than their pre-outbreak densities. Indeed, by 2016 Evasterias had become the numerically dominant sea star in the intertidal zone in Burrard Inlet, at least during the winter when intertidal sea stars are abundant. Although the rise of Evasterias lags the fall of Pisaster, it is possible that the two are linked, especially when considering the timing of the loss of large Pisaster (which are known ecological dominants [42,49], and are especially aggressive towards Evasterias [43]) and likely demographic time lags following Evasterias' own response to disease. In other words, it is possible that this is a case of competitive release. If Evasterias is less susceptible to SSWD, or if its recovery dynamics are more rapid, the competitive release hypothesis could explain the switch in numerical dominance. Below, we consider patterns in differential susceptibility, and then explore the evidence for interspecific competition.
(b). Interspecific differences in vulnerability to wasting disease
In our experiments, disease transmission was rapid, as were its consequences. Susceptible hosts showed visible signs of wasting less than two weeks after initial exposure to the diseased hosts, regardless of species, and 72% of the 50 sea stars exposed to wasting disease died from wasting disease within 28 days. However, the response of the two species was not identical; Evasterias took longer to reach 50% infected and 50% mortality following the introduction of a diseased individual. Once visibly symptomatic, however, Pisaster lived for nearly twice as long. In the field, dead individuals are removed from the intertidal zone by waves in all but the most protected habitats, so the prolonged presence of live but sick Pisaster could therefore increase the length of time available for disease transmission relative to more rapidly removed Evasterias. This potential effect was preserved in our experiments, where we only removed infected sea stars (both disease agent and newly infected stars) once they had died. The difference in transmission windows, along with differences in innate vulnerability, could explain why SSWD effects were more severe for Pisaster in our experiments.
Transmission rate is an important parameter that influences the ability of a parasite or pathogen to infect and persist within host populations [50]. However, little is known about the factors influencing transmission in SSWD [19,32]. Our interpretation of the experimental results rests on the assumption that a sea star must be exhibiting signs of wasting (e.g. lesions and/or deterioration of the body wall) in order to transmit the disease. Thus, we assume that the increased time that Pisaster remains symptomatic translates to higher transmission. However, it is unclear whether the increased time between exposure and disease display represents an increase in resistance to infection or tolerance of early stages of infection. If the host does not need to be symptomatic to transmit SSWD, then the extended asymptomatic period found in Evasterias could represent transmission potential similar to that of Pisaster.
Because the amount of time that a host spends exhibiting signs of disease will affect the probability of detecting disease within the population, our results also have implications for the way field-collected disease incidence data are interpreted. The duration of the symptomatic period can vary with temperature [26] and among species (our study). Taken together, these results suggest that caution should be applied when attempting to interpret drivers of disease transmission using infection prevalence surveys alone. For example, in our laboratory experiments, although the total infection over the experiment was 100% for Pisaster and 92% for Evasterias, the mean daily infection prevalence was 40.7 ± 9.6 s.e. % for Pisaster and only 14.5 ± 4.8 s.e. % for Evasterias. Thus, even in our exaggerated laboratory conditions, total infection prevalence does a poor job of reflecting the full impacts of the disease, and infrequent field sampling could miss symptomatic individuals and perhaps entire outbreaks when mortality sets in soon after signs of disease become apparent. In addition, caution should be applied when setting the lengths of exposure trials, as our results demonstrate that the time from exposure to disease onset can vary substantially between species. For example, the viral challenge experiments evaluating WAaDs in Pisaster and Evasterias presented data from approximately 7 days following disease challenge [25]. In our study, none of the sea stars had signs of disease at 6 days following exposure to SSWD, and at 9 days, none of the Evasterias had signs of disease and only 4 out of 25 Pisaster showed disease signs.
Our results suggest that disease development may vary between host species, and while we have found that two host species have different rates of disease development, there are at least 20 host species that can be infected by SSWD. Indeed, field surveys and laboratory experiments have demonstrated that different species have differing levels of susceptibility, prevalence, and population response [19,21,33]. If interspecific variation allows for a range of levels of vulnerability to SSWD, as demonstrated with Evasterias and Pisaster, then host diversity and community composition within a location could have strong effects on disease spread and persistence. There is a wealth of debate around how host diversity can influence the risk of disease in communities (e.g. [51]), with some suggesting that less susceptible or viable hosts can lead to dilution effects for the community [52,53]. Further work evaluating the variability in disease transmission between hosts, and the effect of local sea star diversity on infection spread and persistence, could provide valuable insights into the debate on the effect of biodiversity on disease transmission.
(c). The role of competition
If Evasterias is better able to withstand the population-level effects of SSWD, perhaps the decline in Pisaster led to the documented increase in Evasterias. We had strong reason to believe that Pisaster would be the competitive dominant in this pairing; Pisaster is regarded as a competitively dominant sea star in the intertidal [42,54] and has asymmetrically agonistic effects on Evasterias [43]. Despite the strong overlap in dietary composition we found, Evasterias and Pisaster did not show evidence of asymmetric resource competition for their dominant food source, the mussel M. trossulus, in our laboratory experiments (electronic supplementary material, S1.3).
Taken at face value, our laboratory results suggest that interspecific competitive release was unimportant. However, our work does not rule out the possibility of direct resource competition between Evasterias and Pisaster. First, size is an important determinant of competitive interactions, and in our experiments the size of the Pisaster (approx. 5–6 cm arm length) were close to the threshold size (4 cm) below which no agonistic interactions were observed by [43]. Larger sea stars were generally unavailable at the time of our study, but prior to the outbreak of SSWD, larger size classes (greater than 10 cm arm length) were common. Thus, SSWD could have altered the competitive outcome between Pisaster and Evasterias by removing the larger, competitively dominant Pisaster from the population [32,34,43]. Second, our experiments were effectively subtidal, but in the field the two species are competing in the intertidal where the interaction could play out differently. Finally, even in the absence of asymmetric interference competition, Pisaster would almost certainly affect the availability of prey for Evasterias over the long term via exploitation competition. This is particularly true as Pisaster can be found in the Burrard Inlet intertidal year-round, albeit at reduced densities in summer, whereas Evasterias only exploits intertidal habitats in the winter (see the electronic supplementary material). The seasonal use of intertidal areas by Evasterias may be a response to reduced avian predation (see [39]) associated with the night-time timing of lower low tides during winter.
The seasonal migration of Evasterias raises an additional possible competitive release scenario—one that we were not able to explore here. Evasterias can also be abundant in the subtidal zone [41], and this is certainly true in Burrard Inlet during the summer. Because we did not monitor subtidal populations, there could be other species interactions we have not taken into consideration that may have triggered Evasterias' population increase and allowed Evasterias to occupy the relatively Pisaster-free intertidal ecosystem. Declines of the sunflower sea star, Pycnopodia, are well documented in the Salish Sea and elsewhere [5,55], with evidence of high susceptibility to wasting disease [5,27]. In Howe Sound (adjacent to Burrard Inlet), Pycnopodia abundance decreased by 89% after wasting disease, and that species is now rarely seen in the subtidal [44]. Evasterias diet when in the subtidal can overlap with Pycnopodia [41], and declines in Pycnopodia may have benefitted seasonally or permanently subtidal populations of Evasterias in and around Burrard Inlet. The role of ecological interactions in the subtidal, particularly as they relate to wasting disease, warrant further investigation. Even if Pycnopodia declines acted as an initial trigger to an increase in subtidal Evasterias, the concurrent declines in Pisaster may have allowed Evasterias to move into the then relatively underexploited intertidal zone.
Finally, an element of apparent competition could be acting in this system. Although the term apparent competition has been applied with varying degrees of specificity (e.g. [13]), the strict interpretation of apparent competition assumes no direct competition between the host species [14]. If we relax the assumption of no competition, then both competitive release and apparent competition mechanisms could act in the same system. Indeed, indirect effects of increased Evasterias density, and thus a potential increase in SSWD persistence or transmission, could be keeping the more susceptible Pisaster populations suppressed. If we expand our consideration of shared hosts of SSWD into the subtidal, the complexity quickly increases, with many sea star species interactions to consider, all likely with varying susceptibility and transmission (as discussed in §4b). Future work exploring the interactions across habitats and among the full complement of the sea star community could provide fruitful insight into the dynamics of this disease.
(d). Implications of Evasterias population increase and emergent properties of wasting disease
Ultimately, our study documents a major shift within the intertidal sea star assemblage following the recent outbreak of SSWD. Evasterias is an understudied sea star and its role in providing top-down control in the intertidal zone has not been well documented. If Evasterias can effectively control the mussel cover of the rocky intertidal by fulfilling a similar top down role Pisaster holds in the intertidal, the expansion of Evasterias in the intertidal zone could mitigate the effects of Pisaster declines on mussel cover.
It is currently unclear the extent to which our results extend beyond Burrard Inlet. However, increases in Evasterias have also been documented in the Strait of Georgia subtidal [56], and post-wasting increases have also been documented for Dermasterias imbricata across the Salish Sea [55]. These simultaneous increases of Evasterias and Dermasterias, despite their radically different diets [41], provide evidence of population expansions in sea stars following wasting disease, and suggests that this phenomenon may not be a property of single species, but that sea star community shifts are probably occurring wherever wasting disease has caused severe declines in dominant and competing sea stars.
Overall, our study demonstrates the possibility that a major disturbance like SSWD can have both direct and indirect ecological effects, and may indirectly benefit certain species even if they are susceptible. In the example of wasting disease, mass mortalities of key consumers have occurred and will probably cause ecological shifts within the predator guild and across trophic levels. Diseases affect their hosts within the context of their communities, and our work highlights the need to evaluate the consequences of disease within the context of entire communities.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We acknowledge and are grateful to have worked on the unceded territories of the Musqueam, Squamish and Tsleil-Waututh First Nations. We thank the fieldwork volunteers: Amelia Hesketh, Sarah Tan, Daniel Laronde, Graham Brownlee, Sarah Dada, Cassandra Konecny, Sandra Emry, Emily Lim, Kelsey Flynn, Tamara Kay, Xicalli Boles, and Cameron Bullen. We thank Colin MacLeod, Nora Brown, and Kat Anderson for input on statistical analyses, Cassandra Konecny for maps, and the Harley laboratory and Tad Dallas for discussions. We thank Drew Harvell and an anonymous reviewer for their insightful and constructive reviews.
Data accessibility
Data for this article can be found in the Dryad Digital Repository: https://doi.org/10.5061/dryad.5b2k785 [57].
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by a University of British Columbia Science Undergraduate Research Experience award to S.W.C.K., a National Science Foundation Postdoctoral Research Fellowship in Biology and a Hakai Postdoctoral position funded by the Tula Foundation to A.-L.M.G., and a Natural Sciences and Engineering Research Council Discovery Grant to C.D.G.H.
References
- 1.Holdo RM, Sinclair ARE, Dobson AP, Metzger KL, Bolker BM, Ritchie ME, Holt RD. 2009. A disease-mediated trophic cascade in the Serengeti and its implications for ecosystem C. PLoS Biol. 7, e1000210 ( 10.1371/journal.pbio.1000210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell EN, Ashton-Alcox KA, Kraeuter JN, Ford SE, Bushek D. 2008. Long-term trends in oyster population dynamics in Delaware Bay: regime shifts and response to disease. J. Shellfish Res. 27, 729–755. ( 10.2983/0730-8000(2008)27[729:LTIOPD]2.0.CO;2) [DOI] [Google Scholar]
- 3.Sutherland KP, Berry B, Park A, Kemp DW, Kemp KM, Lipp EK, Porter JW. 2016. Shifting white pox aetiologies affecting Acropora palmata in the Florida Keys, 1994–2014. Phil. Trans. R. Soc. B 371, 20150205 ( 10.1098/rstb.2015.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tompkins DM, White AR, Boots M. 2003. Ecological replacement of native red squirrels by invasive greys driven by disease. Ecol. Lett. 6, 189–196. ( 10.1046/j.1461-0248.2003.00417.x) [DOI] [Google Scholar]
- 5.Harvell CD, et al. 2019. Disease epidemic and a marine heat wave are associated with the continental-scale collapse of a pivotal predator (Pycnopodia helianthoides). Sci. Adv. 5, eaau7042 ( 10.1126/sciadv.aau7042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miner MC, et al. 2018. Large-scale impacts of sea star wasting disease (SSWD) on intertidal sea stars and implications for recovery. PLoS ONE 13, e0192870 ( 10.1371/journal.pone.0192870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowakowski AJ, Whitfield SM, Eskew EA, Thompson ME, Rose JP, Caraballo BL, Kerby JL, Donnelly MA, Todd BD. 2016. Infection risk decreases with increasing mismatch in host and pathogen environmental tolerances. Ecol. Lett. 19, 1051–1061. ( 10.1111/ele.12641) [DOI] [PubMed] [Google Scholar]
- 8.Cohen JM, Venesky MD, Sauer EL, Civitello DJ, McMahon TA, Roznik EA, Rohr JR. 2017. The thermal mismatch hypothesis explains host susceptibility to an emerging infectious disease. Ecol. Lett. 20, 184–193. ( 10.1111/ele.12720) [DOI] [PubMed] [Google Scholar]
- 9.Hatcher MJ, Dick JT, Dunn AM. 2012. Diverse effects of parasites in ecosystems: linking interdependent processes. Front. Ecol. Environ. 10, 186–194. ( 10.1890/110016) [DOI] [Google Scholar]
- 10.Wood CL, Johnson PTJ. 2015. A world without parasites: exploring the hidden ecology of infection. Front. Ecol. Environ. 13, 425–434. ( 10.1890/140368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decaestecker E, Verreydt D, De Meester L, Declerck SAJ. 2015. Parasite and nutrient enrichment effects on Daphnia interspecific competition. Ecology 96, 1421–1430. ( 10.1890/14-1167.1) [DOI] [PubMed] [Google Scholar]
- 12.Grenfell BT, Dobson AP. 1995. Ecology of infectious diseases in natural populations, pp. 1–16. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 13.Hudson PJ, Greenman J. 1998. Competition mediated by parasites: biological and theoretical progress. Trends Ecol. Evol. 13, 387 ( 10.1016/S0169-5347(98)01475-X) [DOI] [PubMed] [Google Scholar]
- 14.Holt RD, Pickering J. 1985. Infectious disease and species coexistence: a model of Lotka-Volterra form. Am. Nat. 126, 196–211. ( 10.1086/284409) [DOI] [Google Scholar]
- 15.Paine RT. 1969. A note on trophic complexity and community stability. Am. Nat. 103, 91–93. ( 10.1086/282586) [DOI] [Google Scholar]
- 16.Hatcher MJ, Dick JTA, Dunn AM. 2006. How parasites affect interactions between competitors and predators. Ecol. Lett. 9, 1253–1271. ( 10.1111/j.1461-0248.2006.00964.x) [DOI] [PubMed] [Google Scholar]
- 17.Martin LB, Hopkins WA, Mydlarz LD, Rohr JR. 2010. The effects of anthropogenic global changes on immune functions and disease resistance. Ann. N. Y. Acad. Sci. 1195, 129–148. ( 10.1111/j.1749-6632.2010.05454.x) [DOI] [PubMed] [Google Scholar]
- 18.Altizer SM, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD. 2013. Climate change and infectious diseases: from evidence to a predictive framework. Science 341, 514–519. ( 10.1126/science.1239401) [DOI] [PubMed] [Google Scholar]
- 19.Hewson I, et al. 2014. Densovirus associated with sea-star wasting disease and mass mortality. Proc. Natl Acad. Sci. USA 111, 17 278–17 283. ( 10.1073/pnas.1416625111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates A, Hilton B, Harley CD. G. 2009. Effects of temperature, season and locality on wasting disease in the keystone predatory sea star Pisaster ochraceus. Dis. Aquat. Org. 86, 245–251. ( 10.3354/dao02125) [DOI] [PubMed] [Google Scholar]
- 21.Eckert GL, Engle JM, Kushner DJ. 2000. Sea star disease and population declines at the Channel Islands. In Proceedings of the Fifth California Islands Symposium, 29 March-1 April 1999, Santa Barbara, CA, pp. 390–393. Camarillo, CA: US Department of the Interior, Minerals Management Service. [Google Scholar]
- 22.Kohl WT, McClure TI, Miner BG. 2016. Decreased temperature facilitates short-term sea star wasting disease survival in the keystone intertidal sea star Pisaster ochraceus. PLoS ONE 11, e0153670 ( 10.1371/journal.pone.0153670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd MM, Pespeni MH. 2018. Microbiome shifts with onset and progression of sea star wasting disease revealed through time course sampling. Sci. Rep. 8, 16476 ( 10.1038/s41598-018-34697-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bucci C, Francoeur M, McGreal J, Smolowitz R, Zazueta-Novoa V, Wessel GM, Gomez-Chiarri M. 2017. Sea star wasting disease in Asterias forbesi along the Atlantic Coast of North America. PLoS ONE 12, e0188523 ( 10.1371/journal.pone.0188523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hewson I, Bistolas KSI, Quijano Cardé E. M., Button JB, Foster PJ, Flanzenbaum JM, Kocian J, Lewis CK. 2018. Investigating the complex association between viral ecology, environment, and northeast Pacific sea star wasting. Front. Mar. Sci. 5, 245 ( 10.3389/fmars.2018.00077) [DOI] [Google Scholar]
- 26.Eisenlord ME, et al. 2016. Ochre star mortality during the 2014 wasting disease epizootic: role of population size structure and temperature. Phil. Trans. R. Soc. B 371, 20150212 ( 10.1098/rstb.2015.0212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gudenkauf BM, Hewson I. 2015. Metatranscriptomic analysis of Pycnopodia helianthoides (Asteroidea) affected by sea star wasting disease. PLoS ONE 10, e0128150 ( 10.1371/journal.pone.0128150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harley CDG. 2011. Climate change, keystone predation, and biodiversity loss. Science 334, 1124–1127. ( 10.1126/science.1210199) [DOI] [PubMed] [Google Scholar]
- 29.Paine RT. 1966. Food web complexity and species diversity. Am. Nat. 100, 65–75. ( 10.1086/282400) [DOI] [Google Scholar]
- 30.Paine RT. 1974. Intertidal community structure: experimental studies on the relationship between a dominant competitor and its principal predator. Oecologia 15, 93–120. ( 10.1007/BF00345739) [DOI] [PubMed] [Google Scholar]
- 31.Cerny-Chipman EB, Sullivan JM, Menge BA. 2017. Whelk predators exhibit limited population responses and community effects following disease-driven declines of the keystone predator Pisaster ochraceus. Mar. Ecol. Prog. Ser. 570, 15–28. ( 10.3354/meps12121) [DOI] [Google Scholar]
- 32.Menge BA, Cerny-Chipman EB, Johnson A, Sullivan J, Gravem S, Chan F. 2016. Sea star wasting disease in the keystone predator Pisaster ochraceus in Oregon: insights into differential population impacts, recovery, predation rate, and temperature effects from long-term research. PLoS ONE 11, e0153994 ( 10.1371/journal.pone.0153994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gravem SA, Morgan SG. 2017. Shifts in intertidal zonation and refuge use by prey after mass mortalities of two predators. Ecology 98, 1006–1015. ( 10.1002/ecy.1672) [DOI] [PubMed] [Google Scholar]
- 34.Moritsch MM, Raimondi PT. 2018. Reduction and recovery of keystone predation pressure after disease-related mass mortality. Ecol. Evol. 8, 3952–3964. ( 10.1002/ece3.3953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiesecker JM, Blaustein AR. 1999. Pathogen reverses competition between larval amphibians. Ecology 80, 2442–2448. ( 10.1890/0012-9658(1999)080[2442:PRCBLA]2.0.CO;2) [DOI] [Google Scholar]
- 36.Park T. 1948. Interspecies competition in populations of Trilobium confusum Duval and Trilobium castaneum Herbst. Ecol. Monogr. 18, 265–307. ( 10.2307/1948641) [DOI] [Google Scholar]
- 37.Grant PR. 1972. Convergent and divergent character displacement. Biol. J. Linn. Soc. Lond. 4, 39–68. ( 10.1111/j.1095-8312.1972.tb00690.x) [DOI] [Google Scholar]
- 38.Hardin G. 1960. The competitive exclusion principle. Science 131, 1292–1297. ( 10.1126/science.131.3409.1292) [DOI] [PubMed] [Google Scholar]
- 39.Rogers TL, Elliott JK. 2012. Differences in relative abundance and size structure of the sea stars Pisaster ochraceus and Evasterias troschelii among habitat types in Puget Sound, Washington, USA. Mar. Biol. 160, 853–865. ( 10.1007/s00227-012-2139-7) [DOI] [Google Scholar]
- 40.Lambert P. 2000. Sea stars of British Columbia, Southeast Alaska, and Puget Sound. Vancouver, Canada: UBC Press. [Google Scholar]
- 41.Mauzey KP, Birkeland C, Dayton PK. 1968. Feeding behavior of asteroids and escape responses of their prey in the Puget Sound region. Ecology 49, 603–619. ( 10.2307/1935526) [DOI] [Google Scholar]
- 42.Menge JL, Menge BA. 1974. Role of resource allocation, aggression and spatial heterogeneity in coexistence of two competing intertidal starfish. Ecol. Monogr. 44, 189–209. ( 10.2307/1942311) [DOI] [Google Scholar]
- 43.Rogers TL, Schultz HK, Elliott JK. 2018. Size-dependent interference competition between two sea star species demographically affected by wasting disease. Mar. Ecol. Prog. Ser. 589, 167–177. ( 10.3354/meps12461) [DOI] [Google Scholar]
- 44.Schultz JA, Cloutier RN, Côté IM. 2016. Evidence for a trophic cascade on rocky reefs following sea star mass mortality in British Columbia. PeerJ 4, e1980 ( 10.7717/peerj.1980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harley CDG, Pankey MS, Wares JP, Grosberg RK, Wonham MJ. 2006. Color polymorphism and genetic structure in the sea star Pisaster ochraceus. Biol. Bull. 211, 248–262. ( 10.2307/4134547) [DOI] [PubMed] [Google Scholar]
- 46.Held MBE, Harley CDG. 2009. Responses to low salinity by the sea star Pisaster ochraceus from high- and low-salinity populations. Invertebr. Biol. 128, 381–390. ( 10.1111/j.1744-7410.2009.00175.x) [DOI] [Google Scholar]
- 47.Witman JD, Genovese SJ, Bruno JF, McLaughlin JW, Pavlin BI. 2003. Massive prey recruitment and the control of rocky subtidal communities on large spatial scales. Ecol. Monogr. 73, 441–462. ( 10.1890/01-4073) [DOI] [Google Scholar]
- 48.Gooding RA, Harley CDG, Tang E. 2009. Elevated water temperature and carbon dioxide concentration increase the growth of a keystone echinoderm. Proc. Natl Acad. Sci. USA 106, 9316–9321. ( 10.1073/pnas.0811143106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Menge BA. 1974. Effect of wave action and competition on brooding and reproductive effort in the seastar, Leptasterias hexactis. Ecology 55, 84–93. ( 10.2307/1934620) [DOI] [Google Scholar]
- 50.Anderson RM, May R. 1981. The population dynamics of microparasites and their invertebrate hosts. Phil. Trans. R. Soc. Lond. B 291, 451–524. ( 10.1098/rstb.1981.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wood CL, Lafferty KD, DeLeo G, Young HS, Hudson PJ, Kuris AM. 2014. Does biodiversity protect humans against infectious disease? Ecology 95, 817–832. ( 10.1890/13-1041.1) [DOI] [PubMed] [Google Scholar]
- 52.Keesing F, Holt RD, Ostfeld RS. 2006. Effects of species diversity on disease risk. Ecol. Lett. 9, 485–498. ( 10.1111/j.1461-0248.2006.00885.x) [DOI] [PubMed] [Google Scholar]
- 53.Ostfeld RS, Keesing F. 2012. Effects of host diversity on infectious disease. Annu. Rev. Ecol. Evol. Syst. 43, 157–182. ( 10.1146/annurev-ecolsys-102710-145022) [DOI] [Google Scholar]
- 54.Menge BA. 1972. Competition for food between two intertidal starfish species and its effect on body size and feeding. Ecology 53, 635–644. ( 10.2307/1934777) [DOI] [Google Scholar]
- 55.Montecino-Latorre D, Eisenlord ME, Turner M, Yoshioka R, Harvell CD, Pattengill-Semmens CV, Nichols JD, Gaydos JK. 2016. Devastating transboundary impacts of sea star wasting disease on subtidal asteroids. PLoS ONE 11, e0163190 ( 10.1371/journal.pone.0163190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schultz JA. 2018. Sea star wasting disease—update! Ocean Watch B.C. Coast Edition. 1–323. [Google Scholar]
- 57.Kay SWC, Gehman A-LM, Harley CDG. 2019. Data from: Reciprocal abundance shifts of the intertidal sea stars, Evasterias troschelii and Pisaster ochraceus, following sea star wasting disease Dryad Digital Repository. ( 10.5061/dryad.5b2k785) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Kay SWC, Gehman A-LM, Harley CDG. 2019. Data from: Reciprocal abundance shifts of the intertidal sea stars, Evasterias troschelii and Pisaster ochraceus, following sea star wasting disease Dryad Digital Repository. ( 10.5061/dryad.5b2k785) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data for this article can be found in the Dryad Digital Repository: https://doi.org/10.5061/dryad.5b2k785 [57].