Abstract
Objectives
To assess predictive factors for rheumatoid arthritis interstitial lung disease (RA-ILD) in two early rheumatoid arthritis (RA) inception cohorts with a focus on methotrexate (MTX) exposure.
Design
Multicentre prospective early RA inception cohort studies; the early RA study (ERAS) and the early RA network (ERAN).
Setting
Secondary care, ERAS nine centres, ERAN 23 centres in England, Wales and Ireland.
Participants
Patients with new diagnosis of RA, n=2701. Standardised data including demographics, drug therapies and clinical outcomes including the presence of RA-ILD were collected at baseline, within 3–6 months, at 12 months and annually thereafter.
Primary and secondary outcome measures
Primary outcome was the association of MTX exposure on RA-ILD diagnosis. Secondary outcomes were the association of demographic, comorbid and RA-specific factors on RA-ILD diagnosis and the association of MTX exposure on time to RA-ILD diagnosis.
Results
Of 92 eligible ILD cases, 39 occurred in 1578 (2.5%) MTX exposed and 53 in 1114 (4.8%) non-MTX exposed cases. The primary analysis of RA-ILD cases only developing after any conventional synthetic disease-modifying antirheumatic drug treatment (n=67) showed MTX exposure not to be associated with incident RA-ILD (OR 0.85, 95% CI 0.49 to 1.49, p=0.578) and a non-significant trend for delayed ILD diagnosis (OR 0.54, 95% CI 0.28 to 1.06, p=0.072). In an extended analysis including RA-ILD cases present at RA diagnosis (n=92), MTX exposure was associated with a significantly reduced risk of incident RA-ILD (OR 0.48, 95% CI 0.3 to 0.79, p=0.004) and longer time to ILD diagnosis (OR 0.41, 95% CI 0.23 to 0.75, p=0.004). Other independent baseline associations with incident RA-ILD were higher age of RA onset, ever smoking, male gender, rheumatoid nodules and longer time from first RA symptom to first outpatient visit.
Conclusions
MTX treatment was not associated with an increased risk of RA-ILD diagnosis. On the contrary, evidence suggested that MTX may delay the onset of ILD.
Keywords: rheumatoid arthritis, interstitial lung disease, methotrexate
Strengths and limitations of this study.
Multicentre prospective early rheumatoid arthritis (RA) inception cohort study recruiting 2701 patients.
Standardised data collection with up to 25-year follow-up.
Diagnosis of rheumatoid arthritis interstitial lung disease (RA-ILD) made by participating rheumatology centres, or from death certification, without independent verification.
Univariate, multivariate, time varying and time-to-event Cox proportional hazards analyses assessed methotrexate exposure, demographic and RA-specific factors associated with RA-ILD diagnosis.
High proportion of missing smoker status data from early RA study patients recruited from 1986 to 2001.
Introduction
Methotrexate (MTX) is now firmly established globally as the anchor drug for the management of rheumatoid arthritis (RA), recommended for first line use, to which other conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), targeted synthetic disease-modifying antirheumatic drugs and biological disease-modifying antirheumatic drugs (bDMARDs) are generally added.1 2 In addition to an excellent ability to suppress synovitis and restore physical function, there is compelling data demonstrating a beneficial effect on long-term cardiovascular disease3 and hence restoration of life expectancy to that of the normal population.
A hypersensitivity pneumonitis is a rare adverse effect of MTX described in 0.43%,4 generally subacute in presentation, with progression of characteristic symptoms over a period of days to weeks.5 This usually occurs early, within the first year of treatment, but has been reported up to 3 years after starting MTX.4 5 This organ-specific hypersensitivity reaction has led to a creeping concern in routine practice that MTX may also be associated with an increased incidence or exacerbation of the interstitial lung disease (ILD) that is associated with RA and may be a reason to withhold MTX from RA patients with any lung disease. RA-ILD is an uncommon but significant life-threatening extra-articular manifestation, clinically significant in up to 5% of patients with RA, with subclinical high-resolution computed tomography (HRCT) evidence in 33% or more, a median survival from diagnosis of approximately 3 years, contributing to the overall excess mortality of RA.2 6–12 MTX is contraindicated if a patient has insufficient respiratory reserve to survive hypersensitivity pneumonitis. However, evidence is lacking that would deter initiation of MTX treatment in people with mild respiratory disease on grounds of an adverse effect on any other form of lung injury such as ILD. Indeed, the considerable benefits of MTX are such that a decision to withhold it as a treatment option for RA should be reluctantly made and only for sound reasons.
Evidence that MTX may cause or have an adverse impact on RA-ILD is sparse. Meta-analysis of randomised controlled trials (RCTs) of MTX in RA has reported an increased risk of all adverse respiratory events and respiratory infections but not of death due to lung disease or non-infectious respiratory events, with follow-up duration of 24–104 weeks.13 Due to inherent difficulties separating RA-ILD from putative MTX-related ILD, a meta-analysis of MTX versus placebo or active comparator agents in RCTs from non-malignant inflammatory disorders not themselves associated with ILD is of interest.14 This has shown no MTX-associated risk of lung disease in studies ranging from 16 weeks to 52 weeks follow-up. These relatively short duration analyses, in patients preselected for RCTs, are reassuring but require substantiation from RA inception cohorts or patient registries with all-comers included and longer follow-up. Sequential lung function tests in cohorts of MTX-treated patients with RA followed prospectively for up to 5 years have shown a sequential decline, with inconsistent interpretation that this is in keeping with,15 or in excess of,16 expected age-related changes. Interpretation of these studies is limited by a lack of inclusion of non-MTX-treated control patients with RA. In another cohort comparing 55 MTX-treated with 73 non-MTX-treated patients with established RA, there was no adverse influence of MTX on pulmonary function tests over 2 years, including a subanalysis of those found to have largely subclinical pulmonary fibrosis on HRCT.17
We report the association of MTX exposure and other demographic and RA-specific factors with incident cases of RA-ILD in two large multicentre RA inception cohorts: the early rheumatoid arthritis study (ERAS) and the early rheumatoid arthritis network (ERAN), recruiting from 1986 to 2012 with review up to 25 years.
Methods
Patient databases
The study used data from ERAS (1986–2001) and ERAN (2002–2012), two multicentre early RA inception cohorts. ERAS recruited 1465 patients (<2 years disease duration, no prior csDMARD therapy) from nine district general hospitals in England, followed yearly for up to 25 years (median follow-up 10 years). ERAN recruited 1236 patients (<3 years disease duration) from 23 centres in England, Wales and Ireland, followed yearly for up to 10 years (median follow-up 6 years). All participants gave informed consent. STROBE reporting cohort guidelines have been followed (von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies).
Recruitment into ERAS and ERAN was based on clinician diagnosis with 70% of patients fulfilling the minimum ARA criteria18 for RA at baseline and 96% by last visit. Patients subsequently reclassified as non-RA were excluded from the study.
Patient and public involvement
A patient representative from the National Rheumatoid Arthritis Society (NRAS) was involved in the design and conduct of ERAN, including decisions concerning which RA outcomes should be collected. By consenting to recruitment, patients were aware that the purposes of the study included examining the consequences of a range of RA outcomes. As patients were recruited at the time of, or very soon after, the diagnosis of RA they had limited experience of the disease to determine their priorities for ERAS/ERAN analyses. ILD was one of many outcomes that patients knew were to be studied. As ILD is established as one of the most severe complications of RA, and one of the leading causes of premature death, patients were interested in any findings that might add to our knowledge of this complication.
Patients were not involved in the recruitment process to ERAS/ERAN. Patients were not specifically involved in the statistical design of the analysis of ERAS/ERAN data on associations of factors with RA-ILD.
At recruitment, patients were informed that ‘the results of this study would be made available to participating clinicians and will be the subject of international presentations and articles in peer-reviewed scientific journals’. Participants will not be notified of the results individually, but we will request that a summary of the findings be made available to all patients with RA via the patient newsletter of NRAS.
Clinical and laboratory measures
Information on demographic, clinical, treatment, laboratory and functional features was recorded in both cohorts at baseline, between 3 and 6 months, at 12 months and then once yearly on standardised case report forms (CRFs) as previously described.19–21 The disease activity score (DAS) was calculated according to the original three-variable method22 in ERAS and the four-variable DAS28 method23 in ERAN. A transformation formula was used to make DAS and DAS28 comparable.24 Source data verification was undertaken by an experienced nurse practitioner at visits to each centre. Combined analysis of ERAS and ERAN is possible since they are consecutive inception cohorts with similar design, including the variables captured, timing of assessments and patient recruitment. Follow-up across ERAS and ERAN was relatively high given the long term of both prospective studies, and of those not followed to death or closure, cases lost to follow-up for no known reason were only 13.7% overall (12.5% ERAS, 15% ERAN). Full details of reasons for discontinuation in ERAS and ERAN are shown in online supplementary table 1.
bmjopen-2018-028466supp001.pdf (260.9KB, pdf)
Treatment profiles
Patients were treated according to usual care in each of the ERAS and ERAN centres, without specific protocols, strategies or other external influences. All centres followed the 1992 good practice guidance outlined by the British Society for Rheumatology and Royal College of Physicians.25 Treatment details were entered onto the CRF at each ERAS/ERAN data collection visit. At baseline, all patients in ERAS were csDMARD naïve and in ERAN 13.5% had commenced a csDMARD within a few weeks of first secondary care visit. In ERAS and ERAN, initial csDMARD use was as monotherapy with/without steroids in >90%, favouring sulphasalazine (SSZ) from 1986 to 2001, with a switch to MTX monotherapy such that SSZ and MTX were used in equal proportions as first csDMARD in 2002, and thereafter in ERAN MTX became the most likely initial choice,20 21 this reflecting contemporary best practice. Combination csDMARDS were generally used for more severe RA and were introduced at earlier time-points in the later years of ERAS and in 25% of those who received any csDMARDs in ERAN.20 21 In ERAN, the most frequently used combinations of csDMARDs were MTX/SSZ, MTX/SSZ+hydroxychloroquine (HCQ) and MTX/HCQ.21 Only a small proportion of patients received bDMARDs, which were available from 2002 onwards (<2% by 1 year and <10% by 3 years).
Median time from RA symptom onset to first rheumatology outpatient visit (baseline assessment) was 6 months in both cohorts and to first csDMARD initiation 8 months in ERAS and 7 months in ERAN.
ILD identification
Comorbidities including respiratory disease were entered on the CRF at each visit. Death certification was received from the National Health Service (NHS) medical research information service and subsequently the NHS Health and Social Care Information Center for all recruited patients 4 monthly with last data inclusion for this analysis June 2018. The diagnosis of ILD at each centre was according to standard practice, with confirmatory evidence from standard investigations including pulmonary function tests, chest radiographs and HRCT scans. ILD was deemed to be present if the terms pulmonary fibrosis or ILD were listed on the CRF or the death certificate using international classification of diseases 10th revision (ICD-10) criteria.
As the development of RA-ILD is insidious, setting a time of onset had to be pragmatic. In cases where ILD was recorded on the baseline CRF, the time of diagnosis was taken as synchronous with RA onset (n=25). In cases where the first record of ILD was on a CRF, the date of onset was taken as then (n=52). Where the only record of ILD was on the death certificate, and not on the last CRF, the date taken as onset of ILD was recorded as last CRF +1 year if the time from last CRF to death was 2 years or less (n=5), and recorded as last CRF +2 years if this interval was more than 2 years (n=10).
MTX-exposed ILD group
Patients were included in the analysis as MTX-exposed ILD if they were recorded on the CRF as starting MTX at any time prior to the first record of ILD, either on the CRF or the death certificate.
Non-MTX-exposed ILD group
Patients were included in the analysis as non-MTX-exposed ILD if they were recorded as having ILD on the CRF without record of prior MTX treatment. As the analysis was concerned with the onset of ILD, patients who started MTX at any time point after ILD was first recorded on the CRF remained in the non-exposed group, as ILD was first diagnosed before MTX treatment. Patients who were recorded as having ILD on the death certificate but not on the last CRF were included in the non-MTX exposed group if the time interval between last CRF and death was less than 2 years, and no MTX treatment had been recorded on the CRFs throughout ERAS/ERAN data collection. As the development of RA-ILD is slow, it was considered that this was too short a time for any potential but unknown MTX use after the last CRF to have had an effect had it been introduced. If this time interval was 2 years or longer, patients were excluded from the analysis as they could have been exposed to MTX for the first time after last CRF and before the first record of ILD on the death certificate, and as such any potential MTX exposure during this time could have had an effect. Patients where the first record of ILD and MTX use was on the same CRF were considered non-MTX exposed as the maximum time MTX could have been used since the preceding CRF was 1 year, and this was considered too short to have had an effect on the development of ILD within the same period.
Statistical analysis
All analysis used statistical software Stata/IC V.15.1. The primary analyses included all cases of incident RA-ILD reported after any csDMARD exposure. An extended analysis was performed on the entire cohort of incident RA-ILD including patients where ILD was recorded at the baseline visit. These additional cases had developed ILD either preceding or synchronously with RA and prior to any csDMARD use for RA. First univariate analyses were performed for associations of baseline covariates with RA-ILD development. Next multivariate binary logistic models were fitted to the data to determine independent baseline predictors of RA-ILD. As there were multiple data collection points across the ERAS and ERAN follow-up period, multivariate time-varying analysis using Cox proportional hazards models were created to include multiple data entries for covariates with repeated measures. Finally, Cox regression time-to-event analysis was used to assess the relation between first RA symptoms and time of ILD diagnosis in MTX exposed and non-MTX exposed ILD cases and with respect to other baseline covariates.
A detailed description of the univariate and multivariate model analyses is given in online supplementary material.
Results
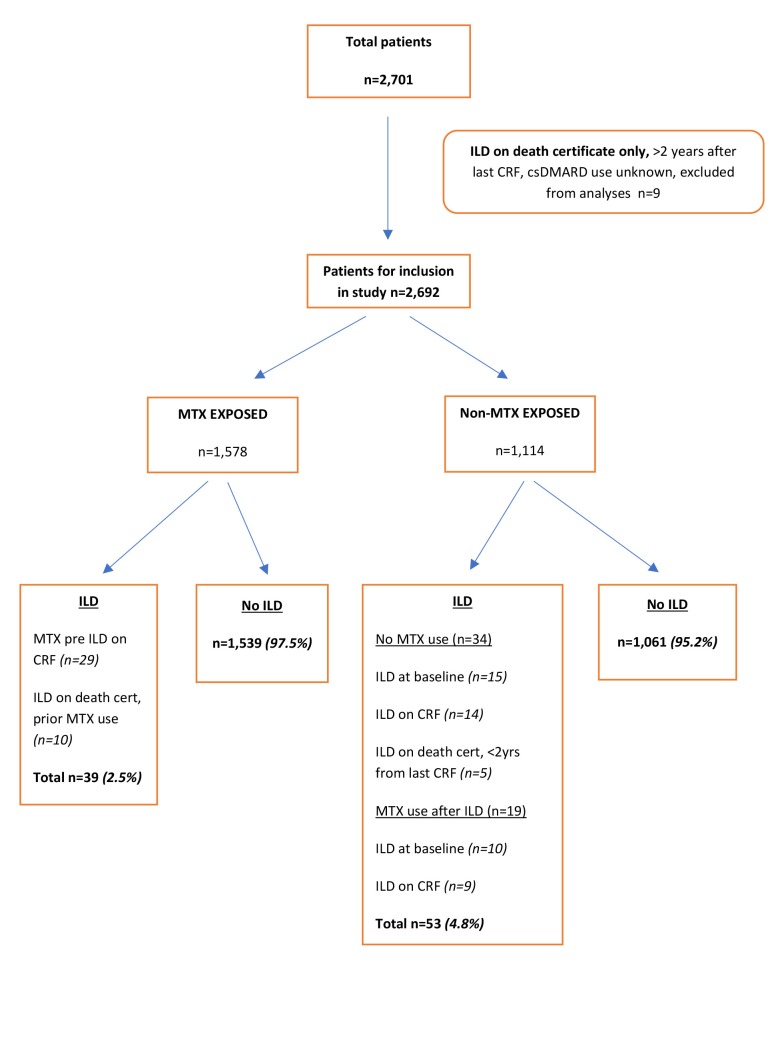
A flow chart of patient selection in shown in figure 1. From 2701 patients, a total of 101 cases of ILD were recorded (3.7%), of which 25 were present at baseline (25%). None of the baseline ILD cases had been treated with csDMARDs prior to first CRF. Nine ILD cases were excluded from analysis because the only record of ILD was on the death certificate and over 2 years had elapsed between this and the last CRF, during which time csDMARD treatment was unknown. There were 1578 MTX-exposed cases of whom 1539 (97.5%) were not and 39 (2.5%) were diagnosed with ILD, and 1114 non-MTX exposed cases of whom 1061 (95.2%) were not and 53 (4.8%) were diagnosed with ILD. Of the 53 non-MTX-exposed ILD cases, 19 (35%) were treated with MTX after ILD was diagnosed.
Figure 1.
Diagram showing patient selection and allocation to MTX-exposed and non-MTX-exposed groups. CRF, case report form; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; ILD, interstitial lung disease; MTX, methotrexate.
Demographic features of the ERAS and ERAN cohorts are shown in table 1. These were generally similar across both cohorts; however, there were significant differences in age of RA onset (older in ERAN), baseline smoking status (more current and ex smokers in ERAN) and MTX use (79% ERAN vs 41% ERAS). The prevalence of ILD was 3.2% in ERAN and 4.2% in ERAS (n.s.). The median dose of MTX across both cohorts was 12.5 mg, but following contemporary practice, this increased with time: ERAS 10 mg and ERAN 20 mg per week. Table 2 shows demographic features of MTX-exposed and non-MTX-exposed cases, where MTX-exposed patients were significantly more likely to have developed RA at a younger age, be in a higher DAS category, rheumatoid factor (RF) positive, nodular, male and borderline more likely to be current or ex-smokers. In the MTX-exposed cases, the median time from exposure to MTX to the first record of ILD was 45 months (ERAS 47 and ERAN 26 months). The mean DAS28 score at first record of RA-ILD in MTX-exposed cases was 3.77 and in non-MTX-exposed cases 4.27 (t-test p=0.30).
Table 1.
Demographic features of the ERAS and ERAN cohorts
| Number | Total | ERAS | ERAN | χ2 p value | ||||
| 2701 | 1465 | 1236 | ||||||
| Gender | Male | 893 | 33.1% | 492 | 33.6% | 401 | 32.4% | 0.530 |
| Female | 1808 | 66.9% | 973 | 66.4% | 835 | 67.6% | ||
| Age of RA onset | <55 | 1146 | 42.4% | 659 | 45.0% | 487 | 39.4% | 0.013 |
| 56–64 | 728 | 27.0% | 380 | 25.9% | 348 | 28.2% | ||
| 65+ | 827 | 30.6% | 426 | 29.1% | 401 | 32.4% | ||
| Baseline Smoker status | Never | 995 | 36.8% | 528 | 36.0% | 467 | 37.8% | <0.001 |
| Current | 594 | 22.0% | 179 | 12.2% | 415 | 33.6% | ||
| Ex-Smoker | 518 | 19.2% | 209 | 14.3% | 309 | 25.0% | ||
| Other | 26 | 1.0% | 26 | 2.1% | ||||
| Missing | 568 | 21.0% | 549 | 37.5% | 19 | 1.5% | ||
| MTX exposure | No | 1114 | 41.2% | 857 | 58.5% | 257 | 20.8% | <0.001 |
| Yes | 1578 | 58.4% | 602 | 41.1% | 976 | 79.0% | ||
| Missing | 9 | 0.3% | 6 | 0.4% | 3 | 0.2% | ||
| ILD diagnosis | No | 2600 | 96.3% | 1404 | 95.8% | 1196 | 96.8% | 0.206 |
| Yes | 101 | 3.7% | 61 | 4.2% | 40 | 3.2% | ||
ERAN, early rheumatoid arthritis network; ERAS, early rheumatoid arthritis study; ILD, interstitial lung disease; MTX, methotrexate; RA, rheumatoid arthritis.
Table 2.
Demographic features of MTX-exposed and non-MTX-exposed cases
| Total | Total | Non-MTX exposed | MTX exposed | χ2 p value | ||||
| 2692 | 1114 | 1578 | ||||||
| Gender | Male | 1804 | 67.0% | 721 | 64.7% | 1083 | 68.6% | 0.034 |
| Female | 888 | 33.0% | 393 | 35.3% | 495 | 31.4% | ||
| Age of RA onset | <55 | 1144 | 42.5% | 436 | 39.1% | 708 | 44.9% | <0.001 |
| 55–64 | 723 | 26.9% | 277 | 24.9% | 446 | 28.3% | ||
| 65+ | 825 | 30.6% | 401 | 36.0% | 424 | 26.9% | ||
| Baseline smoking status | Never | 991 | 36.8% | 346 | 31.1% | 645 | 40.9% | 0.058 |
| Current | 594 | 22.1% | 179 | 16.1% | 415 | 26.3% | ||
| Ex-smoker | 518 | 19.2% | 172 | 15.4% | 346 | 21.9% | ||
| Other | 25 | 0.9% | 2 | 0.2% | 23 | 1.5% | ||
| Missing | 564 | 21.0% | 415 | 37.3% | 149 | 9.4% | ||
| Baseline erosions | No erosions | 1883 | 69.9% | 808 | 72.5% | 1075 | 68.1% | 0.117 |
| Erosions | 699 | 26.0% | 276 | 24.8% | 423 | 26.8% | ||
| Missing | 110 | 4.1% | 30 | 2.7% | 80 | 5.1% | ||
| Baseline RF | Negative | 977 | 36.3% | 462 | 41.5% | 515 | 32.6% | <0.001 |
| Positive | 1633 | 60.7% | 628 | 56.4% | 1005 | 63.7% | ||
| Missing | 82 | 3.0% | 24 | 2.2% | 58 | 3.7% | ||
| Baseline nodules | None | 2515 | 93.4% | 1057 | 94.9% | 1458 | 92.4% | 0.010 |
| Nodules | 177 | 6.6% | 57 | 5.1% | 120 | 7.6% | ||
| Baseline DAS | <1.6 | 31 | 1.2% | 5 | 0.4% | 26 | 1.6% | <0.001 |
| 1.6–2.59 | 298 | 11.1% | 163 | 14.6% | 135 | 8.6% | ||
| 2.6–3.2 | 345 | 12.8% | 180 | 16.2% | 165 | 10.5% | ||
| >3.2–4.19 | 543 | 20.2% | 243 | 21.8% | 300 | 19.0% | ||
| 4.2–5.1 | – | – | – | – | – | – | ||
| >5.1 | 1415 | 52.6% | 503 | 45.2% | 912 | 57.8% | ||
| Missing | 60 | 2.2% | 20 | 1.8% | 40 | 2.5% | ||
DAS, disease activity; MTX, methotrexate; RA, rheumatoid arthritis.
As we were specifically interested in the relation of ILD onset to MTX exposure, the primary analysis was restricted to cases where ILD was only diagnosed after any csDMARD exposure (n=67), excluding 25 cases with ILD recorded at baseline. Univariate analyses of the relation between new diagnosis of RA-ILD and a range of binary and continuous variates are shown in online supplementary table 2a and 2b. This shows no association of MTX exposure and incident RA-ILD: OR 0.96, 95% CI 0.57 to 1.63, p=0.872. Male gender (p=0.034), RF positivity (p=0.01), ever smoking (p=0.004), rheumatoid nodules (p=0.005), age of RA onset (p=0.004) and baseline erythrocyte sedimentation rate (ESR) (p=0.014) were all significantly associated with incident RA-ILD. Longer time between first RA symptoms and the first outpatient appointment (p=0.053), respiratory comorbidities (p=0.056) and minor comorbidities (p=0.053) were borderline significant. Patients who developed ILD were at RA onset a mean 5.14 years older and had a mean baseline ESR score of 8.64 mm/hour higher than patients who did not develop ILD.
Table 3 shows the covariates independently associated with ILD diagnosis in the best fit multivariate model. This confirms higher age of RA onset, ever smoking, RF positivity and longer time from first RA symptom to first OPD visit were independently associated with incident RA-ILD, and there remained no evidence that MTX exposure was associated with RA-ILD onset (OR=0.85, 95% CI 0.48 to 1.49, p=0.578). Unlike univariate analysis in this model baseline major comorbidities (excluding respiratory) were protective. This group of conditions included malignancies, cardiac disease, other non-cardiac cardiovascular conditions (eg, hypertension and cerebrovascular disease), diabetes, thyroid disease, osteoarthritis, spinal disorders and gastrointestinal conditions as defined by ICD-10 criteria.
Table 3.
Multivariate logistic analysis showing covariates independently associated with RA-ILD development
| Primary analysis, RA-ILD onset after any csDMARD exposure (n=67) | Wald test | Extended cohort, including RA-ILD onset prior to any csDMARD (n=92) | Wald test | |
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Methotrexate exposure | 0.85 (0.49 to 1.49) | 0.578 | 0.48 (0.3 to 0.79) | 0.004 |
| Age RA onset | 1.04 (1.02 to 1.06) | <0.001 | 1.04 (1.02 to 1.06) | <0.001 |
| Smoking, ever, baseline | 2.21 (1.21 to 4.03) | 0.01 | 1.91 (1.13 to 3.25) | 0.016 |
| Male gender | 1.44 (0.83 to 2.48) | 0.193 | 1.74 (1.05 to 2.86) | 0.03 |
| RF positive, baseline | 2.02 (1.07 to 3.82) | 0.029 | n.s. | |
| RA nodules, baseline | n.s. | 2.19 (1.08 to 4.41) | 0.029 | |
| Onset − OPD | 1.04 (1.00 to 1.07) | 0.027 | 1.03 (1.0 to 1.07) | 0.04 |
| Baseline major comorbidities* | 0.62 (0.40 to 0.95) | 0.027 | 0.67 (0.46 to 0.98) | 0.037 |
| Baseline ESR | - | n.s. | 1.01 (1.0 to 1.02) | 0.047 |
Onset − OPD: time from first RA symptoms to first hospital out patient appointment.
Note: variables not reported did not reach statistical significance in the respective models.
*Excluding respiratory.
csDMARD, conventional synthetic disease-modifying antirheumatic drugs; RA-ILD, rheumatoid arthritis interstitial lung disease; RF, rheumatoid factor.
Extending the analysis to all 92 RA-ILD cases, including 25 recorded at baseline prior to any csDMARD use, produced similar results on univariate analysis (see online supplementary table 3a, 3b) with male gender (p=0.002), baseline positive RF (p=0.038), ever smoking (p=0.002), presence of rheumatoid nodules (p=0.003), age of RA onset (p<0.0001) and baseline ESR (p=0.001) all associated with incident RA-ILD. MTX exposure was associated with a significantly reduced OR of developing ILD (O.R. 0.51, 95% CI 0.32 to 0.79, p=0.001). Patients who developed ILD were at RA onset a mean 6.93 years older and had a mean baseline ESR score of 10.51 mm/hour higher than patients who did not develop ILD. In the multivariate model (table 3), higher age of RA onset, ever smoking, male gender, baseline rheumatoid nodules, higher baseline ESR and longer time from first RA symptom to first OPD visit were independently associated with incident RA-ILD. MTX exposure (O.R. 0.48, 95% CI 0.3, 0.79) and baseline major comorbidities (excluding respiratory) were associated with significantly reduced odds of RA-ILD onset.
As there was a large number of patients in ERAS with missing smoking status at baseline (n=549), a sensitivity analysis of the primary cohort was performed by running the multivariate analysis in smokers, non-smokers and those with missing smoking status data (see online supplementary table 4). This continued to show no association between MTX use and incident RA-ILD in smokers (OR=1.56, 95% CI 0.74 to 3.29, p=0.240) and in those with missing smoking data (OR=1.35, 95% CI 0.33 to 5.52, p=0.681), but MTX use was associated with a reduction in incident RA-ILD in non-smokers (OR=0.24, 95% CI 0.08 to 0.70, p=0.009).
The multivariate time-varying analysis, incorporating multiple data entries for covariates measured at each follow-up visit (eg, DAS, individual DAS components, health assessment questionnaire (HAQ), full details in online supplementary materials), resulted in similar covariate associations with incident RA-ILD. The best fit model (see online supplementary table 5) with the lowest Akaike information criterion score showed significant associations with age of RA onset (p=0.002), HAQ (p=0.007) and ESR (p=0.01) and continued to show no association with MTX exposure (HR 0.96, 95% CI 0.82 to 1.12, p=0.629).
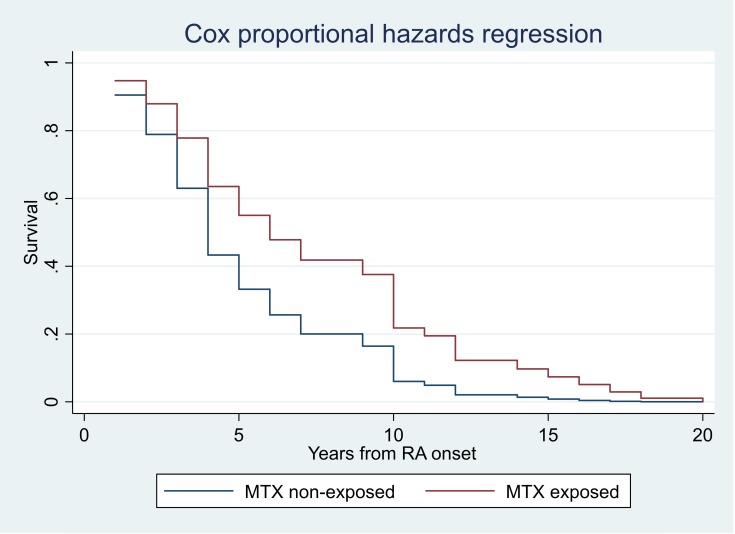
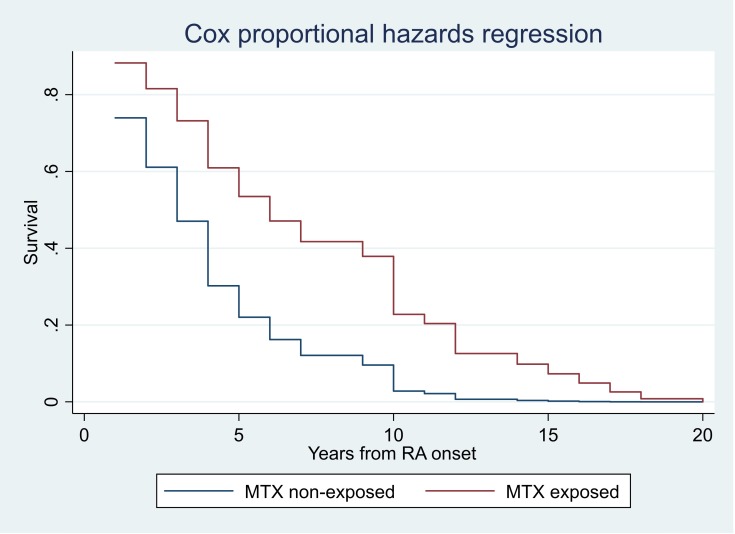
The relation between time to RA-ILD diagnosis after first RA symptoms in the MTX-exposed and non-MTX-exposed groups is shown in figure 2 (primary analysis) and figure 3 (extended cohort). The MTX-exposed ILD group included 10 cases where RA-ILD was only recorded on the death certificate and a mean 6.6 (range 3–11) years had elapsed between this and last CRF. For these cases, the time of ILD diagnosis was unknown and pragmatically was recorded as last CRF +2 years, introducing a bias to earlier record of time of RA-ILD diagnosis. The primary analysis, excluding 25 cases with RA-ILD diagnosed at baseline pre-csDMARD treatment, showed higher age of RA onset associated with earlier RA-ILD diagnosis (HR 1.03, 95% CI 1.0 to 1.06, p=0.048) and a non-significant trend for longer time to RA-ILD diagnosis in MTX exposed cases (HR 0.54, 95% CI 0.28, 1.06, p=0.072). The extended cohort analysis showed a significantly longer time to diagnosis of RA-ILD in MTX-exposed compared with non-MTX-exposed cases (HR 0.41, 95% CI 0.23 to 0.75, p=0.004) and the same effect of higher age of RA onset and earlier diagnosis (HR 1.03, 95% CI 1.0 to 1.06, p=0.028), but no influence of any of the other covariates independently associated with RA-ILD onset in the multivariate model (see table 4).
Figure 2.
Cox proportional time-to-event analysis showing time of onset of RA-ILD from first joint symptoms of RA in MTX-exposed and non-MTX-exposed groups. Primary analysis: cases with RA-ILD first recorded after any csDMARD exposure (n=67). csDMARD, conventional synthetic disease-modifying antirheumatic drug; MTX, methotrexate; RA-ILD, rheumatoid arthritis interstitial lung disease.
Figure 3.
Cox proportional time-to-event analysis showing time of onset of RA-ILD from first joint symptoms of RA in MTX-exposed and non-MTX-exposed groups. Extended cohort: all cases with RA-ILD including those diagnosed at baseline before any csDMARD exposure (n=92). csDMARD, conventional synthetic disease-modifying antirheumatic drug; MTX, methotrexate; RA-ILD, rheumatoid arthritis interstitial lung disease.
Table 4.
Cox regression time-to-event analysis showing associations of methotrexate exposure and baseline covariates with time from RA first symptoms to RA-ILD onset
| Primary analysis, RA-ILD onset after any csDMARD exposure (n=67) | Extended cohort, including RA-ILD onset pre any csDMARD (n=92) | P value | ||
| HR (95% CI) | P value | HR (95% CI) | ||
| Methotrexate exposure | 0.54 (0.28 to 1.06) | 0.072 | 0.41 (0.23 to 0.75) | 0.004 |
| Age RA onset | 1.03 (1 to 1.06) | 0.048 | 1.03 (1 to 1.06) | 0.028 |
| Smoking, ever, baseline | 1.09 (0.52 to 2.26) | 0.817 | 1.16 (0.61 to 2.22) | 0.654 |
| Male gender | 1.02 (0.51 to 2.03) | 0.966 | 0.85 (0.47 to 1.54) | 0.587 |
| RF positive, baseline | 0.96 (0.69 to 1.32) | 0.799 | 1.08 (0.85 to 1.38) | 0.512 |
| Onset – OPD | 0.98 (0.94 to 1.03) | 0.424 | 0.98 (0.94 to 1.02) | 0.276 |
| Baseline major comorbidities* | 1.26 (0.69 to 2.28) | 0.452 | 1.09 (0.63 to 1.9) | 0.762 |
Onset – OPD: time from first RA symptoms to first hospital outpatient appointment.
*Excluding respiratory.
csDMARD, conventional synthetic disease-modifying antirheumatic drug; RA-ILD, rheumatoid arthritis interstitial lung disease; RF, rheumatoid factor.
Discussion
We report an overall prevalence of RA-ILD of 3.7% in ERAS and ERAN, two large RA inception cohorts, recruiting between 1986 and 2012 with maximum follow-up of 25 years. These findings extend the earlier report of RA-ILD from the ERAS cohort alone8 and are in keeping with previous studies, including the UK BRILL network, which reported 2%–3% prevalence across its recruiting centres.10–12 ILD was already present at baseline assessment in 25 patients, representing 24.7% of the entire ILD group, these cases developing ILD either before or synchronously with first joint symptoms. This is similar to the UK BRILL cohort where 10% developed ILD before joint disease and 7% synchronously,10 and consistent with our earlier report from ERAS alone where ILD was present at baseline in 12/52 (23%) cases.8 Discrepancies may reflect the method of detection as demonstrated by Gabbay et al 9, who studied 36 patients with early RA and found abnormalities consistent with RA-ILD using a range of clinical, physiological and imaging modalities in 58%, but this was clinically significant in only 14%.
The results of the multvariate analysis concurred with other studies8–11 in finding an association of incident RA-ILD with increasing age of RA onset, ever smoking and positive RF in the primary analysis and also male gender, baseline rheumatoid nodules and ESR on the extended analysis. As was found with our earlier report from ERAS alone,8 there was no association in the primary analysis between MTX exposure and incident RA-ILD, either on univariate or multivariate analyses. On the contrary, MTX exposure was associated with significantly less RA-ILD in the extended analysis. This concurs with the meta analyses of RCTs by Conway et al, who found no association of MTX use and ILD in RA and non-RA inflammatory diseases,13 14 with the prospective 2-year study reported by Dawson et al of 128 RA patients with established disease,17 and a recent report from the same group in 106 patients with RA commencing MTX and followed for 10 years.26 The implication is to be especially vigilant for the development of RA-ILD in male patients who are RF positive, have nodules, a history of ever smoking and older age of RA diagnosis. MTX should only be witheld from RA patients with insufficient respiratory reserve to make it unlikely that they would survive hypersensitivity pneumonitis. Our findings refute concerns among clinicians that there is an association with MTX exposure and RA-ILD onset and provide no justification to delay or deny patients MTX for fear of inducing RA-ILD while seeking specialist opinions or further investigations of potential respiratory disease or other comorbid features. Such delays are likely to worsen RA outcomes by unnecessarily denying patients the anchor csDMARD for this disease. Reassurance of the benign effect of MTX on established RA-ILD comes from no association found between MTX and hospitalised severe ILD episodes in USA National Databank for Rheumatic Diseases27 and mortality from ILD in the USA Veterans Affairs Rheumatoid Arthritis Registry.28 Furthermore, a retrospective analysis of prognostic factors in 78 cases of RA-ILD, where MTX was specifically used as a therapeutic agent in 67%, found this to be strongly associated with survival.29
Of interest is the finding of a significant association between incident RA-ILD and increased time from RA symptom onset to first outpatient visit on multivariate analysis in both the primary analysis and extended cohort. Both of the other two measures of time to secondary care intervention, first RA symptom to first csDMARD and first outpatient appointment to first csDMARD, were consistent with this association, with the interval being a mean 3.42 and 2.56 months longer, respectively, in patients who subsequently developed ILD in the primary analysis. This is perhaps supportive of the so-called window of opportunity whereby a delay in treatment leads to worse outcomes. The explanation for the protective effect of baseline major comorbidities (excluding respiratory) on incident RA-ILD in both the primary analysis and the extended cohort is not immediately apparent. We speculate that this might be explained by treatment differences, for example, as malignancy was one of the more common major comorbidities, previous cancer therapies may have afforded immunosuppressive effects.
Interestingly, there was a trend for ILD to be less prevalent in the later 2002–2012 ERAN cohort (3.2%) than the 1986–2001 ERAS cohort (4.2%) based on a sample size of 101 cases. This is in contrast to a report of increasing prevalence over time among US veterans from 1985 to 2006, assumed due to increased awareness and investigation of respiratory symptoms coupled with increased survival.30 31 The reason for an apparent decrease in incident RA-ILD in ERAN is unclear; however, it is noteworthy that this was seen despite a significant increase in two exacerbating factors, age of RA onset and current smoking, in ERAN compared with ERAS. MTX use was higher in ERAN, raising the intriguing question whether MTX may have had a protective effect on ILD development despite the higher risk factors in ERAN. This is supported by the time-to-event analysis where MTX exposure was associated with a significantly longer time to RA-ILD onset in the extended analysis and a trend supporting this in the primary analysis with fewer cases. A protective effect of MTX could have been due to better overall RA disease control than in the non-MTX-exposed group, where the majority received SSZ and a minority HCQ or leflunomide.20 21 This is supported by the lower DAS28 score at first record of ILD in MTX-exposed compared with non-MTX-exposed cases, although the difference was not significant. A positive influence of MTX is also supported by Rojas-Serrano et al 29, who found a strong survival effect of MTX on established RA-ILD. Further evidence for an association between RA-ILD and worse disease control comes from the USA Rochester cohort followed up to 2006, where parameters indicative of more severe RA, such as ESR, nodules and destructive joint changes were associated with ILD.11 Similarly, in the UK BRILL cohort, anti-cyclic citrullinated peptide (anti-CCP) antibodies showed the strongest association with ILD,10 this being a recognised marker of disease severity. Unfortunately, there were insufficient numbers of ERAS/ERAN patients with baseline anti-CCP to assess the impact of this on ILD. However, we found higher baseline ESR to have a significant association with incident RA-ILD on univariate and multivariate analysis, this being mean 8.64 mm Hg higher in patients who developed ILD in the primary analysis. Although the ERAS/ERAN cohorts were not designed to compare treatment effects, the conclusion from our findings is that MTX has no association with the development of RA-ILD and may lead to a delayed onset and lower incidence of RA-ILD perhaps as a consequence of better overall RA disease control or specific lung-mediated immune suppression.
Strengths and limitations of this study
The strength of this study is inherent in the nature of ERAS and ERAN, two real-world large inception early RA cohorts, recruiting all-comers, treated according to contemporary best practice, with the rigour of regular standardised assessments and data collection allowing data to be pooled and analysed collectively. In contrast to RCTs, the data from ERAS and ERAN are not restricted to defined RA populations with strict inclusion and exclusion criteria, nor to treatment strategies confined by protocol. ERAS and ERAN are also unique in size recruiting 2701 patients compared for example to 582 in the Rochester cohort,11 and in the long duration of follow-up, adding to the strength of these analyses. The primary analysis of 67 incident RA-ILD cases allowed us to focus on the association with MTX by excluding cases with ILD occurring before any csDMARD use.
It is possible that treatment decisions, being at the discretion of each centre, were influenced by channelling bias, whereby patients perceived to be at higher risk of RA-ILD, such as those with lung disease, might have been excluded from MTX exposure. However, there was no difference in MTX exposure between those with and without baseline respiratory comorbidities, and MTX-exposed patients were more likely to be current and ex-smokers (table 2) so this seems unlikely. We have assumed that all cases of ILD occurring at baseline were RA related. This would seem the most likely aetiology, especially in those where the onset was synchronous with joint disease, but potentially other causes might have explained ILD. As ERAS closed to follow-up in 2011 and ERAN in 2013, it is possible that new cases of RA-ILD were missed after last CRF and predeath. However, we have reported RA-ILD survival to be a median of 3 years (5-year survival 38.8%) in ERAS8 and with last CRF 2011–2013 and latest death certification reports included up to June 2018, it is not likely that many new cases in this period have been missed. Nonetheless, we have had to exclude nine RA-ILD cases from analysis, as we could not confirm that they remained non-MTX exposed, given lack of follow-up data between last CRF +2 years and death when RA-ILD was first notified. Our incident RA-ILD cases available for analysis are therefore lower than reality. A further limitation of the data is the lack of external confirmation of ILD case verification; this being dependent on the reporting of ILD by each centre on the CRF, or the doctor completing the death certificate. While the diagnosis of ILD is strongly influenced by investigations, with incrementally increasing detection from clinical signs to pulmonary function tests and HRCT images, and much subclinical disease being present,5 9 we believe that the specific diagnostic features of ILD and thoroughness of clinical work up by recruiting centres were sufficient to have confidence in the accuracy of ILD reporting. Furthermore, credibility of ILD reporting in ERAS/ERAN is gained from the prevalence being in keeping with other cohorts where it was possible to independently verify the diagnosis for each case.9–12 Another limitation is that smoking status was missing in a large proportion of ERAS patients, because its importance was not appreciated at the time data were collected in the 1980s. However, the sensitivity analysis, running the multivariate model stratified by smoking status at baseline did not change the lack of association between MTX and RA-ILD onset.
In conclusion, we report a prevalence of RA-ILD in the ERAS/ERAN cohorts of 3.7% with independent significant incident associations in line with other studies, namely older age of RA onset, ever smoking, nodules, RF positivity, male gender and high ESR. We also show a significant association of incident RA-ILD with a longer time from first RA symptoms to secondary care intervention supporting the ‘window of opportunity’. We have found no association between MTX treatment and incident RA-ILD and on the contrary provide evidence suggestive that MTX-exposed patients with RA may have a delayed onset of ILD. There seems no reason to confuse the association of MTX and hypersensitivity pneumonitis with the onset of RA-ILD. Assuming baseline lung function is sufficient to withstand an episode of hypersensitivity pneumonitis, there are no other respiratory contraindications to the use of this very effective ‘anchor’ csDMARD in patients with RA.
Key messages
In early rheumatoid arthritis study/early rheumatoid arthritis network, incident rheumatoid arthritis interstitial lung disease (RA-ILD) is significantly associated with older age of rheumatoid arthritis (RA) onset, ever smoking, nodules, rheumatoid factor positivity, male gender, ESR and a longer time from first RA symptoms to first secondary care visit.
There is no association between incident RA-ILD and methotrexate (MTX) treatment.
MTX may have a protective role in delaying the onset of RA-ILD.
Supplementary Material
Acknowledgments
All early rheumatoid arthritis study (ERAS) and early rheumatoid arthritis network (ERAN) recruiting centres. National Rheumatoid Arthritis Society for patient involvement in conduct of ERAN.
Footnotes
Twitter: @pkiely500 @ElenaNikiUK
Contributors: PK: conception of work, data interpretation and manuscript drafting. ADB and KS: statistical analysis, data interpretation and manuscript revision. EN and AY: statistical support, data interpretation and manuscript revision. DAW, PC and JD data interpretation and manuscript revision.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Ethics approval: Ethical approval was obtained from East Hertfordshire local research ethics committee (ERAS) and the Trent research ethics committee (ERAN).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. 10.1136/annrheumdis-2016-210715 [DOI] [PubMed] [Google Scholar]
- 2. Sokka T, Kautiainen H, Toloza S, et al. QUEST-RA: quantitative clinical assessment of patients with rheumatoid arthritis seen in standard rheumatology care in 15 countries. Ann Rheum Dis 2007;66:1491–6. 10.1136/ard.2006.069252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol 2011;108:1362–70. 10.1016/j.amjcard.2011.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis 2009;68:1100–4. 10.1136/ard.2008.093690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Imokawa S, Colby TV, Leslie KO, et al. Methotrexate pneumonitis: review of the literature and histopathological findings in nine patients. Eur Respir J 2000;15:373–81. 10.1034/j.1399-3003.2000.15b25.x [DOI] [PubMed] [Google Scholar]
- 6. Young A, Koduri G, Batley M, et al. Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology 2007;46:350–7. 10.1093/rheumatology/kel253 [DOI] [PubMed] [Google Scholar]
- 7. Iqbal K, Kelly C. Treatment of rheumatoid arthritis-associated interstitial lung disease: a perspective review. Ther Adv Musculoskelet Dis 2015;7:247–67. 10.1177/1759720X15612250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koduri G, Norton S, Young A, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology 2010;49:1483–9. 10.1093/rheumatology/keq035 [DOI] [PubMed] [Google Scholar]
- 9. Gabbay E, Tarala R, Will R, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med 1997;156(2 Pt 1):528–35. 10.1164/ajrccm.156.2.9609016 [DOI] [PubMed] [Google Scholar]
- 10. Kelly CA, Saravanan V, Nisar M, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics--a large multicentre UK study. Rheumatology 2014;53:1676–82. 10.1093/rheumatology/keu165 [DOI] [PubMed] [Google Scholar]
- 11. Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 2010;62:1583–91. 10.1002/art.27405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richman NC, Yazdany J, Graf J, et al. Extraarticular manifestations of rheumatoid arthritis in a multiethnic cohort of predominantly Hispanic and Asian patients. Medicine 2013;92:92–7. 10.1097/MD.0b013e318289ce01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conway R, Low C, Coughlan RJ, et al. Methotrexate and lung disease in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheumatol 2014;66:803–12. 10.1002/art.38322 [DOI] [PubMed] [Google Scholar]
- 14. Conway R, Low C, Coughlan RJ, et al. Methotrexate use and risk of lung disease in psoriasis, psoriatic arthritis, and inflammatory bowel disease: systematic literature review and meta-analysis of randomised controlled trials. BMJ 2015;350:h1269 10.1136/bmj.h1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beyeler C, Jordi B, Gerber NJ, et al. Pulmonary function in rheumatoid arthritis treated with low-dose methotrexate: a longitudinal study. Br J Rheumatol 1996;35:446–52. 10.1093/rheumatology/35.5.446 [DOI] [PubMed] [Google Scholar]
- 16. Khadadah ME, Jayakrishnan B, Al-Gorair S, et al. Effect of methotrexate on pulmonary function in patients with rheumatoid arthritis--a prospective study. Rheumatol Int 2002;22:204–7. 10.1007/s00296-002-0227-6 [DOI] [PubMed] [Google Scholar]
- 17. Dawson JK, Graham DR, Desmond J, et al. Investigation of the chronic pulmonary effects of low-dose oral methotrexate in patients with rheumatoid arthritis: a prospective study incorporating HRCT scanning and pulmonary function tests. Rheumatology 2002;41:262–7. 10.1093/rheumatology/41.3.262 [DOI] [PubMed] [Google Scholar]
- 18. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- 19. Nikiphorou E, Carpenter L, Morris S, et al. Hand and foot surgery rates in rheumatoid arthritis have declined from 1986 to 2011, but large-joint replacement rates remain unchanged: results from two UK inception cohorts. Arthritis Rheumatol 2014;66:1081–9. 10.1002/art.38344 [DOI] [PubMed] [Google Scholar]
- 20. Young A, Dixey J, Williams P, et al. An evaluation of the strengths and weaknesses of a register of newly diagnosed rheumatoid arthritis, 1986-2010. Rheumatology 2011;50:176–83. 10.1093/rheumatology/keq318 [DOI] [PubMed] [Google Scholar]
- 21. Kiely P, Williams R, Walsh D. Young A for the Early Rheumatoid Arthritis Network (ERAN). Contemporary patterns of care and disease activity outcome in early rheumatoid arthritis; the ERAN cohort. Rheumatology 2009;48:57–60. [DOI] [PubMed] [Google Scholar]
- 22. Van der Heijde DM. van’ t Hof M, van Riel PL, van de Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol 1993;20:579–81. [PubMed] [Google Scholar]
- 23. Prevoo ML. van’ t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 24. van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum 1998;41:1845–50. [DOI] [PubMed] [Google Scholar]
- 25. Joint working group of British Society of Rheumatology, & Royal College of Physicians: guidelines and audit measures for the specialist supervision of patients with rheumatoid arthritis. J Roy Coll Phys Lond 1992;26:76–82. [PMC free article] [PubMed] [Google Scholar]
- 26. Dawson JK, Earnshaw BAM, Rahiman I, et al. 246 No evidence that pulmonary fibrosis is a complication of long term methotrexate use: 10 year follow up data of patients treated with methotrexate for inflammatory arthritis. Rheumatology 2018;57:246 10.1093/rheumatology/key075.470 28541488 [DOI] [Google Scholar]
- 27. Wolfe F, Caplan L, Michaud K. Rheumatoid arthritis treatment and the risk of severe interstitial lung disease. Scand J Rheumatol 2007;36:172–8. 10.1080/03009740601153774 [DOI] [PubMed] [Google Scholar]
- 28. England BR, Sayles H, Michaud K, et al. Chronic lung disease in U.S. Veterans with rheumatoid arthritis and the impact on survival. Clin Rheumatol 2018;37:2907–15 10.1007/s10067-018-4314-9 10.1007/s10067-018-4314-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rojas-Serrano J, Herrera-Bringas D, Pérez-Román DI, et al. Rheumatoid arthritis-related interstitial lung disease (RA-ILD): methotrexate and the severity of lung disease are associated to prognosis. Clin Rheumatol 2017;36:1493–500. 10.1007/s10067-017-3707-5 [DOI] [PubMed] [Google Scholar]
- 30. Bartels CM, Bell CL, Shinki K, et al. Changing trends in serious extra-articular manifestations of rheumatoid arthritis among United State veterans over 20 years. Rheumatology 2010;49:1670–5. 10.1093/rheumatology/keq135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O’Dwyer DN, Armstrong ME, Cooke G, et al. Rheumatoid Arthritis (RA) associated interstitial lung disease (ILD). Eur J Intern Med 2013;24:597–603. 10.1016/j.ejim.2013.07.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-028466supp001.pdf (260.9KB, pdf)